First report of Sclerotinia sclerotiorum on potato plants in Iran
M. R. OjaghianA Department of Plant Protection, College of Agriculture, Bu-Ali Sina University, Hamadan, Iran.
B Corresponding author. Email: smro59@gmail.com
Australasian Plant Disease Notes 4(1) 39-41 https://doi.org/10.1071/DN09016
Submitted: 9 March 2009 Accepted: 31 March 2009 Published: 15 April 2009
Abstract
After observing severe symptoms of Sclerotinia sclerotiorum on the lower parts of potato plants (cultivar Agria), some sclerotia were observed and sampled. During sexual and asexual stages of this fungus, some morphologic characteristics of this pathogen were studied and its pathogenesis on cultivar Marfona was proved by a new method. This is the first report of Sclerotinia sclerotiorum on potato in Iran.
Potato (Solanum tuberosum) is one of the most economic and climatically compatible crops in Hamadan, a province in the west of Iran. In early autumn 2007 and during some plant-disease assessments, severe symptoms were observed on the lower parts of potato plants (cultivar Agria) in some fields. The symptoms first appeared as water-soaked lesions in the intersections between the stem and branches, or on branches and stems in contact with the soil. These lesions became quickly covered with a white cottony mycelium that spread rapidly to nearby stems and leaves and expanded to girdle stems under wet conditions but when conditions became dry, the lesions dried out and turned beige, tan or bleached white in colour and papery in appearance. As infected tissue decayed, hard irregularly shaped sclerotia formed on the inside and sometimes on the outside of decaying tissue. Sclerotia were usually 0.6 to 1.3 cm in diameter, initially white to cream in colour and gradually turning black with age. Sclerotia eventually fell to the ground as infected stems dried out but no symptoms were observed on below-ground tissues. These symptoms were sufficient to identify the causal agent as Sclerotinia sclerotiorum. Although this pathogen can cause an important disease on potato called stem rot, reports on its economic impact on potato are scarce (Atallah and Johnson 2004).
Center-pivot sprinkler irrigation and excess nitrogen fertilisation are linked to increased stem rot incidence in several crops (Atallah and Johnson 2004) and these factors have recently become customary in potato fields at Hamadan. This pathogen is a cosmopolitan, homothallic and necrotrophic fungus with an extremely wide host range and was first described as Peziza sclerotiorum by Libert in 1837 (Purdy 1979).
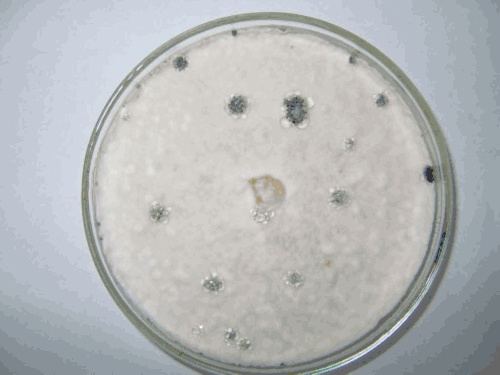
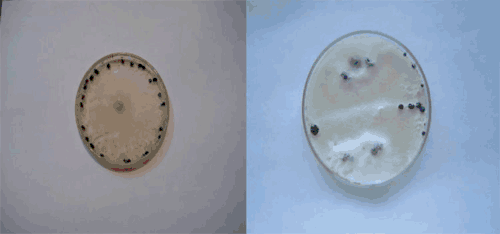
For more assessments, some sclerotia were sampled from different areas of potato fields (Fig. 1) and after disinfection in 3% sodium hypochlorite for 2 min and under sterile conditions, were cultured on PDA (39 g/L; Merck, Darmstadt, Germany) and myceliogenic germination produced septate and hyaline hyphae, a white colony (Fig. 2) and elliptical to globose sclerotia in vitro. Different isolates showed different arrangement of sclerotia on the culture medium (Fig. 3).

