Effect of reduced lairage duration on beef quality
D. M. Ferguson A B E F , F. D. Shaw A C and J. L. Stark A DA Cooperative Research Centre for Cattle and Beef Quality.
B CSIRO Livestock Industries F. D. McMaster Laboratory, Armidale, NSW 2350, Australia.
C PO Box 10, Cannon Hill, Qld 4170, Australia.
D Food Science Australia, Cannon Hill, Qld 4170, Australia.
E Present address: Locked Bag 1, Armidale, NSW 2350, Australia.
F Corresponding author. Email: drewe.ferguson@csiro.au
Australian Journal of Experimental Agriculture 47(7) 770-773 https://doi.org/10.1071/EA05212
Submitted: 11 April 2005 Accepted: 4 January 2006 Published: 2 July 2007
Abstract
A study involving two groups of feedlot cattle (n = 84 and 112) was undertaken to compare the effect of two preslaughter lairage (L) durations (3 h v. 18 h) on carcass and meat quality properties. The cattle were grainfed for 150 days before slaughter and had a mean carcass weight of 347.0 ± 25.4 kg. Cattle from the same feedlot pen were randomly allocated to the two treatments on the day before slaughter. One group was transported to the abattoir the day before slaughter and held overnight (L–18 h) whereas the other group remained at the feedlot and was transported the following morning and remained in the lairage for 3 h before slaughter (L–3 h). After slaughter, meat quality was evaluated on a subset of 15 carcasses/lairage treatment from the two slaughter groups. Objective meat quality measures were made on unaged and 14-day-aged striploins (longissimus lumborum) from these carcasses. Cattle from the reduced lairage treatment had heavier bled bodyweights at slaughter (P < 0.05) but there was no effect (P > 0.05) on carcass weight, muscle glycogen concentration, pH3h, ultimate pH, shear force or Minolta lightness values. Significant interactions (P < 0.05) between lairage duration and aging were observed for cooking loss percentage and Minolta a* and b* values but these were relatively small in magnitude. There were no differences in the incidence of ingesta contamination or rumen rupture between the lairage treatments. It was concluded that shortening holding times in lairage from 18 to 3 h for cattle that have travelled <6 h would not affect carcass or beef quality.
Introduction
The Australian Standing Committee on Agriculture and Resource Management (SCARM) welfare code of practice for the slaughter of livestock requires that on arrival, cattle be rested for a minimum of 2 h before slaughter (Anon. 2001). This applies specifically to cattle that have travelled less than 6 h and show no obvious signs of stress or exhaustion. Longer preslaughter rest periods are recommended for cattle travelling 6 h or longer. In practice, the standard lairage time for slaughter cattle typically ranges between 12–24 h. Given the desire to increase existing utilisation capacity, some abattoirs are now considering reducing the time in lairage in order to facilitate greater throughput. Although this is allowable under the code, questions have been raised with respect to the impact on beef quality.
Although the research to date is not exhaustive or conclusive (see review by Ferguson et al. 2001), the results tend to indicate that reducing the time in lairage may yield benefits in terms of reducing the incidence of dark cutting (Purchas 1992), particularly in bulls (Fabiansson et al. 1984) and could lead to improvements in beef palatability (Jeremiah et al. 1988a, 1988b).
The present study was undertaken to test the hypothesis that reduced lairage duration would not affect meat quality attributes in feedlot cattle. In addition, we examined whether reduced lairage leads to increased problems such as contamination during evisceration because of the increased rumen volume at slaughter.
Materials and methods
This experiment was approved by the Food Science Australia (Cannon Hill, Queensland), Animal Ethics Committee (No. 2002/2).
Sample
Two groups of steers (n = 84 and n = 112) were sourced from a commercial feedlot near Dalby in south-east Queensland. The steers were a stabilised composite based on Bos indicus × Bos taurus breeding and were fed on a commercial feedlot ration for 150 days. The cattle were transported to the abattoir ~150 km from the feedlot. The groups were slaughtered on different days ~1 month apart (Group 1 on 9 April 2002; Group 2 on 15 May 2002).
Lairage treatments
On the day before slaughter, each group was split into two subgroups and randomly allocated to one of two lairage (L) treatments; the conventional overnight lairage of 18 h (L–18 h) and the reduced lairage treatment of 3 h (L–3 h). The L–18 h subgroup was transported from the feedlot at about 1800 hours on the day before slaughter, whereas the L–3 h subgroup left at about 0600 hours the morning of slaughter. After their arrival at the abattoir, the subgroups were placed in lairage pens in groups of 10–12 steers and maintained with access to water in their pens until slaughter. The overall period of fasting was 14 h for the L–3 h group and 27 h for the L–18 h group.
Slaughter
Alternate pens of cattle from each subgroup were walked from the lairage area to the knocking box. The animals were stunned using a captive bolt and bled immediately. Conventional electrical stimulation was not applied; however, the carcasses did receive electric inputs at two points on the slaughter-chain. The carcasses were immobilised immediately after stunning and bleeding and also during mechanical hide removal. Together these inputs provided effective stimulation given that the loin pH was <6.0 by 3 h postslaughter. The carcasses were placed in the same chiller for overnight chilling.
Sample preparation
