Kaolinitic clays as a potential source of dioxins in the Noosa River catchment, Queensland, Australia
Suzanne Vardy A * , Jacob Gruythuysen B and Brenda Baddiley
A * , Jacob Gruythuysen B and Brenda Baddiley  A
A
A Water Quality and Investigations, Science and Technology Division, Department of Environment and Science, Yuggera, Ecosciences Precinct, GPO Box 2454, Brisbane, Qld 4001, Australia.
B Aquatic Ecosystem Health, Science and Technology Division, Department of Environment and Science, Yuggera, Ecosciences Precinct, GPO Box 2454, Brisbane, Qld 4001, Australia.
Environmental Chemistry 19(1) 1-12 https://doi.org/10.1071/EN21163
Submitted: 22 December 2021 Accepted: 17 February 2022 Published: 6 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Dioxins have been found along the east coast of Australia in agricultural areas where there is no obvious source of dioxins. These dioxins have an unusual signature that does not match common industrial sources, but it has been suggested that they may be associated with pesticide use. This study found a strong correlation between dioxins with this unique signature and the amount of a kaolinitic clay in the sediments sampled.
Abstract. The presence, concentrations and profiles of 2,3,7,8-substituted polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in sediment were investigated in this study with the aim of determining whether dioxin-like compounds were present and, if found, a likely source could be deduced. The sampled catchment lies within an area of high conservation value. Sediments from seven lake sites were sampled as possible sinks for any contamination from historical pesticide use. PCDD/Fs were measured in all the lake sediments. The 2,3,7,8-substituted congener profile was dominated by octachlorodibenzo-p-dioxin (OCDD) with furans at or below the limit of detection, a profile that has been associated with kaolinitic clays from around the world. A strong and significant correlation was found between the total dioxin concentration and the percentage of kaolinitic clay in the sediments. The lack of furans in the 2,3,7,8-substituted PCDD/F profile indicates pesticides or pentachlorophenol (PCP) are unlikely to be the source in the catchment. Further, the concentration of total dioxins and OCDD tended to be higher than those measured outside the study area, even though, overall, the study area is likely to have had less pesticide use than the other intensive agricultural areas previously studied. The results presented in this paper indicate that caution should taken when attributing the presence of dioxins in soil and sediment to anthropogenic sources.
Keywords: dioxins, furans, kaolinitic clay, Noosa Catchment, pentachlorophenol, pesticides, sediment.
Introduction
Much attention has been placed on the sources of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) found in soils and marine sediments from the eastern coast of Queensland (Müller et al. 1999; Gaus et al. 2001; Hermanussen et al. 2004; Camenzuli et al. 2015). A characteristic profile of 2,3,7,8-substitued polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (hereafter referred to as PCDD/Fs) has previously been identified along the eastern coast of Queensland, a mostly agricultural landscape with few obvious anthropogenic sources of these compounds. Müller et al. (1999) undertook one of the first systematic studies of dioxin-like compounds in marine sediments in Queensland and reported that PCDDs were detected in all samples. As with the riverine sediments, the marine samples also showed a characteristic PCDD/F congener profile. The marine profiles were dominated by the octachlorodibenzodioxin (OCDD) congener, with furans at or below the limit of detection.
Prior to these studies, and because Queensland is predominantly non-industrial in its land use (e.g. agricultural), it was assumed that no significant sources of dioxins would be found away from the highly urbanised south-east and the state’s capital Brisbane, and therefore, dioxins would be at very low levels. However, the concentrations of OCDD found in soil and sediment samples along the Queensland coast indicated a significant source of dioxin-like compounds, either historic or recent (anthropogenic) (Müller et al. 1999; Gaus et al. 2001). The elevated OCDD concentrations and the distinct congener pattern identified were apparently confined to the coastal region, with lower concentrations of OCDD measured in inland Australia, west of the Great Dividing Range (Prange et al. 2002). Elevated levels of dioxin-like compounds have also been measured in marine sediment of the Great Barrier Reef (GBR) Lagoon, with OCDD concentrations decreasing with increasing distance from each river mouth (Gaus et al. 2001) indicating the source of dioxin contamination was land-based. The characteristic dioxin profile has also been found in sediment cores dated to pre-European settlement (Gaus et al. 2001).
