Evaluation by grafting technique of changes in the contribution of root-to-shoot development and biomass production in soybean (Glycine max) cultivars released from 1929 to 2006 in China
Xiaoning Cao A B C * , Tingting Wu A * , Shi Sun A * , Cunxiang Wu A , Caijie Wang A , Bingjun Jiang A , Jinlu Tao A , Weiwei Yao A , Wensheng Hou A , Wenyu Yang B E , Kadambot H. M. Siddique D and Tianfu Han A E
A * , Shi Sun A * , Cunxiang Wu A , Caijie Wang A , Bingjun Jiang A , Jinlu Tao A , Weiwei Yao A , Wensheng Hou A , Wenyu Yang B E , Kadambot H. M. Siddique D and Tianfu Han A E
A MARA Key Laboratory of Soybean Biology (Beijing), Institute of Crop Sciences, The Chinese Academy of Agricultural Sciences, Beijing, China 100081.
B College of Agronomy, Sichuan Agricultural University, Chengdu, Sichuan Province, China 611130.
C Institute of Crop Germplasm Resources, Shanxi Academy of Agricultural Sciences, Taiyuan, Shanxi Province, China 030031.
D The UWA Institute of Agriculture and School of Agriculture and Environment, The University of Western Australia, M082, LB 5005, Perth, WA 6001, Australia.
E Corresponding authors. Email: hantianfu@caas.cn; mssiyangwy@sicau.edu.cn
Crop and Pasture Science 70(7) 585-594 https://doi.org/10.1071/CP19052
Submitted: 21 June 2018 Accepted: 4 June 2019 Published: 26 July 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Root traits are essential for optimising nutrient and water absorption and anchorage. However, changes in root traits and the contribution of root-to-shoot growth and development of soybean (Glycine max (L.) Merr.) across a century of breeding are poorly documented. In this study, we adopted a grafting technique, using 55 cultivars released in the three main soybean-production regions in China as rootstocks in a pot experiment and 24 cultivars from the Yellow-Huai-Hai Valley (YHH) region as rootstocks in a field experiment, with cv. Zigongdongdou as the common scion. Changes in soybean roots, including dry weight (DW) of roots, lateral root number (LRN) and taproot length (TRL), and their contribution to shoot development and biomass formation, including shoot DW, plant height and node number, were evaluated under optimal conditions in 2011. Aboveground traits declined with year of release in the YHH region and did not vary over time in the northern Heilongjiang province and mid-south Heilongjiang region except for shoot DW. The root traits root DW, LRN and TRL were similar over years of release in the pot and field experiments. The results suggest that the newer cultivars have lesser shoot growth and root capacity but the same amount of root growth as older cultivars. Root traits did not change during selection, suggesting that improvement in soybean root traits should be an aim in future breeding.
Additional keywords: evolutionary change, root traits, shoot, soybean.
Introduction
Root traits are essential for optimising nutrient and water absorption, and for anchorage (Laplaze et al. 2007; Manavalan et al. 2010). The soybean (Glycine max (L.) Merr.) root system includes primary roots (taproot) and lateral branches (lateral roots). The taproot plays an important role in deep rooting ability (Kaspar et al. 1984); increased root length can delay drought stress and other abiotic stresses (Fenta et al. 2014). Root architecture determines the spatial distribution of roots in soil, including their shape and structure. Roots are quantified by measurements such as root length, lateral root numbers and root depth (Hodge et al. 2009).
Photosynthesis in the shoots and water and nutrient absorption in the roots are integrated processes for crop growth, such that the shoot and root systems interact during plant growth (Yan 2007). Differences in root traits between cultivars may result from differences in shoot capacity and vice versa. In a study by Liang et al. (2009), total root length, root surface area, root dry weight and root phosphorus (P) content of two contrasting soybean genotypes, cv. BD2 and cv. BX10, and their 106 F9-derived recombinant inbred lines (RILs) were closely associated with aboveground biomass and yield. Cardwell and Polson (1972) grafted a single cultivar to rootstocks of a diverse collection of genotypes and showed that the scion played an important role in determining seed weight, oil and protein content, and maturity whereas the root had a determinant role in lodging, height and seed yield.
Numerous methods have been developed for studying root systems, including phenotypic root scoring, hydraulic soil excavation, automatic root-volume location method, soil core sampling, root box method, volumetric method, and isotope tracer method (Gross 1995; Lindsey et al. 1995; Pantalone et al. 1996; Liao et al. 2001, 2004; Lontoc-Roy et al. 2005; Yan 2007; Pivetta et al. 2011). However, excavation of roots is labour-intensive and time-consuming, and finding a non-destructive and large-scale technique remains challenging for the assessment of root traits (Fenta et al. 2014). Measuring soil-water content by using buried sensors and probes can infer root activity, and has been adapted in many studies (Han et al. 2012; Shanahan et al. 2015; White et al. 2015; Whalley et al. 2017). Establishing a method for phenotyping the root system is a prerequisite for root research, and phenotyping root function rather than root morphology has a promising future. The intact soybean root system has been used in studies of different aspects of root gene function and nutrient uptake. Liao et al. (2006) applied a uniform hydroponic medium to the entire root system to study P and aluminium (Al) interactions in soybean. Intact soybean roots were also studied in the overexpression and knockdown of a homogeneous transformation system for gene (GmEXPB2) function in root growth and P uptake in response to inorganic P deficiency (Guo et al. 2011). In addition, the intact root system was used for studying kinetics of potassium (K) and magnesium (Mg) uptake (Joseph and Hai 1976a) and phosphate uptake (Joseph and Hai 1976b) in soybean, as well as for studying the degradation and utilisation of exogenous allantoin in a hydroponic growth system (Imsande 1986).
