Recovery of nitrogen fertiliser by drill-sown rice crops using best management practice: a 15N-labelled urea study
Terry J. Rose A B * , Lee J. Kearney A B , Brian W. Dunn
A B * , Lee J. Kearney A B , Brian W. Dunn  C and Tina S. Dunn C
C and Tina S. Dunn C
A Faculty of Science and Engineering, Southern Cross University, PO Box 157, Lismore, NSW 2480, Australia.
B Southern Cross Plant Science, Southern Cross University, PO Box 157, Lismore, NSW 2480, Australia.
C NSW Department of Primary Industries, Yanco Agricultural Institute, PMB, Yanco, NSW 2703, Australia.
Crop & Pasture Science 73(11) 1245-1252 https://doi.org/10.1071/CP21754
Submitted: 5 November 2021 Accepted: 19 April 2022 Published: 12 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Optimising nitrogen (N) management strategies for drill-sown rice crops is essential for minimising input costs for growers and reducing the environmental impact of rice production.
Aims: The study aimed to determine the recovery of fertiliser-N in drill-sown Australian rice crops, following current N fertiliser recommendations where two-thirds of the N is applied at sowing (pre-flood) and one-third at panicle initiation.
Methods: 15N-labelled urea was used to quantify N recovery by field-grown rice crops on a Sodosol and a Vertosol, and to determine the contributions of fertiliser-N applied pre-flood vs that applied at panicle initiation to total N fertiliser recovery on the Vertosol.
Results: Recovery of 15N fertiliser in grain + straw was ∼50% of applied N on both soils, with a further 20% recovered from roots and soil to a depth of 30 cm. Recovery of N fertiliser applied at panicle initiation (59%) was significantly higher than of N fertiliser applied pre-flood (43%), likely due to the presence of actively growing roots and higher plant N demand. Crops that received N fertiliser took up more native soil N than unfertilised crops on the Vertosol; hence, apparent fertiliser N recoveries were 10–15% higher than N fertiliser recovery determined using 15N-labelled urea.
Conclusions: The recovery of 50% of fertiliser-N in aboveground plant material indicates that N fertiliser use efficiency in drill-sown rice is similar to that of dryland cereal crops in Australia when best management practice guidelines for N fertiliser use are followed.
Keywords: direct seeding, fertiliser efficiency, isotope, nitrogen recovery, nitrogen use efficiency, temperate rice crops, water savings, water use efficiency.
Introduction
Over 70% of the world’s rice (Oryza sativa L.) is grown under flooded (paddy) conditions, making the global rice industry a large user of irrigation water. In recent years there has been a push to reduce water usage in rice production owing to costs and competing demands for water (Rejesus et al. 2011). In turn, this has led to adoption of management strategies including ‘alternate wetting and drying’ (AWD), which integrates an aerobic growth phase in the crop cycle to reduce water use (Carrijo et al. 2017). In the Australian rice industry, AWD strategies involving aerobic growth between panicle initiation (PI) and anthesis are not generally feasible because deep water (>25 cm) is needed to protect the crop from cold temperature stress, which can cause severe yield losses (Williams and Angus 1994). As such, water savings in Australian rice crops are typically achieved by drill sowing crops instead of sowing into flooded fields, and growing the crop aerobically until the 3–4-leaf stage (‘drill sowing’) or until 2–3 weeks before PI (delayed permanent water, DPW) (Dunn et al. 2014). Adoption of DPW has been shown to increase water productivity by 17% compared with standard drill sowing practices under Australian conditions (Dunn and Gaydon 2011).
Nitrogen (N) fertiliser management in drill-sown or DPW crops is also critical because broadcasting N fertiliser into floodwater is highly inefficient, with crops recovering as little as 28% of applied fertiliser-N (Humphreys et al. 1987). Higher N fertiliser recoveries in drill-sown crops are achieved when N is applied immediately prior to permanent flooding (Humphreys et al. 1987; Norman et al. 2009). Broadcasting urea onto the soil immediately prior to permanent flooding is therefore currently recommended for both drill-sown and DPW crops in Australia (Dunn et al. 2014, 2016). Using this N application method, apparent N recoveries (i.e. the amount of N accumulated in shoots of an N-fertilised crop minus N accumulated in shoots of an unfertilised crop, divided by the amount of N fertiliser applied) in drill-sown crops range from ∼50% to 70% (Dillon et al. 2012; Dunn et al. 2014).
