An inter-catchment comparison of groundwater biota in the cotton-growing region of north-western New South Wales
K. L. Korbel A B D , R. P. Lim A B and G. C. Hose A CA Cotton Catchment Communities CRC.
B School of the Environment, University of Technology Sydney, PO Box 123, Broadway, Sydney, NSW 2007, Australia.
C Department of Biological Sciences, Macquarie University, NSW 2109, Australia.
D Corresponding author. Email: kkorbel@optusnet.com.au
Crop and Pasture Science 64(12) 1195-1208 https://doi.org/10.1071/CP13176
Submitted: 16 May 2013 Accepted: 13 September 2013 Published: 4 December 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Groundwater is essential to crop production in many parts of the world, and the provision of clean groundwater is dependent on healthy groundwater ecosystems. To understand better the functioning of groundwater ecosystems, it is necessary to understand how the biota responds to environmental factors, and so distinguish natural variation from human induced changes. This study compares the groundwater biota of the adjacent Gwydir and Namoi River alluvial aquifers, both in the heartland of Australia’s cotton industry, and investigates the relative importance of environmental, anthropogenic, geological, and evolutionary processes on biotic distribution.
Distinct differences in biotic assemblages were recorded between catchments at a community level. However, at a functional level (e.g. microbial activity, stygofauna abundances and richness) both ecosystems were similar. The distribution of biota in both catchments was influenced by similar environmental variables (e.g. geology, carbon availability, season, and land use). Broad trends in biotic distribution were evident: stygofauna responded most strongly to geological variables (reflecting habitat) and microbes to water quality and flow. Agricultural activities influenced biota in both catchments. Although possessing different taxa, the groundwater ecosystems of the two aquifers were functionally similar and responded to similar environmental conditions.
Introduction
Groundwater is essential to agricultural production in many parts of the world. Around 900 km3 of groundwater is used for agriculture each year, supporting global agricultural production estimated at AU$210–230 billion annually (Shah 2007). The cotton industry in Australia relies heavily on groundwater to irrigate its crops, with >80% of Australian cotton grown under irrigation (derived from surface and subsurface sources), using a combination of techniques such as trench, drip, and aerial irrigation (CRC 2008).
Although the importance of groundwater to agricultural production is clear, it is often overlooked that the provision of clean, useful groundwater is aided by the ecosystem services provided by the microbes and invertebrate fauna (stygofauna) that live within. Groundwater biota actively removes nitrates, degrades contaminants, and aids water flow within an aquifer (Boulton et al. 2008), making the water available and fit for use in agricultural industries. Consequently, there is a direct link between the health and condition of groundwater ecosystems and the productivity of agricultural industries dependent on groundwater. Despite their importance to agriculture and biodiversity, groundwater ecosystems the world over are generally poorly understood.
Understanding how groundwater ecosystems vary over space and time is key to managing them. In particular, it is important to be able to distinguish natural variation in groundwater assemblages from that due to anthropogenic effects. Furthermore, it is important to know whether groundwater ecosystems in different locations respond similarly to different factors so that consistent management approaches may be implemented, or whether management actions must be tailored to specific locations.
Biotic distribution within aquifers is influenced by several environmental factors. Geological history is thought to have a major influence on stygofauna composition, due to its role in supporting evolutionary divergence of species through isolation (Boulton et al. 2003; Humphreys 2008; Griebler and Lueders 2009). Geology and water quality influence biotic distribution as they determine water flow, energy exchange, and habitat structure (Griebler 2001; Hahn 2006; Hancock and Boulton 2009; Griebler and Lueders 2009). Other influences on the distribution of groundwater biota include climate, vegetation, water quality, links with the hyporheic zone, and human activities (Galassi et al. 1999a, 1999b; Cho and Kim 2000; Jasinska and Knott 2000; Schmidt et al. 2007; Tomlinson 2008; Griebler and Lueders 2009; Hahn and Fuchs 2009; Stein et al. 2010; Korbel et al. 2013). Consequently, aquifers with similar geology, history, and environment might be expected to have similar groundwater faunas.
Globally, several studies have demonstrated that groundwater ecosystems vary with geology and aquifer type (e.g. karst, alluvial, fracture rock; Galassi et al. 2009a; Hahn and Fuchs 2009); however, few studies have examined the relationships between biota and environmental conditions over local (within aquifer) and regional (between similar aquifers) scales (e.g. Schmidt et al. 2007; Malard et al. 2009; Stoch and Galassi 2010).
In this study, the spatial distribution of fauna across two adjacent alluvial aquifers with similar environmental and geological histories, in north-western New South Wales (NSW) is examined. Given the similarities in history, geology, climate, and land uses within these catchments, we expect similar biota to occur in each catchment, and that the biota will respond similarly to environmental factors.
Materials and methods
Study site
The Gwydir River and Naomi River catchments (hereafter the Gwydir and Namoi catchments), in north-western NSW, are approximately parallel tributaries of the Darling River (Fig. 1). The catchments support the primary cotton-growing region in Australia, with large-scale cotton production ongoing since the 1960s. As a result of agricultural activities such as irrigation, both the Namoi and Gwydir regions have experienced reductions and fluctuations in groundwater levels (McLean 2003; Barrett 2009; Kelly and Carr 2010), as well as some contamination of surface- and groundwater by agricultural chemicals (Muschal and Warne 2003; Korbel and Hose 2011; Korbel et al. 2013).
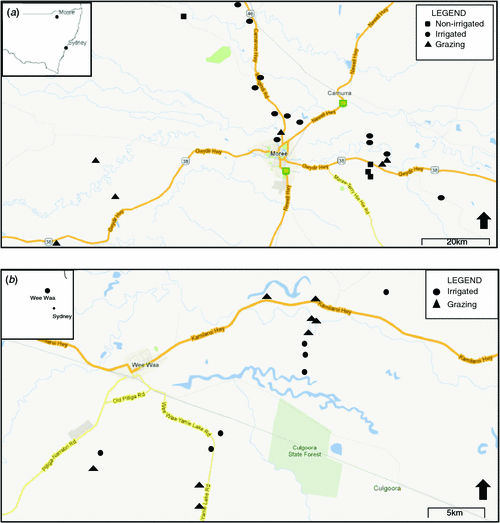
|
Both catchments have similar climatic conditions, being semi-arid with average winter minimum temperatures of 3−5°C and average summer maximum temperatures of 33°C (BOM 2011). Rainfall in the region is highly variable, summer-dominant, and generally of high intensity (Milne-Home et al. 2007).
The Gwydir and Namoi River aquifers have similar geological histories, with the weathering of pre-Quaternary kaolinised profiles in both catchments responsible for the kaolinitic clays in the west of the catchments (Young et al. 2002). Alluvium was widely deposited throughout both catchments in the Quaternary period and it dominates the aquifers (Kelly et al. 2007; Milne-Home et al. 2007; Barrett 2009). Accordingly, the size and shape of alluvial sediments are also similar (Riley 1975). It is expected that the adjacent catchments experienced the same climatic variations during the Neogene and Quaternary periods, with the transition from a wet to dry climate within this period influencing the sediment structure throughout the region, as noted in the distribution of sands/gravels and clays (Kelly et al. 2012).
The hydrology of the shallow aquifers of both catchments is dominated by surface interactions with upwellings and outwellings of groundwater in channel beds and gravel bars (McLean 2003; Kelly et al. 2007; Barrett 2009; Kelly and Carr 2010; Kelly et al. 2012). Significant recharge pulses occur after major rain events (Barrett 2009; Kelly and Carr 2010). However, hydraulic connectivity within and between the two catchments is still poorly understood (Kelly and Carr 2010; Kelly et al. 2012).
