Surficial geochemistry and bioaccessibility of tellurium in semiarid mine tailings
Sarah M. Hayes A C and Nicole A. Ramos B
A C and Nicole A. Ramos B
A Eastern Mineral and Environmental Resources Science Center, US Geological Survey, Reston, VA 20192, USA.
B Department of Chemistry and Biochemistry, University of Alaska Fairbanks, Fairbanks, AK 99775, USA.
C Corresponding author. Email: shayes@usgs.gov
Environmental Chemistry 16(4) 251-265 https://doi.org/10.1071/EN18215
Submitted: 12 October 2018 Accepted: 11 February 2019 Published: 20 March 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Environmental context. Tellurium can be more toxic than arsenic, but its fate in the surficial environment is poorly understood. We studied tellurium behaviour in semiarid mine tailings and found that most tellurium is associated with iron (oxy)hydroxides as tellurate (TeVI), the less toxic oxidation state. Iron (oxy)hydroxides are likely to control the fate of Te in the surficial environment and may effectively sequester Te oxyanions released by oxidative weathering.
Abstract. Tellurium (Te) is a critical element owing to its use in solar technology. However, some forms are highly toxic. Few studies have examined Te behaviour in the surficial environment, thus little is known about its potential human and environmental health impacts. This study characterises two physicochemically distinct Te-enriched mine tailings piles (big and flat tailings) deposited by historic gold (Au) mining in the semiarid Delamar mining district, Nevada, USA. The big tailings are characterised by smaller particle size and higher concentrations of potentially toxic elements (up to 290 mg Te kg−1), which are enriched at the tailings surface. In contrast, the flat tailings have larger particle size and properties that are relatively invariant with depth. Based on the sulfate to sulfide ratio, the tailings were determined to be sulfate dominated, which suggested a high degree of weathering, although the flat tailings did contain significant amounts of sulfides (~40 %). Tellurium X-ray absorption spectroscopy of the big tailings indicates that tellurate, the less toxic Te species, is the principal form of Te. Electron microscopy indicates that most of the Te present at the site is associated with iron (oxy)hydroxides, and sometimes with other potentially toxic elements, especially lead and antimony. Physiologically-based extraction tests indicate that substantially more Te is solubilised in synthetic stomach fluids than in lung fluids, with gastric bioaccessibility ranging from 13 to 31 % of total Te. This points to low to medium bioaccessibility, which is common for iron (oxy)hydroxide-associated elements. Together, these results represent a preliminary assessment of Te surficial behaviour in a semiarid environment and indicate that Te in these tailings represent a moderate health concern.
Introduction
Tellurium (Te) is a critical element owing to its use in a variety of high-technology applications, primarily CdTe-based photovoltaics and thermoelectric-based devices (Anderson 2015; Hayes and McCullough 2018). Despite the high toxicity of some forms, relatively little is known about the behaviour of Te in surficial environments owing to a low average upper crustal abundance of ~5 µg Te kg−1 (Taylor 1996; Wedepohl 1995). Thus, concerns exist that increased industrial demand and geographic redistribution of Te may result in elevated environmental concentrations that could affect human and environmental health (Cobelo-Garcia et al. 2015).
As has been well established for other potentially toxic elements, Te toxicity is influenced by its speciation (e.g. Plumlee et al. 2005). Rat mortality studies indicate that sodium tellurite (Na2TeO3) is 10 times more toxic than sodium tellurate (Na2TeO4). Further, Na2TeO3 can also be more toxic than sodium selenite (Na2SeO3) and sodium arsenite (Na2HAsO3) (Franke and Moxon 1936; Taylor 1996). Exposure to either Te oxyanion induced decreased growth rates and weight loss in rats (Franke and Moxon 1937). One limited human-based study, in which Te was administered at doses that did not produce acute toxic symptoms, demonstrated that the fraction of intestinal absorption was highly variable by individual and form in which each compound was administered (Kron et al. 1991). This underscores the importance of speciation on biological toxicity and lends insight into the potential toxicity of relevant forms of Te in the surficial environment.
In the ore-forming environment, depending on the hypogene conditions (e.g. pH, temperature and pressure, oxidation-reduction potential, oxygen fugacity, and proton availability), Te is typically deposited as telluride minerals, in solid solution or as nano-inclusions in sulfides, or as native Te (Keith et al. 2018; Kelley and Spry 2016; Reich et al. 2005). Oxidative weathering, either within the ore deposit or in mine wastes post-deposition, will transform these primary minerals to more thermodynamically favoured, TeIV and TeVI oxyanion-containing, secondary phases. However, the identity and formation controls of these stable secondary species are largely unknown.
There is a paucity of studies measuring thermodynamic properties upon which to develop models to predict the behaviour of Te, even at standard conditions (25 °C and 1 bar). An example of this are the Pourbaix diagrams shown in Fig. 1, in which two thermodynamic datasets predicted dramatically different stability fields of aqueous Te oxyanions under the same conditions (Brookins 1988; McPhail 1995). The discrepancy between the Pourbaix diagrams highlights the necessity for additional work to constrain the fundamental thermodynamics of Te behaviour, which is critical given the differences in the toxicity of the Te oxyanions. Laboratory-based thermodynamic studies must be complemented by direct field observations of Te speciation, like the work presented here.

|
Tellurium concentrations in environmental samples have been recently reviewed (Belzile and Chen 2015); however, few studies report Te in mining-impacted environments (Cowgill 1988; Moreno et al. 2007; Perkins 2011; Qin et al. 2017). The most pertinent of these studies examined Te speciation in soils from an abandoned mine site using micro-focused X-ray fluorescence (XRF) mapping and micro-focused X-ray absorption near edge structure (XANES) spectroscopy (Qin et al. 2017). Based on micro-focused XRF elemental co-location, this study found that Te is associated primarily with FeIII hydroxides, but that Te can also be associated with other minerals (e.g., illite). Micro-focused XANES revealed that all points interrogated contained a mixture of tellurite (TeIV) and tellurate (TeVI), which indicated that both oxidation states can co-exist in a solid phase at a micro-scale. In a related study, extended X-ray absorption fine structure (EXAFS) analysis demonstrated that both TeIV and TeVI formed inner-sphere surface complexes with FeIII (oxy)hydroxides in an artificially contaminated soil matrix (Harada and Takahashi 2008). Extreme Te enrichment, up to 50 000 times the average crustal abundance, has been observed in ferromanganese nodules and crusts (Hein et al. 2003). EXAFS analysis of natural marine crusts and nodules, and laboratory-generated sorption samples have also demonstrated the strong affinities of Te oxyanions for Mn and FeIII (oxy)hydroxides (Kashiwabara et al. 2014). However, additional evidence of Te speciation in a variety of surficial environmental conditions is warranted.
The Te oxidation state and properties of secondary Te-bearing phases, which are largely unknown, directly control the mobility and bioaccessibility of Te in the surficial environment and the concomitant community and ecosystem health risks (Jamieson 2011). In this study, Te-containing mine tailings from semiarid Delamar, Nevada, USA, were examined using bulk and grain-scale techniques to assess the solid Te-bearing phases. Bulk elemental composition, mineralogy, sulfur (S) and Te speciation, Te-bearing phases, and Te bioaccessibility were determined to provide insight into the surficial behaviour of Te in a semiarid environment. These results contribute to resolving the fundamental questions of the dominant Te oxidation state in the surficial environment and aid in assessing human and environmental health risks associated with Te-rich geomedia. Further, this system serves as a proxy for future high technology industry wastes that may be released into a similar surficial environment.
Experimental
Site description
The historic Delamar mining district (Lincoln County, Nevada, USA) is located in a cold semiarid climate (Köppen-Geiger class: BSk) ~48 km south-west of the nearest city, Caliente, and ~340 km north-east of Las Vegas (Fig. 2a; Chau and Ralph 1993; Kottek et al. 2006). Almost all of the deposits mined in the Delamar (or Ferguson) district were epithermal fissure veins hosted by the Prospect Mountain Quartzite (Tschanz and Pampeyan 1970). The ores consisted of: (1) primarily quartzite breccia cemented by cherty quartz near mafic dikes; (2) cherty quartz veins; (3) silicified quartzite; (4) bedded quartzite; and (5) volcanic breccia and rhyolite dikes brecciated and cemented by comb quartz (Callaghan 1937; Tschanz and Pampeyan 1970). Free Au and Ag were reported (Au : Ag ratio 1 : 3), if seldom observed, in addition to a variety of sulfide and sulfosalt minerals, including pyrite, chalcopyrite, bornite, chalcocite, tetrahedrite, tennantite, sphalerite, and galena. Barite and some fine grained unidentified telluride minerals were also reported in mineralised veins (Callaghan 1937; Emmons 1902). At the Delamar Mine, the principal mine in the district, ore grades increased from the surface to the 7th level and then decreased sharply below the 10th level where unoxidised pyritic ore was encountered, which indicated supergene enrichment processes and oxidation of the ore-body before extraction (Emmons 1902; Tschanz and Pampeyan 1970).

