Research and practice: environmental action for improving water quality in cotton catchments since 1990
I. R. Kennedy A C , M. T. Rose B , A. Crossan A and M. Burns AA Faculty of Agriculture and Environment, University of Sydney, Sydney, NSW 2006, Australia.
B School of Chemistry, Monash University, Melbourne, Vic. 3800, Australia.
C Corresponding author. Email: ivan.kennedy@sydney.edu.au
Crop and Pasture Science 64(12) 1095-1111 https://doi.org/10.1071/CP13091
Submitted: 17 March 2013 Accepted: 13 September 2013 Published: 18 December 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
In the modern era, agriculture must seek to be environmentally sustainable, an obligation now considered as a social contract. This demands that its activities do no significant harm, where the natural resources sustaining it are fully safeguarded, but of necessity in the context of profitable agriculture. The requirement to minimise the environmental impact of the necessary agrochemicals and pesticides in waterways is especially demanding. In the past 20 years, the Australian cotton industry has approached this obligation in various ways, needing extensive planning, learning from past experiences, but it can be legitimately claimed, with significant success. This success has been achieved at some cost, requiring large numbers of personnel, time and resources. This review aims to document the strategies that have been employed, how these required effective research management and how the research data generated was applied. To the extent that this complex program of participatory action has succeeded, while also acknowledging some dramatic failures, other areas of agriculture can also benefit by identification of the key factors contributing to success.
Additional keywords: Diuron, Endosulfan, ELISAs, gin trash, GM cotton, environmental risk assessment, environmental stewardship, pesticide analysis.
Introduction
By 1990, the Australian cotton industry had 30 years of successful expansion in New South Wales (NSW) and Queensland (Qld) as a significant legacy. The availability of sufficient quantities of irrigation water to sustain the production of high quality cotton had established the industry as a major Australian agricultural exporter, about third or fourth in national value – often exceeding a value of $AUD1 billion of exports.
However, despite its financial success, by 1990 the industry was under significant political pressure because of issues related to water quality and contamination of wildlife and livestock. In particular contamination of the northern rivers of NSW and in Qld with agrochemicals was a major source of criticism, both from government agencies defending the environment, from other agricultural industries such as the beef industry and from the public at large now more concerned with protecting the environment.
The use of the organochlorine DDT had been discontinued from 1982 when the NSW Department of Agriculture through its regulatory function withdrew its availability for agriculture; but not before widespread contamination of soil in the river catchments. This legacy is particularly the case in the Namoi Valley, where the cotton industry had existed the longest. Figure 1 shows graphically contours of residual DDT, obtained by ELISA analysis of soil coupled with GIS (geographical information systems) (Shivaramaiah et al. 2002). The presence of significant DDT residues remains at similar levels today (Weaver et al. 2012); but because of the chemical properties of DDT and DDE (Kennedy et al. 2011), their transport is limited and more than 90% of the DDT residues remain in the top 10 cm. Despite a half-life probably greater than 10 years in vertisols used for growing cotton, the extremely low solubility in water reduces aquatic risk (Kennedy et al. 2011). Although some movement of DDE residues occurs down the soil profile to greater depth (Weaver et al. 2012), this almost certainly represents transport on soil particles in these highly cracking soils and not leaching. Because of the extremely low water solubility of DDT/DDE (Kennedy et al. 2011), it is almost certain that residues do not represent a threat to livestock by contaminating ground water, despite their (Weaver et al. 2012) suggestion. The real risk relates to soil biota that may consume soil organic matter, or their predators such as birds. Using ELISA to analyse stomach contents for various pesticides, including DDT, Sánchez-Bayo et al. (1999) demonstrated that detectable DDT residues were still being ingested by birds in cotton-growing areas. It can be anticipated that 15 years later, the risk of such exposure would have declined to half this level.

|
By the time the use of DDT was discontinued, it had lost its effectiveness as an insecticide, requiring more frequent sprays; this was a result of insect mutation and the build-up of pest resistance to the chemical. However, the very persistent DDT did have the advantage that it was relatively immobile in soil and tended largely to stay on farms where it was applied, unless there was significant sediment carried in farm runoff to the river system.
During the 1980s DDT was replaced by other more effective insecticides such as the new synthetic pyrethroids, or the far less persistent organochlorine, endosulfan; endosulfan had an environmental half-life of a few weeks or about 3 months for its main breakdown product endosulfan sulfate (Kennedy et al. 2001), compared with the much longer half-life of DDT in the environment. In contrast to DDT, that could bio-accumulate in cattle throughout their lifetime, livestock with endosulfan residues could be decontaminated fairly rapidly in a few weeks given the high rate of detoxification and excretion in mammalian species – once the source of contamination such as water or pasture was removed. However, this was expensive and not an effective solution, prevention being by far the best option.
Despite early hopes that the resistance developed by insects to DDT would not be found with the newer chemicals, this was hardly the case. In a few years both the synthetic pyrethroids and endosulfan were soon found to induce resistance in insect pests; such a response is now regarded as inevitable in pest management wherever selection pressure is exerted. In response, an effective resistance management was developed in the ‘pyrethroid resistance strategy’ involving staged temporal use of chemical classes limiting the high doses of exposure of insect species to less than a single generation time (Forrester et al. 1993).
However, a complex rotation of chemicals was now needed for cotton production, including endosulfan, pyrethroids, organophosphorus compounds and insect growth regulators such as chlofluazuron (Helix). Most of these compounds could be found in river water in cotton-growing regions, particularly immediately after the period of high insect pressure during rapid growth of the cotton plant. The levels found in the January to March period of intensive spraying of cotton often exceeded environmental guidelines for river water by a large margin.
Furthermore, given the high sensitivity of some fish species to chemicals like endosulfan (LC50 <1 µg/L), the common occurrence of large-scale fish kills in the river systems was a matter of concern, for which the cotton industry was held responsible although other factors such as oxygen deprivation in water can contribute.
To its credit, through its research and development agencies such as the Australian Cotton Growers Research Association (ACGRA) and the Cotton Research and Development Corporation (CRDC), the cotton industry responded well (see CRDC 1991–93) to this perceived crisis. Several initiatives were generated, most of which have had far-reaching results that are currently very obvious.
A theme of this article is that good science and effective research can only thrive when the social environment is conducive to such outcomes. No matter how well a research project succeeds or how effective a solution to a major problem can be shown to be, unless there is willingness for an industry to make the correct choices and to implement these results or solutions, the scientific research however apt will be to little avail. The filtering of information used by the Australian cotton industry, such as by the ACGRA and involving a strong involvement by farmers themselves in drawing attention to the research initiatives needed, has been a feature of the response, as this article will show.
Initiatives in which the industry planned are discussed below.
The initial response: understanding the issues
The Central and North West Rivers Water Quality Program
Given frequent media reports of fish kills and other incidents, a strong demand existed from the public at large for more information about the extent of river contamination. The cotton farming industry itself also needed reassurance about the magnitude of the problems and the urgency for action; however, wiser people were well aware there was such a need.
A joint enterprise by the NSW Water Resources Commission (WRC) and the NSW Irrigators Council, with liaison provided by CRDC board members such as Dr Vic Edge of NSW Department of Agriculture established a long-term monitoring program for the northern rivers of NSW flowing inland into the Murray–Darling system. A working committee comprising members from the WRC, CRDC and the Faculty of Agriculture at the University of Sydney guided the development of analytical protocols for pesticides and other chemicals such as nitrogen and phosphorus as well as biological factors. This monitoring program was to rely on the experience on WRC officers in selecting more than 100 river sites where water gauging data could also be recorded. The monitoring program itself would be funded by equal contributions from the State Treasury and from the Irrigators Council, continuing for almost 15 years managed by the succeeding Department of Land and Water Conservation (Muschal 2000).
In particular, it was considered important to establish an annual baseline of data on concentrations of contaminants. This could then form a reference from which seasonal and other factors controlling the data could be gauged, as well as allowing the effect of any changed farming practices to be measured. Relevant data from this program are given in this paper (Fig. 2). In NSW, as a statutory requirement irrigation water must be recycled on-farm and is not allowed to be returned to the river system. This acted as a measure to minimise contamination from farm water. Farms were also required to capture a significant level of runoff in farm dams as an additional measure to prevent contamination from runoff carried on suspended solids.

|
As an initial part of the program, those in the cotton industry observed a more rigorous approach to preventing deliberate or inadvertent leakage from farms into the river system was undertaken. Perhaps a hundred sites were closed as a discretionary result, over several years. Such measures depend very much on local knowledge, taking into account the likelihood that farmers might consider it necessary to prevent overloading of their farms with excess megalitres of water threatening to cause major engineering damage; severe ‘blow-outs’ could lead to major flows into the rivers of water and suspended sediments. Alternatively, such sediments could be minimised if flow rates could be slowed down by broadening channels. Any such events resulting from very heavy summer rainfall can be very costly to farmers and to water managers; sometimes deliberate breaching of retaining dykes was needed to prevent more serious damage occurring elsewhere on farms. As a consequence of such valid motives for being tempted to breach the law, a variety of WRC measures, ranging from gentle persuasion to vigorous legal measures including rare prosecutions were employed. However, the program had largely succeeded by 1993–94 and such deliberate breeches are now rare.
Environmental audits
Another substantial measure organised by the Australian Cotton Foundation (ACF, now Cotton Australia) in 1991 was an independent environmental audit, covering a large range of on- and off-farm issues involved in cotton production.
A professional firm of consultants based in the United Kingdom was commissioned by ACF to conduct the audit at a world class standard. Once the firm had adjusted their thinking to the vast size of some Australian cotton farms, a set of very useful recommendations were made (see CRDC 1991–93), for possible implementation by the industry.