Gwydir River catchment characteristics
Within the Gwydir catchment, the study focus is around the town of Moree (29°28′S, 149°54′E; population 8083; ABS 2006; Fig. 1a). There are two aquifer systems in the region: a shallow, unconfined (10–30 m); and a deeper, confined aquifer system (35–100 m). The upper, unconfined aquifer is known as the Narrabri formation (McLean 2003; Milne-Home et al. 2007; Barrett 2009). The aquifer matrix is predominately shale and sandstone with seams of clay, sand, gravel, and limestone (Kelly et al. 2007; Barrett 2009). Good local hydraulic connectivity exists between sand and gravel substrates of the upper aquifer, with hydraulic connectivity throughout most of the catchment (Kelly and Carr 2010). Groundwater levels in the region are highly influenced by regulated flow in the Gwydir River and flood events (Barrett 2009; Kelly and Carr 2010).
Namoi River catchment characteristics
The Namoi catchment is immediately south of, and adjacent to, the Gwydir catchment. This study focussed on the lower Namoi catchment around the town of Wee Waa (Fig. 1b). The major town in the region is Narrabri (30°19′S, 149°47′E; population 6100; ABS 2006), ~40 km east of Wee Waa (Fig. 1b). Similar to the Gwydir catchment, the Namoi catchment has both a shallow, unconfined and a deeper, confined aquifer. The upper, unconfined aquifer contains the same ‘Narrabri formation’ as the Gwydir catchment and has soil profiles showing sequencing of clays, sands, and gravels, with considerable mixing of these sediments (McLean 2003; Milne-Home et al. 2007; Kelly et al. 2012). Knowledge of hydraulic connectivity in the aquifer is limited; however, recent studies indicate significant connectivity in the alluvial aquifer at a local scale (Kelly et al. 2012).
Field sampling
Groundwater assemblages were studied in both aquifers during summer 2007 and winter 2008, a period of drought in most of inland Australia. In total, 35 sites were sampled on the two occasions, 20 sites in the Gwydir catchment and 15 in the Namoi catchment.
Bores sampled in both catchments were government-owned and constructed with 50-mm-diameter polyvinyl chloride (PVC) pipe as the bore casing. The casings were completely enclosed except for a discrete section near the bottom of the pipe that was slotted to allow the entrance of aquifer water. To maintain consistency between sites and to ensure similar unconfined aquifers were being sampled, only bores with a slot depth 10–30 m were sampled. Sites with electrical conductivities (EC) >3000 µS cm–1 were avoided, as previous studies indicated that high EC may be a limiting factor for stygofauna (Hancock and Boulton 2008).
At each site, environmental attributes were assessed using field observations and quantitative techniques (Table 1). Land-use classifications were allocated to each site based on visual assessments. ‘Irrigated’ sites were situated either within or adjacent to irrigated farms (predominantly cotton farms) with irrigational infrastructure evident (e.g. canals and trenches). Irrigated cropping was the only land use visible at these sites, generally for several kilometres surrounding the sites.

|
Assessment of sediment type and volume was conducted in the field from sediment collected by pumping 300 L of water from the bores during stygofauna sampling. Classification of sediment particle size was based on the Wentworth scale for soils (Wentworth 1922). The proportion of fine sediment (‘silt/clay’ on the Wentworth scale) in samples was visually assessed after a representative sample (250-mL sample of combined sediment and water collected in the field) had been allowed to settle for 48 h. We accept that some contamination of sediments occurs during bore construction; however, this approach provides our best available estimate of the nature of fine sediments on the aquifer and is necessary because of the absence of completed bore logs and limited knowledge of the aquifers in the region (Milne-Home et al. 2007; Kelly and Carr 2010).
Water quality sampling
Groundwater was pumped from each bore using a motorised inertia pump (Waterra Powerpump II; Waterra Pumps Ltd, Mississauga, Ontario). Prior to sampling at each site, the pump tubing was sterilised internally using potassium hypochlorite and externally using 70% ethanol.
Field meters were used to measure dissolved oxygen (YeoKal 609; YeoKal Electronics, Brookvale, NSW), EC (TPS LC84), and pH/oxidation–reduction potential (ORP)/temperature (TPS LC80A; TPS Pty Ltd, Springwood, Qld) of bore water samples on site. Field measurements were taken after 10, 150, and 300 L of water was pumped from the bore. Water samples were collected in sterile amber glass or clean plastic containers for chemical (including dissolved organic matter, agrochemicals, and nutrients) and microbial analyses and refrigerated or frozen until analysed. Chemical analyses were conducted at the NSW Office of Water Environmental Laboratory in Arncliffe, NSW. Details of chemical analyses and methods are presented in Appendix 1.
Microbial sampling and analysis
Microbial activity was compared between sites using the cotton strip assay method (CSA; Lategan et al. 2010). Cotton strips made of 4 cm by 10 cm sections of calico were deployed in each bore after sampling and left for 6 weeks. Loss of tensile strength of the cotton strips was tested for each site in triplicate using a pneumatic tensiometer Universal Testing Machine (UTM Instron 6022 10-kN load frame; Instron Corporation, Norwood, MA, USA) with flat plate grips and specialised software (DOLI EDC120; DOLI Elektronik GmbH, Munich, Germany). See Lategan et al. (2010) for detailed methods.
Microbial assemblages were assessed using Biolog EcoPlates™ (Biolog, Hayward, CA, USA), which measure microbial activity through carbon utilisation (Preston-Mafham et al. 2002). The EcoPlates were inoculated with 150 µL of the sample per well and incubated in the dark at 20°C (Fliermans et al. 1997). Colour development was measured at 590 nm after 6 days of incubation; see Korbel et al. (2013) for detailed methods.
Stygofauna sampling and analysis
Groundwater (300 L) was pumped and passed through a 63-μm mesh sieve to collect stygofauna following Hancock and Boulton (2009). The sieve contents were preserved in 100% ethanol, stained with Rose Bengal, and later sorted under a microscope (×60 magnification). Specimens were identified to the lowest taxonomic level using relevant keys (e.g. Serov 2002) and identifications were confirmed by experts in taxonomy. In most cases, identification was limited to higher taxonomic levels due to the poor knowledge of the taxonomy of Australian stygofauna, particularly in eastern Australia (Hancock and Boulton 2008; Humphreys 2008).
Due to large differences in the mite assemblages between catchments and poor taxonomic knowledge of this group, this taxon was identified to Class only. Stygofauna taxa were allocated a category determined by size, with small (63 μm–0.25 mm), medium (0.25–0.5 mm), or large (>0.5 mm) animals in length, based on the average sizes of stygofauna.
Data analyses
Differences in cotton tensile strength, stygofauna richness and abundance, and environmental variables were compared using analysis of variance (ANOVA). Data were tested for homogeneity of variance using Bartlett’s test and log-transformed if necessary to satisfy this assumption and approximate normality. Relationships between cotton tensile strength, environmental variables, and stygofauna abundance and richness were examined using product moment correlation.
Patterns in stygofauna assemblages between catchments were visualised using non-metric multidimensional scaling (nMDS) incorporating the Bray–Curtis similarity coefficient. Patterns in microbial functional assemblages between catchments were visualised using principal components analysis. Differences between catchments were tested using one-way permutational ANOVA (PERMANOVA; Anderson et al. 2008).
Relationships between environmental attributes (including water quality) and biotic assemblages were investigated using distance-based linear models (DistLM) (Anderson et al. 2008). Prior to analysis of microbial and stygofauna assemblages, data were log-transformed to standardise and improve distribution. Environmental variables that were strongly correlated (r >0.95) were selectively removed from the dataset before analysis (Clarke 1993).
In all analyses, data were log-transformed and analysed using Bray–Curtis similarity for stygofauna data and Euclidean distance for microbial data. For the stygofauna assemblage data, a dummy variable of 1 was added to all samples to facilitate the inclusion of otherwise empty (zero abundance) samples (Clarke et al. 2006). All multivariate analyses were done using E-Primer (version 6.1.11; Primer-E Ltd, Ivybridge, UK) and univariate analysis completed using Minitab (v 16.1; Minitab Inc. 2010). All univariate and multivariate inferential analyses were conducted with significance level (α) of 0.05.
