Additives and polymer composition influence the interaction of microplastics with xenobiotics
Darius Hummel A , Andreas Fath B , Thilo Hofmann
A , Andreas Fath B , Thilo Hofmann  C D and Thorsten Hüffer
C D and Thorsten Hüffer  C D E
C D E
A Department of Soft Matter Science and Dairy Technology, Institute of Food Science and Biotechnology, University of Hohenheim, Garbenstrasse 21, 70599 Stuttgart, Germany.
B Department of Medical and Life Sciences, Furtwangen University, Jakob-Kienzle-Strasse 17, 78054 VS-Schwenningen, Germany.
C Department of Environmental Geosciences, Centre for Microbiology and Environmental Systems Science, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria.
D Research Platform Plastics in the Environment and Society (PLENTY), University of Vienna, Althanstrasse 14, 1090 Vienna, Austria.
E Corresponding author. Email: thorsten.hueffer@univie.ac.at
Environmental Chemistry 18(3) 101-110 https://doi.org/10.1071/EN21030
Submitted: 15 March 2021 Accepted: 11 May 2021 Published: 2 June 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Environmental context. The effects of the presence of polymer additives and polymeric structure on sorption of xenobiotics to microplastics remain unclear. Our results combined data from experimental sorption batch experiments using three environmentally relevant model sorbates with confocal microscopy. This provides clear evidence that both factors play a major role in sorption strength and the underlying sorption process, affecting sorption onto the particle surface and partitioning into the bulk polymer.
Abstract. Microplastics are particulate contaminants of global concern. Interactions of microplastics with organic contaminants are frequently studied with commercially available polymer materials as surrogates. The influence of the polymer structure (i.e. internal 3D polymer geometry and monomer chain length) and the presence of additives on their interactions with xenobiotics remains unclear. This work investigates sorption of three sorbates of environmental concern to two polyamide (PA) and two polyvinyl chloride (PVC) sorbents of different molecular composition and additive content, respectively. Sorption was studied using complementary data from sorption isotherms and confocal laser-scanning microscopy. The additives in PVC increased sorption affinity owing to an increased sorbent hydrophobicity and a higher void volume within the polymer. Surface area normalisation indicated surface adsorption for unplasticised PVC and absorption for 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH)-plasticised PVC, which were confirmed using confocal laser-scanning microscopy. The strong sorption to PA was mainly driven by hydrogen-bond interactions. The contribution depended on the molecular features of the sorbent and the sorbate. Confocal laser-scanning microscopy showed that PA6 was taking up more sorbate into its bulk polymer matrix than PA12, the two being different in their chemical composition. This difference could be attributed to the higher swelling capability of PA6. The results emphasise that the molecular structure of the polymer and the presence of additives have to be taken into consideration when sorption of organic substances to plastics is investigated.
Keywords: sorption, phthalates, endocrine disrupting chemicals, confocal microscopy.
Introduction
The production and use of plastic have been continuously increasing since their introduction into the mass market in the middle of the 20th century (Hahladakis et al. 2018), reaching a global production of 359 Mt in 2018 (Plastics Europe 2020). In recent years, societal concern regarding environmental plastic pollution has grown, with a particular focus on micro- and more recently nanoplastics, which among other factors has led to increasing activism directed at reducing the use and disposal of plastic products in everyday life, for instance in the ‘zero waste movement’ (Müller and Schönbauer 2020). Despite the fact that research into the occurrence and effects of these particulate contaminants has significantly increased, the debate on how to define and classify environmental plastic debris is mainly driven by their size and less by the diversity of plastic contaminants with respect to their physicochemical properties, for instance their composition (Hartmann et al. 2019a, 2019b; Rochman et al. 2019). Most plastics that enter the environment are expected to be highly persistent and thereby accumulate in the environment, increasing potential impacts on ecosystems, for example, harming wildlife, entering the food web or changing the phase distribution of contaminants (Wagner et al. 2014; Horton et al. 2017). An in-depth understanding of the processes affecting the fate of organic compounds in environmental systems is crucial in several different fields, not least in ecotoxicology: contaminant transport, bioavailability and bioaccumulation, toxic effects on organisms, and transformation reactions are all strongly dependent on sorption (Schwarzenbach et al. 2002).
While the impact of plastic on the global transport of persistent organic contaminants seems of minor importance (Gouin et al. 2011; Koelmans et al. 2016), the number of papers investigating the interactions of microplastics with organic compounds has continued to increase in recent years. Experimental sorption data are available nowadays for a large diversity of organic compounds and polymer particles. These include compounds of environmental concern, for example pharmaceuticals (Guo et al. 2019a, 2019b), polycyclic aromatic hydrocarbons (Fries and Zarfl 2012; Sørensen et al. 2020) and flame retardants (Xu et al. 2019). Endocrine-disrupting compounds, another class of environmental concern, may cause effects on different organisms even at low concentrations and have been used as sorbates in studying sorption to microplastics (Liu et al. 2019). More process-oriented studies on sorption to different polymers have been conducted using small organic molecular probe compounds (Hüffer and Hofmann 2016; Seidensticker et al. 2018; Uber et al. 2019a, 2019b).
Regarding the main properties of plastics, available surface area, glass transition temperature and monomeric composition have been suggested to mainly govern the molecular interactions and the sorption mode with organic compounds (Xing and Pignatello 1997; Guo et al. 2012; Hüffer and Hofmann 2016). Recently, studying the influence of polymer aging on the physico-chemical properties of the sorbent and on their interactions with organic compounds have brought sorption studies closer to environmental reality (Hüffer et al. 2018; Müller et al. 2018; Liu et al. 2020). Little is known about how the chain length of a polymer or the presence of additives, such as functional additives, colourants or fillers, alter the interactions of microplastics with organic compounds (Hahladakis et al. 2018). This is of particular importance because most plastics contain additives to adjust their properties for a particular application. Additives change the internal 3D geometry of polymers and consequently their interaction with organic substances may also be affected (Endo et al. 2005). Polyvinyl chloride (PVC) contains over 60 % of all the additives used in plastic production (The Freedonia Group 2014). Large quantities of phthalates (up to 60 wt-%) are added to the polymer resin during PVC production to increase its flexibility and elasticity (Teuten et al. 2009; Fath 2019). Owing to increased restrictions on the use of phthalates, for instance bis(2-ethylhexyl) phthalate (DEHP) (EU Commission 2011, 2015), 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH) has been used as an alternative since 2002, mainly as a substitute for DEHP. Owing to its similar molecular structure (the phthalate aromatic ring is replaced by a cyclohexane ring), it has similar physical properties, but it is less toxic (Environmental Specimen Bank (ESB) 2020). The present work was designed to test the following hypotheses: (1) the presence of DINCH plasticiser changes the sorption behaviour of organic compounds to PVC; and (2) the chain length of polyamide (PA) changes the sorption behaviour of organic compounds to microplastics. Isotherms were determined for sorption of three hormonally active substances as model sorbates in combination with confocal fluorescence microscopy.