One issue with apparent N fertiliser recovery estimates is the inherent assumption that the N-fertilised crop takes up the same amount of native soil N as the unfertilised crop, and thus the difference between their shoot N accumulation values represents the amount of fertiliser-N taken up by the fertilised crop. However, this assumption is not always valid because the addition of N fertiliser may stimulate greater crop growth and root exploration to take up more native soil N, or can lead to soil N priming, where more native soil N is mineralised (Kuzyakov et al. 2000). Either or both of these processes can lead to N-fertilised plants acquiring more N from the native soil N pool than unfertilised plants. The use of 15N-labelled fertiliser can overcome this obstacle to accurate estimation by directly measuring the uptake of N from the labelled N fertiliser source.
In a study on a Sodosol (Australian Soil Classification; Isbell 1996) in south-eastern Australia, there was no difference in crop recovery of N from 15N-urea fertiliser between crops grown under full flood or DPW watering regimes, with ∼27% of fertiliser-N recovered in grain and straw at maturity under both regimes (Rose et al. 2016). However, all N fertiliser was supplied at sowing (for full flood) or pre-flood (for DPW) in that study, whereas splitting N fertiliser applications between pre-flood and PI is currently recommended for drill-sown rice crops (Dunn et al. 2014, 2016). Therefore, we wished to know whether there is a difference in crop recovery of N from 15N-urea fertiliser depending on when the fertiliser is applied. The present study investigated the recovery of 15N-urea by drill-sown rice crops grown on a Sodosol when all N was applied at immediately prior to permanent water (PW), or with an extra application at PI. We further investigated N fertiliser recovery on a Vertosol where the fertiliser was applied in total at PW or as a 2:1 split between PW and PI, and specifically examined the recovery of N from 15N-labelled urea applied at PW versus PI.
Materials and methods
Field sites and experimental layout
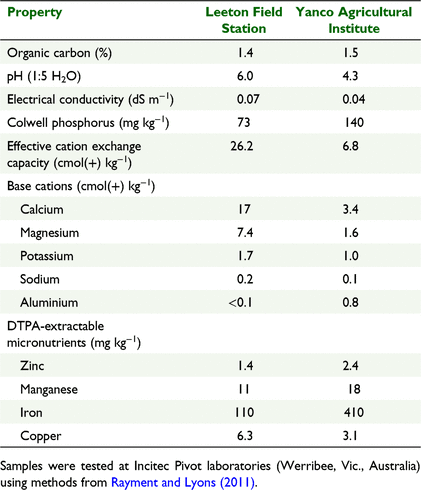
Two field trials were conducted in the Murrumbidgee Irrigation Area of southern New South Wales, Australia, in the 2016–17 rice growing season. One field trial was established on a Sodosol at Yanco Agricultural Institute (YAI) (−34.613181, 146.419479) and another was established on a Vertosol at Leeton Field Station (LFS) (−34.605339, 146.362144). Selected soil chemical properties of the 0–150 mm layer of the soils are presented in Table 1.
|
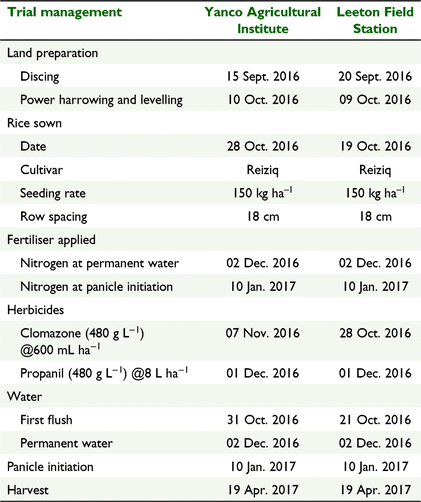
At both trial sites, microplots were established in rice fields in an area that did not receive any N fertiliser, by inserting 300-mm-diameter metal rings to a depth of 150 mm in the soil after the first ‘flush’ irrigation. Details of the rice crop management and timing of permanent water and N applications for each trial site are given in Table 2. For PW applications, 15N-labelled urea (5.1 atom % 15N) was applied by hand to each microplot in appropriate treatments, and for application at PI, by hand directly into floodwater within the rings.
