Phosphorus responses of Trifolium pallescens and T. occidentale, the progenitors of white clover (T. repens)
Shirley N. Nichols A * , James R. Crush A , Vanessa M. Cave B and Warren M. Williams C
A * , James R. Crush A , Vanessa M. Cave B and Warren M. Williams C
A AgResearch, Ruakura Research Centre, Hamilton, New Zealand.
B University of Auckland, Faculty of Science, Statistics, Auckland, New Zealand.
C AgResearch, Grasslands Research Centre, Palmerston North, New Zealand.
Crop & Pasture Science 74(9) 911-923 https://doi.org/10.1071/CP22254
Submitted: 21 July 2022 Accepted: 1 March 2023 Published: 4 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Rock phosphate reserves are decreasing, and requirements to mitigate environmental impacts of farming soils with excess phosphorus (P) are increasing.
Aims: White clover is an allotetraploid hybrid between Trifolium pallescens and T. occidentale. Understanding the P response characteristics of these progenitor species will allow selection of germplasm for development of synthetic white clovers with improved phosphorus-use efficiency (PUE).
Methods: Shoot and root weights and P nutrition characteristics were compared for three Trifolium pallescens, six T. occidentale accessions, and a white clover cultivar in a glasshouse experiment using a low P soil amended with five rates of P.
Key results: White clover had the highest and most P responsive shoot and root dry weights (DW), and T. pallescens was least responsive to P. In the highest P treatment, T. pallescens had the highest shoot and root %P and the lowest shoot DW:total plant P ratio. There was significant variation among progenitor accessions. Spanish accessions of T. occidentale had comparable shoot DW to white clover and higher PUE. Traits of T. pallescens indicated strong adaptation to low P but low productivity, while traits of T. occidentale were more aligned with the white clover cultivar.
Conclusions: The substantial variation in P nutrition characteristics within the progenitor species of white clover could be exploited in breeding programs.
Implications: Comparing the P responses of a wider range of progenitor accessions could inform breeding to improve white clover’s low soil P tolerance through development of synthetic white clovers.
Keywords: evolution, hybridisation, phosphorus acquisition, phosphorus utilisation, progenitors, synthetic white clover, Trifolium occidentale, Trifolium pallescens, white clover.
Introduction
White clover (Trifolium repens L.) is a recent (15 000–28 000 years before present) allotetraploid hybrid between T. pallescens (Schreb.) and T. occidentale (Coombe; Ellison et al. 2006; Williams et al. 2012) and is found globally throughout temperate grasslands. The progenitor species are currently geographically isolated. Trifolium occidentale (western clover) has a maritime distribution, growing within 100 m of the coast on beaches, sand dunes and cliff tops in Brittany, Spain, Portugal, Cornwall and Ireland (Coombe 1961; Coombe and Morisset 1967; Akeroyd 1983). Trifolium pallescens (pale clover) is a European alpine species, found above 1800 m altitude (Zohary and Heller 1984), particularly on glacial forelands (Raffl et al. 2008). The two species maintain their compatibility and can be hybridised to create synthetic white clover (Williams et al. 2012). The progenitor genomes within white clover reside as independent subgenomes/homoeologues and their integrity has been largely maintained, enabling retention of a ‘genetic toolbox’ likely to underpin the adaptability of white clover to diverse environments (Griffiths et al. 2019). Whereas most genes in white clover showed consistent expression of one homoeologue relative to the other across tissues, the few genes that switched between homoeologues in different tissues were associated with flavonoid synthesis, indicating an adaptive role for these transcriptional changes (Griffiths et al. 2019).
White clover is an important component of mixed swards in temperate pastoral agriculture, but it has a higher requirement for soil phosphorus (P) than do its companion grasses (Dunlop and Hart 1987) and some other forage legumes e.g. Lotus pedunculatus (Crush 1974). This is due in part to poor competitive ability against companion grasses for P, i.e. P-acquisition efficiency (Simpson et al. 2014), and partly because of the internal P physiology of the plant, i.e. P-utilisation efficiency (Dunlop and Hart 1987). The root competition is driven by grasses having finer, more branching roots, with longer root hairs (Jackman and Mouat 1972a, 1972b; Evans 1977) and more rapid root-turnover rates, so that the grass roots are constantly growing into fresh soil (Reid and Crush 2013; Reid et al. 2015). There is increasing concern in New Zealand about the environmental impacts of farming soils with elevated P status (Monaghan et al. 2007), and the external environmental footprint of importing phosphate rock. Interspecific hybridisation is being investigated as a strategy for improving the tolerance of white clover to low soil P. Hybrids with the wild relative T. uniflorum L. have been shown to have higher growth than does white clover at low P supply under glasshouse conditions (Nichols et al. 2014a, 2014b; Nichols and Crush 2015).
The P responses of the two progenitor species of white clover are unknown. Here, we report on the P response characteristics of some contemporary T. occidentale and T. pallescens populations compared with a commercial white clover cultivar. Understanding the origins of the P response characteristics of white clover should allow targeted hybridisation for improved P-use efficiency, utilising selected accessions of the progenitor species.
Methods
Plant material
Seeds of six accessions of T. occidentale and three accessions of T. pallescens were obtained from the Margot Forde Germplasm Centre (MFGC), Palmerston North, New Zealand. T. occidentale accessions were from three geographical regions, with two accessions per region collected from beach, cliff top, or other littoral sites (Table 1). T. pallescens accessions were from three sites across the geographical range of the germplasm available in the MFGC for this research. The cultivar ‘Grasslands Kopu II’ was chosen as the white clover control because it has been used in other research on P responses of Trifolium germplasm and as a backcross parent for interspecific hybridisation (Nichols et al. 2014b; Crush et al. 2015; Nichols and Crush 2015). This is a large-leaved cultivar bred for dairying pastures. In related white clover cultivars released over a 57-year period, including Kopu II, internal phosphorus-use efficiency (PUE) was higher in the newer cultivars but there were no differences among the cultivars in the measured root traits. This suggested that positive selection pressure for PUE had occurred in conjunction with selecting for yield (Crush et al. 2015). Because Kopu II was the newest cultivar from that related sequence available at the time of this experiment, we would expect it provided a relatively high PUE control compared with older cultivars.

