First record of Turnip mosaic virus in Pachycladon spp. (Brassicaceae): an endangered native plant species in New Zealand
J. D. Fletcher A C , R. A. Lister A , S. R. Bulman A and P. B. Heenan BA The New Zealand Institute for Plant & Food Research Limited, Private Bag 4704, Christchurch, New Zealand.
B Manaaki Whenua-Landcare Research, PO Box 40, Lincoln 7640, New Zealand.
C Corresponding author. Email: john.fletcher@plantandfood.co.nz
Australasian Plant Disease Notes 5(1) 9-10 https://doi.org/10.1071/DN10004
Submitted: 26 September 2009 Accepted: 15 December 2009 Published: 1 February 2010
Abstract
Pachycladon comprises nine native species from the South Island, New Zealand, including several threatened species. Virus-like disease symptoms were observed in a Pachycladon species collection. This is the first record of a virus in Pachycladon and a further report of Turnip mosaic virus (TuMV) infections in New Zealand native hosts. This may be the first record of association between the aphid species Aulacorthum solani and Pachycladon.
Pachycladon comprises nine species endemic to the South Island, New Zealand, with a single species occurring in Tasmania, Australia. Most of the species occur in the alpine zone of the Southern Alps where they grow on shaded, south-facing bluffs. The genus has been the subject of taxonomic and evolutionary studies for over 14 years (e.g. Heenan 1999; Heenan et al. 2002; Heenan and Mitchell 2003), and for this research an extensive collection of wild-sourced plants has been cultivated in glasshouses at Landcare Research, Lincoln. Several species have restricted distributions and are classified as threatened or uncommon (de Lange et al. 2009).
Five Pachycladon spp. exhibiting virus-like symptoms in a glasshouse at Manaaki Whenua-Landcare Research, Lincoln, were submitted for diagnosis in November 2008 (Table 1, Fig. 1). The affected plants were infested by Aulacorthum solani (Kaltenbach), the potato or fox glove aphid (CM Till, pers. comm.).
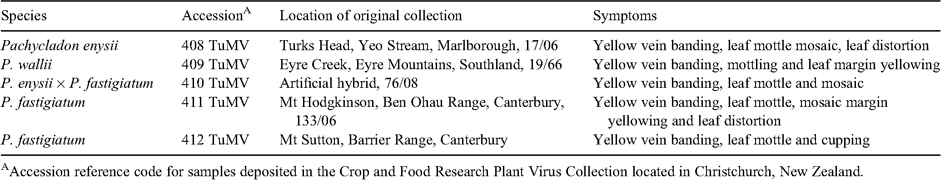
|