After chilling, the longissimus lumborum (LL; 11–12th thoracic to the 5–6th lumbar vertebra) of one side from a randomly selected subset (n = 15) of carcasses within each treatment or slaughter group was removed for meat quality determination. The samples were sent via refrigerated transport to the Food Science Australia Cannon Hill laboratory for further preparation. The LL was cut into two sections that were randomly allocated to aging treatments of 1 or 14 days (postslaughter). The 1-day aged sample was placed in a polyethylene bag and immediately frozen at –20°C. The 14-day aged samples were vacuum packaged and held at 0–1°C for 14 days and then frozen at –20°C.
Measurements
Body and carcass measurements
Bled bodyweight was recorded immediately after bleeding. This was achieved via a load cell located on the carcass rail. The normal hot dressed carcass weight was measured at the conclusion of the slaughter-chain before entry to the chiller. The standard AUS-MEAT carcass descriptors including P8 fat depth and dentition were also recorded.
Rumen, liver and carcass contamination
The effect of differences in rumen volume and gutfill on viscera and carcass contamination was evaluated. All carcasses from the first group and subset of 56 carcasses from the second group were evaluated. An observer was located on the slaughter floor in the vicinity of the evisceration area. The number of livers and rumens condemned by the Australian Quarantine and Inspection Service inspection staff for ingesta contamination following rupture of the rumen were recorded. A record was also made of carcasses placed on a retain rail for further trimming due to ingesta contamination.
The following measurements were made on the randomly selected subset of 15 carcasses or treatment group.
pH and temperature
The pH (pH3h) and temperature (Temp3h) of the LL was measured 3 h post mortem using a TPS WP-81 pH meter with an Ionide IJ42S electrode and automatic temperature-compensating probe (TPS Pty Ltd).
Muscle glycogen
At ~45 min postslaughter, muscle biopsies (0.5–1 g) were removed from the LL and immediately frozen in liquid nitrogen. The glycogen and lactate concentrations were determined according to the methods of Chan and Exton (1976) and Noll (1985), respectively. The preslaughter glycogen level in the muscle (expressed as μmol/g of wet muscle tissue) was derived from the glucose concentration + (2 × lactate concentration).
Meat quality
The frozen meat samples were thawed at 5°C for 48 h. The preparatory and measurement procedures described in detail by Perry et al. (2001) were applied to obtain the meat quality measures of ultimate pH (pHu), Minolta colour values (L*, a* and b*), shear force, compression and cooking loss percentage (CL%). Briefly, after thawing, the samples were trimmed of all fat and epimysial tissue and reduced to a size of 250 g for cooking. pHu and the Minolta colour measurements (bloomed surface) were made before cooking. Samples were cooked in a water bath for 1 h at 70°C and reweighed. After overnight refrigeration, the cooked samples were prepared for modified Warner–Bratzler shear force and compression measurements.
Statistical analyses
The data were analysed using the linear mixed effects model in S-PLUS v6.2 (Insightful Corporation). For the variables of body and carcass weight, muscle glycogen, pH3h and Temp3h, the model included the terms of lairage duration as a fixed effect and slaughter group as a random effect. For the analysis of the meat quality variables, the initial models contained the fixed effects of lairage duration and aging plus the interaction term and the random terms of slaughter group and animal within slaughter group. Any non-significant interactions were removed from the final models. The effect of lairage duration was tested on the between animal error term and effect of aging and the interaction between lairage duration × aging was tested on the animal within slaughter group error term.
Results
The pooled sample (n = 196) had a mean carcass weight of 347.0 ± 25.4 kg and mean P8 fat depth of 14.5 ± 5.1 mm.
The lairage treatment groups significantly differed in their bled bodyweights where the L–3 h group was 2.7% heavier at slaughter (Table 1). However, carcass weight was not found to be different between the lairage treatments. Similarly, no treatment differences were observed for LL glycogen concentration at slaughter or the 3 h pH and temperature measurements.
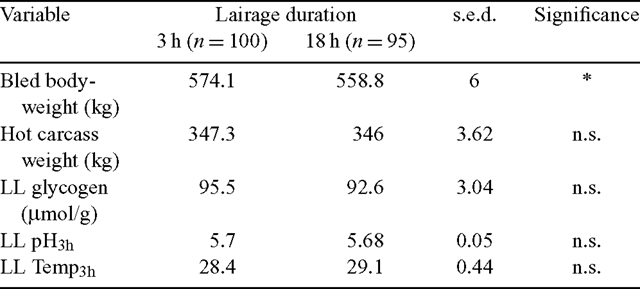
|
For the meat quality variables (Table 2), a significant lairage duration × aging interaction was observed for CL% (P < 0.05) and Minolta a* and b* values (P < 0.01). The CL% increased with aging for the L–3 h treatment but the opposite was observed for the L–18 h treatment. The 14-day aged L–3 h samples had significantly lower a* and b* values compared with the aged L–18 h samples. Overall, these interactions were relatively small in magnitude. Notwithstanding these interactions, meat quality was not significantly affected by lairage duration treatment. In contrast to the negligible effect of lairage duration, aging the samples 14 days significantly improved shear force (P < 0.001) and resulted in significantly higher L* value (P < 0.001) on the meat surface (i.e. lighter coloured meat). The compression measurements were not significantly affected by either the lairage duration or aging treatments.
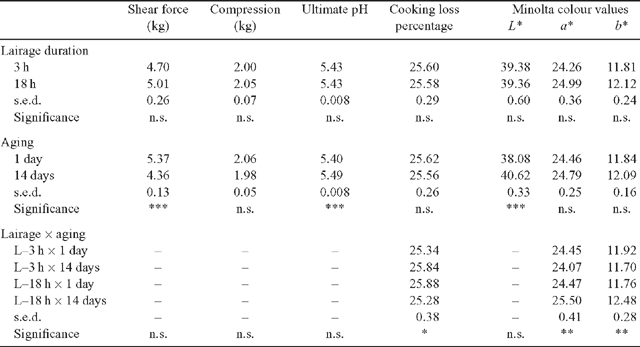
|
The results for liver and rumen condemnations due to pathological lesions or contamination by bile are shown in Table 3. As can be seen, there were very few cases of ingesta contamination following rumen rupture and no clear effect due to lairage treatment.

