Analytical pitfalls when using inhibitors in specific nitrification assays
Guillaume Humbert A B D , Mathieu Sebilo
A B D , Mathieu Sebilo  A C , Marion Chorin B , Véronique Vaury A and Anniet M. Laverman
A C , Marion Chorin B , Véronique Vaury A and Anniet M. Laverman  B
B
A Sorbonne Université, CNRS, INRAE, IRD, UPD, UPEC, Institute of Ecology and Environmental Sciences – Paris, iEES, 75005 Paris, France.
B Université de Rennes 1, Centre National de la Recherche Scientifique (CNRS), ECOBIO – UMR 6553, Université de Rennes, 35042 Rennes, France.
C Université de Pau et des Pays de l’Adour, E2S UPPA, IPREM (Institut des Sciences Analytiques et de Physico-Chimie pour l’Environnement et les Matériaux), 64000 Pau, France.
D Corresponding author. Email: g.humbert86@gmail.com
Environmental Chemistry 18(7) 295-299 https://doi.org/10.1071/EN21118
Submitted: 7 September 2021 Accepted: 13 October 2021 Published: 18 November 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Environmental context. Specific inhibitors of biological reactions in the nitrogen cycle can be used to determine the origin of reactive nitrogen species; these nitrogen species potentially degrade water quality or influence climate. However, inhibitors can potentially interfere with methods for the analysis of stable isotope ratios and concentrations of ammonium, nitrite and nitrate. The effect of this interference on several commonly used methods was investigated. These findings should help avoid the use of inappropriate analytical methods and improve data quality in studies of the nitrogen cycle.
Abstract. Characterisation of the reaction steps involved in nitrification can help determine the processes that produce potentially harmful environmental pollutants such as nitrite, nitrate and nitrous oxide (N2O). The use of nitrification inhibitors can uncouple the reactions and therefore assist in their mechanistic and isotopic characterisation. However, nitrification inhibitors can interfere with the methods for determining the concentrations and stable isotope ratios of ammonium, nitrite and nitrate. The interference of allylthiourea, hydrazine or sodium chlorate in colorimetric methods and stable isotope measurements were assessed. Ammonium concentrations were measured with the salicylate method. Nitrite and nitrate were measured with the Griess reaction, with nitrate first being reduced to nitrite with vanadium (III) chloride. For the stable isotope analysis, nitrite was reduced to N2O in a 1 : 1 sodium azide and acetic acid buffer solution; preceded, when necessary, by ammonium oxidation to nitrite by hypobromite or nitrate reduction to nitrite on an activated cadmium column. Sodium chlorate did not interfere with any of the analyses and none of the inhibitors interfered with the stable isotope ratios determination of nitrate. Allylthiourea interfered with ammonium and nitrate quantification. Both allylthiourea and hydrazine also clearly interfered in the determination of the nitrogen stable isotope ratio of ammonium, while only allylthiourea interfered in the determination of nitrogen and oxygen stable isotope ratios of nitrite. Although we suggest methods to overcome some of these interferences, our study demonstrated that the analytical methods used in combination with allylthiourea or hydrazine as nitrification inhibitors should be considered with caution when designing experiments.
Keywords: colorimetry, stable isotope, ammonium, nitrite, nitrate, allylthiourea, hydrazine, sodium chlorate.
During nitrification, potentially environmentally harmful compounds, such as nitrite (NO2−), nitrate (NO3−) and nitrous oxide (N2O), are produced directly and indirectly (Bothe et al. 2007). Nitrification is the oxidation of ammonium (NH4+) to NO2− via the intermediate hydroxylamine (NH2OH) by ammonia oxidizers, and the subsequent oxidation of NO2− to nitrate (NO3−) by nitrite oxidizers. The N2O can be produced as a reaction side-product from hydroxylamine oxidation in biotic, abiotic or hybrid processes (Caranto et al. 2016; Heil et al. 2015; Terada et al. 2017). Although nitrification consists of sequential oxidative reactions, these all occur simultaneously, with the product of one step being the substrate of the other. Therefore, inhibition of the reaction steps is used to uncouple and investigate them individually.