Various hypotheses have been put forward to explain the elevated dioxin levels and the characteristic dioxin congener pattern in Queensland. The most obvious source of the PCDD/F in regional areas, bushfires, has been ruled out (Prange et al. 2003). PCDD/F contamination of soil and sediment from the historical use of pesticides has also been suggested as an explanation for the elevated levels of OCDD found in eastern coast agricultural areas of Queensland (Camenzuli et al. 2013). In particular, PCDD/F contamination associated with the use of pentachlorophenol (PCP) in Queensland has been theorised as a source of the unknown dioxin contamination (Gaus et al. 2002a), as the PCDD/F signature associated with PCP is similar to the signature found in kaolinitic clay. Unfortunately, records of the use of PCP in agriculture in Queensland are not available (Camenzuli et al. 2013). The PCDD/F pattern in sediments and soils from Queensland resembles those found in kaolinitic clays elsewhere in the world (Prange et al. 2002; Horii et al. 2008, 2011; Schmitz et al. 2011).
The present study area lies in the Noosa River catchment, a southern coastal region of the State of Queensland, Australia. It is a catchment with a large proportion of high conservation value land use and a history of forestry that employed herbicidal weed control. Pesticides of historical concern include 2,4,5-T, which has been banned in Australia since the late 1980s, and other chlorinated herbicides (i.e. 2,4-D and PCP). The association between dioxin-like compounds and pesticide use has caused concern to the local community, particularly around the potential for contamination of waterways.
Between 2020 and 2021, a study was undertaken with the aims of (1) determining whether dioxin-like compounds were present in the sediment of lakes of the Noosa River catchment, as potential sinks of contamination, and (2) if present, to assess the source of these dioxin-like compounds.
Methods
Study area
The Noosa River has its headwaters in the Great Sandy National Park and flows through, or is connected to, a number of lakes until it reaches the mouth at Noosa Heads. The coastal catchment is predominantly covered with conservation and natural areas (79%), with 5% of the catchment currently used for forestry and 2% for agriculture, although more forestry used to occur in the upper catchment. The remaining 14% is dominated by urban land use (Fig. 1). Five sites in the Noosa River catchment were chosen for sediment sampling (two sites in Lake Cootharaba, one site in each of Lakes Cooroibah, Doonella and Weyba) as well as two control sites in the Great Sandy National Park (Lakes Coolamera and Poona). Lakes were chosen as they are likely to be sinks for contaminants. Lake Weyba is connected to the Noosa River at the downstream end of the river. There is no history of significant agriculture or forestry in the Lake Weyba area, although logging did occur in the vicinity historically. Extensive bushfires occurred around Lakes Cootharaba, Cooroibah and Weyba in 2019.
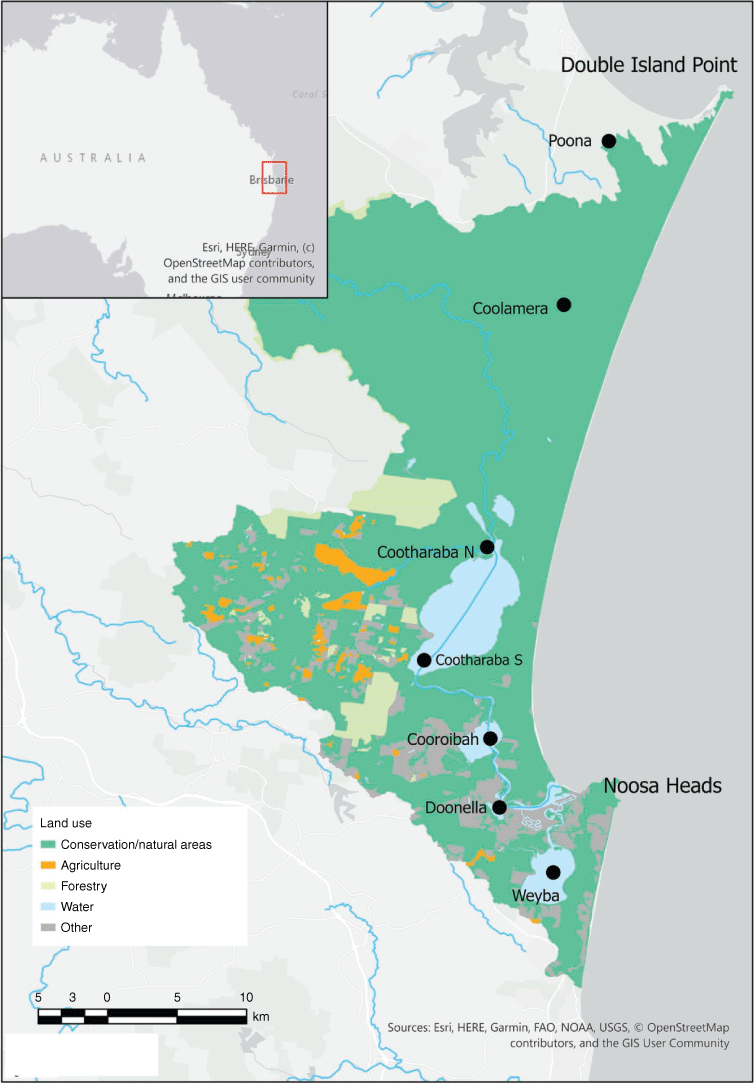
|
Sampling
Sediment sampling occurred in February and November 2020 and April 2021. Sediments were collected in the same manner as outlined in the National Dioxin Program (Müeller et al. 2004). Equipment was washed with Decon 90 and Milli-Q water and wiped with lab-grade methylated spirits before each site was sampled. Ten sub-samples were collected at each site using a standardised aluminium coring device (15 cm length, 2.8 cm diameter). These were collected in a triangular formation and were approximately 25 m apart. After collection, the sub-samples were composited by mixing thoroughly with a stainless steel spoon in a stainless steel bowl, and then placed in laboratory-provided sampling containers and bags for analysis. Samples were double-bagged and placed on ice for transport to the laboratories.
