Agronomic assessment of the durum Rht18 dwarfing gene in bread wheat
G. J. Rebetzke A * , A. R. Rattey B , W. D. Bovill A , R. A. Richards A , B. J. Brooks A and M. Ellis C
A * , A. R. Rattey B , W. D. Bovill A , R. A. Richards A , B. J. Brooks A and M. Ellis C
A CSIRO Agriculture and Food, PO Box 1700, Canberra, ACT 2601 Australia.
B Formerly CSIRO now Intergrain, 19 Ambitious Link, Bibra Lake WA 6162 Australia.
C Formerly CSIRO now 8 Avenue Piaton, Villeurbanne, France.
Crop & Pasture Science 73(4) 325-336 https://doi.org/10.1071/CP21645
Submitted: 29 August 2021 Accepted: 3 November 2021 Published: 14 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The wheat Green Revolution Rht-B1b and Rht-D1b dwarfing alleles are associated with increased grain yields but also with reduced early growth and seedling emergence, especially if sowing conditions are unfavourable. The gibberellic acid-responsive, mutagen-derived Rht18 dwarfing gene was backcrossed from durum wheat (Triticum turgidum subsp. durum L.) cv. Icaro into tall bread wheat (Triticum aestivum L.) cv. Halberd using phenotypic selection for reduced plant height. The Rht18 allele was confirmed among homozygous BC1F2-derived, F5:7 recombinant inbred lines by using a chromosome 6AS-linked, microsatellite molecular marker (Xwms4608), and then assessed for agronomic performance across multiple field sites ranging in yield from 3.6 to 6.4 t/ha. The Rht18-containing lines were significantly (P < 0.05) shorter in height (−24%) and reduced in plant lodging (−51%) compared with tall sister lines. Reductions in plant height were associated with significant increases in grain yield (+16%), reflecting increases in grain number (+21%), number of spikes (+7%) and number of grains per spike (+12%). Coleoptile length, early shoot biomass and ground cover percentage were unaffected by the presence of the Rht18 dwarfing gene. Comparisons of effects of gibberellic acid-insensitive Rht-B1b and Rht18 on early growth and agronomic performance were assessed separately for a set of 30 BC5F6-derived Halberd near-isogenic lines in the field in 2015. Ground cover and coleoptile length were significantly greater for Rht18 lines, whereas plant height, lodging, harvest index, grain number and yield were similar for Rht-B1b and Rht18 sister lines. Reduced lodging and increased grain number and yield, together with greater coleoptile length, indicate a potentially useful role for Rht18 in improving wheat performance.
Keywords: coleoptile, dwarf, early vigour, establishment, germplasm, harvest index, lodging, physiology.
Introduction
Dwarfing genes have been a major driver of improved adaptation and performance with breeding and domestication across all of the major cereals (Hedden 2003). In wheat, the identification and deployment of genes for semi-dwarf stature has promoted the commercial release and global adoption of wheat cultivars with greater yield potential and stability (Perry and D’Antuono 1989; Mathews et al. 2006). Selection for reduced crop height remains a key objective of wheat breeding programs worldwide owing to semi-dwarfs being less prone to lodging and producing greater numbers of grain to increase harvest index and crop yields (Hedden 2003).
Despite the availability of >20 dwarfing genes (McIntosh et al.1998; Ellis et al. 2004), the deployment of the Rht-B1b and Rht-D1b dwarfing alleles from the Japanese wheat cultivar Norin 10 has been remarkably widespread in the selection of reduced plant height globally. Indeed, ∼70% of the commercial wheat cultivars grown worldwide contain either Rht-B1b or Rht-D1b (Evans 1998) and both genes were pivotal in yield increases arising out of the Green Revolution (Hedden 2003). However, their value in improving performance in water-limited environments is less clear (e.g. Butler et al. 2005; Mathews et al. 2006). The Rht-B1b and Rht-D1b alleles are the most widely deployed of a group of gibberellic acid-insensitive (GAI) dwarfing genes that are unique in reducing cell-expansion in response to endogenous gibberellins (Hoogendoorn et al. 1990; Botwright et al. 2005). Reduced cell elongation contributes to reduction in cell length and width without affecting final cell number (Keyes et al.1989; Botwright et al. 2005). Reduction in cell size contributes to reductions in internode length, including the peduncle, to affect final plant height (Hoogendoorn et al. 1990). This reduction in cell size is ubiquitous in above-ground tissues and contributes to reductions in coleoptile and sub-crown internode lengths, coleoptile tiller frequency and size, and individual leaf area to reduce overall seedling vigour (Allan 1989; Botwright et al.2005; Rebetzke et al.2014).
Establishment is a key phase in the development of high-yielding cereal crops (Rebetzke et al. 2007). Optimal plant densities and early leaf area development rely on the seedlings’ ability to elongate and emerge with deep sowing, and are important objectives of breeding programs targeting adaptation to water-limited environments (Richards 1992). Improved establishment and early vigour are likely to be more important with climate change and predicted variability with changes in the ‘break’ for sowing and increasingly greater reliance of deep soil moisture from summer rains (Flohr et al. 2021). Greater seedling vigour has also been suggested as a trait for improving weed competitiveness (Coleman et al.2001; Zerner et al.2016) and nutrient uptake (Pang et al. 2014; Ryan et al.2015). The constraining influence of GAI alleles such as Rht-B1b and Rht-D1b on early growth has limited the capacity for breeders developing more-vigorous GAI wheats (Allan 1989; Rebetzke et al. 2007, 2014). Several major, gibberellic acid-responsive (GAR) dwarfing genes have been identified with potential to reduce plant height without affecting seedling vigour (Ellis et al. 2004; Rebetzke et al. 2011, 2012). Many of these genes are available in bread wheat and include Rht5 (Daoura et al. 2014), Rht8 (Rebetzke and Richards 2000), Rht12 (Chen et al. 2013), and Rht13 (Rebetzke et al. 2011). These genes have been reported to reduce plant height and increase grain yields through greater partitioning of carbon to growing ears, and thereby increase floret fertility to increase grain number (e.g. Rebetzke et al. 2012). Reduced height mutants in durum wheat have been reported to contain three GAR dwarfing genes: Rht14, Rht16 and Rht18 (Haque et al. 2011).