Among others, these recommendations included:
-
To conduct a program of field research to obtain a better understanding of the behaviour of chemicals used in cotton growing and how their impacts and risks could be better managed.
-
To introduce new technologies, such as genetically engineering cotton to contain Bacillus thuringiensis toxins in the foliage – i.e. Bt cotton. Based on overseas experience, this biotechnology had the potential to substantially reduce the need for the use of chemical insecticides such as endosulfan and pyrethroids needed to control chewing insect pests such as Helicoverpa punctigera and armigera.
The outcomes of these recommendations are discussed below.
Minimising the impact of pesticides on the riverine environment (LWRRDC-CRDC-MDBC)
In 1993–98, a large program of research funded by Land and Water Resources Research and Development Corporation (LWRRDC), the CRDC and the Murray–Darling Basin Commission was initiated. This involved a suite of 35 significant research projects (Cox Inall Communications 1998) conducted at various sites in NSW and Qld. This is likely to be the most intensive study of the environmental impact of pesticides ever conducted worldwide, examining their fate and transport of pesticides, by aerial drift or vapour or on dust, in hydrological ‘runoff’ water, as well as their ecotoxicology, using mesocosms in the laboratory and the field. In the early research, there was a focus on endosulfan as the chemical of most immediate concern but also because it was seen as a good model for other chemicals, given its toxicity and its capacity to be transported into the riverine environment by all these mechanisms.
In the very midst of this research program, in 1994 the Helix crisis occurred, an event that must be considered a significant failure of best practice. This growth regulator insecticide, a product marketed by Imperial Chemical Industries (ICI), was favoured by cotton farmers as a highly effective finishing spray (Cotton Pest Management Guide 1992). Unfortunately, it was not understood that the active ingredient in this product, chlorfluazuron an organohalogen compound, was extremely fat-soluble comparable to DDT and highly capable of contaminating the fatty tissues of beef cattle fed cotton trash. Indeed, during the preceding drought period cotton gin trash (CGT) had been employed as emergency stockfeed, either directly or pelleted.
A vigilant analyst in Lismore’s Department of Agriculture observing some strange gas chromatogram peaks in beef samples, had their identity confirmed by mass spectra. Under export treaty obligations, the Australian government as required informed importers (South Korea and the United States) of the levels observed of chlorfluazuron. This resulted in the suspension of the export program for about 6 months, resulting in the loss of hundreds of millions of dollars of beef produce. Livestock found to be contaminated were immediately destroyed with owners required to pay for the cost of disposal. As the result of a legal class action (Bushby 1997), several hundreds of millions of damages were awarded to livestock producers against ICI, largely on the grounds that ICI (Australia) had failed to heed a warning that research was necessary to establish that chlorfluazuron would not contaminate livestock. Such research was not conducted in Australia until too late and the attitude was taken that, as a growth regulator preventing pupation rather than a nerve poison, the Helix would be benign.
The Helix crisis must be considered a major failure in the industry, given that proper consideration of its properties and its high Kd-value for fat could have provided adequate warning that such cotton trash should not be fed to livestock. Cooperative multidisciplinary discussions that could have led to this conclusion were not held, although a warning was made by an officer from the Qld Department of Primary Industries.
As a response to this commercial disaster, the cotton industry has maintained a pledge ever since never to feed cotton trash, a major product of cotton gins, to livestock.
Many substantial publications generated from this program eventually appeared in the peer-reviewed literature in 2001 (Connolly et al. 2001; Kennedy et al. 2001; Kumar and Chapman 2001; Leonard et al. 2001; Raupach et al. 2001a, 2001b; Woods et al. 2001) and in a book published by Oxford University Press for the American Chemical Society in 2007 (Schofield et al. 2007; Crossan et al. 2007; Kennedy et al. 2007; Silburn and Kennedy 2007; Simpson 2007). But the periodic reports and workshops conducted as part of the program by the management team enabled serious discussion and the relevance of the research to the industry to be much better understood, publicised more broadly as The Cotton Model (Cox Inall Communications 1998). No agrochemical has ever been studied more intensively than endosulfan as in this research program and several of the papers published as a result are outstanding in their quality. For example, the two papers by Raupach et al. (2001a, 2001b) (Figs 3 and 4) dealt with the relative magnitude of the possible mechanisms of endosulfan’s transport off sites of application – as aerial drift, vapour, runoff or on dust. Mathematical models were prepared and validated with intensive data sampling at various distances from sites of application.
This study revealed that endosulfan transport as vapour in air, 70% of that applied, could equilibrate with water bodies downwind, reaching levels of concern up to several kilometres from the site of application in shallow water bodies. The relative importance of runoff (dependent on significant rainfall events) or transport as vapour downwind was highlighted. Extensive studies using rain simulators (Silburn et al. 2002; Silburn and Kennedy 2007) showed the factors controlling wash-off of pesticides from foliage or their transport in suspended sediments. The application of ELISA for pesticides like endosulfan and diuron (Lee et al. 1997) allowed rapid analysis of samples, allowing on-site decisions to be made. Although endosulfan is now no longer registered for use in the cotton industry, this study has provided ongoing benefits as a model for many other chemicals and their better management.
In one revealing forum in at the Australian Cotton Research Institute at Myall Vale, it became evident from a comment made by a senior University researcher that the research projects approved up to that point need not automatically lead to on-farm solutions to the chemical risk. On the contrary, the research outputs related more to necessary background data on mechanisms of transport and to fate properties of key chemicals, such as half-life and binding constants (Kd-values) to soil type, but not directly generating the practical measures needed to protect the environment and livestock. Initially a brief cause of dismay to the program manager, realising this proved very important; an extension to the multi-million dollar program was able to focus on the management practices needed to minimise risk. Indeed, this was the genesis of the voluntary Best Management Practices (BMP) scheme of the Australian cotton industry that now as myBMP integrates many modules enabling safer, more efficient cotton production, including the issues raised in the LWRRDC-CRDC-MDBC program (Cox Inall Communications 1998), such as pesticide drift management (Woods et al. 2001).
Implementing change in the cotton industry to reduce environmental risks
Best Management Practices
The original purpose of the Australian Cotton BMP Manual was to ‘provide farm owners, farm managers, farm consultants and service agents with information to assist them in identifying the critical components of their farming operation and in developing environmental policies, farm plans and recording charts for cotton farm operations’ (Williams and Williams 2000; p. ix). The manual contains three ‘best practice’ booklets, which outline the current best knowledge and recommendations to growers regarding pesticide use:
-
Integrated Pest Management (to reduce application of pesticide)
-
-
Increased monitoring of beneficial and pest insect numbers
-
Removal of host plants for insect pests
-
Planting of refuge crops for beneficial insects
-
Control of over-wintering larvae
-
-
Application of pesticides (to limit pesticide transport off-farm in air)
-
-
Increased timing and care in the application of pesticides to prevent drift
-
Buffer zones to reduce effect of drift on ecologically sensitive area
-
Use of band spraying by ground rig where possible
-
-
Farm design and management (to limit pesticide transport off-farm in water):
-
-
Field levelling and design to limit erosion
-
Compulsory recycling of tailwater on-farm (also to gain the maximum economic benefit from a given volume of water)
-
Designation/construction of stormwater overflow areas away from natural watercourses.
-
An initial audit of the BMP program was conducted in 1999 to obtain a baseline of practices at that time (Holloway and Roth 2003). In 2003, a second audit was conducted to determine the scale of compliance with BMP among growers (Macarthur-Agribusiness 2004), with a view to obtain greater participation. Further to this, an external, industry-wide environmental audit was conducted in 2003 to assess the response to the original environmental audit in 1991 (GHD 2003). Overall, the BMP program was highly commended by this audit, as it indicated ‘a high level of stewardship by the cotton industry’ (GHD 2003; p. 3). The risk of acute pesticide toxicity to off-farm ecosystems in cotton areas was significantly reduced by BMP implementation through a decrease in both pesticide concentration and exposure (Schofield et al. 2007).
Nevertheless, at the time, a number of cotton-growing properties in Qld had insufficient tailwater collection systems, and the majority of dryland farmers could not adequately prevent stormwater runoff into local watercourses (GHD 2003). A recommendation was made that the BMP guidelines be fully implemented along with more frequent external audits to improve transparency in the industry. Furthermore, although pesticide application frequency had begun to decline with the promotion of integrated pest management (IPM, particularly within the cotton industry due to the introduction of transgenic cotton), periods and areas of high pesticide concentration remained (Crossan 2002). Indeed, the levels of pesticides in tailwater returning to water storages were up to 100 times more concentrated than that found in off-farm watercourses during that period (Crossan 2002; Sánchez-Bayo et al. 2002; Silburn et al. 2002). The majority of cotton growers did not regularly monitor tailwater and storage water (Macarthur-Agribusiness 2004) and even if they did, the effect of any residues on local fauna would not be known.
More attention was now required to address the issue of on-farm pesticide residues. In the early 2000s, the cotton industry began to invest in research into the value of cotton farms as habitat for native flora and fauna, and the ecosystem services provided by on-farm biodiversity (Reid et al. 2003). It was already known that cotton farm water storages attracted over 40 different waterbird species, including four species listed under the Threatened Species Conservation Act 1995 (Jarman and Montgomery 2001). Many cotton growers were actively involved in bird-watching and indicated that water storage areas were extensively used by birds, especially in drought conditions when storages were sometimes the only local water available (GHD 2003). In fact, one of the recommendations of the 1991 audit was that ‘growers should be encouraged to incorporate features to promote wildlife in water storage lagoons insofar as this is compatible with the primary function of the lagoons’ (GHD 2003; p. 173). In order to protect, and indeed promote, on-farm biodiversity, there was a need to continue to improve the quality of water on-farm while remaining vigilant about potential contamination of off-site ecosystems. Developments in genetic-modification of cotton to confer insect and herbicide resistance were promising tools that could reduce the reliance on both insecticides and persistent herbicides, while renewed interest in re-designing farms for ecosystem services offered a further opportunity to reduce pesticide residues.