|
Experimental
Subsoil of an allophanic ash Horotiu silt loam (Hewitt 2010; USDA Classification: Typic Udivitrand) collected from a former dairy farm was screened through a 1 cm sieve, analysed for Olsen P concentration and plant macronutrients. The soil had an Olsen P concentration of 7 mg L−1, and the soil pH was 6.4. It was amended with CaHPO4 to provide five soil P treatments (P1–P5) with Olsen P concentrations of 7, 8, 9, 11 or 27 mg L−1. The values from 7–11 mg L−1 are common on east coast New Zealand hill country sheep and beef farms (e.g. Gillingham et al. 2007) and 27 mg L−1 is within the target range for dairying on ash soils (Roberts and Morton 2016). Details of the methodology have been described in Nichols et al. (2014b). The treated soils were transferred into 1.8 L pots with potassium sulfate (0.8 g) and magnesium sulfate (0.53 g) added to each pot to bring concentrations of these elements up to recommended levels for dairy farms on the soil type (Roberts and Morton 2016). The potassium and magnesium were applied in 100 mL pot−1 of Long Ashton trace-element solution (Hewitt 1966) containing boron (B,) chlorine (Cl), cobalt (Co), copper (Cu), manganese (Mn), molybdenum (Mo) and zinc (Zn) to avoid any micronutrient limitations to plant growth. The experimental design was generated using GenStat, 16th edition (VSN International 2011). The pots were arranged in a split-plot design across 10 glasshouse tables (replicates), with five main plots (five phosphate treatments) per table, and 10 subplots in each main plot (one plant of each clover line). On each table, the pots were laid out in a 10 row by five column grid, with the columns constituting the main plots. The soil was taken through two cycles of wetting and drying to ensure adsorption of the added P before planting.
Seeds were scarified with sandpaper and germinated on damp filter paper in Petri dishes. Germinated seeds were then transplanted to the experimental pots when the radicles were 1 cm long and inoculated with a suspension of Rhizobium leguminosarum bv trifolii strain TA1 in 10% (w/v) aqueous sucrose solution.
The experiment ran from 1 April 2013 to 1 October 2013 in a temperature-controlled glasshouse with mean day/night temperatures of 19.4°C/12.9°C. Two 400 W day extension lights were used per replicate block, from 0600–0800 hours and 1600–1800 hours, to maintain a 12 h photoperiod and the plants were watered daily. Two weeks prior to the end of the experiment, the second fully expanded leaf from one stolon on each plant was collected, scanned with an Epson Expression 1680 flatbed scanner and analysed for leaf area. Leaves were then dried at 60°C for 24 h and weighed, and specific leaf area (cm2 mg−1) was calculated for each leaf. At harvest, soil was washed off the roots, then the roots, shoots and flowers were separated and dried for 24 h at 60°C before being weighed. Dried root and shoot samples were ground and then analysed for %P by RJ Hill Laboratories Ltd, Hamilton, New Zealand. Dry weight (g) and %P concentrations were used to calculate the root P content, shoot P content, and total P content (mg) for each plant ((DW × 1000) × (%P / 100)). P content data was used to calculate shoot DW per unit total plant P content ratio (shoot DW:total plant P) as an alternative measure of P efficiency, shoot P content per unit root DW, and total plant P content per unit of root DW as a measure of P uptake.
Data analysis
All variables were analysed using a linear mixed model fitted using residual maximum likelihood in GenStat 16th edition (VSN International 2011). The fixed model comprised clover line (a factor with 10 levels), P treatment (five levels) and clover line by P treatment interaction. At harvest, root tubers of parasitic broomrape (Orobanche minor) were found attached to roots of some plants; so, an additional binary factor was included in the fixed model to adjust for their presence. The model accounting for the randomisation structure of the split-plot design comprised the following three random terms: tables, main plots within tables, and subplots within main plots. Residual diagnostic plots were inspected for evidence of departure from the assumptions of normality, independence and constant variance. All variables were natural log-transformed prior to analysis to stabilise the variance. Back-transformed predicted means and least significant ratios were calculated. A principal-component analysis on the correlation matrix was generated in DeltaGen (Jahufer and Luo 2018) by using the back-transformed means for all traits.
Results
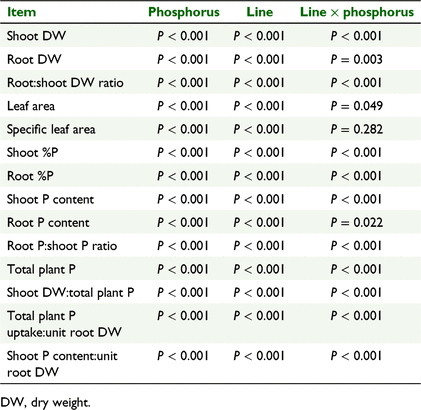
There were significant effects of clover line and phosphate treatment level on all the vegetative traits (shoot and root dry weight (DW), root:shoot DW ratio, leaf area, specific leaf area) and all the phosphorus-related traits (shoot and root %P, shoot and root P content, root P:shoot P content ratio, total plant P, shoot DW:total plant P; Table 2). There were significant clover line × phosphate concentration interactions for all factors except specific leaf area (Table 2).