The Rht18 dwarfing gene was first deployed in a semi-dwarf durum cv. Icaro as a direct selection following fast-neutron mutagenesis of the tall wheat Anhinga (Konzak 1988). The Rht18 gene codes for the GA2oxA9 protein to lower bioactive gibberellic acid content to reduce stem elongation and plant height (Ford et al.2018). There are only two reports of the value of Rht18 in improving grain yield in wheat, and these represent performance in very small plots in single environments (Yang et al.2015; Tang 2016). The aims of the studies reported herein were to report the transfer of the Rht18 dwarfing allele from durum into bread wheat and then to assess the effect of this gene on plant height and agronomic performance in backcross-derived, Rht18, Rht-B1b and tall progeny evaluated in large plots across multiple field environments.
Materials and methods
Transfer of Rht18 into hexaploid wheat and development of recombinant inbred lines
Hybridisation was undertaken between the Rht18-containing, Italian durum wheat cv. Icaro (PI503555) and the tall, non-Rht18 Australian commercial bread wheat cv. Halberd (PI377885). The F1 pentaploid seed was harvested, sown and then backcrossed to Halberd to generate BC1F1 seeds, which were then self-pollinated to produce BC1F2 progeny. Only fertile wheat heads were retained because these were presumed to be fully fertile and therefore genetically hexaploid. This process was repeated for three generations to produce ∼120 BC1F2-derived, F5 recombinant inbred lines (RILs). Seeds from individual F4:5 plants were sown into rows and a single head was harvested from rows homogeneous for plant height. Harvested heads were threshed and sown into rows in the field for plant height assessment in subsequent generations. About 120 BC1F2-derived, F5:7 RILs varying for height were identified and bulk harvested. All lines were then sown into a summer nursery for further seed increase. Two inbred lines were discarded based on visual evidence for partial fertility in the summer nursery. For all sowings, the KCD NIL set, containing wheat height near-isogenic lines (NILs: semi-dwarf, Rht-B1b/D1b; doubled-dwarf, Rht-B1d/D1d; and tall, Rht-B1a/D1a), as described in Richards (1992) was sown to aid in height classification.
Genotyping
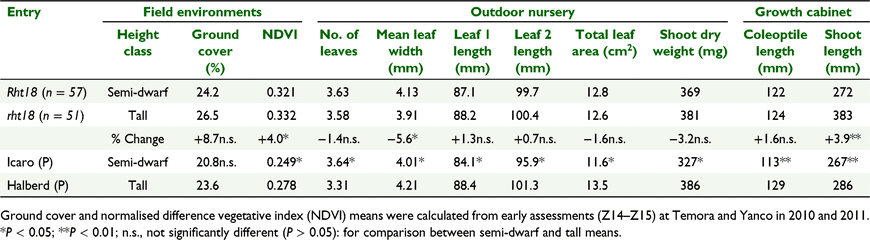
The simple sequence repeat (SSR) marker Xwms4608 tightly linked to Rht18 was used to genotype BC1F5:6 RILs (see Ford et al. 2018). In total, 51 RILs homozygous for a 220 bp allele of Xwms4608 (Halberd allele) averaged mature plant heights of 113 cm, compared with 57 RILs homozygous for the 239 bp Icaro allele averaging 89 cm (−27%) plant height when assessed in a favourable nursery environment.
Agronomic evaluation of all lines
Experiments were undertaken at Temora and Yanco, New South Wales, in 2010, and at Yanco in 2011, 2013, 2014 and 2015. In all experiments, BC1F5:7 RILs were sown at an optimal 3–5 cm sowing depth in 6-m-long, 0.17-m-spaced, 5- or 10-row plots at a seeding rate of ∼200 seeds/m2. Lines were replicated in a row–column experimental design containing partial (p = 1.5) or full (p = 2) replication. Nutrients were supplied at sowing as Starter 15 (14:12.7:11 N:P:S; Incitec Pivot, Melbourne, Vic., Australia) applied at 103 kg/ha and then as commercial urea topdressed at 80 kg/ha at early stem elongation. A pre-sowing irrigation followed by supplemental irrigations as required were supplied to maintain potential growth, and sowing was managed to be free of diseases and weeds with the application of appropriate fungicide and herbicide control measures.
The lines sown included the 108 BC1-derived Rht18 RILs and several control entries including: the original parents Icaro and Halberd; and a set of Rht F5-derived sib-based NILs varying for Rht-B1b and Rht-D1b alleles in CIMMYT-based genetic backgrounds of cvv. Aconchi, Galvez, Kauz, Nesser, Pavon and Seri (Singh et al. 2001).
For each plot, phenological development was recorded using the Zadoks development scale (Zadoks et al. 1974). Lodging was scored at multiple times throughout grain-filling in each plot (1 = perpendicular to 9 = prostate to the soil surface), and plant height was determined at physiological maturity as the distance from the soil surface to the top of the ear (awns excluded) at three random positions in each plot. At harvest maturity, ∼100 culms were hand-cut at ground level using a 40-cm-long quadrat oriented across four rows. Numbers of spikes were counted and samples air-dried at 35°C for 3 days, after which they were weighed and threshed, and grain was weighed. Harvest index calculated as the ratio of grain weight to total aboveground biomass. Plots were end-trimmed to ∼5.0 m length and the outside border rows removed before machine harvesting.
Grain size was determined as 100-grain weight from a random sample of grain from the harvest index cuts. Grain number (per area) and grain number per spike were subsequently calculated from grain size and plot yields. For two environments (Yanco 2013 and 2014), percentage shrivelled grain (‘screenings’), test weight and grain protein concentration were also determined. Nitrogen (N) yield was calculated from grain protein and grain yield, and assuming a grain protein-N correction factor of 5.7.
Early growth assessment
Three separate experiments were undertaken under field and controlled environment conditions for assessment of early vigour:
-
Ground cover was estimated on the BC1F2-derived RILs from digital images in three environments (Temora in 2010 and Yanco in 2010 and 2011) and encompassing one to three development stages (Z16, Z22 and Z23) representing early, mid and late vegetative growth. Digital images were converted to estimates of ground cover percentage using the vegetation-cover prediction software CanopyCover with parameters iterated before setting to minimise distortion from background soil (Li et al. 2010). Normalised difference vegetative index (NDVI) was measured using a GreenSeeker (Trimble, Sunnyvale, CA, USA) reflectance unit at the same time as ground cover images were taken.