Experimental
Materials
PVC containing DINCH was obtained from Hansgrohe SE (Schiltach, Germany). PVC granules were ground with a cryo-mill (CryoMill, Retsch, Haan, Germany) under the following conditions: precooling 90 s, 5 Hz; milling 300 s, 30 Hz, two cycles; intercooling 30 s, 5 Hz. The stainless-steel milling container was cooled with liquid nitrogen (–196 °C) to prevent chemical changes in the polymers due to heat accumulation. Unplasticised PVC (uPVC) powder was obtained by subsequent liquid extraction of the plasticised PVC using cyclohexane. Soxhlet extraction was performed using 4.8 g of ground DEHP-PVC and 100 mL cyclohexane for 6 h. The polymer was dried overnight at ambient temperature. No plasticiser residues could be detected using FTIR (Tensor 27, Bruker, Billerica, MA, USA) with a Golden Gate™ diamond attenuated total reflection (Specac, Orpington, England) sample holder. The absence of plasticisers in PVC was confirmed by chemical analysis following dissolution and precipitation of the polymer and subsequent analysis using an Agilent 8890 gas chromatograph coupled to a 7000D gas chromatograph-triple quadrupole mass spectrometer system (Agilent Technologies, Santa Clara, CA, USA) (Henkel et al. 2019). Polyamide PA6 (Ultramid®) was obtained as a powder from BASF SE (Ludwigshafen, Germany) and polyamide PA12 (DuraForm) as a powder from 3D-Systems GmbH (Darmstadt, Germany). Selected physico-chemical properties of polymer particles are listed in Table 1.

|
Oestrone (E1), 17-α-ethinyloestradiol (EE2) and norethisterone (NOR) were purchased from Sigma–Aldrich (Darmstadt, Germany). Stock solutions of sorbates were prepared in methanol (≥99.5 % from VWR, Darmstadt, Germany) and stored at 4 °C for no longer than 3 weeks. The physico-chemical properties of probe sorbates are shown in Table 2. A background solution was prepared using 10 mM CaCl2 (≥98.0 %, Merck, Darmstadt, Germany) and bi-distilled water with a conductivity of 0.054 µS at 25 °C. The pH of the background solution was below the pKa value of the sorbates, ranging between 7.1 and 7.4.

|
For confocal microscopy, Estradiol Glow and the fluorescence dyes sulfo-cy-5-azide and 3-azido-7-hydroxycoumarine were purchased from Jena Bioscience (Jena, Germany). Fluorescent dyes were used to label EE2. The molecular structures of the dyes are shown in Fig. 1.
Sorbent characterisation
The specific surface area and pore volume of the polymer particles were measured using N2-BET (Brunauer–Emmett–Teller) gas adsorption (Nova 2000e analyser, Quantachrome Industries, Boynton Beach, FL, USA). The relative pressure (p/p0) ranged from 0.074 to 0.297 for determination of surface area and the pore volume was measured with (p/p0) = 0.989. Gas adsorption data did not show evidence of any polymers being porous but an underestimation of the porosity and surface area of the materials cannot be excluded since absorption might be limited, for example, owing to N2 condensation inside the capillaries (Pignatello 1998; Sigmund et al. 2017).
Particle size distributions were determined using dynamic laser light diffraction with an LS13320 particle size analyser (Beckman Coulter, Brea, CA, USA). Polymer particles were measured in distilled water to which several drops of detergent were added to lower surface tension and disperse particles more easily. Polymer particles were added into the mixing chamber until obscuration was high enough (6–8 %) for accurate measurement. The pump speed was set to 50 %. The measurements were conducted with refractive indices of 1.52 for PA6 and PA12 (Wypych 2016), 1.54 for PVC and DINCH-PVC (Wypych 2016), and 1.33 for distilled water (Daimon and Masumura 2007). The refractive index of uPVC was assumed since the initial amount of plasticiser in PVC was not known. For each measurement, particle sizes were determined in triplicate. Particle size distributions were evaluated using the median volume based-diameters (d50).
Sorption batch experiments
Aliquots of 15–40 mg of sorbent material were weighed into 20 mL amber glass vials. Background solution (18 mL) containing 10 mM CaCl2 was added. Samples were prepared in triplicate. Methanolic working solution was added in different amounts to obtain sorbate concentrations ranging over two to three orders of magnitude, not exceeding 10−1 of the sorbate solubility (methanol did not exceed 0.5 % to avoid co-solvent effects). Vials were then closed with screw caps with butyl/PTFE-lined septa. The samples were shaken at 125 rpm (GFL, model 3018, Burgwedel, Germany) at 25 °C. Samples were shaken in the absence of light for up to 17 days for equilibration. Equilibration times were pre-determined and are in line with the literature (Xing and Pignatello 1997; Hüffer and Hofmann 2016). Prior to quantification, samples were centrifuged at 750g for 30 min (CR 4 22, Jouan Inc., Winchester, VA, USA) to separate plastic particles from the liquid phase. Centrifugation of the samples for solid–liquid separation was chosen over filtration, since strong sorption to different membrane materials like PA66, polyethersulfone, cellulose acetate and mixed nitrocellulose occurs during microfiltration for EE2 and E1 (Han et al. 2013b). Supernatant aliquots of 1 mL were taken with a microlitre glass syringe and transferred into a 1.5 mL HPLC glass vial. Aqueous sorbate concentrations were determined using an external calibration with an HPLC-FD system (Agilent, 1100 series, Santa Clara, CA, USA) with a C18 column (Agilent ZORBAX Eclipse Plus C18; 3.5 µm; 4.6 × 150 mm) and an analytical guard column (Eclipse XDB-C18; 4.6 × 12.5 mm; 5 µm). A mixture of acetonitrile and water (50/50 v/v) was used as eluent. Oven temperature was set at 30 °C. A 20 µL sample volume was injected with an eluent flow of 1 mL min−1. Sample preparation was performed only using glass or stainless steel to prevent high sorption of probe sorbates on plastic laboratory devices (Walker and Watson 2010).