|
The Delamar district was mined from 1892 through 1909 and produced a total of 18 t Au; the Delamar Mine produced more than 5.7 t Au and 11.3 t Ag (Callaghan 1937; Mason and Arndt 1996; McFaul et al. 2000; Tschanz and Pampeyan 1970). After a brief period of Au recovery by chlorination (1895), ore was nearly exclusively leached using cyanide (1895–1909) with good Au recoveries (94–97 % reported in 1896). In 1903, the mill was refitted and began to treat free Au ores separate from telluride- and sulfide-bearing ores. Mining permanently ceased at Delamar in 1909 leaving two large tailings piles, termed the circular tailings and big tailings. From 1932 to 1940, regrinding and cyanide leaching were performed on 532 000 t of the richer early tailings. The tailings were redeposited on site creating a third tailings pile, known as the flat tailings (Callaghan 1937; Tschanz and Pampeyan 1970). There is no report that flotation was used as a processing method in this district. At least 408 000 t of mine tailings remain at the site in several physically and chemically distinct piles. This study focuses on the big and flat tailings piles, located at 37.45763, −114.77478 and 37.45892, −114.77739, respectively using NAD83 spheroid and WGS84 datum (Fig. 2).
Sampling
Samples collected included big tailings (a surficial grab sample and a 60 cm core), flat tailings (4 grab samples at a variety of depths and a 120 cm core), surficial sediments (0–2 cm depth, collected from 8.0, 8.1, and 22 km downstream of the tailings piles), and an off-site control collected 1.8 km to the NW of the tailings. Sample identification numbers and their collection depth are tabulated in Table 1. Grab samples were collected in Ziploc bags and core samples were collected in plastic sleeves before being frozen in the field using dry ice and placed in Anaerojars (grab samples) or large Ziploc bags (cores) with Anaeropac oxygen scrubbers and indicators (Mitsubishi Gas Chemical, Japan). All samples remained frozen and isolated from atmospheric oxygen before analysis or preservation by freeze-drying. Frozen cores were quickly sectioned in 5-cm intervals using a Dremel tool to cut open the plastic core tube in an aerobic environment before freeze drying each subsample. Samples were either thawed for analysis of physical properties and pH (all samples except off-site control), kept frozen for synchrotron experiments (grab sample of big tailings), or freeze-dried before X-ray fluorescence (all samples), X-ray diffraction (XRD; surficial grab samples of tailings and deepest core samples), physiologically-based extraction tests (PBETs; grab samples of tailings and downstream sediments), and preparation of thin sections.
Physicochemical properties
Percent moisture was determined by mass loss resulting from 24 h drying at 100 °C. Colour was determined on thawed, field capacity samples and on oven-dried samples using a Munsell soil colour chart (Munsell Color; Grand Rapids, MI, USA). Particle size analysis was performed on selected samples at the University of Arizona Center for Environmental Physics and Mineralogy (Tucson, AZ, USA) using an LS 13 320 Laser Diffraction Particle Size Analyser (Beckman Coulter; Brea, CA, USA). Additional size fractionation was performed by dry sieving (Cole-Parmer sieves; Vernon Hills, IL, USA) to recover <250 µm and <38 µm size fractions for gastric and lung PBETs. Tailings pH was measured in triplicate using a 1 : 1 solid to solution ratio reacted for 24 h in an end-over-end mixer before separation using centrifugation, filtration, and measurement of supernatant pH and electrical conductivity (EC).
Bulk mineralogy
Bulk mineralogy of flat tailings (surficial 362 and 358 A-D, and lowest depth 361 D-F) and big tailings (surficial 368 and 366 A-D, and lowest depth 367 D-F) were examined using XRD at the US Geological Survey in Reston, VA, USA. Sample splits were micronised in isopropyl alcohol using a McCrone microniser (Westmont, IL, USA) before being loaded into 3-cm side-loaded Al sample holders. Samples were analysed using a PANalytical X’Pert PRO powder diffractometer (The Netherlands) with Co Kα radiation at 45 kV and 40 mA for all samples. Patterns were collected from 3 to 70 ° (2θ) with a step size of 0.0167 ° (2θ) and counting times of 200 s per point. Pattern interpretation was conducted using Reitveld refinements with X’Pert HighScore Plus software (PANalytical; version 4.7) and reference patterns, which included structure data, from the ICSD Database FIZ Karlsruhe 2013.
Oriented clay XRD was performed at the University of Arizona Center for Environmental Physics and Mineralogy (Tucson, AZ, USA) on samples 362, 363, 364, 365, and 368. Clay-size fractions were separated by gravity and prepared as oriented clay slides (Moore and Reynolds 1989). Analyses were performed using an X’Pert Pro MPD X-ray diffractometer (PANalytical; The Netherlands) with Cu Kα radiation at 45 kV and 40 mA. Samples were scanned from 2 to 35 ° (2θ) at a step size of 0.04 ° (2θ) with a counting time of 3 s per step. Mineral phase identification was performed using X’Pert HighScore Plus software.
Elemental composition
Analysis of major elements was performed by wavelength dispersive XRF (WDXRF) on ground samples pressed into pellets using 1.37 × 108 Pa pressure for 2 min with a polyvinyl alcohol binder. Analyses were performed on an Axios WDXRF (PANalytical; Westborough, MA, USA), equipped with a LiF 220 crystal, scintillation detector, and 150 µm collimator operating at 60 keV and 66 mA, located in the Advanced Instrumentation Laboratory (AIL) at University of Alaska Fairbanks (UAF). A pressed pellet of NIST 2780 was also analysed and values agreed to within 11 % for all elements reported, except Te. Tellurium was measured at 10 mg kg−1 and has a reported provisional value of 5 mg kg−1, which is near the estimated detection limit of the technique. The elements quantified included major elements reported as oxides (Al2O3, BaO, CaO, Fe2O3, K2O, Na2O, MgO, MnO, P2O5, SiO2, TiO2) and minor elements (Ag, As, Bi, Cu, Mo, Ni, Pb, S, Sb, Se, Te, Zn); however, only selected elements were reported. The sum of the components totalled between 91 wt-% and 104 wt-%. The values reported for SiO2 were normalised to the totals for each sample to mitigate the apparent differences in SiO2 content arising from the differences in the sum of all components measured by WDXRF. Total and inorganic carbon (C) were analysed at the Cold Regions Research and Engineering Laboratory (CRREL; Fairbanks, AK, USA) using a TOC-L analyser (Shimadzu; Columbia, MD, USA) equipped with a Solid Sample Combustion Unit (SSM-5000A). A 5-point external calibration was performed daily using anhydrous dextrose (BDH) for total C and sodium bicarbonate (BDH) for inorganic C. Quality control checks were performed using potassium hydrogen phthalate (Alpha Aesar) every 10 samples. Elemental correlations were examined using SAS JMP statistical software (version 14.0.0; Cary, NC, USA).
Physiologically-based extraction tests
Surficial Delamar tailings, streambed sediments and several reference minerals were subjected to PBETs to determine the fraction of sample that would dissolve in simulated gastric and alveolar fluids (Drysdale et al. 2012; EPA 2012; Takaya et al. 2006). Each reference mineral, CdTe (Alpha Aesar), Na2TeO3 (Alpha Aesar) and Na2TeO4 (Alpha Aesar), was diluted 50× in quartz before PBETs to better replicate the sample matrix (Schaider et al. 2007). Briefly, samples were reacted in a 1 : 100 solid to solution ratio with a preheated synthetic gastric (0.4 M glycine solution adjusted to pH 1.5 using OmniTrace HCl) or alveolar fluid (freshly prepared modified Gamble’s solution; Takaya et al. 2006), and incubated and shaken at 37 °C at 60 rpm in the dark for 1 h for the gastric and 7 days for the lung extraction. Experiments were terminated by centrifuging (8500 g for 10 min) before decanting and filtering the supernatant using an acid washed 0.2-µm polypropylene filter (Acrodisc GHP). The pH of the supernatant was measured before acidification to pH <2 using OmniTrace nitric acid. Quantification of metal(loid)s in aqueous supernatants was performed using inductively coupled plasma-mass spectrometry (ICP-MS). Quantification of metal(loid)s in triplicate aqueous supernatants, quantitatively diluted by mass, was performed using an Agilent 7500ce ICP-MS (Santa Clara, CA, USA) located in AIL at UAF. Appropriate external and internal (i.e. Ge72, Y89, Rh103, and Ir193) calibration was used and a blank and calibration check were performed every 10 samples. Each session, the standard reference materials (SRMs) SLRS-5 and NIST-1640 were analysed in addition to a Te calibration check of a known concentration, since the SRMs do not report Te values. After verifying the data passed quality control measures, triplicate data were averaged, the method blank was subtracted, and error associated with the triplicate measurements was propagated.
Sulfur and tellurium speciation
Data collection
XANES was conducted at the Stanford Synchrotron Radiation Lightsource (SSRL) on beam lines 4–3 for S, and 4–1 and 11–2 for Te. Sulfur samples were prepared in a glove box by applying a thin layer of sample to S-free Mylar tape with a thin polypropylene cover before being individually transported to the beam line in Ziploc bags filled with N2 for immediate analysis. Data were collected at a maximum beam current of 500 mA with a Si (111) phi = 0 monochromator using 2-mm vertical slits in fluorescence geometry using a passivated implanted planar silicon (PIPS) detector. Sulfur XANES measurements were conducted in a He atmosphere at room temperature and scans were acquired between 2445 eV and at least 3200 eV using 0.15 eV steps in the XANES region. Energy calibration was checked between every sample and performed by setting the maximum of the first peak of sodium thiosulfate to 2472.0 eV.