For quality control, samples were split into three at three sites in February 2020 (Cootharaba North, Cootharaba South and Weyba) and two sites in April 2021 (Cootharaba North and Weyba). Of these, blind duplicates were sent to the main analytical laboratory, Australian Laboratory Services (ALS), and the third sub-sample sent to a secondary laboratory, National Measurement Institute (NMI) (see Supplementary material for results). Analyses for dioxin-like compounds, clay identification, quantitative X-ray diffraction (XRD), particle size distribution (PSD) and total organic carbon (TOC) were undertaken during three separate sampling runs. The analyses performed on samples from each run are indicated below (Table 1).

|
Dioxin analysis
Samples were solvent-extracted by accelerated solvent extraction (ASE) for soils (based on US Environmental Protection Agency (USEPA) Method 3545). Sample extracts were then concentrated, and subjected to a series of chromatographic clean-ups, including acid gel clean-up, Florisil clean-up and alumina clean-up (based on USEPA Method 1613) to remove co-extracted organics such as hydrocarbons and pesticides. Sample extracts were analysed by gas chromatography/high-resolution mass spectrometry (GC/HRMS) using an Agilent 6890 gas chromatograph and a Waters Autospec Ultima Magnetic Sector mass spectrometer) and were quantitated by isotope dilution. Specifically, isotopically labelled versions of 15 of the 17 dioxins and furans analysed were added to the sample prior to extraction, and these compounds were used to quantify the native compounds present in the sample (based on USEPA Method 1613).
Particle size analysis
This method was developed in-house by the Department of Environment and Science Chemistry Centre (based on Thorburn and Shaw 1987). Sediment samples were disaggregated in an aqueous solution by means of chemical reagents and mechanical dispersion. Sediment was shaken with water containing sufficient Calgon (sodium hexametaphosphate) to separate soil peds into their constituent particles. Primary particle sizes are classified according to four ranges defined as follows: coarse sand, 200–2000 µm; fine sand, 20–200 µm; silt, 2–20 µm and clay, <2 µm. Coarse and fine sand fractions were determined gravimetrically, and the silt and clay fractions were determined using a hydrometer.
Total organic carbon
Total organic carbon (TOC) in sediment samples was analysed using a LECO TruMac automated analyser. The carbon content of the sample was determined by analysing the quantity of CO2 produced by combustion via an infrared detection system.
Mineralogical analysis
Sub-samples were accurately weighed, and specimens were prepared for XRD analysis by the addition of a corundum (Al2O3) internal standard at 20 wt-%. The specimens were micronised in a McCrone XRD-mill using zirconium oxide beads and ethanol, then dried in an oven overnight at 40°C. The resultant homogeneous powders were back-pressed into sample holders. A small portion of the crushed samples was dispersed in water. After sonication (5 min) and settling for 5 min, the fine fraction (nominally <5 μm in suspension) was transferred via pipette to a low-background plate and allowed to settle and dry. Step-scanned X-ray diffraction patterns were collected for 1 h per sample using a Bruker D8 Advance powder diffractometer and cobalt Kα radiation operating in Bragg–Brentano geometry. The collected data were analysed using JADE (V2010, Materials Data Inc.), EVA (V5, Bruker) and X’Pert Highscore Plus (V4, PANalytical) with various reference databases (PDF4+, AMCSD, COD) for phase identification. Rietveld refinement was performed using TOPAS (V6, Bruker).