-
Seed from the Yanco 2011 field harvest was sized and sown for early vigour determinations after Rebetzke and Richards (1999). Briefly, good-quality seeds free of any visible damage (particularly shrivelling) and weighing 40–45 mg were obtained for early vigour assessment of each NIL, RIL and parent (Icaro and Halberd). These were sown into wooden seedling trays (600 by 300 by 120 mm) containing a fertile, compost-based potting mix. The experiment was a row–column, partial replicate (p = 1.5) design constraining genotypes from being sown in the same position across replicates. After sowing, trays were placed in an outdoor nursery during the winter until ∼3.5-leaf stage, whereupon seedlings were harvested and assessed for numbers of main-stem leaves, and width and length of the first three main-stem leaves. Total leaf area was estimated using a planimeter and dry weights were determined after drying in a 70°C oven for 3 days.
-
The same seed from the Yanco 2011 field harvest was sized and sown for coleoptile/shoot length determinations after Rebetzke et al. (2007, 2014). As for Expt 2, good-quality seeds weighing 40–45 mg were obtained of each RIL, parents Icaro and Halberd, and the CIMMYT height NILs, and sown into wooden trays. Trays were then placed in a darkened growth cabinet with soil temperatures set at a constant 15°C. Seedlings were harvested at 200 degree-days and assessed for length of both coleoptile and the entire shoot (as the entire length from the seed to the tip of leaf 1).
Validation of Rht18 and Rht-B1b dwarfing gene NILs
A set of NILs varying for Rht-B1b and Rht18 was developed in the Halberd background for comparing the effects of the two genes in the field. The Rht18 NILs were developed from crossing the Rht18-containing line HI25M (a selection from the Halberd × 2/Icaro population described above) and genotyping F1 progeny with the SSR marker Xwms4608 (see above). This was repeated five times before single-seed descent to generate 15 BC5F6-derived Rht18-containing NILs. Halberd was also crossed to the Rht-B1b-containing cv. Lang and backcrossed five times with selection of Rht-B1b containing F1 progeny using the KASP (Kompetitive allele specific PCR) Rht-B1b marker (after Ellis et al. 2004). Fifteen BC5F6-derived NILs were then developed following single-seed descent. All 30 lines and the tall recurrent parent Halberd were grown under favourable conditions in the glasshouse, and mature seed was harvested before threshing and sowing into deep wooden seedling trays for assessment of coleoptile length (as above). These same 15 Rht-B1b and Rht18 NILs, and recurrent parent Halberd, were sown in a partial-replicated (p-rep = 1.5), row–column experimental design at the Yanco Managed Environment Facility (Rebetzke et al. 2013) in 2015 only. Plots were grown in rainfed and irrigated treatments, with diseases and nutrition managed to maximise crop growth. Sowing depth was confirmed at 4 cm (data not shown). Measurements were made at Z13 and Z14 of NDVI, and at maturity of plant height, number of spikes, grain yield, total biomass, harvest index (as grain yield/total biomass), grain size, grain number, grain protein concentration and N yield.
Statistical analyses
Data were analysed statistically after first checking for normality and error variance heterogeneity across environments. For lodging, the sampling date representing the largest lodging-score mean was used in statistical analysis. All data were analysed as measured except ground cover percentage, which was arcsine transformed prior to statistical analysis. Thereafter, a two-stage, mixed model approach was employed (Cullis et al. 1996) using the spatial models procedures in Genstat 14th Edition (VSN International, Hemel Hempstead, UK). Each environment was analysed separately with the best spatial models being determined after first fitting the experimental design and then modelling the residual variation with autoregressive row and column terms. Significant spatial effects were then identified, and residuals assessed before determinations were made as to the need for fitting of other (e.g. linear) effects (Cullis et al. 1996). The best linear unbiased estimates (BLUEs) for each environment were then used as inputs in the subsequent cross-environment analyses (after McIntosh 1983).
Analysis of the effect of each dwarfing gene on agronomic performance was assessed using: (a) a t-test for testing dwarf vs tall allele(s) in the CIMMYT-derived Rht-B1b and Rht-D1b semi-dwarfs with their corresponding CIMMYT tall NILs, and Halberd-derived Rht18 semi-dwarf RILs with their corresponding tall siblings; and (b) a Dunnett’s test for comparisons of the Halberd-derived dwarfing gene NILs with tall recurrent parent M808S and Halberd, respectively. Genetic correlations were estimated for plant height and selected traits after Holland (2006). Unless otherwise stated, statistical significance under all null hypotheses of no entry or dwarfing gene difference was at α = 0.05.
Results
Weather conditions
Experiments experienced good in-crop rainfalls ranging between 279 and 410 mm across years. Rainfall was particularly high in 2010 and 2013, exceeding long-term averages for the Yanco site (Fig. 1). Years 2011 and 2014 experienced good rainfall before or early in crop growth but were drier from flowering onward, in turn requiring up to 90 mm irrigation during grain-filling. Temperatures were reasonably consistent across years except for above-average temperatures during flowering and grain-filling in 2015 (Fig. 1).
|
|
Dwarfing gene contrasts
Halberd-derived Rht18 and tall RILs, and CIMMYT dwarfing NILs
Agronomic performance
Environments in each site × year combination were considered favourable, with grain yields exceeding an average 5 t/ha in all but one environment (Table 1). Average plant height varied significantly among environments, ranging from 90 to 108 cm, and with ranges in plant height across lines within environments of up to 80 cm (data not shown). Grain number and size were commonly large, consistent with the commonly wet and mild conditions experienced in the sampled environments. One site (Temora 2010) was sown to all lines but they were not harvested owing to prolonged rainfall during harvest damaging the mature grain. Notwithstanding, data for plant height and lodging score were collected (Table 1) and have been included in statistical analyses of these traits (Tables 1 and 2).