Results
Water quality
Not surprisingly, several water quality variables were strongly correlated (r >0.95): EC and chloride; calcium, chloride, and magnesium; and total nitrogen, ammonia, and oxidised nitrogen. Aluminium, manganese, and sulfate varied little between sites. As a result, only oxidised nitrogen, bicarbonate, pH, EC, total phosphorus, and dissolved organic carbon (DOC) were used for further analysis of the impact of water quality on biota. Calcium was included in the microbial analysis only. Considering all water quality variables together, there was no significant difference in water quality between catchments (PERMANOVA; P = 0.230).
The average EC for the Gwydir and Namoi catchments was 1011 and 621 µS cm–1, respectively. The herbicide atrazine was detected in two samples from the Gwydir catchment (both adjacent to irrigated cotton/grain farms) and four samples from both grazing and irrigated cotton/grain farming areas in the Namoi catchment. Diethyl atrazine was detected in two samples and diuron in one sample from the Namoi catchment.
Microbial assemblages
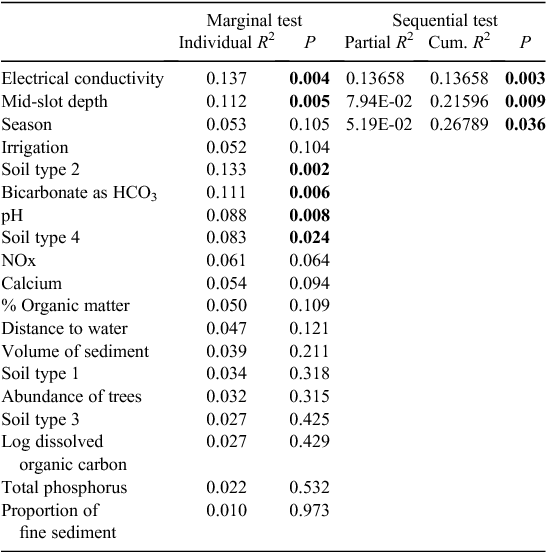
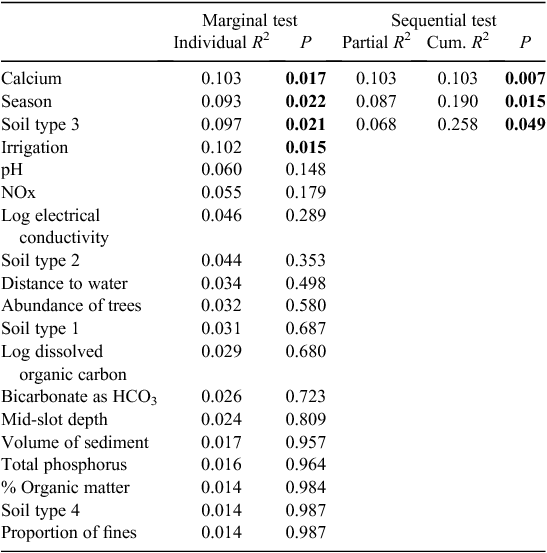
The microbial assemblages (indicated by EcoPlates) differed significantly between catchments (PERMANOVA, P = 0.001). This difference is evident as the clear separation of samples from the two catchments (Fig. 2). Analysed separately, microbial assemblages from both catchments were influenced strongly by season (Tables 2, 3). The presence of irrigation at a site was also significantly related to microbial assemblages in both catchments, but this variable did not explain unique variation in the respective stepwise models. Water quality variables, particularly dissolved ions (EC, calcium) and soil type, were correlated with microbial assemblage composition in both catchments, although in the stepwise models, soil type was significant only in the Namoi catchment (Table 3). Sampling depth was a significant factor in the Gwydir catchment (Table 2).
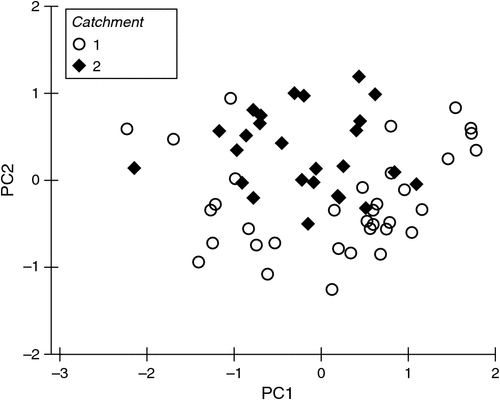
|
|
|
There was no significant difference in overall microbial activity (CSA method) between catchments (P = 0.206), nor was there a significant interaction between tensile strength and time (P > 0.05). Cotton strip tensile strength differed significantly with soil type (ANOVA, P = 0.008; Fig. 3) and volume of sediment extracted within a sample (ANOVA, P = 0.003), with activity increasing with coarseness of particle size and volume of sediment retrieved from a bore. Cotton tensile strength was positively correlated with abundance of stygofauna (P < 0.05) in both catchments.
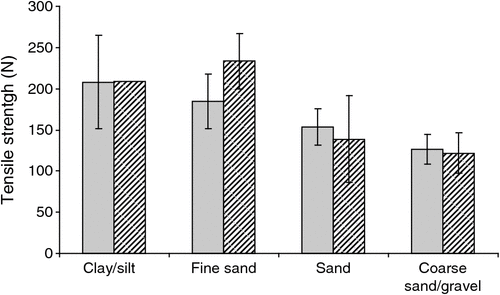
|
Stygofauna communities
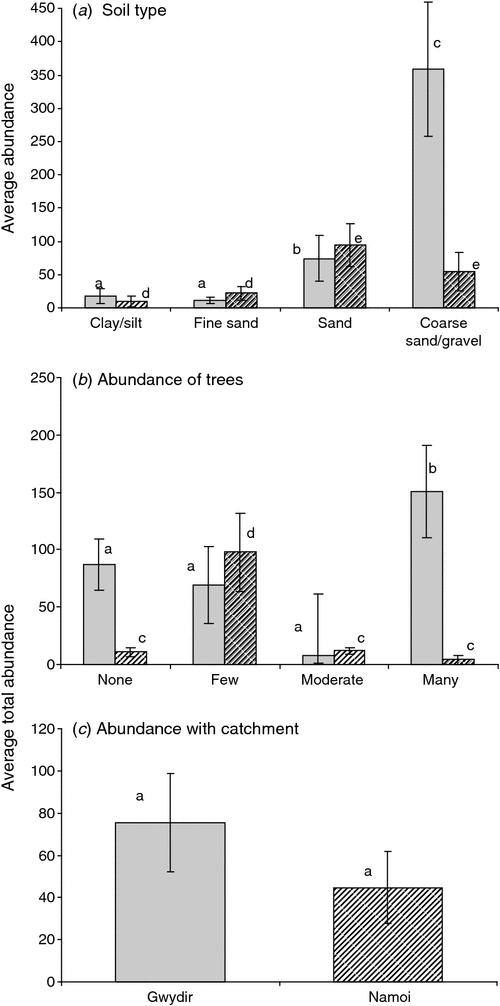
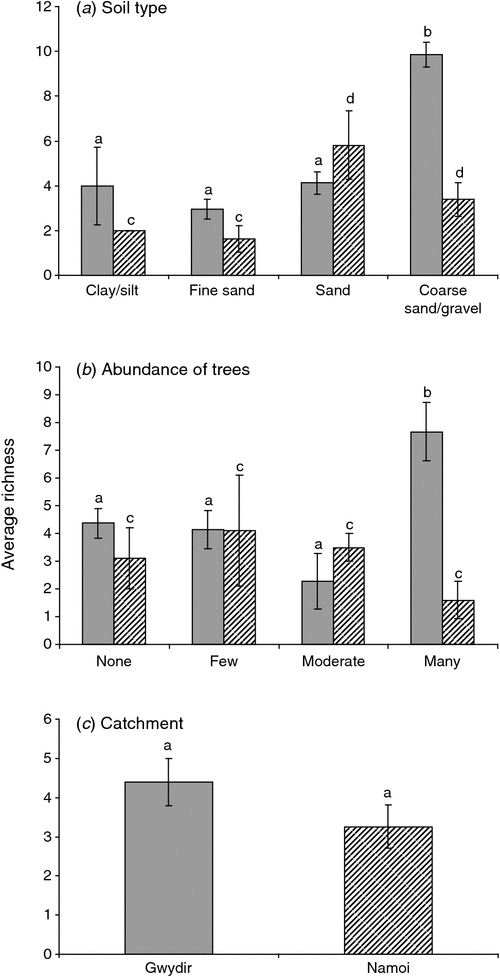
Stygofauna assemblages differed significantly between the Gwydir and Namoi catchments (P = 0.001), evident as a clear separation in the MDS ordination (Fig. 4). The Gwydir catchment contained 20 taxa, and the Namoi catchment 14 taxa (see Appendix 2); however, there was no significant difference in the average abundance (Fig. 5c) or richness (Fig. 6c) of stygofauna per sample between the catchments.