Three inhibitors are commonly used to inhibit and investigate the reaction steps involved in nitrification. Allylthiourea (ATU) chelates the copper in the active site of the ammonia monooxygenase, ultimately hindering the oxidation of NH4+ to NH2OH through a non-competitive process (Bédard and Knowles 1989). Hydrazine inhibits the specific oxidation of NH2OH to NO2− driven by the hydroxylamine oxidoreductase through a competitive process where it acts as an alternative substrate (Nicholas and Jones 1960). Similarly, chlorate (ClO3−) inhibits the oxidation of nitrite to nitrate through its competitive reduction to chlorite (ClO2−) by nitrite oxidoreductase (Hynes and Knowles 1983). In contrast to commercial nitrification inhibitors such as dicyandiamide, nitrapyrin and pronitradine, which are frequently used in agriculture to limit nitrogen loss into the environment, allylthiourea, hydrazine and sodium chlorate have been widely employed in research to inhibit, isolate and study the specific reactions involved in nitrification.
The use of inhibitors in combination with stable isotope techniques could help decipher the processes regulating the N cycle in ecosystems. Stable isotope analysis is widely applied to identify the source of nitrate pollution in groundwater, rivers or oceans (Xue et al. 2009). Before being reduced through denitrification, NO3− can originate from direct inputs (synthetic fertilizers, atmospheric deposition) or from the mineralisation of organic matter and its subsequent nitrification (Sebilo et al. 2013; Vitòria et al. 2004). In addition to source characterisation, this approach requires the characterisation of the N isotope fractionation that accompanies NO3− production through nitrification and which results from the difference in equilibrium constant or reaction rate observed between the heavier and lighter isotopes. The use of inhibitors could help to uncouple and characterise fractionation during the different reaction steps involved in nitrification.
However, to date, the combined use of nitrification inhibitors and stable isotope approaches to analyse nitrogen fractionation during the different nitrification steps has not been tested. In addition to specific inhibition issues, allylthiourea was reported to interfere in the colorimetric quantification of NH4+ (Tatari et al. 2017). Additional interference of inhibitors in other colorimetric methods and stable isotope techniques may occur and should thus be tested before these are used together in environmental studies (Tsikas 2007). The aim of this study was to: (i) report possible interference of inhibitors in measuring concentrations and N or O stable isotope ratios of ammonium, nitrite and nitrate; (ii) assess the ability of colorimetric methods (i.e. the salicylate method and the vanadium chloride reduction followed by Griess reaction) to overcome the potential matrix effect caused by nitrification inhibitors in solution; and (iii) assess sample preparation methods used in isotope ratio mass spectrometry (IRMS) analysis.
The issues associated with the use of nitrification inhibitors, allylthiourea or sodium chlorate, in experimental studies were assessed for two colorimetric analytical methods. The NH4+ and NOx− (NO2− and NO3−) concentrations were measured using the salicylate method and the Griess test respectively with the automated chemistry analyser Gallery Plus (Thermo Fisher). Detection and quantification limits were 0.9 and 3 µM respectively for NH4+, 0.1 and 0.3 µM for NO2−, and 3.6 and 10.7 µM for NO3−. Ammonia reacted with hypochlorite ions generated by the alkaline hydrolysis of sodium dichloroisocyanurate to form monochloramine. This reacted with salicylate ions in the presence of sodium nitroprusside at approximately pH 12.6 to form an indophenol-like blue compound. The absorbance of this compound formed by the salicylate method was measured spectrophotometrically at 660 nm.
Nitrate was reduced to nitrite by vanadium (III) chloride at 37 °C. Nitrite then formed a pink azo compound through diazotization with sulfanilamide combined with N-(1-naphthyl)-ethylenediamine dihydrochloride at acidic pH. The absorbance of this compound formed by the Griess reagents was measured spectrophotometrically at 540 nm.
