Managing glyphosate resistance in Australian cotton farming: modelling shows how to delay evolution and maintain long-term population control
David Thornby A C , Jeff Werth A and Steven Walker BA Queensland Department of Agriculture, Fisheries and Forestry, PO Box 2282, Toowoomba, Qld 4350, Australia.
B University of Queensland, Queensland Alliance for Agriculture and Food Innovation, PO Box 2282, Toowoomba, Qld 4350, Australia.
C Corresponding author. Email: david.thornby@daff.qld.gov.au
Crop and Pasture Science 64(8) 780-790 https://doi.org/10.1071/CP13109
Submitted: 4 April 2013 Accepted: 19 August 2013 Published: 29 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Glyphosate resistance is a rapidly developing threat to profitability in Australian cotton farming. Resistance causes an immediate reduction in the effectiveness of in-crop weed control in glyphosate-resistant transgenic cotton and summer fallows. Although strategies for delaying glyphosate resistance and those for managing resistant populations are qualitatively similar, the longer resistance can be delayed, the longer cotton growers will have choice over which tactics to apply and when to apply them. Effective strategies to avoid, delay, and manage resistance are thus of substantial value. We used a model of glyphosate resistance dynamics to perform simulations of resistance evolution in Sonchus oleraceus (common sowthistle) and Echinochloa colona (awnless barnyard grass) under a range of resistance prevention, delaying, and management strategies.
From these simulations, we identified several elements that could contribute to effective glyphosate resistance prevention and management strategies. (i) Controlling glyphosate survivors is the most robust approach to delaying or preventing resistance. High-efficacy, high-frequency survivor control almost doubled the useful lifespan of glyphosate from 13 to 25 years even with glyphosate alone used in summer fallows. (ii) Two non-glyphosate tactics in-crop plus two in-summer fallows is the minimum intervention required for long-term delays in resistance evolution. (iii) Pre-emergence herbicides are important, but should be backed up with non-glyphosate knockdowns and strategic tillage; replacing a late-season, pre-emergence herbicide with inter-row tillage was predicted to delay glyphosate resistance by 4 years in awnless barnyard grass. (iv) Weed species’ ecological characteristics, particularly seed bank dynamics, have an impact on the effectiveness of resistance strategies; S. oleraceus, because of its propensity to emerge year-round, was less exposed to selection with glyphosate than E. colona, resulting in an extra 5 years of glyphosate usefulness (18 v. 13 years) even in the most rapid cases of resistance evolution.
Delaying tactics are thus available that can provide some or many years of continued glyphosate efficacy. If glyphosate-resistant cotton cropping is to remain profitable in Australian farming systems in the long-term, however, growers must adapt to the probability that they will have to deal with summer weeds that are no longer susceptible to glyphosate. Robust resistance management systems will need to include a diversity of weed control options, used appropriately.
Additional keywords: cotton, Echinochloa colona, glyphosate, herbicide resistance, modelling, Sonchus oleraceus.
Introduction
Weed control in Australian cotton farming has become substantially less diverse over recent decades (Werth et al. 2008), owing to the imperatives of minimising tillage for soil and water conservation, regulatory and environmental pressures, post-patent price reductions of the system’s most useful herbicide (glyphosate), and the broad adoption of glyphosate-resistant crops. All of these factors have increased the use and importance of glyphosate for weed control in Australian cotton production, and at the same time reduced the use of other herbicides and tillage. Cotton production in Australia is especially reliant on glyphosate for weed control; well over 90% of the area planted to cotton each year carries a glyphosate-resistance trait. In recent years, cotton has been planted on up to 600 000 ha per season in New South Wales and Queensland (ABARES 2012). Crops may be planted annually if irrigation is available, or biennially or less frequently without irrigation. In dryland situations, cotton has typically been grown in rotation with other crops, usually with winter cereals (Walker et al. 2005). Summer fallows are used between winter crops, and between cotton crop years in dryland cropping, to allow incorporation and retention of soil moisture for use by subsequent summer crops. Where summer fallows are included in the cropping rotation, glyphosate is also the main method of weed control (Walker et al. 2005; Osten et al. 2007). Therefore, whether summer cropping is annual, biennial, or even less frequent, summer-active weeds are under substantial selection pressure for glyphosate resistance. Loss of control of summer annual weed species, including through resistance, is a substantial threat to cotton production in this system.
Over-reliance on glyphosate in subtropical Australian farming has resulted in the selection of both glyphosate-tolerant weeds (Werth et al. 2010) and glyphosate-resistant biotypes of Echinochloa colona (L.) Link. (awnless barnyard grass), a usually susceptible summer annual grass (Nguyen Thai et al. 2012). Both resistant and tolerant species make good control of the weed spectrum with glyphosate difficult, and since managing weeds with a diversity of methods is both more complex and more expensive than using glyphosate alone, resistance (and indeed even the need for strategies to delay the onset of resistance) threatens the long-term viability of many cotton farms.