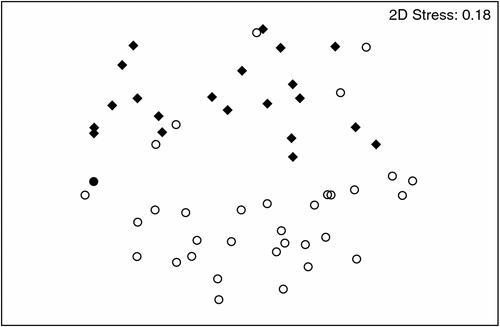
|
|
|
In the Gwydir catchment, soil type, distance to surface water, the presence of irrigation activity, tree abundance, volume and organic matter content of sediments, and season of sampling all influenced the stygofauna assemblage, and together accounted for 46% of the variability in the stygofauna assemblage (Table 4). All of these variables accounted for a unique component of the variation in the stygofauna assemblage structure and each was significantly correlated with assemblage structure. There was no significant relationship between any of the water quality variables and stygofauna assemblage structure (Table 4).
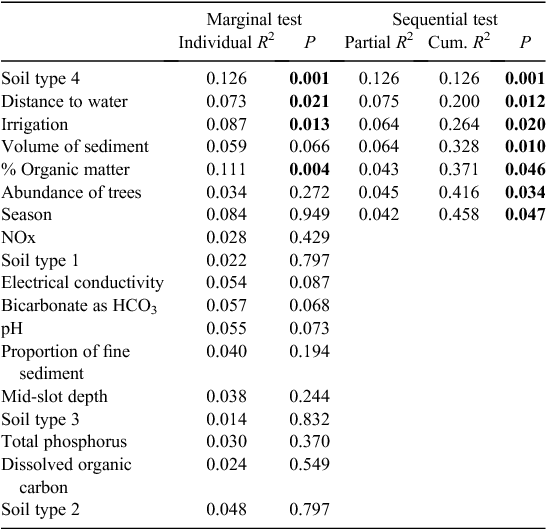
|
Soil type (coarse soil) and the presence of irrigation were also significant correlates of stygofauna assemblage structure in the Namoi catchment (Table 5). In addition, DOC concentration and depth of sampling both explained a unique component of the variation in the stygofauna assemblages. Other soil types and the proportion of fine sediments in the samples were each correlated with the stygofauna assemblage structure but were not significant in the stepwise model. Other than DOC, water quality variables did not have a significant relationship with stygofauna assemblage structure.
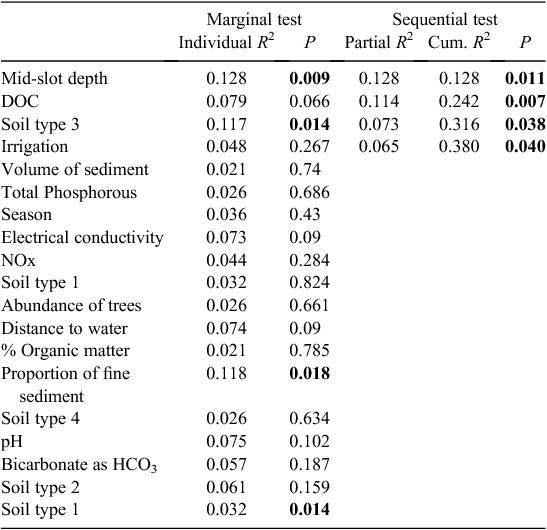
|
In the Gwydir and Namoi catchments, stygofauna abundance was influenced by soil type (ANOVA, P = 0.049, P < 0.001, respectively; Fig. 5a) and abundance of trees (P = 0.032, 0.004, respectively; Fig. 5b). Stygofauna richness in both catchments was influence by soil type (Gwydir, P = 0.042; Namoi, P = 0.001; Fig. 6a), with richness increasing with soil coarseness.
Across both catchments, no large animals (e.g. Amphipoda and some Syncarida) were found in substrates consisting of fine sand/silt or clays (Fig. 7a), with medium and small animals found in all soil types (Fig. 7b, c). Among the Syncarida, Chilibathynella and Family A were found exclusively in the Gwydir catchment. Amphipods were also more abundant in the Gwydir catchment than in the Namoi (see Appendix B for taxa located in each catchment). The abundance of copepods, oligochaetes, bathynellids, and Notobathynella sp. were similar in both catchments; however, the abundance of mites was higher in the Namoi catchment than in the Gwydir.
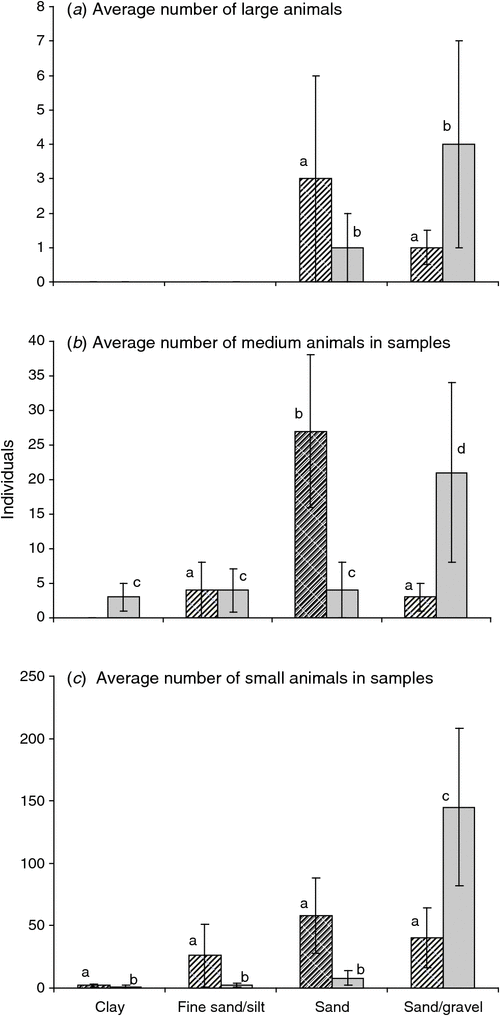
|
Discussion
Variation in biota between catchments
Microbial and stygofaunal assemblages varied significantly between the two catchments, indicating unique biotic communities. However, biotic assemblages in both the Gwydir and Namoi catchments were correlated with similar environmental variables and displayed functional similarities. Stygofauna communities in both catchments were most strongly correlated with variables reflecting sediment and aquifer matrix structure, and site attributes such as the presence of trees and irrigation, whereas, microbial communities were influenced most strongly by season and dissolved ion concentrations.