As the centre of origin for soybean, China is also a primary centre for modern soybean breeding. In the past century through to 2015, more than 2400 soybean cultivars were developed in China (Wang et al. 2016). During this time, yield, agronomic and phenological traits of soybean have markedly improved (Zheng et al. 2006; Tian et al. 2007; Zhao et al. 2008, 2012; Xu et al. 2011; Wang et al. 2015; Wu et al. 2015; Qin et al. 2017). Modern soybean varieties tend to have greater root weight, root volume, root surface and lateral root length than older varieties (Yang et al. 2001). The weight of root exudates has also increased with year of release (Sun et al. 2012). However, few studies have documented the improvements in root features that contribute to shoot growth and development, and biomass production.
In this study, we adopted a grafting system to examine the overall capacity of roots to contribute to shoot growth, using the same scion to exclude genetic differences in performance, for a range of soybean cultivars released from 1929 to 2006 in China. The objectives of this study were: (i) to establish a method for studying the intact root system by using the same scion in a grafting technique; and (ii) to evaluate changes in soybean root traits and their contribution to shoot development and biomass formation during the last century of breeding in China. Our results will provide theoretical and technical support for the improvement of soybean varieties.
Materials and methods
Plant materials
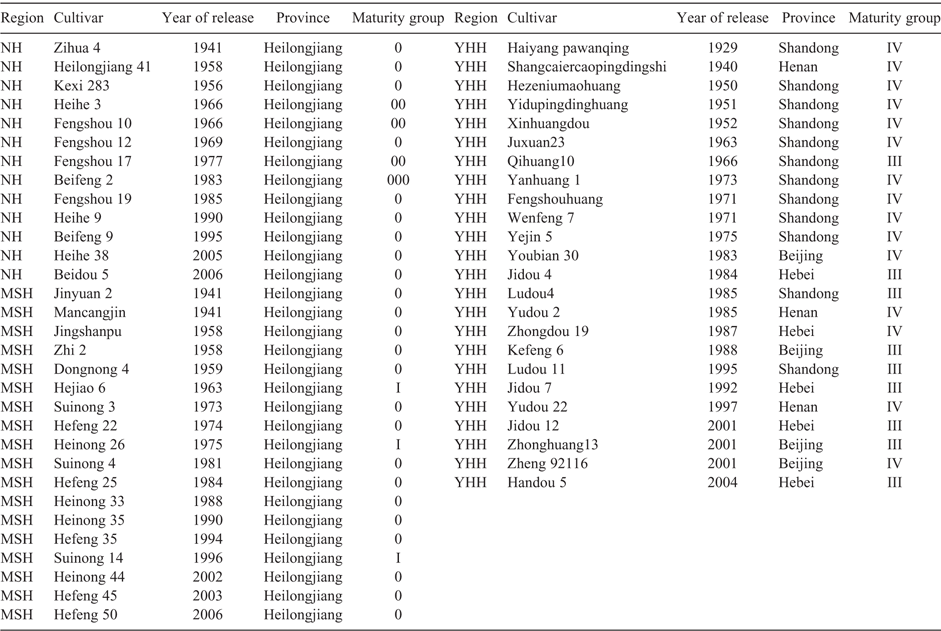
Fifty-five widely grown soybean cultivars released from 1929 to 2006 in China were used as rootstocks (Table 1). These cultivars originated from three major soybean-producing regions in China: northern Heilongjiang province (NH) (13 cultivars), mid-south Heilongjiang region (MSH) (18 cultivars), and Yellow-Huai-Hai Valley region (YHH) (24 cultivars) (Wang et al. 2013, 2015, 2016). A late-maturing cultivar, Zigongdongdou (ZGDD), from South China (maturity group (MG) X) (Wu et al. 2006; Jia et al. 2011), was used as the common scion (Supplementary Materials fig. S1, available at the journal’s website). ZGDD could retain vegetative growth throughout the experiment, and could therefore uniformly reflect biomass production without the influence of energy consumption of the reproductive period, as well as avoiding interference from aboveground differences, thereby accurately reflecting the function of root system.
Cut-in grafting method
The cut-in grafting method has been documented previously (Pantalone et al. 1999; Cao et al. 2013). After the cotyledons of the rootstock had expanded, the apical meristem of the seedlings was removed, and a bamboo toothpick was inserted into the hypocotyl of the young stem to 2 cm to make a hole (Supplementary Materials fig. S2a–c). A section of scion cultivar stem 1.5–2.0 cm long with a wedge-shaped end (Supplementary Materials fig. S2d–e) was inserted into the top centre of the rootstock. The grafting joint zone was wrapped with laboratory film (Parafilm; Beamis, Neenah, WI, USA) (Supplementary Materials fig. S2f), and the grafted seedlings were covered with plastic bags to recover for 3–4 days. The mean survival rate of cut-in grafts was >90% (Supplementary Materials fig. S3a, b) (Cao et al. 2013). After recovery, the grafted seedlings were transplanted into larger pots or the field.
Experimental design
Pot experiment
A pot experiment was performed at the Chinese Academy of Agricultural Sciences (CAAS) campus and followed a randomised complete block design with three replications. Five seeds for the scion and rootstock plants were sown in pots (9 cm top diameter, 9 cm height) filled with vermiculite on 5 May and 9 May 2011, respectively. After the grafting process and recovery, five grafted plants with uniform height and establishment from each pot were transplanted to larger pots (21 cm top diameter, 18 cm height) (Supplementary Materials fig. S3). The soil volume was uniform from pot to pot. The plants were managed routinely to prevent water stress and pest attack. The experiment was concluded on 18 August 2011, when the scion was still in vegetative growth. At that time, the aboveground and belowground parts of the grafting union were cut and separated.
