Impact of waterlogging on the nutrition of cotton (Gossypium hirsutum L.) produced in sodic soils
K. Dodd A , C. N. Guppy B D , P. V. Lockwood B and I. J. Rochester CA Environmental Resources Management, #10-01 120 Robinson Road, Singapore 068913.
B School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
C CSIRO Plant Industry, Australian Cotton Research Institute, LB 59, Narrabri, NSW 2390, Australia.
D Corresponding author. Email: cguppy@une.edu.au
Crop and Pasture Science 64(8) 816-824 https://doi.org/10.1071/CP13093
Submitted: 20 March 2013 Accepted: 21 August 2013 Published: 29 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Sodicity in Vertosols used for agricultural production can adversely affect the growth and nutrition of cotton (Gossypium hirsutum L.) plants. Cotton produced in sodic soils has reduced dry matter and lint yield and can develop toxic plant tissue concentrations of sodium (Na) but limited tissue concentrations of phosphorus (P,) potassium (K), and micronutrients. Crops produced on sodic soils frequently suffer from aeration stress after an irrigation or rainfall event, and it was hypothesised that the adverse physical and/or chemical conditions of sodic soils may exacerbate the effects of waterlogging. We measured the impacts of sodicity on the growth, nutrition, and root recovery time of cotton during and after waterlogging in two experiments. In the first, cotton plants were subjected to a 7-day period of inundation in Grey Vertosols with a range of exchangeable sodium percentage (ESP) values from 2 to 25%; 32P was placed in the pots and its accumulation in the plant was used to indicate root activity and recovery after the waterlogging event. In a second experiment, agar was dissolved in nutrient solutions with a range of Na concentrations (9, 30, and 52 mm) matching soil solution Na concentrations in sodic soils, in order to simulate a waterlogging event. Following the waterlogging event, the solutions were labelled with 32P, in order to determine the effect of sodic soil solution chemistry on the rate of recovery of cotton root function after the event. Plant nutrient analysis was used to determine the effects of sodicity and waterlogging on cotton nutrition. In both experiments, waterlogging reduced root activity and reduced the uptake and transport of labelled P by the cotton plants, decreased plant P and K concentrations, and increased the plant Na concentrations. Sodicity exacerbated the effects of waterlogging on root function and cotton nutrition in the soil experiment but not in the nutrient solution experiment, suggesting that any contribution of waterlogging to the patterns of nutrient accumulation in cotton crops produced in sodic fields occurs due to soil physical factors rather than soil solution chemistry.
Additional keywords: phosphorus, potassium, sodium, soil solution, Vertosols.
Introduction
Almost 30% of the Australian landmass, including 80% of the irrigated agricultural area, is occupied by sodic soils (Rengasamy and Olsson 1993). More than 50% of soils used for high-value crops such as cotton (Gossypium hirsutum L.) are affected by sodicity (Rengasamy 2002). Despite the prevalence of sodic soils in cotton production systems, the nature of their impact on cotton plants is poorly understood. Cotton crops produced on sodic soils commonly have reduced concentrations of phosphorus (P) and potassium (K) and increased concentrations of sodium (Na) in their tissues, although the mechanisms behind these patterns of nutrient accumulation are not fully understood (Rochester 2010).
The dispersive nature of sodic soils can result in a low level of macroporosity and, thus, reduced hydraulic conductivity. Crops produced on sodic soils frequently suffer aeration stress after irrigation or rainfall (Jayawardane et al. 1987). Restricted water intake during rainfall or irrigation results in waterlogging in the surface soil layers on flat lands, and restricted internal drainage results in waterlogging in sodic subsoils (McIntyre 1979; McIntyre et al. 1982).
Gases diffuse slowly in solution, and so waterlogging results in several changes in the soil, including decreases in oxygen and increases in ethylene concentration, and increases in CO2 concentrations (Wiengweera and Greenway 2004). The consequences of waterlogging for the plant may include reduced or ceased growth, the death of root apices, and changes to the patterns of nutrient accumulation (Trought and Drew 1980a, 1980b, 1980c). In corn grown under aerobic conditions, roots actively accumulate P and K and partially exclude Na over a wide range of Na chloride concentrations. Under anoxic conditions, uptake of P and K is severely reduced, and increased levels of Na accumulate in plant tissues (Drew and Dikumwin 1985; Drew and Lauchli 1985). It has been hypothesised that the active components of P and K uptake and Na exclusion are eliminated by root anoxia due to a rapid decline in root ATP levels. Extended periods of anoxia have an impact on the root cell membrane integrity, and thus ions pass through non-selectively (Trought and Drew 1980a).