|
|
|
Genotypic variation was large and statistically significant for plant height measured across all entries (Table 2, Fig. 2). Genotype × environment interaction was small relative to among-genotype genetic variance (data not shown), contributing to high entry-mean repeatabilities for plant height across experiments. Accordingly, the average rank correlation for plant height across environments (rg) was high at 0.89. The range in genotype means for plant height across all environments was 78–133 cm (Fig. 2). Lines containing the 6AS molecular marker linked to Rht18 were significantly shorter (−24%) than their tall rht18 counterparts, and were commonly as short as, or shorter than, Rht-B1b- and Rht-D1b-containing CIMMYT NILs grown in the same experiments (Fig. 2, Table 2). The shortest Rht18-containing lines were significantly shorter than the Rht18 donor Icaro (cf. Fig. 2 and Table 2) (the two shortest Rht18 lines were 78 and 81 cm), whereas the tallest lines were significantly taller than the standard height parent Halberd (the two tallest lines were 131 and 132 cm in height). Reduction in plant height with Rht18 was associated with a significant reduction in (stem) lodging (Table 2), with the shortest Rht18-containing lines producing among the highest standability scores in the study (data not shown).
Lines containing the Rht18-linked marker had on average significantly greater grain yield across all environments than their tall siblings (Table 2). This increase in yield largely reflected significant increases in harvest index with little change in total biomass. Anthesis date was similar for semi-dwarf and tall lines (<1 day difference), and grain number was larger (+21%) in lines containing the Rht18 dwarfing allele (Table 2). Number of spikes and number of grains per spike were greater for lines containing the dwarfing Rht18 allele (+7% and 12%, respectively), and grain size was significantly smaller (−4%) for semi-dwarf lines (Table 2).
Compared with the tall parent (Halberd), the significantly reduced grain yields of the tall BC1 derivatives (Table 2) reflects their greater heights and reduced harvest index, and their reduced proportion of Halberd ‘adaptation’ alleles (∼75%). Further, unlike the substantial testing and selection involved in the development of the older but successful cultivar Halberd, these tall progeny lines were random samples from a large genetic population selected only for plant height and not for grain yield or any other agronomic attribute.
Presence of the Rht-B1b and Rht-D1b dwarfing alleles was associated with significant height reduction in the CIMMYT-based NILs (Table 2). Differences in height between Rht-B1b and Rht-D1b NILs were small and, in most cases, not statistically significant (data not shown). In the CIMMYT NILs, the GAI dwarfing alleles were associated, on average, with a 22% reduction in plant height. Grain yield was significantly greater for Rht-B1b- and Rht-D1b-containing NILs, with differences in grain yield being comparable with yield differences in the Halberd-derived Rht18 lines (Table 2). Greater yield was associated with significantly higher harvest index determined largely though increases in grain number, and particularly number of grains per spike. Total biomass was unchanged between semi-dwarf and tall CIMMYT NILs.
Physical grain quality
The Rht18 dwarfing gene was associated with a small but non-significant (P > 0.05) reduction in average grain protein concentration compared with tall, non-Rht18 RILs (Table 3). When coupled with the increased grain yield of Rht18-containing lines, N yield was significantly greater for semi-dwarf progeny. Grain protein concentration was significantly lower for the GAI, semi-dwarf CIMMYT NILs; however, their greater yield contributed to increased N yield compared with the CIMMYT tall NILs. Screenings percentage was significantly greater and test weight smaller for Rht18 than rht18 RILs (Table 3). Consistent with Rht18 RILs, test weight was significantly smaller for Rht-B1b and Rht-D1b CIMMYT-derived NILs. This small difference in screenings between classes of CIMMYT NILs may reflect the greater average grain weight of both the semi-dwarf and tall CIMMYT NILs (Table 2).
Coleoptile length
Repeatabilities were high for coleoptile (85 ± 7%) and shoot (78 ± 9%) lengths, respectively, for lines assessed in the controlled environment. The tall, long-coleoptile parent Halberd produced significantly (P < 0.01) longer coleoptiles and shoots than the Rht18 donor Icaro (Table 4), with Halberd’s greater length consistent with its longer coleoptile size in previous studies (e.g. Rebetzke et al. 2007) and with the size of Rht-B1a and Rht-D1a tall CIMMYT NILs in the same growth cabinet experiment (cf. Fig 3 and Table 4). In turn, the coleoptiles and shoots of Icaro were longer than those of the Rht-B1b and Rht-D1b CIMMYT NILs (cf. Table 4 and Fig. 3). The range in coleoptile and shoot lengths was similar for lines with and without the Rht18-linked marker (Fig. 3). The range was commonly large for both genotype classes with evidence for transgressive segregation of both shorter and longer shoots. Mean coleoptile and shoot lengths were similar and not statistically different for short Rht18 and tall genotypes (Table 4).
|
Early vigour
The influence of Rht18 on early growth was assessed in RILs phenotyped in both field and controlled nursery environments in Canberra. Parent variety Halberd produced significantly longer and wider seedling leaves than Icaro to increase total leaf area per plant at the 3.5-leaf stage (Table 4). This contrasted with their progeny, where Rht18 lines had greater mean leaf width than rht18 lines but were not statistically different for leaf length, plant leaf area or biomass. Numbers of leaves were similar for tall and Rht18-containing lines, but the latter produced significantly more tillers at sampling (1.92 and 1.78 tillers for Rht18 and rht18 lines, respectively) (data not shown).
Multiple field observations were made of ground cover percentage and NDVI representing different seasons and sites and sometimes multiple growth stages (Fig. 4). The genetic correlation (±s.e.) of ground cover and NDVI was 0.88 (±0.04) at Yanco and 0.85 (±0.04) at Temora at the 5.0-leaf stage, and the pooled genetic correlation across sites was 0.86 (±0.03). The strong correlations reflected the consistent performance of lines for both characteristics across sample dates and environments (Fig. 4). Indeed, genotype × environment and genotype × sample date interactions were small relative to the large genetic variance for ground cover and NDVI (data not shown).