Field experiment
The field experiment was carried out at the Changping Experimental Station of the Institute of Crop Sciences, CAAS, in a completely randomised design with three replications. Twenty-four cultivars from the YHH region were used as rootstocks (Table 1). The soil type in Changping is fluvo-aquic (Avery 1973). Eight seeds of the scion cultivar and five seeds of each rootstock plant were sown in each pot (replication) on 22 May and 26 May 2011, respectively. After the grafting process and recovery, uniform plants were transplanted to the field on 18 June 2011, with a row spacing of 0.9 m and hill spacing of 0.9 m, with five plants in each hill (Supplementary Materials fig. S3d). Plants were harvested on 19 September 2011.
Data collection and measurements
At the end of each experiment, all plants in each pot in the pot experiment or in the field experiment were harvested for measurement of plant height (PH), node number (NN) and shoot dry weight (SDW). PH referred to the height of the grafted part from the cotyledonary node to the terminal growing point, and NN was the number of nodes between the grafting cotyledon node and the top of the terminal growing point. All aboveground parts were collected, and a subset of samples was taken for measuring SDW (biomass) after oven drying at 80°C for 48 h to a constant weight. In the pot experiment, roots were soaked for 2 h in a basin, and soil and impurities were carefully removed from roots by rinsing with clean water. In the field experiment, the roots of five plants in each hill were excavated in a soil block 90 cm in diameter and 45 cm deep for washing and drying. Taproot length (TRL) was measured from the cotyledonary node of stock to the end of the main root tip; lateral root number (LRN) was determined by counting the branched roots emerging from tap and ≥3 cm in length; and root dry weight (RDW) was determined after oven drying at 80°C for 48 h (Pantalone et al. 1996; Fenta et al. 2014).
Data analyses
All measured traits were analysed by analysis of variance (ANOVA), using the GLM model. In the pot experiment, region and cultivar nested within each region were considered fixed factors, and replication a random factor. In the field experiment, cultivar was considered a fixed factor and replication a random factor. A linear regression analysis of trait means with their respective year of stock cultivar release was performed within each region from the pot and field experiments. Pearson’s correlation coefficients were estimated for the aboveground and belowground traits. Data were analysed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Changes in aboveground traits of the scion with year of release of rootstock cultivars from different regions
The ANOVA showed significant differences among the rootstock cultivars for aboveground and below-ground traits in both the pot and field experiments (Supplementary Materials table S1). From the histograms in Fig. 1, node number and plant height showed in a right-skewed distribution, whereas other traits showed approximately normal distribution with a slightly left skew; the self-grafted control ranked in the upper half of each trait. To investigate the effect of rootstock cultivars on aboveground biomass accumulation and genetic improvement over time, the SDW of the common scion ZGDD was regressed against the release year of stock cultivars. In the pot experiment, SDW declined significantly with release year of stock cultivars from the NH region (R2 = 0.41*), with an annual decrease of 0.14 g plant–1 (Fig. 2a). From this region, SDW ranged from 9.67 g (cv. Fengshou19, released 1985) to 25.45 g (cv. Zihua 4, 1941). From the MSH and YHH regions, SDW did not vary over time. In the field experiment, SDW declined with year of release of cultivars from the YHH region (R2 = 0.18*), with an annual decrease of 0.35 g plant–1 (Fig. 2b). From this region, SDW ranged from 29.09 g (cv. Ludou 4, 1985) to 89.32 g (cv. Juxuan 23, 1963).
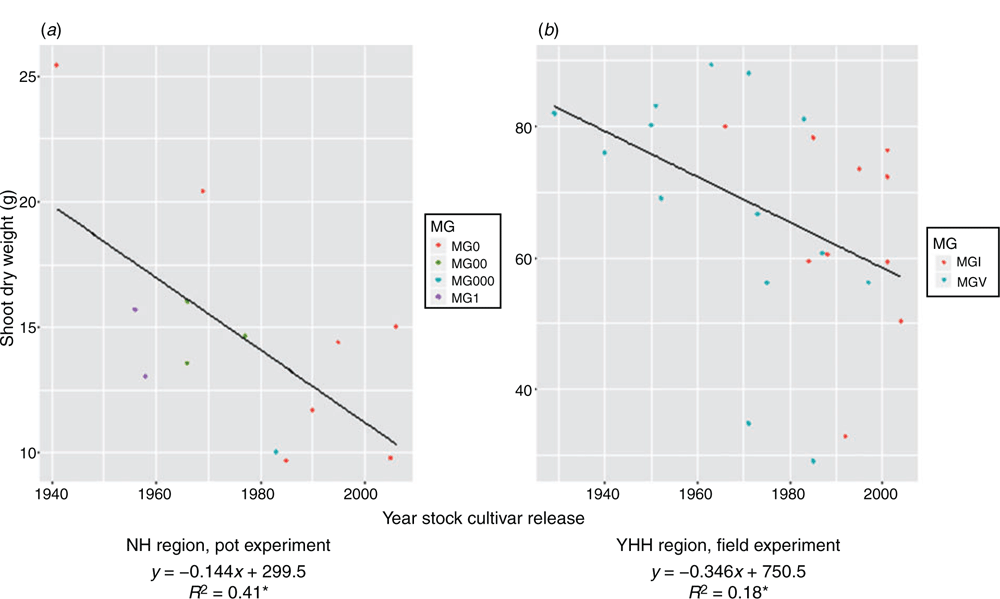
In the pot experiment, PH ranged from 52.13 cm (cv. Beifeng 2, released 1983) to 88.80 cm (cv. Fengshou 19, 1985) from the NH region; 18.00 cm (cv. Heinong 44, 2002) to 85.75 cm (cv. Hejiao 6, 1963) from the MSH region; and 61.37 cm (cv. Zhongdou 19, 1987) to 97.83 cm (cv. Wenfeng 7, 1971) from the YHH region. However, the trends with year of release were not significant for any region. In the field, PH ranged from 57.25 cm (cv. Jidou7, 1992) to 93.63 cm (cv. Hezeniumaohuang, 1950) from the YHH region and decreased with year of release of rootstock cultivar (R2 = 0.44**), with an annual decrease of 0.32 cm (Fig. 3), indicating that older cultivars were taller than newer ones.