There have been some discrepancies between the patterns of nutrient accumulation observed in cotton produced in sodic field situations (Rochester 2010) and that produced under experimental glasshouse situations (Dodd et al. 2013), with the field-grown plants having significantly higher concentrations of Na and lower concentrations of P in their tissues than those produced in the glasshouse. This inconsistency is hypothesised to be due to the interaction between the sodic soil conditions and waterlogging events. The soil solution Na concentration at which the plant becomes impaired may be appreciably lower in waterlogged conditions than in aerobic conditions due to increased Na accumulation. The rate of recovery of the plant from waterlogging may be lower in sodic soils due to the poor physical condition of the soil or to increased levels of root damage occurring under conditions of high Na. An understanding of the impact of anoxic soil/solution conditions on the nutrient uptake and growth of cotton and its interaction with high levels of soil Na may aid in understanding the patterns of nutrient accumulation that we observe in cotton crops grown under sodic conditions.
This paper reports two glasshouse experiments, one using a Grey Vertosol with an artificially created range of sodicity levels and one using nutrient solutions with a range of Na levels. A waterlogging treatment was applied in each experiment and the application of 32P to the soil/nutrient solution after the completion of the waterlogging period allowed assessment of the impact of sodicity on the length and rate of recovery of the plants from waterlogging. The impact of waterlogging on nutrient accumulation at different levels of sodicity was also assessed through plant nutrient analysis. We hypothesised that plants waterlogged under sodic soil conditions would take longer to recover and accumulate less P and K and more Na, and that higher solution Na concentrations would further exacerbate problems associated with the growth and nutrition of cotton.
Methods
Experiment 1
The soil used in this experiment was created in 50-kg, exchangeable sodium percentage (ESP) treatment batches according to the method outlined in Dodd et al. (2010b). The soil was equilibrated in batches with a range of solutions of various sodium adsorption ratio (SAR) values (0, 45, 100 and 200) before the removal of excess salt by equilibration with solutions of equivalent SAR but lower total cation concentrations, to create soils with a consistent salinity level. The experiment consisted of four ESP treatments (~2, 12, 16 and 25%) with six 2.5-kg replicates of each control and waterlogging treatment.
The experiment was carried out in a glasshouse at the University of New England, Armidale, New South Wales. The temperature in the glasshouse was maintained within the range 20−35°C. Before planting, fertiliser was incorporated in the soil for each replicate; 100 mg/kg of nitrogen (N) as urea, 10 mg/kg of P as mono-ammonium phosphate, and 1 mg/kg of zinc as ZnSO4. The soil for each replicate was then weighed into free-draining pots 25 cm in height and 10 cm in diameter. The soil and pot were weighed and the soil was brought to field capacity (~42% w/w). Ten cotton seeds (variety Sicot 289BRR, CSD Pty Ltd, Wee Waa, NSW) were planted in each pot, and the pot was then covered with a plastic bag to prevent excessive evaporation. Upon plants reaching the 2-leaf stage, the plastic bags were removed and the plants were thinned to six per pot.
Throughout the first 4 weeks of the experiment, each pot was watered by weight to field capacity every second day. When the plants had reached a height of 20 cm, half of the replicates of each ESP treatment were waterlogged by placing each pot in a 10-L plastic bucket filled with de-ionised water. The remaining replicates continued to be watered by weight to field capacity every day. After 7 days, the waterlogged plants were removed from the buckets. Two replicates from each sodicity and waterlogging treatment were harvested by cutting their stems just above the soil level. The non-waterlogged pots continued to be watered by weight to field capacity, and this process was re-applied to the waterlogging pots once a water content of ~30% w/w was reached.
Preparation of 32P and application
A 0.37 MBq mL–1 solution of 32P in deionised water was made. To apply the isotope to the soil 35 days after planting, five fine holes were placed in each pot to a depth of 10 cm with a metal skewer ~3 mm in diameter. In each hole, 1 mL of 32P was applied with a syringe and 10-cm tip. The cotton plants were assessed for 32P uptake daily by placing a Geiger counter on the youngest mature leaf (YML).
Plant harvest and nutrient determination
Fourteen days after the removal of the waterlogging treatment, all remaining plants were harvested by cutting the stems just above the soil surface. The plants were dried in a fan-forced oven at 80°C, weighed, ground to <2 mm, and digested with perchloric acid and hydrogen peroxide, using the sealed chamber method outlined by Anderson and Henderson (1986). The total nutrient composition of the samples was determined using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Isotope activity was measured using standard methods in a liquid scintillation counter.
Experiment 2
Three nutrient solutions were developed, varying in Na concentration (9, 30, and 52 mm) (Table 1). Phosphorus was included in the nutrient solutions at concentrations ~10 times those found in the soil to avoid the rapid depletion of P commonly observed in hydroponic plant culture where low P concentrations are used. A maximum electrical conductivity (EC) of 5 dS m–1 was chosen because it was below the level of 7.7 dS m–1 at which ionic strength reduces the growth of cotton by 10% (Maas and Hoffman 1977). A maximum Na : calcium (Ca) molar ratio of 46 : 1 was chosen as it allowed the inclusion of the range of Na concentrations found in the soil solutions of Vertosols (Table 2) at commonly occurring soil ESP values of 2–25% (Norrish et al. 2001; Dang et al. 2004; Rochester 2010), while maintaining Ca concentrations to prevent Ca deficiency (Blair and Taylor 2004).