Averaged across all environments, ground cover was not different between Rht18 and tall lines, whereas NDVI was significantly greater for tall genotypes, although the difference was small (0.321 and 0.332 for Rht18 and tall lines, respectively). Despite the differences between genotype classes, the overlap in individual genotype values was large for both ground cover and NDVI scores (Fig. 4), highlighting that more vigorous Rht18 lines could be readily identified. Indeed, the two best performing lines for ground cover and NDVI were reduced in height and contained the Rht18 gene (data not shown).
Halberd-derived Rht-B1b and Rht18 NILs
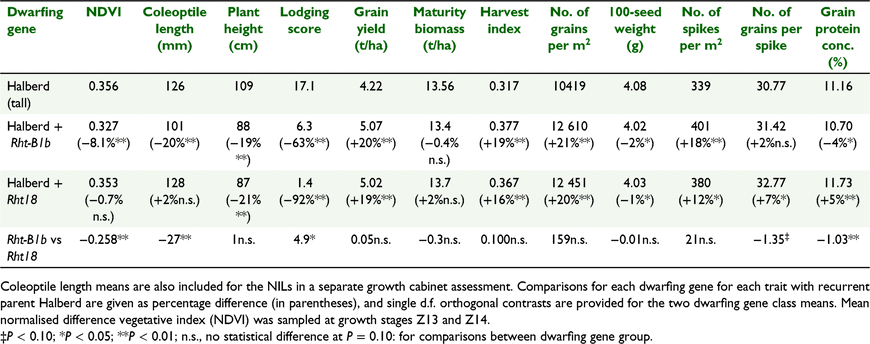
A high water-limited grain yield potential of 5.0 t/ha was observed for the field assessment of the BC5 Rht-B1b and Rht18 Halberd NILs at Yanco in 2015 (Table 5). Grain yields for rainfed and irrigated treatments were 3.6 and 5.9 t/ha, respectively. Significant differences were observed for plant height, with the Rht18 and Rht-B1b single-dwarfs reduced by 21% and 19% of the height of the tall recurrent parent Halberd, respectively, whereas the Rht-B1b and Rht18 NILs were themselves not statistically different. Crop lodging score was significantly higher for Halberd, and the Rht-B1b NILs had a significantly higher lodging score than the Rht18 NILs. Harvest index and grain yield of the two semi-dwarf sets of NILs were significantly greater than those of Halberd but were themselves not significantly different (Table 5). The greater grain yields of the semi-dwarf NILs reflected significantly larger grain number, but they were smaller in average grain size. Number of spikes and number of grains per spike were significantly greater for both semi-dwarf NILs than for Halberd. Number of spikes was the same for Rht-B1b and Rht18 NILs, whereas number of grains per spike was larger for the Rht18 NILs (P < 0.10). Maturity biomass was the same for the Rht-B1b and Rht18 NILs. Grain protein concentration was significantly greater for the Rht18 NILs, but smaller for the Rht-B1b NILs, than for Halberd (Table 5). Grain N yield was significantly greater for both Rht18 (102 kg/ha, +20%) and Rht-B1b (94 kg/ha, +11%) than for Halberd, and significantly greater for Rht18 (+9%) than for Rht-B1b NILs.
|
Number of plants per m2 was not significantly different for Rht18 (138 plants/m2) and Rht-B1b (132 plants/m2) NILs, or recurrent parent Halberd (151 plants/m2). Early ground cover was assessed as NDVI at crop development stages Z13–14 (Table 5), with Rht18 NILs being similar in NDVI to recurrent parent Halberd, whereas both Rht18 lines and Halberd were significantly more vigorous than the Rht-B1b NILs. Consistent with NDVI estimates, coleoptile lengths were similar for Rht18 and Halberd, and both were significantly longer than for Rht-B1b NILs.
Discussion
The Rht18 dwarfing gene had been deployed previously in commercial durum cv. Icaro where it performed well in producing higher grain yields and improved grain quality (Konzak 1988). In our own unpublished glasshouse and field studies, comparisons between Icaro (Rht18) and both cvv. Casteloporziano (Rht14) and Edmore M1 (Rht16) indicated that Icaro was more extreme in height reduction, consistent with the comparisons reported in Haque et al. (2011). Further, genetic studies at CSIRO have highlighted Rht18 as different from the majority of other dwarfing genes in that reduced plant height was inherited as a dominant trait (and thereby had dominant gene action) in F1 and subsequent segregating generations (G. J. Rebetzke, unpubl. data). In turn, physiological mechanisms contributing to reduction in plant height might be different from other height-reducing genes. Indeed, Ford et al. (2018) has reported the Rht18 gene as encoding the GA2oxA9 protein to lower bioactive gibberellic acid content. This differs from the Rht-B1b and Rht-D1b Green Revolution dwarfing genes, which are DELLA mutants rendering plant cells insensitive to endogenous gibberellins.
Large and repeatable genotypic differences were observed for plant height, largely reflecting variation due to Rht18. The large height range in the progeny encompassed variation in the parents (the Rht18-containing Icaro and the tall parent Halberd), with a number of progeny significantly exceeding either parent. The large range reflects combinations of both the Rht18 major dwarfing gene from Icaro and minor height-reducing alleles contributed from Halberd. The average 24% height reduction in the Rht18-containing lines was comparable to the height reductions of Rht-B1b and Rht-D1b NILs in the CIMMYT NILs (22% reduction) and in the direct height comparison with the Halberd-derived BC5 Rht-B1b NILs. The observed height reduction with Rht18 was consistent with results reported previously for both GAR (e.g. Loskutova 1998; Rebetzke et al. 2012; Daoura et al.2014) and GAI (e.g. Brandle and Knott 1986; Flintham et al. 1997; Rebetzke et al.2001; Ellis et al.2002) dwarfing genes.