|
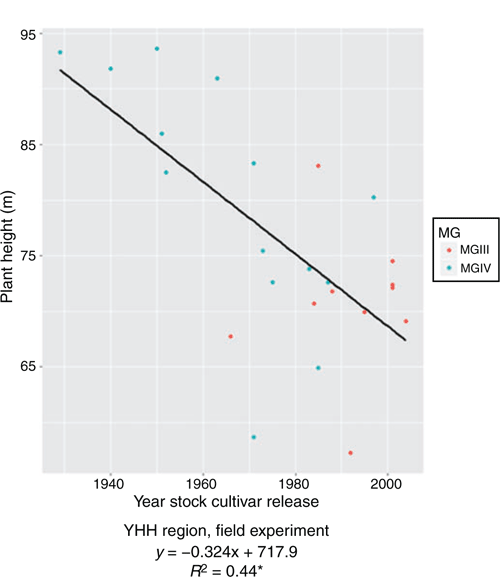
In the pot experiment, NN ranged from 9.25 (cv. Beifeng 2, released 1983) to 19.13 (cv. Fengshou 10, 1966) from the NH region; 5.00 (cv. Heinong 44, 2002) to 22.50 (cv. Hejiao 6, 1963, and cv. Hefeng 45, 2002) from the MSH region; and 15.80 (cv. Shangcaiercaopingdingshi, 1940) to 20.65 (cv. Kefeng 6, 1988) from the YHH region. However, the trends with year of release were not significant in any region. In the field experiment, NN ranged from 14.4 (cv. Fengshouhuang, 1971) to 21.8 (cv. Haiyangpawanqing, 1929) from the YHH region, and decreased significantly with year of release (R2 = 0.23*), with an annual decrease of 0.04 (Fig. 4), suggesting that older cultivars have more nodes than newer ones.
|
Changes in root traits of rootstock cultivars with year of release
Root traits were regressed against the release year of the rootstock cultivar. The traits TRL, LRN and RDW did not change significantly with year of release in the pot or field experiment, indicating that these root traits have not been used as selection criteria in soybean breeding in China. This result emphasises the need to continue to improve the soybean root system.
The rootstock soybean cultivars, encompassing 55 widely grown soybean cultivars from three regions in China, had normal root growth at harvest (Fig. 5). Interestingly, root longevity did not differ when using cultivars ranging from MG 000 to MG IV as rootstock. For instance, Beifeng 2 (MG 000), an extremely early-maturing and spring-planting cultivar survived and grew for as long as Yuejin 5 (MG IV), a summer-planting cultivar from the YHH region (Fig. 5a, f).
Correlation between aboveground and belowground traits
The correlations between shoot and root traits in both the pot and field experiments were positive for all traits (Table 2). In the pot experiment, SDW had significant positive correlations with TRL (0.45, P < 0.01), LRN (0.48, P < 0.01) and RDW (0.75, P < 0.01), suggesting that greater dry weights of shoots are associated with longer taproot, more lateral roots and greater dry weights of roots. RDW was positively correlated with NN (0.37, P < 0.01), TRL (0.48, P < 0.01), LRN (0.53, P < 0.01), indicating that greater dry weights of roots are associated with more nodes, longer taproot and more lateral roots.

|
The field experiment produced associations similar to, but less significant than, the pot experiment. SDW had significant positive relationships with TRL (0.50, P < 0.05) and RDW (0.53, P < 0.01), and TRL was positively correlated with NN (Table 2). Our results indicate that root growth is highly associated with shoot growth.
In both the pot and field experiments, SDW was positively and highly correlated with RDW. Therefore, breeding programs can concurrently select RDW and SDW, and RDW can be evaluated by measuring SDW without destroying the root system.
Discussion
Advantages of grafting technique for studying crop root function
The grafting technique is a satisfactory means for assessing the effects of scion and rootstock factors on several physiological aspects of plant growth and development (Lawn et al. 1974; Lawn and William 1974; Lawn and Bushby 1982; Abd-alla et al. 1998). For soybean, grafting technology has been used to assess photoperiod responses (Borthwick and Parker 1938; Przepiorkowski and Martin 2003) and interactions between shoots and roots (Balatti and Pueppke 1990; Ookawa et al. 2001). Grafting techniques have shown that shoots and roots play different roles in the nodulation process (Kleese and Smith 1970; Delves et al. 1986). Grafting has also been used to study the influence of rootstocks on the scion and the scion on rootstocks in inducing flowering in soybean (Hamner 1969).
In the present study, the seedling cut-in grafting technique was used as a platform for different rootstocks with the same scion to exclude interference caused by different shoots on the root system. By grafting, we can directly observe the overall function of roots and evaluate the contribution of the root to the shoot as an entity. Grafting provides a non-destructive and highly efficient approach for large-scale phenotyping of the soybean root system and has advantages for evaluating root features and capacities.
Evolutionary changes in the supporting capacity of the root system for aboveground parts in widely grown soybean cultivars
In the present study, scion SDW—a good indicator of the comprehensive capability of a root system—was negatively correlated with the year of release of rootstock cultivars from the NH region in the pot experiment and the YHH region in the field experiment. Less dependence of shoot biomass on the root system in these cultivars may explain the reduced contribution of roots.
According to our results, plant height and node number of the scion cultivar (cv. Zigongdongdou) decreased when grafted to newer cultivars than to older cultivars of soybean. Communication between rootstock and scion is bidirectional. The rootstock affects scion growth through transmissible substances (water, nutrient and hormone) and the effect of scion on rootstock is dependent on supply of photosynthates and signals (Albacete et al. 2015).