Australian cotton is predominantly grown in subtropical north-eastern Australia. Although some growers in this region are now faced with glyphosate-resistant populations of E. colona (Preston 2012), in the majority of fields, growers are still likely to be trying to maximise the time remaining before glyphosate susceptibility is lost in this weed. There are also various other locally important species that are not yet resistant. Therefore, attempting to create substantial delays in the onset of resistance remains a viable goal for most growers for at least some fields, even while the management of resistant populations is rapidly becoming the major imperative.
In Australia, transgenic glyphosate-resistant cotton cropping is subject to regulation via a Crop Management Plan (CMP) (Monsanto Australia 2013). The CMP mandates that growers must take action to prevent seed set in any weeds that survive glyphosate applications, but does not include specific instructions on how this is done. Substantial variability likely exists in how dedicated and effective individuals are at finding survivors, and when and how they attempt to kill them. We used an updated version of our glyphosate resistance model (Thornby and Walker 2009) to explore how this variability might affect the usefulness of the survivor-control approach.
Through mode-of-action labelling on products, the CMP, and best management practice advice in general, growers are encouraged to use a variety of methods to control weeds in an integrated weed management (IWM) program (McGillion and Storrie 2006). Several pre-emergent and post-emergent herbicides applied at various timings, and inter-row cultivation, are available in-crop, and tillage plus a wider range of herbicides can also be used in summer fallows (Maas 2012). We used our updated model to predict the effectiveness of the range of IWM tools available in cotton cropping systems in Australia, both in-crop and in-fallow.
In order to explore the effects of these variations on evolution of glyphosate resistance, we simulated the number of years of glyphosate susceptibility for two key weed species, E. colona and Sonchus oleraceus L. (common sowthistle), under a range of different management strategies in irrigated and non-irrigated cotton systems. Both of these weeds are frequently observed to be among the most prevalent species in subtropical north-eastern Australia generally (as in Osten et al. 2007), or on Australian cotton farms specifically (Werth et al. 2010). Thus, loss of control of these weeds would be (or is, in the case of E. colona) a widespread threat to the sustainable use of glyphosate-resistant cotton in Australia. Both species scored in the top six of all weeds listed on glyphosate product labels in Australia for propensity to evolve herbicide resistance (Werth et al. 2011). We evaluated the outcome of each weed control strategy in terms of delaying resistance, the effects of ecological differences between species on the effectiveness of each strategy, and the impact on long-term weed population density. We aimed to use the model’s predictions to develop a set of strategies that could guide grower decision-making towards minimising the rate of evolution of glyphosate resistance in E. colona and S. oleraceus, and maximising weed population control over the long-term, even where resistance occurs.
Materials and methods
Glyphosate resistance model
To simulate the evolution of resistance in Australian cotton farming systems, we updated our glyphosate resistance model to include representations of all the typical weed control options used in herbicide-resistant and conventional cotton in subtropical north-eastern Australia. The model is an age- and stage-structured population model that simulates weed population density and changes in allele frequencies for a resistance-conferring gene. The model is implemented in Vensim 5 (Ventana Systems, Inc., Harvard, MA, USA), linked to the Agricultural Production Systems Simulator (APSIM; Keating et al. 2003). Through APSIM, the model runs on a daily time-step and allows for variation in weather conditions and therefore weed control requirements from year to year.
Various APSIM modules are used to provide functionality around plant growth, competition between weeds and crops, and resource availability for growth and development such as water and nutrients. The APSIM manager module also provides for on-event scripting for weed management and flags for mating and germination events. The Vensim extensions (as outlined in Thornby and Walker 2009) model resistance genetics, mating between genotypes, and seed bank dynamics, since APSIM does not directly model population persistence between seasons. Resistance is assumed to be due to a single, partially dominant gene conferring moderate levels of resistance, as has been found in the resistant E. colona populations studied to date (Nguyen Thai et al. 2012) and in other species with glyphosate resistance (Powles and Preston 2006). Figure 1 provides a schematic view of the breakdown between population dynamics equations in Vensim and APSIM’s plant growth and development code and management scripting. A summary of the model is given below, summarised from Thornby and Walker (2009).

|
There are three seed bank fractions, defined as age classes: <1 year old; 1–2 years old; ≥3 years old; and three genotypes (homozygous susceptible, homozygous resistant, heterozygous). The size S of the seed bank fractions i for genotype g in any year n is determined as:
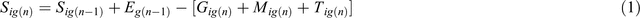
where E is the number of seeds entering the seed bank fraction, either from seed rain for the first fraction or as transport from younger fractions (that is, ageing) for fractions two and three; G is the number of germinants from a seed bank fraction; M is the seed bank mortality for a fraction; and T is the transport out from a fraction to the next oldest fraction (again simulating ageing), if applicable, at the end of the season. Seed bank mortalities are applied as a simple multiplier per fraction.