Confocal fluorescence microscopy
Samples were prepared using 1 mL aqueous phosphate buffered saline buffer solution (pH 7.4) using one buffer salt tablet (Merck) containing 13.7 mM NaCl, 0.27 mM KCl, 1.0 mM Na2HPO4 and 0.18 mM KH2PO4 dissolved in distilled water. Ethanolic Estradiol Glow working solution was prepared at a concentration of 1 mg L−1 Estradiol Glow. One spatula tip (~1 mg) of polymer powder was added to the solution before the vials were sealed and shaken vigorously for 1 week at room temperature using a vortex shaker (VWR International GmbH, VV3, Darmstadt, Germany). Reference samples were prepared using polymer and background solution, to differentiate between autofluorescence and fluorescence of the sorbate. Estradiol Glow was used for sorption experiments to identify sorption sites in and on the polymers. Considering sorption depends on the overall structure of the sorbate molecule and its interaction with the sorbent, the fluorophore part of the labelled hormone may alter the sorption properties of Estradiol Glow compared with the hormone probe sorbates (EE2, NOR, E1). To clarify whether the fluorophore part of the labelled hormone influences the sorption site on the particle, another experiment with EE2 was conducted: an aqueous suspension of PA6 and EE2 was prepared as for determination of the sorption isotherms. After 1 week of sorption, polymer particles were transferred into a microscopy well plate (Laboratory Tek II, eight chambers, Thermo Fisher Scientific Inc., Waltham, USA) consisting of polystyrene and borosilicate glass. The EE2 was labelled using 3-azido-7-hydroxycoumarine and sulfo-cy5-azide (Kunz 2009). Chemically bound and free coumarin derivatives showed different fluorescence intensities, as the delocalised electron system expands with cycloaddition (Du et al. 2010). For catalysis of the cycloaddition, EE2 (6.76 × 10−9 M) was added to 200 µL of reaction kit (4.93 × 10−8 M 3-azido-7-hydroxycoumarine) fluorescent dye mixture while fluorescence was continuously measured. More experimental details regarding reference and blank samples are given in the Supplementary material S1.
DINCH-PVC, PA6 and PA12 particles were analysed using a Leica TCS SP8 scan head system (Leica Microsystems GmbH, Wetzlar, Germany). An HC PL APO CS2 63×/1.40 OIL objective was used in combination with immersion oil (Leica, Type F). Polymer suspensions prepared with sulfo-cy-5-azide and Estradiol Glow were scanned bidirectionally and polymer suspensions prepared with 3-azido-7-hydroxycoumarine and L-thyroxin unidirectionally with a scan speed of 400 Hz. Samples containing Estradiol Glow were excited at a wavelength of 458 nm (Coherent, Chameleon, Dieburg, Germany) and fluorescence was detected with a highly sensitive hybrid detector in the range from 585 to 638 nm. Sulfo-cy5-azide samples were excited at 514 nm and emission detected in the range 651–689 nm. The microscope operated with the software Leica application suite X 3.1.5.16308 and data processing was carried out with version 2.0.0.14332. Unplasticised PVC particles were prepared with Estradiol Glow as sorbent as mentioned before. Pictures were taken using a confocal laser scanning microscope (CLSM) (Eclipse C1; Nikon GmbH, Düsseldorf, Germany) with a 63× oil immersion objective. The microscope was controlled via the software NIS-Elements Confocal (Nikon). The fluorescence analysis of L-thyroxine was carried out with a PerkinElmer LS50 B luminescence spectrometer. L-thyroxine was excited at 400 nm, scanning emission between 200 and 800 nm.
Sorption data analysis
Experimental sorption isotherm data were fitted to three sorption models: Freundlich, Langmuir, Polanyi–Manes. Details of the model equations and parameters can be found elsewhere (Hüffer and Hofmann 2016). All statistical tests and model fits were performed using Sigma Plot 12.0 for Windows. Standard errors for the model parameters were computed using reduced chi-square and weighted regressions, with weighting to reciprocal y2.
Results and discussion
The presence of DINCH as polymer additive altered sorption to PVC
The isotherms for PVC showed stronger sorption when DINCH was present within the polymer (Fig. 2). Independently of the molecular features of the sorbate, the sorption affinity to PVC containing the additive with an octanol–water partitioning constant (log Kow) of 9.82 (Pence and Williams 2010) on average doubled (as the Freundlich affinity coefficient, KF; see Table 3). The molecular interactions involved in sorption to microplastics depend on the properties of the polymer particles as well as sorbate properties and hydrochemical factors (Fred-Ahmadu et al. 2020). Given the neutral nature of the sorbates, these interactions include in principle non-specific van der Waals, hydrophobic and π–π electron donor–acceptor interactions. The latter are expected to be of minor importance regarding sorption to PA and PVC as they do not contain conjugated π-electron systems. Hydrophobic interactions have been shown to play a major role in sorption to PVC (Hüffer and Hofmann 2016), but the presence of additives in microplastics and PVC in particular has hardly been addressed. The increase in sorption between unplasticised and DINCH-PVC can be assumed to stem from the stronger hydrophobic interaction between the sorbates and the additive-containing PVC in comparison with the polymer without DINCH (log Kow 9.82 for DINCH and 1.62 for vinyl chloride, the PVC monomer (Pence and Williams 2010)). The distribution coefficients of the sorbates, log Kd (calculated at 10−4 of their aqueous solubility), increased NOR < E1 < EE2 for both DINCH-PVC (2.23, 2.76, 3.06, respectively) and uPVC (1.56, 1.88, 2.66, respectively). This increase in sorption follows the trend of octanol–water partitioning constants for the sorbates (Table 2), which further supports that hydrophobic interactions play a major role in sorption to PVC. Although the particle size distributions of DINCH-containing PVC and uPVC were in a similar range, the mean particle sizes (d50 in Table 1) were slightly larger for uPVC. Sorption of triclosan to PVC of two different size fractions showed higher distribution coefficients and sorption capacity for smaller PVC particles, resulting from higher specific surface area and stronger hydrophobicity (Ma et al. 2019). The extraction of the additive from PVC resulted in a larger available surface area (Table 1). It must be kept in mind that the surface area determined using N2 BET analyses can be underestimated owing to diffusion-limited access and capillary condensation of N2 in the pores of the sorbent material (Pignatello 1998). To investigate if the differences in sorption between the two PVC sorbents resulted from differences in available surface area, surface-normalised distribution coefficients were calculated (Table 3). Following surface normalisation, the distribution coefficients of the DINCH-containing PVC with the smaller available surface area remained higher compared with the distribution coefficients calculated for the uPVC with greater surface area. The differences in the distribution coefficients between DINCH-PVC and uPVC remained even following surface normalisation. This indicates that surface-governed adsorption is not the dominating sorption mode for DINCH-containing PVC.