Evolutionary trends of root traits in widely grown soybean cultivars
From all three regions, TRL, LRN and RDW of soybean rootstocks did not change significantly with year of cultivar release. For the NH and MSH regions, in the northern part of North East China, this finding could be attributed to the narrow-row planting technique and machinery widely used in North East China, particularly in the NH region (Hu 2008; Hang et al. 2009a, 2009b). Consequently, the cultivars are characterised by narrow leaves, few branches and small seeds, as well as small root volume and RDW, and yield increase is mainly attributed to high plant density (Yang 1982; Du and Lyu 2011). The governmental policy of a family-contract responsibility system was applied in the early 1980s, which enhanced the level of field management (increase in plant density and fertiliser application) (Wang 1993). We suggest that the increased fertiliser application provides adequate nutrition to topsoil roots and attenuates the absorbing capacity and root depth of modern soybean cultivars (Zheng et al. 2015); thus, no large root system for new soybean cultivars.
In the YHH region, innovations in field management techniques and increased fertiliser application provide adequate nutrition to topsoil roots and attenuate the absorbing capacity and root depth of modern soybean cultivars. Because seed yield is the primary target in soybean breeding, more upright and shorter plants with higher yields over time were observed in our previous study (Wang et al. 2016). Genetic selection of cultivars with smaller shoot and root systems may align with modern breeding objectives of high-plant-density cultivation. For instance, Jidou 7, released in the 1990s in Hebei province (YHH region), has a compact plant architecture (PH 57.25 cm, NN 15.0, SDW 32.93 g) and small root system (TRL 10.83 cm, LRN 15.0, RDW 4.00 g in the field experiment). By contrast, Haiyangpawanqing, an obsolete cultivar developed in 1929 in Shandong province (YHH region), has an extensive plant architecture (PH 93.63 cm, NN 21.8, SDW 81.97 g) and large root system (TRL 14.90 cm, LRN 19.8, RDW 4.91 g in the field experiment). In the present study, lower shoot biomass and relatively smaller root systems in the newer cultivars as rootstocks than the older cultivars is indicative of attenuated root functionality. Enhanced field-management techniques (increased plant density and fertiliser application) since the 1980s in China have dramatically improved topsoil nutrition, which may have led to a shallower rooting system in the modern cultivars (Wang 1993). Previous studies in rice (Oryza sativa L.) showed that redundant root growth may utilise excess energy source and thus cause yield loss (Wang et al. 2004). Appropriate pruning or cutting is effective to inhibit redundant root growth and reduce unnecessary consumption of carbohydrates in wheat (Triticum aestivum L.) and soybean (Shi et al. 1999, 2000; Ma et al. 2008). In the present study, root longevity did not differ among rootstocks of different maturity groups, suggesting that the scion plays a key role in the determination of root longevity. A similar result was found in the study of Li et al. (2017) with 11 scion cultivars of different maturity groups released in different years and two rootstock cultivars, in which agronomic traits such as pod numbers, seed numbers, 100-seed mass and yield were correlated with release years of scion cultivars.
Suggestions for root-trait improvement in soybean breeding
Our previous studies on changing trends in agronomic traits in these widely grown soybean cultivars indicated that, in North East China, newer cultivars had earlier flowering, shorter plants with fewer branches, higher lodging resistance and higher yields per plant than older cultivars, and in the YHH region, modern cultivars had shorter plants, fewer nodes, higher lodging resistance, more seeds per pod and heavier seeds than older cultivars (Wu et al. 2015; Wang et al. 2016). These results may shed light on strategies for soybean root improvement in different production regions. Because root longevity is mainly controlled by the shoots, the growing environments for the shoot and root systems need to improve through cultivation and farming practices to prevent premature senescence of roots and improve yield. Improvement of soybean root features, using conventional breeding and marker-assisted selection, has the potential to improve yield significantly. This study demonstrated the genetic progress in root traits and root contributions to shoots during a century-long breeding process. It also offers a methodology for studying intact root systems by observing aboveground growth, which will facilitate breeding for improved soybean root traits to boost soybean production.
Conflicts of interest
The authors declare that no competing or conflicts of interest exist.
Acknowledgements
This work was funded by the grants from the National Key R&D Program of China (2017YFD0101400) to TH and the China Agriculture Research System (CARS-04) to TH and WY.
References
Abd-alla MH, Vuong T, Harper J (1998) Genotypic differences in nitrogen fixation response to NaCl stress in intact and grafted soybean. Crop Science 38, 72–77.| Genotypic differences in nitrogen fixation response to NaCl stress in intact and grafted soybean.Crossref | GoogleScholarGoogle Scholar |
Albacete A, Martínez-Andújar C, Martínez-Pérez A, Thompson A, Dodd I, Pérez-Alfocea F (2015) Unravelling rootstock × scion interactions to improve food security. Journal of Experimental Botany 66, 2211–2226.
| Unravelling rootstock × scion interactions to improve food security.Crossref | GoogleScholarGoogle Scholar | 25754404PubMed |
Avery BW (1973) Soil classification in the soil survey of England and Wales. European Journal of Soil Science 24, 324–338.
