First report of Pepper leaf curl Bangladesh virus strain associated with bitter gourd (Momordica charantia L.) yellow mosaic disease in India
S. K. Raj A C , S. K. Snehi A , M. S. Khan A , A. K. Tiwari B and G. P. Rao BA Division of Virology, National Botanical Research Institute, Rana Pratap Marg, Lucknow, 226 001, Uttar Pradesh, India.
B Sugarcane Research Station Kunraghat, Gorakhpur, 273 008, Uttar Pradesh, India.
C Corresponding author. Email: skraj2@rediffmail.com
Australasian Plant Disease Notes 5(1) 14-16 https://doi.org/10.1071/DN10006
Submitted: 19 October 2009 Accepted: 26 January 2010 Published: 17 February 2010
Abstract
A severe yellow mosaic disease with a significant disease incidence was observed on bitter gourd (Momordica charantia L.) during a survey of different locations around Gorakhpur, Uttar Pradesh, India in 2008. Polymerase chain reaction (PCR) was carried out using total DNA isolated from infected leaf samples and a pair of begomovirus-specific primers. The expected size (~1300 bp) amplicon was detected from all four symptomatic samples but not from samples of healthy plants, indicating the presence of a begomovirus infection. A PCR amplicon was cloned and sequenced (GenBank Accession reference EU888908). Basic local alignment search tool analysis revealed the newly derived sequence had the highest identities (99–97%) with Pepper leaf curl Bangladesh virus (PepLCBV) sequences. The phylogenetic analysis of the virus sequences with selected begomovirus sequences also revealed the closest relationship with PepLCBV. These results suggest an association of PepLCBV with yellow mosaic disease of bitter gourd; PepLCBV on bitter gourd (M. charantia) is a new record in India.
Bitter gourd (Momordica charantia L.) of the family Cucurbitaceae, also known as bitter melon is widely grown in China, India and throughout South-east Asia. It is also grown in small acreages in California and Florida USA, as a food and for medicine. Bitter gourd has been used for centuries in the ancient traditional medicines of India, China, Africa and Latin America. Its extract possesses anti-oxidant, anti-microbial, anti-viral, anti-hepatotoxic and anti-ulcerogenic properties and has the ability to lower blood sugar levels (Behera et al. 2009).
Bitter gourd is extensively cultivated in the north-eastern region of Uttar Pradesh (UP), India as one of the major vegetable crops and has great economic importance (Yadav et al. 2004). It has been reported to be a natural host of many viruses which affects its cultivation worldwide. During a survey in August 2008, a severe yellow mosaic disease was observed on bitter gourd growing in different locations of eastern UP, India. The incidence of the disease within crops was significant (10–20%) and symptoms consisted of a severe yellow mosaic and slight curling of leaf tissues (Fig. 1). Infected plants had less fruit, which were smaller in size as compared with those produced by healthy plants. An abundant population of the whitefly species Bemisia tabaci was also observed on the crop and also in the vicinity. The characteristic disease symptoms and presence of whitefly indicated the possibility of a begomovirus infection. Leaf samples from infected and healthy bitter gourd plants were collected.