|
The sorption isotherm of uPVC was almost linear (Freundlich exponent, n = 0.955–1.089). This differs from the results of our previous study, where sorption to uPVC using organic probe molecules of similar hydrophobicity and measuring isotherms of comparable aqueous concentrations was non-linear and the Freundlich exponent ranged from 0.593 to 0.866 (Hüffer and Hofmann 2016). The Freundlich exponents obtained from the sorption isotherms for DINCH-containing PVC ranged between 0.976 and 1.014 (Table 3). Literature-reported linearity for sorption of organic substances to PVC ranges depending on the sorbate considered from highly non-linear (Li et al. 2018; Wang and Wang 2018; Qiu et al. 2019) to fully linear (Li et al. 2018; Ma et al. 2019). Differences in sorption linearities may stem, among others factors, from the experimental aqueous concentration range within which sorption was determined. The physico-chemical properties of the sorbent play an important role for the distribution of sorption site energies and consequently in sorption linearity. Among the properties of plastics influencing sorption are surface charge, surface area, molecular chain arrangement (i.e. linear, cross-linked, branched and network), and functional groups present (Hüffer et al. 2018; Müller et al. 2018; Fred-Ahmadu et al. 2020). Sorption of organic compounds to polyethylene (PE) has been shown to be substantially different depending on the type of PE considered (i.e. low- or high-density PE) (Uber et al. 2019b). Pore-filling (i.e. surface adsorption) leading to heterogeneous distribution of sorption site energies can explain non-linear sorption isotherms to glass-like PVC (Xing and Pignatello 1997). Sorption linearity is often used as an indication of the dominating sorption mode (ad- or absorption). The more linear the sorption isotherms, the more sorption is assumed to be governed by absorption (Walker and Watson 2010). Sorption to glass-like PVC is typically considered to be dominated by adsorption to the surface (Pignatello et al. 2006; Hüffer and Hofmann 2016). Additionally, sorbate molecules can undergo adsorption-like interactions with the PVC surface as pore-filling results from cohesion within condensed PVC segments (Xing and Pignatello 1997). Assuming that the extraction of plasticiser from PVC has made surface pore spaces more easily accessible for the sorbates to interact with compared with DINCH-PVC raises questions on the role of additives on the dominant sorption mode of PVC.
Confocal laser scanning images support the aforementioned observation of absorption being the dominant sorption mode for DINCH-PVC. Fluorescence of the probe molecule Estradiol Glow is observed not only at the particle surface but throughout the whole cross-sectional area of the particle (Fig. 3), indicating absorption of the molecule into the bulk polymer. There are no specific regions of fluorescence maxima or minima. The plasticiser makes the internal polymer void volume more accessible to the sorbate. The high hydrophobicity of DINCH increases the sorption of PVC, even when Kd is normalised against surface area. Since the probe substance is evenly distributed over the cross-sectional area of the particle, it can be concluded that particle size for DINCH-PVC is less important for sorption compared with uPVC. Our findings are supported by results from previous work on the influence of type (DINCH, DEHP, DEHT (di(ethylhexyl) terephthalate) and tris(2-ethylhexyl)trimellitate) (TOTM)) and the concentration of the plasticiser in PVC on the sorption of diazepam showing that sorption of diazepam increased with increasing polymer film thickness and content of plasticiser in the range of 10–40 % m/m plasticiser. For ratios of plasticiser above 40 % m/m present in polymers, the partitioning coefficient decreased, which was explained by a phase separation between the polymer–plasticiser blend and coalescing of the plasticiser (Al Salloum et al. 2015).

|
uPVC showed highly intense fluorescence at regions close to the particle surface, decreasing with increasing penetration depth (Fig. 3). The absence of plasticiser decreases the accessibility of the particle to the sorbate, allowing the conclusion to be drawn that the dominant mode of sorption for uPVC is adsorption. Comparison of the results from sorption batch experiments and confocal microscopy emphasises the importance of direct evidence on the dominant sorption mode (e.g. from microscopic or spectroscopic method) or indirect indication from sorption linearity. In previous studies, adsorption was considered to be dominant for PVC based on its glass transition temperature (Pignatello et al. 2006; Hüffer and Hofmann 2016; Ma et al. 2019). The confocal microscopy images (Fig. 3) show little penetration of the dye into the polymer. UPVC only shows little swelling in water at low percentages (0.04–0.4 % m/m) (Wypych 2016). It seems unlikely the fluorescence observed in the areas close to the particle surface is a result of water uptake by the particles. The partial absorption of the probe molecule is more likely to be based on an underestimated pore volume of the particles, because of limited access of the N2 during the BET surface determination. Overall, the combination of sorption batch experiments with confocal microscopy suggests that absorption is the dominant process of sorption for DINCH-PVC while adsorption dominates sorption to uPVC.