Microbes
Cotton strength loss is a reliable surrogate for general heterotrophic microbial activity (Lategan et al. 2010), and here, it was similar between catchments, suggesting that total microbial activity was also similar between catchments. Microbes are major ecosystem service providers in terms of water quality improvement via denitrification and contaminant degradation (Griebler and Schmidt 2009). Microbes are the basis of the aquifer food web, capturing dissolved and any particulate carbon and providing a food source for higher trophic-level organisms (Gibert et al. 1994; Humphreys 2006). Microbial activity was correlated with stygofauna abundance, suggesting a strong trophic link.
Although levels of activity were similar, microbial communities (as measured by EcoPlates) differed between the two adjacent catchments. EcoPlates show that the microbial assemblages from the different catchments are able to utilise different carbon sources, with the inference that the taxonomic composition of those assemblages is also different. Microbial communities respond quickly to environmental changes such as rainfall, season, and contamination (Feris et al. 2004; Griebler and Lueders 2009; Velasco Ayuso et al. 2009), so it is possible that the differences between catchments reflect differences in the conditions around the time of sampling. Indeed, the ‘everything everywhere’ paradigm of groundwater microbiology (Griebler and Lueders 2009) would suggest that differences in composition, at least at this regional scale, were not expected, particularly given the similarities in the water quality, land use, and geology between the catchments. Indeed, it may be that many microbial taxa are present but not metabolically active (Chapelle 2001) and, thus, undetectable by the methods chosen. Consequently, our methods may underestimate the similarity of microbial assemblages between the catchments.
Stygofauna
Stygofauna abundance and richness, and size of organisms, were similar between catchments. Each provides a measure of biomass and ecosystem productivity (see Korbel and Hose 2011) and they suggest similarities in ecosystem function between the catchments. Furthermore, assemblages in each catchment shared all higher taxa. The similarity in composition, size, and abundance of those fauna is likely evidence of similar levels of ecosystem service provision and functional capacity of the assemblages (Boulton et al. 2008).
Although stygofauna richness and abundance were similar between catchments, assemblage composition differed at the level of Family and below. Limited taxonomic knowledge of Australian groundwater fauna (e.g. Hancock and Boulton 2008; Guzik et al. 2010) hampered the further identification of the animals collected, and our attempts to use molecular methods were stifled by difficulties with amplification of DNA, such as are common in analyses of stygofauna (see Asmyhr and Cooper 2012). Nevertheless, there were clear differences in the stygofauna assemblages at even the coarse taxonomic levels used. These differences between catchments were most evident within syncarids, with Chilibathynella sp. and Family A being restricted to the Gwydir River catchment. This finding may reflect the limited distribution of these taxa, but equally could reflect the lesser sampling effort in the Namoi.
Environmental relationships with biota
Water quality
Our results suggest that water quality is not primary determinant of the distribution of groundwater biota, at least over the range of conditions that we encountered. This has been suggested elsewhere (Dumas et al. 2001; Hahn 2006; Dole-Olivier et al. 2009a). The EC and calcium influenced microbial assemblages in both catchments, but the relative importance of these variables differed. Both EC and calcium reflect the overall dissolved ion concentrations in the aquifers. The EC was similar within the two catchments ranging from ~200 to 2000 µS cm–1 (Kelly et al. 2007; Barrett 2009), and well below the range (>3000 µS cm–1) at which effects on stygofauna are expected (Hancock and Boulton 2008). The overall similarities in ion concentrations between the two catchments are likely to be due to similar geology, rainfall, and land uses in the two catchments.
Geology
Microbial activity (CSA) and assemblage (EcoPlates) were influenced by geology in both catchments, with activity increasing with grain size. Particle size influences the flow of water, with larger particles providing greater interstitial spaces as habitat, and allowing greater movement of water through the aquifer matrix than is possible through finer sediments (Strayer et al. 1997; Mösslache 1998; Hahn 2006; Dole-Olivier et al. 2009a, 2009b; Griebler and Schmidt 2009). Most groundwater microbes are attached to sediment particles rather than being free-living (Griebler et al. 2002), and generally, bacterial activity and abundance is greater in sediments with larger than with smaller particles (Fredrickson et al. 1997; Zhang et al. 1998; Velasco Ayuso et al. 2009). The increased hydraulic conductivity associated with larger sized particles allows for a greater supply of waterborne oxygen, carbon, and other nutrients, which may explain the relationship between microbial activity with sediment size (Zhou et al. 2002; Lehman et al. 2001; Velasco Ayuso et al. 2009).
Sediment particle size was among the most important variables influencing stygofauna distribution in both catchments, with larger animals more prevalent, and overall abundance and richness of stygofauna were greatest at sites with coarse sediments. These findings relate to habitat availability and hydrological conductivity and are consistent with previous studies in the region (Korbel and Hose 2011; Korbel et al. 2013), and elsewhere (e.g. Dole-Olivier et al. 2009a, 2009b). With both microbial activity (CSA) and stygofauna abundance positively correlated with particle size, it is unclear which of these is directly causal or due to secondary associations (Gounot 1994; Brunke and Fisher 1999; Chapelle 2001).
Availability of carbon
Stygofauna abundance, richness, and community assemblages were all associated with the abundance of trees at a site, although results were variable throughout the catchments. These findings support recent suggestions that tree roots are responsible for increased taxon richness and abundances of stygofauna (Hancock and Boulton 2008), as they provide both potential habitat (Jasinska and Knott 2000) and a source of bioavailable, dissolved carbon (Bonkowski 2004) in groundwater. However, the presence of trees was not related to differences in the microbial assemblages, which may be expected if the trees were indeed an important source of bioavailable carbon for the ecosystem. The inverse responses of stygofauna abundance and microbial activity could imply that stygofauna influence the microbial assemblages.
In the Namoi catchment, stygofauna and microbial assemblages were influenced by DOC. This relationship was not evident in the Gwydir catchment, and it is unclear why this was the case, particularly since DOC concentrations were similar in both catchments. Concentrations of DOC have been correlated with microbial and stygofaunal activity in groundwater elsewhere (Mauclaire et al. 2000; Datry et al. 2005), and organic enrichment is known to impact stygofauna and microbial communities (see Korbel and Hose 2011). However, changes in invertebrate and microbial assemblages are often difficult to detect over low to medium gradients of DOC, with relationships only evident in cases of substantial DOC enrichments (e.g. Mosslacher et al. 2001; Goldscheider et al. 2006; Masciopinto et al. 2006).
Land uses
In both the Namoi and Gwydir catchments, sites being used for irrigated cropping had higher abundances of stygofauna than those under other land uses. Irrigation enhances the connectivity of surface practices to groundwater due to deep drainage (Korbel et al. 2013). It is unclear whether the apparent differences in stygofauna communities between land uses are due to water-quality changes or periodic alterations in water levels often associated with irrigation (Tomlinson 2008), all of which are likely to influence stygofauna communities (Tomlinson 2008; Korbel et al. 2013; Stumpp and Hose 2013). The influence of land practices in both catchments is consistent with recent findings in the Gwydir catchment (Korbel et al. 2013) and elsewhere (Schmidt et al. 2007).
Irrigation activities were related to changes in microbial assemblages in the Namoi catchment. Microbial communities in groundwater change in response to rainfall events (Velasco Ayuso et al. 2009), and the leaching of irrigation water through the soil profile to groundwater may have similar effects. The prevalence of pesticide and fertiliser use with irrigation, and the potential for leaching of those chemicals to groundwater, may also lead to changes in the microbial assemblages (see Griebler and Lueders 2009), although with few incidences of agrochemicals detected, such impacts may be limited in these catchments at the time of our study. The greater prevalence of irrigated cropping in the Namoi compared with the Gwydir may explain why the impacts of irrigation on microbes are only evident in that catchment.