|
Discussion
Cattle receiving the 3 h lairage duration, had heavier (P < 0.05) bled bodyweights at slaughter but did not differ in their carcass weights compared with the more conventional overnight (18 h) lairage treatment. The latter result was anticipated as differences in carcass weight are more apparent after longer preslaughter fasting periods (typically >24 h) (Ferguson et al. 2001) although there have been exceptions to this trend (Purchas et al. 2002). The bodyweight difference can largely be attributed to differences in gutfill given that the preslaughter fasting period was considerably longer for the L–18 h group (~27 h) compared with the L–3 h group (~14 h). Despite the difference in gutfill, there was no difference between the lairage treatments in the incidence of ingesta contamination or rumen rupture during evisceration. It has generally been accepted that a period of rest without feed is a necessary pre-slaughter requirement (Gracey et al. 1999). This is largely predicated on the belief that the reduction in gutfill will minimise the risk of ingesta contamination and rumen rupture during evisceration. Unfortunately, there is a paucity of published evidence relating to this issue. However, the available evidence (Wythes and Shorthose 1984; Wythes et al. 1984) does not indicate that increased gutfill always leads to increased dressing problems, as confirmed in the present study.
Glycolytic rate as determined by the measure pH3h or pHu was not affected by lairage treatment. The result for the latter was not unexpected given the high muscle glycogen levels. Similarly, no difference in the objective meat texture measures of shear force and compression was found between the lairage treatments. A significant interaction between lairage treatment and aging duration was observed for CL% and the Minolta a* and b* values. However, these differences were relatively small in magnitude for both and thus it is unlikely that they are important in terms of the colour or functional properties of the meat. The muscle glycogen content of the cattle was high but consistent with other published data for grainfed cattle (Pethick et al. 1999). The stressors that apply to cattle during the preslaughter period (e.g. handling, transport, fasting and exposure to novel environments) lead to inevitable losses in muscle glycogen (Tarrant 1989). In this instance, the additional 15 h in lairage has not resulted in any further depletion of the muscle glycogen reserves. This is not surprising given the fact that depletion rates in cattle during fasting, at rest are relatively low (1.3 μmoles/g.day) (McVeigh and Tarrant 1982).
The observation that shear force was not affected by lairage duration was consistent with the findings of Jones et al. (1986) who evaluated cattle slaughtered within 4 h of leaving the farm compared with 24 h. However, it is important to note that in their 24-h treatment, the cattle were also mixed and deprived of water. In contrast, Jeremiah et al. (1988a, 1988b) evaluated similar experimental preslaughter treatments to those of Jones et al. (1986) and reported significant improvements in the sensory texture and flavour attributes of beef following 4 h of lairage. Given the confounded nature of their 24 h treatment, it is difficult to conclude whether the effect was simply due to lairage duration. (J. C. Petherick, V. J. Doogan, D. M. Fergsuson, R. G. Holroyd, P. Quinn, B. K. Venus, unpubl. data) applied the same lairage treatments (3 h v. 18 h) used here in their study involving 144 grainfed (78 days) B. indicus × B. taurus composite cattle and found no difference in Meat Standards Australia sensory panel tenderness, juiciness or flavour scores. Thus, it would appear that any reduction to the conventional overnight lairage period is unlikely to influence tenderness and eating quality.
Conclusions
In grainfed cattle, reducing the holding time in lairage from 18 to 3 h had no effect on any of the meat quality variables measured. Cattle given reduced lairage time before slaughter will have more gutfill, however, this is unlikely to lead to increased problems during evisceration. Therefore, it is concluded that relative to the conventional overnight lairage, any reduction in lairage will not affect carcass or meat quality in cattle that comply with the SCARM code of ‘<6 h transport and showing no obvious signs of exhaustion’.
Acknowledgements
The authors would like to acknowledge the financial support of the CRC for Cattle and Beef Quality. We are also extremely grateful for the support and cooperation of staff from the Northern Australian Pastoral Company and Valley Beef Abattoir. The technical assistance of Donna Knox is gratefully acknowledged.
Chan TM, Exton JH
(1976) A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Analytical Biochemistry 71, 96–105.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fabiansson S,
Erichsen I, Reutersward AL
(1984) The incidence of dark cutting beef in Sweden. Meat Science 10, 21–33.
| Crossref | GoogleScholarGoogle Scholar |