Standard curves were plotted to quantify ammonium concentrations ranging from 3.6 to 71.4 and from 35.7 to 714 µM (low and high [NH4+] respectively); nitrite concentrations ranging from 1.4 to 35.7 and from 17.9 to 357 µM (low and high [NO2−] respectively) and nitrate concentrations ranging from 35.7 to 143 and 71.4 to 1.43 × 103 µM (low and high [NO3−] respectively). The effect of inhibitors on ammonium, nitrite and nitrate determination was assessed by pairwise comparison of standard curves performed with and without addition (i.e. inhibitor-free) of allylthiourea or sodium chlorate at 100 or 10000 µM respectively. Each standard curve was plotted from the relationship between the measured absorbance and the known concentrations of standards that were prepared. Regression analysis and calculation of the coefficient of determination (r2) was used to assess the quality of the standard curves.
The % absolute error (|error|) that an inhibitor-free standard curve would contribute to determining concentrations in a sample containing an inhibitor was calculated as follows:
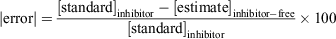
where [standard]inhibitor is the known concentration of the standard prepared in the presence of the inhibitor and [estimate]inhibitor-free is an estimate of the concentration determined from the absorbance of inhibitor-containing standard used in the standard curve established from the inhibitor-free standards.
The mass spectrometry analysis of N or O stable isotope ratios of ammonium, nitrite and nitrate was the second analytical method assessed in the presence of allylthiourea, hydrazine and sodium chlorate. The isotope ratios of reference samples without inhibitor addition were compared with those obtained from samples with 80, 640 or 8000 µM of allylthiourea, hydrazine or sodium chlorate respectively. The international standard IAEA-N2, an environmental sample and a local standard of potassium nitrate were used as reference samples to determine the N or O stable isotope ratios of ammonium, nitrite and nitrate respectively.
Atmospheric N2 and Vienna Standard Mean Ocean Water (VSMOW) were the references used for N and O isotope ratios respectively, expressed in conventional δ notation, in per mil (‰). In the method used, the substrate was converted (ammonium or nitrite or nitrate) into dissolved N2O. Nitrogen and oxygen stable isotope ratios of nitrate and nitrite were determined separately following a modified protocol of McIlvin and Altabet (McIlvin and Altabet 2005; Semaoune et al. 2012). Nitrate was reduced to nitrite by flowing the sample through a granular cadmium-filled column, after setting at 20 µM NO3− in 40 mL of a buffered solution of imidazole (pH = 8.5). The nitrogen stable isotope ratio of ammonium was determined following the protocol of Zhang et al. (2007). Then, 20 mL of 10 µM ammonium was oxidised to nitrite by hypobromite before arsenite addition to remove excess BrO−. Finally, 15 mL of 1 µM nitrite was reduced to N2O by a 1 : 1 sodium azide and acetic acid buffer solution. The δ15N values of ammonium, and the δ15N and δ18O values of nitrite and nitrate were then determined from a standard curve created by a combination of nitrate or ammonium standards that underwent the same chemical conversion as the samples (USGS-32, δ15NO3− = 180 ‰, δ18O-NO3− = 25.7 ‰; USGS-34, δ15N-NO3− = −1.8 ‰, δ18O-NO3− = −27.9 ‰; USGS-35, δ15N-NO3− = 2.7 ‰, δ18O-NO3− = 57.5 ‰; IAEA-N1, δ15N-NH4+ = 0.4 ‰; IAEA-305A, δ15N-NH4+ = 39.8 ‰; USGS-25, δ15N-NH4+ = −30.4 ‰). The quality of the calibration curve was assessed with additional international standards (IAEA-NO-3, δ15N-NO3− = 4.7 ‰, δ18O-NO3- = 25.6 ‰; IAEA-N2, δ15N-NH4+ = 20.3 ‰). The precision for δ15N ranged between ± 0.3 and ± 0.8 ‰ and between ± 0.5 and ± 1 ‰ for δ18O.
The effectiveness of the cadmium-filled column used for converting nitrate to nitrite was verified in addition to the conversion of inhibitor-containing samples. The isotope values were compared among the NO3− standards that passed through the column before and after processing the inhibitor-containing samples.
The ammonium and nitrate concentrations were underestimated when determined in the presence of allylthiourea (ATU) using both the salicylate colorimetric method and the vanadium (III) chloride and Griess reagents (Fig. 1).