|
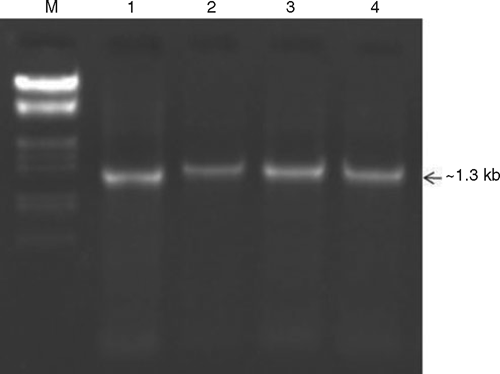
To determine the identity of begomovirus, total nucleic acids were extracted from 100 mg of leaf tissue from samples of infected and healthy plants by a method described by Dellaporta et al. (1983) and resuspended in 20 μL TE (Tris + EDTA) buffer, quantified by spectrophometry then stored at –20°C. The extracts were tested by polymerase chain reaction (PCR) using the begomovirus genus-specific primers, PALIv 1978 and PARIc 496 (Rojas et al. 1993). Each 50 µL PCR contained 20 ng DNA, 1× PCR buffer (Bangalore Genei Pvt. Ltd, Bangalore, India), 200 µM of each dNTP, 1.5 mM MgCl2, 25 pmole of each forward and reverse primers and 3 U Taq DNA polymerase (Bangalore Genei Pvt. Ltd). Amplifications were performed in a Peltier thermal cycler (MJ Research, Watertown, MA, US), using an incubation regime of 94°C for 5 min once, followed by 30 cycles of 94°C for 50 s, 52°C for 60 s and 72°C for 90 s then a final incubation of 7 min at 72°C. The amplified DNA fragments were evaluated by electrophoresis with DNA marker (Lambda DNA double digested with HindIII and EcoRI; Bangalore Genei Pvt. Ltd) using a 1% agarose gel. An expected size amplicon of ca.1300 bp was observed from all four symptomatic samples (Fig. 2) but not from healthy samples collected from the same location.
|
The amplicon obtained from one of the symptomatic samples was cloned into a pGEM-T easy cloning vector (Promega, Madison, WI, USA) and three clones were sequenced from both directions. The 1313 bp consensus sequence without ambiguities was deposited in NCBI GenBank under Accession reference EU888908. The translation of this into amino acids indicated presence of partial rep protein (AC1), complete C4 protein (AC4) in complementary sense and pre-coat protein (AV2) and partial coat protein (AV1) genes in virion sense.
Basic local alignment search tool (BLASTn) analysis of the 5′ end (1–811) of the newly derived sequence (GenBank EU888908) was 99% identical with Pepper leaf curl Bangladesh virus (PepLCBV, GenBank AF314531), 92% with Chilli leaf curl Pakistan virus isolate Multan (ChLCPV, GenBank DQ116877), ChLCPV isolate Khanewal (GenBank DQ116879 and DQ114477) and 89–88% with PepLCBV (GenBank DQ116881, AM404179). While sequence identities of 3′ end sequences (812–1313) of the sequence were 97% with PepLCBV (GenBank AF314531), 94% with Chilli leaf curl virus-[Jessore] (GenBank AM087118 and AM087119) and 91–90% with Tomato leaf curl Joydebpur virus (GenBank DQ673859, AJ875159, FJ345401, EU431116 and FJ345402).
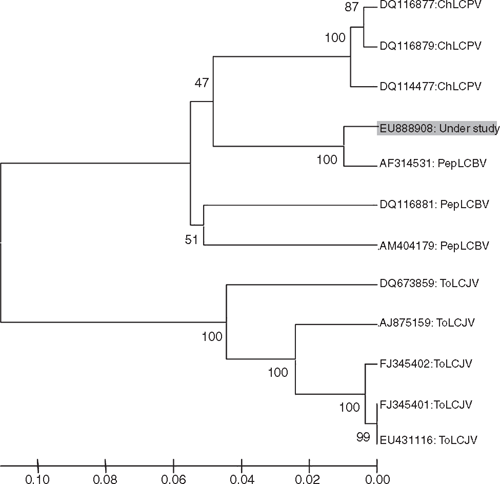
Phylogenetic analysis of nucleotide sequence of the virus isolate with selected begomovirus isolates which were all at least 88% identical, was performed using the molecular evolutionary genetics analysis (MEGA) version 4.0 software (Tamura et al. 2007). The analyses revealed closest relationship of the virus isolate with PepLCBV (GenBank AF314531) and also close relationships with two isolates of PepLCBV (GenBank DQ116881 and AM404179) and three isolates of ChLCPV (GenBank DQ116877, DQ116879 and DQ114477). However, it did not show any relationship with Tomato leaf curl Joydebpur virus isolates considered in this study (Fig. 3). The criteria for identification of begomoviruses lists a demarcation value of 89%, above which virus isolates are considered to belong definitively to a single species (Fauquet et al. 2008). Given the newly derived sequence clearly exceeds the demarcation value for species identification, as it is 99% and 97% identical at the 5′ end and at 3′ end sequence, respectively, to known PepLCBV sequences, the virus detected in bitter gourd with yellow mosaic disease was identified as an isolate of Pepper leaf curl Bangladesh virus (PepLCBV).
|
The possibility of other viruses, particularly potyviruses which are commonly found infecting cucuribits, was checked by sap inoculations on three seedlings each of M. charantia, Cucurbita maxima, Cucumis sativus, Nicotiana tabacum cv. White Burley, Lycopersicon esculentum and Datura metal. No local or systemic symptoms were observed in any of the test plants, indicating an absence of other viruses.
Many viruses have been reported to naturally infect bitter gourd. These include Papaya ring spot virus (Dahal et al. 1997), Watermelon mosaic virus (Tomar and Jitendra 2005), begomovirus (Khan et al. 2002; Raj et al. 2005), Bitter gourd yellow mosaic virus (Rajinimala et al. 2005), Tomato leaf curl New Delhi virus (Tahir and Haider 2005), Indian cassava mosaic virus (Rajinimala and Rabindran 2007) and Melon yellow spot virus (Takeuchi et al. 2009). However, no report of PepLCBV on M. charantia or any other host exists in India; therefore, it may be considered as a new report.
Dahal G,
Lecoq H, Albrechtsen SE
(1997) Occurrence of Papaya ringspot potyvirus and Cucurbit viruses in Nepal. The Annals of Applied Biology 130, 491–502.
| Crossref | GoogleScholarGoogle Scholar |