|

|
Basal macronutrients were applied at the following concentrations (mm): K 0.3, Mg 1.2, P 0.3, S 0.8. These nutrients were included as a mixture of nitrate, sulfate, phosphate, and chloride salts. The solution Cl– concentrations were maintained at relatively constant levels (±5 mm) of 40 mm. Ammonium-N and nitrate-N were applied at 0.03 and 5.3 mm, respectively. Micronutrients were applied at the following concentrations (µm): Fe 65, Cu 28, B 83, Zn 11, Mo 0.7. The above nutrient treatments were each dissolved in 260 L of distilled water. The pH values of the nutrient solutions were maintained at 6.2–6.8 using a balance of NO3– and NH4+ ions taken up by roots, with the optimum level of NH4+ ions needed to stabilise the pH of the solution found to be ~0.8% of total solution N (mm).
The oxic control treatments were established by bubbling air through the nutrient solutions. The nutrient solutions for the waterlogging treatments were created according to the method outlined by Wiengweera et al. (1997) by dissolving 0.1% (w/w) agar (Difco Bacto, Difco Laboratories Pty Ltd, Mt Pritchard, NSW) in each of the nutrient solutions. This process was undertaken by heating the agar and nutrient solution with a magnetic stirrer. Both the aerated and waterlogging treatment nutrient solutions were then autoclaved at 120°C for 15 min and then cooled with magnetic stirring to prevent the formation of lumps.
The experiment was a randomised block design, with cotton grown in 36 jars containing nutrient solutions with three different Na levels. Two aeration regimes were applied during the experiment, including a control treatment that was aerated throughout the experiment and a waterlogging treatment that was treated with agar for a period of 7 days. Two replicates of each sodicity and waterlogging treatment were harvested immediately after the waterlogging event and the remaining four were harvested 2 weeks later.
Plant culture
This experiment was conducted in a glasshouse at the University of New England. The temperature range of the glasshouse during the experimental period was maintained at 20−35°C. Seeds of cotton cv. Sicot 289BRR were germinated in moist sand in the glasshouse. After 7 days, the seedlings were transferred into tubs of solutions containing all of the basal nutrients but no Na. The plants were suspended above the nutrient solutions using a wooden board and plastic cups filled with non-wetting cotton wool.
On day 24 after germination, the plants were transferred to individual foil-wrapped jars filled with 750 mL of treatment solution. The plants were suspended above the nutrient solutions using a small hole cut in the jar lid and non-wetting cotton wool. Air was bubbled through each jar using a small pump and plastic tubing. Twelve replicates of each treatment solution were used and the plants were allowed to grow under aerated conditions for 12 days. The nutrient solutions for all treatments were renewed after 6 days.
On day 37 after germination, the nutrient solutions of the six replicates of each Na level in the control treatments were renewed and the application of aeration was continued. In the six replicates of each Na level in the waterlogging treatments, agar-treated nutrient solutions were applied and aeration was removed. The plants were allowed to grow in these solutions for 7 days.
Preparation and application of 32P
Following the 7-day period of waterlogging, two plants from each Na level in the control and waterlogging treatments were harvested. The nutrient solutions of all of the remaining plants were renewed with non-agar-treated solutions and aeration was applied to all plants. Labelled P was applied to each replicate immediately following the renewal of the nutrient solution. In total, 0.15 MBq of 32P was applied to each treatment by adding 10 mL of an 0.015 MBq mL–1 isotope solution to each replicate. The uptake of the 32P by the cotton plants was assessed every 1.5 h by placing a Geiger counter on the YMLs, with the rest of the plant shielded behind a Perspex screen. The plants were allowed to grow until all replicates were producing Geiger counter readings of >10 counts s–1, which took ~24 h.
Plant harvest and nutrient determination
An Australasian Soil and Plant Analysis Council (ASPAC) plant sample was included in the analysis, in order to ensure the accuracy of the results. Isotope activity, dry weight, and tissue concentrations were measured as described in Expt 1.
Statistical analyses
The replicates in the experiments were arranged in a randomised block design. Analyses of variance (ANOVA) were used to test the significance of treatment differences (P < 0.05), with nutrient solution Na/ESP and waterlogging as factors, and two replicate plants per treatment at the initial harvest date and four plants per treatment at the final harvest date. Where interactions between the effects of Na and the effects of waterlogging were found, differences between individual treatment combinations were further evaluated by least significant difference (l.s.d.) (P < 0.05).Statistical analyses were carried out using the Genstat program (7th edn, Lawes Agricultural Trust) (Payne 1987).
Results
Experiment 1
Soil sodification produced ESP values ranging from 2 to 25% (P < 0.001) but without changing effective cation exchange capacity (ECEC) (P = 0.61) (Table 3). The exchangeable Ca and Mg concentrations of the soil decreased with increasing sodicity (P < 0.001), but there was no difference in exchangeable K between the soils (P = 0.11). The pH of the soil increased with increasing soil sodicity (P < 0.001), although this increase was smaller when measured in an appropriate solution matrix with matched Ca and Na concentrations. There was no difference in the EC of the soils among the four sodicity treatments (P = 0.11).