|
Shoot and root dry weight
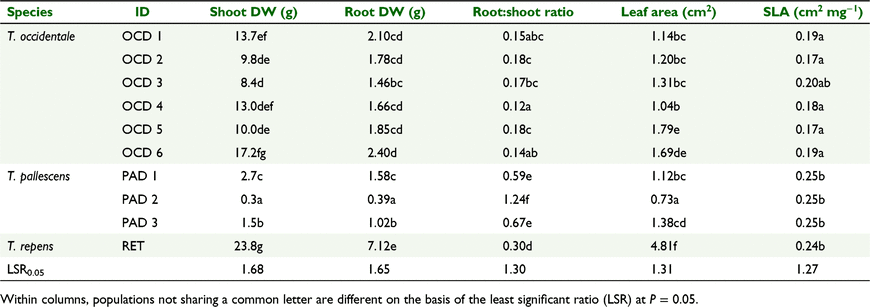
Differences in plant dry weight were most apparent in the highest (P5) phosphate treatment (Fig. 1a). In this treatment, white clover shoot DW was heavier than for all but the largest T. occidentale line, and all T. pallescens lines were lighter than the T. occidentale lines (Table 3). Among the six T. occidentale lines, shoot DW varied by a factor of two, with differences among the heavier and lighter lines. There were no consistent effects of source location on shoot DW in the P5 treatment for T. occidentale. Shoot DW of T. pallescens differed among all three lines in the P5 treatment (Table 3), with a nine-fold variation between the lightest and heaviest lines. PAD 2 had minimal response to additional P supply (Fig. 1a, Table 3).
|
|
Root DW P-response patterns were essentially similar to those for shoot DW, with white clover having the heaviest and most P-responsive root system (Fig. 1b, Table 3), which was heavier than all the T. occidentale lines. T. pallescens had the lightest and least P-responsive root systems (Fig. 1b). Among the T. pallescens lines, roots of all three lines differed from each other in the P5 treatment, with PAD 2 being considerably lighter than the other two lines (Table 3). In the P5 treatment, the only difference in root DW among the T. occidentale lines was between the heaviest (OCD 6) and the lightest (OCD 3) lines (Table 3).
Root:shoot DW ratios decreased with increasing soil P concentrations for all three species (Fig. 2). In the P5 treatment, all the T. pallescens lines had higher root:shoot ratios than did white clover and T. occidentale, especially for PAD 2, with its particularly small root mass (Table 3). White clover also had a higher root:shoot DW ratio than all the T. occidentale lines in the P5 treatment (Table 3). There was a narrow range of root:shoot DW ratios (0.12–0.18) among the T. occidentale lines, with differences only between the lines with the highest (OCD 2 and 5) and lowest (OCD 4) values (Table 3).
|
|
Leaf area
Individual leaf area of the white clover cultivar was substantially greater than that of lines of the two progenitor species (Fig. 3a, Table 3), and leaf area increased in all the populations as soil P increased. In the P5 treatment, the Spanish T. occidentale lines OCD 5 and OCD 6 had larger leaf areas than did the other T. occidentale lines (Table 3). Among the T. pallescens lines, line PAD 2 had smaller leaves than did the other two lines (Fig. 3a, Table 3). Specific leaf area (cm2 mg−1) of five of the six T. occidentale lines was lower than Kopu II and T. pallescens, which did not differ from each other (Fig. 3b, Table 3).
|
|
Phosphorus in roots and shoots
In the P5 treatment, T. pallescens had higher shoot P concentrations than did T. occidentale and white clover (Table 4) and PAD 2 had higher shoot %P than did PAD 1. Shoot %P in both PAD 1 and PAD 3 increased strongly from the P4 treatment to the P5 treatment, unlike for PAD 2 (Fig. 4a). Among the T. occidentale lines, OCD 1 and 2 (from Ireland) and OCD 3 and 4 (France) did not differ for shoot %P, but their concentrations were higher than for OCD 5 and OCD 6 (Spain) (Table 4, Fig. 4a). Shoot %P of OCD 5 and OCD 6 did not differ from white clover, which was, in turn, lower than the other T. occidentale lines and T. pallescens. There was a similar pattern of results for root P concentrations, with higher values for T. pallescens than for T. occidentale, and lower values for white clover than for all the T. occidentale lines (Table 4, Fig. 4b). The response patterns for root P concentration in the T. pallescens lines were very similar to those observed for shoot %P. There was little variation in root %P among the T. occidentale lines, in contrast to shoot %P results.
|
|
Shoot P content (mg P) was generally lower in T. pallescens than in T. occidentale and white clover (Table 4). The exception was PAD 1, which had a shoot P content similar to that of OCD 3 and OCD 5, and a much higher shoot P content than that of the other two PAD lines. There was no evidence of a difference in the shoot P contents of white clover and OCD Lines 1, 4, and 6. The root P content of white clover was substantially higher than for all the other lines (Table 4). PAD 2 had a lower root P content than did four of the six OCD lines, and that of PAD 1 was higher than that of four of the OCD lines.
Ratios of root P content:shoot P content generally decreased as soil P concentration increased (Fig. 5a). They were highest in T. pallescens, particularly in PAD 2 (Fig. 5a, Table 4), which had a higher ratio than the other two T. pallescens lines, and higher in white clover than in all the T. occidentale lines. The ratio also varied among the T. occidentale lines (Fig. 5a, Table 4), with the French line collected from the beach (OCD 1) having a lower ratio than the line collected from the cliff-top site (OCD 2); however, this pattern was reversed in the Irish lines (OCD 3 and 4, Table 4). The two Spanish lines (OCD 5 and 6) did not differ from each other, and OCD 5 from the beach site had a higher root P:shoot P ratio than for all the Irish and French lines, whereas OCD 6 had a higher ratio than did OCD 1 and 4.
|
|
The ratio of shoot DW per unit total plant P content was higher in T. occidentale and white clover than in T. pallescens (Fig. 5b, Table 4). It was also higher in white clover and the two Spanish accessions of T. occidentale than in the French and Irish accessions of T. occidentale. The T. occidentale accessions further segregated into types where this ratio either increased (the Spanish lines, OCD 5 and 6) or decreased (OCD 1, 2, 3, and 4) in the P5 treatment (Fig. 5b). Shoot DW:total plant P also increased between the P4 to P5 treatments in white clover (Fig. 5b), but decreased in PAD 1 and 3. For PAD 2, the soil P treatment level had very little effect.