|
|
|
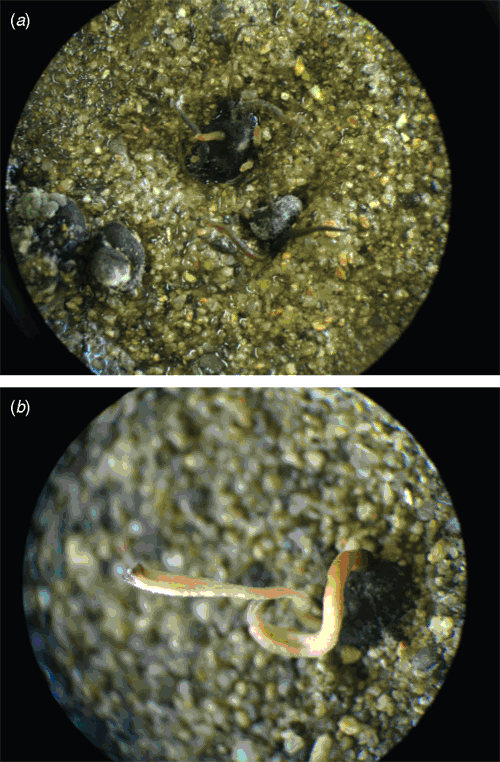
To fulfill Koch’s Postulates and appraise its pathogenesis on other common potato cultivars grown in Hamadan (Marfona), after preparing wheat inoculum (Jones et al. 2003), some inoculated wheat grains were tied by cellophane to the stems of 10 2-month potato plants grown in five flowerpots and maintained in a greenhouse. To provide humid conditions, a wet transparent plastic bag completely covered each pot at a temperature below 24°C. After 5 days, white cottony mycelium and sclerotia of the pathogen were observed on the plants (Fig. 4). In S. sclerotiorum, this is an efficient method to make or measure disease on plants in pots in greenhouse trials without any mist-producing equipment and is reported for the first time. The sexual stage of this fungus (carpogenic germination of sclerotia) was conducted in five replicates (Cobb and Dillard 2004) and after 2 months in 40% of sclerotia, stipitate and funnel-shaped apothecia were formed together (Fig. 5) or rarely singly. The host, size of sclerotia, sexual stage and the collective growth of apothecial stipes confirmed the identification of this pathogen as S. sclerotiorum (Whetzel 1945; Kohn 1979). The family Sclerotiniaceae have generally brown apothecia that are borne on long stipes that arise from stromata or sclerotia (Alexopoulos et al. 1996). Sclerotinia minor is another species in the family Sclerotiniaceae that is likely to attack potato but sclerotia are 0.5–3 mm in diameter and carpogenic germination is rarely seen in nature (Abawi and Grogan 1979).

|
|
Some control measures such as crop rotation, sanitation, irrigation methods, deep ploughing, the use of protectant chemicals (Hubbard et al. 1997; Subbarao 1998; Bardin and Huang 2001), steam sterilisation and fumigation (Jones et al. 2003) have been suggested to combat this pathogen on crops but because of the wide host range, longevity of sclerotia (Gerlagh et al. 1999), the lack of resistant cultivars in crops including potato (Atallah and Johnson 2004) and economic or environmental concerns, no simple and effective control measures are currently available to manage this disease economically and consistently and this has led to an increase in the search for biological control agents of sclerotia. This is the first report of Sclerotinia sclerotiorum attacking potato in Iran.
Abawi GS, Grogan RG
(1979) Epidemiology of diseases caused by Sclerotinia species. Phytopathology 69, 899–904.
| Crossref | GoogleScholarGoogle Scholar |

Atallah ZK, Johnson DA
(2004) Development of Sclerotinia stem rot in potato fields in south-central Washington. Plant Disease 88, 419–423.
| Crossref | GoogleScholarGoogle Scholar |

Bardin SD, Huang HC
(2001) Research on biology and control of Sclerotinia diseases in Canada. Canadian Journal of Plant Pathology 23, 88–98.

Gerlagh M,
Goossen-van de Geijn HM,
Fokkema NJ, Vereijken PFG
(1999) Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology 89, 141–147.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hubbard JC,
Subbarao KV, Koike ST
(1997) Development and significance of dicarboximide resistance in Sclerotinia minor isolates from commercial lettuce fields in California. Plant Disease 81, 148–153.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Jones EE,
Mead A, Whipps JM
(2003) Evaluation of different Coniothyrium minitans inoculum sources and application rates on apothecial production and infection of Sclerotinia sclerotiorum sclerotia. Soil Biology & Biochemistry 35, 409–419.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Kohn LM
(1979) A monographic revision of the genus Sclerotinia. Mycotaxon 9, 365–444.

Purdy LH
(1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 69, 875–880.
| Crossref | GoogleScholarGoogle Scholar |

Subbarao KV
(1998) Progress toward integrated management of lettuce drop. Plant Disease 82, 1068–1078.
| Crossref | GoogleScholarGoogle Scholar |

Whetzel HH
(1945) A synopsis of the genera and species of the Sclerotiniaceae, a family of stromatic inoperculate discomycetes. Mycologia 37, 648–689.
| Crossref | GoogleScholarGoogle Scholar |



