Vernalisation and photoperiod responses of diverse wheat genotypes
Maxwell T. Bloomfield A G * , Corinne Celestina
A G * , Corinne Celestina  A H , James R. Hunt
A H , James R. Hunt  A H , Neil Huth B , Bangyou Zheng C , Hamish Brown D , Zhigan Zhao E , Enli Wang E , Katia Stefanova F , Jessica Hyles E , Tina Rathjen E and Ben Trevaskis E
A H , Neil Huth B , Bangyou Zheng C , Hamish Brown D , Zhigan Zhao E , Enli Wang E , Katia Stefanova F , Jessica Hyles E , Tina Rathjen E and Ben Trevaskis E
A Department of Animal, Plant and Soil Sciences, La Trobe University, 5 Ring Road, Bundoora, Vic. 3086, Australia.
B CSIRO Agriculture and Food, 203 Tor Street, Toowoomba, Qld 4350, Australia.
C CSIRO Agriculture and Food, Queensland Biosciences Precinct, 306 Carmody Road, St Lucia, Qld 4067, Australia.
D The New Zealand Institute for Plant & Food Research Limited, Private Bag 4704, Christchurch, New Zealand.
E CSIRO Agriculture and Food, GPO Box 1700, Canberra, ACT 2601, Australia.
F Statistics for the Australian Grains Industry West, Curtin University, Perth, WA, Australia.
G Present address: Field Applied Research Australia, 2/63 Holder Road, Bannockburn, Vic. 3331, Australia.
H Present address: School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Melbourne, Vic. 3052, Australia.
Crop & Pasture Science 74(5) 405-422 https://doi.org/10.1071/CP22213
Submitted: 20 June 2022 Accepted: 15 December 2022 Published: 8 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Wheat (Triticum aestivum L.) adaptation is highly dependent on crop lifecycle duration, particularly the time at which flowering occurs in a specific environment. Frost, low solar radiation, heat and drought can significantly reduce yield if a crop flowers too early or late. Wheat genotypes have different lifecycle durations determined by plant responses to temperature (thermal time accumulation and vernalisation) and photoperiod. These responses are largely controlled by five phenology genes (two PPD1 and three VRN1 genes). Advances in crop phenology modelling suggest that flowering time under field conditions could be accurately predicted with parameters derived from photoperiod and vernalisation responses obtained in controlled environments.
Aims: This study quantified photoperiod and vernalisation responses of 69 Australian wheat genotypes selected for diversity at the PPD1 and VRN1 loci.
Methods: Spring and winter genotypes were grown in four controlled environments at a constant temperature of 22°C with photoperiod (17 or 8 h) and vernalisation (0 or 8 weeks) treatments as factors.
Key results: Thermal time from coleoptile emergence to flowering in spring genotypes was typically decreased more by long photoperiod than by vernalisation; the opposite was true for winter genotypes. Spring genotypes that were sensitive to vernalisation contained a sensitive allele at the Vrn-A1 locus.
Conclusions: There is large diversity in phenological responses of wheat genotypes to photoperiod and vernalisation, including among those with matching multi-locus genotype.
Implications: Data from this study will be used to parameterise and test a wheat phenology model in a future study.
Keywords: flowering time, G x E, phenology, photoperiod, thermal time, Triticum aestivum L., vernalisation, wheat.
Introduction
Domestication of wheat (Triticum aestivum L.) has led to its adaptation to a wide range of environments, primarily through adjustment of the duration of the crop life cycle (phenology). All environments have a seasonal period most conducive for wheat growth, defined by favourable temperatures, water availability and radiation (Flohr et al. 2017). Yield is maximised when these environmental optima and crop critical growth periods align (Hunt et al. 2021). Flowering is an important marker of crop critical period (Sadras and Dreccer 2015); flowering too early will reduce yield through increased frost risks and insufficient radiation interception, whereas flowering too late will reduce yield through drought and heat stresses (Flohr et al. 2017). Flohr et al. (2017) define an environment’s optimal flowering period as the time of year when combined yield loss from these stresses are minimised.
As a temperate long-day plant, wheat progresses through its lifecycle in response to increasing temperature and photoperiod. The cardinal temperature range in which wheat develops spans from ~0°C to 35–40°C, with an optimal temperature of ~22–27°C (Porter and Gawith 1999; Parent and Tardieu 2012; Wang et al. 2017). Photoperiod response occurs at ~8–16 h daylength with some genotypic variation (Slafer and Rawson 1995). For spring wheat, warm temperatures and long days reduce time to flowering, whereas cool temperatures and shorter days delay flowering. Winter wheat has an additional obligate requirement for vernalisation (a prolonged cool period, i.e. winter), which triggers progression from the vegetative to reproductive phase (Chouard 1960). Vernalisation is upregulated in the cardinal temperature range from ~−1°C to 16°C, with an optimal temperature of ~5°C (Porter and Gawith 1999). Davidson et al. (1985) demonstrated that winter wheat requires 4–8 weeks of constant vernalising temperature to fully satisfy vernalisation; they also showed that some spring genotypes exhibit a facultative response to vernalisation.
There are three major phenophases in wheat that are genetically regulated in response to environment from sowing to harvest: vegetative (sowing to floral initiation), reproductive (floral initiation to anthesis), and grain-filling (anthesis to harvest) (Slafer et al. 2014). Key development stages occur within each phase, and the timing of these stages and the lengths of the phases relative to environmental conditions will have a large impact on grain yields. For example, an extended stem elongation subphase (during the reproductive phase) can lead to yield increase associated with increased grain number per unit area (Miralles et al. 2000; Slafer et al. 2001; González et al. 2005; Acuña et al. 2019).
Regulation of VERNALISATION1 (VRN1) and PHOTOPERIOD1 (PPD1) genes largely controls phenology in response to the environment (i.e. temperature and daylength). It is further finetuned by other minor genes, broadly termed earliness per se genes (EPS), that regulate the circadian clock and light perception and interact with VRN1 and PPD1 genes (see review by Hyles et al. 2020; and references therein).
Alleles at the VRN1 genes Vrn-A1, Vrn-B1 and Vrn-D1, located on chromosomes 5A, 5B and 5D, respectively, determine a genotype’s vernalisation requirement and whether it is of winter or spring habit (Trevaskis et al. 2003; Yan et al. 2004; Fu et al. 2005; Chen et al. 2009; Santra et al. 2009; Shcherban et al. 2012; Zhang et al. 2012). Recessive wild type alleles at all three VRN1 loci confer winter habit, and these multi-locus genotypes develop from the vegetative to reproductive phase significantly faster when vernalisation is satisfied. Genotypes carrying a dominant mutant allele at one or more of the VRN1 loci have spring habit and vernalisation has less to nil effect on development rate.
The major genes controlling daylength response are the PPD1 genes Ppd-B1 and Ppd-D1, located on chromosomes 2B and 2D, respectively (Pugsley 1966; Beales et al. 2007; Guo et al. 2010; Díaz et al. 2012). Daylength sensitivity is conferred by wild type recessive alleles at both the Ppd-D1 and Ppd-B1 loci. A recessive allele at the Ppd-D1 locus and a dominant increased copy number allele at the Ppd-B1 locus confer photoperiod sensitivity, and dominant mutant alleles at the Ppd-D1 locus confer photoperiod insensitivity regardless of which allele is present at the Ppd-B1 locus (Shaw et al. 2012; Cane et al. 2013). Genetically photoperiod ‘insensitive’ wheat genotypes still exhibit a short but significant delay to flowering under short days relative to long days, but to a lesser degree than sensitive genotypes (Law et al. 1978; Gomez et al. 2014; Steinfort et al. 2017; Bloomfield et al. 2018).
In Australia, wheat accounts for ~10–13 Mha (~50%) of the winter cropping area sown each year, and ~65–70% of the grain is exported (ABARES 2020). The winter cropping regions of Australia, historically termed the Australian wheatbelt, cover a diverse range of climates. Environments include high rainfall (>500 mm year−1) temperate, semi-arid (250–400 mm year−1) Mediterranean areas (in the south-east and south-west where most of Australia’s wheat is grown), areas with evenly distributed annual rainfall in the mid-east, and subtropical summer-dominant rainfall areas in the north-east (Richards et al. 2014). Owing to relatively mild winter temperatures, spring wheat is most commonly sown in late April to early June following the first major autumn rainfall events of each season (referred to as the autumn break). However, the timing of the autumn break has become later and the quantity of rainfall has decreased in recent decades because of anthropogenic climate change (Pook et al. 2009; Cai et al. 2012; Cai and Cowan 2013; Flohr et al. 2021). This means that growers require access to phenologically diverse cultivars that can be sown anywhere from mid-March to mid-June depending on the timing of the break, and in environments ranging from latitude 23°S to 42°S with mean annual temperature of 23–10°C.