|
At LFS, five treatments were established that differed with regard to timing of N application (applied in full at PW, or split 2:1 between PW and PI) and, for split applications, whether the N fertiliser was unlabelled or 15N-labelled urea. The five treatments (kg N ha−1, application PW/PI) were: (i) nil/nil (i.e. control); (ii) 180 kg as 15N-labelled urea/nil (18015N/0N); (iii) 120 kg/60 kg, both as 15N-labelled urea (12015N/6015N); (iv) 120 kg as unlabelled urea/60 kg as 15N-labelled urea (120N/6015N); and (v) 120 kg as 15N-labelled urea/60 kg as unlabelled urea (12015N/60N). Three replicate microplot rings per treatment were positioned in a 3 × 5 layout with 5 m between rings in the unfertilised area of the field, with treatments positioned randomly.
At YAI, three treatments (kg N ha−1, application IPPW/PI) were established: (i) nil/nil (control); (ii) 120 kg as 15N-labelled urea/nil (12015N/0N); and (iii) 120 kg/60 kg, both as 15N-labelled urea (12015N/6015N). Three replicate microplot rings per treatment were positioned in a 3 × 3 layout with 5 m between rings in the unfertilised area of the field, with treatments positioned randomly. Unfortunately, however, the control plots received 60 kg N ha−1 as unlabelled urea at PI (0N/60N), and therefore did not represent a nil-N control. A parallel incubation experiment was conducted in the laboratory at YAI to determine whether addition of the unlabelled urea affected soil 15N abundance. The 90-day incubation experiment used unfertilised soil adjacent to the YAI trial, with soil samples (20 g) placed in 200-mL containers and incubated in the dark at 25°C. Addition of 60, 120 or 180 kg N ha−1 to soil (on a weight basis, calculated using soil bulk density and an assumed urea penetration depth of 100 mm into soil) had no significant effect on the 15N abundance of the soil after 90 days (mean 15N abundance +7.5‰ (±0.09‰)). Plant and soil material from the 0N/60N microplots was thus used as ‘nil-N’ material for calculations below.
Measurements
At crop maturity, plant height was measured with a ruler before all aboveground material within the rings was harvested by severing shoots at ground level, and grain was manually separated from the straw. Rings were removed by digging away the surrounding soil with a shovel, and the top 300 mm of the soil and roots was retained, comprising the top 150 mm of soil + roots within the inserted ring and the next layer (150–300 mm) of soil + roots below the ring. The two layers of soil + roots were kept separate. Roots were then separated from soil by dry sieving to remove the bulk of soil, followed by hand-washing through multiple sieves to clean the roots. Root material from the two soil layers was combined for each plot, whereas soil material from the two layers was kept separate. All plant tissue and soil material was then dried for 3 days at 60°C. Plant material was then weighed before all plant and soil material was finely ground for analysis of total N concentration by using a TruMAC CNS analyser (LECO, MI, USA) and quantification of N isotope ratios via a Thermo Delta V Plus isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) following combustion on a Thermo Flash EA 1112 elemental analyser (Thermo Fisher Scientific). For total N concentrations, subsamples (200 mg) were combusted for all material, whereas for N isotope ratios, 1 mg was combusted for plant material and 4 mg was combusted for soil material.
Calculations
Aboveground biomass was calculated by summing grain and straw biomass, and total plant biomass was calculated by summing root, straw and grain biomass. Harvest index was calculated by dividing grain biomass by aboveground biomass. The N content of root, straw and grain tissues was calculated by multiplying the biomass by the respective tissue N concentration. Plant aboveground N content was calculated by summing grain N content and straw N content. Total plant N content was calculated by summing root, straw and grain N contents.