Individual lines had similar responses to increasing soil P for both P uptake rate (Fig. 6a) and shoot P content:unit root DW (Fig. 6b), and relative differences among lines were similar for both of these traits. The exception was PAD 2, where P uptake rate was considerably higher than shoot P content:unit root DW, reflecting the higher proportion of P retained in the roots.
|
|
P uptake rates per unit root DW generally increased with an increasing soil P, although for most lines these changes were small across P1 to P4, with the largest responses occurring at P5 (Fig. 6). The exception again was PAD 2, which was varied little across all P concentrations. Between P4 and P5, the increase in uptake rates per unit of root dry weight was generally as follows: PAD 1 and PAD 3 > OCD > Kopu II > PAD 2 (Table 4).
At P5, the total P uptake rate per unit root DW was lower in Kopu II than in the other lines (Table 4). Among T. occidentale lines, OCD 5 had the lowest total P uptake rate and OCD 1 and OCD 4 had the highest. There was no evidence of a difference in total P uptake rate among the remaining T. occidentale or T. pallescens lines. Although the total P uptake rate of PAD 2 was similar to most other OCD and PAD lines, its shoot P content per unit root DW was lower, as was that of Kopu II. PAD 1 and PAD 3 also had lower shoot P contents per unit root DW than did the OCD lines, except for OCD 5. Differences among the OCD lines were similar to those for total P uptake rate, being lowest in OCD 5 and generally highest in OCD 1 and 4.
Principal-component analysis
Principal-component analysis on the means of all traits at P5 showed clear separation among the three species (Fig. 7), with overall trait combinations reflecting the relative differences among lines for individual traits (Tables 3, 4). T. pallescens exhibited low DWs, low shoot DW:total plant P, high %P concentrations, high partitioning of DW and P to roots, and low leaf size, but high specific leaf area. Kopu II had high DWs and shoot DW:total plant P, low %P concentrations, low P uptake rates, and higher partitioning to roots compared with T. occidentale. T. occidentale had high P uptake rates, low partitioning of DW and P to roots, and above-average shoot DW and shoot DW:total plant P. Within T. occidentale and T. pallescens, there were some differences in the alignment of individual lines to particular traits (Fig. 7), and when leaf area and SLA were removed from the PCA, these lines formed more clusters (data not shown).
At lower P treatment levels, the position of the vectors and the plant lines relative to the vectors were very similar to the P5 treatment, including separation of the species and overall trait combinations, but there was more separation among lines within the progenitor species (Fig. 8). This reflects the differences for individual traits shown in Tables 3, 4. For example, at P4 the T. occidentale lines separated into two clusters, one containing OCD 1, 2 and 4, and one containing OCD 3, 5 and 6 (Fig. 8). Among T. pallescens lines, PAD 2 formed a separate cluster from PAD 1 and 3.
Discussion
Individual traits, and combinations of traits, showed distinctive differences within and among species, which likely reflect edaphic adaptation. This included greater separation among lines within progenitor species in lower soil P treatments. Knowledge of these differences will contribute to improved understanding of the influence of the T. occidentale and T. pallescens progenitors on the soil P requirements of white clover. The variation within the progenitor species for P nutrition-related traits indicates that there is considerable potential for future development of synthetic white clover with an increased PUE.
Root:shoot DW ratios were much higher in T. pallescens than in white clover, and were lowest in T. occidentale. This suggests that T. pallescens is adapted to survive on young, low available-P glacial outwash soils through high investment in root growth. Available-P concentrations in these alpine soils are not often recorded, but one Austrian site with T. pallescens, near the source of PAD 2, had 0.06 μg P g−1 soil (acetate–lactate extraction; Tscherko et al. 2005). This is grossly P-deficient soil by agricultural standards, equivalent to an Olsen test value of <1 mg L−1 compared with recommended phosphate test values of 20–30 for New Zealand sheep/beef farms on sedimentary soils (Morton and Roberts 2018). Another wild relative of white clover, Trifolium uniflorum L., has also been shown to have higher root:shoot DW ratios than white clover in low-P experimental soils (Nichols et al. 2014a, 2014b; Nichols and Crush 2015), but with values usually <0.8, which contrasts with values in the current study of up to 1.24 in PAD 2. Root:shoot DW ratios generally declined in all three species as soil P concentration increased and this is the normal response pattern (Lynch and Brown 2008; Nichols et al. 2014b). However, in the P5 treatment the root:shoot DW ratios of the T. pallescens lines were still clearly greater than those for white clover or T. occidentale. There were also significant differences in root:shoot ratio within T. pallescens, with particularly high values for PAD 2. Seeds of this line were collected from an alpine site in the Austrian Tyrol (Table 1). The young alpine soils that are the typical habitat for T. pallescens on the glacier forelands are characterised by extremely localised variation in edaphic conditions (Burga et al. 2010) where flexible root:shoot ratios would be an adaptive advantage. There was little variation within the quite tightly clustered root:shoot ratios for T. occidentale, with all values being lower than in white clover but much closer to white clover than T. pallescens. The shallow soils of T. occidentale’s natural habitat are prone to rapid drying and it flowers much earlier than does white clover growing at the same location (Coombe 1961). This suggests that T. occidentale uses early flowering to mitigate soil-moisture deficits rather than development of large root systems, which would not necessarily be effective on shallow, drying soils. In the current study, the patterns of allocation of resources to roots or shoots under P deprivation had similar trajectories in all three species, but T. pallescens had much higher root:shoot ratios, suggesting it may have a stronger adaptation to low-P soils.
Shoot and root P concentrations were also much higher in T. pallescens than in white clover and T. occidentale. This may have resulted in part from growth dilution of tissue P in the faster-growing species, but also suggests lower internal P-use efficiency in T. pallescens, which was supported by lower values for shoot DW:total plant P. As it is an alpine species, it is possible that T. pallescens produces antifreeze proteins (AFP) as many species do (Gupta and Deswal 2014). If this is the case, the AFP would be responsible for some of the elevated shoot and root P concentrations, and lower shoot DW:total plant P.