Seedling cohorts are germinated as a coarse estimated accumulation of staggered germinations over time. Up to four cohorts may germinate per year for any species, each on a single day in the model, with contributions from each age fraction of the seed bank for each genotype. The size of a cohort C of genotype g emerging from seed bank age fraction i is determined thus:

where Cgmax is the maximum number of seeds that could germinate in a cohort (a fixed proportion of the current seed bank); Drain is a discount factor for rainfall amounts accumulated in APSIM over 5 days that fall between the minimum and the optimum; Dtherm is a discount factor for thermal time in degree-days (base 12°C) accumulated since the beginning of the season or the time of the last cohort; and Dseed is a discount factor that reduces potential germination in older seed bank age fractions. All discount factors D have values 0 < D ≤ 1 (Table 1).

|
As in the previous version of the model, we developed a set of parameters for APSIM’s generic weed module in order to model additional species, including S. oleraceus (Table 1). Parameter values for E. colona were also altered to reflect more recent data.
Simulations
We developed a range of scenarios of resistance evolution in cotton, in three categories: (a) crop rotations; (b) frequency and efficacy of controlling glyphosate survivors in transgenic cotton crops; and (c) IWM strategies, varying diversity of weed management actions in crops and summer fallows.
In each of these simulations, the population is assumed to have had no prior selection by glyphosate. Each scenario is simulated over 30 years. A baseline strategy of glyphosate-only applications in annual glyphosate-resistant cotton crops is common to all three sets of simulations a, b, and c.
In all simulations, E. colona emerges after rainfall between September and March and is sprayed with glyphosate after each flush in fallows if the population is greater than the population density threshold of 0.5 plants m–2 (Table 1), plus other controls as specified for each simulation (see Tables 2, 3, 4). Sonchus oleraceus can emerge year-round (Widderick et al. 2010), and is controlled after emergence in fallows with one application of glyphosate plus a broadleaf-selective herbicide (a common control measure in the real system), followed by glyphosate alone at 4-week intervals, if the population is >0.5 plants m–2, plus other controls as specified for each scenario. In all simulations, the non-glyphosate controls were applied regardless of weed density.

|

|
In all three sets of simulations, we analysed annual and biennial cotton cropping. In biennial cropping, the non-cotton summers remain fallow. Management of summer fallows is critically important for maximising crop yields in this system, so we have incorporated scenarios to predict the difference between using glyphosate alone and glyphosate plus other tactics in summer fallows.
Crop rotation
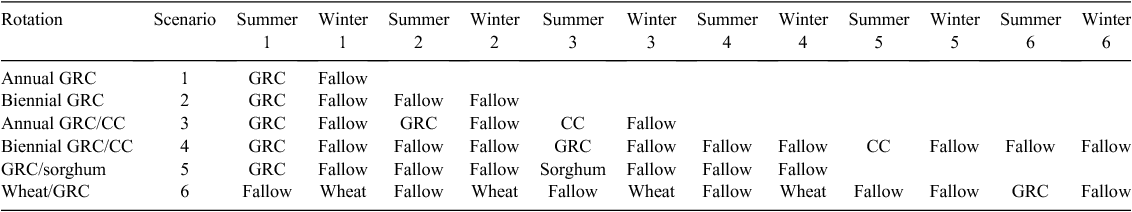
We used the model to analyse several subtropical, north-eastern Australian, mixed-crop rotations for comparison with cotton-only rotations (Table 2). Many of this region’s non-irrigated growers are highly responsive to changes in commodity prices and available soil moisture, and consequently grow diverse rotations including cotton and winter and summer grains and pulses. We tested several rotations as examples of Australian cropping systems, both representative of current practice, and speculative: (i) glyphosate-resistant cotton alone; (ii) two glyphosate-resistant followed by one conventional cotton; (iii) glyphosate-resistant cotton and sorghum in alternate years; (iv) four winter wheat crops followed by a long fallow and one glyphosate-resistant cotton crop in the subsequent summer.
Weed-control tactics for the rotation scenarios were as follows. In glyphosate-resistant cotton crops, only glyphosate was used. In conventional cotton, pre-emergence herbicides (i.e. soil-applied herbicides used to control weeds not yet emerged) were applied before sowing and mid-season, and glyphosate was applied at planting. In sorghum crops, a pre-emergence herbicide and glyphosate were used before sowing, and glyphosate was applied before harvest. In wheat, glyphosate was applied at sowing, and one broadleaf herbicide application was made in-crop. In summer fallows between wheat crops, a pre-emergence herbicide was used before first emergence of summer weeds, and glyphosate was applied to subsequent emergences over the population density threshold. In each case of the use of pre-emergence herbicide, we assumed that the herbicide used was one appropriate to the soil, climate, and plant-back situation in which it is applied. Efficacy was 90% on the day of application, falling to zero over a period of 8 weeks.
Controlling glyphosate survivors
We used a set of simulations to test the effects of applying a follow-up tactic to control survivors of glyphosate applications at different frequencies in glyphosate-resistant cotton cropping (Table 3). The follow-up action was at either 80% kill rate or 99.9% kill rate, and was applied after every glyphosate application, after one glyphosate application per crop, or after one glyphosate application every second crop. We tested annual and biennial cotton rotations as defined above, so that in the least-frequent case, one follow-up application was being applied every 4 years.