Genetically modified (GM) cotton and IPM
Prior to the introduction of GM cotton in the late 1990s, profitable growth of cotton in Australia invariably required applications of large quantities of insecticides. Any monoculture tends to favour the development of infestations of pests and this was particularly the case with cotton. Indeed, without chemical control an industry would have been impossible, given the intense insect pressure experienced in cotton crops grown in sub-tropical conditions. In some areas of northern Australia, such as the valley of the Ord River where a large dam for irrigation was established in the 1960s as the modern cotton industry was being established in Australia, insect pressure was one of the main reasons why a putative Ord River cotton industry failed.
The availability in the 1980s of transgenic technology using Bacillus thuringiensis genes for toxins active against chewing insects was therefore of great interest to Australian cotton growers. As explained by Fitt (2003), the cotton industry’s response, after approaches to Monsanto and CSIRO, was to attempt to introduce this biotechnology. This was carefully planned, involving initial generation of transgenic cultivars suitable for Australian conditions and their subsequent release for field tests, for the primary stage of Ingard Cotton with one Bt gene, is summarised in Table 1. Adequate research funding was provided by CRDC and the new federal system for regulation of transgenic technology approved this development under strict conditions. The fact that cotton was not regarded as human food played a critical role in minimising political opposition to GM. This was not to be the case for later attempts to introduce herbicide resistance to cotton and other crops, with political opposition succeeding in imposing State moratoria, significantly delaying its introduction.
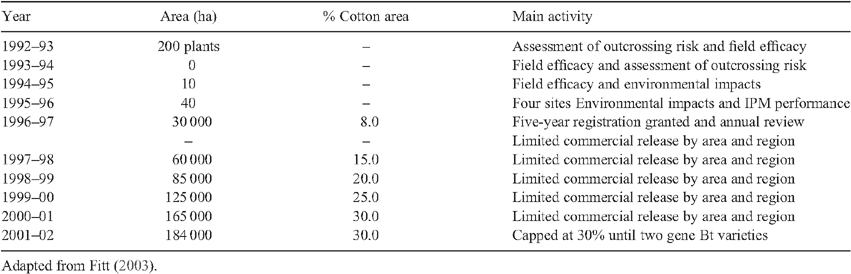
|
Subsequently, Bollgard II with two separate Bt toxin genes was developed to the point where conventional cotton seed is now rarely employed by cotton growers. The benefits from GM cotton are both environmental and economic. This has been a feature of the introduction of Roundup Ready Cotton (Crossan and Kennedy 2004) and now Roundup Ready Flex.
A major development once GM cotton was economically established was the subsequent development of IPM. Until insect pressure was substantially reduced, IPM was merely an aspiration, given the need to spray chemicals. Now that chemical use could decline, technologies using alternative measures such as the promotion of beneficial predators of pests could be applied (Fitt 2000). This extends to the deliberate encouragement of beneficial insects such as food sprays or the growth of companion crops as a source of beneficial predators (Mensah and Singleton 2004; Wilson et al. 2004). IPM is often offered as an alternative to chemical control, but this is not true. Chemicals remain in the background, to be used only if other means fail. It is most important that crops like cotton should not fail from insect attack, for farming must be profitable to be sustainable. Up till now, the introduction of GM technology for cotton in Australia has been remarkably successful. But this is not guaranteed. In recent years similar success is being claimed in India (Krishna and Qaim 2012), where lessons learned in Australia were applied (see Table 1).
Bioremediation
To complement the reductions in pesticide applications afforded by GM technology, the Australian cotton industry also invested heavily in methods for cleaning up any pesticide residues that remained on-farm. Until this time most efforts to clean up pesticide residues in the environment were directed at high concentration point sources and involved the use of physico-chemical methods such as filtration, precipitation, oxidation, acid or alkaline hydrolysis or ultraviolet irradiation. The benefits of these techniques include rapid remediation, relative insensitivity to heterogeneity in the contaminant matrix, and the ability to function over a wide range of oxygen, pH, pressure, temperature, and osmotic potentials (Cunningham et al. 1997). Unfortunately, their implementation for the clean up of diffuse residues on cotton farms was highly unlikely because of their generally high cost and technical inflexibility.
In response to the problems associated with industrial remediation techniques, especially high cost, the use of biological systems for contaminant remediation emerged. Although bioremediation technologies had been proposed as long as physico-chemical methods, and had found application in some areas, implementation remained restricted due to a limited understanding of the biological processes involved and related legal requirements (Cunningham et al. 1997). With the interest in increasing biodiversity on-farm and the need for low cost solutions, the Australian cotton industry, with financial assistance from the National Heritage Trust, commenced a program to investigate the potential to use native microorganisms and plants for accelerating pesticide removal from cotton farm waterways.
The use of microorganisms for the detoxification of pesticides capitalises on the ability of microbial species to acquire novel genes that allow them to adapt to polluted environments (Shelton and Karns 1998). The process of selection and enrichment of pesticide-degrading microbial communities often occurs unknowingly, but naturally on farms, as repeat applications of a pesticide can provide a niche for the selection of microbes able to use the pesticide as a carbon or energy source. The result for the farmer is a gradual decrease in the efficacy of the pesticide as these microbial communities multiply and accelerate pesticide breakdown (R. Harris, pers. comm. 2002).
Early research showed that microorganisms capable of degrading cotton pesticides could be isolated from cotton soils after a brief period of enrichment in the laboratory (Van Zwieten and Kennedy 1995). It was proposed that these microbes could be used established in a bioreactor type-design on-farm, similar to those found in sewage treatment plant; however, it was quickly realised that unknowns in regulation and the capability to operate these systems without continual monitoring would likely limit their use on all but the largest and most intensive farms. Related research showed some pesticide-degrading genes from the isolated strains could be transferred to ‘rhizosphere-competent’ microbial strains, such as Rhizobium and Azospirillum, which allowed these strains to survive, multiply and detoxify pesticide residues in association with plant roots (Feng et al. 1994; Van Zwieten and Kennedy 1995; Van Zwieten et al. 1995; Feng and Kennedy 1997). But despite the theoretical potential, the research necessary to bring promising early results to an applied stage for multiple pesticides was deemed too costly and time-consuming.
An alternative to the use of whole, live microorganisms to clean up pesticide residues was proposed by researchers at CSIRO: pesticide-degrading strains (or transformants) could be cultured on a large scale in the laboratory and the enzymes responsible for pesticide degradation extracted and applied directly to contaminated runoff in the field. One of the main benefits of this technique is the ability to grow large numbers of microbes under controlled, optimal conditions in the laboratory instead of introducing them directly into the field environment where their survival (and function) is uncertain. By using non-living enzymes, this technique also avoids possible regulatory hurdles regarding the large-scale introduction of live microbial cells into the environment. Early trials of an organophosphate-degrading enzyme showed that levels of the insecticide parathion-methyl cotton farm irrigation runoff could be reduced by an order of magnitude in 10 minutes (Russell et al. 2001). This and improved enzyme technology for destroying organophosphate and carbamate pesticides (Cheesman et al. 2007) has now been commercialised and is available worldwide. Continued interest and research in this area has enabled the development of new remediation enzymes targeting different pesticides, including the herbicide atrazine (Scott et al. 2010).
Wetlands and healthy waterways on cotton farms
As research on microbial bioremediation progressed, complementary experiments were undertaken to assess the potential for using plants to assist in mopping up pesticide residues – a process known as ‘phytoremediation’. Initial screening experiments showed that certain native wetland plant species, such as Persicaria spp., were able to enhance the removal of pesticide mixtures from water (Rose et al. 2001). Building on these glasshouse experiments, a pilot-scale treatment wetland was constructed on a cotton farm in northern NSW to quantify the rates of pesticide dissipation from vegetated versus non-vegetated water storages (Rose et al. 2006). The majority of water storages at this time were deliberately kept free of vegetation because of concerns they would harbour pests, disrupt water movement around the farm, and increase water loss through transpiration. On the contrary, it was hypothesised that the introduction of vegetation into normally barren water storages would stimulate microbial activity and increase pesticide breakdown (Rose et al. 2007).
Monitoring and modelling of the pilot-scale wetland over three seasons of operation showed some surprising results. Vegetation markedly increased the removal of suspended sediments and pesticide residues associated with those sediments, and these sediment-associated pesticide residues did not accumulate from season to season (Rose et al. 2006). Modelling also demonstrated that vegetation increased the surface area of natural biofilms, which help to bind and degrade dissolved pesticide residues (Rose et al. 2008). Nevertheless, under some circumstances open (non-vegetated) areas of water were more effective for removing certain pesticides. For example, herbicides susceptible to breakdown by sunlight (e.g. phenylureas such as diuron), were more effectively degraded by non-vegetated, unshaded areas, but only if the turbidity of water was low (Rose et al. 2008). These results enabled some practical recommendations to be made to farmers in the form of manipulating the design of water storages to maximise pesticide breakdown. These included breaking up larger storage areas into small storages to increase the area of biofilm surface area, and the use of vegetated ponds or strips to maximise sediment removal before water flow into non-vegetated areas where pesticide breakdown by sunlight is favoured.