Appropriate phenology is especially important for wheat yields across Mediterranean grain-growing environments, owing to the hot dry conditions in spring and summer. Spring wheat cultivars can be sown at different times to alter flowering time, whereas winter wheat cultivars have a relatively stable flowering time across a range of sowing dates (Hunt 2017; Flohr 2018). Winter cultivars have a strong requirement for low temperatures (vernalisation) to induce flowering. Once vernalisation requirement is satisfied, alleles at the PPD1 loci and other minor genes affecting development per se will determine whether a winter cultivar flowers in the optimal flowering period of a given environment. Relative to spring cultivars, winter cultivars are therefore suited to a narrower range of environments in which their flowering time falls within the optimum period (Cann et al. 2020).
In addition to less reliable autumn rainfall, phenologically diverse cultivars are also required because farm sizes have increased (Fletcher et al. 2016), and in turn, sowing occurs over a longer period. Spring wheat cultivars have only a ~2-week sowing window, and multiple cultivars with differing phenology are required to ensure optimal flowering across the whole farm if sowing extends beyond 2 weeks. However, accurate sowing guides are not available when a new cultivar is released because of the large costs associated with conducting multi-year time-of-sowing field experiments across the broad range of cropping environments in Australia. It can take at least 2 years for growers and advisers to optimise sowing times of new cultivars to their environments to ensure that the crops flower during the optimal period, by which time much opportunity has been missed and new cultivars have superseded the older ones.
An opportunity exists to use crop simulation software to better inform growers’ decision making of cultivar and sowing date for their given environments. Wheat simulation models have been developed and improved over many years, but issues about their accuracy to predict phenology and/or yields outside of the environments in which they were parameterised remain prevalent. It has been proposed that genetically derived parameters could be incorporated into physiological models to predict phenology accurately across a broad range of environments based on alleles at the PPD and VRN genes (White et al. 2008; Brown et al. 2013; Zheng et al. 2013). However, a genetically derived parameter estimate model used by White et al. (2008) accounted for less of the variation in flowering time than the conventional model, and a model used by Zheng et al. (2013) required additional experimentally derived cultivar-specific parameters to predict heading time accurately. The results from these models indicate that further genetic information is required to derive parameters to predict flowering time accurately. Brown et al. (2013) incorporated molecular concepts based on the literature of VRN genes into a physiological model framework to predict flowering time for a spring isoline and a winter isoline of wheat cv. Batten. The release of the Agricultural Production Systems sIMulator Next Generation software (APSIM NG; Holzworth et al. 2014, 2018) and the plant modelling framework (PMF; Brown et al. 2014) that models growth and development provides a new and innovative approach to crop modelling. The wheat PMF phenology module simulates development through a series of phases of which the start and end points are characterised by important physiological stages, and the length of each phase is determined by environment (temperature and photoperiod) and a genotype’s sensitivity to vernalisation and photoperiod (Brown et al. 2018). The major benefit of the PMF is that genotype-specific parameters can be derived from controlled-environment experiments that record development over time in response to vernalisation and photoperiod. This avoids the need for expensive and time-consuming field experiments to parameterise genotypes, particularly when a new cultivar is released. In theory, the model should be able to predict phenology across a much broader range of environments than traditional physiological models (e.g. APSIM Classic; Keating et al. 2003), which have historically performed poorly outside of environments in which they were parameterised.
A secondary benefit of crop simulation models is that they provide useful insights into the growth and development dynamics of crop species in different environments. They can inform management decisions for growers and advisers (e.g. Yield Prophet; Hochman et al. 2009) and can be used by researchers to simulate different scenarios such as examining genotype responses to different environments and/or management practices; crop rotations; and mixed cropping–livestock systems (e.g. APSIM Classic and APSIM NG; Keating et al. 2003; Holzworth et al. 2014, 2018). Of particular importance to future global food security is the role of crop models to predict development and growth in changing climates (Hunt et al. 2019; Collins and Chenu 2021).
We sought to improve understanding of the phenological diversity of contemporary Australian wheat cultivars by studying a diverse group of Australian wheat cultivars and near-isogenic lines (NILs), herein termed the Australian Phenology Panel (APP). Genotypes were grown in four controlled environments (photoperiod of 17 or 8 h and vernalisation of 0 or 8 weeks) to quantify the timing of important phenological traits in response to photoperiod and vernalisation treatments. Data presented here have been used to derive genotype-specific parameters for a new version of the APSIM NG wheat phenology model, the derivation and validation of which will be described in a subsequent publication.
Materials and methods
This experiment was conducted in controlled-environment rooms at AgriBio, Centre for AgriBioscience, Bundoora, Victoria, Australia (−37.724253, 145.053287).
Genotype selection
Forty-seven elite commercial wheat cultivars and 17 NILs were selected to form the APP. The cultivars were selected based on diversity of allele variation at the PPD1 and VRN1 loci, popularity among Australian growers, and variation in phenology under field conditions. The NILs were selected to match the alleles of multi-locus genotypes (MLGs) of the five major PPD1 and VRN1 genes of cultivars where available. The NILs, as described by Steinfort et al. (2017), were developed by introgressing target alleles of PPD1 and VRN1 into the recurrent parent (cv. Sunstate) through five rounds of recurrent crossing. The APP and an additional four commercial cultivars and one breeding line (Condo, LRPB Nighthawk, Illabo, DS Bennett and ADV08.0008) were selected for this experiment (Table 1). The additional four commercial cultivars and the breeding line were selected for their use in previous time-of-sowing field experiments (Porker et al. 2019, 2020) and for future use in optimisation and development of the APSIM NG phenology model. Seeds of the cultivars were sourced from the Australian Winter Cereals Collection and/or breeding companies, and the NILs were provided by one of the authors (Dr B Trevaskis, CSIRO Agriculture and Food, Canberra). All cultivars were grown in a glasshouse at 22°C during daylight hours (14 h natural irradiance and supplemental sodium halide lamps when natural irradiance fell below 170 W m−2) and 14°C at night (10 h), in order to provide seed grown in a consistent maternal environment for the experiment.

|
Molecular marker analysis
Four seeds per genotype of APP seed stock used in the experiment were grown in a glasshouse, and leaf tissue was collected during the seedling stage. DNA was extracted in a high-throughput genotyping facility (CSIRO, Canberra) by using the protocol of Ellis et al. (2005) with a Microlab NIMBUS (Hamilton, Reno, NV, USA) robotic liquid-handling procedure. Major wheat genes and alleles were assayed by using perfect markers and, where possible, conversion to KASP (Kompetitive Allele-Specific PCR; LGC, Teddington, UK; as reported in Bloomfield et al. 2018). KASP markers developed in other studies (Dreisigacker et al. 2016; Grogan et al. 2016; Sukumaran et al. 2016) were also deployed. Sequential screening of the bi-allelic KASP markers (based on a process of elimination) was used to confirm the alleles present. The high-throughput genotyping facility comprised Meridian and Kube instrumentation (LGC) and CFX qPCR machine/software (Bio-Rad, Hercules, CA, USA) for single nucleotide polymorphism (SNP) calling.
Genotyping results conducted on a separate stock of APP seed of the NILs CSIROW011, CSIROW023, CSIROW027 and CSIROW102 are included in Table 1 because of insufficient quantities of seed from the APP stock used in this experiment. Seven genotypes were mixed at one locus and one at two loci (Table 1; see Supplementary Table S1 for the genotypes and allele frequencies). All four replicates of Janz appeared heterozygous for both the Ppd-B1b and c alleles. CSIROW027 had heterozygous alleles appear for some replicates of both Ppd-B1 and Vrn-B1. All other genotypes showed differing homozygous alleles between replicates. This could have been caused by segregation during seed bulk-up. Alleles for Ppd-B1 could not be identified for Condo, Ellison, Forrest and Kelalac.