Tellurium XANES samples were prepared for analysis as packed powders in thin aluminium sample holders; references were prepared as powder smears on kapton tape. Data were collected at a maximum beam current of 350 or 500 mA with a Si (220) phi = 0 and phi = 90 monochromator crystal on beamline 4–1 and 11–2, respectively. The beam line was configured with 2-mm vertical slits in transmission or fluorescence geometry using an ion chamber, a Lytle detector, or a 13- or 100-element Ge array detector. The samples were maintained at <77 K using a liquid nitrogen cryostat (Debye temperature of Te metal is 139 K; Walford et al. 1968) and XANES data were acquired between 31580 eV and at least 32474 eV (k = 13) using 0.05-eV steps in the XANES region. Energy calibration was performed by assigning the maximum of the first derivative of TeO2 to 31814 eV. Calibration spectra were collected for each scan using in-line calibration, with a TeO2 reference standard placed behind the sample between the second and third ion chambers, and using lead shielding to isolate the fluorescence detector from scattered X-rays.
Data reduction and analysis
SIXPACK (version 0.58) was used to process and analyse all XANES data (Webb 2005). The energy calibrated and averaged spectra were normalised to an edge step of one using a linear pre-edge region −200 to −15 for S and −300 to −130 for Te that was extended through the data and a quadratic post edge beginning at 40 for S and 100 for Te that continued through to the end of the data.
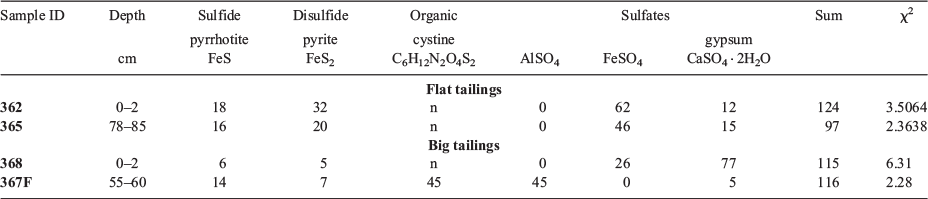
A linear combination fitting (LCF) routine was applied to both S and Te XANES using SIXPACK. Sulfur LCFs were performed using S reference spectra from Hayes et al. (2014), which were collected under similar conditions. Matrix fits were used for initial fits of specific data regions, including the sulfide region (2465–2480 eV), the sulfate region (2480–2515 eV) and the final fit range (2465–2487 eV). A uniform subset of references was selected for subsequent fits of all samples based on matrix fit statistics and visual inspection of the spectra.
Specific references selected were meant to represent S oxidation states, and may not indicate the presence of a specific mineral itself. Final fits were performed over the entire data range using pyrrhotite to represent sulfides, pyrite to represent disulfides, cystine to represent an organic S-species, and aluminium, copper, and ferrous iron sulfates and gypsum to represent sulfate minerals. Thus, extensive sensitivity testing was performed using linear combination fits of various energy regions and combinations of reference spectra. Throughout this sensitivity testing, the ratios of sulfate, sulfide and organic S were consistent for each sample. The percentage of sulfates, sulfides and organic S fit varied by ±12 %, ±9 %, and ±6 % respectively between fits. The consistent ratios of S oxidation states observed pointed to robust fits, and the variation was similar to those previously reported for LCF fits of laboratory-prepared mixtures (Prietzel et al. 2011).
Linear combination fits of Te spectra were performed on both the XANES and the first derivative of the XANES spectra. These fits were complicated by the small energy shift observed between the white lines of Na2TeO3 and Na2TeO4, and limitations in the energy reproducibility at the beam line, which necessitated the use of an in-line energy calibration standard. Sensitivity testing was performed by using both 1- and 2-component fits of the spectra.
Grain-scale investigation of tellurium
Data collection
Electron microprobe analysis (EMPA) was performed on thin sections prepared as pucks by vacuum impregnation with EPOTEK 301–2FL epoxy at room temperature. Thin sections were generated from these pucks by Spectrum Petrographics (Vancouver, WA, USA) by sectioning to 30 µm, double polishing and mounting on a fused quartz slide. Thin sections were carbon coated (Edwards Carbon Coating System) before analysis with a JXA-8530F HyperProbe Electron Probe Microanalyser (JEOL; Peabody, MA, USA) at UAF AIL. The instrument was equipped with a field emission source, and a Thermo System 7 silicon drift detector (SDD) energy dispersive spectrometer (EDS). Point analyses were collected using variable magnification, a 20 kV accelerating voltage and beam current (range 15–72 nA).
The Feature Sizing software package of the Thermo-NSS software (version 4.0) was leveraged to efficiently locate Te-rich particles, as these particles are bright in backscattered images (owing to a high average atomic number). After adjusting the brightness and contrast to highlight high Z particles, the stage was automated to scan large areas of the thin sections for bright particles, and collect a brief (5 s) EDS spectrum from each particle. For particles containing >2 wt-% Te, based on the Te L lines, the software recorded a particle image and collected an additional 30 s EDS spectrum.
Processing Feature Sizing data
The EDS-determined X-ray spectrum for each Te-containing grain identified by Feature Sizing was quantified using the Thermo software standardless routine and manually quality controlled to ensure correct peak identification, especially in the case of known overlapping peaks (e.g. Pb, S, Bi). Data were sorted and any element present at >10 % was highlighted.
Unequivocal mineral identification was not possible in most cases owing to the small particle size (exposed surface area in the thin sections ranged from 8 to 18 000 μm2; median 18 μm2) and heterogeneity of many of the Te-containing particles. The sample area probed by the focused electron beam was ~2 μm2 and inevitably interacted with more than just the mineral grains of interest. This meant that each X-ray spectrum contained information about the phase of interest in addition to an unknown dilution factor of unknown composition (epoxy or other minerals). Thus, the presence of primary rock-forming elements in the X-ray spectrum was ignored (e.g. Si, Al, K, Ca). Further, mole ratios were calculated and used in tentative mineral identification in an effort to circumvent signal dilution from the matrix. Each data point was sorted and grouped based on elemental composition and potential minerals were identified using the International Mineralogical Association (IMA) database of mineral properties (Downs 2007).
Results
The two tailings collected from Delamar, NV, USA, were distinct based on the field observations. The flat tailings were topographically flat with sparse vegetation while the big tailings were deposited in an elongated mound that has steep topography, no vegetation and significant evidence of surface water erosion. The flat tailings were characterised by loose grains while the big tailings were cemented together, which made digging difficult. Samples were collected to depths of 120 cm and 60 cm for the flat and big tailings respectively. Neither tailings pile showed any obvious colour change with depth, based on a Munsell colour chart, which could indicate a redox gradient or the presence of reducing conditions at depth.
Physicochemical properties
The flat and big tailings samples, collected as a function of depth, were characterised by light grey to light brownish grey colour in the surficial flat tailings transitioning to dull brown and dull orange at depth. As shown in Table 1, all tailings also exhibited low moisture content (0.2–7.2 wt-%), circum-neutral to slightly basic pH (pH = 7.4–8.6) and low total S (0.01–0.53 wt-% S). These characteristics are not unusual for soils in semiarid regions or mine tailings from low sulfidation deposits (e.g. Lottermoser 2003; Mendez and Maier 2008). The EC measured in the surficial big tailings (19.7 dS m−1) was an order of magnitude higher than those measured deeper in the big tailings (1.4 dS m−1 at 45–60 cm), and 2–3 orders of magnitude higher than flat tailings (0.07–0.12 dS m−1; Table 1).
The median particle sizes of the surficial (0–2 cm depth) flat and big tailings were 210 and 43 μm respectively (Table 2). As such, the surficial flat tailings contained a much larger fraction of sand-size particles (8 % clay and silt-sized particles) relative to the big tailings (52 % clay and silt-sized particles), likely as a result of differences in ore treatment. The particle size distribution of the flat tailings was relatively invariant with depth, although the fraction of sand-size particles decreased slightly with increasing depth (92 to 87 %; Table 2).

|
Bulk mineralogy
The mineralogy of the Delamar tailings was largely invariant across the samples analysed. The tailings were primarily composed of quartz (>95 %) with minor amounts of feldspars, mostly potassium feldspars and some plagioclase. XRD indicated that there was less feldspar in the big tailings relative to the flat tailings. Other tentatively identified trace minerals included several clay minerals, rutile, ilmenite, orthopyroxene and garnet-group minerals. Iron oxides were also likely present in all samples, based on the electron microscopy, but could not be unambiguously identified by XRD. This may have arisen from the short-range order of some iron (oxy)hydroxides or their low abundance, indicated by low total Fe content. The XRD results are broadly consistent with the chemical composition of the tailings, with very high Si content; relatively low amounts of alkali and alkali earth metals; low Al, Ti, Mn, Fe and S contents. Further, the presence of only low amounts of mafic minerals are consistent with both literature on Delamar geology and the bulk chemistry (Table 1; Callaghan 1937).
Variable minor amounts of 10-Å clays were present in all samples, but were highest in the big tailings samples (estimated at ~0.5–2 wt-% of bulk), consistent with the presence of mica/illite. Oriented clay XRD results were consistent with bulk XRD in identifying quartz and 10-Å clay minerals, but they also identified small amounts 7-Å kaolinite group clays in all samples examined. The big tailings also contained a higher fraction of quartz in the clay-size fraction, which could have resulted from being ground to a finer particle size at the time of Au extraction.