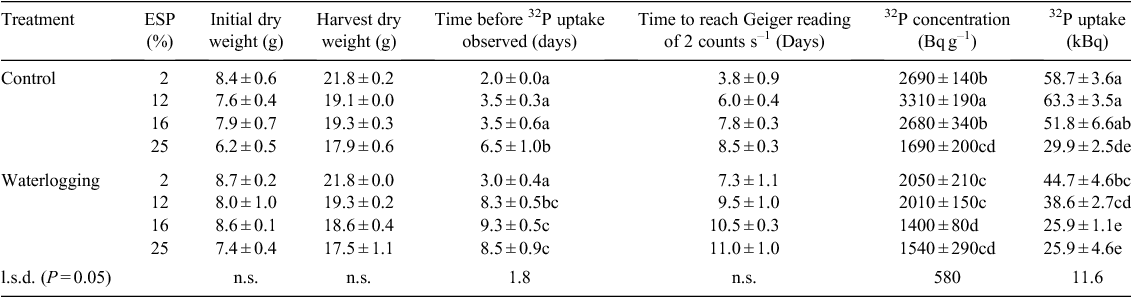
Increasing soil ESP reduced the dry matter accumulation of cotton at the initial harvest (P = 0.05) (Table 4). At the final harvest, cotton dry matter accumulation was reduced by ESP (P < 0.001), decreasing by 19% between the 2% and 25% ESP treatments when averaged over waterlogging regimes. Waterlogging did not interact with ESP to reduce dry matter accumulation further (P > 0.05).
Rate of recovery from waterlogging
Uptake of labelled P was observed 2 days after application in the aerated non-sodic pots and at increasing intervals with increasing soil sodicity (P < 0.001) (Table 4). As soil ESP increased, uptake of labelled P took 225% longer in the control treatments and 183% longer in the waterlogged treatments. Waterlogging also delayed the uptake of labelled P (P < 0.001). There was an interaction of sodicity × waterlogging on the time taken for uptake of labelled P to be observed (P < 0.001), with waterlogging reducing P uptake greatest in the moderately sodic soils. The time to reach a Geiger counter reading of 2 counts s–1 increased with increasing soil sodicity (P < 0.001) and waterlogging (P < 0.001) (Table 4). There was no sodicity × waterlogging interaction on the time taken to reach the Geiger counter reading of 2 counts s–1 (P > 0.05). Both increasing soil sodicity and waterlogging decreased the concentrations (P < 0.001) and total accumulation (P < 0.001) of 32P in the cotton plants (Table 4). There were interactions of sodicity × waterlogging on the concentrations (P = 0.05) and accumulation (P = 0.04) of 32P in the cotton plants. Isotope recoveries were ~50% lower in the highest ESP treatments regardless of waterlogging, but were also lower where ESP was 16% when waterlogged.
Nutrient concentrations and accumulation
Cotton plant Na concentrations and total accumulation increased with increasing soil sodicity in both the control and waterlogged treatments, at the first and second harvest dates (P < 0.001) (Table 5), and accumulated more Na between the initial and final harvest date (P < 0.001). Total Na uptake was 40–50% higher when cotton was waterlogged at 16 and 25% ESP (Table 5).
Sodicity decreased the K concentrations of cotton at both the first (P = 0.003) and second (P < 0.001) harvest dates (Table 5). The total K accumulation of cotton between the initial and final harvests also decreased with higher sodicity (P < 0.001). Waterlogging decreased the cotton K concentrations at both the initial (P = 0.01) and final (P < 0.001) harvests and reduced the accumulation of K by the cotton plants during this period (P < 0.001). Tissue K concentrations were reduced more by moderate sodicity when waterlogged (Table 5).
Waterlogging reduced the P concentrations of cotton at both the initial and final harvest date and the total accumulation of P during this period (P < 0.001) (Table 5). At the final harvest, waterlogging reduced cotton P concentrations by an average of 20% and P accumulation by an average of 28%, across the range of ESP levels. Although the 16% ESP initial P concentration in the control treatment was elevated, overall tissue P concentrations displayed no regular pattern.