In the biennial cotton simulations, we tested the frequency and efficacy of survivor control against a background of either glyphosate-only summer fallows or ‘IWM fallows’, in which a pre-emergent herbicide is applied shortly before first summer weed emergence, a ‘double-knock’ (glyphosate followed a few days later by paraquat) is applied to the first-emerging cohort, and glyphosate is used on subsequent emergences if the trigger weed density threshold is exceeded.
Integrated Weed Management in cotton
In this set, we varied the number of non-glyphosate actions in crop and fallow in glyphosate-resistant cotton (Table 4). The in-crop, non-glyphosate tactics were pre-plant and midseason (known as ‘layby’) pre-emergent herbicides, full disturbance tillage at planting, and tillage between rows (affecting an estimated 80% of the weed population). The layby herbicide is assumed to have minimal impact on the crop’s competitiveness with weeds. Potential tillage effects on subsequent emergences or on soil structure are not modelled. Summer fallow strategies used in biennial cotton were either with IWM (a spring-applied, pre-emergence herbicide and a double-knock, plus glyphosate up to every 4 weeks thereafter if required), or with glyphosate alone, as in the survivor control simulations described above.
In all simulations, glyphosate is applied three times over the top of every glyphosate-resistant cotton crop.
Results
Biennial cropping with glyphosate-resistant cotton and without non-glyphosate tactics in summer fallows was, overall, the worst-case scenario for E. colona (scenario 1, Table 5). Glyphosate resistance was predicted to evolve in 13 years, and long-term seed-bank density was the highest of all scenarios. Results for S. oleraceus were less clear; biennial cropping with glyphosate alone resulted in resistance after 18 years, but several other scenarios had similar results and two were slightly faster (see below).
Crop rotations
In annual cotton cropping scenarios, switching between glyphosate-resistant and conventional cotton varieties had little effect on either rate of resistance evolution or long-term seed bank population size, adding only a year at most to the useful lifespan of glyphosate (from 18 to 19 years to resistance in S. oleraceus, scenarios 1 and 3, Table 5). In biennial situations, moderate benefits for slowing resistance were predicted for both weeds (scenarios 2–6). Two years were added to time-to-resistance where glyphosate was used alone in summer fallows (up to 15 years in E. colona and 20 years in S. oleraceus). Greater benefits were obtained by adding a pre-emergence herbicide and a double-knock to summer fallows, however. Switching between glyphosate-resistant and conventional cotton and using two non-glyphosate actions in the summer fallows increased the predicted useful lifespan of glyphosate on E. colona to 20 years, an increase of almost 50% over the worst case, and on S. oleraceus by >60% (scenario 3). Seed bank density was similarly reduced; very low-density seed banks were predicted for S. oleraceus when non-glyphosate actions were added to summer fallows (7–187 seeds/m2). Seed banks were reduced by ~40% for E. colona, compared with the worst case, although the levels achieved (>3000 seeds m–2) would still be termed inadequate long-term control.
Switching to a cotton–sorghum rotation (scenario 5) was not predicted to offer any benefits over glyphosate-resistant cotton alone for S. oleraceus, but was of substantial benefit for long-term E. colona control, reducing long-term seed bank density from >5000 to <500 seeds m–2, and delaying resistance by up to 7 years, where non-glyphosate actions were used in summer fallows. The cotton–sorghum rotation was the only one of this set of scenarios in which satisfactory long-term control of E. colona seed banks was obtained.
The wheat–cotton rotation was marginally better than cotton alone for E. colona (scenario 6). For S. oleraceus, long-term seed bank control was particularly improved (down to 4–287 seeds m–2), and where IWM measures were used in summer fallows, this was one of only a few simulations in the whole experiment in which resistance did not come to dominate the population within 30 years.
For both weeds, there was a substantial difference between scenarios with glyphosate-only summer fallows and those with IWM used in summer fallows. Resistance evolution was delayed by up to 9 years in E. colona and ≥9 years in S. oleraceus. Long-term average seed-bank densities were reduced by ~10–80% in E. colona and by 90–99% in S. oleraceus, just by adding two non-glyphosate actions to summer fallows.
