First report of the seed-borne nature of root and collar rot disease caused by Rhizoctonia solani in sunflower from India
N. Lakshmidevi A D , J. Sudisha B , S. Mahadevamurthy C , H. S. Prakash B and H. Shekar Shetty BA Department of Microbiology, Manasagangotri, University of Mysore, Mysore 570 006, Karnataka, India.
B Department of Biotechnology, Manasagangotri, University of Mysore, Mysore 570 006, Karnataka, India.
C Department of Microbiology, Yuvaraja’s College, University of Mysore, Mysore 570 005, Karnataka, India.
D Corresponding author. Email: lakshmiavina@rediffmail.com
Australasian Plant Disease Notes 5(1) 11-13 https://doi.org/10.1071/DN10005
Submitted: 8 December 2009 Accepted: 6 January 2010 Published: 1 February 2010
Abstract
This is the first report of the seed-borne nature of root and collar rot disease caused by Rhizoctonia solani in sunflower from India. The disease incidence has increased from 17% in 2006 to 21% in 2008. Consequently, disease monitoring and management measures need to be taken.
Introduction
Sunflower (Helianthus annuus L.), one of the important oil seed crops, faces the problem of crop loss due to diseases caused by microorganisms. Root and collar rot disease (Rhizoctonia solani) occurs destructively wherever sunflowers are grown. Severely infected plants show damping off, seedling necrosis at the 2–4-leaf stage, rotting of the collar region and presence of dark brown spots on the stem. Pathogenicity of the fungus revealed delayed sprouting of seeds and emergence of the seedlings in artificially infested soil. The seedling mortality was 100% in trays that contained 5% and 10% of R. solani inoculum. In all the infected plant parts, the pathogen R. solani was successfully reisolated. The pathogen was found to be present in the seed coat, endosperm and cotyledons.
Host symptoms and fungal morphology
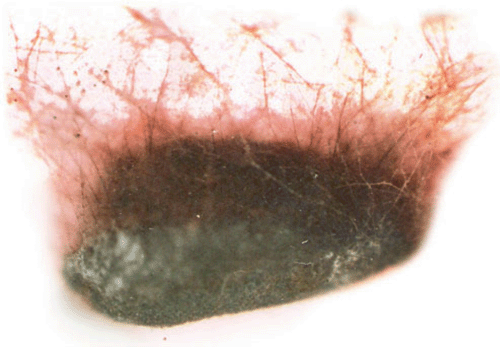
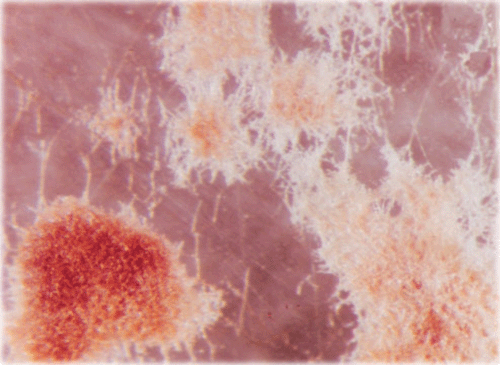
A sunflower (Helianthus annuus L.) field survey was conducted in 2005–2007 at Gandhi Krishi Vignana Kendra (GKVK Agricultural Research Station), Bangalore, India and Doddaballapura (Agricultural Research Station), India. In GKVK 30% of the hypocotyl seedlings exhibited ‘Soreshine’ symptoms (Fig. 1) and these seedlings collapsed, resulting in poor plant stand. In Doddaballapura, 42% of the plants showed similar symptoms of ‘Soreshine’ at the pre-flowering stage. Disease symptoms included severe damping off, seedling necrosis at the 2–4-leaf stage, rotting of the collar region and presence of dark brown spots on the stem. A fungus was isolated from diseased seed (Fig. 2), seedlings (Fig. 3), root (Fig. 4) and stem by plating surface-sterilised plant tissues onto potato dextrose agar (PDA) medium incubated for 7 days under 12 : 12 h light : dark (L : D) at 23 ± 2°C. Resultant cultures on PDA produced flocose aerial mycelia which were light brown to dark brown in colour, macroscopic dark brown sclerotia were also found of irregular shape (Fig. 5), mycelia were sparce and fleecy. For identification of the anastomosis groups, the culture was incubated in 25 ± 2°C for 24 h on Stewart’s medium and on PDA. On incubation on Stewart’s medium, mycelial contact stained with lactophenol cotton blue and hyphal anastomosis was observed under the light microscope with ×400 and ×1000 magnifications. Isolates were consistently and uniformly brown. On PDA, the colony appearance of the isolates was less characteristic. As a result, anastomosis group AG-3 was found with a frequency of 31.7% (Castro et al. 1983). Based on the symptoms, culture morphology and colony habit, the fungus was identified as Rhizoctonia solani (Michail et al. 1977); the pathogen causes root and collar rot in sunflower. To confirm Koch’s postulates, sterilised soil was amended with a 7-day-old wheat bran culture of the R. solani inoculum densities (1, 2, 3, 4, 5 and 10% R. solani inoculum in sterilised soil (w/w) and placed in plastic trays (30 × 25 cm). Sterilised non-amended soil without the R. solani inoculum served as control. Ten seeds of susceptible cultivar MSFH 6 were sown at 3-cm depth in R. solani inoculated trays, covered with plastic bags and maintained under ambient conditions (25 ± 2°C and 90% relative humidity with a 12-h L : D photoperiod). The trays were watered every other day. The experiment was replicated four times with 10 seedlings/replicate. At 15 days after sowing (DAS) the sprouting of seeds and emergence of the seedlings were delayed in the infested soil. The seedling mortality was 100% in trays that contained 5% and 10% of R. solani inoculum. Infected plant parts were collected and plated on PDA plates, on PDA it produced macroscopic dark brown sclerotia which were irregular in shape and mycelia were sparse and fleecy which revealed the presence of R. solani, and was correlated with the observation of Castro et al. (1983). To locate R. solani in different seed tissues, the component plating method was adopted, the pathogen was detected in all the seed components viz., seed coat (7–69%), endosperm (2.5–41%) and cotyledon (2–29%) indicating the internal and external seed-borne nature of the pathogen. Although the pathogen was previously reported from sunflower across the world, the present study clearly demonstrated the seed-borne nature and re-establishment of R. solani which causes root and collar rot of sunflower, and is now reported for the first time in India.

|
|

|

|
|
Castro C,
Davis JR, Wiese MV
(1983) Differential medium for identification of Rhizoctonia solani. Plant Disease 67, 1069–1071.
| Crossref | GoogleScholarGoogle Scholar |

Michail SH,
Mathur SB, Neergaard P
(1977) Seed health testing for Rhizoctonia solani on blotters. Seed Science and Technology 5, 603–661.