Experiment 2
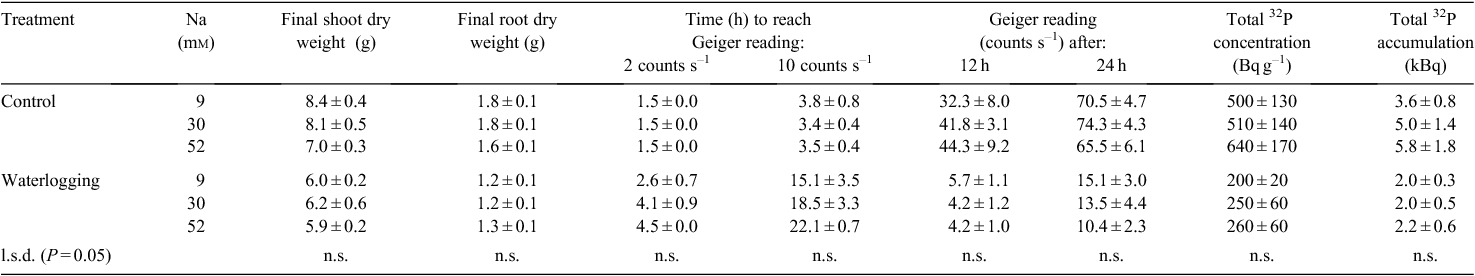
Nutrient solution Na concentrations did not have any effect on the shoot (P = 0.13) or root (P = 0.87) dry weight of cotton at the final harvest (Table 6). Waterlogging decreased both the shoot and root dry weights of cotton (P < 0.001).
Rate of recovery from waterlogging
Waterlogging greatly reduced (P < 0.001) cotton root activity, with the plants in the control treatment all generating Geiger counter readings of 2 counts s–1 within the first 1.5 h after application of the isotope, compared with 2.6–4.5 h for plants in the waterlogged treatments (Table 6). Waterlogging also reduced uptake by 6–8 times after 12 or 24 h (P < 0.001) and this was reflected in less than half the isotope activity in cotton tissue (P < 0.001) (Table 6). There was no significant effect of nutrient solution Na on the time taken for the cotton plants to generate Geiger counter readings of 2 (P = 0.13) or 10 (P = 0.26) counts s–1, or on Geiger counts after 12 or 24 h, or on isotope concentration and accumulation (Table 6).
Nutrient concentrations and accumulation
Cotton plant Na concentrations increased with increasing nutrient solution Na concentrations, at both the initial and final harvest (P < 0.05) (Table 7). Although there was no initial interaction of waterlogging × solution Na, at final harvest Na concentrations were 34% higher at 30 mm Na when waterlogged. Tissue K concentration was reduced by waterlogging (P < 0.001). However, the interaction of waterlogging × solution Na on total K was to increase K uptake initially in the controls, but reduce it when waterlogged (Table 7). Waterlogging initially reduced P concentrations, but there were no treatment effects at final harvest associated with solution Na or waterlogging on tissue P (Table 7).
Discussion
This experiment aimed to identify whether the adverse effects of waterlogging on root growth and function were exacerbated by increasing soil ESP or by increasing solution Na concentrations directly associated with sodic soils. The use of isotopes as an indicator of recovery of root function demonstrated that activity and uptake of P took up to twice as long when roots were waterlogged in sodic environments. This delay in recovery is most likely associated with soil physical conditions and delayed drainage of micropores, as elevated solution Na concentrations had minimal effect on root P uptake. When a soil becomes waterlogged, the pore space in the soil structure that usually allows the exchange of gas between the soil and atmosphere is filled with water, and diffusion of oxygen is limited. Root and microorganism respiration can then totally deplete the soil of oxygen within 24 h and build up CO2, ethylene, and H2S concentrations (Trought and Drew 1980a). These anaerobic conditions in the root environment can lead to a cessation of root growth, changes to the patterns of nutrient accumulation, and root death (Jackson and Drew 1984; Wiengweera and Greenway 2004). Use of 32P is an appropriate technique to measure the recovery of the root from these conditions, as more isotope will be taken up by healthy, functioning, and actively growing roots.
The observed impact of waterlogging on the accumulation of 32P by cotton provides evidence of reduced growth and/or functioning of the cotton roots. Accumulation of 32P by waterlogged cotton plants never reached levels equivalent to the control treatments in the 2-week recovery period. The lack of a measured increase in the rate of 32P accumulation in the latter part of the recovery period indicates that, although these plants accumulated 32P at the same rate as the other treatments at this stage, they could not compensate for the reduced rates of uptake immediately after the waterlogging events due to the delay in the return of oxic conditions. Milroy et al. (2009) also observed that waterlogging early in cotton development resulted in a consistent reduction in nutrient accumulation over the life of the crop compared with waterlogging later in growth, where redistribution presumably mitigates the adverse root-uptake environment. The frequency of cotton irrigation under a field situation is commonly as low as 7–8 days during the peak of the growing season, and as such, these plants could expect to be inundated again within the 2-week recovery period, and P accumulation would never reach the control levels.
Soil sodicity was a significant factor in determining the root activity of cotton after a waterlogging event. Although none of the plants affected by waterlogging achieved the 32P accumulation of their control counterparts, the sodic soils were more affected. The 2% ESP treatment accumulated more 32P than the 12% treatment, and this treatment accumulated more 32P than the most sodic treatments. The 32P accumulation of the cotton produced in the ESP treatments of 16 and 25% in the waterlogged treatments was not different, and low throughout the measurement period.