Geological history
Distinct differences in both stygofauna and microbial assemblages were evident between catchments; however, the two ecosystems were similar at a functional level (e.g. microbial activity, stygofaunal abundances, and animal size). The divergence of the invertebrate and microbial assemblages between catchments may reflect the separation of once common taxa by changes in the landscape following uplift of the Lachlan fold belt and the formation of the current parallel river valleys. Unfortunately, little is known of the formation of the landscape of the region; however, their geological similarities, particularly the deposits of alluvium, may indicate that the alluvium was once linked (W. Timms, University of NSW, pers. comm.).
Several processes may have led to the differences in the groundwater biota of the Gwydir and Namoi Valleys. These include independent colonisation from surface ancestors (e.g. Leys et al. 2003; Murphy et al. 2009), divergence due to geological isolation (e.g. Boulton et al. 2003; Murphy et al. 2009), and diversity due to vicariance (e.g. Byrne et al. 2008; Guzik et al. 2010). Investigations into the evolution of Australian stygofauna have been largely limited to the arid central and western regions of the country (e.g. Leys et al. 2003, 2010; Murphy et al. 2009), and little is known of the evolutionary relationships among taxa in eastern Australia (Guzik et al. 2010).
The differences in microbial communities between the adjacent Namoi River and Gwydir River catchments are attributed to the ability of microbes to respond rapidly to environmental change (Griebler and Lueders 2009). The short generation times for microbes means that communities can change quickly (Griebler and Lueders 2009); thus, the overall patterns in microbial functional assemblages may only indicate different conditions present at the time of sampling and may not necessarily reflect a long-term difference. Stygofauna are arguably more stable than microbial assemblages, which is reflected in the seasonal differences in the microbial but not stygofaunal assemblages.
The shallow aquifers of the Namoi and Gwydir Rivers support diverse invertebrate and microbial assemblages. While there were taxonomic differences in the fauna between the catchments, the assemblages shared similarities in terms of functional roles of fauna and other attributes of ecosystem functioning and energy turnover. Furthermore, the microbial and stygofauna assemblages in each catchment responded to a similar suite of environmental variables. For stygofauna, it was land use, soil type, and carbon availability; and for microbial assemblages, it was ionic water quality and time of sampling (season). The inherent functional similarities in the biota of these aquifers suggest that approaches for managing, assessing, and monitoring similar, nearby groundwater ecosystems may not require catchment-specific targets or approaches.
Acknowledgements
We thank the Cotton Catchment Communities CRC and the School of Environmental Science, University of Technology Sydney for funding this project, including the post-graduate scholarship for K. Korbel. NSW DWE provided access to bores and analysis of water samples; P. Hancock provided assistance with the identification of taxa in this project. Norman Booth provided assistance with cotton strength testing. Josie Lategan, Sarah Stephenson, Peter Hancock, and Chris Whetters assisted with fieldwork. We are grateful for the considered comments of the editor and two anonymous reviewers.
References
ABS (2006) ‘National Census.’ (Australian Bureau of Statistics, Australian Government: Canberra, ACT)Anderson M, Gorley RN, Clarke KR (2008) ‘PERMANOVA+ for PRIMER: Guide to software and statistical methods.’ (PRIMER-E: Ivybridge, UK)
Asmyhr M, Cooper S (2012) Difficulties barcoding in the dark: the case of crustaceans stygofauna from eastern. Australia. Invertebrate Systematics 26, 583–591.
| Difficulties barcoding in the dark: the case of crustaceans stygofauna from eastern. Australia.Crossref | GoogleScholarGoogle Scholar |
Barrett K (2009) Lower Gwydir Groundwater Source: Groundwater Management Area 004, Groundwater Status Report 2008. NSW Department of Water and Energy, Sydney.
BOM (2011) Climate data online. Bureau of Meteorology, Australian Government, Canberra, ACT. Available at: www.bom.gov.au/climate/data/
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytologist 162, 617–631.
| Protozoa and plant growth: the microbial loop in soil revisited.Crossref | GoogleScholarGoogle Scholar |
Boulton AJ, Humphreys W, Eberhand S (2003) Imperilled subsurface waters in Australia: Biodiversity, threatening processes and conservation. Aquatic Ecosystem Health & Management 6, 41–54.
| Imperilled subsurface waters in Australia: Biodiversity, threatening processes and conservation.Crossref | GoogleScholarGoogle Scholar |
Boulton AJ, Fenwick G, Hancock P, Harvey M (2008) Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebrate Systematics 22, 103–116.
| Biodiversity, functional roles and ecosystem services of groundwater invertebrates.Crossref | GoogleScholarGoogle Scholar |
Brunke M, Fisher H (1999) Hyporheic bacteria – relationships to environmental gradients in a prealpine stream. Archiv fur Hydrobiologie 146, 189–217.
Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MA, Cooper SJB, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll KH (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar |
Chapelle F (2001) ‘Groundwater microbiology and geochemistry.’ (Wiley Press: New York)
Cho J, Kim S (2000) Increases in bacteria community diversity in subsurface aquifers receiving livestock wastewater input. Applied and Environmental Microbiology 66, 956–965.
| Increases in bacteria community diversity in subsurface aquifers receiving livestock wastewater input.Crossref | GoogleScholarGoogle Scholar |
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18, 117–143.
| Non-parametric multivariate analyses of changes in community structure.Crossref | GoogleScholarGoogle Scholar |
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. Journal of Experimental Marine Biology and Ecology 330, 55–80.
| On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages.Crossref | GoogleScholarGoogle Scholar |
CRC (2008) Cotton Catchment Communities CRC Annual Report 2007–2008. Cotton Catchment Community Cooperative Research Centre, Narrabri, NSW. Available at: www.cottoncrc.org.au/industry/About_Us/Annual_Reports/Cotton_Catchment_Communities_CRC_Annual_Reports
Datry T, Malard F, Gibert J (2005) Response of invertebrate assemblages to increased groundwater recharge in a phreatic aquifer. Journal of the North American Benthological Society 24, 461–477.
Dole-Olivier MJ, Malard F, Martin D, Lefébure T, Gibert J (2009a) Relationships between environmental variables and groundwater biodiversity at the regional scale. Freshwater Biology 54, 797–813.
| Relationships between environmental variables and groundwater biodiversity at the regional scale.Crossref | GoogleScholarGoogle Scholar |
Dole-Olivier MJ, Castellarini F, Coineau N, Galassi DMP, Martin P, Mori N, Valdecasas A, Gibert J (2009b) Towards and optimal sampling strategy to assess groundwater biodiversity: comparison across six European regions. Freshwater Biology 54, 777–796.
| Towards and optimal sampling strategy to assess groundwater biodiversity: comparison across six European regions.Crossref | GoogleScholarGoogle Scholar |
Dumas P, Bou C, Gibert J (2001) Groundwater macrocrustaceans as natural indicators of the Ariege Alluvial Aquifer. International Review of Hydrobiology 86, 619–633.
| Groundwater macrocrustaceans as natural indicators of the Ariege Alluvial Aquifer.Crossref | GoogleScholarGoogle Scholar |
Feris KP, Ramsey PW, Frazar C, Rillig M, Moore JN, Gannon JE, Holben WE (2004) Seasonal dynamics of shallow-hyporheic-zone microbial community structure along a heavy-metal contamination gradient. Applied and Environmental Microbiology 70, 2323–2331.
| Seasonal dynamics of shallow-hyporheic-zone microbial community structure along a heavy-metal contamination gradient.Crossref | GoogleScholarGoogle Scholar |
Fliermans CB, Franck MM, Hazen TC, Gorden RW (1997) Ecofunctional enzymes of microbial communities in ground water. FEMS Microbiology Reviews 20, 379–389.