Elemental composition
The majority of both tailings piles was composed of Si, with a higher amount in the flat tailings (range = 95–97 wt-% SiO2), relative to the big tailings (range = 91–93 wt-% SiO2). There was also slightly more Fe in the big tailings (average = 1.0 wt-% Fe) relative to the flat tailings (average = 0.7 wt-% Fe). Both elements were invariant with depth. Overall S content was low, as is expected in tailings generated from a low sulfidation deposit, but the big tailings contained about an order of magnitude more total S (Table 1). Sulfur content was largely invariant with depth in the flat tailings (range = 0.03–0.08 wt-%), although there was a slight enrichment in the highest moisture sample (15–30 cm). Surficial big tailings contained about twice as much S (0.52 wt-%) as samples collected from below the surface (range = 0.22–0.28 wt-% S in samples from below 15 cm). Total C was relatively low, but increased with depth in the flat tailings (85–900 mg kg−1); in most cases, about half the total C was present as inorganic C (44 to 522 mg kg−1). The Delamar tailings contained only low amounts of organic C (6 to 566 mg kg−1), as calculated from the difference between total and inorganic carbon.
Tellurium was highly enriched (by factors of 3000 to 58 200) relative to average crustal abundance. Other selected potentially toxic elements, as shown in Table 1, were also enriched to a much lesser degree (e.g. Sb, As, Cu, Pb and Bi). In some cases, these values exceeded the levels set for residential soils (all tailings for Sb and As and surficial for Pb) and domestic animal toxicity levels (all tailings for Pb and big tailings for Cu). Some trace element concentrations, especially Te, Cu and Pb, were much higher in the big tailings relative to the flat tailings. The concentrations of elements were relatively consistent in the flat tailings as a function of depth, with a slight increase in Te, Cu and Pb at the highest moisture content sample (15–30 cm) and in the lowest depth sampled. In the big tailings, Te and Pb, and to a lesser extent Bi, had a slight trend of surficial enrichment, but only As had a significant trend of increasing concentration with increasing depth. Further, the surficial big tailings grab sample (sample 368, collected 0–2 cm) tended to have higher concentrations of all potentially toxic elements relative to the corresponding core sample (sample 366A-D, collected from 0–15 cm), which suggested that the surficial enrichment was constrained to a thin layer at the surface of the tailings.
The playa sample was distinctly different from the tailings samples, while the streambed samples are of intermediate composition. The playa had much more total and inorganic C and Fe relative to the tailings and contained less SiO2 and potentially toxic elements. The off-site control contained similar SiO2 and Fe to the tailings, but much lower levels of potentially toxic elements (Table 1). The lack of surficial enrichment of potentially toxic elements in the control sample relative to the surficial tailings suggested that these elements originated in the tailings rather than being deposited from the atmosphere.
Several elements exhibited strong positive correlations with Te, which were statistically significant, including Pb, Cu and S with correlations of 0.99, 0.98 and 0.94 respectively (P < 0.0001; n = 20 samples). Antimony and As were correlated with one another (0.85) and to Bi with correlations of 0.92 and 0.77 respectively (P <0.0001). These elements, Sb and As, were also most strongly correlated with depth (0.52 and 0.53, respectively), although these correlations are only significant at P <0.02, and thus were much weaker than the other correlations observed.
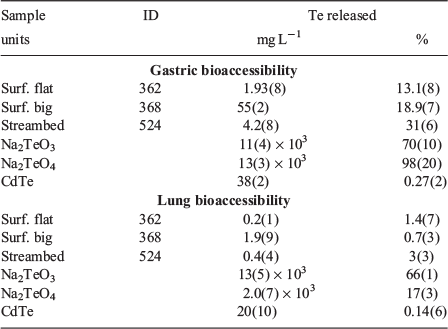
Bioaccessibility
Tellurium bioaccessibility was assessed for selected surficial samples using PBETs to simulate the physiological conditions present in the stomach and lungs. The fraction of Te released by the low pH gastric PBETs varied from 31 %, 19 % and 13 % for the streambed, surficial big and surficial flat tailings respectively (Table 3). The PBETs employing circum-neutral pH simulated lung fluids released a substantially smaller fraction of Te, 0.7 to 3 %. Reference minerals were also subjected to the same PBET extractions for comparison. The simulated gastric fluids liberated 70 %, 98 % and 0.27 % and lung PBET solutions solubilised 66 %, 17 % and 0.14 % of Na2TeO3, Na2TeO4 and CdTe respectively. The reference mineral PBETs may aid in interpreting the sample results, but represent an upper limit since they are not fully matrix matched and would not have the same potential complications and kinetic limitations for large particles or from rinding or encapsulation.
|
Sulfur speciation
Sulfur XANES, shown in Fig. 3, was used to assess the ratio of sulfides to sulfates as a proxy for the overall extent of mineral weathering, which may also serve as a useful indicator of Te behaviour. The reference minerals used during fitting are intended to represent classes of minerals (e.g. sulfides, disulfides, sulfates), rather than specific minerals (Table 4). Together, the results indicated that all tailings are sulfate-dominated with a significant amount of sulfides, especially in the flat tailings. The lowest big tailings sample also contained a substantial amount of organic S. The flat tailings contained the highest sulfide content that was relatively invariant with depth (50 and 36 % in the surficial and 78–85 cm sample respectively). In contrast, the big tailings fits contained mostly sulfates and only 11 and 21 % sulfides in the surficial and deep samples respectively. Surprisingly, sample 367F, the deepest big tailing sample collected, also contained peaks attributed to organic S, specifically a cystine-type moiety (C6H12N2O4S2), which was not observed in the other S XANES spectra, and thus not included in the other fits.

|
Tellurium speciation
Tellurium speciation was only examined in the surficial big tailings owing to Te in the flat tailings being below the detection limit of the instrument. Linear combination fits of Te K-edge XANES and XANES first derivative spectra indicated that Te was primarily present as the less toxic TeVI in the surficial big tailings (Fig. 4). The two-component fits indicated the best fit for both the XANES and first derivative XANES spectra were achieved with 74 % Na2TeO4 and 26 % Na2TeO3 (XANES fit χ2 = 0.0001; first derivative χ2 = 6 × 10−6). The two-component fits represented a significant (>10 % reduction to χ2) improvement over the single component fits with only Na2TeO4 (XANES fit with 91 % scalar and χ2 = 0.0003; first derivative χ2 = 9 × 10−6). Previous investigation of Te speciation in mine tailings also found that most Te was present in mixed-valance phases on the micro-scale (Qin et al. 2017).

|
Grain-scale investigation of tellurium
Backscatter images, shown in Fig. 5, were representative of the diversity of Te-bearing particles identified in the Delamar tailings. Several massive iron (oxy)hydroxide particles were identified, including the particle in Fig. 5a which contained an iron (oxy)hydroxide with 63 wt-% Fe, 7 % Te and 3 % Bi, and apparent zonation and several fractures. Fig. 5b shows another iron (oxy)hydroxide which contained 58 % Fe and 11 % Te, which was intergrown with a lower average atomic number phase, likely a silicate. Fig. 5c shows cervelleite (Ag4TeS) inclusions in quartz that were texturally related to Sn and Cu sulfide minerals and Fig. 5d exhibits hessite (Ag2Te) inclusions in quartz. Fig. 5e displays an aggregate of Sb, Te, Pb and O, which could be a mixture of PbTeO3 (fairbankite or plumbotellurite) with Pb2Sb2O7 (bindheimite, monimolite or oxyplumboromeite), since no mixed Pb-Sb-Te-O minerals have been recognised by IMA. Fig. 5f shows micrometre-sized native Te and Al2O3 particles. Many other sub-micrometre-sized high average atomic number (bright) particles, such as those shown in Fig. 5a, were not analysed owing to their small size, but could contain Te. Further, the small size of many of the Te-bearing particles identified has important implications for transport and weathering rates of Te-bearing materials.
Particles containing iron (oxy)hydroxides with secondary textures were much more abundant than the phases discussed above. For example, Fig. 5g, h shows an Fe content (~60 wt-%) consistent with Fe (oxy)hydroxides and significant Te concentrations (12 % and 5 %), respectively. Fig. 5i also illustrates secondary, lathe-like textures and a calculated stoichiometry of Mn : 2Te : 3O. This may potentially be spiroffite (Mn2Te3O8; the only exclusively Mn-Te-O mineral), although this does not match the calculated stoichiometry, or may be an intergrowth of Te or TeO2 and Mn-oxides. These point analyses demonstrated that the Delamar tailings had a wide variety of primary and secondary Te-bearing phases.