Because the greatest risk of pesticide exposure to native fauna exists immediately after tailwater exits the field (Rose et al. 2005), sub-surface flow wetlands, in the form of gravel bed filters, were also investigated for their potential integration into cotton farming systems. A pilot-scale sub-surface flow system built on a cotton farm in northern NSW delayed, reduced and spread out high peak concentrations of pesticide in runoff and substantially reduced sediment concentrations (Crossan et al. 2007). However, repeated use of the sub-surface flow (SSF) system caused some of the pesticides trapped in the early irrigation treatments to be re-dissolved in later, less contaminated irrigation treatments (Crossan et al. 2007). It was concluded that more work was necessary, with recommendations that several SSF beds could be constructed to enable cycling of each system to allow for greater time between irrigations for pesticide breakdown within the gravel beds. From this research program several recommendations were provided to farmers and industry stakeholders on ways to increase the environmental value of water storage areas and waterways on farms, summarised in Fig. 5. The practice of constructing artificial wetlands in agricultural landscapes for pesticide decontamination and water quality improvement is now becoming commonplace around the world (Gregoire et al. 2009; O’Geen et al. 2010).
|

|
Ecological risk assessment (ERA) in Australian cotton: a framework to measure and document change
From the late 1990s, internationally accepted approaches to ERA were applied in research for sustainable cotton production. The approach involves three phases (see Fig. 6): problem formulation, estimation of actual pesticide exposure by organisms in the ecosystem and documented toxic effects followed by characterisation of risk and risk management (ECOFRAM 1992; US EPA 1998). The more advanced forms of ERA involve probabilistic risk assessment, requiring extensive monitoring data from the field as well as well founded indices of chemical hazard. If these overlap frequently regarding concentrations of chemical residues in water there is clearly a problem and management to remove the overlap is required. Here, we will describe several actual case studies as examples of application of ERA.

|
Problem formulation involves the identification of the hazardous toxicant – the potential for contamination or ecological harm. For example, the use of particular chemicals might harm ecological species that are found in a nearby wetland. Ecological relative risk (EcoRR) (Sánchez-Bayo et al. 2002) was a development within the Cotton CRC to estimate risk from cotton chemicals sprayed on a farm bordering the RAMSAR-registered nature reserve located in the Macquarie Marshes, NSW, in the Murray–Darling Basin. This methodological tool has since been used internationally in teaching programs to illustrate the ERA methodology. Although EcoRR was able to show that a rearrangement of the farm field plan would have advantages in substantially reducing the ecological risk from cotton farming, the irrigation water licenses of the farm involved were the subject of ‘buy-back’ in the Murray–Darling Basin as part of scheme to provide more water to the environment.
Regarding concerns of CGT contaminating waterways and natural ecosystems
Problem formulation often involves a priori knowledge regarding chemical use or the potential problem might be identified by a third party. This was the case in the Cotton Gin Trash project (CRDC US 156C, 2004), where the NSW Department of Environment and Conservation (DEC, formerly EPA) tabled data revealing concentrations of chemicals in stockpiled CGT. The DEC required the cotton industry to show that it was not hazardous waste and therefore subject to expensive and ongoing procedures regarding its disposal.
A risk assessment was undertaken regarding the potential for the concentration of pesticide residues in cotton gin trash to pose a ‘hazardous’ risk because of their potential to leach into natural ecosystems at toxic levels of concern (Crossan et al. 2006). CGT is the aggregated particulate waste removed during the cleaning of lint during the ginning process, amounting to ~20% of its mass. CGT mainly consists of soil particles, leaf, pieces of woody plants, broken and seeds not suitable for pressing, and poor quality lint. The assessment and characterisation of waste is stipulated by waste management guidelines, of which 1 of the 27 pesticides analysed, commonly used in cotton production were characterised.
Monitoring of trash ginned from cotton during the 2002 season occurred over a 2-year period at three gin sites from different valleys (Crossan and Kennedy 2008). In total, 14 pesticide residues were detected in gin trash; 13 commonly used pesticides of cotton and DDE, the main aerobic breakdown product of DDT (Kennedy et al. 2011). The concentrations of these residues were then characterised for risk of contaminating water using standard methodology (ECOFRAM 1992; Norton et al. 1992) and the EPA guidelines (EPA 1999). The degradation rates of the residues were also measured. It was found that the concentration of residues in gin trash, and therefore the ecological hazard, was dependent on the mass of the gin trash, which was decaying (Crossan and Kennedy 2008). As the gin trash decayed the concentration of the pesticides residues were observed to increase, an effect most pronounced in the case of historical DDE residues, given their dissipation rates were lower than the rate of decay of the organic trash.
The concentrations of residues in gin trash were not found to pose a hazardous risk, also unlikely to be effectively leached. Furthermore, the chemical residues being strongly bound to organic matter were unlikely to be available to cause toxic action. The significant residues in gin trash disappeared after storage, in less than a year if composted, except for the DDE found only in samples from the Namoi Valley reflecting the historical use. Based upon the characterisation guidelines for chlorpyrifos (EPA 1999), CGT was found to be ‘Solid Waste’, two management categories lower than ‘Hazardous Waste’. There was no evidence to suggest these chemical residues detected should be considered a risk of causing toxic action in the future. Furthermore, future classification was expected to reduce CGT to an ‘inert’ waste, because of decreased pesticide use with increased use of Bollgard II cotton varieties. Hassall and Associates (2005) independently estimated (see CRDC archives) the cost-benefit ratio of this research project of ~10 000 : 1, in compliance costs of $AUD1.2 billion, discounted over the next 20 years.
Roundup Ready cotton
Two projects were undertaken assessing the introduction of Roundup Ready Cotton: a preliminary risk assessment, then several years later a review of hazard with respect to the more residual herbicide programs being used as shown by the pattern of herbicide usage data following the introduction of the new technology.
The first project commenced in 1999 and consisted of a risk assessment using modelled data, which involved a series of theoretical herbicide application programs, and a field study involving herbicide treatment programs for weed control in cotton (Crossan and Kennedy 2004; Crossan et al. 2007). Three analyses were undertaken – assessment of the risk of exceeding water quality guidelines based on the runoff concentrations from the field study; relative risk to ecosystems based on data from the field trials; and the analysis of relative risk of the herbicide programs likely to be used with Roundup Ready Cotton new technology compared with conventional programs.
Glyphosate (the key active ingredient in Roundup and variants) posed the smallest probability of exceeding the water quality guidelines, a result of its relatively low mobility and low detectable concentrations in runoff samples, taken with its low toxicity (Crossan et al. 2007).
In assessing the conventional versus GM herbicide programs it was concluded that the use of prometryn and fluometuron in a GM program instead of trifluralin and diuron would result in a reduction of risk associated with the overall GM program when ecological endpoint were considered. The analysis for the combined herbicide programs supported this finding. Herbicides, such as glyphosate, prometryn and fluometuron, were routinely found to present ‘negligible’ or ‘low risk’ of either exceeding water quality guidelines or threatening the ecosystem species assessed. This reinforced the suggestion and prediction that certain chemicals, such as glyphosate, metolachlor, 2,4-D, pyrithobac-sodium and clethodim, could be included in a ‘low risk’ herbicide program if their use has replaced higher risk herbicides such as trifluralin and diuron.
In 2008, a follow-up study used the Environmental Impact Quotient (EIQ) method to review the herbicide use associated with the introduction of Roundup Ready (Kovach et al. 1992). An EIQ (see also Knox et al. 2006, 2012) is a score based upon physical, chemical and toxicological characteristics of pesticides and includes farm-worker occupational health and safety and consumer components that provides capability to report social indicators.
These data indicated that on an industry scale, the used of herbicides following the introduction for Roundup Ready had not changed significantly on average for all forms of cotton, largely because of greater use on conventional cotton (Crossan et al. 2007; Kennedy et al. 2011). These results were surprising, but consistent with the previous predictions regarding the selection of particular herbicides. Indeed, it was recently estimated that the introduction of herbicide-resistant GM crop technology led to a 239-million-kilogram increase in herbicide use in the United States between 1996 and 2011 (Benbrook 2012). Whether or not this equated to increased ecological risk was not systematically ascertained. In Australia, it was expected that the widespread introduction of Roundup Ready Flex from 2008, a more effective two-gene version of the technology, would enable greater reduction in use of higher risk residual herbicides. However, the emergence of glyophosate-resistant weed populations requiring alternative herbicides for control (Werth et al. 2008; Green 2012) will need to be accounted for in future risk assessments. An updated risk analysis is now necessary to confirm more recent changes in herbicide use in the Australian cotton industry.
Trends in environmental risk of insecticide and herbicide use with the introduction of GM cotton
A University of Sydney honours project made use of the Cotton Consultants Australia historical pesticides application dataset from 1995–96 through to 2005–06. Insecticide use for each cotton-growing catchment was totalled for each season included in the available dataset and an EIQ was calculated for each chemical and the total EIQ was determined for each catchment (Kovach et al. 1992). EIQ were also calculated to compare conventional and GM cotton varieties.
This was the first overall risk assessment for cotton production in Australia and the results were largely as expected, based upon the observation of substantially reduced pesticide application (Fig. 7). Although the assessment used a simple scoring system (not a measure of actual risk because data on exposure was not quantified), it was used to compare catchments and seasonal trends. It was found (Crossan et al. 2007) that the EIQ and therefore the environmental ‘footprint’ with respect to insecticide use generally declined from 1998 to 2006 (Fig. 7, Namoi catchment). Such data need to be presented together with rainfall precipitation records, which we consider to be a precursor to insect pressure. However, it is clear that the GM programs provided reduced potential impact on cotton farms when analysed on a per-hectare basis. Not only does this analysis show improvements to on-farm water quality but includes the broader social aspect of worker safety with increasing adoption of GM technologies.