Balatti PA, Pueppke S (1990) Cultivar-specific interactions of soybean with Rhizobium fredii are regulated by the genotype of the root. Plant Physiology 94, 1907–1909.
| Cultivar-specific interactions of soybean with Rhizobium fredii are regulated by the genotype of the root.Crossref | GoogleScholarGoogle Scholar | 16667934PubMed |
Borthwick HA, Parker M (1938) Photoperiodic perception in Biloxi soybeans. Botanical Gazette 100, 374–387.
| Photoperiodic perception in Biloxi soybeans.Crossref | GoogleScholarGoogle Scholar |
Cao X, Sun S, Wu C, Han T, Yang W (2013) Grafting techniques for root–shoot relationship study in soybean. Chinese Journal of Oil Crop Sciences 35, 579–583. [in Chinese]
Cardwell VB, Polson DE (1972) Response or ‘Chippewa 64’ soybean scions to roots of different genotypes. Crop Science 12, 217–219.
| Response or ‘Chippewa 64’ soybean scions to roots of different genotypes.Crossref | GoogleScholarGoogle Scholar |
Delves AC, Mathews A, Day D, Carter A, Carroll B, Gresshoff P (1986) Regulation of the soybean–Rhizobium nodule symbiosis by shoot and root factors. Plant Physiology 82, 588–590.
| Regulation of the soybean–Rhizobium nodule symbiosis by shoot and root factors.Crossref | GoogleScholarGoogle Scholar | 16665072PubMed |
Du J, Lyu J (2011) Effect of fertilizing amount and planting density on soybean root characteristics in plough layer. Journal of Heilongjiang Bayi Agricultural University 23, 1–6. [in Chinese]
Fenta BA, Beebe SE, Kunert KJ, Burridge JD, Barlow KM, Lynch JP, Foyer CH (2014) Field phenotyping of soybean roots for drought stress tolerance Agronomy 4, 418–435.
| Field phenotyping of soybean roots for drought stress toleranceCrossref | GoogleScholarGoogle Scholar |
Gross R (1995) Construction application of hydraulic soil excavation. In ‘Trees and building sites. Proceedings International Society of Arboriculture’. Savoy, IL. (Eds G Watson, D Weeley) pp. 177–184. (International Society of Arboriculture: Atlanta, GA, USA)
Guo W, Zhao J, Li X, Qin L, Yan X, Liao H (2011) A soybean β‐expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. The Plant Journal 66, 541–552.
| A soybean β‐expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses.Crossref | GoogleScholarGoogle Scholar | 21261763PubMed |
Hamner KC (1969) Glycine max (L.) Merrill. In ‘The induction of flowering: some case histories’. (Ed. LT Evans) pp. 62–89. (Cornell University Press: Ithaca, NY, USA)
Han T, Cao X, Sun S, Wu C, Jia Z, Hou W, Jiang B (2012) A method for evaluating root traits and the capacity of yield contribution of root to shoot. China. Patent No. ZL 201210089067.8. 2012-03-29.
Hang J, Song Y, Shen X, Liu Z, Wang Q, Jia H, Liu F, Zhao G (2009a) Soybean yield structure under the super-high yield cultivation mode with narrow row and dense seeded semi-dwarf cultivar. Soybean Science 28, 636–640. [in Chinese]
Hang J, Zheng D, Shen X, Wang Q, Liu Z, Jia H, Liu F, Du J, Zhao G, Song Y (2009b) Soybean population growth dynamic under the super-high yield cultivation mode with narrow row and dense seeded semi-dwarf cultivar. Soybean Science 28, 842–845. [in Chinese]
Hodge A, Berta G, Doussan C, Crespi M (2009) Plant root growth, architecture and function. Plant and Soil 321, 153–187.
| Plant root growth, architecture and function.Crossref | GoogleScholarGoogle Scholar |
Hu X (2008) Cultivation technique system of high-yield and superior quality soybeanhigh-yield and superior quality narrow-compact planting technique system for Hefeng No. 42. Heilongjiang Agricultural Sciences 2008, 45–47. [in Chinese]
Imsande J (1986) Degradation and utilization of exogenous allantoin by intact soybean root Physiologia Plantarum 68, 685–688.
| Degradation and utilization of exogenous allantoin by intact soybean rootCrossref | GoogleScholarGoogle Scholar |
Jia Z, Wu C, Wang M, Sun H, Hou W, Jiang B, Han T (2011) Effects of leaf number of stock or scion in graft union on scion growth and development of soybean. Acta Agronomica Sinica 37, 650–660.
| Effects of leaf number of stock or scion in graft union on scion growth and development of soybean.Crossref | GoogleScholarGoogle Scholar | [in Chinese]
Joseph RA, Hai TV (1976a) Kinetics of potassium and magnesium uptake by intact soybean roots. Physiologia Plantarum 36, 233–235.
| Kinetics of potassium and magnesium uptake by intact soybean roots.Crossref | GoogleScholarGoogle Scholar |
Joseph RA, Hai TV (1976b) Uptake of phosphate by intact soybean roots: mediated by a single multiphasic mechanism. Zeitschrift für Pflanzenphysiologie 78, 222–227.
| Uptake of phosphate by intact soybean roots: mediated by a single multiphasic mechanism.Crossref | GoogleScholarGoogle Scholar |
Kaspar TC, Taylor H, Shibles R (1984) Taproot-elongation rates of soybean cultivars in the glasshouse and their relation to field rooting depth. Crop Science 24, 916–920.
| Taproot-elongation rates of soybean cultivars in the glasshouse and their relation to field rooting depth.Crossref | GoogleScholarGoogle Scholar |
Kleese RA, Smith L (1970) Scion control of genotypic differences in mineral salts accumulation in soybean (Glycine max L. Merr.) seed. Annual Botany 34, 183–188.
| Scion control of genotypic differences in mineral salts accumulation in soybean (Glycine max L. Merr.) seed.Crossref | GoogleScholarGoogle Scholar |
Laplaze L, Benkova E, Casimiro I, Maes L, Steffen V, Swaru R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, Offringa R, Graham N, Doumas P, Friml J, Bogusz D, Beeckman T, Bennett M (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell 19, 3889–3900.
| Cytokinins act directly on lateral root founder cells to inhibit root initiation.Crossref | GoogleScholarGoogle Scholar | 18065686PubMed |
Lawn RJ, Bushby HVA (1982) Effect of root, shoot and Rhizobium strain on nitrogen fixation in four Asiatic Vigna species. Crop Science 92, 425–434.