The presence of ATU at 100 µM in standard samples led to large errors in the determination of ammonium and nitrate concentrations, which ranged from 23 to 37 % for low [NH4+] and >88 % for low and high [NO3−]. These results confirmed previous findings that interference increased with increased inhibitor concentration (Tatari et al. 2017). Both the amine and thiourea functional groups in ATU may interfere in indophenol blue compound formation (Ngo et al. 1982).
Our study suggests, however, that this interference can be overcome either by using a specific calibration curve established at given ATU concentrations or by working with high [NH4+]. Similar to the ATU-free standard curves, linear relationships for the standard curves were obtained in the presence of ATU with determination coefficients r2 > 0.99 (Fig. 1a, d). Quality controls (QCs) were analysed to assess how the matrix effect was corrected in the standard curves established in the presence of ATU. These QCs showed an appropriate correction of the matrix effect in ammonium measurements with errors <10 %. Finally, less than 12 % error was observed for [NH4+] > 143 µM; decreasing below 10 % in the middle range of the standard curve established for high [NH4+].
The quantification of NO3− plus NO2− using the vanadium (III) chloride and Griess reagents should also be reconsidered in the presence of ATU (Fig. 1c, f). Taylor et al. (2010) observed a similar interference of ATU in nitrate quantification, although the authors used Szechrome reagent as the colorimetric method. Here, comparison of the interference in the quantification of NO2− and NO3− revealed that the chemical reduction of nitrate to nitrite by vanadium (III) chloride in the presence of ATU presented a major issue that cannot be overcome using a specific calibration curve. The [NO3−] determined for QCs with standard curves corrected for ATU effects displayed high variability (CV > 13%) and accuracy values <95 %.
Finally, when no other analytical method (e.g. ionic chromatography, autoanalyzer, oxygen uptake rate) is available, our results suggest that NO2− should be preferably quantified with the Griess reaction to study nitrification in the presence of ATU. This should be carried out either by using a specific calibration curve established at given ATU concentrations or by working with nitrite concentrations >35.7 µM (Fig. 1b, e). ATU at 100 µM in standard samples resulted in absolute errors ranging from 11 % to 26 % for low nitrite concentrations (between 1.4 and 35.7 µM). Similar to ATU-free standard curves, linear relationships described the standard curves obtained in the presence of ATU with determination coefficients r2 > 0.99. The QCs analysed with this standard curve showed an appropriate correction of the matrix effect in determining nitrite concentration with errors <10 %. Further, less than 12 % error was observed for nitrite concentrations ranging from 17.9 to 357 µM and this decreased to below 5 % in the middle range of the standard curve established for high [NO2−].
Our study revealed additional interference by inhibitors in N and O isotope ratios analysis (δ15N and δ18O respectively) of ammonium and nitrite (Table 1). Allylthiourea and hydrazine clearly interfered in the determination of δ15N-NH4+, while no interference was observed for δ15N-NH4+ determination in the presence of sodium chlorate. Both ATU and hydrazine contain an amine functional group that may interfere in the chemical oxidation of NH4+ to NO2− with hypobromite. Although this interference is negligible when working with environmental samples, up to 30 % of N-containing organic compounds (i.e. glycine) can be oxidised with this method (Zhang et al. 2007). However, the yield of ammonium to nitrite conversion in the presence of ATU or hydrazine never exceeded 100 %, which makes it unlikely that ammonium from the crystalline inhibitor stocks was contributing to the observed results. The study of specific interferences reported here would require additional investigations. Our results also showed that ATU interferes in the determination of δ15N and δ18O of NO2−, although we cannot fully explain this interference. Surprisingly, ATU did not interfere in the determination of δ15N and δ18O of NO3−. This may results from the dilution of the sample with the imidazole buffer solution before passage on the cadmium column. The reduction of nitrate on the activated Cd column was performed by using 40 mL samples, while only 15 mL of the sample was used in the direct determination of nitrite isotopes. This was also consistent with the negligible difference observed in net isotope ratio measurements between NO3− reference materials prior and post conversion of inhibitor-containing samples; i.e. mean difference of –0.3 ± 0.2 ‰ and –0.4 ± 0.3 ‰ for δ15N and δ18O respectively (n = 4). More generally, the dilution step in NO3− sample preparation can explain the lack of interference in the determination of δ15N and δ18O of NO3−, regardless of the inhibitor tested. Hence, the processing of inhibitor-containing samples did not alter the performance of the cadmium column.