The low shoot P concentrations in the Spanish accessions of T. occidentale (OCD 5 and OCD 6) also suggested that they had a higher shoot-level PUE than did the French and Irish accessions and similar efficiency to white clover. For the white clover cultivar, both shoot (0.047–0.059%) and root (0.08–0.088%) P concentrations were low compared with the concentrations previously reported from pot experiments, such as, for example, shoots 0.19–0.32 %P, roots 0.24–0.34 %P (Crush et al. 2015). This reflects the low soil P treatment levels that were chosen to test whether the progenitor species were adapted to infertile, non-agricultural soils, and which were representative of on-farm Olsen P concentrations in farm systems where improved PUE would be particularly valuable.
White clover populations tolerant of low-P soils have higher shoot P concentrations than do those from high-P soils (Snaydon and Bradshaw 1962). This suggests that gene expression from T. pallescens may be contributing more than that from T. occidentale in low soil P-adapted ecotypes of white clover. In its natural environment T. pallescens has a thick taproot, with only a few thin lateral roots (Kuen and Erschbamer 2002). This is probably an adaptation to surviving frost-heave but would not be very effective for P capture, which is optimised with long, fine, branching roots. No information was located on root characteristics of T. occidentale growing in its natural habitat; however, observations from potted plants (Williams et al. 2011) have suggested it may have thinner roots than does T. pallescens. However, the current results showed that P uptake rates per unit of root DW were higher in T. occidentale and T. pallescens than in Kopu II. The low values for Kopu II may be a result of the large root system in this cultivar bred for high yield in fertile soils. There is ecotypic variation in white clover root systems related to soil P concentrations (Caradus and Snaydon 1988). Types adapted to low-P soils have higher proportions of fine roots, which would improve the efficiency of P acquisition. A comparison of root morphology and architecture in multiple populations of both progenitor species and a range of white clover ecotypes and cultivars would help our understanding of the relative contribution of the progenitor species to P-acquisition characteristics of white clover root systems. Because there is substantial genotype-level variation in root-trait morphology in white clover (Jahufer et al. 2008), comparing roots of progenitor genotypes with those of synthetic white clover derived from them would be an alternative research strategy.
Root P content:shoot P content ratios were higher in T. pallescens than in the other species, which suggests that T. pallescens may store P in the roots as an adaptation to low soil P. Similar adaptations have been reported in white clover ecotypes from New Zealand hill country pastures where soil P is often low (Caradus 1986), and in a small-leaved white clover cultivar bred from New Zealand hill country ecotypes (Chapman and Hay 1993), as well as in T. uniflorum, which also shows adaptation to low-P soils (Nichols and Crush 2015). In pastures, storage of P below grazing height enables internal P reserves to be conserved and later mobilised for shoot growth. The presence of this trait in T. pallescens may provide a source of genetics for improving performance of white clover in P-deficient environments. T. occidentale stored proportionally less P in its roots than did T. pallescens and white clover, but the higher root P:shoot P in Spanish T. occidentale accessions than in the other lines shows that there is some variation for this trait in this species.
Shoot DW per unit plant P can indicate the potential P fertiliser costs of growing a plant for forage. Values were low in T. pallescens, reflecting its slow growth and high internal P concentrations compared to other lines. The highest values were in the white clover cultivar and the Spanish T. occidentale Lines OCD 5 and OCD 6, all of which had low shoot %P concentrations compared to other lines, and moderate to high growth rates. This Spanish germplasm could be a useful resource for hybridising with white clover to investigate P-use efficiency. Values for shoot DW:total plant P of the other four T. occidentale populations were tightly clustered, with maximum values in the P3 soil treatment and lower values in the P5 treatment. The reasons for this pattern are not immediately evident but may indicate optimal soil P concentrations for P-use efficiency. The white clover result was expected because clover breeding programs have resulted in increased shoot dry weight per unit P absorbed, i.e. an increase in internal P-use efficiency (Crush et al. 2015). Snaydon and Bradshaw (1962) also reported lower shoot P concentrations (i.e. higher P efficiency) in white clover adapted to high-P soils. This may offset the lower P uptake rates per unit root DW for Kopu II than for most T. occidentale and T. pallescens lines. Results indicated that T. occidentale and T. pallescens had higher uptake of P per unit root DW, but used P less efficiently than did Kopu II, except for OCD 5 and OCD 6, which had a similar efficiency. In T. pallescens, this may be driven by the high proportion of P stored in roots, as well as possible sequestration in shoot tissues (e.g. anti-freeze proteins). In contrast, the T. occidentale lines had lower storage of P in roots and higher shoot P content:root DW compared with T. pallescens and Kopu II. Given their differences in P efficiency, this indicated that the French and Irish accessions of T. occidentale may be sequestrating P in shoot tissues (e.g. storage as inorganic P in vacuoles), which prevents the conversion of higher P uptake into growth. Among T. occidentale lines, P uptake results also indicated that the Spanish accessions were taking up similar or lower amounts of P per unit root DW, but using it more efficiently than did the other lines, possibly reflecting the absence of shoot sequestration of P. Generally, OCD 2 and 3 also exhibited similar responses when compared with OCD 1 and 4, taking up less P per unit root DW, but using it equally efficiently.
Shoot and root weights and leaf size of the white clover cultivar Kopu II were larger than those of both progenitor species. This was expected because of selection for large plant and leaf size during development of Kopu II as a dairy pasture type cultivar (Woodfield et al. 2001), and the tetraploid nature of white clover compared with the diploid progenitors. Leaf area was similar in both wild species, and this smaller leaf size is also a common feature in white clover ecotypes adapted to infertile conditions (Caradus 1994). The Spanish accessions of T. occidentale (OCD 5 and OCD 6) had larger leaves than did the Irish and French accessions.
Specific leaf area (SLA, cm2 mg−1) was identical in Kopu II white clover and T. pallescens. Species with lower growth rates, such as the T. pallescens in this experiment, generally have a lower SLA than than do faster-growing species such as white clover (Poorter et al. 2009), and species from highly productive habitats usually have a higher SLA than do those from sites of low productivity (Poorter and De Jong 1999). It is not at all clear why SLA was very similar in the T. pallescens and white clover, but there is considerable variation reported in the relationships among SLA, relative growth rate, and the productivity of plants’ natural habitat (Poorter and De Jong 1999; Poorter et al. 2009).