| Ecofunctional enzymes of microbial communities in ground water.Crossref | GoogleScholarGoogle Scholar |
Fredrickson JK, McKinley JP, Bjornstad BN, Long PE, Ringelberg DB, White DC, Krumholz LR, Suflita JM, Colwell FS, Lehman RM, Phelps TJ, Onstott TC (1997) Pore-size constraints on the activity and survival of subsurface bacteria in a late Cretaceous shale-sandstone sequence, northwestern New Mexico. Geomicrobiology Journal 14, 183–202.
| Pore-size constraints on the activity and survival of subsurface bacteria in a late Cretaceous shale-sandstone sequence, northwestern New Mexico.Crossref | GoogleScholarGoogle Scholar |
Galassi DMP, De Laurentiis P, Dole Olivier MJ (1999a) Nitocrellopsis rocuhi sp.n.: a new ameirid harpacticoid from phreatic waters of France (Copepoda: Harpacticoida: Ameiridae). Hydrobiologia 412, 177–189.
| Nitocrellopsis rocuhi sp.n.: a new ameirid harpacticoid from phreatic waters of France (Copepoda: Harpacticoida: Ameiridae).Crossref | GoogleScholarGoogle Scholar |
Galassi DMP, Dole Olivier MJ, De Laurentiis P (1999b) Phylogeny and biogeography of the genus Pseudectinosoma and description of P. janineae sp. n. (Crustacea, Copepoda, Ectinsomatidae). Zoologica Scripta 28, 289–303.
| Phylogeny and biogeography of the genus Pseudectinosoma and description of P. janineae sp. n. (Crustacea, Copepoda, Ectinsomatidae).Crossref | GoogleScholarGoogle Scholar |
Galassi D, Huys R, Reid J (2009a) Diversity ecology and evolution of groundwater copepods. Freshwater Biology 54, 691–708.
| Diversity ecology and evolution of groundwater copepods.Crossref | GoogleScholarGoogle Scholar |
Gibert J, Stanford JA, Dole-Oliver MJ, Ward J (1994) Basic attributes of groundwater ecosystems and prospects for research. In ‘Groundwater ecology’. (Eds J Gibert, D Danielopol, J Stanford) pp. 7–40. (Academic Press: San Diego, CA)
Goldscheider N, Hunkeler D, Rossi P (2006) Review: Microbial biocenoses in pristine aquifers and an assessment of investigation methods. Hydrogeology Journal 14, 926–941.
| Review: Microbial biocenoses in pristine aquifers and an assessment of investigation methods.Crossref | GoogleScholarGoogle Scholar |
Gounot AM (1994) Microbial ecology of groundwaters. In ‘Groundwater ecology’. (Eds J Gibert, D Danielopol, J Stanford) pp. 189–216. (Academic Press: San Diego, CA)
Griebler C (2001) Microbial Ecology of Subsurface Ecosystems. In ‘Groundwater ecology: A tool for management of water resources’. (Eds C Griebler, D Danielopol, J Gibert, HP Nachtnebel, J Notenboom) pp. 81–108. (Official Publication of the European Communities: Luxembourg)
Griebler C, Lueders T (2009) Microbial biodiversity in groundwater ecosystems. Freshwater Biology 54, 649–677.
| Microbial biodiversity in groundwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Griebler C, Schmidt SI (2009) Groundwater ecosystem functioning and assessment of the ecological status. SILnews 54, 16–17.
Griebler C, Mindl B, Slezak B, Geiger-Kaiser M (2002) Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers, studied with an in situ sediment exposure microcosm. Aquatic Microbial Ecology 28, 117–129.
| Distribution patterns of attached and suspended bacteria in pristine and contaminated shallow aquifers, studied with an in situ sediment exposure microcosm.Crossref | GoogleScholarGoogle Scholar |
Grimshaw HM, Allen SE, Parkinson JA (1989) Nutrient elements. In ‘Chemical analysis of ecological materials’. 2nd edn. (Ed. SE Allen) (Blackwell Scientific Publications: Oxford, UK)
Guzik MT, Austin AD, Cooper SJB, Harvey MS, Humphreys WF, Bradford T, Eberhand SM, King RA, Leys R, Muirhead KA, Tomlinson M (2010) Is the Australian subterranean fauna uniquely diverse? Invertebrate Systematics 24, 407–418.
| Is the Australian subterranean fauna uniquely diverse?Crossref | GoogleScholarGoogle Scholar |
Hahn HJ (2006) The GW-Fauna-Index: A first approach to a quantitative ecological assessment of groundwater habitats. Limnologica 36, 119–137.
| The GW-Fauna-Index: A first approach to a quantitative ecological assessment of groundwater habitats.Crossref | GoogleScholarGoogle Scholar |
Hahn HJ, Fuchs A (2009) Distribution patterns of groundwater communities across aquifer types in south-western Germany. Freshwater Biology 54, 848–860.
| Distribution patterns of groundwater communities across aquifer types in south-western Germany.Crossref | GoogleScholarGoogle Scholar |
Hancock PJ, Boulton AJ (2008) Stygofauna biodiversity and endemism in four alluvial aquifers in eastern Australia. Invertebrate Systematics 22, 117–126.
| Stygofauna biodiversity and endemism in four alluvial aquifers in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hancock PJ, Boulton AJ (2009) Sampling groundwater fauna: efficiency of rapid assessment methods tested in bores in eastern Australia. Freshwater Biology 54, 902–917.
| Sampling groundwater fauna: efficiency of rapid assessment methods tested in bores in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Humphreys WF (2006) Aquifers: the ultimate groundwater dependent ecosystem. Australian Journal of Botany 54, 115–132.
| Aquifers: the ultimate groundwater dependent ecosystem.Crossref | GoogleScholarGoogle Scholar |
Humphreys WF (2008) Rising from Down Under: Developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebrate Systematics 22, 85–101.
| Rising from Down Under: Developments in subterranean biodiversity in Australia from a groundwater fauna perspective.Crossref | GoogleScholarGoogle Scholar |
Jasinska E, Knott B (2000) Root driven faunas in cave waters. In ‘Ecosystems of the world. Vol. 30: Subterranean ecosystems’. (Eds H Wilkens, D Culver, W Humphreys) pp. 287–307. (Elsevier: Amsterdam)
Kelly BFJ, Carr JR (2010) Gwydir Catchment groundwater hydrographs. Final Report. Cotton Catchment Communities CRC, Narrabri, NSW. Available at: www.insidecotton.com/handle/1/285
Kelly B, Merrick N, Dent B, Milne-Home W, Yates D (2007) Groundwater knowledge and Gaps in the Namoi Catchment Management Area. Report NCGM 2007/1. National Centre for Groundwater Management, University of Technology, Sydney.
Kelly BFJ, Giambastiani B, Larsen J, Ralph T, Timms W, Baker W (2012) Paleoclimate impacts on aquifer architecture and hydraulic connectivity. In ‘Proceedings 38th Symposium on the Geology of the Sydney Basin’. Hunter Valley, May 2012. (NSW Trade and Investment: Sydney)
Korbel KL, Hose GC (2011) A tiered framework for assessing groundwater ecosystem health. Hydrobiologia 661, 329–349.
| A tiered framework for assessing groundwater ecosystem health.Crossref | GoogleScholarGoogle Scholar |
Korbel KL, Hancock PJ, Serov P, Lim P, Hose GC (2013) Groundwater ecosystems vary with land use across a mixed agricultural landscape. Journal of Environmental Quality 42, 380–390.
| Groundwater ecosystems vary with land use across a mixed agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Lategan M, Korbel KL, Hose GC (2010) Is cotton-strip tensile strength a surrogate for microbial activity in groundwater? Marine and Freshwater Research 61, 351–356.
| Is cotton-strip tensile strength a surrogate for microbial activity in groundwater?Crossref | GoogleScholarGoogle Scholar |
Lehman RM, Colwell FS, Bala G (2001) Attached and unattached microbial communities in a simulated basalt aquifer under fracture-and porous-flow conditions. Applied and Environmental Microbiology 67, 2799–2809.
| Attached and unattached microbial communities in a simulated basalt aquifer under fracture-and porous-flow conditions.Crossref | GoogleScholarGoogle Scholar |
Leys R, Watts CHS, Cooper SJB, Humphreys WF (2003) Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834.