|
We have summarised the methods used to assess risk from pesticides used on cotton, documenting the expected improvement to water quality associated with the introduction of GM cotton as a clear result. Nevertheless, the period of introduction of the GM technology coincided with reduced rainfall and a marked reduction in available water to grow cotton. It is therefore legitimate to warn the industry that the drought period assisted the transition into GM more successfully; this reduced the likelihood of the GM technology failing from resistance development from higher insect pressure and providing immediate feedback to growers that chemical applications could be reduced without significant risk to yield.
Based on the assumption that greater regional precipitation would generate greater insect pressure on cotton crops, analysis of the rates of insecticide application and precipitation was interesting. Such an analysis was presented in Crossan et al. (2007) confirming this correlation – a strong trend could be observed between precipitation and insecticide use, averaged across the industry. While decreases in the use of insecticides on per-hectare basis could be easily reported for GM crops there were actually increases for conventional cotton crops in wetter years. Hence, the proportion of the total crop planted with GM was identified to be critically important to assess the success of the technology with respect to insecticide application, given these countervailing results. From this analysis it appeared that at least 80% of the total crop needed to be GM in order to observe the benefits of reduced insecticide use. Whatever the detailed explanation for the data (Crossan et al. 2007) may be, the story for GM introduction is a successful one with respect to the decreased in total insecticide use and shift to more selective products as defined by the series of hazard and risk assessments presented.
Changes in chemical use still need to be reviewed, monitored and managed appropriately. Every pesticide application poses an inherent hazard at the site and to adjacent ecosystems and waterways. Management systems such as BMP and tools to review practice site specific practices are more recently becoming available to complete a robust package of scientific enquiry and subsequent application to improve practice.
Environmental stewardship: towards systems’ perspectives
Water quality tests and quick tests
A critical feature of research on the environmental fate of pesticides has the availability of reliable analytical techniques. During the 20-year period of this review, significant developments in such procedures have occurred from which research has benefited. For example, the reliance of gas chromatography-electron capture detection, most useful for organochlorines, has been replaced by mass spectrometric methods such as liquid chromatography bi-dimensional mass spectrometry (LC-MSMS), where residues are identified by their mass structure. Quality assurance was a significant feature of the LWRRDC-CRDC-MDBC project of the 1990s (Kennedy et al. 1998). Many research students became expert at such analyses (Feng et al. 1994, Feng and Kennedy 1997; Van Zwieten and Kennedy 1995; Van Zwieten et al. 1995; Crossan et al. 2002; Shivaramaiah et al. 2005; Shivaramaiah and Kennedy 2006; Burns et al. 2008), also participating in the development of anti-body based technology as used in ELISA (Lee et al. 1995, 1997; Wang et al. 1997, 2003, 2007; Lee and Kennedy 2001; Yuan et al. 2011). This research has culminated in Cotton Catchment Communities CRC research to develop rapid water testing technology, using immunogold to visualise water quality with respect to herbicides such as diuron, prometryn and fluometuron (see Fig. 8). The work was conducted in collaboration with Tianjin University of Science and Technology, where Shuo Wang, a former CRDC research student is now University President. This technology as Quick Tests allows the presence of contaminants or their absence to be established within 5–10 min, allowing on-site management decisions. As recently as 2012, this technology was successfully validated (Crossan and Kennedy 2012) using instrumental analyses (LC-MSMS) for water samples taken from the Namoi River in NSW and the Fitzroy River at Emerald in Qld. The CRC technology is now being commercialised by a spin-off company, Quick Test Technologies.

|
Catchment-scale ecological risk assessment
Australian studies often characterise the ‘risk’ from pesticides but generally lack the use of a consistent method in the assessments. The Australian cotton industry invested in the development of a catchment-based ecological risk model that would be capable of highlighting aquatic ecosystems in cotton catchments at risk from the use of pesticides on adjacent farms. Characterising both the range of approaches capable of characterising the risk of pesticides in agricultural catchments, ecotoxicological responses and catchment-scale modelling available, a PhD thesis (Burns 2011) focussed on the use of such a framework in the Gwydir River catchment.
A probabilistic characterisation of risk using available river concentration obtained from the NSW Department of Water and ecotoxicity data identified ‘hotspot’ areas. Through joint probabilistic analysis of concentrations of diuron in the reaches of the Gwydir River catchment and ecotoxicity information using the method of Solomon et al. (2000), hotspots were identified (see Burns 2011; for details). The risks from pesticides were related to adjacent agricultural land uses. Qualitative uncertainties identified from this analysis included uncertainty in the concentrations of pesticides that occurred in the rivers outside of the sampling times and the sources of chemical loading in the sub-catchments. Time-variable concentrations and identification of potential sources of chemical loading was further investigated using the PRZM-RIVWQ (Williams et al. 2004; Carousel et al. 2005) spatial model framework. Using readily available spatial data and a review of cropping practices, modelling scenarios were executed. A summary of the application of this framework and potential outputs for the Gwydir River catchment is given in Fig. 9.
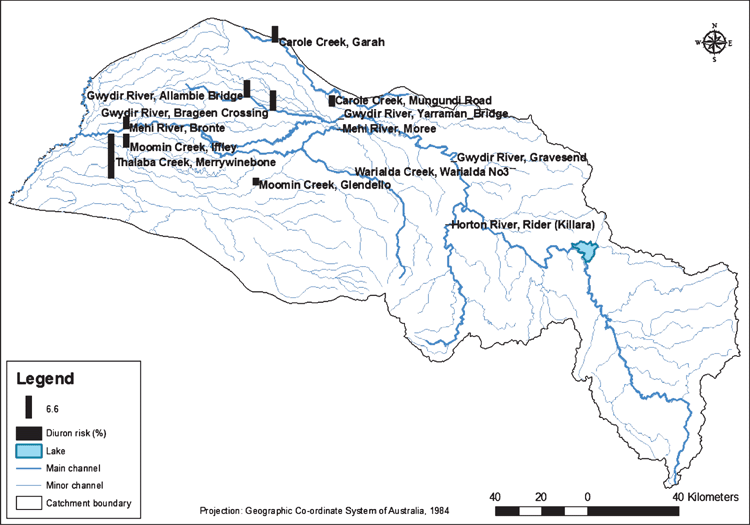
|
These methods provide a simple solution to actively managing the risk from pesticides in aquatic ecosystems. Such tools could be readily utilised by catchment managers and industry bodies in the active management of pesticides. The participation of the Australian cotton industry in supporting the development of such tools highlights their proactive nature in seeking solutions to using and managing pesticides in a sustainable manner. However, more resolve as a matter of policy may be needed to proceed to this logical stage of more effective risk management.
Whole catchment management and participatory action
The importance of government and other statutory agencies assisting in the regulation of the environment must also be acknowledged. For example, the Australian Pesticides and Veterinary Medicines Authority (APVMA) that regulates the registration and conditions of use of agricultural and veterinary chemicals must be acknowledged. The APVMA ensures that registrants meet strict standards regarding efficacy of action of products and their human and environmental safety. In so doing it must achieve a broad consensus that is bound to displease extreme points of view regarding precautionary environmental protection and minimum regulation.
This role was evident during the recent process of review of the registration of the residual herbicide diuron (Armbrust et al. 2013) where viewpoints were sought from all sectors of society in achieving its evidence-based decisions requiring even stricter conditions of use, often at lower rates than formerly, but partly cognisant of the need to consider unintended consequences of complete bans. This process showed the value of field research since data gained years before in the 1990s (e.g. Baskaran and Kennedy 1999; Silburn and Kennedy 2007; Simpson 2007) was key to their decisions. Earlier, during the period of greater potential risk when much larger quantities of chemicals were applied by the cotton industry, the APVMA kept a watching brief to ensure that risks to human health were adequately considered, seeking evidence about risk from government agencies and also depending on government departments of health for monitoring statistical clustering of potential effects. For example, the APVMA state confidently based on its sources of evidence that the very toxic insecticide endosulfan was being applied safely, even up to the point where its registration was withdrawn in response to the listing of endosulfan as a persistent organic pollutant by the United Nations Stockholm Convention. Such a conclusion was based on information of the strict conditions under which this chemical was applied, involving expert aerial application using GPS recording of every metre of cotton plants to which this chemical was applied. In addition, it was clear from field evidence that this chemical was not persistent under Australian conditions of use (Kennedy et al. 2011; Armbrust et al. 2013) and that its listing by the Stockholm Convention as persistent was driven by eco-politics rather than science since it clearly does not meet the Convention’s criteria for persistence (see Armbrust et al. 2013). For the sake of its credibility, the Stockholm Convention would better have redefined its criteria rather than provide a flawed decision with disordered results. With its enhanced knowledge of how to manage risk from endosulfan, the Australian cotton industry would have preferred to retain access to its unique chemistry, as an important part of its strategy for managing pest resistance; that access to endosulfan may still be required under special licence conditions if GM cotton shows signs of losing its effectiveness.