For each soil layer (0–150 and 150–300 mm), the N content (kg N ha−1) was calculated by multiplying the weight of the layer per ha by the %N concentration in the layer. The weight of soil in each layer (kg ha−1) within the microplot was calculated by using the bulk density of the layer.
All 15N data were expressed as the atom % excess, corrected for background abundance (0.36765%). The percentage of N derived from fertiliser (%Ndff; derived from the 15N-labelled urea portion of applied N) in plant and soil samples was calculated using the equation:
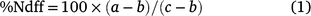
where a is the atom % 15N in the treatment plant or soil sample; b is the % 15N in the unfertilised (control) plant or soil sample; and c is the atom % 15N in the fertiliser (5.1%). Note that for the YAI trial, the b value was obtained from plant or soil that had received 60 kg N as unlabelled urea at PI.
The percentage of applied 15N fertiliser recovered in each plant tissue and soil layer (%NFR) was calculated as:
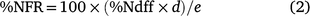
where %Ndff is as previously defined, d is the kg N ha−1 in plant tissue or soil layer, and e is the amount of N fertiliser applied (kg N ha−1).
The percentage of fertiliser-N recovered in aboveground plant material was calculated by summing the per cent recovery in grain and straw tissue, and the per cent recovery in plants was calculated by summing the per cent recovery of root, straw and grain tissue. Finally, the total fertiliser N recovery in the system was calculated by summing the per cent recovery in plants and per cent recovery in each soil layer.
Apparent N fertiliser recovery for each of the N-fertilised treatments at LFS was calculated by subtracting the aboveground N content from the nil-N plots from the aboveground N content of treated plots, and dividing by the amount of N fertiliser applied (in kg N ha−1) and expressing as a percentage. No apparent N fertiliser recovery could be calculated at YAI because nil-N plots received 60 kg N ha−1 as unlabelled urea at PI.
Statistical analyses
For each trial site, plant biomass, grain yield, harvest index and grain N data, and %Ndff and %NFR data were analysed by one-way analysis of variance fitting N fertiliser treatment in GenStat Release 16.1 (VSN International, Hemel Hempstead, UK), using a probability level of 0.05. Significance of differences between treatment means at each trial site was tested by using Duncan’s multiple range test at P = 0.05.
Results
Biomass and grain yields
Grain yield at YAI increased significantly (P < 0.05), by >5 t ha−1, in response to N fertiliser application at PW (12015N/0N) compared with application only at PI (0N/60N), with a further significant increase of 2 t ha−1 when an additional 60 kg N ha−1 was applied at PI (12015N/6015N) (Table 3). This trend was also reflected in total aboveground biomass and total biomass (Table 3). At LFS, grain yield in the nil N control (5.4 t ha−1) was significantly lower than in all of the treatments with N applied, where yields were >13 t ha−1 and not significantly different from each other.

|
Harvest index and grain N
The harvest index did not differ among treatments at either site, with a mean of ∼0.5 in all treatments at both field sites. Similarly, grain N percentage did not differ between treatments at either site, with a mean of ∼1% for all treatments at both sites (Table 3).
Plant N accumulation and percentage of N derived from fertiliser in plant tissues
At YAI, the 12015N/6015N treatment resulted in significantly higher %Ndff than the 12015N/0N treatment for grains (39.5% vs 27.1%) and straw (33.2% vs 23.5%), resulting in higher overall %Ndff in aboveground plant material (37.6% vs 26.0%) and all plant material (30.8% vs 22.4%) (Table 4). This was reflected in a significantly greater amount of aboveground plant N derived from 15N-labelled fertiliser in the 12015N/6015N treatment than the 12015N/0N treatment (93 vs 58 kg N ha−1) (Fig. 1a). Ultimately, uptake of N from soil and unlabelled N fertiliser did not differ among treatments (mean of ∼163 kg N ha−1). Aboveground N accumulation increased significantly with increasing N application from 60 to 180 kg N ha−1 (Fig. 1a), which mirrored the trend in grain yields.