Results and discussion
Dioxins were measured in sediment in all the sampled Noosa River lakes. The highest concentrations were measured at Lake Weyba in both 2020 and 2021 (Table 2), with the sum of all dioxin and furan homologues being 8794 and 18 879 pg g−1 dry weight (dw), respectively. Of the total 2,3,7,8-substituted PCDD/Fs congeners, OCDD was the dominant dioxin, ranging between 89 and 91% of the total. OCDD was measured between 1210 pg g−1 dw (WHO-TEQ) 0.36 pg g−1 dw) (Lake Doonella) and 9895 pg g−1 dw (i.e. WHO-TEQ = 2.97 pg g−1 dw) (Lake Weyba) (Table 2). WHO-TEQ refers to the World Health Organization toxic equivalence which expresses the toxicity of OCDD in terms of the most toxic dioxin 2,3,7,8-tetrachloro-p-dioxin (TCDD). The concentrations of OCDD congener from the sediments collected in the Noosa River catchment lakes are generally higher than those reported in sediments from agricultural areas throughout the state. Müller et al. (1999) reported OCDD concentrations in marine and estuarine sediment adjacent to intensive agriculture in Queensland ranging between 14 and 2860 pg g−1 dw (i.e. WHO-TEQ 0.0042–0.804 pg g−1 dw), and in marine sediments from the mouths of rivers in rural areas ranging between 49 and 1200 pg g−1 dw (i.e. WHO-TEQ 0.015 and 0.36 pg g−1 dw). All congener and homologue results are presented in the Supplementary material.
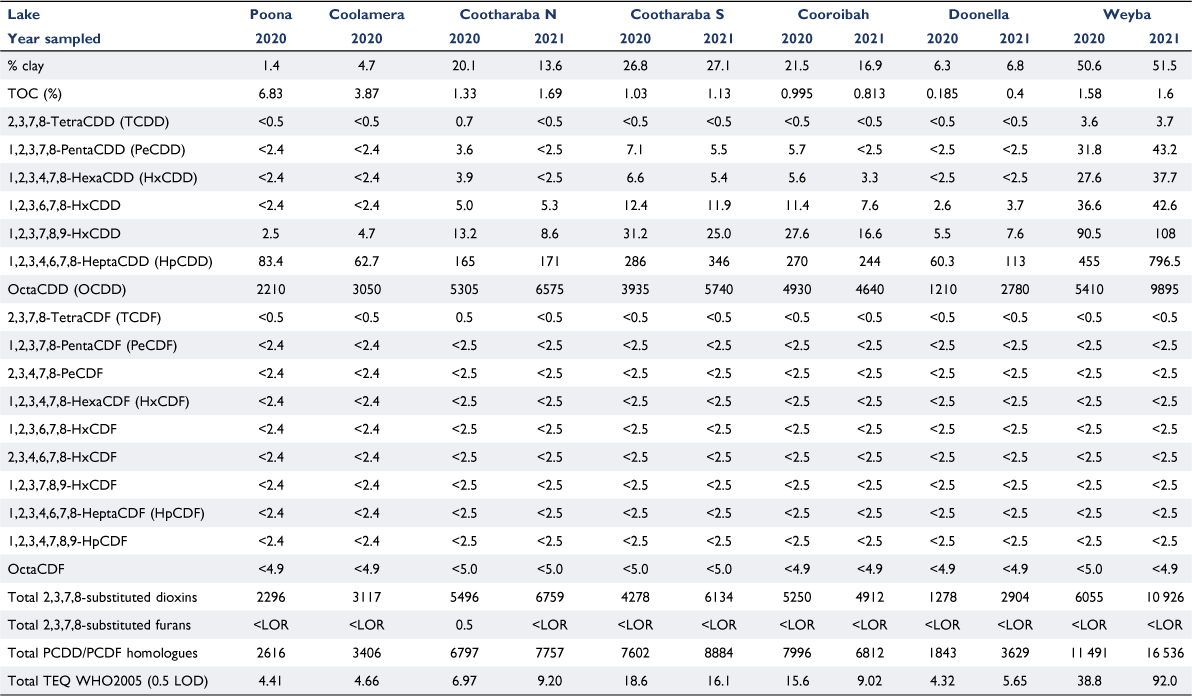
|
Dioxin-like compounds have low solubility in water and bind strongly to particulate organic matter (Gatehouse 2004), and these properties play an important role in the transportation, distribution and sorption of dioxin-like compounds (Baran et al. 2020 and reference cited within). The two sites with the highest organic carbon concentration had the lowest PCDD/F concentrations. No correlation was found between TOC and total PCDD/PCDF concentration in the sediment of the Noosa River lakes (Fig. 2a), but there was a significant (P < 0.05) and strong (0.95) Pearson correlation between total PCDD/PCDF concentration and kaolinite (Fig. 2b). The dominant clays in the Noosa River lake sediments were kaolinite and illinite/mica (Table 3). Total PCDD/PCDF concentrations were compared with percentage of kaolinite instead of individual 2,3,7,8-congeners. Horii et al. (2011) noted that specific congener profiles with predominant 1,4,6,9-substitutions were found kaolin clays, and by looking at total PCDD/PCDF, these additional congeners (not individually analysed for in the present study) are accounted for.