Conclusion
In retrospect, the effectiveness of the response by the Australian cotton industry to environmental risk arising from its activities in cotton catchments has been formidable. In particular, the improved quality of water in the north-eastern rivers of Australia is testimony to this outcome. This is well recognised by agencies such as the federal and state departments of the environment that were active partners in the transition. In effect, a situation in 1990 that threatened the continued existence of the industry was reversed so that the industry is now credited as being strongly pro-active in this area. The significant reduction in the need for using agrochemicals through GM of cotton as well as their better management, linked with integrated pest management, have been the most impressive developments. This has been testimony to the benefit that can flow from genetic modification of crops. Achieving this has required a cast of thousands, farmers’ organisations, research managers, researchers and research students, needed in both planning and execution. These successes are not fully appreciated in urban political viewpoints, but they exist irrespective of such recognition, as shown by various monitoring studies outlined in this paper (see Fig. 10). In a liberal democracy, the ability of farmers to use their resources of land and water in the most profitable manner possible, consistent with adequate environmental protection, has led them to choose cotton as a their most favoured product on sound economic grounds.

|
The Australian system of jointly funded research shared between government and industry has played a strong role during the past quarter century. The CRDC and the three successive Cooperative Cotton Research Centres funded between 1994 and 2012 have provided a continuity of effort that has been essential for this success. This is justified on the basis that environmental research has benefits for both production and ecosystem services. Although the era of cooperative research centres between universities, CSIRO, government departments and industry may be about to end, it is clear that this system has served rural Australia well. This does not deny that a vigilant approach to environmental protection is still needed.
Science is a human social activity. Applying it successfully requires a range of social skills as well as technological skills. Its extension in society and in rural communities is an ongoing process that is never complete. This paper has attempted to illustrate the real nature of this sometimes visionary activity.
Acknowledgements
We are grateful to the three Australian Cotton CRCs, CRDC, LWRRDC, GRDC and the Australian Centre for International Agricultural Research (ACIAR) for funding this research. We also thank Dr Vic Edge, Bruce Pyke and Ralph Schulze of the CRDC for their ongoing feedback and encouragement. Ralph’s advice that ‘we will make the decisions if you provide us with the scientific facts’ has been a guiding principle of the relationship between industry and researchers. Cotton CRC CEOs, Greg Constable, Gary Fitt, Guy Roth and Philip Armytage all earned our respect. We are also very pleased to acknowledge strong research cooperation with Professors Ron Harris and Keith Solomon at the University of Guelph, and Don Mackay at Trent University, Canada since the 1990s. We also acknowledge the contributions of Nazir Ahmad, Lukas Van Zwieten, Lu Feng, Shuo Wang, Alice Lee, Lyndal Hugo, Honna Shivaramaiah and Mark Silburn – all PhD graduates of the University of Sydney – Stephen Kimber, Sebastian Southan, Helen Beasley, Nicholas Woods, Gary Dorr, Drs Francisco Sánchez-Bayo, Sundaram Baskaran, Yajuan Shi, John Skerritt and Barry Noller, Professors Shuo Wang and Yan Zhang at the Tianjin University of Science and Technology, with many others, for their enthusiastic involvement. Permission to reproduce figs 1–4, 6 and 10 from the American Chemical Society is also acknowledged.
References
Armbrust K, Burns M, Crossan AN, Fischhoff DA, Hammond LE, Johnston JJ, Kennedy I, Rose MT, Seiber JN, Solomon K (2013) Perspectives on communicating risks of chemicals. Journal of Agricultural and Food Chemistry 61, 4676–4691.| Perspectives on communicating risks of chemicals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsVCjs7k%3D&md5=59a0e99b88f60a59b9746d0e908ef231CAS | 23662936PubMed |
Baskaran S, Kennedy IR (1999) Sorption and desorption kinetics of diuron, fluometuron, prometryn and pyrithiobac sodium in soils. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes 34, 943–963.
| Sorption and desorption kinetics of diuron, fluometuron, prometryn and pyrithiobac sodium in soils.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FjtVKkug%3D%3D&md5=ab0be20b882779b8b2f39c43c3fd6e5fCAS | 10565420PubMed |
Benbrook CM (2012) Impacts of genetically engineered crops on pesticide use in the U.S. – the first sixteen years. Environmental Sciences Europe 24, Art. No. 24
| Impacts of genetically engineered crops on pesticide use in the U.S. – the first sixteen years.Crossref | GoogleScholarGoogle Scholar |
Burns M (2011) Catchment-scale ecological risk assessment of pesticides. PhD Thesis, University of Sydney, NSW, Australia.
Burns M, Crossan AN, Kennedy IR, Rose MT (2008) Sorption and desorption of endosulfan sulfate and diuron to composted cotton gin trash. Journal of Agricultural and Food Chemistry 56, 5260–5265.
| Sorption and desorption of endosulfan sulfate and diuron to composted cotton gin trash.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVynt7k%3D&md5=3186c1601b6a4a70d93720df8ec28407CAS | 18543928PubMed |
Bushby J (1997) The Helix case. Released from Clayton Utz in September 1997. Available at: http://www.tved.net.au/PublicPapers/September_1997,_Lawyers_Education_Channel,_The_Helix_Case.html
Carousel RF, Imhoff JC, Hummel PR, Cheplick JM, Donigian AS, Suarez LA (2005) ‘Pesticide Root Zone Model (PRZM)-3. PRZM: a model for predicting pesticide and nitrogen fate in the crop root and unsaturated soil zones.’ 3.12.2 edn. (USEPA: Athens, GA)
Cheesman MJ, Horne I, Weir KM, Pandey G, Williams MR, Scott C, Russell RJ, Oakeshott JG (2007) Carbamate pesticides and their biological degradation – prospects for enzymatic bioremediation. In ‘Rational environmental management of agrochemicals: risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 320–337. (Oxford University Press: Washington, DC)
Connolly RD, Kennedy IR, Silburn DM, Simpson BW, Freebairn DM (2001) Simulating endosulfan transport in runoff from cotton fields in Australia using the GLEAMS model. Journal of Environmental Quality 30, 702–713.
| Simulating endosulfan transport in runoff from cotton fields in Australia using the GLEAMS model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsLc%3D&md5=3c7a2657979c1cc0730c657fe964916aCAS | 11401260PubMed |
Cotton Pest Management Guide (1992) ‘Cotton pest management guide.’ (Ed. AJ Shaw) (NSW Department of Primary Industries: Orange, NSW)
Cox Inall Communications (1998) ‘The cotton model. Minimising the impact of pesticides on the riverine environment.’ (Land and Water Resources Research and Development Corporation: Canberra)
CRDC (Cotton Research and Development Corporation) (1991–93) Annual Reports, 1991–93. Narrabri. Available at: www.CRDC.com.au
CRDC US 156C (2004) Waste classification of cotton gin trash. Final Report. Cotton Research and Development Corporation, Narrabri, NSW, Australia.
Crossan AN (2002) Remediation of pesticides on cotton farms: studies of the environmental distribution, transport and fate of five pesticides. PhD Thesis, The University of Sydney, NSW, Australia.
Crossan A, Kennedy I (2004) ‘A snapshot of Roundup Ready cotton in Australia.’ (The University of Sydney: Sydney)
Crossan A, Kennedy IR (2008) Calculation of pesticide degradation in decaying cotton gin trash. Bulletin of Environmental Contamination and Toxicology 81, 355–359.
| Calculation of pesticide degradation in decaying cotton gin trash.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFajurzL&md5=81dc85a5875d5bd5e94742cecd325d34CAS | 18651089PubMed |
Crossan AN, Kennedy IR (2012) Monitoring diuron in two cotton catchments. Cotton Research and Development Corporation Report, Narrabri, NSW, Australia.
Crossan AN, Lee N, Sharma R, Kennedy IR, Beckett IR (2002) Assessment of the distribution of pesticides on soil particle fractions in simulated irrigation run-off using centrifugal split-flow thin-channel fractionation and ELISA. Analytica Chimica Acta 468, 199–208.
| Assessment of the distribution of pesticides on soil particle fractions in simulated irrigation run-off using centrifugal split-flow thin-channel fractionation and ELISA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFyhu7c%3D&md5=224322ff1ec1d5d393060ae045887ab5CAS |
Crossan A, Sanchez-Bayo F, Kennedy I, Bodnaruk K (2006) Risk of pesticide contamination in cotton seed and livestock. The Australian Cottongrower, February–March 2006.
Crossan AN, Rose MT, Kennedy IR (2007) Pesticide risk reduction by management practices: an environmental case study of the Australian cotton industry. In ‘Rational environmental management of agrochemicals: risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 320–337. (Oxford University Press: Washington, DC)
Cunningham SD, Shann JR, Crowley DE, Anderson TA (1997) Phytoremediation of contaminated water and soil. In ‘Phytoremediation of soil and water contaminants’. (Eds EL Kruger, TA Anderson, JR Coats) (American Chemical Society: Washington, DC)
ECOFRAM (Framework for Ecological Risk Assessment) (1992) Environmental Protection Agency, EPA/630/R-92/001, Washington, DC, USA.
EPA (1999) ‘Environmental Guidelines: assessment, classification and management of liquid and non- liquid wastes.’ (NSW Environmental Protection Agency: Sydney)
Feng L, Kennedy IR (1997) Biodegradation and plant protection from the herbicide 2,4-D by plant-microbial associations in cotton production systems. Biotechnology and Bioengineering 54, 513–519.
| Biodegradation and plant protection from the herbicide 2,4-D by plant-microbial associations in cotton production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtVWqtbk%3D&md5=4f95acc8d13bee14223e8dc3b59bfcabCAS | 18636407PubMed |
Feng L, Rolfe BG, Gartner E, Van Zwieten L, Kennedy IR (1994) Expression of the 2,4-D degrading plasmid pJP4 of Alcaligenes eutrophus in Rhizobium trifolii. Acta Biotechnologica 14, 119–129.
| Expression of the 2,4-D degrading plasmid pJP4 of Alcaligenes eutrophus in Rhizobium trifolii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmsFSqu7Y%3D&md5=536c2e175523ef21704061925a50c39cCAS |
Fitt GP (2000) An Australian approach to IPM in cotton: integrating new technologies to minimise insecticide dependence. Crop Protection 19, 793–800.
| An Australian approach to IPM in cotton: integrating new technologies to minimise insecticide dependence.Crossref | GoogleScholarGoogle Scholar |
Fitt GP (2003) Implementation and impact of transgenic Bt cottons in Australia. In ‘ICAC Recorder’. Vol. 21. (Ed. M Rafiq Chaudhry) pp. 14–19. (International Cotton Advisory Committee: Washington, DC)
Forrester NW, Cahill M, Bird LJ, Layland JK (1993) Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bulletin of Entomological Research (Suppl No. 1), 132–145.