Strong sorption to PA was influenced by monomer chain length
The isotherms for sorption to PA showed stronger sorption of the sorbates to PA12 compared with PA6 (Fig. 4). This difference was up to almost an order of magnitude for EE2 and NOR. Taking the specific surface of the polymer powders into consideration, higher sorption to PA12 is likely to be based on the higher specific surface. The average particle size of PA12 (d50 57.65 ± 0.98 µm) is smaller than that of PA6 (d50 94.59 ± 0.48 µm), which corresponds to a surface area for PA6 of 0.241 m2 g−1 and for PA12 of 5.315 m2 g−1 (Table 1). Normalising the partitioning coefficient against the surface area of the polymer sorbents, the partitioning coefficient is higher for PA6. Previously performed filtration experiments with PA66 membranes and EE2 and E1 as probe sorbates have shown higher sorption of the probe molecules in filters with smaller pore size, as reported earlier (Han et al. 2013b), which emphasises the importance of available surface area for sorption to PAs.

|
To understand the difference in sorption between the two sorbents and the sorption mechanism involved, it is necessary to note the physical and chemical structure of the sorbent material. As an aliphatic polymer, PA is composed of a monomer in which an amine and a carbonyl group are linked by a hydrocarbon chain. For PA6, this chain contains five methylene fragments and for PA12, the chain consists of 11 methylene fragments. This increase in hydrocarbon chain length makes the sorbent material more hydrophobic, which in turn may explain the stronger sorption to PA12 over PA6. Stronger sorption of the antibiotic triclosan to PA12 over PA6 was recently explained by a combination of adsorption of the sorbate onto the surface of PA12 via hydrophobic interaction and absorption of the sorbate molecules into the polymer matrix of PA via strong hydrogen bonds on accessible amide groups (Han et al. 2013a). The strong interactions of polyamide, especially PA12, has led to the suggestion for this material to be highly promising for water treatment in comparison with high-surface-area sorbents typically used for such applications, such as activated carbon or polystyrene-polyvinyl butyral (PS-PVB) resins.
The increase in sorption among the sorbates investigated did not follow their trends in hydrophobicity. EE2 showed significantly strong sorption in comparison with the other sorbates, while sorption of E1 to PA was relatively weak in comparison with the other sorbates. This can again be explained by looking more closely at the molecular moieties of the two sorbates that can result in differences in the relevant molecular interactions. Unlike many other microplastics, PA in addition to hydrophobic, van der Waals and electron donor–acceptor interactions can undergo hydrogen-bond and Lewis acid–base interactions (Kohan 1995). The electron-deficient proton at the phenolic C1 position of E1 and EE2 has high affinity and can form strong hydrogen bonds with the electron-rich region of the oxygen-containing carbonyl functional groups in polyamide, which could explain the strong sorption of EE2 to PA (Liu et al. 2019). Additionally, the molecules of NOR and EE2 contain an electron-deficient proton in the hydroxyl group at C17 in the cyclopentane ring that can also undergo strong hydrogen-bond interaction with PA. The monosubstituted alkylene moiety at C17 of EE2 and NOR may also contribute to the hydrogen-bond interaction with PA (Liu et al. 2019). The molecules of E1 contain a carbonyl group (C17). The high electron density at the oxygen of this functional group makes this moiety a potential proton acceptor (Schäfer et al. 2011), which in turn may interact with the electron-deficient proton at the amine group of PA (Han et al. 2013b). In aqueous environments, this hydrogen-bond interaction of PA may be outcompeted by water molecules, which preferentially interact with amine protons in PA via hydrogen-bond interactions (Han et al. 2012). According to this argument, sorption to PA can be correlated with proton donating moieties of the sorbate molecule as follows: E1 (one strong donor) < NOR (one strong and one weak donor) << EE2 (two strong and one weak donor).
Sorption to both PA sorbents did not follow a strictly linear pattern, as indicated by the Freundlich coefficients (n), which ranged between 0.636 and 0.915 for PA6 and 0.869 and 0.907 for PA12. There was no general trend apparent in sorption linearity (based on the properties of the probe sorbates, i.e. their hydrophobicity and molar volumes). Owing to non-linear sorption to the two PA sorbents, the experimental isotherm data were fitted to non-linear isotherm models commonly used to fit sorption data to microplastics (Schwarzenbach et al. 2002; Hüffer and Hofmann 2016), i.e. to Freundlich model (FM), Langmuir model (LM), and Polanyi–Manes model (PMM). The fitting parameters obtained from the sorption model fit are shown in the Supplementary material (Tables S1–S3). For all sorbates, the FM and the PMM fitted the experimental data better than the LM, as indicated by the lowest standard error of estimates, SEE (Table 4). The FM is actually a special case of the PMM (when fitting parameter d = 1) (Condon 2000), which explains why both models may have yielded similar fits. Akaike’s Information Criterion (AIC) was calculated to ensure that the observed increase in goodness-of-fit was not the result of overparameterisation due to the increase in number of degrees of freedom from two-parameter models to three-parameter models (i.e. from the FM or LM to the PMM). The AIC can be used to quantify discrepancies between models (Kah et al. 2011; Hüffer et al. 2013) while taking into the consideration the degrees of freedom between models. The AIC values calculated for the data fit for all sorbates were FM ≤ PMM < LM (Table 4). In addition to the AIC values, the model probabilities and evidence ratios indicate that for the sorbates investigated, the FM and the PMM are superior to the LM (data not shown). As stressed earlier, the significance of the statistical fit of the experimental data and the conclusion that the data are better fitted using one sorption model rather than another clearly depend, among other factors, on the quality of the experimental data and the concentration range over which the sorption isotherms were determined. A comprehensive understanding of the models applied to fitting sorption isotherms is necessary to avoid not only misapplication but also inaccurate conclusions. The results of the model fit show a heterogeneous distribution of the sorption site energies of the sorbents investigated and indicate no saturation-limited sorbate monolayers had formed on the sorbent surfaces.