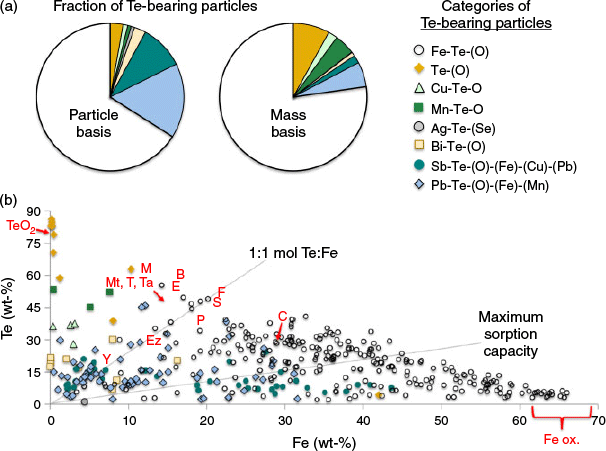
To better assess the relative abundance of Te elemental associations, large areas of several samples were scanned for bright particles using the backscattered electron images and interrogated using EDS to determine elemental compositions and identify Te-bearing particles (Fig. 6). The 391 Te-bearing particles identified were categorised on the basis of elemental associations. The relative abundance of these groups are shown in pie charts on both a particle and mass basis (Fig. 6a); 66 % of Te-bearing particles identified by Feature Sizing were associated with Fe (oxy)hydroxides, which accounted for 77 % of the Te mass. Native Te or TeO2 was also identified, which contributed 3 % based on particle counts but 8 % of Te by mass owing to the large mass fraction of Te. A small population of Te associated with Cu and O was also isolated as having consistent stoichiometric ratios, tentatively identified as frankhawthornite (Cu2TeO4(OH)2). Similarly, another group containing Te, Mn and O with stoichiometric ratios similar to spiroffite (Mn2Te3O8) was identified, but these particles could also be intergrowths of MnO2 and Te or TeO2. A few silver tellurides and selenides were also identified: hessite (Ag2Te), cervelleite (Ag4TeS) and Te-associated naumannite (Ag2Se).
|
Complex and inconsistent stoichiometric associations with other trace elements (especially Pb, Bi and Sb) were observed, but could not be identified as specific mineral phases. The inconsistent stoichiometry of these phases suggested intergrown mineral phases, coprecipitation or Te substitution, such as Te substitution into sulfate or sulfosalt minerals that have been previously postulated or reported (George et al. 2017; Konaka et al. 2008; Trudu and Knittel 1998). The Feature Sizing software poorly identified and quantified S in the presence of Bi and Pb, which complicated the identification of sulfosalt minerals. Thus, sulfosalt minerals may have represented a portion of these elemental co-association categories and have been previously reported at the site (Callaghan 1937). However, these particles tended to have low mass fractions of Te, which reduced their overall importance on a mass basis.
The weight percent of Fe versus Te was plotted to further illustrate the complex stoichiometric relationships (Fig. 6b), with IMA-recognised Te-Fe-containing minerals indicated on the plot. Also plotted were lines representing a 1 : 1 Te : Fe mole ratio and the maximum sorption capacity line, calculated based on all anion-available sorption sites of ferrihydrite being occupied by Te oxyanions (Dzombak and Morel 1990). This line clearly represents an upper limit as ferrihydrite has the highest sorption capacity of the FeIII (oxy)hydroxide minerals and competition with other ions for the sorption sites was ignored. The detection limit for Te during EDS analysis was set at 2 % Te, thus there was likely some amount of Te sorbed to Fe (oxy)hydroxides that was not detected with Feature Sizing, thereby underrepresenting the contribution of sorbed Te. Additionally, various Fe-Te-bearing minerals may have been present in the samples based on the wide range of observed Te : Fe mole ratios (range = 1.7 to 0.02). IMA-recognised Te-Fe-bearing phases include: blakeite [Fe2(TeO3)3], burkhardtite [Pb2(Fe, Te) (AlSi3O8)O6], cuzticite (Fe2TeO6·3H2O), emmonsite [Fe2(TeO3)3·2H2O], eztlite [Pb2Fe6(TeO3)3(TeO6) (OH)10·8H2O], ferrotellurite (FeTeO4), mackayite [FeTe2O5(OH)], metatamboite [Fe3(OH) (H2O)2(SO4) (TeO3)3(TeO(OH)2) (H2O)], poughite [Fe2(TeO3)2SO4·3H2O], sonoraite [FeTeO3(OH)·H2O], tamboite [Fe3(OH) (H2O)2(SO4) (TeO3)3(TeO(OH)2) (H2O)3], telluromandarinoite [Fe2(TeO3)3·6H2O], tellurite and paratellurite (TeO2), walfordite [(Fe, Te, Ti, Mg)Te3O8)], and yecoraite [Fe3Bi5O9(TeO3) (TeO4)2·9H2O)].
Indeed, the total Te concentration calculated from the Feature Sizing data was substantially lower than the bulk Te concentrations measured by WD-XRF. Feature Sizing was able to account for 0.7, 1.3, 3.8 and 22 % of Te measured by WD-XRF in samples 523, 362, 368 and 367F respectively. This indicated that Te associated with Fe (oxy)hydroxides or other phases at concentrations below the detection limit of the Feature Sizing was an important reservoir of Te. Indeed, the fraction of Te accounted for by the Feature Sizing was lowest in the samples with low total Te, which potentially suggested that sorption was an important mechanism, especially at lower bulk Te concentrations.
Tellurium mineralogy in these tailings was quite complex. Iron (oxy)hydroxide-associated Te was perhaps the most important phase controlling Te mobility, which accounted for 77 % of the Te by mass detected using Feature Sizing. However, other Te-Fe-(Sb)-(Pb)-(Bi) phases and other minor Te-bearing minerals, including native Te, were observed as well. The distribution of these minerals between samples was variable and may lend insight into Te fate in the environment. Fig. 7 shows the distribution of elemental association categories for each sample. Only 15 Te-bearing particles were identified in the surficial flat tailings, including a micron-sized Ag telluride (Fig. 5c), three <10-micron-sized Pb-Te-Fe-bearing particles likely encapsulated in silicates based on high Si values, and a variety of Fe-Te-(O) particles (e.g. Fig. 5a, b). The streambed sample (sample 523) had the fewest particles identified (n = 6), which included three Bi-Te-(O) particles that were likely bismuth tellurides encapsulated in silicates, the complex Fe (oxy)hydroxide particle shown in Fig. 5i, a Pb phosphate particle, and the Ag-telluride inclusions in quartz (Fig. 5h). Many of these particles likely survived since deposition, owing to encapsulation in silicate phases (e.g. Fig. 5c, h), thereby sequestering them from the weathering environment.

|
The surficial and deepest big tailings samples (samples 328, 367F) had the most Te-bearing particles identified and the highest bulk Te content by WD-XRF. Thus, these results dominated and bias the data shown in Fig. 6 towards the big tailings. Sample 368 (0–2 cm) contained the largest fractions of the Sb- and Pb-containing Te-oxide phases, while sample 367F (55–60 cm) contained more native Te or TeO2-particles, which perhaps indicated less oxidative weathering of Te had occurred at depth.
Discussion
Weathering characteristics
The flat tailings were characterised by sparse vegetation, flat topography and lower levels of potentially toxic elements. The pH and elemental profiles were relatively invariant with depth, with perhaps a slight increase in total and inorganic C with increasing depth. The larger particle size in the flat tailings would allow less solute transport under low moisture conditions (Brady and Weil 2004), perhaps explaining the relatively consistent elemental and EC profiles as a function of depth. The highest EC values in the flat tailings were lower in the tailings profile, potentially indicating downward solute movement (Brady and Weil 2004). The larger median particle size also likely resulted in less mineral weathering since deposition, which could account for the larger amounts of feldspars and lower amounts of clay minerals in these tailings. Further, these larger particles may encapsulate sulfide minerals, which allows them to persist in these tailings and accounts for the higher fraction of sulfide minerals in the flat tailings. Encapsulation may also affect Te-bearing phases, as Feature Sizing results suggested a higher fraction of tiny encapsulated Te-bearing particles in addition to the Fe (oxy)hydroxide-associated Te (Figs 5c and 7). Encapsulation was also consistent with the flat tailings having the lowest bioaccessible concentrations of Te solubilised in gastric fluids (Table 3; Ruby et al. 1999). Further, there was no direct measurement of Te speciation in the flat tailings, but higher sulfide concentrations relative to the big tailings may indicate that Te has also undergone less oxidative weathering.
The big tailings were characterised by steep, unvegetated slopes with extensive surface water erosion indicated by visible rills. The median particle size (43 µm) indicated that a large fraction of the particles could be wind-transportable (Gillette and Walker 1977; Kok et al. 2012). However, these tailings were highly aggregated, forming a cemented layer that persisted to at least 30 cm, which may help the tailings resist transport. The smaller median particle size also suggests that the big tailings would be more amenable to pore water and solute transport under low water conditions than the flat tailings (Brady and Weil 2004). The larger exposed surface area would also likely lead to faster rates of mineral weathering in these tailings, consistent with the higher abundance of clay minerals and lower amounts of feldspars observed. The big tailings had more 10-Å mica-group clays than the flat tailings, which, along with Fe (oxy)hydroxides, may have contributed to the higher degree of aggregation. This may explain the more consolidated nature of the big tailings, despite other similarities in bulk mineralogy and C concentrations with the flat tailings.
Particle size may also help explain why the big tailings were characterised by the highest EC and concentrations of potentially toxic metal(loid)s in the top 2 cm. This surficial enrichment (enrichment factor ~1.6 for Te) may point to the upward movement of solute-rich pore waters through capillary action under low moisture conditions and possibly the formation of efflorescent salts. Other studies have reported high EC measurements in semiarid tailings (up to 22 dS m−1) arising from high evapotranspiration and low precipitation (e.g. Mendez and Maier 2008; Meza-Figueroa et al. 2009). The presence of efflorescent salts in the 0–2 cm region of the big tailings was supported by the highest S concentrations, which S XANES indicated were gypsum and other sulfates (Fig. 3; Table 4), although sulfates were not identified by XRD. These sulfate minerals may accommodate isostructural tetrahedral metatellurate ions (TeO42−; Konaka et al. 2008).