At LFS, %Ndff in whole plants was in the order 18015N/0N = 12015N/6015N > 12015N/60N > 120N/6015N, and this same trend was observed for all tissues and aboveground plant material (Table 4). It is noteworthy that the sum of %Ndffplant of the treatments 12015N/60N (22.0%) and 120N/6015N (14.4%) of 36.4% was similar to the treatments where 180 kg 15N ha−1 was applied in total (37.3% for 18015N/0N and 38.7% for 12015N/6015N). Ultimately, ∼43% of aboveground N was derived from 15N-labelled fertiliser in treatment 18015N/0N, where all N was applied as 15N labelled fertiliser at PW, compared with 25% and 17% in treatments 12015N/60N and 120N/6015N, where 15N-labelled fertiliser was applied at PW and PI, respectively (Table 4). This was reflected in the proportion of aboveground N content derived from 15N-labelled fertiliser, where 83 and 90 kg N ha−1 was derived from 15N fertiliser in the 18015N/0N and 12015N/6015N treatments, respectively, compared with 52 kg N ha−1 in the 12015N/60N treatment and 35 kg N ha−1 in the 120N/6015N treatment (Fig. 1b). Notably, 81 kg N ha−1 was taken up from the soil in the nil-N control treatment, but where 180 kg N ha−1 was added as 15N-labelled urea (i.e. 18015N/0N and 12015N/6015N), native soil N uptake was significantly higher at ∼115 kg N ha−1 (Fig. 1b). The differences in non-15N-labelled urea uptake between the 18015N/0N and 12015N/6015N treatments and the 12015N/60N and 120N/6015N treatments are attributed to uptake of N from unlabelled urea as opposed to differences in native soil N uptake, because the total aboveground N content did not differ between treatments where a total of 180 kg N ha−1 was added (Fig. 1b).
15N fertiliser recovery in plants and soil
At YAI there was no significant difference in 15N recovery in plant or soil material between the 12015N/0N and 12015N/6015N treatments (Table 5). Approximately 50% of applied N was recovered in aboveground plant material, with a total N fertiliser recovery (i.e. all plant and soil material) of ∼70%.

|
At LFS, a significantly higher proportion of the 15N was recovered in aboveground plant material when applied only at PI (120/6015N, 62.3%) than when 15N was applied only at PW (12015N/60N, 47.3%; 18015N/0N, 51.9%) (Table 5). This was due to significantly greater 15N recovery in grains (44.8% when 15N was applied only at PI vs 32.2% in 12015N/60N and 33.2% in 18015N/0N), because recovery of 15N in straw and root tissue did not differ significantly among treatments (Table 5). Notably, when 15N was applied at both PW and PI (12015N/6015N), the per cent recovery of 15N in grains (38.4%) and whole plants (55.4%) was intermediate to, and not significantly different from, recovery when 15N was applied solely at PI or solely at PW. Despite a higher per cent recovery of 15N in plants when applied solely at PI, there was no significant difference in the total recovery of 15N in the plant–soil system (%NFRtotal) between any two treatments (mean 73.0%) owing to lower recovery of 15N applied at PI in the soil (9.6% at 0–150 mm and 0.4% at 150–300 mm) (Table 5). Finally, apparent recovery of N fertiliser did not differ among treatments (mean 68.7%) but was substantially higher than the mean aboveground plant 15N recoveries, which ranged from 43.0% in 12015N/60N to 58.5% in 120N/6015N (Table 5).