GHD (2003) ‘Second Australian Cotton Industry Environmental Audit.’ (Cotton Research and Development Corporation: Narrabri)
Green JM (2012) The benefits of herbicide-resistant crops. Pest Management Science 68, 1323–1331.
| The benefits of herbicide-resistant crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFeitL7N&md5=8420ba381c3e7d19a1a96ac046b5dac2CAS | 22865693PubMed |
Gregoire C, Elsaesser D, Huguenot D, Lange J, Lebeau T, Merli A, Mose R, Passeport E, Payraudeau S, Schütz T, Schulz R, Tapia-Padilla G, Tournebize J, Trevisan M, Wanko A (2009) Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems. Environmental Chemistry Letters 7, 205–231.
| Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFaltrs%3D&md5=d2bf5e3428e5e38e603ace10dc621672CAS |
Hassall and Associates (2005) ‘Value of research investment relating to the waste classification of cotton gin trash.’ (Cotton Research and Development Corporation: Narrabri, NSW)
Holloway R, Roth G (2003) Grower feedback on cotton BMP auditing. The Australian Cottongrower 25, 20–22.
Jarman PJ, Montgomery J (2001) ‘Waterbirds and irrigation storages in the Lower Gwydir Valley, NSW.’ (The University of New England: Armidale, NSW)
Kennedy IR, Ahmad N, Beasley H, Chapman J, Hobbs J, Simpson BW, Woods N (1998) ‘Quality assurance in pesticide sampling and analysis.’ Occasional Paper 14/98. (Land and Water Resources Research and Development Corporation: Canberra, ACT)
Kennedy IR, Sánchez-Bayo F, Kimber SW, Hugo L, Ahmad N (2001) Off-site movement of endosulfan from irrigated cotton in New South Wales. Journal of Environmental Quality 30, 683–696.
| Off-site movement of endosulfan from irrigated cotton in New South Wales.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsLk%3D&md5=ae8b7dd78aeca9845a8b94112dddb14aCAS | 11401258PubMed |
Kennedy IR, Solomon KR, Gee SJ, Crossan A, Wang S, Sánchez-Bayo F (2007) Achieving rational use of agrochemicals: environmental chemistry in action. In ‘Rational environmental management of agrochemicals: risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 2–13. (Oxford University Press: Washington, DC)
Kennedy I, Crossan AN, Burns M, Shi Y (2011) Fate and transport of agrochemicals in the environment. In ‘Kirk-Othmer encyclopedia of chemical technology’. (Ed. A Seidel) pp. 1–33. (John Wiley & Sons: New York)
Knox OGG, Constable GA, Pyke B, Gupta VVSR (2006) Environmental impact of conventional and Bt insecticidal cotton expressing one and two Cry genes in Australia. Australian Journal of Agricultural Research 57, 501–509.
| Environmental impact of conventional and Bt insecticidal cotton expressing one and two Cry genes in Australia.Crossref | GoogleScholarGoogle Scholar |
Knox OGG, Walker RL, Booth EJ, Hall C, Crossan AN, Gupta VVSR (2012) Capitalizing on deliberate, accidental, and GM-driven environmental change caused by crop modification. 6th European Workshop on Leaf Senescence. Journal of Experimental Botany 63, 543–549.
| Capitalizing on deliberate, accidental, and GM-driven environmental change caused by crop modification. 6th European Workshop on Leaf Senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xos1Crsw%3D%3D&md5=ed5a29933dcbc32f0d3cecf5659676bfCAS |
Kovach J, Petzoldt C, Regni J, Tettle J (1992) A method to measure the environmental impact of pesticides. New York Food Life Sciences Bulletin 139, 1–18.
Krishna VV, Qaim M (2012) Bt cotton and sustainability of pesticide reductions in India. Agricultural Systems 107, 47–55.
| Bt cotton and sustainability of pesticide reductions in India.Crossref | GoogleScholarGoogle Scholar |
Kumar A, Chapman J (2001) Profenofos residues in wild fish from cotton-growing areas of New South Wales, Australia. Journal of Environmental Quality 30, 740–750.
| Profenofos residues in wild fish from cotton-growing areas of New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsbw%3D&md5=4babb0b44a1d99e362c19adcc12722f2CAS | 11401263PubMed |
Lee NA, Kennedy IR (2001) Environmental monitoring of pesticides by immunochemical techniques: validation, current status, and future perspectives. Journal - Association of Official Analytical Chemists 84, 1393–1406.
Lee N, Skerritt JH, Kennedy IR (1995) Chemistry protecting the environment: field testing for pesticide residues. Chemistry in Australia 62, 14–17.
Lee N, Beasley HL, Kimber SWL, Silburn M, Woods N, Skerritt JH, Kennedy IR (1997) Application of immunoassays to studies of the environmental fate of endosulfan. Journal of Agricultural and Food Chemistry 45, 4147–4155.
| Application of immunoassays to studies of the environmental fate of endosulfan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtlKksbs%3D&md5=1bfd8392bf16ce58061c554ff74be9e6CAS |
Leonard AW, Hyne RV, Lim RP, Leigh KA, Le J, Beckett R (2001) Fate and toxicity of endosulfan in Namoi River water and bottom sediment. Journal of Environmental Quality 30, 750–759.
| Fate and toxicity of endosulfan in Namoi River water and bottom sediment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsb0%3D&md5=db114aa03c2354f58adb435fdb32612eCAS | 11401264PubMed |
Macarthur-Agribusiness (2004) ‘Evaluation of the Australian Cotton Industry Best Management Practices Program.’ (Cotton Research and Development Corporation: Narrabri)
Mensah RK, Singleton A (2004) Development of IPM in cotton in Australia: establishment and utilization of natural enemies and integration with biological and synthetic insecticides. In ‘Proceedings World Cotton Conference – 3. Cotton production for the new millennium’. Cape Town, 2003. (Ed. A Swanepoel) pp. 941–951. (ARC, Institute for Industrial Crops: Pretoria, South Africa)
Muschal M (2000) Central and North West Regions Water Quality Program: 1999–2000 report on pesticides monitoring. NSW Department of Land and Water Conservation, Parramatta, NSW.
Norton SB, Rodier DJ, Gentilke JH, van der Schalie WH, Wood WP, Slimak MW (1992) A framework for ecological risk assessment at the EPA. Environmental Toxicology and Chemistry 11, 1663–1672.
| A framework for ecological risk assessment at the EPA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmsFKqu7s%3D&md5=94a8be276d9d516c3985b1061961ba50CAS |
O’Geen AT, Budd R, Gan J, Maynard JJ, Parikh SJ, Dahlgren RA (2010) Mitigating nonpoint source pollution in agriculture with constructed and restored wetlands. Advances in Agronomy 108, 1–76.
| Mitigating nonpoint source pollution in agriculture with constructed and restored wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFKgurrP&md5=0296670c5619a9b0514f99555bd181b0CAS |
Raupach MR, Briggs PR, Ford PW, Leys JF, Muschal M, Cooper B, Edge VE (2001a) Endosulfan transport: I. Integrative assessment of airborne and waterborne pathways. Journal of Environmental Quality 30, 714–728.
| Endosulfan transport: I. Integrative assessment of airborne and waterborne pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsb4%3D&md5=61a3ee5b5b2d5c1543d2fb9d7ca68047CAS | 11401261PubMed |
Raupach MR, Briggs PR, Ahmad N, Edge VE (2001b) Endosulfan transport: II. Modeling airborne dispersal and deposition by spray and vapor. Journal of Environmental Quality 30, 729–740.
| Endosulfan transport: II. Modeling airborne dispersal and deposition by spray and vapor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsb8%3D&md5=8565a03ffd5fd6aa377459aaaf155e4bCAS | 11401262PubMed |
Reid N, O’Shea G, Silberbauer L (2003) A review of biodiversity research in the Australian Cotton Industry. Final report to the Cotton Research and Development Corporation, December 2003, The University of New England, Armidale, NSW.