The sodicity × waterlogging interaction resulted in the greatest decrease in uptake where sodicity was up to 16%. This includes the range of sodicity values commonly observed in Vertosols used for cotton production in Australia. Where ESP was 25%, the adverse environment reduced uptake regardless of whether it was under prolonged inundation. This result was reflected in tissue P concentrations where non-sodic soil and highly sodic soil both had similar P concentrations, and the greatest difference occurred at ESP 12 and 16%. A secondary outcome of monitoring the 32P uptake of cotton allowed observation of the effect of sodicity on P uptake under optimal moisture. Root recovery was slowed even under optimal conditions of moisture, where access and uptake of the applied isotope took 2–3 times longer as sodicity increased (Table 4). Only under the highest sodicity level was the delay significant enough to reduce total accumulated isotope.
That these effects are associated with slower drainage and adverse physical conditions for plant growth are best illustrated by the absence of any effect of increased solution Na concentration on isotope uptake under either aerated or anaerobic solution culture conditions (Table 6). Waterlogging again increased the time taken for roots to acquire 32P, but soil solution chemistry did not exacerbate that delay. This is consistent with the solution culture experiment of Dodd et al. (2010a), in which solution chemistry had minimal effects on growth of cotton in sodic solutions, but contrasts with studies of other species where elevated solution Na concentration changed internal P requirements (Awad et al. 1990).
Plant dry matter
Waterlogging of cotton reduces dry matter accumulation in mature cotton plants (Hocking et al. 1987; Bange et al. 2004). However, no impact of waterlogging on the early season dry matter accumulation of cotton has been reported in field experiments (Hodgson 1982). Thus, it is not surprising that no impact of waterlogging on the dry matter accumulation of cotton was observed in this experiment. Soil sodicity has also been reported to reduce the dry matter accumulation of cotton plants, even at low ESP levels, but these reductions are not generally apparent until fruit production has begun (Dodd et al. 2013). The significant decrease in dry matter accumulation that occurred in this experiment, regardless of waterlogging, with increasing ESP was not expected, given the early growth stage of the harvests. A likely explanation for this discrepancy is that multiple plants and smaller soil volumes used in this experiment increased the rate of water depletion from the pots and thus the required frequency of irrigation events and exposure of the plants to conditions outside their non-limiting water range.
Nutrient concentrations and accumulation
Sodicity increased tissue Na concentrations, as expected by a species that commonly increases Na uptake and sequesters it as a tolerance mechanism (Lauchli and Stelter 1982; Leidi and Saiz 1997). Waterlogging increased Na uptake at sodicity values >12% initially, and >16% at the final harvest, most likely due to breakdown in the energy-dependent Na exclusion mechanisms (Drew and Sisworo 1979). Values of Na in mature YML >0.20% are often associated with reduced growth (Dodd et al. 2013; Rochester 2010), and tissue Na concentrations above this concentration were reached in these, admittedly younger, plants at ESP values >12, and were considerably higher in waterlogged conditions. This reduced cotton growth may have been related to K nutrition, as only when ESP were >12 did tissue K concentrations fall significantly in both control and waterlogged pots (Table 5). However, very high tissue Na in the solution culture experiment did not affect tissue K (or P) concentrations in the same manner (Table 7). This provides evidence that the effect of sodicity on K and P nutrition does not act competitively through soil solution composition at the root surface reducing K (or P) uptake, but acts through physical conditions that reduce root extension or reduce aerobic respiration and hence K and P uptake. These processes may act independently of plant Na status, and even soil P and K availability. Similar conclusions were alluded to by Milroy et al. (2009) where elevated tissue Na and reduced tissue P and K were observed, but K and P status were more sensitive to waterlogging.
Conclusion
Amelioration of the adverse effects of waterlogging, exacerbated by soil physical conditions associated with sodicity, is of considerable interest to cotton producers. Modifying soil physical conditions is more difficult than applying extra P and K fertiliser to cope with reduced root function and uptake during waterlogging. A recent study reported improved cotton growth due to K supplementation during prolonged waterlogged conditions, in what appears to be a non-sodic soil (Ashraf et al. 2011). However, in furrow/flood irrigated conditions, the results of this study suggest that root activity recovers more slowly as sodicity increases, and that this is further exacerbated by waterlogging due to the longer time taken for aerobic conditions in the root-zone to return. We consider it unlikely that simply adding more fertiliser to yield-limited, sodic cotton fields will overcome effects that are more closely related to soil physical conditions. It should be noted that these results were gathered on young cotton plants grown in pots, and field verification is warranted. However, further research is required into cost-effective means of improving the soil physical condition of sodic soils where furrow/flood irrigation practices are likely to continue.
Acknowledgements
The authors thank the Cotton, Catchment Communities Cooperative Research Centre for supporting the PhD the lead author undertook in completing this research. The authors would also like to acknowledge Dr Ivan McLeod whose unpublished PhD thesis suggested innovative paths of investigation for this problem.