Compared with Kopu II and T. pallescens, SLA was much lower in T. occidentale, probably owing to the additional layers of leaf palisade mesophyll cells that characterise the species (Coombe 1961). Low SLA is also characteristic of many species from arid areas (Garnier et al. 2019). Coombe (1961) reported that leaves of T. occidentale were thicker than those of white clover, suggesting an adaptation to moisture-stress conditions experienced in sandy littoral soils. In contrast, Kopu II and T. pallescens, both with thinner leaves, would be better adapted to the moister conditions of temperate New Zealand pastures and the European Alps. The lower altitudinal limit for T. pallescens is determined by increased seedling mortality caused by drying of soils during the growing season (Hilligardt 1993), which is consistent with a requirement for moister conditions.
White clover has largely retained progenitor genome integrity (Griffiths et al. 2019) and the contribution from both parents can be seen in its vegetative characteristics. For example, SLA in the current study was similar in T. pallescens and white clover, and lower in T. occidentale, which has additional mesophyll palisade layers. Parameters related to P nutrition (e.g. shoot and root %P, shoot DW:total plant P and root P:shoot P ratio) were generally more closely aligned in the white clover cultivar and T. occidentale and different in T. pallescens, suggesting that the relevant genes from T. occidentale were expressed in the white clover, whereas those from T. pallescens were not. This may be an artefact from the use of Kopu II as the white clover control because this cultivar was bred for intensive lowland dairy pastures (Caradus and Woodfield 1997) that characteristically have high soil fertility. In contrast, small-leafed white clover ecotypes grown under low soil P conditions (Caradus 1986) show many of the P-response characteristics of T. pallescens, which in this experiment had many traits indicative of adaptation to low soil P (high %P, low shoot DW:total plant P, high root:shoot and high root P:shoot P), including low utilisation of acquired P. Although P uptake rates per unit of root DW of the T. pallescens lines were similar or lower than those of the T. occidentale lines at P5, those for PAD 2 were much higher at lower-P treatments more representative of low on-farm Olsen P concentrations. This may indicate a strategy of high acquisition of P under limiting soil P conditions. Although the uptake rates of this line did not respond greatly to increasing soil P overall, this may be a further indication of adaptation to low soil P. Similar rates of P uptake among the T. pallescens lines at P5, combined with lower shoot P content per unit root DW for PAD 2, reflect the higher proportion of total plant P sequestered to roots by this line.
White clover arose from multiple hybridisation events (Griffiths et al. 2019) and the ecotypes of the ancestral progenitors are unknown. There was population-level variation for P traits, and combinations of traits, in both progenitor species (e.g. Spanish accessions of T. occidentale and PAD 2) and this has also been observed in white clover (Snaydon and Bradshaw 1962; Caradus and Snaydon 1986). Faville et al. (2020) found wide genomic diversity in a screen of global ecotype collections of white clover, of which New Zealand accessions represented only a small portion of diversity. Comparing the P-response traits of a range of white clover ecotypes with those of a wider range of progenitor populations would be informative and could support breeding targeted at the progenitor subgenomes of white clover for improvement of low soil P tolerance. This could be implemented either via interspecific hybridisation with existing cultivars or creation of new synthetic white clover populations. In addition to overall differences in trait combinations among species, principal-component analysis showed that separation within progenitor species differed between higher and lower P treatments, indicating the importance of screening across a range of relevant on-farm plant-available soil P concentrations.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Personnel and general operating costs were covered by AgResearch Ltd. Costs of the phosphorus analyses were met by Pastoral Genomics, a joint venture co-funded by DairyNZ, Beef+Lamb New Zealand, Dairy Australia, AgResearch Ltd, DEEResearch, and the Ministry of Business, Innovation and Employment (New Zealand).
Acknowledgements
We thank Lily Ouyang for technical assistance.
References
Akeroyd JR (1983) Further notes on Trifolium occidentale D.E.Coombe in Ireland. Irish Naturalists’ Journal 21, 32–34.Burga CA, Krüsi B, Egli M, Wernli M, Elsener S, Ziefle M, Fischer T, Mavris C (2010) Plant succession and soil development on the foreland of the Morteratsch glacier (Pontresina, Switzerland): straight forward or chaotic? Flora – Morphology, Distribution, Functional Ecology of Plants 205, 561–576.
| Plant succession and soil development on the foreland of the Morteratsch glacier (Pontresina, Switzerland): straight forward or chaotic?Crossref | GoogleScholarGoogle Scholar |
Caradus JR (1986) Variation in partitioning and percentage nitrogen and phosphorus content of the leaf, stolon, and root of white clover genotypes. New Zealand Journal of Agricultural Research 29, 367–379.
| Variation in partitioning and percentage nitrogen and phosphorus content of the leaf, stolon, and root of white clover genotypes.Crossref | GoogleScholarGoogle Scholar |
Caradus JR (1994) Selection for improved adaptation of white clover to low phosphorus and acid soils. Euphytica 77, 243–250.
| Selection for improved adaptation of white clover to low phosphorus and acid soils.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, Snaydon RW (1986) Response to phosphorus of populations of white clover 1. Field studies. New Zealand Journal of Agricultural Research 29, 155–162.
| Response to phosphorus of populations of white clover 1. Field studies.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, Snaydon RW (1988) Effect of grass competition, cutting frequency, and soil type on the phosphorus response of semi-natural populations of white clover. New Zealand Journal of Agricultural Research 31, 95–103.
| Effect of grass competition, cutting frequency, and soil type on the phosphorus response of semi-natural populations of white clover.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, Woodfield DR (1997) Review: world checklist of white clover varieties II. New Zealand Journal of Agricultural Research 40, 115–206.
| Review: world checklist of white clover varieties II.Crossref | GoogleScholarGoogle Scholar |
Chapman DF, Hay MJM (1993) Translocation of phosphorus from nodal roots in two contrasting genotypes of white clover (Trifolium repens). Physiologia Plantarum 89, 323–330.
| Translocation of phosphorus from nodal roots in two contrasting genotypes of white clover (Trifolium repens).Crossref | GoogleScholarGoogle Scholar |
Coombe DE (1961) Trifolium occidentale, a new species related to T. repens L. Watsonia 5, 68–87.