Experimental design and setup
The experiment was conducted in four controlled-environments, where each environment was a combination of one of two photoperiods (17 or 8 h) and two vernalisation treatments (vernalisation period 0 or 8 weeks). The same set of 69 genotypes was used in each environment, with three replications. A complete spatial grid of 27 columns × eight rows was maintained by replicating three randomly selected genotypes twice in each block. One-directional blocking (randomised complete block design) was used, where the first nine columns × eight rows comprised Replicate (block) one, the next nine columns × eight rows Replicate two, and the final nine columns × eight rows Replicate three.
Environments
The four controlled-environment treatments were the same as those used by Bloomfield et al. (2018): short-day, not vernalised (SN); short-day, vernalised (SV); long-day, not vernalised (LN); long-day, vernalised (LV).
Six seeds of each genotype per environment were germinated on three Whatman 70-mm filter papers (Whatman, Maidstone, UK) with reverse-osmosis water (3 mL) in 90-mm Petri dishes for 48 h (24 h at 5°C and 24 h at 22°C) to break potential dormancy. Six germinated seeds for each vernalised treatment (SV, LV) were directly sown into seedling trays containing a standard potting mix with slow-release fertiliser. They were then grown in a Humiditherm temperature and humidity-controlled growth cabinet (Thermoline, Wetherill Park, NSW, Australia) at 5°C for 8 weeks to fulfil vernalisation requirements. For non-vernalised treatments (SN, LN), two germinated seeds per replicate were directly sown in 90-mm olive pots in standard potting mix and transferred to a controlled-environment room with the applicable daylength. One seedling was removed from each pot after 7 days so that only one plant per pot remained. Following the 8-week vernalisation period for the vernalised treatments, one seedling of each genotype per replicate was transplanted into 90-mm olive pots containing standard potting mix and placed in the appropriate controlled-environment room.
Photosynthetically active radiation (PAR) was 300 µmol m−2 s−1 at pot height in the controlled-environment rooms and 180 µmol m−2 s−1 in the growth cabinet. Controlled-environment rooms were set to a constant temperature of 22°C, and air (at pot height) and soil temperatures were monitored at 30-min intervals with Tinytag Plus 2 data loggers (Gemini Data Loggers, Chichester, UK) in radiation shields. There was minimal difference between air and soil temperatures, and so only air temperatures were used in calculations of thermal time (degree-days) (see below).
Phenology measurements
Progressive leaf number was recorded as Haun stage (HS) twice per week on all plants, following Haun’s scale (Haun 1973). All plants were monitored at least 5 days per week during busy periods (i.e. when emergence, flag leaf appearance, heading and flowering were occurring for most plants) so as to record the following: emergence date, coleoptile emerged above soil surface; final leaf number (FLN), total number of leaves on main stem; flag leaf date, flag leaf fully emerged from preceding leaf collar on main stem; heading date, whole spike fully emerged above flag leaf collar on main stem, or on first culm if main stem has died; median heading date, spikes fully emerged on 50% of culms on a plant; flowering date, first visible anthers extruded from spikelets or white/yellow anthers observed within spikelets on main spike, or on first culm if main stem has died; and median flowering date, flowering on 50% of spikes.
Statistical analyses
Daily thermal time (DTT) was calculated for each environment using mean daily temperature from the half-hourly temperature (HT) logger data (Eqn 1). Eqn 1 is a simple equation that does not account for base temperature (assumed 0°C) or maximum temperature (assumed 35°C) because the controlled-environment temperatures for vernalisation in the growth cabinet (5°C) and development in the controlled-environment room (22°C) are the optimal values and falling within the cardinal range for wheat (Porter and Gawith 1999):

Accumulated thermal time (TT) was calculated as the sum of DTT from date of coleoptile emergence to a given date of interest (n, Eqn 2). Accumulated TT was used to standardise the time from emergence to the key phenological stages measured over time rather than calendar days:
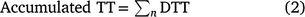
Linear mixed model techniques were used to analyse data from the controlled environments. The model accounted for the blocking structure, treatment structure and the extra sources of variation such as spatial trends and extraneous variation (Gilmour et al. 1997; Stefanova et al. 2009). FLN and TT from coleoptile emergence date to flag leaf date (TTFL), heading date and flowering date (TTF), and from flag leaf to flowering dates (TTFL–F), were analysed by fitting genotype, environment and their interaction as fixed effects, a random blocking term to account for the randomisation process, and additional random or fixed terms to model the spatial variability within each environment. This model allowed comparative study of the performance of genotypes for the listed traits. In order to assess the magnitude of the variance components, genotype and genotype:environment were modelled as random, and a diagonal variance–covariance structure was fitted on genotype: environment, while assuming different genotypic variances for each environment. Statistical software ASReml-R (Butler et al. 2009) and R (R Core Team 2020) were used to conduct the analyses. Additionally, a regression model with a grouping factor of environment was used to calculate phyllochron (degree-days leaf−1) as the slope of accumulated TT vs progressive leaf number (HS) between Leaves 3 and 7 in Genstat Edn 19 (VSN International, Hemel Hempstead, UK). Graphs were created in R using the packages ‘ggplot2’ (Wickham 2016) and ‘gridExtra’ (Auguie 2017).
Results
Phyllochron
The phyllochron (TT between appearance of successive leaf tips from Leaves 3 to 7) varied among genotypes and environments (Fig. 1). Both of the long-day environments (LN and LV) decreased phyllochron for nearly all genotypes compared with the short-day environments (SN and SV). Environment LV decreased phyllochron for most genotypes, but increased it for a handful of spring genotypes, compared with LN. Environment SV had varying effects on phyllochron compared with SN. No genotypes had a phyllochron <100 degree-days leaf−1 in the SN or SV environments, and none of the winter types achieved a phyllochron ≤100 degree-days leaf−1 in the LN environment, whereas 27 of the spring types did have a phyllochron <100 degree-days leaf−1 in the LN environment. The impact of vernalisation on phyllochron was greater under long days than under short days. Vernalisation accelerated leaf emergence under long days for most genotypes and under short days for winter genotypes, but its impact on spring genotypes was variable.
Final leaf number
Both the spring and winter types produced ≤9 leaves in the LV environment, with spring NIL CSIRO105 producing the fewest (5) leaves (Fig. 2). FLN was markedly higher for winter types in the LN and SN environments than in LV, and all winter genotypes except CSIROW007 and DS Bennett had at least one more leaf in the SV environment than in LV. Of the spring types, 13 genotypes in LN and seven genotypes in SV differed by less than one leaf from their values in the LV environment.
Thermal time to flag leaf
The TTFL also varied among genotypes and environments (Fig. 3). TTFL was typically lower for spring types than winter types in the non-vernalised environments (LN and SN). TTFL of all genotypes was delayed in the SN and SV environments compared with LV, and TTFL of all winter types was delayed in the LN environment compared with LV. TTFL of 22 spring types in the LN environment was within 156 degree-days of that in LV, with two spring NILs reaching flag leaf >100 degree-days quicker in the LN environment than in LV.
Thermal time to flowering
Data for TT to heading and flowering were similar, and therefore, only TTF is presented. TTF was delayed considerably for the winter types in all three limiting environments compared with the LV environment (Fig. 4). TTF was also delayed for the spring types in the short-day environments (SN and SV) compared with LV. Nineteen genotypes in SV and 43 genotypes in SN had TTF delayed by >1000 degree-days compared with LV. TTF of 34 of the spring types in the LN environment was within 260 degree-days of that in LV. Of those 34 spring types, 14 reached anthesis quicker in the LN environment.
Thermal time from flag leaf appearance to flowering
The TTFL–F occurred within a narrow range in both long-day environments (LN and LV) for both spring and winter types, and variation was much greater in the short-day environments (SN and SV) (Fig. 5). All but five genotypes had a lower TTFL–F in the LN environment than in LV. Of these, Magenta showed the greatest difference, flowering 233 degree-days quicker after flag leaf emergence in LN than LV. Five winter and 13 spring genotypes flowered >226 degree-days slower after flag leaf emergence in the SV environment than LV. Fifteen of the 19 genotypes that had TTF delayed by >1000 degree-days (Fig. 4) were in this group.
Vernalisation and photoperiod effects
The individual and combined effects of vernalisation and photoperiod on each trait in each genotype are shown in Fig. 6. Vernalisation effects were measured as the values of the traits in the LN environment compared with values in LV. Photoperiod effects were measured as the values of the traits in the SV environment compared with LV. Combined effects of both vernalisation and photoperiod were measured as the values of the traits in the SN environment compared with LV.