Discussion
Recovery of fertiliser-N by flooded rice crops has traditionally been low (20–40%), owing to a combination of N2 losses and NH3 volatilisation losses (Vlek and Byrnes 1986). However, agronomic research over the past three decades has led to optimisation of both the rate and timing of N fertiliser application to minimise N losses and maximise crop yields. In Australia, it is currently recommended that two-thirds of N be applied IPPW in drill-sown crops, with the final third applied at PI (Dunn et al. 2016). With a standard N rate of 180 kg ha−1 split between IPPW and PI applications, recovery of 15N fertiliser in aboveground tissue (grain and straw) was ∼50% of applied N on both the Sodosol (YAI) and Vertosol (LFS), with total N recovery (all plant material and soil recovery to 30 cm depth) of almost 70% (Table 5). Given an average aboveground recovery of 15N fertiliser of 44% (±14%) by dryland crops in Australia (Angus and Grace 2017), our results indicate that high-yielding, drill-sown rice crops can have similar fertiliser-N recoveries to those of dryland crops when optimised N fertiliser management strategies are employed.
The 48.1% recovery of 15N in aboveground tissue when 120 kg N ha−1 was applied only IPPW at YAI (12015N/0N) was slightly lower (although not significantly so) than recovery in the 12015N/6015N treatment (51.8%), but substantially greater than the 29% recovery reported by Rose et al. (2016) when 120 kg N ha−1 was applied pre-flood in a drill-sown crop grown on a similar Sodosol. We suggest the difference in N fertiliser recoveries between the present study and the earlier study was largely due to lower crop yields in the earlier study, where aboveground biomass and grain yields were 19 t and 10 t ha−1, respectively, compared with 30 t and 15 t ha−1 in the present study (Table 3). The poorer growth in the study by Rose et al. (2016) may have diminished crop N demand and, therefore, crop recovery of fertiliser-N, although differences in rice variety or other soil or seasonal conditions may also have contributed. Similar results have been reported for drill-sown rice crops in southern USA, where 15N fertiliser recovery in grain and straw from split N application (100 kg N ha−1 pre-flood + 34 kg N ha−1 at PI) was 35% in one season but was 48% in the same field in the subsequent season, when rice biomass production and grain yields were higher (Bollich et al. 1994).
Although the overall recovery of 15N fertiliser in straw and grain was ∼50% in the 12015N/6015N treatments at both field sites (Table 5), at LFS the recovery and partitioning of 15N applied at IPPW differed from 15N applied at PI. A greater proportion of 15N was recovered by plants when applied solely at PI (58.5% for aboveground material and 62.3% for whole plants) than when applied only at IPPW (43.0% for aboveground material and 47.3% for whole plants). This is consistent with earlier reports that N fertiliser applied at PI is recovered more efficiently by rice crops than N applied pre-flood (Westcott et al. 1986; Bacon and Heenan 1987). This greater efficiency of fertiliser-N uptake at PI has generally been attributed to greater plant N demand at this stage than at earlier growth stages (Westcott et al. 1986) rather than any specific conditions in the soil at PI that reduce the chance of N loss through denitrification.
Owing to a lower proportion of 15N being recovered in the soil when applied at PI, there was no significant difference in overall system recovery of 15N, presuming that the 15N in the soil would still be present and available for subsequent crops. The higher per cent recovery of 15N in the 120N/6015N treatment was due greater recovery in grain tissue (Table 5), suggesting that N applied at PI was also partitioned among plant tissues differently from N applied at IPPW. This is supported by the fact that 18% of grain N in the 120N/6015N treatment was derived from the 60 kg N ha−1 as 15N-labelled fertiliser, whereas only 25% of grain N was derived from 15N-labelled fertiliser when 120 kg 15N ha−1 was applied at IPPW (12015N/60N; Table 4). Although the lack of difference in per cent grain N derived from fertiliser between the 18015N/0N and 12015N/6015N treatments appears at odds with this, these numbers are not directly comparable because grain yields differed between these treatments (i.e. lower on average by ∼1 t ha−1 in the 18015N/0N treatment, although not significant at P = 0.05). The lack of a significant yield difference between the N-fertilised treatments also highlights the fact that higher recovery of 15N at PI is not necessarily associated with higher yields, and as noted by Bollich et al. (1994), split N applications can actually lead to yield losses when insufficient N is applied earlier in the season. This relationship between crop phenology and N supply is also cultivar dependent (Bollich et al. 1994), and emphasises the importance of ongoing agronomic research to optimise N fertiliser rate and timing for new cultivars as they are released from breeding programs.