Coombe DE, Morisset P (1967) Further observations on Trifolium occidentale. Watsonia 6, 271–275.
Crush JR (1974) Plant growth responses to vesicular-arbuscular mycorrhiza VII. Growth and modulation of some herbage legumes. New Phytologist 73, 743–749.
| Plant growth responses to vesicular-arbuscular mycorrhiza VII. Growth and modulation of some herbage legumes.Crossref | GoogleScholarGoogle Scholar |
Crush JR, Ouyang L, Nichols SN (2015) Root morphology and architecture, and internal phosphate use efficiency, in related white clover cultivars of different ages. New Zealand Journal of Agricultural Research 58, 302–310.
| Root morphology and architecture, and internal phosphate use efficiency, in related white clover cultivars of different ages.Crossref | GoogleScholarGoogle Scholar |
Dunlop J, Hart AL (1987) Mineral nutrition. In ‘White clover’. (Eds MJ Baker, WM Williams) pp. 153–183. (CAB International: Wallingford, UK)
Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL (2006) Molecular phylogenetics of the clover genus (Trifolium – Leguminosae). Molecular Phylogenetics and Evolution 39, 688–705.
| Molecular phylogenetics of the clover genus (Trifolium – Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Evans PS (1977) Comparative root morphology of some pasture grasses and clovers. New Zealand Journal of Agricultural Research 20, 331–335.
| Comparative root morphology of some pasture grasses and clovers.Crossref | GoogleScholarGoogle Scholar |
Faville MJ, Griffiths AG, Baten A, Cao M, Ashby R, Ghamkhar K, Hong W, Larking A, Williamson M, Webber Z (2020) Genomic assessment of white clover and perennial ryegrass genetic resources. Journal of New Zealand Grasslands 82, 27–34.
| Genomic assessment of white clover and perennial ryegrass genetic resources.Crossref | GoogleScholarGoogle Scholar |
Garnier E, Vile D, Roumet C, Lavorel S, Grigulis K, Navas M-L, Lloret F (2019) Inter- and intra-specific trait shifts among sites differing in drought conditions at the north western edge of the Mediterranean Region. Flora 254, 147–160.
| Inter- and intra-specific trait shifts among sites differing in drought conditions at the north western edge of the Mediterranean Region.Crossref | GoogleScholarGoogle Scholar |
Gillingham AG, Morton JD, Gray MH (2007) Pasture responses to phosphorus and nitrogen fertilisers on East Coast hill country: total production from easy slopes. New Zealand Journal of Agricultural Research 50, 307–320.
| Pasture responses to phosphorus and nitrogen fertilisers on East Coast hill country: total production from easy slopes.Crossref | GoogleScholarGoogle Scholar |
Griffiths AG, Moraga R, Tausen M, Gupta V, Bilton TP, Campbell MA, Ashby R, Nagy I, Khan A, Larking A, Anderson C, Franzmayr B, Hancock K, Scott A, Ellison NW, Cox MP, Asp T, Mailund T, Schierup MH, Andersen SU (2019) Breaking free: the genomics of allopolyploidy – facilitated niche expansion in white clover. Plant Cell 31, 1466–1487.
| Breaking free: the genomics of allopolyploidy – facilitated niche expansion in white clover.Crossref | GoogleScholarGoogle Scholar |
Gupta R, Deswal R (2014) Antifreeze proteins enable plants to survive in freezing conditions. Journal of Biosciences 39, 931–944.
| Antifreeze proteins enable plants to survive in freezing conditions.Crossref | GoogleScholarGoogle Scholar |
Hewitt EJ (1966) ‘Sand and water culture methods used in the study of plant nutrition.’ Technical Communication No. 22. (Commonwealth Bureau of Horticulture and Plantation Crops: East Maling, UK)
Hewitt AE (2010) ‘New Zealand soil classification.’ (Manaaki Whenua Press: Lincoln, New Zealand)
Hilligardt M (1993) Durchsetzungs- und reproduktionsstrategien bei Trifolium pallescens Schreb. und Trifolium thalii Vill. II. Untersuchungen zur populationsbiologie. Flora 188, 175–195.
| Durchsetzungs- und reproduktionsstrategien bei Trifolium pallescens Schreb. und Trifolium thalii Vill. II. Untersuchungen zur populationsbiologie.Crossref | GoogleScholarGoogle Scholar |
Jackman RH, Mouat MCH (1972a) Competition between grass and clover for phosphate I. Effect of browntop (Agrostis tenuis Sibth.) on white clover (Trifolium repens L.) growth and nitrogen fixation. New Zealand Journal of Agricultural Research 15, 653–666.
| Competition between grass and clover for phosphate I. Effect of browntop (Agrostis tenuis Sibth.) on white clover (Trifolium repens L.) growth and nitrogen fixation.Crossref | GoogleScholarGoogle Scholar |
Jackman RH, Mouat MCH (1972b) Competition between grass and clover for phosphate II. Effect of root activity, efficiency of response to phosphate, and soil moisture. New Zealand Journal of Agricultural Research 15, 667–675.
| Competition between grass and clover for phosphate II. Effect of root activity, efficiency of response to phosphate, and soil moisture.Crossref | GoogleScholarGoogle Scholar |
Jahufer MZZ, Luo D (2018) DeltaGen – a comprehensive decision support tool for plant breeders. Crop & Pasture Science 58, 1118–1131.