Variation in plant height among Halberd-derived progeny was strongly genetically correlated with reductions in crop lodging in the RIL population (rg = 0.63, P < 0.01). Similarly, both the Rht18- and Rht-B1b-containing Halberd NILs scored significantly lower than Halberd for lodging, whereas the Rht18-containing NILs scored significantly lower than Rht-B1b-containing Halberd NILs. The reduced lodging with both Rht-B1b and Rht18 was consistent with the influence of height reduction on lodging reported for GAI dwarfing genes elsewhere (Allan 1989). However, the even greater reduction in lodging associated with Rht18 was quite remarkable given the similarity in plant height with both Rht-B1b and Rht18 NILs (Table 5). The increased grain yield associated with Rht18 largely reflected increases in harvest index, with little change in aerial biomass. In turn, increases in harvest index and thereby grain yield were largely due to increases in grain number (rg = 0.84, P < 0.01) through the production of significantly more spikes and greater numbers of grains per spike (i.e. fertility). Increases in grain number were partly compensated by a reduction in average grain size. Increases in harvest index and grain number for Rht18 were consistent with the Rht-B1b Halberd NILs, and consistent with reports elsewhere for Rht-B1b and Rht-D1b (Fischer and Stockman 1986; Richards 1992; Flintham et al. 1997; Butler et al. 2005) and the alternative GAR dwarfing genes (e.g. Rebetzke and Richards 2000; Rebetzke et al. 2012). In three winter biparental populations, Rht18 was associated with reductions in height of 12–25% depending on population (Yang et al. 2015). Grain yield and mature plant biomass (on a single-plant basis) were reduced in Rht18-containing progeny, whereas harvest index was increased relative to the tall parents. Similarly, the influence of Rht18 on numbers of spikelets and grains per spike and grain size also varied across populations. In turn, the results of both Tang (2015) and Yang et al. (2015) must be treated with caution owing to plot type and size (Rebetzke et al.2014).
The Rht18 dwarfing gene was associated with small but non-significant reductions in grain protein concentration and a significant increase in grain screenings and test weight compared with non-Rht18 talls in the RIL population. However, the Rht18-containing Halberd NILs produced significantly greater grain protein concentration than both the recurrent parent Halberd and the Rht-B1b-containing NILs in the 2015 field study. In all cases, the higher yields associated with Rht18 were associated with greater N yield. Reductions in grain protein concentration have been observed elsewhere for Rht-B1b and Rht-D1b (e.g. Brandle and Knott 1986; Flintham et al. 1997; McCartney et al. 2006).
The lines developed and reported herein served as key parents in genetic studies reported by Flohr et al. (2021), Ford et al. (2018) and Tang (2015), and have been used elsewhere in germplasm development for delivery to breeding programs. In the present study, the Rht18 dwarfing gene did not reduce seedling leaf area, biomass or coleoptile length compared with either the tall recurrent parent Halberd or tall RIL siblings, and Rht18 NILs had significantly greater coleoptile length than Rht-B1b NILs. Both ground cover and NDVI were marginally smaller throughout vegetative growth for Rht18 and tall Halberd BC1-derived RIL progeny, whereas BC5-derived Rht18 NILs had greater NDVI than Rht-B1b NILs. Vigorous growth and rapid leaf area development are important in achieving a good plant stand and large canopy early in the season. The shorter coleoptile and reduced NDVI of the Rht-B1b NILs is consistent with previous reports for GAI semi-dwarf wheats (e.g. Allan 1989; Rebetzke et al. 2007, 2014), and the neutral effect of Rht18 on coleoptile length and early biomass is consistent with previous observations for other GAR dwarfing genes (e.g. Ellis et al. 2004; Addisu et al. 2009; Rebetzke et al. 2012). In the work of Yang et al. (2015), coleoptile lengths of Rht18 dwarf sibs were, on average, similar to those of tall parents in two populations while being ∼20% shorter in a third population. The GAI Rht-B1b and Rht-D1b dwarfing genes decrease cell elongation to reduce leaf length and shoot biomass (Ellis et al. 2004; Botwright et al. 2005), whereas Rht8 and other GAR dwarfing genes do not affect cell elongation and seedling growth relative to tall, non-dwarfing-gene-containing NILs (Ellis et al. 2004; Rebetzke et al. 2012). Despite the reduction in leaf length, Rht18 was not associated with any reduction in leaf area in either the field or controlled environment studies, and maintenance of seedling leaf area and biomass was consistent across the two contrasting genetic backgrounds. In the study of Yang et al. (2015), coleoptile length was similar for Rht18 sibs and tall parents, whereas Flohr et al. (2021) reported that coleoptiles of Rht18 BC3 NILs were ∼30% longer and emerged significantly better than their Rht-B1b near-isogenic siblings.
In conclusion, the Rht18 dwarfing allele has been stably transferred from durum to bread wheat to reduce plant height and lodging and increase grain yield across multiple genetic backgrounds. Presence of Rht18 increases harvest index and grain number to increase grain yield, consistent with the established influence of the Green Revolution Rht-B1b dwarfing gene. This increase reflects increases in spike number and increased spike fertility to increase numbers of grains produced in each spike. The Rht18 gene appears to reduce seedling leaf length but this does not compromise leaf area owing to commensurate increases in leaf width and greater tillering early in development. The chromosomal location of the Rht18 gene on the A-genome of wheat and access to linked molecular markers should facilitate ready selection of this allele in bread and durum wheat populations targeting improved adaptation to favourable environments. Further studies are needed to confirm that benefits of this allele extend to improved performance in less favourable environments than those sampled in this study and to develop greater understanding of physiological mechanisms underpinning reduction in plant height by this genetically dominant allele.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive specific funding that requires acknowledgement.
Acknowledgements
We acknowledge the dedicated technical support of Bronwyn Matheson and the assistance of staff at the CSIRO Ginninderra Experiment Station, Canberra, ACT. We also thank Kathryn Bechaz and the NSWDPI Managed Environment Facility team at Yanco, NSW, and Dr Tony Fischer and anonymous reviewers for useful comments on the manuscript.