Apparent N fertiliser recovery in drill-sown crops ranges from 50% to 70% (Dillon et al. 2012; Dunn et al. 2014); however, experimental comparisons in rice suggest that N fertiliser recoveries derived by using 15N-labelled fertiliser are substantially lower than the apparent N fertiliser recoveries (Humphreys et al. 1987). This was observed at LFS in our study, where the mean apparent N fertiliser recovery in the split N treatments (12015N/6015N, 12015N/60N and 120N/6015N) was ∼70%, whereas the mean 15N recovery of these three treatments was ∼50% (Table 5). This difference is attributed to greater uptake of native soil N where N fertiliser was added, as indicated by additional 30–35 kg ha−1 of native soil N accumulated in 18015N/0N and 12015N/6015N treatments (Fig. 1). Whether this was due to greater root exploration or soil N priming, or a combination of both, is not known. Ultimately, the crop N uptake data from LFS also indicated that >50% of crop N uptake in the 18015N/0N and 12015N/6015N treatments was derived from the soil, clearly demonstrating the reliance of flooded rice crops on native soil N sources for much of their N demand (see also Bacon and Heenan 1987; Cassman et al. 1998).
Finally, it is acknowledged that the rings used for the microplots in the study were inserted only 150 mm into the soil, and it is possible that some of 15N-labelled urea moved deeper into the soil and out of the sampling area. However, previous 15N studies on flooded rice in Australia (Humphreys et al. 1987) and elsewhere (Westcott et al. 1986; Bollich et al. 1994) have all used similar methodology; therefore, our results are directly comparable to these earlier studies.
Conclusions
The recovery of ∼50% of applied N fertiliser in rice plants (grain + straw) suggests that following best management practice for N application in drill-sown rice crops leads to more effective N capture than typically observed in traditional flooded rice crops (20–40%; Vlek and Byrnes 1986). The most effective recovery of N fertiliser occurs when N is applied at PI, likely due to greater root surface area and crop N demand at this time compared with N application at IPPW. Ultimately, the 50% fertiliser-N recovery in straw + grain material demonstrates that N fertiliser use efficiency in drill-sown rice is similar to that of dryland cereal crops in Australia when current best practice guidelines for N fertiliser use are followed.
Data availability
Data are available from the corresponding author upon request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive and specific funding.
Acknowledgements
We thank Environmental Analysis Laboratory at Southern Cross University for assistance with sample processing and analysis.
References
Angus JF, Grace PR (2017) Nitrogen balance in Australia and nitrogen use efficiency on Australian farms. Soil Research 55, 435–450.| Nitrogen balance in Australia and nitrogen use efficiency on Australian farms.Crossref | GoogleScholarGoogle Scholar |
Bacon PE, Heenan DP (1987) Nitrogen budgets for intensive rice growing in Southern Australia. In ‘Efficiency of nitrogen fertilisers in rice’. (Eds JR Freney, R Wetselaar, ACF Trevitt, JR Simpson) pp. 89–95. (International Rice Research Institute: Los Baños, Philippines)
Bollich PK, Lindau CW, Norman RJ (1994) Management of fertiliser nitrogen in dry-seeded, delayed-flood rice. Australian Journal of Experimental Agriculture 34, 1007–1012.
| Management of fertiliser nitrogen in dry-seeded, delayed-flood rice.Crossref | GoogleScholarGoogle Scholar |
Carrijo DR, Lundy ME, Linquist BA (2017) Rice yields and water use under alternate wetting and drying irrigation: a meta-analysis. Field Crops Research 203, 173–180.
| Rice yields and water use under alternate wetting and drying irrigation: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Cassman KG, Peng S, Olk DC, Ladha JK, Reichardt W, Dobermann A, Singh U (1998) Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Research 56, 7–39.
| Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems.Crossref | GoogleScholarGoogle Scholar |
Dillon KA, Walker TW, Harrell DL, Krutz LJ, Varco JJ, Koger CH, Cox MS (2012) Nitrogen sources and timing effects on nitrogen loss and uptake in delayed flood rice. Agronomy Journal 104, 466–472.