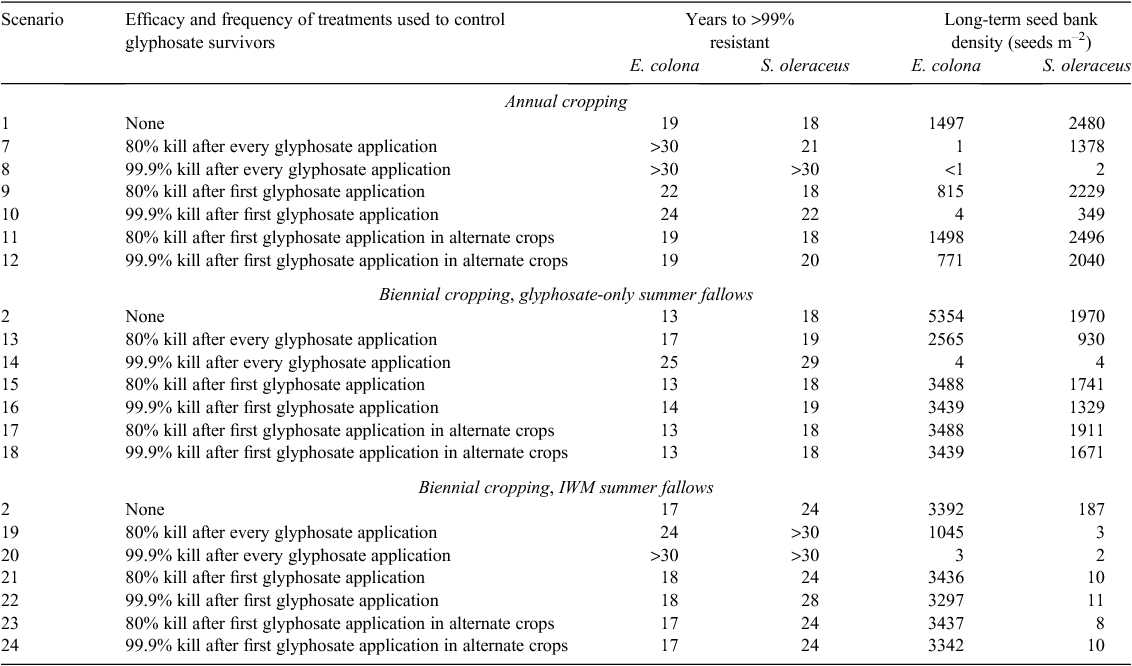
Controlling glyphosate survivors
The model predicted that substantial reductions in the rate of evolution of glyphosate resistance could be obtained by deliberately controlling the survivors of glyphosate applications (Table 6). Echinochloa colona was more likely to respond to these delaying tactics than S. oleraceus, including delays of up to 12 years (almost doubling the worst-case lifespan of glyphosate) in biennial cropping with glyphosate-only summer fallows (scenario 14), and delays in resistance beyond 30 years in annual cropping scenarios (7 and 8) and one biennial cropping scenario with IWM tactics used in summer fallows (scenario 20). Long-term seed bank control in biennial cropping was adequate for E. colona (reduced from ~5000 to 1000 seeds m–2) or very good (down to 3 seeds m–2) where multiple applications of survivor-control methods were made per season, for glyphosate-only and IWM summer fallows, respectively (scenarios 19 and 20). Similar seed-bank size predictions were found for S. oleraceus except that summer fallow IWM added much greater benefits in biennial cropping scenarios. While efficacy was important in delaying resistance and providing long-term control of population size, using any tactic at high frequency was better than using very high efficacy tactics infrequently.
Integrated Weed Management in cotton
The model predicted that varying levels of success could be obtained by using combinations of non-glyphosate tactics in-crop and in summer fallows (Table 7). Notably, there were substantial differences between annual and biennial cropping in the case of E. colona. In annual cropping scenarios, every strategy using more than one non-glyphosate action in every crop resulted in resistance evolution being pushed beyond the 30-year time limit of the model (scenarios 27, 37, 40, 46, 49). Where non-glyphosate controls were applied in every crop (scenarios 27, 37, 40, 46), long-term seed-bank control was excellent (<60 seeds m–2) except in the case of adding a single pre-planting, pre-emergence herbicide (scenario 25), in which case, long-term seed-bank control was still substantially better than using glyphosate alone, at 876 seeds m–2. Results for S. oleraceus, conversely, were not substantially different from the use of glyphosate alone, except for some seed-bank size reduction where two inter-row tillage operations were combined with an early pre-emergence herbicide (scenario 46).
Where summer fallows in biennial cropping contained only glyphosate, results from using any type, combination, or frequency of non-glyphosate tactics were diluted substantially. In particular, none of the simulations with glyphosate used alone in fallows predicted good long-term control of resistant seed banks of either weed; seed banks varied between ~3400 and 5000 seeds m–2 for E. colona and between 1800 and 2000 seeds m–2 for S. oleraceus where only pre-emergence herbicides were added in-crop (scenarios 32, 33, 34, 36). Some reasonable reductions over the worst-case scenario were found for E. colona where frequent inter-row tillage was used (scenarios 39 and 42), down to as low as 780 seeds m–2 where inter-row tillage was combined with an early-season, pre-emergence herbicide (scenario 48).
The most robust strategy (an early-season, pre-emergence herbicide plus two inter-row cultivations) when used annually resulted in good long-term seed-bank control in concert with IWM in fallows (scenario 47), and was predicted to offer substantial increases (of >17 years for E. colona and 11 years for S. oleraceus) in the useful lifespan of glyphosate.
Discussion
It is not surprising that more frequent use of non-glyphosate tactics equates generally to greater delay before populations become dominated by resistance, and to better long-term control of resistant populations. In order to develop useful strategies for cotton growers, however, we must look for nuances in the simulation results.