Ferguson DM,
Bruce HL,
Thompson JM,
Egan AF,
Perry D, Shorthose WR
(2001) Factors affecting beef palatability – farmgate to chilled carcass. Australian Journal of Experimental Agriculture 41, 879–891.
| Crossref | GoogleScholarGoogle Scholar |

Jeremiah LE,
Newman JA,
Tong AKW, Gibson LL
(1988a) The effects of castration, pre-slaughter stress and Zeranol implants on beef: 1. The texture of loin steaks from bovine males. Meat Science 22, 83–101.
| Crossref | GoogleScholarGoogle Scholar |

Jeremiah LE,
Newman JA,
Tong AKW, Gibson LL
(1988b) The effects of castration, pre-slaughter stress and Zeranol implants on beef: 2. Cooking properties and flavour of loin steaks from bovine males. Meat Science 22, 103–121.
| Crossref | GoogleScholarGoogle Scholar |

Jones SDM,
Newman JA,
Tong AKW,
Martin AH, Robertson WM
(1986) The effects of two shipping treatments on the carcass characteristics of bulls implanted with Zeranol and unimplanted steers. Journal of Animal Science 62, 1602–1608.
| PubMed |

McVeigh JM, Tarrant PV
(1982) Glycogen content and repletion rates in beef muscle, effect of feeding and fasting. The Journal of Nutrition 112, 1306–1314.
| PubMed |

Perry D,
Shorthose WR,
Ferguson DM, Thompson JM
(2001) Methods used in the CRC program for the determination of carcass yield and beef quality. Australian Journal of Experimental Agriculture 41, 953–958.
| Crossref | GoogleScholarGoogle Scholar |

Pethick DW,
Cummins L,
Gardner GE,
Knee BW,
McDowell M,
McIntyre BL,
Tudor G,
Walker PJ, Warner RD
(1999) The regulation by nutrition of glycogen in the muscle of ruminants. Recent Advances in Animal Nutrition in Australia 12, 145–152.

Purchas RW,
Burnham DL, Morris ST
(2002) Effects of growth potential and growth path on tenderness of beef longissimus muscle from bulls and steers. Journal of Animal Science 80, 3211–3221.
| PubMed |

Wythes JR,
Smith PC,
Arthur RJ, Round PJ
(1984) Feeding cattle at abattoirs: the effect on carcass attributes and muscle pH. Animal Production in Australia 15, 643–646.