|

|
The congener profile associated with PCP use is reported to be similar to that found in kaolinitic clays, particularly in respect to the lack of PCDFs (Fig. 3; Hoogenboom et al. 2020). Records on the amount of PCP used in Queensland, and for what purpose, are limited. Dioxin-like compounds are associated with PCP in two ways: dioxins may be formed during the manufacture of PCP, or PCP itself may be a dioxins precursor (Holt et al. 2008). Gaus et al. (2002b) hypothesised that the presence of dioxin-like compounds with the Queensland profile in deep marine sediments off the coast of Queensland may be due to surfactant-mediated transport of PCP through the sediment profile, and then in situ conversion of the PCP to dioxins. Although the profiles of dioxins associated with PCP and kaolinitic clays are reported to be similar, there is no evidence PCP are the source of contamination in kaolinitic clays worldwide. A study of carbon isotopes undertaken by Horii et al. (2008) indicated a distinct difference between dioxins formed through anthropogenic processes and those found in kaolinitic clays. Further, studies into commercial PCP found that the PCDD/PCDF ratio is between 1 and 82 (Rappe and Andersson 2000 and references cited within; Masunaga et al. 2001) indicating a high contribution of PCDFs to the total dioxin concentration, and that the dominant HxCDD is 1,2,3,6,7,8-HxCDD (Müller et al. 1999; Rappe and Andersson 2000 and references cited within).

|
Data obtained from the Noosa River lakes sediments were compared with the dioxin congener patterns of kaolinitic clays from the USA and Japan (Fig. 4), as well as patterns associated with pesticides known to have been or currently used in the catchment (i.e. 2,4,5-T and 2,4-D) (Fig. 5). Each toxic congener was expressed as the contribution to total TEQ in order to overcome the dominance of the OCDD congener (Hoogenboom et al. 2020). Neither the 2,4,5-T/2,4-D nor any of the 2,4-D dioxin TEQ congener profiles matched those found in the sediment in the Noosa River lakes, but the TEQ congener profile from Lake Weyba in the Noosa River was similar to that found kaolinitic clays from Japan and the USA (Horii et al. 2011) (Fig. 4).

|

|
To assess the potential for the dioxins in the Noosa River lakes sediment to have come from PCP use, a local source of PCDD/F contamination associated with the likely use of PCP was investigated. Data were obtained from a dioxin soil study from an abandoned sawmill (data provided by Noosa Shire Council). PCP is known to have been used as a fungicide in the wood industry in Australia and around the world, with the environment surrounding sawmills commonly contaminated with dioxin-like compounds. Although the dioxin fingerprint from this sawmill had some similarities to the reported congener pattern associated with kaolinitic clay (Table 4), the presence of a range of furans was remarkably different to the congener pattern identified in the Noosa River lakes sediment (Figs 4, 5). In addition, in the sawmill samples, 1,2,3,6,7,8-HxCDD was not dominant as reported for the ‘natural formation pattern’, but it was measured in approximately the same concentrations as 1,2,3,7,8,9-HxCDD (Ferrario et al. 1997; Gadomski et al. 2002; Horii et al. 2009, 2010, 2011; Schmitz et al. 2011) (Table 4). In contrast, in the Noosa River lakes sediment samples, 1,2,3,7,8,9-HxCDD concentration was 2–4 times higher than that of 1,2,3,6,7,8-HxCDD (Table 5).