Best management strategies are those that deliver good results (i.e. long-term delays in resistance evolution) and fit well with the cropping system. Some authors posit that it is more efficient to use up all of the available susceptibility to a given herbicide and then switch to another option. This is particularly the case in bioeconomic analyses of resistance where herbicide susceptibility is assumed to be a non-renewable resource simply or linearly depleted by every application of the herbicide (as discussed in Weersink et al. 2005). It is not clear from our results that the system is so simply reducible, particularly when frequent, highly effective, non-glyphosate weed control tactics are used. Furthermore, the bioeconomic position assumes that the only difference between glyphosate and other options is price, which is demonstrably untrue; glyphosate has advantages over all currently available alternatives in one or more of ease and safety of use, broadness of spectrum, environmental credentials, and reliability. One application of glyphosate foregone now could be of greater value if made later, depending on changes in weed flora, crop rotation, or even the regulatory environment. Retaining the ability for ‘emergency control’ of weeds that for reasons of climate or crop timing are unable to be controlled with ‘less forgiving’ herbicides than glyphosate is of substantial practical value. A system that maximises the number of available control options for the maximum amount of time can be demonstrated to be optimal if the values to be optimised are not solely price-related, and thus maximising the length of time before populations become mostly glyphosate-resistant is consistent, in our view, with a best management approach.
Controlling survivors: timing and efficacy
Deliberate, well-timed control of the survivors of glyphosate sprays is predicted to be the best tactic for delaying and managing resistance (Table 6). This was true for both weed species, although several permutations of IWM tactics used on S. oleraceus were almost as effective (Table 7). In annual cotton cropping, frequent use of even relatively low-efficacy options for controlling survivors was predicted to be sufficient to provide good long-term control. Where crops are grown in alternate years (and by extrapolation, less frequently), higher efficacy and the addition of some non-glyphosate actions in fallows are required to provide good results (Table 6). We tested at 80% and 99.9% efficacy levels, which could be implemented as inter-row cultivation or shielded spraying with a knockdown herbicide (80%), or as some combination of those with additional chipping, hand pulling, or spot spraying (99.9%).
The efficacy of non-glyphosate treatments was important to the effectiveness of resistance-delaying strategies. In particular, strategies that relied only on pre-emergence herbicides (which, due to variations in timing between application and germination, often had quite low efficacies) were less effective than strategies including knockdown herbicides (used as double-knock applications here) or tillage. However, the results for controlling-survivor simulations in cotton (Table 6) show that applying moderately effective control multiple times per season is better than using more effective tactics less often.
Crop frequency, summer fallows and IWM
The simulations investigating the effects of varying IWM tactics in crop (Table 7) demonstrate the importance of also including non-glyphosate actions between crops. Summer fallows where only glyphosate is used seriously dilute the beneficial effects of even the most frequent in-crop, non-glyphosate tactics. The particular tactics used in-crop had some bearing on the outcome; inter-row tillage, while not the most efficacious treatment available, could be performed at any time in the model, catching late germinations more effectively than pre-emergence herbicides. Pre-emergence herbicides were also prone to losing efficacy if germinations did not occur close to the time of application, so in general terms, while the best strategies used a mixture of tactics, inter-row tillage was a more robust option especially late in-crop.
The substantial differences for E. colona outcomes between annual and biennial cropping indicate that annual cropping (i.e. irrigated cropping) is predicted to receive large benefits from adding non-glyphosate actions in-crop. This is likely to be due to a combination of the increased frequency of non-glyphosate tactics in these scenarios and the effects of annual, substantial crop competition on E. colona seed production.
Species differences
The differences between S. oleraceus and E. colona demonstrate that species ecology is of real importance in resistance management. In the cotton system studied here, summer-dominant species such as E. colona appear to be both more susceptible to glyphosate resistance and more responsive to strategies that incorporate some use of non-glyphosate tactics, especially in the case of annual cropping. Since S. oleraceus emerges in both winter and summer in the model, a substantial proportion of the annual emerged weed population is either not affected by weed control (for simulations with winter cropping) or affected by winter applications of glyphosate plus a selective broadleaf herbicide. Thus, interactions between biology and agronomy appear to result in different rates of resistance evolution. Strategies for managing resistant populations should therefore be devised with reference to species ecology, rather than generically.
Long-term management
Growers in subtropical northern Australian cropping are almost certainly not dealing with completely unselected weed populations; that is, they may have some non-trivially elevated level of resistance-conferring alleles in weed populations even where glyphosate resistance is still invisible. For non-irrigated cotton growers in Australia who have been using reduced tillage practices and planting glyphosate-resistant cotton for >10 years, our simulations paint a grim picture, suggesting that only a few years, if any, of efficacy may be left on key species. However, they also show that long-term seed-bank management is possible where a robust strategy is applied before the population becomes mostly or entirely resistant. In particular, biennial cropping scenarios showed that using IWM in summer fallows resulted in consistently lower long-term seed-bank densities of both weeds.