| Nitrogen sources and timing effects on nitrogen loss and uptake in delayed flood rice.Crossref | GoogleScholarGoogle Scholar |
Dunn BW, Gaydon DS (2011) Rice growth, yield and water productivity responses to irrigation scheduling prior to the delayed application of continuous flooding in south-east Australia. Agricultural Water Management 98, 1799–1807.
| Rice growth, yield and water productivity responses to irrigation scheduling prior to the delayed application of continuous flooding in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Dunn BW, Dunn TS, Beecher HG (2014) Nitrogen timing and rate effects on growth and grain yield of delayed permanent-water rice in south-eastern Australia. Crop & Pasture Science 65, 878–887.
| Nitrogen timing and rate effects on growth and grain yield of delayed permanent-water rice in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Dunn BW, Dunn TS, Orchard BA (2016) Nitrogen rate and timing effects on growth and yield of drill-sown rice. Crop & Pasture Science 67, 1149–1157.
| Nitrogen rate and timing effects on growth and yield of drill-sown rice.Crossref | GoogleScholarGoogle Scholar |
Humphreys E, Chalk PM, Muirhead WA, Melhuish FM, White RJG (1987) Effects of time of urea application on combine-sown Calrose rice in south-east Australia. III. Fertiliser nitrogen recovery, efficiency of fertilisation and soil nitrogen supply. Australian Journal of Agricultural Research 38, 129–138.
| Effects of time of urea application on combine-sown Calrose rice in south-east Australia. III. Fertiliser nitrogen recovery, efficiency of fertilisation and soil nitrogen supply.Crossref | GoogleScholarGoogle Scholar |
Isbell RF (1996) ‘The Australian Soil Classification.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biology and Biochemistry 32, 1485–1498.
| Review of mechanisms and quantification of priming effects.Crossref | GoogleScholarGoogle Scholar |
Norman RJ, Wilson CE, Slaton NA, Griggs BR, Bushong JT, Gbur EE (2009) Nitrogen fertilizer sources and timing before flooding dry-seeded, delayed-flood rice. Soil Science Society of America Journal 73, 2184–2190.
| Nitrogen fertilizer sources and timing before flooding dry-seeded, delayed-flood rice.Crossref | GoogleScholarGoogle Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Rejesus RM, Palis FG, Rodriguez DGP, Lampayan RM, Bouman BAM (2011) Impact of the alternate wetting and drying (AWD) water-saving irrigation technique: evidence from rice producers in the Philippines. Food Policy 36, 280–288.
| Impact of the alternate wetting and drying (AWD) water-saving irrigation technique: evidence from rice producers in the Philippines.Crossref | GoogleScholarGoogle Scholar |
Rose TJ, Erler DV, Farzana T, van Zwieten L (2016) Delayed permanent water rice production systems do not improve the recovery of 15N-urea compared to continuously flooded systems. European Journal of Agronomy 81, 46–51.
| Delayed permanent water rice production systems do not improve the recovery of 15N-urea compared to continuously flooded systems.Crossref | GoogleScholarGoogle Scholar |
Vlek PLG, Byrnes BH (1986) The efficacy and loss of fertilizer N in lowland rice. Fertilizer Research 9, 131–147.
| The efficacy and loss of fertilizer N in lowland rice.Crossref | GoogleScholarGoogle Scholar |
Westcott MP, Brandon DM, Lindau CW, Patrick HW (1986) Effects of seeding method and time of fertilization on urea-nitrogen-15 recovery in rice. Agronomy Journal 78, 474–478.
| Effects of seeding method and time of fertilization on urea-nitrogen-15 recovery in rice.Crossref | GoogleScholarGoogle Scholar |
Williams RL, Angus JF (1994) Deep floodwater protects high nitrogen rice crops from low temperature damage. Australian Journal of Experimental Agriculture 34, 927–932.
| Deep floodwater protects high nitrogen rice crops from low temperature damage.Crossref | GoogleScholarGoogle Scholar |