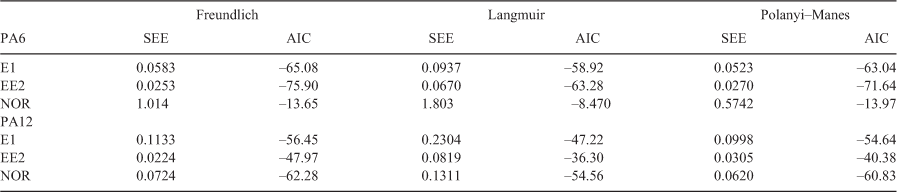
|
Comparing the images obtained from confocal light microscopy for PA6 and PA12 using Estradiol Glow as the probe molecule, the fluorescence intensities of PA6 particles are higher than the intensity of PA12 particles (Fig. 5). Fluorescence of PA6 particles is more evenly distributed over the particle surface compared with the PA12 particles. Furthermore, the PA6 particles did not only show fluorescence on the surface but also inside the particle. The fluorescence intensity was highest at the particle surface and decreased with increasing penetration depth. PA6 possesses a higher water absorption capacity (7–10 % w/w) compared with PA12 (1 % w/w), due to a shorter hydrocarbon chain length of the monomer caprolactam compared with the PA12 monomer laurolactam making the polymer more hydrophilic (Wypych 2016). Swelling of PA6 particles makes the peripheral regions of the particles accessible to the probe molecule. Evenly distributed fluorescence over the PA6 particles, and increased penetration depth of the sorbent compared with PA12 can be related to increased water swelling of the PA6 particles. To ensure that the sorption sites on the particles were not different for Estradiol Glow and EE2, a click reaction was performed for samples with EE2 after an equilibrium time. Fluorescence was detected at the particle surface and in the peripheral region as was shown with Estradiol Glow.

|
Conclusion
Polymer type and sorbent particle size are the two most frequently investigated factors influencing the interactions of plastics with xenobiotics. The results of this study clearly provide evidence that the presence of additives and the polymer structure (i.e. internal 3D polymer geometry and monomer chain length) additionally play an important role in sorption. Both factors are shown to alter sorption strength and also sorption mode in terms of adsorption onto the sorbent surface and partitioning into the bulk polymer. This has clear implication because for many effects of micro- and nanoplastics discussed in the scientific and general community, for instance the uptake and transport of both organic solutes, both factors are largely unaddressed. Future work should further explore the influence of additive presence and polymer structure on sorption to microplastics by extending, for example, the variety of molecular properties of sorbates, and type and quantity of additives in the sorbent. The strong interactions of organic substances to PA underline the importance of this material as highly promising for water treatment in comparison with high surface-area sorbents typically used for these applications such as activated carbon or PS-PVB resins.
Supplementary material
Experimental details on the confocal microscopy and fitting parameters of sorption isotherm models are available on the Journal’s website.
Conflicts of interest
Thilo Hofmann is a guest Associate Editor of Environmental Chemistry, but was blinded from the peer review process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We would like to acknowledge Dr Nikolaus Nestle and Dr Markus Rueckel from BASF SE (Ludwigshafen, Germany), for providing equipment, support for the CLSM measurements and supplying PA6 polymer. We further thank Charlotte Henkel from the University of Vienna for chemical analysis of the polymer additives.
References
Al Salloum H, Saunier J, Aymes-Chodur C, Barakat H, Yagoubi N (2015). Impact of the nature and concentration of plasticizers on the ability of PVC to sorb drug. International Journal of Pharmaceutics 496, 664–675.| Impact of the nature and concentration of plasticizers on the ability of PVC to sorb drugCrossref | GoogleScholarGoogle Scholar | 26561727PubMed |
Condon JB (2000). Equivalency of the Dubinin–Polanyi equations and the QM based sorption isotherm equation. B. Simulations of heterogeneous surfaces. Microporous and Mesoporous Materials 38, 377–383.
| Equivalency of the Dubinin–Polanyi equations and the QM based sorption isotherm equation. B. Simulations of heterogeneous surfacesCrossref | GoogleScholarGoogle Scholar |
Daimon M, Masumura A (2007). Measurement of the refractive index of distilled water from the near-infrared region to the ultraviolet region. Applied Optics 46, 3811–3820.
| Measurement of the refractive index of distilled water from the near-infrared region to the ultraviolet regionCrossref | GoogleScholarGoogle Scholar | 17538678PubMed |
Du L, Ni N, Li M, Wang B (2010). A fluorescent hydrogen peroxide probe based on a ‘click’ modified coumarin fluorophore. Tetrahedron Letters 51, 1152–1154.
| A fluorescent hydrogen peroxide probe based on a ‘click’ modified coumarin fluorophoreCrossref | GoogleScholarGoogle Scholar | 20204162PubMed |
Endo S, Takizawa R, Okuda K, Takada H, Chiba K, Kanehiro H, Ogi H, Yamashita R, Date T (2005). Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Marine Pollution Bulletin 50, 1103–1114.
| Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differencesCrossref | GoogleScholarGoogle Scholar | 15896813PubMed |
Environmental Specimen Bank (ESB) (2020). Environmental specimen bank (ESB). Available at https://www.umweltprobenbank.de/de/documents/profiles/analytes/25391 [verified 28 May 2020]
EU Commission (2011). Commission Regulation (EU) No 143/2011, 2011. Annex XIV to Regulation (EC) No. 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (‘REACH’).
EU Commission (2015). Commission Delegated Directive (EU) 2015/863, 2015. Annex II to Directive 2011/65/EU of the European Parliament and of the Council as Regards the List of Restricted Substances.