Dellaporta SL,
Wood J, Hicks JB
(1983) A plant DNA minipreparation: Version III. Plant Molecular Biology Reporter 1, 19–21.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Fauquet CM,
Briddon RW,
Brown JK,
Moriones E,
Stanley J,
Zerbini M, Zhou X
(2008) Geminivirus strain demarcation and nomenclature. Archives of Virology 153, 783–821.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Khan JA,
Siddiqui MK, Singh BP
(2002) The association of begomovirus with bitter melon in India. Plant Disease 86, 328.
| Crossref | GoogleScholarGoogle Scholar |

Raj SK,
Khan MS,
Singh R,
Nisha K, Prakash D
(2005) Occurrence of yellow mosaic geminiviral disease on bitter gourd (Momordica charantia) and its impact on phytochemical contents. International Journal of Food Sciences and Nutrition 56, 185–192.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rajinimala N, Rabindran R
(2007) First report of Indian cassava mosaic virus on bittergourd (Momordica charantia) in Tamil Nadu, India. Australasian Plant Disease Notes 2, 81–82.
| Crossref | GoogleScholarGoogle Scholar |

Rajinimala N,
Rabindran R,
Ramiah M, Kamlakhan A
(2005) Virus vector relationship of Bitter gourd yellow mosaic virus and whitefly Bemisia tabaci germ. Acta Phytopathologica et Entomologica Hungarica 40, 23–30.
| Crossref | GoogleScholarGoogle Scholar |

Rojas MR,
Gilbestron RL,
Russell DR, Maxwell DP
(1993) Use of degenerate primers in the polymerase chain reaction to defect whitefly transmitted geminivirus. Plant Disease 77, 340–347.
|
CAS |

Tahir M, Haider MS
(2005) First report of Tomato leaf curl New Delhi virus infecting bitter gourd in Pakistan. Plant Pathology 54, 807.
| Crossref | GoogleScholarGoogle Scholar |

Takeuchi S,
Shimomoto Y, Ishikawa K
(2009) First report of Melon yellow spot virus infecting balsam pear (Momordica charantia L.) in Japan. Journal of General Plant Pathology 75, 154–156.
| Crossref | GoogleScholarGoogle Scholar |

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Tomar SPS, Jitendra M
(2005) Severe mosaic caused by Watermelon mosaic virus-1 on bitter gourd (Momordica charantia) in western U.P. Journal of Living. WORLD (Oakland, Calif.) 84, 12.

Yadav RK,
Yadav DS, Sarma P
(2004) Diversity of Cucurbitaceous crops in north eastern region, Himalayan Ecology. ENVIS Bulletin 13, 2.