References
Anderson DL, Henderson LJ (1986) Sealed chamber digest for plant nutrient analysis. Agronomy Journal 78, 937–938.| Sealed chamber digest for plant nutrient analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XlvVOisrk%3D&md5=07282cf976a063652fdc2fc742ccc4faCAS |
Ashraf MA, Ahmad MSA, Ashraf M, Al-Qurainy F, Ashraf MY (2011) Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop & Pasture Science 62, 25–38.
| Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXks1Srtw%3D%3D&md5=417c6ab8c7b8a225a6c04b682508dc3fCAS |
Awad AS, Edwards DG, Campbell LC (1990) Phosphorus enhancement of salt tolerance in tomato. Crop Science 30, 123–128.
| Phosphorus enhancement of salt tolerance in tomato.Crossref | GoogleScholarGoogle Scholar |
Bange MP, Milroy SP, Thongbai P (2004) Growth and yield of cotton in response to waterlogging. Field Crops Research 88, 129–142.
| Growth and yield of cotton in response to waterlogging.Crossref | GoogleScholarGoogle Scholar |
Blair LM, Taylor GJ (2004) Maintaining exponential growth, solution conductivity and solution pH in low ionic strength solution culture using a computer-controlled nutrient delivery system. Journal of Experimental Botany 55, 1557–1567.
| Maintaining exponential growth, solution conductivity and solution pH in low ionic strength solution culture using a computer-controlled nutrient delivery system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlOqu70%3D&md5=4962ad142263f091db8e0478c19737d2CAS | 15181105PubMed |
Dang Y, Dalal R, Harms B, Routley R, Kelly R, McDonald M (2004) Subsoil constraints in the grain cropping soils of Queensland. In ‘Supersoil 2004’. Sydney. (Ed. B Singh) (The Regional Institute Ltd: Gosford, NSW)
Dodd K, Guppy C, Lockwood P, Rochester I (2010a) The effect of sodicity on cotton: plant response to solutions containing high sodium concentrations. Plant and Soil 330, 239–249.
| The effect of sodicity on cotton: plant response to solutions containing high sodium concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktFequ7k%3D&md5=528547cafd0082ee305ae18d53ef1c7eCAS |
Dodd K, Guppy CN, Lockwood PV (2010b) Overcoming the confounding effects of salinity on sodic soil research. Communications in Soil Science and Plant Analysis 41, 2211–2219.
| Overcoming the confounding effects of salinity on sodic soil research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1SntrjO&md5=638404da0151b6c93cefab8794316ff3CAS |
Dodd K, Guppy CN, Lockwood PV, Rochester IJ (2013) The effect of sodicity on cotton: does soil chemistry or soil physical condition have the greater role? Crop & Pasture Science 64, 806–815.
Drew MC, Dikumwin E (1985) Sodium exclusion from the shoots by roots of Zea mays (cv. LG 11) and its breakdown with oxygen deficiency. Journal of Experimental Botany 36, 55–62.
| Sodium exclusion from the shoots by roots of Zea mays (cv. LG 11) and its breakdown with oxygen deficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFSitr8%3D&md5=11326a8336023bb82ee5b6155c9616eaCAS |
Drew MC, Lauchli A (1985) Oxygen dependent exclusion of sodium ions from shoots by roots of Zea mays (cv. Pioneer 3906) in relation to salinity damage. Plant Physiology 79, 171–176.
| Oxygen dependent exclusion of sodium ions from shoots by roots of Zea mays (cv. Pioneer 3906) in relation to salinity damage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlvFehs7w%3D&md5=3cc07789a4ccf1a8bc20f3bcbcb45dc4CAS | 16664364PubMed |
Drew MC, Sisworo EJ (1979) The development of waterlogging damage in young barley plants in relation to plant nutrient status and changes in soil properties. New Phytologist 82, 301–314.
| The development of waterlogging damage in young barley plants in relation to plant nutrient status and changes in soil properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXltlWmtbs%3D&md5=7fb6b2cebe059ea324cbbba5a8cfd517CAS |
Hocking PJ, Reicosky DC, Meyer WS (1987) Effects of intermittent waterlogging on the mineral nutrition of cotton. Plant and Soil 101, 211–221.
| Effects of intermittent waterlogging on the mineral nutrition of cotton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXltlensro%3D&md5=149700d05ff936e7b7767cf41c1e56d9CAS |
Hodgson AS (1982) The effects of duration, timing and chemical amelioration of short-term waterlogging in a cracking grey clay. Australian Journal of Agricultural Research 33, 1019–1028.
| The effects of duration, timing and chemical amelioration of short-term waterlogging in a cracking grey clay.Crossref | GoogleScholarGoogle Scholar |
Jackson MB, Drew MC (1984) Effect of flooding on growth and metabolism of herbaceous plants. In ‘Flooding and plant growth’. (Ed. TT Kozlowski) pp. 47–128. (Academic Press: London)
Jayawardane NS, Blackwell J, Stapper M (1987) Effect of changes in moisture profiles of transitional red-brown earth with surface and slotted gypsum applications on the development and yield of a wheat crop. Australian Journal of Agricultural Research 38, 239–251.