Vernalisation effects were pronounced for all winter types, whereas effects on the spring types varied. A clear separation can be seen between spring and winter types where the largest vernalisation effect on TTF for a spring type (LRPB Beaufort, 742 degree-days) was ~200 degree-days lower than for the least affected winter type (CSIROW021, 944 degree-days). A similar distinct separation in TTFL was also observed. Twenty-seven of the 56 spring types flowered ≥230 degree-days quicker when vernalisation was saturated, whereas four of the spring NILs flowered >100 degree-days slower. Twenty-nine spring types were quicker to flag leaf emergence by >156 degree-days when vernalisation was saturated, whereas two of the four previously mentioned spring NILs were slower to flag leaf emergence by >100 degree-days. Most genotypes were marginally delayed in TTFL–F when vernalised, with eight spring types being >100 degree-days slower to flower after flag leaf emergence.
Photoperiod effects were typically distinct in all genotypes for phyllochron, TTFL and TTF. Short photoperiod increased FLN by ≥1 in all but seven genotypes (two winter and five spring types). All genotypes were markedly slower to reach the flag leaf stage (delayed in TTFL), and five winter and 13 spring types were >226 degree-days slower to flower after flag leaf emergence (delayed in TTFL–F) under short photoperiod.
Combined effects of vernalisation and photoperiod showed an increase with short photoperiod and no vernalisation (SN vs LV) for all genotypes in the traits phyllochron, FLN, TTFL and TTF. The main stems on all replicates of winter genotype Whistler died before they were able to produce a flag leaf. TTFL–F was delayed by >226 degree-days in 16 genotypes (seven spring and nine winter types) of the 68 (Whistler excluded). Of these 16, 11 were genotypes that were also delayed >226 degree-days under shorter photoperiod.
All spring genotypes with the Vrn-A1v allele, except CSIROW105, expressed a vernalisation response as shown in their quicker flowering (by 203–726 degree-days; lower TTF) in LV than LN. All but one of the genotypes with the Vrn-A1a allele expressed minimal to no vernalisation response (−156 to 114 degree-days in LV vs LN), the exception being Bolac (329 degree-days quicker), with some genotypes flowering quicker in LN than LV.
Although all genotypes showed strong photoperiod response (SV compared to LV), genotypes with the Ppd-D1a allele had lower photoperiod responses than genotypes with the Ppd-D1b, c or d allele, except for Bolac and Manning. Axe and CSIROW105 were the only two genotypes with TTF in SV within <260 degree-days of that in LV.
Multi-locus genotype
Genotypes with matching MLG of the two PPD1 and three VRN1 genes developed at a similar rate with respect to some traits and environments and not others. There was generally less variation between genotypes with matching MLG in the LV environment than in SN, SV or LN. As examples, Young was slower to flower than CSIROW077 (MLG aaaaa) across all environments. EGA Gregory was quicker than Strzelecki (MLG bavva) in SN and LN but similar in SV and LV. Longsword, EGA Wedgetail and Illabo were quicker than SQP Revenue (MLG bavvv) in LV; Longsword and Illabo were quicker than EGA Wedgetail, which was quicker than SQP Revenue, in SV; they all differed in LN; and Illabo was much quicker than EGA Wedgetail, which was quicker than SQP Revenue and Longsword, in SN.
Variation in TTF within MLGs in the long-day environments was caused by differences in phyllochron and FLN. Phyllochron, FLN and TTFL–F caused variation in TTF in the short-day environments.
Discussion
The controlled-environment conditions used in this study allowed quantification of photoperiod and/or vernalisation sensitivities of a phenologically diverse panel of 69 wheat genotypes. Confounding effects were removed by adopting conditions that completely saturated or limited vernalisation and/or photoperiod while using optimal temperatures.
Vernalisation and photoperiod sensitivities varied greatly in the 69 wheat genotypes. Short photoperiod (SV vs LV) increased phyllochron, delayed TTFL (including when there was minimal difference in FLN), and delayed TTF for all genotypes. Vernalisation (LV vs LN) accelerated TTF for all winter genotypes by ~950–1700 degree-days but did not accelerate it for 26 of the 56 spring genotypes (≤100 degree-days difference). TTF was decreased by ~115–750 degree-days in the 30 spring types that did show a vernalisation response. Similarly, mixed vernalisation responses were exhibited by the spring genotypes for phyllochron, FLN and TTFL.
Vernalisation and photoperiod effects under controlled conditions
As expected, vernalisation accelerated development of all winter types by reducing phyllochron, FLN, TTFL and TTF. Vernalisation also reduced FLN in 43 of the spring genotypes. TTFL increased by >150 degree-days in spring types only when FLN increased by >2 in the LN environment compared with LV. It may be that the introduction of mutant dwarfing alleles (Rht-B1b and Rht-D1b) into Australian wheat breeding programs is responsible for these small differences in TTFL, which reduce cell number and length in leaves (Keyes et al. 1989). This hypothesis would need to be explored further, because there were no older/taller cultivars with the wild-type Rht alleles in this study for comparison. Modern Australian cultivars (i.e. those selected in the APP) typically have smaller flag leaves (Vandeleur and Gill 2004), which could result in a shorter phyllochron to extrude smaller flag leaves (Mossad et al. 1995). Mossad et al. (1995) also showed that some spring genotypes had lower phyllochron when not vernalised than when vernalised, which also occurred in some spring genotypes in this study.
Long photoperiod decreased phyllochron and TTFL of all genotypes, and decreased FLN and TTF for most genotypes. Compared with LV, SV delayed TTF more so than LN for most of the spring types, whereas the opposite occurred for winter types. A decrease in phyllochron associated with long days has also been shown in previous studies (Cao and Moss 1989; Mossad et al. 1995; Slafer and Rawson 1997). Friend et al. (1967) demonstrated an increasing leaf area ratio in spring wheat (cv. Marquis) with decreasing photoperiods (8-, 12-, 16-, 20- and 24-h treatments), and perhaps this photoperiod response in growth dynamics is linked with development rate, but growth traits were not measured in the present study to test this. More recently, Baumont et al. (2019) showed a clear relationship between leaf appearance rate and irradiance, indicating that leaf appearance rate is carbon-limited. They incorporated photothermal quotient in a leaf appearance rate model to account for carbon limitation, and this improved the accuracy of the model. Incorporating a photothermal quotient factor to account for differences in phyllochron of the long- and short-day treatments in the present study may also shed further light on this, given the large number of genotypes used. TTFL–F was delayed by >100 degree-days in 31 genotypes in the SV environment compared with LV, but was similar, and in some cases accelerated, in LN compared with LV. There are complex interactions between photoperiod, vernalisation and development phases. For example, Slafer et al. (2014) stated that some plant species exhibit a short photoperiod-independent ‘juvenile’ phase following seedling emergence, but it is not the case for wheat, which exhibits photoperiod sensitivity from emergence. Conversely, Steinfort et al. (2017) reported that eight wheat NILs differing at the PPD1 and VRN1 loci were more affected by vernalisation earlier during the vegetative phase (emergence to first node), whereas photoperiod affected plants more in the late vegetative to mid-reproductive phases (stem elongation). This was supported by results presented here. Some spring genotypes displayed a higher sensitivity to vernalisation than to photoperiod for FLN, but the lower FLN in SV than LN did not necessarily translate to parallel differences in TTF. For example, Beaufort produced fewer leaves, by 5.3 on average, in SV than LN, and in turn flowered 345 degree-days quicker, whereas Scythe produced fewer leaves, by 3.7 on average, but flowered 605 degree-days later.
‘Short-day vernalisation’, a vernalisation-like response of some winter genotypes grown under short daylength and temperature above the upper limit of vernalisation induction compared with long daylength at the same temperature, has been reported in controlled-environment experiments growing NIL (cv. Batten; Brooking and Jamieson 2002) and doubled haploid (cv. Triple Dirk; Allard et al. 2012) wheat genotypes. Those studies reported lower FLN and TTF in winter lines exposed to an initial period of short daylength for varying periods (10–84 days) before being transferred to long daylength, compared with constant exposure to long daylength. Except for FLN for winter genotype SQP Revenue being the same in both SN and LN environments, winter genotypes in our study developed quicker for all traits in LN than SN; however, we did not experiment with treatments that transferred plants from periods of short to long daylength.