Fath A (2019). ‘Mikroplastik.’ (Springer: Berlin)
Fred-Ahmadu OH, Bhagwat G, Oluyoye I, Benson NU, Ayejuyo OO, Palanisami T (2020). Interaction of chemical contaminants with microplastics: Principles and perspectives. The Science of the Total Environment 706, 135978
| Interaction of chemical contaminants with microplastics: Principles and perspectivesCrossref | GoogleScholarGoogle Scholar | 31864138PubMed |
Fries E, Zarfl C (2012). Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE). Environmental Science and Pollution Research International 19, 1296–1304.
| Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE)Crossref | GoogleScholarGoogle Scholar | 22083414PubMed |
Gouin T, Roche N, Lohmann R, Hodges G (2011). A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environmental Science & Technology 45, 1466–1472.
| A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplasticCrossref | GoogleScholarGoogle Scholar |
Guo X, Wang X, Zhou X, Kong X, Tao S, Xing B (2012). Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environmental Science & Technology 46, 7252–7259.
| Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical compositionCrossref | GoogleScholarGoogle Scholar |
Guo X, Chen C, Wang J (2019a). Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 228, 300–308.
| Sorption of sulfamethoxazole onto six types of microplasticsCrossref | GoogleScholarGoogle Scholar | 31035168PubMed |
Guo X, Liu Y, Wang J (2019b). Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Marine Pollution Bulletin 145, 547–554.
| Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation studyCrossref | GoogleScholarGoogle Scholar | 31590822PubMed |
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P (2018). An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Journal of Hazardous Materials 344, 179–199.
| An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recyclingCrossref | GoogleScholarGoogle Scholar | 29035713PubMed |
Han J, Qiu W, Hu J, Gao W (2012). Chemisorption of estrone in nylon microfiltration membranes: Adsorption mechanism and potential use for estrone removal from water. Water Research 46, 873–881.
| Chemisorption of estrone in nylon microfiltration membranes: Adsorption mechanism and potential use for estrone removal from waterCrossref | GoogleScholarGoogle Scholar | 22189293PubMed |
Han J, Cao Z, Gao W (2013a). Remarkable sorption properties of polyamide 12 microspheres for a broad-spectrum antibacterial (triclosan) in water. Journal of Materials Chemistry. A, Materials for Energy and Sustainability 1, 4941–4944.
| Remarkable sorption properties of polyamide 12 microspheres for a broad-spectrum antibacterial (triclosan) in waterCrossref | GoogleScholarGoogle Scholar |
Han J, Meng S, Dong Y, Hu J, Gao W (2013b). Capturing hormones and bisphenol A from water via sustained hydrogen bond driven sorption in polyamide microfiltration membranes. Water Research 47, 197–208.
| Capturing hormones and bisphenol A from water via sustained hydrogen bond driven sorption in polyamide microfiltration membranesCrossref | GoogleScholarGoogle Scholar | 23127621PubMed |
Hartmann N, Hüffer T, Thompson R, Hassellöv M, Verschoor A, Daugaard A, Rist S, Karlsson T, Brennholt N, Cole M, Herrling M, Hess M, Ivleva N, Lusher A, Wagner M (2019a). Response to the letter to the editor regarding our feature ‘Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris’. Environmental Science & Technology 53, 4678–4679.
| Response to the letter to the editor regarding our feature ‘Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris’Crossref | GoogleScholarGoogle Scholar |
Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, Heß M, Ivleva NP, Lusher AL, Wagner M, Hess MC, Ivleva NP, Lusher AL, Wagner M (2019b). Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science & Technology 53, 1039–1047.
| Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debrisCrossref | GoogleScholarGoogle Scholar |
Henkel C, Hüffer T, Hofmann T (2019). The leaching of phthalates from PVC can be determined with an infinite sink approach. MethodsX 6, 2729–2734.
| The leaching of phthalates from PVC can be determined with an infinite sink approachCrossref | GoogleScholarGoogle Scholar | 31788438PubMed |
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017). Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. The Science of the Total Environment 586, 127–141.
| Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research prioritiesCrossref | GoogleScholarGoogle Scholar | 28169032PubMed |
Hüffer T, Hofmann T (2016). Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environmental Pollution 214, 194–201.
| Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solutionCrossref | GoogleScholarGoogle Scholar | 27086075PubMed |
Hüffer T, Kah M, Hofmann T, Schmidt TC (2013). How redox conditions and irradiation affect sorption of PAHs by dispersed fullerenes (nC60). Environmental Science & Technology 47, 6935–6942.
| How redox conditions and irradiation affect sorption of PAHs by dispersed fullerenes (nC60)Crossref | GoogleScholarGoogle Scholar |
Hüffer T, Weniger A-K, Hofmann T (2018). Sorption of organic compounds by aged polystyrene microplastic particles. Environmental Pollution 236, 218–225.
| Sorption of organic compounds by aged polystyrene microplastic particlesCrossref | GoogleScholarGoogle Scholar | 29414343PubMed |
Kah M, Zhang X, Jonker MTO, Hofmann T (2011). Measuring and modeling adsorption of PAHs to carbon nanotubes over a six order of magnitude wide concentration range. Environmental Science & Technology 45, 6011–6017.
| Measuring and modeling adsorption of PAHs to carbon nanotubes over a six order of magnitude wide concentration rangeCrossref | GoogleScholarGoogle Scholar |
Koelmans AA, Bakir A, Burton GA, Janssen CR (2016). Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environmental Science & Technology 50, 3315–3326.
| Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studiesCrossref | GoogleScholarGoogle Scholar |
Kohan MI (1995). ‘Nylon plastics handbook.’ (Hanser Publications: Liberty Township, OH)
Kunz D (2009). Klick-Chemie. Synthesen, die gelingen. Chemie in Unserer Zeit 43, 224–230.
| Klick-Chemie. Synthesen, die gelingenCrossref | GoogleScholarGoogle Scholar |
Li J, Zhang K, Zhang H (2018). Adsorption of antibiotics on microplastics. Environmental Pollution 237, 460–467.
| Adsorption of antibiotics on microplasticsCrossref | GoogleScholarGoogle Scholar | 29510365PubMed |
Liu X, Xu J, Zhao Y, Shi H, Huang C-H (2019). Hydrophobic sorption behaviors of 17β-estradiol on environmental microplastics. Chemosphere 226, 726–735.
| Hydrophobic sorption behaviors of 17β-estradiol on environmental microplasticsCrossref | GoogleScholarGoogle Scholar | 30959457PubMed |
Liu P, Zhan X, Wu X, Li J, Wang H, Gao S (2020). Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 242, 125193
| Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risksCrossref | GoogleScholarGoogle Scholar | 31678851PubMed |
Ma J, Zhao J, Zhu Z, Li L, Yu F (2019). Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environmental Pollution 254, 113104
| Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chlorideCrossref | GoogleScholarGoogle Scholar | 31472455PubMed |
Müller R, Schönbauer S (2020). Zero Waste – Zero Justice?. Engaging Science, Technology, and Society 6, 416–420.