Leys R, Roudnew B, Watts CHS (2010) Paroster extraordinarius sp. Nov., a new groundwater diving beetle from the Flinders Ranges, with notes on other diving beetles from gravels in South Australia (Coleoptera Dytiscidas). Australian Journal of Entomology 49, 66–72.
| Paroster extraordinarius sp. Nov., a new groundwater diving beetle from the Flinders Ranges, with notes on other diving beetles from gravels in South Australia (Coleoptera Dytiscidas).Crossref | GoogleScholarGoogle Scholar |
Malard F, Boutin C, Camacho A, Ferreira S, Michel G, Sket B, Stoch F (2009) Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshwater Biology 54, 756–776.
| Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe.Crossref | GoogleScholarGoogle Scholar |
Masciopinto C, Semeraro F, La Mantia R, Inguscio S, Rossi E (2006) Stygofauna abundance and distribution in the fissures and caves of the Nardo (southern Italy) fractured aquifer subject to reclaimed water injections. Geomicrobiology Journal 23, 267–278.
| Stygofauna abundance and distribution in the fissures and caves of the Nardo (southern Italy) fractured aquifer subject to reclaimed water injections.Crossref | GoogleScholarGoogle Scholar |
Mauclaire L, Gibert J, Claret C (2000) Do bacteria and nutrients control faunal assemblages in alluvial aquifers? Archiv fur Hydrobiologie 148, 85–98.
McLean WA (2003) Hydrogeochemical evolution and variability in a stressed alluvial aquifer system: Lower Namoi River catchment, NSW. PhD Thesis, University of New South Wales, Sydney.
Milne-Home W, Merrick N, Kelly B, Dent B, Yates D (2007) Groundwater knowledge and gaps in the Border Rivers–Gwydir Catchment Management Area. Report NCGM 2007/5. National Centre for Groundwater Management, University of Technology, Sydney, NSW, Australia.
Minitab Inc. (2010) ‘Minitab® Statistical Software, Version 16.1.’ (Minitab Inc.: State College, PA, USA) Available at: www.minitab.com
Mösslache F (1998) Subsurface dwelling crustacea as indicators of hydrological conditions, oxygen concentration and sediment structure in an alluvial aquifer. International Review of Hydrobiology 83, 349–364.
| Subsurface dwelling crustacea as indicators of hydrological conditions, oxygen concentration and sediment structure in an alluvial aquifer.Crossref | GoogleScholarGoogle Scholar |
Mosslacher F, Griebler C, Notenboom J (2001) Biomonitoring of groundwater systems: Methods, applications and possible indicators among the groundwater biota. In ‘Groundwater ecology: A tool for management of water resources’. (Eds C Griebler, D Danielopol, J Gibert, HP Nachtnebel, J Notenboom) pp. 132–170. (European Communities: Luxembourg)
Murphy NP, Adams M, Austin AD (2009) Independent colonization and extensive cryptic speciation of freshwater amphipods in the isolated groundwater springs of Australia’s Great Artesian Basin. Molecular Ecology 18, 109–122.
Muschal M, Warne MJ (2003) Risk posed by pesticides to aquatic organisms in rivers of northern inland New South Wales, Australia. Human and Ecological Risk Assessment 9, 1765–1787.
| Risk posed by pesticides to aquatic organisms in rivers of northern inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Preston-Mafham J, Boddy L, Randerson PF (2002) Analysis of microbial community functional diversity using sole-carbon-source utilization profiles—a critique. FEMS Microbiology Ecology 42, 1–14.
Riley SJ (1975) Channel shape–grain size relation in Eastern Australia and some paleohydorlogic implications. Sedimentary Geology 14, 253–258.
| Channel shape–grain size relation in Eastern Australia and some paleohydorlogic implications.Crossref | GoogleScholarGoogle Scholar |
Schmidt SI, Hahn HJ, Hatton TJ, Humphreys WF (2007) Do faunal assemblages reflect the exchange intensity in groundwater zones? Hydrobiologia 583, 1–19.
| Do faunal assemblages reflect the exchange intensity in groundwater zones?Crossref | GoogleScholarGoogle Scholar |
Serov PA (2002) ‘A preliminary identification of Australian Syncarida (Crustacea).’ MDFRC Identification Guide No. 44. (CRC Freshwater Ecology: Albury, NSW)
Shah T (2007) Groundwater: a global assessment of scale and significance In ‘Water for food, water for life: A Comprehensive assessment of water management’. Ch. 10. (Ed. D Molden) (Earthscan: London/International Water Management Institute: Colombo) Available at: www.iwmi.cgiar.org
Stein H, Kellermann C, Schmidt SI, Breilmann H, Steube C, Berkhoff SE, Hahn HJ, Thulin B, Griebler C (2010) The potential use of fauna and bacteria as ecological indicators for the assessment of groundwater quality. Journal of Environmental Monitoring 12, 242–254.
| The potential use of fauna and bacteria as ecological indicators for the assessment of groundwater quality.Crossref | GoogleScholarGoogle Scholar |
Stoch F, Galassi DMP (2010) Stygobiotic crustacean species richness: a question of numbers, a matter of scale. Hydrobiologia 653, 217–234.
| Stygobiotic crustacean species richness: a question of numbers, a matter of scale.Crossref | GoogleScholarGoogle Scholar |
Strayer DL, May S, Nielsen P, Wolheim W, Hausam S (1997) Oxygen, organic matter and sediment granulometry as control on hyporheic animal communities. Archiv fur Hydrobiologie 140, 131–145.
Stumpp C, Hose GC (2013) The impact of water table drawdown and drying on subterranean aquatic fauna in in-vitro experiments. PLoS ONE 8, e78502
| The impact of water table drawdown and drying on subterranean aquatic fauna in in-vitro experiments.Crossref | GoogleScholarGoogle Scholar |
Tomlinson M (2008) A framework for determining environmental water requirements for alluvial aquifer ecosystems. PhD Thesis, University of New England, NSW, Australia.
Velasco Ayuso S, Guerrero MC, Monters C, Lopez-Archilla AI (2009) Spatiotemporal distribution of microbial communities in a coastal, sandy aquifer system (Doñana, SW Spain). Geobiology 7, 66–81.
| Spatiotemporal distribution of microbial communities in a coastal, sandy aquifer system (Doñana, SW Spain).Crossref | GoogleScholarGoogle Scholar |
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. The Journal of Geology 30, 377–392.
| A scale of grade and class terms for clastic sediments.Crossref | GoogleScholarGoogle Scholar |
Young RW, Young ARM, Price DM, Wray RAL (2002) Geomorpology of the Namoi alluvial plain, northwestern New South Wales. Australian Journal of Earth Sciences 49, 509–523.
| Geomorpology of the Namoi alluvial plain, northwestern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Zhang C, Palumbo AV, Phelps TJ, Beauchamp JJ, Brockman FJ, Murray CJ, Parsons BS, Swift DJP (1998) Grain size and depth constraints on microbial variability in coastal plain subsurface sediments. Geomicrobiology Journal 15, 171–185.
| Grain size and depth constraints on microbial variability in coastal plain subsurface sediments.Crossref | GoogleScholarGoogle Scholar |
Zhou J, Xia B, Treves D, Wu L, Marsh T, O’Neil R, Palumbo A, Tiedje J (2002) Spatial and resource factors influencing high microbial diversity in soil. Applied and Environmental Microbiology 68, 326–334.
| Spatial and resource factors influencing high microbial diversity in soil.Crossref | GoogleScholarGoogle Scholar |

|