|
Crushed leaf sap from the five plants tested positive for Turnip mosaic virus (TuMV) (Potyviridae) (mean of controls + 3 × s.d.) using double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) (AS0132, DSMZ, Braunschweig, Germany). TuMV infection was further confirmed by reverse transcriptase polymerase chain reaction (RT–PCR) essentially as in Fletcher et al. (2009). RNA was extracted from ~1 cm2 leaf discs of five TuMV-infected plants using the RNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA). RT–PCRs were carried out using the Qiagen OneStep RT–PCR kit (Qiagen) using the TuMVF1 and TuMVR1 primers (Fletcher et al. 2009). The expected ~290 bp DNA fragment was amplified from all five samples, while RT-negative and H2O-negative PCR reactions did not give DNA amplification (Fletcher et al. 2009). The PCR products from the five samples were then sequenced and were confirmed by DNA sequence alignment to be from a single TuMV isolate.
Leaf tissue from the five symptomatic plants was used to mechanically inoculate appropriate herbaceous indicator plants and the TuMV isolate found to infect Brassica rapa ‘Striker’, Brassica pekinensis ‘Wong bok’ and ‘Pak choi’, Chenopodium quinoa, C. amaranticolor, Raphinus sativus ‘Gentle Giant’, R. sativus ‘French Breakfast’ and Sinapsis alba. Symptoms on all hosts appeared characteristic of those for TuMV infection as summarised in Provvidenti (1996).
Turnip mosaic virus is found in New Zealand in a wide range of hosts (Pearson et al. 2006; Fletcher et al. 2009). In Canterbury, large areas of Brassica rapa (turnip) and Brassica napus var. napobrassica (swede) have been grown regularly for sheep and cattle fodder. This is the first record of a virus in Pachycladon spp. and a further report of a TuMV infection in a New Zealand native host (Fletcher et al. 2009).
The aphid species Myzus persicae and Brevicoryne brassicae are the two most common vectors of TuMV in cultivated brassicas (Karl 1971). In 2006, B. brassicae was identified on Pachycladon fastigiatum from Mt. Sutton (Barrier Range, Canterbury) and Myzus cymbalariae on Pachycladon stellatum from Yeo Stream, Marlborough (D.A.J. Teulon, pers. comm.). Myzus cymbalariae is not recorded as a vector of TuMV but is known to vector Shallot yellow stripe potyvirus (Van Dijk 1993). Aulacorthum solani has also been recorded as a vector of TuMV (Kennedy et al. 1962) but has not been recorded as a coloniser of Pachycladon. As such, this also appears to be the first record of an association between A. solani and Pachycladon.
The three Pachycladon species, P. cheesemanii, P. exile, and P. stellatum are classified as threatened (de Lange et al. 2009). Interestingly, specimens of these three species were grown alongside the infected plants examined in this study but did not exhibit disease symptoms, suggesting that they may be resistant to TuMV infection. We believe TuMV infection probably occurred in the glasshouse and although most species of Pachycladon occur in the alpine zone, well away from crop plants, P. cheesemanii and P. exile occur in lowland sites and may be at risk from nearby crops that are potential hosts to both aphids and TuMV. However, it is also of concern that B. brassicae, one of the main vectors of TuMV, has been collected from P. fastigiatum in the alpine zone (~1600 m) at Mt. Sutton, Barrier Range (Canterbury). This raises the possibility of alpine species of Pachycladon that occur some distance from lowland Brassica crops and associated aphid vectors of TuMV also being infected with the virus. If infected with TuMV these endangered plants may either die directly as a result or indirectly from secondary fungal, bacterial or insect attack. There is also a further risk of infection to other native plants from such incursions.
de Lange PJ,
Norton DA,
Courtney SP,
Heenan PB,
Barkla JW,
Cameron EK,
Hitchmough R, Townsend AJ
(2009) New Zealand extinct, threatened and at risk vascular plant list. New Zealand Journal of Botany 47, 61–96.

Fletcher JD,
Bulman SA,
Fletcher PJ, Houliston GJ
(2009) First record of Turnip mosaic virus in Cook’s scurvy grass (Lepidium oleraceum agg.) an endangered native plant in New Zealand. Australasian Plant Disease Notes 4, 9–11.

Heenan PB
(1999) Artificial intergeneric hybrids between the New Zealand endemic Ischnocarpus and Pachycladon (Brassicaceae). New Zealand Journal of Botany 39, 595–601.

Heenan PB, Mitchell AD
(2003) Phylogeny, biogeography, and adaptive radiation of Pachycladon (Brassicaceae) in the mountains of South Island, New Zealand. Journal of Biogeography 30, 1737–1749.
| Crossref | GoogleScholarGoogle Scholar |

Heenan PB,
Mitchell AD, Koch M
(2002) Molecular systematics of the New Zealand Pachycladon (Brassicaceae) complex: generic circumscription and relationships to Arabidopsis s. l. and Arabis s. l. New Zealand Journal of Botany 40, 543–562.

Karl E
(1971) New vectors for some non-persistent viruses. Archiv fur Pflanzenschutz 7, 337–342.

Pearson MN,
Clover GRG,
Guy PL,
Fletcher JD, Beever REB
(2006) A review of the plant virus, viroid and mollicute records for New Zealand. Australasian Plant Pathology 35, 217–252.
| Crossref | GoogleScholarGoogle Scholar |