Relevance to vernalisation and photoperiod effects in the field
Winter cropping environments in Australia cover a large range of latitudes and temperatures. The southern cropping regions experience the shortest and longest days and cooler winters. Conversely, daylength varies less in the northern regions (i.e. winter days are longer but summer days are shorter) and mean winter temperatures are higher. Vernalising temperatures are experienced in all of the major winter cropping areas in Australia, with the possible exception of Central Queensland. Although daylengths vary, the shortest day experienced in the southern areas is ~9.5 h. Therefore, it is harder to quantify a genotype’s photoperiod and vernalisation response under field conditions because environmental conditions will always be at least partially upregulating the vernalisation and photoperiod response. For example, Sadras and Lawson (2011) selected 13 genotypes released over the period from 1958 to 2007 based on their similar phenology in South Australian field conditions in order to quantify traits contributing to yield gains over that time. Three of those genotypes (Janz, Yitpi and Wyalkatchem) were also selected in our study. Sadras and Lawson (2011) observed similar phenology progression for all three genotypes in three field environments, with Yitpi being marginally slower to flower than Janz and Wyalkatchem. Under the controlled environment conditions in our study, Yitpi experienced a much larger delay to flowering in the short-day environments than Janz and Wyalkatchem; Wyalkatchem and Yitpi showed decreased TTF in SV compared with SN more so than Janz; Janz was quickest in LN, whereas Yitpi and Wyalkatchem were slower in LN but had reduced TTF in LV, comparable with Janz. The larger differences in the controlled environments are supported by the allelic variation of these cultivars at the Ppd-D1 and Vrn-A1 loci.
Major development genes
Of interest with regard to crop modelling was that photoperiod and vernalisation responses differed between genotypes that carry the same MLG (the same alleles at the five PPD1 and VRN1 loci). This has also been shown previously in both controlled-environment experiments (Bloomfield et al. 2018) and field experiments (Eagles et al. 2010; Cane et al. 2013; Harris et al. 2017; Christy et al. 2020). Bloomfield et al. (2018) showed that MLG was not able to predict accurately flowering time by growing 13 commercial cultivars paired with matching NILs under the same four controlled environments used in our study. Eagles et al. (2010) showed that alleles of Ppd-D1, Vrn-A1, Vrn-B1 and Vrn-D1 accounted for 45% of the variation in heading date in a large dataset of multiple south-eastern Australian field sites and years (128 in total), and Cane et al. (2013), with addition of the Ppd-B1 gene and additional Ppd-D1 alleles, accounted for 53% of the variation in the same dataset. Harris et al. (2017) found that Ppd-B1, Vrn-A1 and Vrn-B1 accounted for 75% of the variation in anthesis date in a doubled-haploid population that was fixed for the a and v alleles at the Ppd-D1 and Vrn-D1 loci, respectively. In the doubled-haploid population of Harris et al. (2017), spring genotypes differing only at Vrn-A1 and Vrn-B1 (bavav and baavv; cavav and caavv) flowered at the same time. Eagles et al. (2010) found that the presence of Vrn-D1v delayed flowering more than the presence of Vrn-A1v and Vrn-B1v in spring genotypes with different combinations of spring and winter alleles at the other two loci. Genotypes with Vrn-A1v grown by Zheng et al. (2013) showed a larger vernalisation response than genotypes with Vrn-A1a and different combinations of Vrn-B1 and Vrn-D1 a and v alleles when comparing field experiments with and without pre-vernalisation treatments and extended photoperiod, with the exception of the genotype with VRN1 MLG avv, which also exhibited a strong vernalisation response. Our findings concur, all genotypes with the Vrn-A1v allele (except CSIROW105) exhibited medium to strong vernalisation responses (LN vs LV), as well as Sunbri and Bolac (VRN1 MLG avv) and others with Vrn-A1b or w. Nine genotypes with VRN1 MLG avv and one with Vrn-A1w exhibited little to no vernalisation response.
A few alleles at various loci differed in some genotypes compared with those presented in other studies (Eagles et al. 2009; Cane et al. 2013; Zheng et al. 2013; Bloomfield et al. 2018). Some differences were attributed to identification of alleles that were not previously identified. For example, Eagles et al. (2009) and Zheng et al. (2013) were limited to two alleles of Ppd-D1: a for photoperiod insensitivity or b for photoperiod sensitivity. Cane et al. (2013) updated the alleles of genotypes used by Eagles et al. (2009) to include the c and d alleles of Ppd-D1. There are several possible causes. The released commercial cultivars and NILs with discrepancies may not be 100% homozygous, and the heterozygosity leads to ‘biotypes’ with different allele/s. Another possibility is that some markers will not be informative if recombination has occurred, owing to the use of some molecular markers that are not causal for the genetic variant (i.e. imperfect markers that are genetically linked but not diagnostic for an allele). The discrepancies found highlight the importance of genetic screening of material, because it could have detrimental effects on assumptions or models that have genetically derived parameters.
Modelling phenology
A key feature of APSIM NG (Holzworth et al. 2014, 2018) and the plant modelling framework wheat phenology model (Brown et al. 2018) is the ability to allow for cultivar-specific parameter inputs that are derived from controlled-environment experiment data such as presented here. The controlled-environment parameters selected must quantify a genotype’s response to the major environmental factors, vernalisation (not vernalised vs completely vernalised) and photoperiod (saturating long days of 16+ h vs limiting short days of <10 h) and the combinations of them.
In addition to phenotyping genotypes in controlled environments, there is scope to incorporate genetic and/or genomic information into wheat phenology models as minor phenology genes and new quantitative trait loci that contribute to a genotype’s phenology are identified (White et al. 2008; Christy et al. 2020). For example, Zheng et al. (2013) explained 96% of variation in time to heading in a model that incorporated allelic information for the major Ppd-D1, Vrn-A1, Vrn-B1 and Vrn-D1 loci, with the addition of experimentally derived, cultivar-specific thermal time functions to account for earliness per se. Christy et al. (2020) incorporated a photoperiod factor to the thermal time equation in a model that used allele combinations of the four (without Ppd-B1) and five major PPD1 and VRN1 genes to predict phenology of a diverse panel of commercial, NIL and recombinant inbred lines. The model predicted time from sowing to anthesis with an error of 5 days compared with observations taken at sites across a broad range of latitudes in Australia’s western and eastern high-rainfall zones, although sowing times prior to May were not conducted in the validation experiments. Their study complements the findings of Baumont et al. (2019), where predictions from a leaf appearance rate model were improved with the addition of a photothermal quotient factor to account for radiation. With further progress in genome sequencing and genetic marker technology, it would seem likely that the addition of allelic and/or genomic information (e.g. SNP markers) could further quantify genotypic responses to environment – such as in genotypes with different vernalisation and/or photoperiod responses but matching MLGs or photoperiod responses that occur in genotypes with insensitive alleles at PPD1 loci – and identify earliness per se and other genes involved in finetuning a genotype’s phenology in an environment. This could further enhance such a model and remove the need for field and/or controlled-environment phenotyping to derive genotype-specific parameters in the future.
Conclusions
Vernalisation and photoperiod effects on phenology differed greatly in a panel of 69 wheat genotypes when grown in four controlled environments with different treatments of vernalisation (0 or 8 weeks vernalisation) and photoperiod (17 or 8 h daylength). Long photoperiod decreased time to flowering in all genotypes, regardless of whether they had insensitive or sensitive alleles at the PPD1 loci, but those with the Ppd-D1a allele were less sensitive than those with the b, c, or d allele apart from two genotypes. Vernalisation decreased time to flowering in all winter genotypes and in spring genotypes with the Vrn-A1v or b allele. Some genotypes with matching MLG developed at similar rates in some environments, but no MLG pairs or groups displayed similar development rates across all four environments. These results suggest that Australian wheat cultivars have diverse responses to vernalisation and photoperiod that may not be distinguishable under field conditions. Further comparisons between controlled-environment and field experimental data would be useful to investigate this further.
Supplementary material
Supplementary material is available online.
Data availability
Analysed means for all genotypes and environments can be found in Table S2. Values presented in Fig. 6 are available in Table S3. Raw data are available from the La Trobe University research repository (https://doi.org/10.26181/20010428).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The research undertaken as part of this project is made possible by the significant contributions of growers through the support of the Grains Research and Development Corporation (National Phenology Initiative: ULA00011; development of near-isogenic lines: CSP0071, CSP00131, CSP00183) and Statistics for the Australian Grains Industry West (CUR00026).
Acknowledgements
We thank the rest of the GRDC National Phenology Initiative team for their thoughtful discussions around controlled-environment setup and phenotyping methods. MTB thanks La Trobe University and the GRDC for financial support of a PhD RTP scholarship and a top-up stipend, respectively.
