Some notes on Phytophthora syringae and P. multivesiculata in Australia
James H. Cunnington A C , Sri Kanthi de Alwis A and Michael Priest BA Biosciences Research Division, Department of Primary Industries, Knoxfield Centre, Private Bag 15, Ferntree Gully Delivery Centre, Victoria 3156, Australia.
B NSW Department of Primary Industries, Orange Agricultural Institute, Forest Road, Orange, NSW 2800, Australia.
C Corresponding author. Email: james.cunnington@dpi.vic.gov.au
Australasian Plant Disease Notes 4(1) 42-43 https://doi.org/10.1071/DN09017
Submitted: 28 November 2008 Accepted: 2 April 2009 Published: 22 April 2009
Abstract
In June 2008, Phytophthora syringae was baited from soil at the base of a dying pear tree in an orchard in the outer eastern suburbs of Melbourne, Australia. Phytophthora syringae has been rarely reported in Australia. The identification was made using rDNA ITS sequence data. State checklists record P. syringae on Prunus in South Australia and on Pyrus in Victoria. Neither of these records is linked to cultures or herbarium specimens. The only preserved specimen of P. syringae in Australia is from Cymbidium collected in New South Wales in 1991, but this specimen has been re-identified as P. multivesiculata, a species not previously recorded in Australia. These two cultures appear to be the only verifiable records of P. syringae and P. multivesiculata in Australia.
In June 2008 a pear tree with a severe canker at the base of the trunk was submitted to Department of Primary Industries Victoria Crop Health Services in Knoxfield. The tree originated from an orchard in the Dandenong Ranges to the east of Melbourne. Standard isolation techniques failed to recover any plant pathogenic organisms from the canker, probably due to the dry nature of the sample. A Phytophthora isolate was pear baited from the accompanying soil sample taken from the base of the affected tree. From the purified culture, the rDNA internal transcribed spacer region was amplified and sequenced using primers ITS5 and ITS4 (White et al. 1990). The sequence was 0–2 bases different from many P. syringae sequences on GenBank, including only 2 bases from AF266803 (Cooke et al. 2000). Our sequence was deposited in GenBank as accession number FJ766021. The morphological characters observed on V8 agar generally agreed with those described for P. syringae (Erwin and Ribeiro 1996). At 20°C colonies reached a diameter of 7 cm in 1 week. Sporangia produced on solid media were semipapillate, obovoid and measured 37–50 × 20–38 µm. Abundant hyphal swellings were observed in older cultures (Fig. 1). Oogonia were produced sparsely in the original isolation, but were not observed in subsequent subcultures and unfortunately no details were recorded. Phytophthora syringae is reported to produce abundant oogonia (Erwin and Ribeiro 1996). A culture has been lodged in herbarium VPRI as accession 41421.
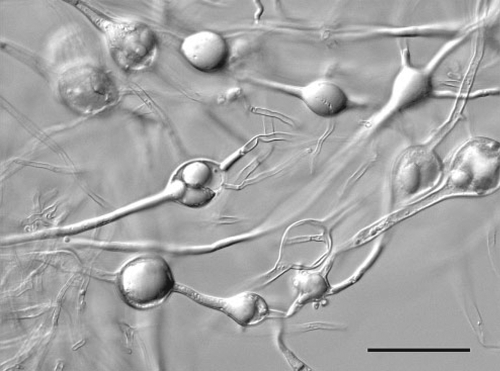
|
In Victoria, P. syringae has previously been reported on Pyrus communis (Washington and Nancarrow 1983) but no further details are listed and no voucher specimens are cited. In South Australia, P. syringae is reported from Prunus avium and P. dulcis (Cook and Dubé 1989). This reference also gives no further details and does not include any reference to voucher specimens. It is interesting to note that Warcup and Talbot (1981) did not list P. syringae in their checklist of plant diseases in South Australia published 8 years earlier.
A search of Australian fungal collections returned only a single preserved specimen and culture of P. syringae. The specimen (DAR 66142) had been isolated from Cymbidium in New South Wales in 1991. This culture was obtained and the rDNA ITS region was sequenced as above. The sequence obtained was identical to the sequence available from the type specimen of P. multivesiculata, GenBank AF266790 (Cooke et al. 2000). Our sequence has been deposited in GenBank as accession FJ766022. The ITS sequence from this isolate (DAR 66142) was also sequenced by Crawford et al. (1996), believing it to be P. syringae. Morphological characters on V8 agar agreed with the description of P. multivesiculata (Ilieva et al. 1998). At 20°C colonies reached ~7 cm in diameter after 1 week. Sporangia were non-papillate or semipapillate, ovoid, 42–54 × 28–34 µm. Oogonia were ~40 µm in diameter. Antheridia were amphigynous; in contrast to Phytophthora syringae which has paragynous antheridia (Erwin and Ribeiro 1996). The abundant hyphal swellings (Fig. 2) are similar to those formed by P. syringae and were presumably the basis for the original identification. Phytophthora multivesiculata was described 7 years after this material was collected.
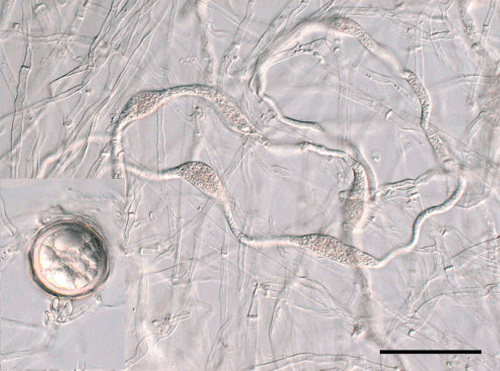
|
Phytophthora syringae was originally described from lilac (Syringa vulgaris), but is better known as a pathogen of members of the Rosaceae in temperate climates (Erwin and Ribeiro 1996). It is primarily responsible for collar rots and stem cankers, but can cause fruit rots as well. Its distribution in Australia is uncertain, given that the only verifiable specimen in Australia is the one reported here.
Phytophthora multivesiculata is a pathogen of Cymbidium (Orchidaceae). It causes blackening of the leaves and stems (Ilieva et al. 1998). Although quite common in New Zealand (Hill 2004), this is the first record of it in Australia. It is interesting that Hall (1989) noted the occurrence of P. erythroseptica on Cymbidium in Perth and New Zealand. Phytophthora erythroseptica also forms chains of hyphal swelling, thus it is possible that these are also mis-identifications of P. multivesiculata.
Cooke DEL,
Drenth A,
Duncan JM,
Wagels G, Brasier CM
(2000) A molecular phylogeny of Phytophthora and related Oomycetes. Fungal Genetics and Biology 30, 17–32.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Crawford AR,
Bassam BJ,
Drenth A,
Maclean DJ, Irwin JAG
(1996) Evolutionary relationships among Phytophthora species deduced from rDNA sequence analysis. Mycological Research 100, 437–443.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Hall G
(1989) Unusual or interesting records of plant pathogenic Oomycetes. Plant Pathology 38, 604–611.
| Crossref | GoogleScholarGoogle Scholar |

Hill CF
(2004) First report of Phytophthora multivesiculata on cymbidium orchids in New Zealand. Australasian Plant Pathology 33, 603–604.
| Crossref | GoogleScholarGoogle Scholar |

Ilieva E,
Man in’t Veld WA,
Veenbaas-Rijks W, Pieters R
(1998)
Phytophthora multivesiculata, a new species causing rot in Cymbidium. European Journal of Plant Pathology 104, 677–684.
| Crossref | GoogleScholarGoogle Scholar |