Developing strategies
Models are particularly effective tools for refining and developing strategies for herbicide resistance prevention and management, since they can be used to test much larger numbers of situations and permutations than could be done in the field, and much more quickly. Where current resistance management strategies are largely generic and qualitative, strategies developed using simulation models such as this one can be quantified and specific to particular regions and industries.
Our simulations suggest the following are important parts of a robust strategy for glyphosate resistance management in subtropical broadacre farming:
-
Deliberately seeking out and controlling glyphosate survivors is the most robust approach to delaying or preventing resistance. Infrequent but very high-efficacy survivor control is not a good substitute for monitoring and controlling survivors frequently.
-
Two non-glyphosate tactics in crop plus two in summer fallows provide long-term delays in resistance evolution. It is especially important to avoid glyphosate-only summer fallows.
-
Pre-emergence herbicides are an important tool, but should be backed up with non-glyphosate knockdowns and strategic tillage, especially for controlling late-season germinations.
-
Resistance prevention and management plans should be devised with reference to species characteristics, particularly including seed bank dynamics.
Monitoring herbicide efficacy after spraying is a critical component of any strategy for weed control and resistance prevention, and our recommendations for controlling survivors rely on robust monitoring practices. It is equally important to follow these guidelines in non-cropped areas on farms as well as in fields.
Our simulations are not spatially explicit. If, in future real incidences of glyphosate resistance in Australian cotton cropping, patches of resistant plants can be identified while still manageably small, zonal management approaches could be taken to minimise the cost of responding to the resistant weed biotype. Future modelling work could usefully aim at describing the spatial dynamics of resistance in patches, to define appropriate, spatially explicit management recommendations.
Acknowledgements
We gratefully acknowledge funding of this work by the Cotton Research and Development Corporation, the Cotton Catchment Communities Cooperative Research Centre, the Grains Research and Development Corporation, and Monsanto Australia.
References
ABARES (2012) ‘Agricultural commodity statistics 2012.’ (Australian Bureau of Agricultural and Resource Economics and Sciences: Canberra, ACT)Boutsalis P, Powles SB (1995) Resistance of dicotyledon weeds to acetolactate synthase (ALS)-inhibiting herbicides in Australia. Weed Research 35, 149–155.
| Resistance of dicotyledon weeds to acetolactate synthase (ALS)-inhibiting herbicides in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXot12ku7o%3D&md5=ca2f7b53ca3f8f0640bef1673acd58f7CAS |
Chauhan BS, Gill G, Preston C (2006) Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Science 54, 854–860.
| Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpvF2rsL0%3D&md5=09016b9f7403982d3198431db66351d6CAS |
Dorado J, Sousa E, Calha IM, González Andújar JL, Fernández-Quintanilla C (2009) Predicting weed emergence in maize crops under two contrasting climatic conditions. Weed Research 49, 251–260.
| Predicting weed emergence in maize crops under two contrasting climatic conditions.Crossref | GoogleScholarGoogle Scholar |
Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z, McLean G, Verburg K, Snow V, Dimes JP, Silburn M, Wang E, Brown S, Bristow KL, Asseng S, Chapman S, McCown RL, Freebairn DM, Smith CJ (2003) An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288.
| An overview of APSIM, a model designed for farming systems simulation.Crossref | GoogleScholarGoogle Scholar |
Maas S (Ed.) (2012) ‘Cotton pest management guide.’ (Greenmount Press: Toowoomba, Qld)
Madsen KH, Valverde BE, Jensen JE (2002) Risk assessment of herbicide-resistant crops: a Latin American perspective using rice (Oryza sativa) as a model. Weed Technology 16, 215–223.
Martinkova Z, Honek A, Lukas J (2006) Seed age and storage conditions influence germination of barnyardgrass (Echinochloa crus-galli). Weed Science 54, 298–304.
McGillion T, Storrie A (Ed.) (2006) ‘Integrated Weed Management in Australian cropping systems—a training resource for farm advisors.’ (CRC for Australian Weed Management: Adelaide)
Mercado BL, Talata RL (1977) Competitive ability of Echinochloa colonum L. against direct-seeded lowland rice. In ‘Proceedings of the 6th Asia-Pacific Weed Science Society Conference’. Indonesia. (Asia-Pacific Weed Science Society)
Monsanto Australia (2013) Roundup Ready Flex® Cotton Crop Management Plan. Monsanto Company. Available at: www.monsanto.com/global/au/products/Documents/Monsanto/Roundup Ready Flex CMP 2012.pdf
Nguyen Thai H, Malone J, Boutsalis P, Preston C (2012). An overview of glyphosate resistance in barnyard grass (Echinochloa colona). In ‘Proceedings of the 18th Australasian Weeds Conference’. (Ed. V. Eldershaw) (Weed Society of Victoria, Inc.: Melbourne)
Osten VA, Walker SR, Storrie A, Widderick M, Moylan P, Robinson GR, Galea K (2007) Survey of weed flora and management relative to cropping practices in the north-eastern grain region of Australia. Australian Journal of Experimental Agriculture 47, 57–70.