|
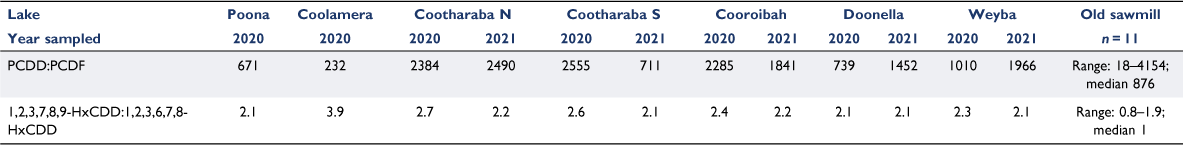
|
There is evidence for the natural formation of dioxin-like compounds in clays, although the actual mechanism is still unknown. The natural formation pattern of dioxin-like compounds has been found in sediment and soils that have been dated to more than 48 000 years (Holmstrand et al. 2006), in areas where there are no known PCDD/F sources. This has led to the hypothesis that an unknown geologic process has caused the formation of dioxin-like compounds in kaolinite (Schmitz et al. 2004). Schmitz et al. (2011) found that total dioxin and furan concentrations measured from in situ kaolin were low to non-detectable; however, they were higher in sedimentary kaolinitic and lignitic clays, indicating that the dioxin-like compounds are formed during transportation and sedimentation. Chlorine isotope analysis of OCDD and black carbon analysis undertaken on Mississippi ball clays indicated an abiotic and non-pyrogenic origin of the kaolinite-associated dioxins (Holmstrand et al. 2006). In situ mineral-catalysed synthesis of dioxin-like compounds has been proposed to explain the presence of dioxins in kaolinite. OCDD was produced when PCP was mixed with montmorillonite clay under ambient conditions (Gu et al. 2011), and 2,4,5-trichlorophenol mixed with montmorillonite clay produced 2,3,7,8-TCDD and 1,2,4,7,8-PeCDD precursors, which can be converted into the dioxins by UV light, moderate heat or biotransformation (Gu et al. 2011). However, no experimental evidence of in situ mineral-catalysed synthesis of dioxin-like compounds with kaolinite has been presented in the literature, and no montmorillonite was identified in the Noosa River lakes sediment. Although a mechanism for the formation of dioxin-like compounds in kaolinite has not been definitively determined, kaolinite is the most ubiquitous clay mineral in Australian soils, and is dominant in the coastal areas compared with inland areas (Viscarra Rossel 2011), which aligns with the findings in the literature.
Another possible source of the dioxin-like compounds in the Noosa River catchment is fires. Extensive bushfires occurred in the Noosa River region in 2019–2020 (i.e. the year prior to sampling). A number of authors who have studied the potential of forest fires to contribute to the dioxin load in landscapes have only reported low concentrations of PCDD/Fs and have suggested most of the dioxins associated with bushfires dissipate into the atmosphere (Holmstrand et al. 2006). A specific study undertaken in Queensland to assess whether bushfires were a potential source of dioxin-like compounds found that it was unlikely, as the overall concentration of the sum of PCDD and PCDF did not increase after fires, nor did the concentration of OCDD (Prange et al. 2003). Deardorff et al. (2008) found that high levels of dioxins and furans can be produced by wildfires, but this was principally in areas where homes were burned, which was not the case in the fires in the Noosa River catchment. Dioxin profiles associated with urban fires tend to contain both dioxins and furans (Holmstrand et al. 2006 and references cited within, Deardorff et al. 2008). Therefore, it is unlikely that fires are the source of the unusual dioxin profile in the Noosa River lake sediments.
Conclusions
Based on a weight of evidence approach, the authors believe that the dioxin-like compounds in the Noosa River lakes are associated with the kaolinitic clay found in the lakes. As well as the fingerprint of the clay being consistent with kaolinitic clays described worldwide, a strong and significant correlation was found between the total dioxin concentration and the kaolinitic clay concentration in the sediments. The lack of furans present in the dioxin profile and the high concentrations of total dioxins would indicate that PCP or fires in the area are unlikely to be the source, although this has previously been thought to be the case in Queensland, Australia. The congener profile associated with dioxin contamination of the pesticide 2,4-D (which is known to be used widely in the area for weed control) is not consistent with the profile in the sediment in the catchment. Further, the concentration of total dioxins and OCDD measured in the Noosa River lake sediments tended to be higher than those measured in other areas in Queensland, even though, overall, the Noosa River catchment is likely to have had less pesticide use than other intensive agricultural areas studied on the eastern coast of Queensland.
Data availability
The data that support this study will be shared on reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was funded by the Department of Environment and Science, Queensland.
Supplementary material
Supplementary material is available online.
Acknowledgements
Many thanks to Dr Reinier Mann for a thorough review of the article, and Dr Christoph Braun for reviewing and formatting. We express our gratitude to the Library Services team at the Ecosciences Precinct (DES) for always providing valuable assistance and obtaining relevant research papers so efficiently. Thank you also to Troy Weigand (DES) who helped with the fabrication of equipment to enable consistent techniques for sampling and to Peter Blow from Australian Laboratory Services for sharing his dioxin expertise. Thanks to QPWS rangers John Dargusch and Kieran Hoey for providing equipment and access to the National Park for sampling.
References
ABARES (2016) The Australian Land Use and Management Classification Version 8, Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra. CC BY 3.0.Baran A, Mierzwa-Hersztek M, Urbaniak M, Gondek K, Tarnawski M, Szara M, Zieliński M (2020). An assessment of the concentrations of PCDDs/Fs in contaminated bottom sediments and their sources and ecological risk. Journal of Soils and Sediments 20, 2588–2597.
| An assessment of the concentrations of PCDDs/Fs in contaminated bottom sediments and their sources and ecological risk.Crossref | GoogleScholarGoogle Scholar |
Camenzuli L, Scheringer M, Gaus C, Hungerbuhler K (2013). Connecting the high field levels of OCDD with historical pesticide use in a rural tropical region. Organohalogen Compounds 75, 804–807.