References
ABARES (2020) Agricultural commodity statistics 2020. Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra, ACT, Australia.Acuña TB, Richards R, Partington D, Merry A, Christy B, Zhang H, O’Leary G, Riffkin P (2019) Extending the duration of the ear construction phase to increase grain yield of bread wheat. Crop & Pasture Science 70, 428–436.
| Extending the duration of the ear construction phase to increase grain yield of bread wheat.Crossref | GoogleScholarGoogle Scholar |
Allard V, Veisz O, Kõszegi B, Rousset M, Le Gouis J, Martre P (2012) The quantitative response of wheat vernalization to environmental variables indicates that vernalization is not a response to cold temperature. Journal of Experimental Botany 63, 847–857.
| The quantitative response of wheat vernalization to environmental variables indicates that vernalization is not a response to cold temperature.Crossref | GoogleScholarGoogle Scholar |
Auguie B (2017) gridExtra: miscellaneous functions for “grid” graphics. R package version 2.3. The R Foundation, Vienna, Austria. Available at https://cran.r-project.org/web/packages/gridExtra/index.html
Baumont M, Parent B, Manceau L, Brown HE, Driever SM, Muller B, Martre P (2019) Experimental and modeling evidence of carbon limitation of leaf appearance rate for spring and winter wheat. Journal of Experimental Botany 70, 2449–2462.
| Experimental and modeling evidence of carbon limitation of leaf appearance rate for spring and winter wheat.Crossref | GoogleScholarGoogle Scholar |
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theoretical and Applied Genetics 115, 721–733.
| A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Bloomfield MT, Hunt JR, Trevaskis B, Ramm K, Hyles J (2018) Ability of alleles of PPD1 and VRN1 genes to predict flowering time in diverse Australian wheat (Triticum aestivum) cultivars in controlled environments. Crop & Pasture Science 69, 1061–1075.
| Ability of alleles of PPD1 and VRN1 genes to predict flowering time in diverse Australian wheat (Triticum aestivum) cultivars in controlled environments.Crossref | GoogleScholarGoogle Scholar |
Brooking IR, Jamieson PD (2002) Temperature and photoperiod response of vernalization in near-isogenic lines of wheat. Field Crops Research 79, 21–38.
| Temperature and photoperiod response of vernalization in near-isogenic lines of wheat.Crossref | GoogleScholarGoogle Scholar |
Brown HE, Jamieson PD, Brooking IR, Moot DJ, Huth NI (2013) Integration of molecular and physiological models to explain time of anthesis in wheat. Annals of Botany 112, 1683–1703.
| Integration of molecular and physiological models to explain time of anthesis in wheat.Crossref | GoogleScholarGoogle Scholar |
Brown HE, Huth NI, Holzworth DP, Teixeira EI, Zyskowski RF, Hargreaves JNG, Moot DJ (2014) Plant modelling framework: software for building and running crop models on the APSIM platform. Environmental Modelling & Software 62, 385–398.
| Plant modelling framework: software for building and running crop models on the APSIM platform.Crossref | GoogleScholarGoogle Scholar |
Brown H, Huth N, Holzworth D (2018) Crop model improvement in APSIM: using wheat as a case study. European Journal of Agronomy 100, 141–150.
| Crop model improvement in APSIM: using wheat as a case study.Crossref | GoogleScholarGoogle Scholar |
Butler DG, Cullis BR, Gilmour AR, Gogel BJ (2009) ‘ASReml-R reference manual.’ (The State of Queensland, Department of Primary Industries and Fisheries: Brisbane, Qld, Australia)
Cai W, Cowan T (2013) Southeast Australia autumn rainfall reduction: a climate-change-induced poleward shift of ocean–atmosphere circulation. Journal of Climate 26, 189–205.
| Southeast Australia autumn rainfall reduction: a climate-change-induced poleward shift of ocean–atmosphere circulation.Crossref | GoogleScholarGoogle Scholar |
Cai W, Cowan T, Thatcher M (2012) Rainfall reductions over Southern Hemisphere semi-arid regions: the role of subtropical dry zone expansion. Scientific Reports 2, 702
| Rainfall reductions over Southern Hemisphere semi-arid regions: the role of subtropical dry zone expansion.Crossref | GoogleScholarGoogle Scholar |
Cane K, Eagles HA, Laurie DA, Trevaskis B, Vallance N, Eastwood RF, Gororo NN, Kuchel H, Martin PJ (2013) Ppd-B1 and Ppd-D1 and their effects in southern Australian wheat. Crop & Pasture Science 64, 100–114.
| Ppd-B1 and Ppd-D1 and their effects in southern Australian wheat.Crossref | GoogleScholarGoogle Scholar |
Cann DJ, Schillinger WF, Hunt JR, Porker KD, Harris FAJ (2020) Agroecological advantages of early-sown winter wheat in semi-arid environments: a comparative case study from southern Australia and Pacific Northwest United States. Frontiers in Plant Science 11, 568
| Agroecological advantages of early-sown winter wheat in semi-arid environments: a comparative case study from southern Australia and Pacific Northwest United States.Crossref | GoogleScholarGoogle Scholar |
Cao W, Moss DN (1989) Daylength effect on leaf emergence and phyllochron in wheat and barley. Crop Science 29, 1021–1025.
| Daylength effect on leaf emergence and phyllochron in wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Chen Y, Carver BF, Wang S, Zhang F, Yan L (2009) Genetic loci associated with stem elongation and winter dormancy release in wheat. Theoretical and Applied Genetics 118, 881–889.
| Genetic loci associated with stem elongation and winter dormancy release in wheat.Crossref | GoogleScholarGoogle Scholar |
Chouard P (1960) Vernalization and its relations to dormancy. Annual Review of Plant Physiology 11, 191–238.
| Vernalization and its relations to dormancy.Crossref | GoogleScholarGoogle Scholar |
Christy B, Riffkin P, Richards R, Partington D, Acuña TB, Merry A, Zhang H, Trevaskis B, O’Leary G (2020) An allelic based phenological model to predict phasic development of wheat (Triticum aestivum L.). Field Crops Research 249, 107722
| An allelic based phenological model to predict phasic development of wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Collins B, Chenu K (2021) Improving productivity of Australian wheat by adapting sowing date and genotype phenology to future climate. Climate Risk Management 32, 100300
| Improving productivity of Australian wheat by adapting sowing date and genotype phenology to future climate.Crossref | GoogleScholarGoogle Scholar |
Davidson JL, Christian KR, Jones DB, Bremner PM (1985) Responses of wheat to vernalization and photoperiod. Australian Journal of Agricultural Research 36, 347–359.
| Responses of wheat to vernalization and photoperiod.Crossref | GoogleScholarGoogle Scholar |
Díaz A, Zikhali M, Turner AS, Isaac P, Laurie DA (2012) Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS ONE 7, e33234
| Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar |
Dreisigacker S, Sehgal D, Reyes-Jaimez AE, Luna-Garrido B, Muñoz-Zavala S, Núñez-Ríos C, Mollins J, Mall S (2016) ‘CIMMYT wheat molecular genetics: laboratory protocols and applications to wheat breeding.’ (CIMMYT: Mexico)
Eagles HA, Cane K, Vallance N (2009) The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop & Pasture Science 60, 646–657.
| The flow of alleles of important photoperiod and vernalisation genes through Australian wheat.Crossref | GoogleScholarGoogle Scholar |
Eagles HA, Cane K, Kuchel H, Hollamby GJ, Vallance N, Eastwood RF, Gororo NN, Martin PJ (2010) Photoperiod and vernalization gene effects in southern Australian wheat. Crop & Pasture Science 61, 721–730.
| Photoperiod and vernalization gene effects in southern Australian wheat.Crossref | GoogleScholarGoogle Scholar |
Ellis MH, Rebetzke GJ, Azanza F, Richards RA, Spielmeyer W (2005) Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theoretical and Applied Genetics 111, 423–430.
| Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat.Crossref | GoogleScholarGoogle Scholar |
Fletcher A, Lawes R, Weeks C (2016) Crop area increases drive earlier and dry sowing in Western Australia: implications for farming systems. Crop & Pasture Science 67, 1268–1280.
| Crop area increases drive earlier and dry sowing in Western Australia: implications for farming systems.Crossref | GoogleScholarGoogle Scholar |
Flohr BM (2018) Stabilising the flowering time of wheat in response to autumn rainfall decline in southern Australia. PhD Thesis, College of Science, Research School of Biology, Division of Plant Sciences, Australian National University, Canberra, ACT, Australia.