The presence of organic S in the deep big tailings was surprising because if 45 % of the S was organic, this would translate to an organic S content of 1035 mg kg−1 (Table 1). Based on the regression for the C : S ratio developed by Fagerbakke et al. (1996), if all organic C were present as microbial biomass, only 8 mg S kg−1 would be expected. This suggested that microbial biomass cannot account for all the organic sulfur observed in the tailings. The biotic oxidation of sulfides can produce intermediate organic S compounds including elemental S, thiosulfate (S2O32−) and tetrathionate (S4O62−), which subsequently oxidise to sulfuric acid (Schippers and Sand 1999). This process could be occurring in microenvironments and the intermediary organic S molecules preserved and detected by S XANES. Alternatively, organic S compounds may have been introduced during the Au extraction process as S-containing flotation collectors or as cyanide substitutes [e.g. thiourea (CH4N2S) or thiocyanate (SCN−)], although there was no evidence that flotation was practiced at Delamar and cyanide substitutes are more common in modern times (Marsden and House 2006). Chemicals thus introduced potentially could have transformed since into the cystine-like form detected. However, neither of these ideas can unequivocally explain the presence of organic S in the deepest big tailings sample.
Tellurium geochemistry in semiarid mine tailings
Various Te-bearing phases were identified in the Delamar tailings, including primary Te-bearing phases such as native Te or, potentially, TeO2 (Fig. 5f) and Ag tellurides (Fig. 5c, d). Many of these occurred as inclusions in silicate matrices, which supported the idea that encapsulation prevented oxidative weathering both before extraction and post-deposition. The encapsulation of these phases and the phases themselves likely have relatively low bioaccessibility (Ruby et al. 1999; Taylor 1996). However, these reduced Te-bearing phases represent only a minor fraction of Te in the Delamar samples either on the basis of the EMPA Feature Sizing analysis (Figs 6 and 7) and Te XANES, which indicated primarily tellurate in the surficial big tailings (Fig. 4). Their likely low bioaccessibility taken together with their relatively low abundance indicate that these phases are likely not to be important concerns in terms of ecosystem and human health.
Most Te in the tailings was associated with Fe (oxy)hydroxides (Fig. 6), which occurred in both potentially primary massive textures (Fig. 5a, b) and secondary heterogeneous aggregates (Fig. 5g, h). These textural differences are likely to be very important, as these phases exhibit different behaviours with respect to Te mobilisation, transportability and bioaccessibility. Further, owing to the detection limit of the EDS (set at 2 % Te), Feature Sizing likely underestimated the amount of Te associated with Fe (oxy)hydroxides, which led to a low calculated total Te from feature size data relative to measured bulk concentrations. Owing to the large fraction of Te associated definitively with Fe (oxy)hydroxides, this phase likely dominates the bioaccessibility of Te in the tailings. Previous work has demonstrated the Fe (oxy)hydroxide-associated elements often exhibit low to medium bioaccessibility (e.g. Ruby et al. 1999), which is consistent with the medium-low bioaccessibility of the Delamar samples.
Although unequivocal mineral identification is often not possible, Feature Sizing revealed Te associations with Cu, Mn (Fig. 5i), Bi, Sb (Fig. 5e) and Pb. Of these, Mn and Cu occurred with consistent stoichiometry, likely indicating a mineral, while the others had variable composition. Bulk Te correlates with Pb, Cu and S, which may have been consistent with the presence of sulfosalts, which often contain Pb, Cu and S, or the substitution of Te oxyanions in sulfates. Although not detected by Feature Sizing in association with Te, S XANES indicated the presence of sulfates, especially in sample 368, which may be capable of incorporating tetrahedral tellurate at levels below the detection limit of Feature Sizing (Konaka et al. 2008). The bioaccessibility of these phases is unknown, but were likely not to be important drivers as, together, these accounted for a small fraction of the Te in the tailings. Possible exceptions were Te associated with Sb and Pb in the surficial big tailings, which accounted for roughly a third of the feature-sizing detectable Te, and the streambed sample, where no Fe (oxy)hydroxide-associated Te was found.
Comparison of the Te released by PBETs and the relative abundances of the different Te-bearing categories identified by individual samples (Table 3, Fig. 7) provided insight into the bioaccessibility of Te-bearing phases. There seemed to be a general trend towards higher bioaccessibility with increased weathering from the flat tailings with higher sulfide content to the sulfate-dominated surficial big tailings to the streambed samples. The streambed sample had the highest bioaccessibility and, like all tailings samples, was dominated by phases not detectable with Feature Sizing, potentially Te associated with Fe (oxy)hydroxides or other phases (sulfates, carbonates). Secondary phases forming or in equilibrium with the lower Te environment of the streambed sediments would likely have lower overall concentrations of Te, thus it made sense that few particles were detectable by Feature Sizing. Iron (oxy)hydroxide texture may also be an important control on the release of Te, as more massive textures may have differing amounts of Te relative to those actively forming under surficial conditions, and may also be less susceptible to weathering owing to a larger particle size. Further, the identity of the iron (oxy)hydroxide and the nature of the association between Te and the Fe (oxy)hydroxides, which may be either sorbed or coprecipitated, will have important implications for Te mobility. These are complexities with natural samples that were not disentangled by this study.
Implications of Te behaviour and health risks
Mine tailings are commonly characterised by low organic C and high concentrations of salts and potentially toxic elements in pore waters, which can stress the heterotopic microbial community (Mendez and Maier 2008). This is particularly concerning as toxic effects of tellurite oxyanions have been observed in bacteria at concentrations as low as 1 mg L−1 (Chasteen et al. 2009). These characteristics also inhibit the formation of normal soil structure and vegetation, which leaves tailings extremely vulnerable to erosion and may impact off-site transport and the potential for human exposure (Mendez and Maier 2008).
Vegetation would reduce the force of rain droplet impact on the tailings surface, thereby reducing the potential for surface water erosion and stabilising soil particles in the case of overland flow during intense precipitation events (Brady and Weil 2004). Despite the aggregation in the big tailings, numerous rills provide evidence of tailings particle transport. Off-site transport of potentially toxic elements by surface water was supported by the Te enrichment of the downstream sediments, relative to average crustal abundance. Despite the higher fraction of bioaccessible Te in these sediments, they had lower total Te concentrations and are unlikely to have a significant environmental impact.
Aeolian transport can be a dominant mechanism of off-site transport in arid and semiarid environments (Breshears et al. 2003) and is facilitated by the lack of vegetation that would otherwise reduce wind speeds at the tailings surface (Brady and Weil 2004; Gillette and Walker 1977; Kok et al. 2012). This area is often used by recreational vehicles, which could loft potentially toxic elements and disturb tailings aggregates, making particles more vulnerable to future transport. This is of particular concern because these recreational vehicles are typically not equipped with air filtration. Thus, human exposure could occur through inhalation of dusts or ingestion of particles by respiration or hand-to-mouth.
It has long been recognised that the total amount of a potentially toxic element in geomedia is not always correlated with health risk (e.g. Davis et al. 1992), since only a fraction will dissolve under physiological conditions (termed bioaccessible) and even less will be absorbed into the organism and be available to do harm (termed bioavailable; Plumlee et al. 2005). Tellurium human and animal model bioavailability studies have similarly shown that Te toxicity is highly variable, although more soluble forms tend to be more bioaccessible. A limited human study found 23 ± 9 % of Na2TeO4 (n = 4), 21.5 % of Na2TeO3 (n = 1), and 10 ± 4 % of metallic Te colloids (n = 3) were absorbed when administered orally (Kron et al. 1991). Animal model studies have also reported similar oral bioavailability values of 25 % gut absorption of TeO2 and lower bioavailability of elemental Te (Taylor 1996). Similarly, the bioaccessibility values of Te reference minerals in this study were broadly consistent with previous work, as much more Na2TeO3 and Na2TeO4 were liberated relative to CdTe liberated by both PBETs. However, it is surprising that a higher fraction of Na2TeO4 was liberated by the gastric extraction relative to the reportedly more toxic and soluble Na2TeO3, perhaps arising from particle size differences because reference minerals were reacted as received. Further, the bioaccessible fraction of Te measured in Na2TeO3 and Na2TeO4 is higher than that of the samples. This could arise from differences in particle size, which is likely smaller for the reference minerals, rinding or encapsulation in samples, or owing to differences in the bioaccessibility of Te-bearing phases.