References
Addisu M, Snape JW, Simmonds JR, Gooding MJ (2009) Reduced height (Rht) and photoperiod insensitivity (Ppd) allele associations with establishment and early growth of wheat in contrasting production systems. Euphytica 166, 249–267.| Reduced height (Rht) and photoperiod insensitivity (Ppd) allele associations with establishment and early growth of wheat in contrasting production systems.Crossref | GoogleScholarGoogle Scholar |
Allan RE (1989) Agronomic comparisons between Rht1 and Rht2 semidwarf genes in winter wheat. Crop Science 29, 1103–1108.
| Agronomic comparisons between Rht1 and Rht2 semidwarf genes in winter wheat.Crossref | GoogleScholarGoogle Scholar |
Botwright TL, Rebetzke GJ, Condon AG, Richards RA (2005) Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.). Annals of Botany 95, 631–639.
| Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 15655105PubMed |
Brandle JE, Knott DR (1986) The effect of a gene for semi-dwarfism (Rht1) on various characters in a spring wheat cross. Canadian Journal of Plant Science 66, 529–533.
| The effect of a gene for semi-dwarfism (Rht1) on various characters in a spring wheat cross.Crossref | GoogleScholarGoogle Scholar |
Butler JD, Byrne PF, Mohammadi V, Chapman PL, Haley SD (2005) Agronomic performance of Rht alleles in a spring wheat population across a range of moisture levels. Crop Science 45, 939–947.
| Agronomic performance of Rht alleles in a spring wheat population across a range of moisture levels.Crossref | GoogleScholarGoogle Scholar |
Chen L, Phillips AL, Condon AG, Parry MAJ, Hu Y-G (2013) GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat. PloS ONE 8, e62285
| GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat.Crossref | GoogleScholarGoogle Scholar | 23658622PubMed |
Coleman RK, Gill GS, Rebetzke GJ (2001) Identification of quantitative trait loci (QTL) for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Australian Journal of Agricultural Research 52, 1235–1246.
Cullis BR, Thomson FM, Fisher JA, Gilmour AR, Thompson R (1996) The analysis of the NSW wheat variety database. II. Variance component estimation. Theoretical and Applied Genetics 92, 28–39.
| The analysis of the NSW wheat variety database. II. Variance component estimation.Crossref | GoogleScholarGoogle Scholar | 24166113PubMed |
Daoura BG, Chen L, Du Y, Hu Y-G (2014) Genetic effects of dwarfing gene Rht5 on agronomic traits in common wheat (Triticum aestivum L.) and QTL analysis on its linked traits. Field Crops Research 156, 22–29.
| Genetic effects of dwarfing gene Rht5 on agronomic traits in common wheat (Triticum aestivum L.) and QTL analysis on its linked traits.Crossref | GoogleScholarGoogle Scholar |
Ellis M, Spielmeyer W, Gale K, Rebetzke G, Richards R (2002) ‘Perfect‘ markers for the Rht-B1b and Rht-D1b dwarfing mutations in wheat (Triticum aestivum L.). Theoretical and Applied Genetics 105, 1038–1042.
| ‘Perfect‘ markers for the Rht-B1b and Rht-D1b dwarfing mutations in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Ellis MH, Rebetzke GJ, Chandler P, Bonnett D, Spielmeyer W, Richards RA (2004) The effect of different height reducing genes on the early growth of wheat. Functional Plant Biology 31, 583–589.
| The effect of different height reducing genes on the early growth of wheat.Crossref | GoogleScholarGoogle Scholar | 32688930PubMed |
Evans LT (1998) Feeding the ten billion: plants and population growth. Cambridge University Press.
Fischer RA, Stockman YM (1986) Increased kernel number in Norin 10-derived dwarf wheat: evaluation of the cause. Australian Journal of Plant Physiology 13, 767–784.
| Increased kernel number in Norin 10-derived dwarf wheat: evaluation of the cause.Crossref | GoogleScholarGoogle Scholar |
Flintham JE, Borner A, Worland AJ, Gale MD (1997) Optimizing wheat grain yield: effects of Rht (gibberellin-insenstive) dwarfing genes. The Journal of Agricultural Science (Camb.) 128, 11–25.
| Optimizing wheat grain yield: effects of Rht (gibberellin-insenstive) dwarfing genes.Crossref | GoogleScholarGoogle Scholar |
Flohr BM, Ouzman J, McBeath TM, Rebetzke GJ, Kirkegaard JA, Llewellyn RS (2021) Spatial analysis of the seasonal break and implications for crop establishment in southern Australia. Agricultural Systems 190, 103105
Ford BA, Foo E, Sharwood R, Karafiatova M, Vrána J, MacMillan C, Nichols DS, Steuernagel B, Uauy C, Doležel J, Chandler PM, Spielmeyer W (2018) Rht18 semidwarfism in wheat is due to increased GA 2-oxidaseA9 expression and reduced GA content. Plant Physiology 177, 168–180.
| Rht18 semidwarfism in wheat is due to increased GA 2-oxidaseA9 expression and reduced GA content.Crossref | GoogleScholarGoogle Scholar | 29545269PubMed |
Haque M, Martinek P, Watanabe N, Kuboyama T (2011) Genetic mapping of gibberellic acid-sensitive genes for semi-dwarfism in durum wheat. Cereal Research Communications 39, 171–178.
| Genetic mapping of gibberellic acid-sensitive genes for semi-dwarfism in durum wheat.Crossref | GoogleScholarGoogle Scholar |
Hedden P (2003) The genes of the green revolution. Trends in Genetics 19, 5–9.
| The genes of the green revolution.Crossref | GoogleScholarGoogle Scholar | 12493241PubMed |
Holland JB (2006) Estimating genotypic correlations and their standard errors using multivariate restricted maximum likelihood estimation with SAS Proc MIXED. Crop Science 46, 642–654.
| Estimating genotypic correlations and their standard errors using multivariate restricted maximum likelihood estimation with SAS Proc MIXED.Crossref | GoogleScholarGoogle Scholar |
Hoogendoorn J, Rickson JM, Gale MD (1990) Differences in leaf and stem anatomy related to plant height of tall and dwarf wheat (Triticum aestivum L.). Journal of Plant Physiology 136, 72–77.
| Differences in leaf and stem anatomy related to plant height of tall and dwarf wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Keyes GJ, Paolillo DJ, Sorrells ME (1989) The effects of dwarfing genes Rht1 and Rht2 on cellular dimensions and rate of leaf elongation in wheat. Annals of Botany 64, 683–690.