Flohr BM, Hunt JR, Kirkegaard JA, Evans JR (2017) Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crops Research 209, 108–119.
| Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Flohr BM, Ouzman J, McBeath TM, Rebetzke GJ, Kirkegaard JA, Llewellyn RS (2021) Redefining the link between rainfall and crop establishment in dryland cropping systems. Agricultural Systems 190, 103105
| Redefining the link between rainfall and crop establishment in dryland cropping systems.Crossref | GoogleScholarGoogle Scholar |
Friend DJC, Helson VA, Fisher JE (1967) Effect of daylength on the growth of wheat. Canadian Journal of Botany 45, 117–131.
| Effect of daylength on the growth of wheat.Crossref | GoogleScholarGoogle Scholar |
Fu D, Szűcs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Molecular Genetics and Genomics 273, 54–65.
| Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat.Crossref | GoogleScholarGoogle Scholar |
Gilmour AR, Cullis BR, Verbyla AP (1997) Accounting for natural and extraneous variation in the analysis of field experiments. Journal of Agricultural, Biological, and Environmental Statistics 2, 269–293.
| Accounting for natural and extraneous variation in the analysis of field experiments.Crossref | GoogleScholarGoogle Scholar |
Gomez D, Vanzetti L, Helguera M, Lombardo L, Fraschina J, Miralles DJ (2014) Effect of Vrn-1, Ppd-1 genes and earliness per se on heading time in Argentinean bread wheat cultivars. Field Crops Research 158, 73–81.
| Effect of Vrn-1, Ppd-1 genes and earliness per se on heading time in Argentinean bread wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
González FG, Slafer GA, Miralles DJ (2005) Pre-anthesis development and number of fertile florets in wheat as affected by photoperiod sensitivity genes Ppd-D1 and Ppd-B1. Euphytica 146, 253–269.
| Pre-anthesis development and number of fertile florets in wheat as affected by photoperiod sensitivity genes Ppd-D1 and Ppd-B1.Crossref | GoogleScholarGoogle Scholar |
Grogan SM, Brown-Guedira G, Haley SD, McMaster GS, Reid SD, Smith J, Byrne PF (2016) Allelic variation in developmental genes and effects on winter wheat heading date in the U.S. Great Plains. PLoS ONE 11, e0152852
| Allelic variation in developmental genes and effects on winter wheat heading date in the U.S. Great Plains.Crossref | GoogleScholarGoogle Scholar |
Guo Z, Song Y, Zhou R, Ren Z, Jia J (2010) Discovery, evaluation and distribution of haplotypes of the wheat Ppd-D1 gene. New Phytologist 185, 841–851.
| Discovery, evaluation and distribution of haplotypes of the wheat Ppd-D1 gene.Crossref | GoogleScholarGoogle Scholar |
Harris FAJ, Eagles HA, Virgona JM, Martin PJ, Condon JR, Angus JF (2017) Effect of VRN1 and PPD1 genes on anthesis date and wheat growth. Crop & Pasture Science 68, 195–201.
| Effect of VRN1 and PPD1 genes on anthesis date and wheat growth.Crossref | GoogleScholarGoogle Scholar |
Haun JR (1973) Visual quantification of wheat development. Agronomy Journal 65, 116–119.
| Visual quantification of wheat development.Crossref | GoogleScholarGoogle Scholar |
Hochman Z, van Rees H, Carberry PS, Hunt JR, McCown RL, Gartmann A, Holzworth D, van Rees S, Dalgliesh NP, Long W, Peake AS, Poulton PL, McClelland T (2009) Re-inventing model-based decision support with Australian dryland farmers. 4. Yield Prophet® helps farmers monitor and manage crops in a variable climate. Crop & Pasture Science 60, 1057–1070.
| Re-inventing model-based decision support with Australian dryland farmers. 4. Yield Prophet® helps farmers monitor and manage crops in a variable climate.Crossref | GoogleScholarGoogle Scholar |
Holzworth DP, Huth NI, deVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, Moore AD, Brown H, Whish JPM, Verrall S, Fainges J, Bell LW, Peake AS, Poulton PL, Hochman Z, Thorburn PJ, Gaydon DS, Dalgliesh NP, Rodriguez D, Cox H, Chapman S, Doherty A, Teixeira E, Sharp J, Cichota R, Vogeler I, Li FY, Wang E, Hammer GL, Robertson MJ, Dimes JP, Whitbread AM, Hunt J, van Rees H, McClelland T, Carberry PS, Hargreaves JNG, MacLeod N, McDonald C, Harsdorf J, Wedgwood S, Keating BA (2014) APSIM – evolution towards a new generation of agricultural systems simulation. Environmental Modelling & Software 62, 327–350.
| APSIM – evolution towards a new generation of agricultural systems simulation.Crossref | GoogleScholarGoogle Scholar |
Holzworth D, Huth NI, Fainges J, Brown H, Zurcher E, Cichota R, Verrall S, Herrmann NI, Zheng B, Snow V (2018) APSIM next generation: overcoming challenges in modernising a farming systems model. Environmental Modelling & Software 103, 43–51.
| APSIM next generation: overcoming challenges in modernising a farming systems model.Crossref | GoogleScholarGoogle Scholar |
Hunt JR (2017) Winter wheat cultivars in Australian farming systems: a review. Crop & Pasture Science 68, 501–515.
| Winter wheat cultivars in Australian farming systems: a review.Crossref | GoogleScholarGoogle Scholar |
Hunt JR, Lilley JM, Trevaskis B, Flohr BM, Peake A, Fletcher A, Zwart AB, Gobbett D, Kirkegaard JA (2019) Early sowing systems can boost Australian wheat yields despite recent climate change. Nature Climate Change 9, 244–247.
| Early sowing systems can boost Australian wheat yields despite recent climate change.Crossref | GoogleScholarGoogle Scholar |
Hunt JR, Kirkegaard JA, Harris FA, Porker KD, Rattey AR, Collins MJ, Celestina C, Cann DJ, Hochman Z, Lilley JM, Flohr BM (2021) Exploiting genotype × management interactions to increase rainfed crop production: a case study from south-eastern Australia. Journal of Experimental Botany 72, 5189–5207.
| Exploiting genotype × management interactions to increase rainfed crop production: a case study from south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hyles J, Bloomfield MT, Hunt JR, Trethowan RM, Trevaskis B (2020) Phenology and related traits for wheat adaptation. Heredity 125, 417–430.
| Phenology and related traits for wheat adaptation.Crossref | GoogleScholarGoogle Scholar |
Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z, McLean G, Verburg K, Snow V, Dimes JP, Silburn M, Wang E, Brown S, Bristow KL, Asseng S, Chapman S, McCown RL, Freebairn DM, Smith CJ (2003) An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288.
| An overview of APSIM, a model designed for farming systems simulation.Crossref | GoogleScholarGoogle Scholar |
Keyes GJ, Paolillo DJ, Sorrells ME (1989) The effects of dwarfing genes Rht1 and Rht2 on cellular dimensions and rate of leaf elongation in wheat. Annals of Botany 64, 683–690.
| The effects of dwarfing genes Rht1 and Rht2 on cellular dimensions and rate of leaf elongation in wheat.Crossref | GoogleScholarGoogle Scholar |
Law CN, Sutka J, Worland AJ (1978) A genetic study of day-length response in wheat. Heredity 41, 185–191.
| A genetic study of day-length response in wheat.Crossref | GoogleScholarGoogle Scholar |
Miralles DJ, Richards RA, Slafer GA (2000) Duration of the stem elongation period influences the number of fertile florets in wheat and barley. Functional Plant Biology 27, 931–940.
| Duration of the stem elongation period influences the number of fertile florets in wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Mossad MG, Ortiz-Ferrara G, Mahalakshmi V, Fischer RA (1995) Phyllochron response to vernalization and photeperiod in spring wheat. Crop Science 35, 168–171.
| Phyllochron response to vernalization and photeperiod in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Parent B, Tardieu F (2012) Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytologist 194, 760–774.
| Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species.Crossref | GoogleScholarGoogle Scholar |
Pook M, Lisson S, Risbey J, Ummenhofer CC, McIntosh P, Rebbeck M (2009) The autumn break for cropping in southeast Australia: trends, synoptic influences and impacts on wheat yield. International Journal of Climatology 29, 2012–2026.