Lawn RJ, William AB (1974) Symbiotic nitrogen fixation in soybeans. III. Effect of supplemental nitrogen and intervarietal grafting. Crop Science 14, 22–25.
| Symbiotic nitrogen fixation in soybeans. III. Effect of supplemental nitrogen and intervarietal grafting.Crossref | GoogleScholarGoogle Scholar |
Lawn RJ, Fischer KS, Williams AB (1974) Symbiotic nitrogen fixation in soybeans. II. Interrelationship between carbon and nitrogen assimilation. Crop Science 14, 17–22.
| Symbiotic nitrogen fixation in soybeans. II. Interrelationship between carbon and nitrogen assimilation.Crossref | GoogleScholarGoogle Scholar |
Li S, Fei T, Yao D, Yao X, Zhang H, Wang H, Song S, Martin SS, Xie F (2017) Agronomic traits of soybean cultivars released in different decades after grafting record‐yield cultivar as rootstock. Plant Breeding 136, 133–138.
| Agronomic traits of soybean cultivars released in different decades after grafting record‐yield cultivar as rootstock.Crossref | GoogleScholarGoogle Scholar |
Liang Q, Yin Y, Yan X, Liao H (2009) Genetic analysis of root characters in soybean using a recombinant inbred line population at two phosphorus levels. Molecular Plant Breeding 7, 321–329.
Liao H, Rubio G, Yan X, Cao A, Brown K, Lynch J (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant and Soil 232, 69–79.
| Effect of phosphorus availability on basal root shallowness in common bean.Crossref | GoogleScholarGoogle Scholar | 11729851PubMed |
Liao H, Yan X, Rubio G, Beebe S, Blair M, Lynch J (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Functional Plant Biology 31, 959–970.
| Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean.Crossref | GoogleScholarGoogle Scholar |
Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiology 141, 674–684.
| Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system.Crossref | GoogleScholarGoogle Scholar | 16648222PubMed |
Lindsey PA, Gross R, Milano R (1995) An investigation to assess the importance of street infrastructure improvement on roots of adjacent cork oak tree. In ‘Trees and building sites’. (Eds G Watson, D Neely) pp. 22–32. (International Society of Agriculture: Savoy, IL, USA)
Lontoc-Roy M, Dutilleul P, Prasher S, Han L, Smith D (2005) Computed tomography scanning for three-dimensional imaging and complexity analysis of developing root systems. Canadian Journal of Botany 83, 1434–1442.
| Computed tomography scanning for three-dimensional imaging and complexity analysis of developing root systems.Crossref | GoogleScholarGoogle Scholar |
Ma S, Xu B, Li F, Huang Z (2008) Effect root pruning on root distribution root efficiency and yield formation of winter wheat in loess plateau. Chinese Journal of Applied Ecology 28, 6172–6173. [in Chinese]
Manavalan LP, Guttikonda S, Nguyen V, Shannon J, Nguyen H (2010) Evaluation of diverse soybean germplasm for root growth and architecture. Plant and Soil 330, 503–514.
| Evaluation of diverse soybean germplasm for root growth and architecture.Crossref | GoogleScholarGoogle Scholar |
Ookawa T, Nishiyama M, Takahiro J, Ishihara K, Hirasaw T (2001) Analysis of the factors causing differences in the leaf senescence pattern between two soybean cultivars, Enrei and Tachinagaha. Plant Production Science 4, 3–8.
| Analysis of the factors causing differences in the leaf senescence pattern between two soybean cultivars, Enrei and Tachinagaha.Crossref | GoogleScholarGoogle Scholar |
Pantalone VR, Rebetzke G, Burton J, Carter T (1996) Phenotypic evaluation of root traits in soybean and applicability to plant breeding. Crop Science 36, 456–459.
| Phenotypic evaluation of root traits in soybean and applicability to plant breeding.Crossref | GoogleScholarGoogle Scholar |
Pantalone VR, Rebetzke G, Burton J, Carter T, Israel D (1999) Soybean PI 416937 root system contributes to biomass accumulation in reciprocal grafts. Agronomy Journal 91, 840–844.
| Soybean PI 416937 root system contributes to biomass accumulation in reciprocal grafts.Crossref | GoogleScholarGoogle Scholar |
Pivetta LA, Castoldi G, Santos G, Rosolem C (2011) Soybean root growth and activity as affected by the production system. Pesquisa Agropecuária Brasileira 46, 1547–1554.
| Soybean root growth and activity as affected by the production system.Crossref | GoogleScholarGoogle Scholar |
Przepiorkowski T, Martin S (2003) The effect of grafting on the flowering of near-isogenic lines of soybean. Crop Science 43, 1760–1763.
| The effect of grafting on the flowering of near-isogenic lines of soybean.Crossref | GoogleScholarGoogle Scholar |
Qin X, Feng F, Li D, Herbert SJ, Liao Y, Siddique KHM (2017) Changes in yield and agronomic traits of soybean cultivars released in China in the last 60 years. Crop & Pasture Science 68, 973–984.
| Changes in yield and agronomic traits of soybean cultivars released in China in the last 60 years.Crossref | GoogleScholarGoogle Scholar |
Shanahan P, Binley A, Whalley WR, Watts CW (2015) The use of electromagnetic induction (EMI) to monitor changes in soil moisture profiles beneath different wheat cultivars. Soil Science Society of America Journal 79, 459–466.
| The use of electromagnetic induction (EMI) to monitor changes in soil moisture profiles beneath different wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Shi Y, Wei D, Yu Z, Yu S (1999) Effects of deep cultivation-root cutting on dry weight of root system after anthesis and yield in dry land wheat of high yield. Acta Agriculturae Boreali-Sinica 14, 91–95.