| The effects of dwarfing genes Rht1 and Rht2 on cellular dimensions and rate of leaf elongation in wheat.Crossref | GoogleScholarGoogle Scholar |
Konzak CF (1988) Genetic analysis, genetic improvement and evaluation of induced semi-dwarf mutants in wheat. In ‘Semi-dwarf cereal mutants and their use in cross-breeding III. Research coordination meeting, 16–20 December 1985’. pp. 76–94. (International Atomic Energy Agency: Vienna, Austria)
Li Y, Chen D, Walker CN, Angus JF (2010) Estimating the nitrogen status of crops using a digital camera. Field Crops Research 118, 221–227.
| Estimating the nitrogen status of crops using a digital camera.Crossref | GoogleScholarGoogle Scholar |
Loskutova NP (1998) The influence of Rht 1–5, Rht 8–9 and Rht 13 genes on morphological characters and yield productivity of wheat. In ‘Proceedings of the 9th International wheat genetics symposium’. (Ed. AE Slinkard) pp. 283–284. (University Extension Press, University of Saskatchewan: Saskatoon, SK, Canada)
Mathews KL, Chapman SC, Trethowan R, Singh RP, Crossa J, Pfeiffer W, van Ginkel M, DeLacy I (2006) Global adaptation of spring bread and durum wheat lines near-isogenic for major reduced height genes. Crop Science 46, 603–613.
| Global adaptation of spring bread and durum wheat lines near-isogenic for major reduced height genes.Crossref | GoogleScholarGoogle Scholar |
McCartney CA, Somers DJ, Lukow O, Ames N, Noll J, Cloutier S, Humphreys DG, McCallum BD (2006) QTL analysis of quality traits in the spring wheat cross RL4452 × ‘AC Domain’. Plant Breeding 125, 565–575.
| QTL analysis of quality traits in the spring wheat cross RL4452 × ‘AC Domain’.Crossref | GoogleScholarGoogle Scholar |
McIntosh MS (1983) Analysis of combined experiments. Agronomy Journal 75, 153–155.
| Analysis of combined experiments.Crossref | GoogleScholarGoogle Scholar |
McIntosh RA, Hart GE, Devos KM, Gale MD, Rogers WJ (1998) Catalogue of gene symbols for wheat. In: ‘Proceedings 9th International Wheat Genetics Symposium, Vol. 5’. Saskatoon: Canada. pp. 1–235
Pang J, Palta JA, Rebetzke GJ, Milroy SP (2014) Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth. Functional Plant Biology 41, 215–222.
| Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth.Crossref | GoogleScholarGoogle Scholar | 32480980PubMed |
Perry MW, D’Antuono MF (1989) Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982. Australian Journal of Agricultural Research 40, 457–472.
| Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Richards RA (1999) Genetic improvement of early vigour in wheat. Australian Journal of Agricultural Research 50, 291–301.
| Genetic improvement of early vigour in wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Richards RA (2000) Gibberellic acid-sensitive dwarfing genes reduce plant height to increase kernel number and grain yield of wheat. Australian Journal of Agricultural Research 51, 235–245.
| Gibberellic acid-sensitive dwarfing genes reduce plant height to increase kernel number and grain yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Appels R, Morrison A, Richards RA, McDonald G, Ellis MH, Spielmeyer W, Bonnett DG (2001) Quantitative trait loci on chromosome 4B for coleoptile length and early vigour in wheat (Triticum aestivum L.). Australian Journal of Agricultural Research 52, 1221–1234.
Rebetzke GJ, Ellis MH, Bonnett DG, Richards RA (2007) Molecular mapping of genes for coleoptile growth in bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics 114, 1173–1183.
| Molecular mapping of genes for coleoptile growth in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 17294164PubMed |
Rebetzke GJ, Ellis MH, Bonnett DG, Condon AG, Falk D, Richards RA (2011) The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crops Research 124, 323–331.
| The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Ellis MH, Bonnett DG, Mickelson B, Condon AG, Richards RA (2012) Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L.). Field Crops Research 126, 87–96.
| Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Chenu K, Biddulph B, Moeller C, Deery DM, Rattey AR, Bennett D, Barrett-Lennard EG, Mayer JE (2013) A multisite managed environment facility for targeted trait and germplasm phenotyping. Functional Plant Biology 40, 1–13.
| A multisite managed environment facility for targeted trait and germplasm phenotyping.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Verbyla AP, Verbyla KL, Morell MK, Cavanagh CR (2014) Use of a large multiparent wheat mapping population in genomic dissection of coleoptile and seedling growth. Plant Biotechnology Journal 12, 219–230.
| Use of a large multiparent wheat mapping population in genomic dissection of coleoptile and seedling growth.Crossref | GoogleScholarGoogle Scholar | 24151921PubMed |
Richards RA (1992) The effect of dwarfing genes in spring wheat in dry environments. I. Agronomic characteristics. Australian Journal of Agricultural Research 43, 517–527.
| The effect of dwarfing genes in spring wheat in dry environments. I. Agronomic characteristics.Crossref | GoogleScholarGoogle Scholar |
Ryan PR, Liao M, Delhaize E, Rebetzke GJ, Weligama K, Spielmeyer W, James R (2015) Early vigour and phosphate uptake in bread wheat. Journal of Experimental Botany 66, 7089–7100.
Singh RP, Huerta-Espino J, Rajaram S, Crossa J (2001) Grain yield and other traits of tall and dwarf isolines of modern bread and durum wheats. Euphytica 119, 241–244.
| Grain yield and other traits of tall and dwarf isolines of modern bread and durum wheats.Crossref | GoogleScholarGoogle Scholar |
Tang T (2016) Physiological and genetic studies of an alternative semi-dwarfing gene Rht18 in wheat. PhD Thesis, University of Tasmania, Hobart, Tas., Australia.
Yang Z, Zheng J, Liu C, Wang Y, Condon AG, Chen Y, Hu Y-G (2015) Effects of the GA-responsive dwarfing gene Rht18 from tetraploid wheat on agronomic traits of common wheat. Field Crops Research 183, 92–101.
| Effects of the GA-responsive dwarfing gene Rht18 from tetraploid wheat on agronomic traits of common wheat.Crossref | GoogleScholarGoogle Scholar |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |
Zerner RK, Gill GS, Rebetzke GJ (2016) Stability of wheat cultivars in weed competitive ability in differing environments in southern Australia. Crop and Pasture Science 67, 695–702.