| Survey of weed flora and management relative to cropping practices in the north-eastern grain region of Australia.Crossref | GoogleScholarGoogle Scholar |
Powles SB, Preston C (2006) Evolved glyphosate resistance in plants: biochemical and genetic basis of resistance. Weed Technology 20, 282–289.
| Evolved glyphosate resistance in plants: biochemical and genetic basis of resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvFKhtr8%3D&md5=c5f9a75db118578021529f17c2646697CAS |
Preston C (2012) The Australian Glyphosate Sustainability Working Group. May 2012. Available at: www.glyphosateresistance.org.au/
Salisbury EJ (1942) ‘The reproductive capacity of plants.’ (G. Bell: London)
Steinmaus SJ, Prather TS, Holt JS (2000) Estimation of base temperatures for nine weed species. Journal of Experimental Botany 51, 275–286.
| Estimation of base temperatures for nine weed species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsVOjs7Y%3D&md5=08d2cf96597ad14707ef6be31dc63ddeCAS | 10938833PubMed |
Thornby DF, Walker SR (2009) Simulating the evolution of glyphosate resistance in grains farming in northern Australia. Annals of Botany 104, 747–756.
| Simulating the evolution of glyphosate resistance in grains farming in northern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtF2ktbjF&md5=d75bc157f347650ba6fc48ce8e6dfddeCAS | 19567415PubMed |
Uremis I, Uygur FN (2005) Seed viability of some weed species after 7 years of burial in the Cukurova Region of Turkey. Asian Journal of Plant Science 4, 1–5.
| Seed viability of some weed species after 7 years of burial in the Cukurova Region of Turkey.Crossref | GoogleScholarGoogle Scholar |
Walker SR, Taylor IN, Milne G, Osten VA, Hoque Z, Farquharson RJ (2005) A survey of management and economic impact of weeds in dryland cotton cropping systems of subtropical Australia. Australian Journal of Experimental Agriculture 45, 79–91.
| A survey of management and economic impact of weeds in dryland cotton cropping systems of subtropical Australia.Crossref | GoogleScholarGoogle Scholar |
Walker S, Wilson B, Wu H, Widderick M, Taylor I (2006) Weed seed persistence with changing farming practices in southern Queensland. In ‘Proceedings of the 15th Australian Weeds Conference’. Adelaide. (Eds C Preston, JH Watts, ND Crossman) (Weed Management Society of South Australia: Adelaide)
Weersink A, Llewellyn RS, Panell DJ (2005) Economics of pre-emptive management to avoid weed resistance to glyphosate in Australia. Crop Protection 24, 659–665.
| Economics of pre-emptive management to avoid weed resistance to glyphosate in Australia.Crossref | GoogleScholarGoogle Scholar |
Werth JA (2007) Weed resistance risk management in glyphosate-resistant cotton. PhD Thesis, University of Adelaide, SA, Australia.
Werth JA, Preston C, Taylor IN, Charles GW, Roberts GN, Baker J (2008) Managing the risk of glyphosate resistance in Australian glyphosate- resistant cotton production systems. Pest Management Science 64, 417–421.
| Managing the risk of glyphosate resistance in Australian glyphosate- resistant cotton production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksVGqur4%3D&md5=592bbd5b09eb9e506e4e680515b3417fCAS | 18080291PubMed |
Werth J, Thornby D, Walker S, Charles G, McDonald C (2010) Species shift and resistance: challenges for Australian cotton systems. In ‘Proceedings of the 17th Australasian Weeds Conference’. (Ed. S Zydenbos) (New Zealand Plant Protection Society: Christchurch, New Zealand)
Werth J, Thornby D, Walker S (2011) Assessing weeds at risk of evolving glyphosate resistance in Australian sub-tropical glyphosate-resistant cotton systems. Crop & Pasture Science 62, 1002–1009.
| Assessing weeds at risk of evolving glyphosate resistance in Australian sub-tropical glyphosate-resistant cotton systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gru7jJ&md5=c6dca04913fc19f4651f9a0340eb5bf2CAS |
Widderick MJ (2002) Ecology and management of the weed common sowthistle. PhD Thesis, University of New England, Armidale, NSW, Australia.
Widderick MJ, Walker SR, Sindel BM, Bell KL (2010) Germination, emergence, and persistence of Sonchus oleraceus, a major crop weed in subtropical Australia. Weed Biology and Management 10, 102–112.
| Germination, emergence, and persistence of Sonchus oleraceus, a major crop weed in subtropical Australia.Crossref | GoogleScholarGoogle Scholar |
Wu H, Walker S, Osten V, Taylor I, Sindel B (2004) Emergence and persistence of barnyard grass (Echinochloa colona L. Link) and its management options in sorghum. In ‘14th Australian Weeds Conference Papers and Proceedings’. (Eds BM Sindel, SB Johnson) pp. 538–541. (Weeds Society of New South Wales: Wahrooga)