Shi Y, Wei D, Yu Z, Yu S (2000) Effects of deep cultivation-root cutting on senescence of root soybean. Chinese Journal of Applied and Environmental Biology 6, 516–519. [in Chinese]
Sun B, Sun M, Xu K, Li D, Zhang Z, Wu Z (2012) Changes of root bleeding sap weight and its correlation with biomass of above-ground organs in soybean cultivars released in different years. Soybean Science 31, 579–583. [in Chinese]
Tian W, Xu K, Bing X, Zhai J, Zhang Z, Chen Z, Wu Z (2007) Study on some agronomic traits of soybean cultivars with year of release in Jilin province. Chinese Journal of Oil Crop Sciences 29, 397–401. [in Chinese]
Wang J (1993) Review on the change of soybean plant types of the main soybean producing regions in China. Journal of Northeast Agriculture College 24, 209–213.
Wang Q, Fan X, Liu F, Li F, Klaus D, Sattemacher B (2004) Effect of root cutting on rice yield by shifting normal paddy to upland cultivation. Chinese Journal of Rice Science 18, 437–442. [in Chinese]
Wang C, Sun S, Wu B, Chang R, Han T (2013) Pedigree analysis of widely planting soybean varieties in China since 1940s. Chinese Journal of Oil Crop Sciences 35, 246–252. [in Chinese]
Wang C, Wu T, Wu C, Jiang B, Sun S, Hou W, Han T (2015) Changes in photothermal sensitivity of widely grown Chinese soybean cultivars due to a century of genetic improvement. Plant Breeding 134, 94–104.
| Changes in photothermal sensitivity of widely grown Chinese soybean cultivars due to a century of genetic improvement.Crossref | GoogleScholarGoogle Scholar |
Wang S, Han T, Liu L (2016) Strategies of sustainable development of soybean seed industry in China. In ‘Research on strategies of sustainable development of modern agricultural industry in China: Soybean Monograph’. (Ed. T Han) pp. 149–176. (China Agriculture Press: Beijing)
Whalley W, Binley A, Watts C, Shanahan P, Dodd IC, Ober ES, Ashton RW, Webster CP, White RP, Hawkesford MJ (2017) Methods to estimate changes in soil water for phenotyping root activity in the field. Plant and Soil 415, 407–422.
| Methods to estimate changes in soil water for phenotyping root activity in the field.Crossref | GoogleScholarGoogle Scholar |
White CA, Sylvester-Bradley R, Berry PM (2015) Root length densities of UK wheat and oilseed rape crops with implication for water capture and yield. Journal of Experimental Botany 66, 2293–2303.
| Root length densities of UK wheat and oilseed rape crops with implication for water capture and yield.Crossref | GoogleScholarGoogle Scholar | 25750427PubMed |
Wu C, Ma Q, Yam K, Cheung M, Xu Y, Han T, Lam HM, Chong K (2006) In situ expression of the GmNMH7 gene is photoperiod-dependent in a unique soybean (Glycine max [L.] Merr.) flowering reversion system. Planta 223, 725–735.
| In situ expression of the GmNMH7 gene is photoperiod-dependent in a unique soybean (Glycine max [L.] Merr.) flowering reversion system.Crossref | GoogleScholarGoogle Scholar | 16208488PubMed |
Wu T, Sun S, Wang C, Lu W, Sun B, Song X, Han X, Guo T, Man W, Cheng Y, Niu J, Fu L, Song W, Jiang B, Hou W, Wu C, Han T (2015) Characterizing changes from a century of genetic improvement of soybean cultivars in northeast China. Crop Science 55, 2056–2067.
| Characterizing changes from a century of genetic improvement of soybean cultivars in northeast China.Crossref | GoogleScholarGoogle Scholar |
Xu Z, Xu K, Zhang Z, Li D, Sun M (2011) Changes of biomass above-ground organs of soybean cultivars with year of release in Jilin Province. Journal of Nanjing Agricultural University 34, 7–12. [in Chinese]
Yan X (2007) ‘Principle and application of root biology.’ pp. 1–16. (Science Press: Beijing)
Yang Q (1982) The primary analysis of the changing trends of agronomic traits in soybean cultivars in Heilongjiang Province. Journal of Northeast Agriculture College 1982, 41–45.
Yang X, Wu Z, Zhang G (2001) Evolution of root characters of soybean varieties of different ages. Scientia Agricultura Sinica 34, 292–295. [in Chinese]
Zhao H, Xu K, Yang G, Yang C, Bian S, Lu J (2008) Changes and correlations between yield and partial physiological characters in leaves of soybean cultivars released from 1923 to 2005 in Jilin Province. Acta Agronomica Sinica 34, 1259–1265.
| Changes and correlations between yield and partial physiological characters in leaves of soybean cultivars released from 1923 to 2005 in Jilin Province.Crossref | GoogleScholarGoogle Scholar | [in Chinese]
Zhao J, Zhang W, Qiu Q, Zhang M, Zhang J, Yan X (2012) Changes of photosynthetic characters and canopy agronomic traits among different maturity groups in soybean genetic improvement. Soybean Science 31, 568–574. [in Chinese]
Zheng H, Xu K, Zhao H, Li D, Wang X, Zhang Z, Yang G, Yang C, Lu J (2006) Study on stature evolution of soybean cultivars in Jilin province. Chinese Journal of Oil Crop Sciences 28, 276–281. [in Chinese]
Zheng H, Zhen J, Luo Y, Li R, Wang H, Li W, Liu W, Qi H (2015) Effects of different tillage layer structures on growth and yield of maize in cropland zone in northeast of China. Agricultural Research in the Arid Areas 33, 41–95. [in Chinese]
* Xiaoning Cao, Tingting Wu and Shi Sun contributed equally to this work