Rose MT, Sánchez-Bayo F, Kennedy IR (2001) Phytoremediation of pesticide residues by wetland plants. In ‘Abstracts, 2nd National Wetlands Conference’. 14–16 November 2001, South Stradbroke Island, Qld. (Eds A Jensen, J O’Loghlin) p. 104. (WetlandCare Australia: Ballina, NSW)
Rose MT, Crossan AN, Kennedy IR (2005) Reducing pesticide exposure risk on floodplain cotton farms by enhancing natural removal processes. In ‘8th International River Symposium’. 6–9 September, Brisbane, Qld. (International WaterCentre: Brisbane) Available at: www.riversymposium.com/about/proceedings/2005-proceedings/
Rose MT, Sánchez-Bayo F, Crossan AN, Kennedy IR (2006) Pesticide removal from cotton farm tailwater by a pilot-scale ponded wetland. Chemosphere 63, 1849–1858.
| Pesticide removal from cotton farm tailwater by a pilot-scale ponded wetland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkslSgu7o%3D&md5=0358ce2d45146fd38778ea7272512354CAS | 16330067PubMed |
Rose MT, Crossan AN, Kennedy IR (2007) Dissipation of cotton pesticides from runoff water in glasshouse columns. Water, Air, and Soil Pollution 182, 207–218.
| Dissipation of cotton pesticides from runoff water in glasshouse columns.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltVylu7Y%3D&md5=8e277f83cf8dc56a4c77a99c4daa237fCAS |
Rose MT, Crossan AN, Kennedy IR (2008) The effect of vegetation on pesticide dissipation from ponded treatment wetlands: quantification using a simple model. Chemosphere 72, 999–1005.
| The effect of vegetation on pesticide dissipation from ponded treatment wetlands: quantification using a simple model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1ShsLo%3D&md5=9a8020ea5e2b6d478565ee95fa18d51bCAS | 18539309PubMed |
Russell RJ, Sutherland TD, Horne I, Oakeshott JG (2001) Enzymatic bioremediation of chemical pesticides. Australasian Biotechnology 11, 24–26.
Sánchez-Bayo F, Ward R, Beasley H (1999) A new technique to measure bird’s dietary exposure to pesticides. Analytica Chimica Acta 399, 173–183.
| A new technique to measure bird’s dietary exposure to pesticides.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Bayo F, Baskaran S, Kennedy IR (2002) Ecological Relative Risk (EcoRR): another approach for risk assessment of pesticides. Agriculture, Ecosystems & Environment 91, 37–57.
| Ecological Relative Risk (EcoRR): another approach for risk assessment of pesticides.Crossref | GoogleScholarGoogle Scholar |
Schofield N, Williams A, Holloway R, Pyke B (2007) Minimising riverine impacts of endosulfan used in cotton farming – A science into practice environmental success story. In ‘Rational environmental management of agrochemicals: Risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 320–337. (American Chemical Society: Washington, DC)
Scott C, Lewis SE, Milla R, Taylor MC, Rodgers AJW, Dumsday G, Brodie JE, Oakeshott JG, Russell RJ (2010) A free-enzyme catalyst for the bioremediation of environmental atrazine contamination. Journal of Environmental Management 91, 2075–2078.
| A free-enzyme catalyst for the bioremediation of environmental atrazine contamination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyjsbnO&md5=0a6e0f656a4c99937c256e57d166cde8CAS | 20570036PubMed |
Shelton DR, Karns JS (1998) Pesticide bioremediation: genetic and ecological considerations. In ‘Pesticide remediation in soils and water’. (Eds PC Kearney, TR Roberts) pp. 181–216. (John Wiley and Sons, Ltd: West Sussex, UK)
Shivaramaiah HM, Kennedy IR (2006) Biodegradation of endosulfan by a soil bacterium. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes 41, 895–905.
| Biodegradation of endosulfan by a soil bacterium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Squrc%3D&md5=fd3dcf2eedbb96650943b1bfbb1ce603CAS | 16893778PubMed |
Shivaramaiah HM, Odeh IOA, Kennedy IR, Skerritt JH (2002) Mapping the distribution of DDT residues as DDE in the soils of the irrigated regions of Northern New South Wales, Australia using ELISA and GIS. Journal of Agricultural and Food Chemistry 50, 5360–5367.
| Mapping the distribution of DDT residues as DDE in the soils of the irrigated regions of Northern New South Wales, Australia using ELISA and GIS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvVOrt74%3D&md5=4de5101beced50a53ff763acfe762e98CAS | 12207475PubMed |
Shivaramaiah HM, Sánchez-Bayo F, Al-Rifai J, Crossan AN, Kennedy IR (2005) The fate of endosulfan in water. Journal of Environmental Science and Health B40, 711–720.
Silburn DM, Kennedy IR (2007) Rain simulation to estimate pesticide transport in runoff. In ‘Rational environmental management of agrochemicals: risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 120–137. (Oxford University Press: Washington, DC)
Silburn DM, Simpson BW, Hargreaves PA (2002) Management practices for control of runoff losses from cotton furrows under storm rainfall. II. Transport of pesticides in runoff. Australian Journal of Soil Research 40, 21–44.
| Management practices for control of runoff losses from cotton furrows under storm rainfall. II. Transport of pesticides in runoff.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitVWnt7o%3D&md5=21b87d2cf4b4bbcc582b43d9d1536554CAS |
Simpson BW (2007) Lessons for achieving effective management from field research on agrochemicals. In ‘Rational environmental management of agrochemicals: risk assessment, monitoring, and remedial action’. ACS 966. (Eds IR Kennedy, KR Solomon, SJ Gee, A Crossan, S Wang, F Sánchez-Bayo) pp. 338–359. (Oxford University Press: Washington, DC)
Solomon K, Giesy J, Jones P (2000) Probabilistic risk assessment of agrochemicals in the environment. Crop Protection 19, 649–655.
| Probabilistic risk assessment of agrochemicals in the environment.Crossref | GoogleScholarGoogle Scholar |
US EPA (United States Environmental Protection Agency) (1998) ‘Guidelines for ecological risk assessment. EPA/630/R095/002F.01 April 1998 Risk Assessment Forum.’ (US EPA: Washington, DC)
Van Zwieten L, Kennedy IR (1995) Rapid degradation of atrazine by Rhodococcus sp. NI86/21 and by an atrazine perfused soil. Journal of Agricultural and Food Chemistry 43, 1377–1382.
| Rapid degradation of atrazine by Rhodococcus sp. NI86/21 and by an atrazine perfused soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlsFels70%3D&md5=317ead314ced4b3850ee478d0dcaed32CAS |
Van Zwieten L, Feng L, Kennedy IR (1995) Colonisation of seedling roots by 2,4-D degrading bacteria: a plant-microbial model. Acta Biotechnologica 15, 27–39.
| Colonisation of seedling roots by 2,4-D degrading bacteria: a plant-microbial model.Crossref | GoogleScholarGoogle Scholar |
Wang S, Kimber SWL, Kennedy IR (1997) The dissipation of lambda-cyhalothrin from cotton production systems. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes 32, 335–352.
| The dissipation of lambda-cyhalothrin from cotton production systems.Crossref | GoogleScholarGoogle Scholar |
Wang S, Beasely HL, Sumpter SR, Kennedy IR (2003) Validation of pyrithiobac-sodium (Staple) ELISA for Australian cotton soils. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes 38, 281–291.
| Validation of pyrithiobac-sodium (Staple) ELISA for Australian cotton soils.Crossref | GoogleScholarGoogle Scholar | 12716046PubMed |
Wang S, Guo AY, Zheng WJ, Zhang Y, Qiao H, Kennedy IR (2007) Development of ELISA for the determination of transgenic Bt-cottons using antibodies against Cry1ac protein from Bacillus thuringiensis HD-73. Engineering in Life Sciences 7, 149–154.
| Development of ELISA for the determination of transgenic Bt-cottons using antibodies against Cry1ac protein from Bacillus thuringiensis HD-73.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvV2itbs%3D&md5=b0c385c28975b7db3152af1d537547d5CAS |
Weaver TB, Ghadiri H, Hulugalle NR, Harden S (2012) Organochlorine pesticides in soil under irrigated cotton farming systems in vertisols of the Namoi Valley, north-western New South Wales, Australia. Chemosphere 88, 336–343.
| Organochlorine pesticides in soil under irrigated cotton farming systems in vertisols of the Namoi Valley, north-western New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltValtLk%3D&md5=51bb393dc13160013fef63718cc02260CAS | 22464189PubMed |
Werth JA, Preston C, Taylor IN, Charles GW, Roberts GN, Baker J (2008) Managing the risk of glyphosate resistance in Australian glyphosate-resistant cotton production systems. Pest Management Science 64, 417–421.
| Managing the risk of glyphosate resistance in Australian glyphosate-resistant cotton production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksVGqur4%3D&md5=592bbd5b09eb9e506e4e680515b3417fCAS | 18080291PubMed |
Williams A, Williams J (2000) ‘Australian cotton industry best management practices manual.’ (Cotton Research and Development Corporation: Narrabri)
Williams WM, Zdinak CE, Ritter AM, Cheplick JM, Singh P (2004) ‘RIVWQ chemical transport model for riverine environments: user manual and program documentation.’ (Waterborne Environmental Inc.: Leesburg)
Wilson LJ, Mensah RK, Fitt GP (2004) Implementing Integrated Pest Management in Australian cotton. In ‘Insect pest management, field and protected crops’. (Eds AR Horowitz, I Ishaaya) pp. 97–118. (Springer-Verlag: Berlin/Heidelberg)
Woods N, Craig IP, Dorr G, Young B (2001) Spray drift of pesticides arising from aerial application in cotton. Journal of Environmental Quality 30, 697–701.
| Spray drift of pesticides arising from aerial application in cotton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFWrsLY%3D&md5=e9d61c9463e175350827646154eb047cCAS | 11401259PubMed |
Yuan M, Liu B, Liu E, Sheng W, Zhang Y, Crossan A, Kennedy I (2011) An immunoassay for phenylurea herbicides: application of molecular modeling and QSAR analysis on antigen-antibody interaction study. Analytical Chemistry 83, 4767–4774.
| An immunoassay for phenylurea herbicides: application of molecular modeling and QSAR analysis on antigen-antibody interaction study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFSns7g%3D&md5=c7433cca21f3036f0c823d979f645319CAS | 21539295PubMed |