| Zero Waste – Zero Justice?Crossref | GoogleScholarGoogle Scholar |
Müller A, Becker R, Dorgerloh U, Simon F-G, Braun U, Müller A, Becker R, Dorgerloh U, Simon F-G, Braun U (2018). The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics. Environmental Pollution 240, 639–646.
| The effect of polymer aging on the uptake of fuel aromatics and ethers by microplasticsCrossref | GoogleScholarGoogle Scholar | 29772514PubMed |
Pence HE, Williams A (2010). ChemSpider database. Available at http://www.chemspider.com
Pignatello JJ (1998). Soil organic matter as a nanoporous sorbent of organic pollutants. Advances in Colloid and Interface Science 76–77, 445–467.
| Soil organic matter as a nanoporous sorbent of organic pollutantsCrossref | GoogleScholarGoogle Scholar |
Pignatello JJ, Lu Y, LeBoeuf EJ, Huang W, Song J, Xing B (2006). Non-linear and competitive sorption of apolar compounds in black carbon-free natural organic materials. Journal of Environmental Quality 35, 1049–1059.
| Non-linear and competitive sorption of apolar compounds in black carbon-free natural organic materialsCrossref | GoogleScholarGoogle Scholar | 16738390PubMed |
Plastics Europe (2020). ‘Plastics – the facts 2019. An analysis of European plastics production, demand and waste data.’ (Plastics Europe: Brussels)
Qiu Y, Zheng M, Wang L, Zhao Q, Lou Y, Shi L, Qu L (2019). Sorption of polyhalogenated carbazoles (PHCs) to microplastics. Marine Pollution Bulletin 146, 718–728.
| Sorption of polyhalogenated carbazoles (PHCs) to microplasticsCrossref | GoogleScholarGoogle Scholar | 31426214PubMed |
Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, Athey S, Huntington A, McIlwraith H, Munno K, De Frond H, Kolomijeca A, Erdle L, Grbic J, Bayoumi M, Borrelle SB, Wu T, Santoro S, Werbowski LM, Zhu X, Giles RK, Hamilton BM, Thaysen C, Kaura A, Klasios N, Ead L, Kim J, Sherlock C, Ho A, Hung C (2019). Rethinking microplastics as a diverse contaminant suite. Environmental Toxicology and Chemistry 38, 703–711.
| Rethinking microplastics as a diverse contaminant suiteCrossref | GoogleScholarGoogle Scholar | 30909321PubMed |
Schäfer AI, Akanyeti I, Semião AJC (2011). Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Advances in Colloid and Interface Science 164, 100–117.
| Micropollutant sorption to membrane polymers: A review of mechanisms for estrogensCrossref | GoogleScholarGoogle Scholar | 21106187PubMed |
Schwarzenbach RP, Gschwend PM, Imboden DM (2002). ‘Environmental organic chemistry.’ (John Wiley & Sons: Hoboken, NJ)
Seidensticker S, Grathwohl P, Lamprecht J, Zarfl C (2018). A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environmental Sciences Europe 30, 30
| A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplasticsCrossref | GoogleScholarGoogle Scholar | 30148026PubMed |
Sigmund G, Hüffer T, Hofmann T, Kah M (2017). Biochar total surface area and total pore volume determined by N2 and CO2 physisorption are strongly influenced by degassing temperature. The Science of the Total Environment 580, 770–775.
| Biochar total surface area and total pore volume determined by N2 and CO2 physisorption are strongly influenced by degassing temperatureCrossref | GoogleScholarGoogle Scholar | 27964990PubMed |
Sørensen L, Rogers E, Altin D, Salaberria I, Booth AM (2020). Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environmental Pollution 258, 113844
| Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditionsCrossref | GoogleScholarGoogle Scholar | 31874435PubMed |
Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Björn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 2027–2045.
| Transport and release of chemicals from plastics to the environment and to wildlifeCrossref | GoogleScholarGoogle Scholar |
The Freedonia Group (2014). ‘Speciality plastic additives – Industry market research for business leaders, strategists and decision makers.’ (The Freedonia Group: Cleveland, OH)
Uber TH, Hüffer T, Planitz S, Schmidt TC (2019a). Sorption of non-ionic organic compounds by polystyrene in water. The Science of the Total Environment 682, 348–355.
| Sorption of non-ionic organic compounds by polystyrene in waterCrossref | GoogleScholarGoogle Scholar | 31125748PubMed |
Uber TH, Hüffer T, Planitz S, Schmidt TC (2019b). Characterization of sorption properties of high-density polyethylene using the poly-parameter linear free-energy relationships. Environmental Pollution 248, 312–319.
| Characterization of sorption properties of high-density polyethylene using the poly-parameter linear free-energy relationshipsCrossref | GoogleScholarGoogle Scholar | 30802745PubMed |
Wagner M, Scherer C, Alvarez-Muñoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T, Rodriguez-Mozaz S, Urbatzka R, Vethaak A, Winther-Nielsen M, Reifferscheid G (2014). Microplastics in freshwater ecosystems: what we know and what we need to know. Environmental Sciences Europe 26, 12
| Microplastics in freshwater ecosystems: what we know and what we need to knowCrossref | GoogleScholarGoogle Scholar | 28936382PubMed |
Walker CW, Watson JE (2010). Adsorption of estrogens on laboratory materials and filters during sample preparation. Journal of Environmental Quality 39, 744–748.
| Adsorption of estrogens on laboratory materials and filters during sample preparationCrossref | GoogleScholarGoogle Scholar | 20176847PubMed |
Wang W, Wang J (2018). Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 193, 567–573.
| Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplasticsCrossref | GoogleScholarGoogle Scholar | 29169132PubMed |
Wypych G (2016). ‘Handbook of polymers, 2nd edn.’ (ChemTec Publishing: Toronto)
Xing B, Pignatello JJ (1997). Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environmental Science & Technology 31, 792–799.
| Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matterCrossref | GoogleScholarGoogle Scholar |
Xu P, Ge W, Chai C, Zhang Y, Jiang T, Xia B (2019). Sorption of polybrominated diphenyl ethers by microplastics. Marine Pollution Bulletin 145, 260–269.
| Sorption of polybrominated diphenyl ethers by microplasticsCrossref | GoogleScholarGoogle Scholar | 31590785PubMed |