Jahufer MZZ, Nichols SN, Crush JR, Ouyang L, Dunn A, Ford JL, Care DA, Griffiths AG, Jones CS, Jones CG, Woodfield DR (2008) Genotypic variation for root trait morphology in a white clover mapping population grown in sand. Crop Science 48, 487–494.
| Genotypic variation for root trait morphology in a white clover mapping population grown in sand.Crossref | GoogleScholarGoogle Scholar |
Kuen V, Erschbamer B (2002) Comparative study between morphology and age of Trifolium pallescens in a glacier foreland of the Central Alps. Flora – Morphology, Distribution, Functional Ecology of Plants 197, 379–384.
| Comparative study between morphology and age of Trifolium pallescens in a glacier foreland of the Central Alps.Crossref | GoogleScholarGoogle Scholar |
Lynch JP, Brown KM (2008) Root strategies for phosphorus acquisition. In ‘The ecophysiology of plant-phosphorus interactions’. (Eds PJ White, JP Hammond) pp. 83–116. (Springer: Dordrecht, The Netherlands)
Monaghan RM, Hedley MJ, Di HJ, McDowell RW, Cameron KC, Ledgard SF (2007) Nutrient management in New Zealand pastures – recent developments and future issues. New Zealand Journal of Agricultural Research 50, 181–201.
| Nutrient management in New Zealand pastures – recent developments and future issues.Crossref | GoogleScholarGoogle Scholar |
Morton JD, Roberts AHD (2018) ‘Fertiliser use on New Zealand Sheep and Beef farms.’ 5th edn. (New Zealand Fertiliser Manufacturers’ Research Association: Auckland, New Zealand)
Nichols SN, Crush JR (2015) Phosphate response of Trifolium uniflorum compared with T. repens and some T. repens × T. uniflorum hybrids. Crop & Pasture Science 66, 857–863.
| Phosphate response of Trifolium uniflorum compared with T. repens and some T. repens × T. uniflorum hybrids.Crossref | GoogleScholarGoogle Scholar |
Nichols SN, Hofmann RW, Williams WM, Crush JR (2014a) Nutrient responses and macronutrient composition of some Trifolium repens × Trifolium uniflorum interspecific hybrids. Crop & Pasture Science 65, 370–381.
| Nutrient responses and macronutrient composition of some Trifolium repens × Trifolium uniflorum interspecific hybrids.Crossref | GoogleScholarGoogle Scholar |
Nichols SN, Crush JR, Ouyang L (2014b) Phosphate responses of some Trifolium repens × T. uniflorum interspecific hybrids grown in soil. Crop & Pasture Science 65, 382–387.
| Phosphate responses of some Trifolium repens × T. uniflorum interspecific hybrids grown in soil.Crossref | GoogleScholarGoogle Scholar |
Poorter H, De Jong R (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist 143, 163–176.
| A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182, 565–588.
| Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Raffl C, Holderegger R, Parson W, Erschbamer B (2008) Patterns in genetic diversity of Trifolium pallescens populations do not reflect chronosequence on alpine glacier forelands. Heredity 100, 526–532.
| Patterns in genetic diversity of Trifolium pallescens populations do not reflect chronosequence on alpine glacier forelands.Crossref | GoogleScholarGoogle Scholar |
Reid JB, Crush JR (2013) Root turnover in pasture species: perennial ryegrass (Lolium perenne L.). Crop & Pasture Science 64, 165–177.
| Root turnover in pasture species: perennial ryegrass (Lolium perenne L.).Crossref | GoogleScholarGoogle Scholar |
Reid JB, Gray RAJ, Springett JA, Crush JR (2015) Root turnover in pasture species: chicory, lucerne, perennial ryegrass and white clover. Annals of Applied Biology 167, 327–342.
| Root turnover in pasture species: chicory, lucerne, perennial ryegrass and white clover.Crossref | GoogleScholarGoogle Scholar |
Roberts AHC, Morton JD (2016) ‘Fertiliser use on New Zealand dairy farms.’ 4th edn. (New Zealand Fertiliser Manufacturers’ Research Association: Wellington, New Zealand)
Simpson RJ, Richardson AE, Nichols SN, Crush JR (2014) Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems. Crop & Pasture Science 65, 556–575.
| Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems.Crossref | GoogleScholarGoogle Scholar |
Snaydon RW, Bradshaw AD (1962) Differences between natural populations of Trifolium repens L. in response to mineral nutrients. I. Phosphate. Journal of Experimental Botany 13, 422–434.
| Differences between natural populations of Trifolium repens L. in response to mineral nutrients. I. Phosphate.Crossref | GoogleScholarGoogle Scholar |
Tscherko D, Hammesfahr U, Zeltner G, Kandeler E, Bӧcker R (2005) Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain. Basic and Applied Ecology 6, 367–383.
| Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain.Crossref | GoogleScholarGoogle Scholar |
VSN International (2011) ‘GenStat for Windows.’ 16th edn. (VSN International: Hemel Hempstead, UK) Genstat.co.uk.
Williams WM, Verry IM, Ansari HA, Hussain SW, Ullah I, Williamson ML, Ellison NW (2011) Eco-geographically divergent diploids, Caucasian clover (Trifolium ambiguum) and western clover (T. occidentale), retain most requirements for hybridization. Annals of Botany 108, 1269–1277.
| Eco-geographically divergent diploids, Caucasian clover (Trifolium ambiguum) and western clover (T. occidentale), retain most requirements for hybridization.Crossref | GoogleScholarGoogle Scholar |
Williams WM, Ellison NW, Ansari HA, Verry IM, Hussain SW (2012) Experimental evidence for the ancestry of allotetraploid Trifolium repens and creation of synthetic forms with value for plant breeding. BMC Plant Biology 12, 55
| Experimental evidence for the ancestry of allotetraploid Trifolium repens and creation of synthetic forms with value for plant breeding.Crossref | GoogleScholarGoogle Scholar |
Woodfield DR, Clifford PTP, Cousins GR, Ford JL, Baird IJ, Miller JE, Woodward SL, Caradus JR (2001) Grasslands Kopu II and Crusader: new generation white clovers. Proceedings of the New Zealand Grassland Association 63, 103–108.
| Grasslands Kopu II and Crusader: new generation white clovers.Crossref | GoogleScholarGoogle Scholar |
Zohary M, Heller D (1984) ‘The genus Trifolium.’ (The Israel Academy of Sciences and Humanities: Jerusalem, Israel)