Camenzuli L, Scheringer M, Gaus C, Grant S, Zennegg M, Hungerbühler K (2015). Historical emissions of octachlorodibenzodioxin in a watershed in Queensland, Australia: estimation from field data and an environmental fate model. Science of the Total Environment 502, 680–687.
| Historical emissions of octachlorodibenzodioxin in a watershed in Queensland, Australia: estimation from field data and an environmental fate model.Crossref | GoogleScholarGoogle Scholar |
Deardorff T, Karch NJ, Holm SE (2008). Dioxins Levels in ash and soil generated in sourthern California fires. Organohalogen Compounds 70, 2284–2288.
Ferrario J, Byrne C, Lorber M, Saunders P, Leese W, Dupuy A, Winters D, Cleverly D, Schaum J, Pinsky P, Deyrup C, Ellis R, Walcott J (1997). A statistical survey of dioxin-like compounds in United States poultry fat. Organohalogen Compounds 32, 245–251.
Gadomski D, Golden E, Irvine RL, Talley JW, Lakhwinder HS (2002). Formation and sources: Field cases. Natural formation of dioxins : a review of trends among four sites. Organohalogen Compounds 59, 2000–2003.
Gatehouse R (2004) Ecological risk assessment of dioxins in Australia National Dioxins Program – Technical Report No. 11, Australian Government Department of Environment and Heritage, Canberra. awe.gov.au/sites/default/files/documents/report-11.pdf
Gaus C, Päpke O, Dennison N, Haynes D, Shaw GR, Connell DW, Müller JF (2001). Evidence for the presence of a widespread PCDD source in coastal sediments and soils from Queensland, Australia. Chemosphere 43, 549–558.
| Evidence for the presence of a widespread PCDD source in coastal sediments and soils from Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 11372838PubMed |
Gaus C, Brunskill GJ, Connell DW, Prange J, Müller JF, Päpke O, Weber R (2002a). Transformation Processes, Pathways, and Possible Sources of Distinctive Polychlorinated Dibenzo-p-dioxin Signatures in Sink Environments. Environmental Science & Technology 36, 3542–3549.
| Transformation Processes, Pathways, and Possible Sources of Distinctive Polychlorinated Dibenzo-p-dioxin Signatures in Sink Environments.Crossref | GoogleScholarGoogle Scholar |
Gaus C, Prange JA, Papke O, Muller JF, Weber R (2002b). Formation and sources: field cases. An alternative hypothesis to natural PCDD formation. Organohalogen Compounds 59, 243–246.
Gu C, Liu C, Ding Y, Li H, Teppen BJ, Johnston CT, Boyd SA (2011). Clay mediated route to natural formation of polychlorodibenzo-p-dioxins. Environmental Science & Technology 45, 3445–3451.
| Clay mediated route to natural formation of polychlorodibenzo-p-dioxins.Crossref | GoogleScholarGoogle Scholar |
Hermanussen S, Limpus C, Papke O, Blanshard W, Connell D, Gaus C (2004). Evaluating spatial patterns of dioxins in sediments to aid determination of potential implications for marine reptiles. Dioxin 66, 1861–1867.
Holmstrand H, Gadomski D, Mandalakis M, Tysklind M, Irvine R, Andersson P, Gustafsson Ö (2006). Origin of PCDDs in ball clay assessed with compound-specific chlorine isotope analysis and radiocarbon dating. Environmental Science & Technology 40, 3730–3735.
| Origin of PCDDs in ball clay assessed with compound-specific chlorine isotope analysis and radiocarbon dating.Crossref | GoogleScholarGoogle Scholar |
Holt E, von der Recke R, Vetter W, Hawker D, Alberts V, Kuch B, Weber R, Gaus C (2008). Assessing dioxin precursors in pesticide formulations and environmental samples as a source of octachlorodibenzo-p-dioxin in soil and sediment. Environmental Science & Technology 42, 1472–1478.
| Assessing dioxin precursors in pesticide formulations and environmental samples as a source of octachlorodibenzo-p-dioxin in soil and sediment.Crossref | GoogleScholarGoogle Scholar |
Holt E, Weber R, Stevenson G, Gaus C (2010). Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) impurities in pesticides: A neglected source of contemporary relevance. Environmental Science & Technology 44, 5409–5415.
| Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) impurities in pesticides: A neglected source of contemporary relevance.Crossref | GoogleScholarGoogle Scholar |
Hoogenboom RLAP, Malisch R, van Leeuwen SPJ, Vanderperren H, Hove H, Fernandes A, Schächtele A, Rose M (2020). Congener patterns of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls as a useful aid to source identification during a contamination incident in the food chain. Science of the Total Environment 746, 141098
| Congener patterns of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls as a useful aid to source identification during a contamination incident in the food chain.Crossref | GoogleScholarGoogle Scholar |
Horii Y, Van Bavel B, Kannan K, Petrick G, Nachtigall K, Yamashita N (2008). Novel evidence for natural formation of dioxins in ball clay. Chemosphere 70, 1280–1289.
| Novel evidence for natural formation of dioxins in ball clay.Crossref | GoogleScholarGoogle Scholar | 17825874PubMed |
Horii Y, Hosono S, Ohtsuka N, Minomo K, Kannan K, Pks L, Yamashita N (2009). Study on natural formation of dioxins: dioxins in kaolin clays from Asia and several other countries. Organohalogen Compounds 71, 2479–2484.
Horii Y, Ohtsuka N, Minomo K, Nojiri K, Hosono S, Yamashita N (2010). A nationwide survey of dioxins in kaolin clays from Japan. Organohalogen Compounds 72, 876–879.
Horii Y, Ohtsuka N, Minomo K, Nojiri K, Kannan K, Lam PKS, Yamashita N (2011). Distribution, Characteristics, and Worldwide Inventory of Dioxins in Kaolin Ball Clays. Environmental Science & Technology 45, 7517–7524.
| Distribution, Characteristics, and Worldwide Inventory of Dioxins in Kaolin Ball Clays.Crossref | GoogleScholarGoogle Scholar |
Masunaga S, Takasuga T, Nakanishi J (2001). Dioxin and dioxin-like PCB impurities in some Japanese agrochemical formulations. Chemosphere 44, 873–885.
| Dioxin and dioxin-like PCB impurities in some Japanese agrochemical formulations.Crossref | GoogleScholarGoogle Scholar | 11482680PubMed |
Müeller J, Muller R, Goudkamp K, Mortimer M, Haynes D, Paxman C, Hyne R, McTaggart A, Burniston D, Symons R, Moore M (2004) Dioxins in Aquatic Environments in Australia, National Dioxins Program Technical Report No. 6, Australian Government Department of Environment and Heritage, Canberra. awe.gov.au/sites/default/files/documents/report-6a.pdf
Müller JF, Haynes D, McLachlan M, Böhme F, Will S, Shaw GR, Mortimer M, Sadler R, Connell DW (1999). PCDDS, PCDFS, PCBS and HCB in marine and estuarine sediments from Queensland, Australia. Chemosphere 39, 1707–1721.
| PCDDS, PCDFS, PCBS and HCB in marine and estuarine sediments from Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 10520488PubMed |
Prange JA, Gaus C, Päpke O, Müller JF (2002). Investigations into the PCDD contamination of topsoil, river sediments and kaolinite clay in Queensland, Australia. Chemosphere 46, 1335–1342.
| Investigations into the PCDD contamination of topsoil, river sediments and kaolinite clay in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 12002459PubMed |
Prange JA, Gaus C, Weber R, Päpke O, Müller JF (2003). Assessing forest fire as a potential PCDD/F source in Queensland, Australia. Environmental Science & Technology 37, 4325–4329.
| Assessing forest fire as a potential PCDD/F source in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Rappe C, Andersson R (2000). Natural formation of dioxins. Сoncentrations of PCDDs in ball clay and kaolin. Organohalogen Compounds 46, 9–11.
Schmitz M, Bernau S, Pernak P, Rotard W, Germann K (2004). Levels in soil and water. Dioxin distribution in a tertiary sedimentary clay profile (Germany). Organohalogen Compounds 66, 1297–1304.
Schmitz M, Scheeder G, Bernau S, Dohrmann R, Germann K (2011). Dioxins in primary kaolin and secondary kaolinitic clays. Environmental Science & Technology 45, 461–467.
| Dioxins in primary kaolin and secondary kaolinitic clays.Crossref | GoogleScholarGoogle Scholar |
Thorburn P, Shaw R (1987). Effects of different dispersion and fine fraction determination methods on the results of routine particle size analysis. Australian Journal of Soil Research 25, 347
| Effects of different dispersion and fine fraction determination methods on the results of routine particle size analysis.Crossref | GoogleScholarGoogle Scholar |
Viscarra Rossel RA (2011). Fine-resolution multiscale mapping of clay minerals in Australian soils measured with near infrared spectra. Journal of Geophysical Research 116, F04023
| Fine-resolution multiscale mapping of clay minerals in Australian soils measured with near infrared spectra.Crossref | GoogleScholarGoogle Scholar |