| Effect of changes in moisture profiles of transitional red-brown earth with surface and slotted gypsum applications on the development and yield of a wheat crop.Crossref | GoogleScholarGoogle Scholar |
Lauchli A, Stelter W (1982) Salt tolerance of cotton genotypes in relation to K/Na selectivity. In ‘International Workshop on Biosaline Research’. La Paz, Mexico. (Ed. A San Pietro) pp. 511–514. (Plenum Press: New York)
Leidi EO, Saiz JF (1997) Is salinity tolerance related to Na accumulation in upland cotton (Gossypium hirsutum) seedlings? Plant and Soil 190, 67–75.
| Is salinity tolerance related to Na accumulation in upland cotton (Gossypium hirsutum) seedlings?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvFyjsb0%3D&md5=001807413fed8504a2bda2cebd2b2165CAS |
Maas EV, Hoffman GJ (1977) Crop salt tolerance - current assessment. Journal of the Irrigation and Drainage Division 103, 115–134.
McIntyre DS (1979) Exchangeable sodium, subplasticity and hydraulic conductivity of some Australian soils. Australian Journal of Soil Research 17, 115–120.
| Exchangeable sodium, subplasticity and hydraulic conductivity of some Australian soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXkvFShtrw%3D&md5=b1cacf297b5dda5cfc5523672b9b9a70CAS |
McIntyre DS, Loveday J, Watson CL (1982) Field studies of water and salt movement in an irrigated swelling clay soil. 1. Infiltration during ponding. Australian Journal of Soil Research 20, 101–105.
| Field studies of water and salt movement in an irrigated swelling clay soil. 1. Infiltration during ponding.Crossref | GoogleScholarGoogle Scholar |
Milroy SP, Bange MP, Thongbai P (2009) Cotton leaf nutrient concentrations in response to waterlogging under field conditions. Field Crops Research 113, 246–255.
| Cotton leaf nutrient concentrations in response to waterlogging under field conditions.Crossref | GoogleScholarGoogle Scholar |
Norrish S, Cornish PS, Moody PW, Jessop RS, Rummery G (2001) Soil fertility and wheat crop response to phosphorus fertiliser on Vertosols in low rainfall areas of the northern grain zone. In ‘10th Australian Agronomy Conference’. Hobart, Tas. (Eds B Rowe, D Donaghy, N Mendham) (Australian Society of Agronomy/The Regional Institute Ltd: Gosford, NSW)
Payne RW (1987) ‘Genstat 5 Reference Manual.’ (Clarendon Press: Oxford, UK)
Rengasamy P (2002) Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Australian Journal of Experimental Agriculture 42, 351–361.
| Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview.Crossref | GoogleScholarGoogle Scholar |
Rengasamy P, Olsson KA (1993) Irrigation and sodicity. Australian Journal of Soil Research 31, 821–837.
| Irrigation and sodicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktlCjt78%3D&md5=e7b06241aa43fdee5e8191fc9bd26777CAS |
Rochester IJ (2010) Phosphorus and potassium nutrition of cotton:interaction with sodium. Crop & Pasture Science 61, 825–834.
| Phosphorus and potassium nutrition of cotton:interaction with sodium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1yqt7zL&md5=d708f3c453dd47d33e7a71e6072357b7CAS |
Trought MCT, Drew MC (1980a) The development of waterlogging damage in wheat seedlings (Triticum aestivum L.). 1. Shoot and root growth in relation to changes in the concentration of dissolved gases and solutes in the soil solution. Plant and Soil 54, 77–94.
| The development of waterlogging damage in wheat seedlings (Triticum aestivum L.). 1. Shoot and root growth in relation to changes in the concentration of dissolved gases and solutes in the soil solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhs1artLk%3D&md5=76f6cd0d0aa4c175c43967c33081b559CAS |
Trought MCT, Drew MC (1980b) The development of waterlogging damage in wheat seedlings (Triticum aestivum L.). 2. Accumulation of nutrients by the shoots. Plant and Soil 56, 187–199.
| The development of waterlogging damage in wheat seedlings (Triticum aestivum L.). 2. Accumulation of nutrients by the shoots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXmtF2ktb4%3D&md5=71187ad291e11f77dd07c3ce8184fc5fCAS |
Trought MCT, Drew MC (1980c) The development of waterlogging damage in young wheat plants in anaerobic solution cultures. Journal of Experimental Botany 31, 1573–1585.
| The development of waterlogging damage in young wheat plants in anaerobic solution cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXhtlKgt7o%3D&md5=f6afcbb857f53792124adfc031e2b220CAS |
Wiengweera A, Greenway H (2004) Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. Journal of Experimental Botany 55, 2121–2129.
| Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnsVyit74%3D&md5=dd79f66de6ab0239cd5b49a2f35a2d4dCAS | 15310817PubMed |
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80, 115–123.
| The use of agar nutrient solution to simulate lack of convection in waterlogged soils.Crossref | GoogleScholarGoogle Scholar |