Simulated gastric extractions of samples resulted in low to moderate Te bioaccessibility, which solubilised 13 %, 19 % and 31 % of Te in the surficial flat tailings, surficial big tailings and streambed sample respectively (Table 3). However, Te concentrations in simulated gastric fluid of up to 55 mg L−1 were concerning when compared with animal model studies. In injection studies using rat models, the 48-h minimum fatal doses of Na2TeO4 and Na2TeO3 were 30 mg kg−1 and 2.5 mg kg−1 respectively. This is consistent with the observation of damage to the kidney, liver and nervous system at oral exposure concentrations of 375–1500 mg kg−1 in mice and rats exposed to TeO2, one of the more toxic forms of Te studied. To absorb a likely fatal dose of 2.5 mg Te kg−1 if all Te were present as tellurite, a 60-kg human would have to ingest at least 2.5 kg tailings with 19 % solubility. This is an underestimation since the value measured 19 % bioaccessibility, which is likely higher than the actual bioavailability. Thus, whole organism acute adverse health effects are unlikely, but ingestion could result in toxic Te concentrations. Given that the gastric PBET liberated the highest fraction and the highest concentrations of Te, it is encouraging that ingestion is a less likely exposure mechanism for these tailings.
Bioaccessibility of Te in lung fluids is very important considering the most likely mechanism of exposure is inhalation of lofted particles. However, only a small fraction of Te (0.7–3 %) was solubilised in simulated lung fluids. Measured Te concentrations were low, ranging from 0.2 to 1.9 mg Te L−1, but are still somewhat concerning based on concentrations found to be toxic to microorganisms and in animal model studies. Based on the potentially toxic element content measured in the off-site control, these lofted particles are unlikely to be transported long distances, but surface water transport is an important mechanism of dispersion. Thus, some concern is warranted regarding exposure events arising from recreational vehicle use at the site, but acute effects are unlikely.
Conclusions
Two Te-enriched but physicochemically distinct tailings piles from semiarid Delamar, Nevada, USA were examined as a function of depth to assess Te behaviour in the surficial environment. After ~75 years of weathering, both tailings piles are sulfate dominated, indicating substantial oxidative weathering. XANES of the big tailings indicate that Te is present in the less toxic TeVI form with moderate and low bioaccessibility in gastric and lung fluids respectively. Most Te identified by electron microscopy is associated with iron (oxy)hydroxides, and sometimes other potentially toxic elements. While this study demonstrates that Te at this site represents a moderate health risk, this type of investigation of Te speciation and bioaccessibility under a wide variety of climatic conditions is warranted for a more complete health risk assessment.
Conflicts of interest
The authors declare no conflicts of interest. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the USA Government.
Acknowledgements
We are grateful for the insights and assistance of Rainer Newberry, Ken Severin, Karen Spaleta, Tom Trainor, Thomas Douglas, Amanda Barker, George Breit and Alan Wallace. We also thank the Stanford Synchrotron Radiation Lightsource staff, Sam Webb, Ryan Davis, Courtney Roach and Matthew Latimer, as portions of this work were performed at SSRL, SLAC National Accelerator Laboratory, which is supported by the USA Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02–76SF00515. Portions of this work were performed at the Advanced Instrumentation Laboratory (AIL) at University of Alaska Fairbanks and at the Cold Regions Research and Engineering Laboratory (CRREL) in Fairbanks, AK. This work was financially supported by USA Geological Survey Mineral Resources External Research Program (USGS/G12AP20054). Student support for this project was provided by the Geological Society of America, Undergraduate Research and Scholarly Activity (URSA) and Biomedical Learning and Student Training (BLaST) programs at UAF. Work reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under three linked award numbers RL5GM118990, TL4 GM 118992 and 1UL1GM118991. The work is solely the responsibility of the authors and the USA Geological Survey, and does not necessarily represent the official view of the National Institutes of Health. This licenced material constitutes ‘a work of the United States Government’ as defined by the USA Copyright Act, 17 U.S.C. sec. 101; 17 U.S.C. sec. 105. A ‘work of the United States Government’ is a work prepared by an officer or employee of the United States Government as part of that person’s official duties and is not subject to copyright protection in the United States. However, copyright in a foreign country may apply.
References
Anderson CS (2015). Selenium and tellurium. ‘Minerals Yearbook, Vol. 1, Metals and Minerals’. (US Geologic Survey: Washington, DC)Belzile N, Chen YW (2015). Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Applied Geochemistry 63, 83–92.
| Tellurium in the environment: A critical review focused on natural waters, soils, sediments and airborne particlesCrossref | GoogleScholarGoogle Scholar |
Brady NC, Weil RR (2004). ‘Elements of the nature and properties of soils.’ (Prentice Hall: Upper Saddle River, NJ).
Breshears DD, Whicker JJ, Johansen MP, Pinder JE (2003). Wind and water erosion and transport in semi-arid shrubland, grassland and forest ecosystems: Quantifying dominance of horizontal wind-driven transport. Earth Surface Processes and Landforms 28, 1189–1209.
| Wind and water erosion and transport in semi-arid shrubland, grassland and forest ecosystems: Quantifying dominance of horizontal wind-driven transportCrossref | GoogleScholarGoogle Scholar |
Brookins DG (1988). ‘Eh-ph diagrams for geochemistry.’ (Springer-Verlag: Berlin)
Callaghan E (1937). Geology of the Delamar District, Lincoln County, Nevada. University of Nevada Bulletin 31, 1–72.
Chasteen TG, Fuentes DE, Tantalean JC, Vasquez CC (2009). Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiology Reviews 33, 820–832.
| Tellurite: history, oxidative stress, and molecular mechanisms of resistanceCrossref | GoogleScholarGoogle Scholar | 19368559PubMed |
Chau LY, Ralph J (1993). Delamar mine, Delamar district, Lincoln Co., Nevada, USA. In Mindat.org (Keswick, VA: Hudson Institute of Mineralogy)
Cobelo-Garcia A, Filella M, Croot P, Frazzoli C, Du Laing G, Ospina-Alvarez N, Rauch S, Salaun P, Schafer J, Zimmermann S (2015). COST action TD1407: Network on technology-critical elements (NOTICE)–from environmental processes to human health threats. Environmental Science and Pollution Research International 22, 15188–15194.
| COST action TD1407: Network on technology-critical elements (NOTICE)–from environmental processes to human health threatsCrossref | GoogleScholarGoogle Scholar | 26286804PubMed |
Cowgill UM (1988). The tellurium content of vegetation. Biological Trace Element Research 17, 43–67.
| The tellurium content of vegetationCrossref | GoogleScholarGoogle Scholar | 2484368PubMed |
Davis A, Ruby MV, Bergstrom PD (1992). Bioavailability of arsenic and lead in soils from the butte, montana, mining district. Environmental Science & Technology 26, 461–468.
| Bioavailability of arsenic and lead in soils from the butte, montana, mining districtCrossref | GoogleScholarGoogle Scholar |
Downs RT (2007). International mineralogical association mineral list. In RRUFF Project. (RRUFF: Tucson, AZ). Available at http://rruff.info/ima/ [verified 7 October 2018]
Drysdale M, Bjorklund KL, Jamieson HE, Weinstein P, Cook A, Watkins RT (2012). Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluid. Environmental Geochemistry and Health 34, 279–288.
| Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluidCrossref | GoogleScholarGoogle Scholar | 21983883PubMed |
Dzombak DA, Morel FM (1990). ‘Surface complexation modeling: Hydrous ferric oxide.’ (John Wiley and Sons: New York, NY)
Emmons SF (1902). The Delamar and the Horn-silver mines:Two types of ore-deposits in the deserts of Nevada and Utah. Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers 31, 658–675.
Environmental Protection Agency (EPA) (2012). Standard operating procedure for an in vitro bioaccessibility assay for lead in soil. (EPA 9200, 2–86: Washington, DC).
Environmental Protection Agency (EPA) (2018). Regionals screening levels (RSL) summary table. (EPA 9200 2–86: Washington, DC). Available at https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables [verified 7 October 2018]
Fagerbakke KM, Heldal M, Norland S (1996). Content of carbon, nitrogen, oxygen, sulfur, and phosphorous in native aquatic and cultured bacteria. Aquatic Microbial Ecology 10, 15–27.
| Content of carbon, nitrogen, oxygen, sulfur, and phosphorous in native aquatic and cultured bacteriaCrossref | GoogleScholarGoogle Scholar |
Franke KW, Moxon AL (1936). A comparison of the minimum fatal doses of selenium, tellurium, arsenic, and vanadium. The Journal of Pharmacology and Experimental Theraputics 58, 454–459.
Franke KW, Moxon AL (1937). The toxicity of orally ingested arsenic selenium tellurium vanadium and molybdenum. The Journal of Pharmacology and Experimental Therapeutics 61, 89–102.