| The autumn break for cropping in southeast Australia: trends, synoptic influences and impacts on wheat yield.Crossref | GoogleScholarGoogle Scholar |
Porker K, Hunt J, Harris F, Noack S, Moodie M, Angel K, Straight M, Clarke G, Bruce D, Wallace A, Fettell N, Brooke G, McMillan H, Haskins B, Brady M, McDonald T, Spriggs B, Buderick S, Warren D (2019) Management of early sown wheat: matching genotype to environment. In ‘Proceedings of the 19th Australian agronomy conference’. 25–29 August 2019, Wagga Wagga, NSW. (Australian Society of Agronomy) Available at http://www.agronomyaustraliaproceedings.org/
Porker K, Straight M, Hunt JR (2020) Evaluation of G × E × M interactions to increase harvest index and yield of early sown wheat. Frontiers in Plant Science 11, 994
| Evaluation of G × E × M interactions to increase harvest index and yield of early sown wheat.Crossref | GoogleScholarGoogle Scholar |
Porter JR, Gawith M (1999) Temperatures and the growth and development of wheat: a review. European Journal of Agronomy 10, 23–36.
| Temperatures and the growth and development of wheat: a review.Crossref | GoogleScholarGoogle Scholar |
Pugsley AT (1966) The photoperiodic sensitivity of some spring wheats with special reference to the variety thatcher. Australian Journal of Agricultural Research 17, 591–599.
| The photoperiodic sensitivity of some spring wheats with special reference to the variety thatcher.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://cran.r-project.org/
Richards RA, Hunt JR, Kirkegaard JA, Passioura JB (2014) Yield improvement and adaptation of wheat to water-limited environments in Australia – a case study. Crop & Pasture Science 65, 676–689.
| Yield improvement and adaptation of wheat to water-limited environments in Australia – a case study.Crossref | GoogleScholarGoogle Scholar |
Sadras V, Dreccer MF (2015) Adaptation of wheat, barley, canola, field pea and chickpea to the thermal environments of Australia. Crop & Pasture Science 66, 1137–1150.
| Adaptation of wheat, barley, canola, field pea and chickpea to the thermal environments of Australia.Crossref | GoogleScholarGoogle Scholar |
Sadras VO, Lawson C (2011) Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop & Pasture Science 62, 533–549.
| Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007.Crossref | GoogleScholarGoogle Scholar |
Santra DK, Santra M, Allan RE, Campbell KG, Kidwell KK (2009) Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1, and Vrn-D1 in spring wheat germplasm from the pacific northwest region of the U.S.A. Plant Breeding 128, 576–584.
| Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1, and Vrn-D1 in spring wheat germplasm from the pacific northwest region of the U.S.A.Crossref | GoogleScholarGoogle Scholar |
Shaw LM, Turner AS, Laurie DA (2012) The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). The Plant Journal 71, 71–84.
| The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar |
Shcherban AB, Efremova TT, Salina EA (2012) Identification of a new Vrn-B1 allele using two near-isogenic wheat lines with difference in heading time. Molecular Breeding 29, 675–685.
| Identification of a new Vrn-B1 allele using two near-isogenic wheat lines with difference in heading time.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Rawson HM (1995) Photoperiod × temperature interactions in contrasting wheat genotypes: time to heading and final leaf number. Field Crops Research 44, 73–83.
| Photoperiod × temperature interactions in contrasting wheat genotypes: time to heading and final leaf number.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Rawson HM (1997) Phyllochron in wheat as affected by photoperiod under two temperature regimes. Functional Plant Biology 24, 151–158.
| Phyllochron in wheat as affected by photoperiod under two temperature regimes.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Abeledo LG, Miralles DJ, Gonzalez FG, Whitechurch EM (2001) Photoperiod sensitivity during stem elongation as an avenue to raise potential yield in wheat. In ‘Wheat in a global environment: proceedings of the 6th international wheat conference’. 5–9 June 2000, Budapest, Hungary. (Eds Z Bedö, L Láng) pp. 487–496. (Springer: Dordrecht, Netherlands) https://doi.org/10.1007/978-94-017-3674-9_64
Slafer GA, Kantolic AG, Appendino ML, Tranquilli G, Miralles DJ, Savin R (2014) Genetic and environmental effects on crop development determining adaptation and yield. In ‘Crop physiology’. 2nd edn. (Eds VO Sadras, DF Calderini) pp. 285–319. (Academic Press: San Diego, CA, USA)
Stefanova KT, Smith AB, Cullis BR (2009) Enhanced diagnostics for the spatial analysis of field trials. Journal of Agricultural, Biological, and Environmental Statistics 14, 392–410.
| Enhanced diagnostics for the spatial analysis of field trials.Crossref | GoogleScholarGoogle Scholar |
Steinfort U, Trevaskis B, Fukai S, Bell KL, Dreccer MF (2017) Vernalisation and photoperiod sensitivity in wheat: impact on canopy development and yield components. Field Crops Research 201, 108–121.
| Vernalisation and photoperiod sensitivity in wheat: impact on canopy development and yield components.Crossref | GoogleScholarGoogle Scholar |
Sukumaran S, Lopes MS, Dreisigacker S, Dixon LE, Zikhali M, Griffiths S, Zheng B, Chapman S, Reynolds MP (2016) Identification of earliness per se flowering time locus in spring wheat through a genome-wide association study. Crop Science 56, 2962–2672.
| Identification of earliness per se flowering time locus in spring wheat through a genome-wide association study.Crossref | GoogleScholarGoogle Scholar |
Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proceedings of the National Academy of Sciences 100, 13099–13104.
| MADS box genes control vernalization-induced flowering in cereals.Crossref | GoogleScholarGoogle Scholar |
Vandeleur RK, Gill GS (2004) The impact of plant breeding on the grain yield and competitive ability of wheat in Australia. Australian Journal of Agricultural Research 55, 855–861.
| The impact of plant breeding on the grain yield and competitive ability of wheat in Australia.Crossref | GoogleScholarGoogle Scholar |
Wang E, Martre P, Zhao Z, Ewert F, Maiorano A, Rötter RP, Kimball BA, Ottman MJ, Wall GW, White JW, Reynolds MP, Alderman PD, Aggarwal PK, Anothai J, Basso B, Biernath C, Cammarano D, Challinor AJ, De Sanctis G, Doltra J, Dumont B, Fereres E, Garcia-Vila M, Gayler S, Hoogenboom G, Hunt LA, Izaurralde RC, Jabloun M, Jones CD, Kersebaum KC, Koehler A-K, Liu L, Müller C, Naresh Kumar S, Nendel C, O’Leary G, Olesen JE, Palosuo T, Priesack E, Eyshi Rezaei E, Ripoche D, Ruane AC, Semenov MA, Shcherbak I, Stöckle C, Stratonovitch P, Streck T, Supit I, Tao F, Thorburn P, Waha K, Wallach D, Wang Z, Wolf J, Zhu Y, Asseng S (2017) The uncertainty of crop yield projections is reduced by improved temperature response functions. Nature Plants 3, 17102
| The uncertainty of crop yield projections is reduced by improved temperature response functions.Crossref | GoogleScholarGoogle Scholar |
White JW, Herndl M, Hunt LA, Payne TS, Hoogenboom G (2008) Simulation-based analysis of effects of vrn and ppd loci on flowering in wheat. Crop Science 48, 678–687.
| Simulation-based analysis of effects of vrn and ppd loci on flowering in wheat.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2016) ‘ggplot2: elegant graphics for data analysis.’ (Springer-Verlag: New York, NY, USA)
Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theoretical and Applied Genetics 109, 1677–1686.
| Allelic variation at the VRN-1 promoter region in polyploid wheat.Crossref | GoogleScholarGoogle Scholar |
Zhang J, Wang Y, Wu S, Yang J, Liu H, Zhou Y (2012) A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response. Theoretical and Applied Genetics 125, 1697–1704.
| A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response.Crossref | GoogleScholarGoogle Scholar |
Zheng B, Biddulph B, Li D, Kuchel H, Chapman S (2013) Quantification of the effects of VRN1 and Ppd-D1 to predict spring wheat (Triticum aestivum) heading time across diverse environments. Journal of Experimental Botany 64, 3747–3761.
| Quantification of the effects of VRN1 and Ppd-D1 to predict spring wheat (Triticum aestivum) heading time across diverse environments.Crossref | GoogleScholarGoogle Scholar |