|
The allylthiourea, hydrazine and sodium chlorate concentrations tested here were consistent with those commonly used in nitrification studies; i.e. 0.001–0.86 mM, 2–100 mM and 1–30 mM for ATU, hydrazine and sodium chlorate respectively (Belser and Mays 1980; Hooper and Terry 1973; Lees and Simpson 1957; Nicholas and Jones 1960; Santoro and Casciotti 2011). In addition, owing to their inhibiting capacity, these have also been used as negative controls in nitrification assays (e.g. Lam et al. 2009; Santoro and Casciotti 2011). Our study demonstrated that the use of ATU or hydrazine as nitrification inhibitors with these specific analytical methods should be considered with caution. No interference between sodium chlorate and the colorimetric methods tested here was observed (data not shown).
The tests presented here were performed on synthetic, non-environmental samples. However, the presence of enzymes in environmental samples may have a mitigating effect. Finally, in addition, when designing experiments, as well as taking into account the choice of analytical method in the presence of inhibitors, the possible interference in analysis and the inhibition efficacy should also be considered, especially because an excess of inhibitors remains a prerequisite for ensuring the complete inhibition of nitrifying reactions.
Data availability statement
All data included in this study are available upon request by contacting the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by the French National Research Agency (grant no. ANR-15-CE04–0014–02).
Acknowledgements
The authors acknowledge the ECOCHIM platform of Rennes University for the spectrophotometric analysis. They would also like to thank Leigh Gebbie (LKG Scientific Editing and Translation, Brisbane, Qld, Australia) for her work to improve the language of this article.
References
Bédard C, Knowles R (1989). Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiological Reviews 53, 68–84.| Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers.Crossref | GoogleScholarGoogle Scholar | 2496288PubMed |
Belser LW, Mays EL (1980). Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Applied and Environmental Microbiology 39, 505–510.
| Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments.Crossref | GoogleScholarGoogle Scholar | 16345525PubMed |
Bothe H, Ferguson SJ, Newton WE (2007). ‘Biology of the nitrogen cycle.’ (Elsevier: Amsterdam)
Caranto JD, Vilbert AC, Lancaster KM (2016). Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proceedings of the National Academy of Sciences of the United States of America 113, 14704–14709.
| Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission.Crossref | GoogleScholarGoogle Scholar | 27856762PubMed |
Heil J, Liu S, Vereecken H, Brüggemann N (2015). Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biology & Biochemistry 84, 107–115.
| Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties.Crossref | GoogleScholarGoogle Scholar |
Hooper AB, Terry KR (1973). Specific inhibitors of ammonia oxidation in Nitrosomonas. Journal of Bacteriology 115, 480–485.
| Specific inhibitors of ammonia oxidation in Nitrosomonas.Crossref | GoogleScholarGoogle Scholar | 4725614PubMed |
Hynes RK, Knowles R (1983). Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination. Applied and Environmental Microbiology 45, 1178–1182.
| Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination.Crossref | GoogleScholarGoogle Scholar | 16346262PubMed |
Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, Gutiérrez D, Amann R, Jetten MSM, Kuypers MMM (2009). Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proceedings of the National Academy of Sciences of the United States of America 106, 4752–4757.
| Revising the nitrogen cycle in the Peruvian oxygen minimum zone.Crossref | GoogleScholarGoogle Scholar | 19255441PubMed |
Lees H, Simpson JR (1957). The biochemistry of the nitrifying organisms. V. Nitrite oxidation by Nitrobacter. The Biochemical Journal 65, 297–305.