George L, Cook N, Ciobanu C (2017). Minor and trace elements in natural tetrahedrite-tennantite: Effects on element partitioning among base metal sulphides. Minerals 7, 17
| Minor and trace elements in natural tetrahedrite-tennantite: Effects on element partitioning among base metal sulphidesCrossref | GoogleScholarGoogle Scholar |
Gillette DA, Walker TR (1977). Characteristics of airborne particles produced by wind erosion of sandy soil, high plains of west texas. Soil Science 123, 97–110.
| Characteristics of airborne particles produced by wind erosion of sandy soil, high plains of west texasCrossref | GoogleScholarGoogle Scholar |
Harada T, Takahashi Y (2008). Origin of the difference in the distribution behavior of tellurium and selenium in a soil-water system. Geochimica et Cosmochimica Acta 72, 1281–1294.
| Origin of the difference in the distribution behavior of tellurium and selenium in a soil-water systemCrossref | GoogleScholarGoogle Scholar |
Hayes SM, McCullough E (2018). Critical minerals: A review of elemental trends in comprehensive criticality studies. Resources Policy 59, 192–199.
| Critical minerals: A review of elemental trends in comprehensive criticality studiesCrossref | GoogleScholarGoogle Scholar |
Hayes SM, Root RA, Perdrial N, Maier RM, Chorover J (2014). Surficial weathering of iron sulfide mine tailings under semi-arid climate. Geochimica et Cosmochimica Acta 141, 240–257.
| Surficial weathering of iron sulfide mine tailings under semi-arid climateCrossref | GoogleScholarGoogle Scholar | 25197102PubMed |
Hein JR, Koschinsky A, Halliday AN (2003). Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of tellurium. Geochimica et Cosmochimica Acta 67, 1117–1127.
| Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of telluriumCrossref | GoogleScholarGoogle Scholar |
Jamieson HE (2011). Geochemistry and mineralogy of solid mine waste: Essential knowledge for predicting environmental impact. Elements 7, 381–386.
| Geochemistry and mineralogy of solid mine waste: Essential knowledge for predicting environmental impactCrossref | GoogleScholarGoogle Scholar |
Kashiwabara T, Oishi Y, Sakaguchi A, Sugiyama T, Usui A, Takahashi Y (2014). Chemical processes for the extreme enrichment of tellurium into marine ferromanganese oxides. Geochimica et Cosmochimica Acta 131, 150–163.
| Chemical processes for the extreme enrichment of tellurium into marine ferromanganese oxidesCrossref | GoogleScholarGoogle Scholar |
Keith M, Smith DJ, Jenkin GRT, Holwell DA, Dye MD (2018). A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geology Reviews 96, 269–282.
| A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processesCrossref | GoogleScholarGoogle Scholar |
Kelley KD, Spry PG (2016). Critical elements in alkaline igneous rock-related epithermal gold deposits. Reviews in Economic Geology 18, 195–216.
Kok JF, Parteli JR, Michaels TI, Bou Karam D (2012). The physics of wind-blown sand and dust. Reports on Progress in Physics 75, 106901
| The physics of wind-blown sand and dustCrossref | GoogleScholarGoogle Scholar | 22982806PubMed |
Konaka S, Ozawa Y, Yagasaki A (2008). Tetrahedral tellurate. Inorganic Chemistry 47, 1244–1245.
| Tetrahedral tellurateCrossref | GoogleScholarGoogle Scholar | 18220344PubMed |
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006). World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15, 259–263.
| World map of the Köppen-Geiger climate classification updatedCrossref | GoogleScholarGoogle Scholar |
Kron T, Hansen C, Werner E (1991). Renal excretion of tellurium after peroral administration of tellurium in different forms to healthy human volunteers. Journal of Trace Elements and Electrolytes in Health and Disease 5, 239–244.
Lottermoser BG (2003). ‘Mine wastes: Characterization, treatment, and environmental impacts.’ (Springer: Berlin)
Marsden J, House I (2006). ‘The chemistry of gold extraction.’ (Society for Mining, Metallurgy, and Exploration, Inc.: Englewood, CO)
Mason GT, Arndt RE 1996. Mineral resources data system (MRDS). US Geological Survey Digital Data Series 20. Available at https://mrdata.usgs.gov/mrds/ [verified 7 October 2018]
McFaul EJ, Mason GT, Ferguson WB, Lipin BR 2000. US Geological survey mineral databases: MRDS and MAS/MILS. US Geological Survey Digital Data Series 52. Available at https://mrdata.usgs.gov/mrds/ [verified 7 October 2018]
McPhail DC (1995). Thermodynamic properties of aqueous tellurium species between 25-degrees-C and 350-degrees-C. Geochimica et Cosmochimica Acta 59, 851–866.
Mendez MO, Maier RM (2008). Phytostabilization of mine tailings in arid and semiarid environments- an emerging remediation technology. Environmental Health Perspectives 116, 278–283.
| Phytostabilization of mine tailings in arid and semiarid environments- an emerging remediation technologyCrossref | GoogleScholarGoogle Scholar | 18335091PubMed |
Meza-Figueroa D, Maier RM, de la O-Villanueva M, Gómez-Alvarez A, Moreno-Zazueta A, Rivera J, Campillo A, Grandlic CJ, Anaya R, Palafox-Reyes J (2009). The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 77, 140–147.
| The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, MexicoCrossref | GoogleScholarGoogle Scholar | 19500816PubMed |
Moore DM, Reynolds RC (1989). ‘X-ray diffraction and the identification and analysis of clay minerals.’ (Oxford University Press: Oxford)
Moreno T, Oldroyd A, McDonald I, Gibbons W (2007). Preferential fractionation of trace metals-metalloids into PM10 resuspended from contaminated gold mine tailings at Rodalquilar, Spain. Water, Air, and Soil Pollution 179, 93–105.
| Preferential fractionation of trace metals-metalloids into PM10 resuspended from contaminated gold mine tailings at Rodalquilar, SpainCrossref | GoogleScholarGoogle Scholar |
National Research Council (NRC) (1980). ‘Mineral tolerances of animals.’ (National Academies Press: Washington, DC)
Perkins WT (2011). Extreme selenium and tellurium contamination in soils–an eighty year-old industrial legacy surrounding a Ni refinery in the Swansea valley. The Science of the Total Environment 412–413, 162–169.
| Extreme selenium and tellurium contamination in soils–an eighty year-old industrial legacy surrounding a Ni refinery in the Swansea valleyCrossref | GoogleScholarGoogle Scholar | 22055656PubMed |
Plumlee GS, Ziegler TL, Lollar BS (2005). The medical geochemistry of dusts, soils, and other earth materials. In ‘Environmental geochemistry’. (Eds HD Holland, KK Turekian) pp. 263–310. (Elsevier: Amsterdam)
Prietzel J, Botzaki A, Tyufekchieva N, Brettholle M, Thieme J, Klysubun W (2011). Sulfur speciation in soil by s k-edge xanes spectroscopy: Comparison of spectral deconvolution and linear combination fitting. Environmental Science & Technology 45, 2878–2886.
| Sulfur speciation in soil by s k-edge xanes spectroscopy: Comparison of spectral deconvolution and linear combination fittingCrossref | GoogleScholarGoogle Scholar |
Qin H-B, Takeichi Y, Nitani H, Terada Y, Takahashi Y (2017). Tellurium distribution and speciation in contaminated soils from abandoned mine tailings: Comparison with selenium. Environmental Science & Technology
| Tellurium distribution and speciation in contaminated soils from abandoned mine tailings: Comparison with seleniumCrossref | GoogleScholarGoogle Scholar |
Reich M, Kesler SE, Utsunomiya S, Palenik CS, Chryssoulis SL, Ewing RC (2005). Solubility of gold in arsenian pyrite. Geochimica et Cosmochimica Acta 69, 2781–2796.
| Solubility of gold in arsenian pyriteCrossref | GoogleScholarGoogle Scholar |
Ruby MV, Schoof R, Brattin W, Goldade M, Post G, Harnois M, Mosby DE, Casteel SW, Berti W, Carpenter M, Edwards D, Cragin D, Chappell W (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science & Technology 33, 3697–3705.
| Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessmentCrossref | GoogleScholarGoogle Scholar |
Rudnick RL, Gao S (2006). Composition of the continental crust. In ‘The crust’. (Ed. RL Rudnick) pp. 1–64. (Elsevier: New York, NY)
Schaider LA, Senn DB, Brabander DJ, McCarthy KD, Shine JP (2007). Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure, and bioavailability. Environmental Science & Technology 41, 4164–4171.
| Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure, and bioavailabilityCrossref | GoogleScholarGoogle Scholar |
Schippers A, Sand W (1999). Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Applied and Environmental Microbiology 65, 319–321.
Takaya M, Shinohara Y, Serita F, Onon-Ogasawara M, Otaki N, Toya T, Takata A, Yoshidda K, Kohyama N (2006). Dissolution of functional materials and rare earth oxides into pseudo alveolar fluid. Industrial Health 44, 639–644.
| Dissolution of functional materials and rare earth oxides into pseudo alveolar fluidCrossref | GoogleScholarGoogle Scholar | 17085926PubMed |
Taylor A (1996). Biochemistry of tellurium. Biological Trace Element Research 55, 231–239.
| Biochemistry of telluriumCrossref | GoogleScholarGoogle Scholar | 9096851PubMed |
Trudu A, Knittel U (1998). Cheminform abstract: Crystallography, mineral chemistry and chemical nomenclature of goldfieldite, the tellurian member of the tetrahedrite solid-solution series. Canadian Mineralogist 36, 1115–1137.
Tschanz CM, Pampeyan EH (1970). ‘Geology of mineral deposits of Lincoln County, Nevada.’ (Mackay School of Mines, University of Nevada: Reno, NV).
Walford LK, Carron GJ, Schoeffe JA (1968). X-ray Debye temperatures of tellurium and cadmium. Materials Research Bulletin 3, 911–918.
| X-ray Debye temperatures of tellurium and cadmiumCrossref | GoogleScholarGoogle Scholar |
Webb SM (2005). Sixpack: A graphical user interface for XAS analysis using ifeffit. Physica Scripta T115, 1011–1014.
| Sixpack: A graphical user interface for XAS analysis using ifeffitCrossref | GoogleScholarGoogle Scholar |
Wedepohl KH (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta 59, 1217–1232.
| The composition of the continental crustCrossref | GoogleScholarGoogle Scholar |