| The biochemistry of the nitrifying organisms. V. Nitrite oxidation by Nitrobacter.Crossref | GoogleScholarGoogle Scholar | 13403908PubMed |
McIlvin MR, Altabet MA (2005). Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Analytical Chemistry 77, 5589–5595.
| Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater.Crossref | GoogleScholarGoogle Scholar | 16131070PubMed |
Ngo TT, Phan APH, Yam CF, Lenhoff HM (1982). Interference in determination of ammonia with the hypochlorite-alkaline phenol method of Berthelot. Analytical Chemistry 54, 46–49.
| Interference in determination of ammonia with the hypochlorite-alkaline phenol method of Berthelot.Crossref | GoogleScholarGoogle Scholar |
Nicholas DJD, Jones OTGJ (1960). Oxidation of hydroxylamine in cell-free extracts of Nitrosomonas europaea. Nature 185, 512–514.
| Oxidation of hydroxylamine in cell-free extracts of Nitrosomonas europaea.Crossref | GoogleScholarGoogle Scholar |
Santoro AE, Casciotti KL (2011). Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation. The ISME Journal 5, 1796–1808.
| Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation.Crossref | GoogleScholarGoogle Scholar | 21562601PubMed |
Sebilo M, Mayer B, Nicolardot B, Pinay G, Mariotti A (2013). Long-term fate of nitrate fertilizer in agricultural soils. Proceedings of the National Academy of Sciences of the United States of America 110, 18185–18189.
| Long-term fate of nitrate fertilizer in agricultural soils.Crossref | GoogleScholarGoogle Scholar | 24145428PubMed |
Semaoune P, Sebilo M, Templier J, Derenne S (2012). Is there any isotopic fractionation of nitrate associated with diffusion and advection?. Environmental Chemistry 9, 158–162.
| Is there any isotopic fractionation of nitrate associated with diffusion and advection?.Crossref | GoogleScholarGoogle Scholar |
Tatari K, Gülay A, Thamdrup B, Albrechtsen H-J, Smets BF (2017). Challenges in using allylthiourea and chlorate as specific nitrification inhibitors. Chemosphere 182, 301–305.
| Challenges in using allylthiourea and chlorate as specific nitrification inhibitors.Crossref | GoogleScholarGoogle Scholar | 28505572PubMed |
Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ (2010). Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Applied and Environmental Microbiology 76, 7691–7698.
| Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils.Crossref | GoogleScholarGoogle Scholar | 20889792PubMed |
Terada A, Sugawara S, Hojo K, Takeuchi Y, Riya S, Harper WF, Yamamoto T, Kuroiwa M, Isobe K, Katsuyama C, Suwa Y, Koba K, Hosomi M (2017). Hybrid nitrous oxide production from a partial nitrifying bioreactor: hydroxylamine interactions with nitrite. Environmental Science & Technology 51, 2748–2756.
| Hybrid nitrous oxide production from a partial nitrifying bioreactor: hydroxylamine interactions with nitrite.Crossref | GoogleScholarGoogle Scholar |
Tsikas D (2007). Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 851, 51–70.
| Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research.Crossref | GoogleScholarGoogle Scholar | 16950667PubMed |
Vitòria L, Otero N, Soler A, Canals A (2004). Fertilizer characterization: Isotopic data (N, S, O, C, and Sr). Environmental Science & Technology 38, 3254–3262.
| Fertilizer characterization: Isotopic data (N, S, O, C, and Sr).Crossref | GoogleScholarGoogle Scholar |
Xue D, Botte J, De Baets B, Accoe F, Nestler A, Taylor P, Van Cleemput O, Berglund M, Boeckx P (2009). Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Research 43, 1159–1170.
| Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater.Crossref | GoogleScholarGoogle Scholar | 19157489PubMed |
Zhang L, Altabet MA, Wu TX, Hadas O (2007). Sensitive measurement of NH4+ 15N/14N (δ15NH4+) at natural abundance levels in fresh and saltwaters. Analytical Chemistry 79, 5297–5303.
| Sensitive measurement of NH4+ 15N/14N (δ15NH4+) at natural abundance levels in fresh and saltwaters.Crossref | GoogleScholarGoogle Scholar | 17567102PubMed |



