Soil organic carbon concentrations and storage in irrigated cotton cropping systems sown on permanent beds in a Vertosol with restricted subsoil drainage
N. R. Hulugalle A B , T. B. Weaver A , L. A. Finlay A and V. Heimoana AA Australian Cotton Research Institute, NSW Department of Primary Industries, Locked Bag 1000, Narrabri, NSW 2390, Australia.
B Corresponding author. Email: nilantha.hulugalle@dpi.nsw.gov.au
Crop and Pasture Science 64(8) 799-805 https://doi.org/10.1071/CP12374
Submitted: 5 November 2012 Accepted: 28 February 2013 Published: 2 April 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Long-term studies of soil organic carbon dynamics in two- and three-crop rotations in irrigated cotton (Gossypium hirsutum L.) based cropping systems under varying stubble management practices in Australian Vertosols are relatively few. Our objective was to quantify soil organic carbon dynamics during a 9-year period in four irrigated, cotton-based cropping systems sown on permanent beds in a Vertosol with restricted subsoil drainage near Narrabri in north-western New South Wales, Australia. The experimental treatments were: cotton–cotton (CC); cotton–vetch (Vicia villosa Roth. in 2002–06, Vicia benghalensis L. in 2007–11) (CV); cotton–wheat (Triticum aestivum L.), where wheat stubble was incorporated (CW); and cotton–wheat–vetch, where wheat stubble was retained as in-situ mulch (CWV). Vetch was terminated during or just before flowering by a combination of mowing and contact herbicides, and the residues were retained as in situ mulch. Estimates of carbon sequestered by above- and below-ground biomass inputs were in the order CWV >> CW = CV > CC. Carbon concentrations in the 0–1.2 m depth and carbon storage in the 0–0.3 and 0–1.2 m depths were similar among all cropping systems. Net carbon sequestration rates did not differ among cropping systems and did not change significantly with time in the 0–0.3 m depth, but net losses occurred in the 0–1.2 m depth. The discrepancy between measured and estimated values of sequestered carbon suggests that either the value of 5% used to estimate carbon sequestration from biomass inputs was an overestimate for this site, or post-sequestration losses may have been high. The latter has not been investigated in Australian Vertosols. Future research efforts should identify the cause and quantify the magnitude of these losses of organic carbon from soil.
Additional keywords: cropping system, greenhouse gas, Haplustert, rotation, stubble retention, Vertisol.
Introduction
Enhancing storage of carbon in agricultural soil has been proposed as a partial solution to offset the accelerated release of greenhouse gases associated with global warming (Lal 2004). Under semi-arid conditions, however, significant and sustained sequestration of carbon has been reported primarily for farming systems with perennial crops and pastures (Luo et al. 2010; Sanderman et al. 2010; Chan et al. 2011; Powlson et al. 2011). Although some benefits have been reported for conservation farming practices such as zero-tillage and crop rotation, carbon sequestration rates have generally been low and subject to variables such as water and nutrient availability, temperature, soil condition and type, and management (Luo et al. 2010; Sanderman et al. 2010; Chan et al. 2011; Powlson et al. 2011). Where relatively high values (~2 Mg C ha–1 year–1 in the surface 0.3 m) have been reported (Follett et al. 2005; Rochester 2011), it has been in irrigated Vertosols.
Cropping systems under which irrigated cotton (Gossypium hirsutum L.) is grown in Australian Vertosols can be broadly classified into three groups: (1) cotton monoculture, where cotton is sown continuously in the same field; (2) long-fallow cotton, where cotton alternates with a bare fallow; and (3) cotton–rotation crop sequences, where cotton alternates with either summer or winter rotation crops (Cooper 1999). The most common rotation crop, used by >75% of cotton growers, is wheat (Cooper 1999). In contrast, 10–15% of cotton growers sow leguminous rotation crops such as faba bean (Vicia faba L.), vetch (Vicia spp.), and chickpea (Cicer arietinum L.), primarily with the objective of improving soil nitrogen (N) stocks (Hulugalle and Scott 2008). The frequency of rotation crops in irrigated systems can vary from 1 : 1 cotton–rotation crop to 2 : 1 (i.e. two cotton crops to one rotation crop). Sowing two or three rotation crops after cotton is, however, unusual. In one of the few studies that investigated including a third crop such as a legume in a two-crop, cotton-based rotation, water conservation and N stocks were enhanced but overall productivity and soil quality was not (Hulugalle et al. 2011, 2012a, 2013; Powell and Scott 2011). In contrast, Power et al. (2011) reported that a third crop (legume) was able to reduce risk, improve water use, and reduce N inputs.
Results from most cotton (Gossypium hirsutum L.) based farming systems in Australian semi-arid Vertosols suggest that a net loss rather than gain of carbon in soil is common (Hulugalle 2000; Hulugalle and Scott 2008; Knowles and Singh 2003). Nonetheless, because of the high aggregation potential, and high clay and silt contents in these soils (Six et al. 2002), it is theoretically possible that with conservation farming practices such as permanent beds, stubble retention/mulching, and crop rotation, carbon sequestration could take place at higher rates than in non-swelling soils. The objective of this study, therefore, was to quantify soil organic carbon dynamics in four irrigated, cotton-based cropping systems sown on permanent beds in a Vertosol with subsoil sodicity and, thus, restricted drainage. One of the cropping systems was a three-crop system, two were two-crop systems, and one was a cotton monoculture.
Materials and methods
Site
The experimental site was at the Australian Cotton Research Institute, near Narrabri (149°47′E, 30°13′S), in New South Wales, Australia. Narrabri has a subtropical, semi-arid climate, BSh (Kottek et al. 2006), and experiences four distinct seasons with a mild winter and a hot summer. The hottest month is January (mean daily maximum 35°C and minimum 19°C) and the coldest is July (mean daily maximum 18°C and minimum 3°C). Mean annual rainfall is 593 mm. The soil at the experimental site is a self-mulching, endohypersodic, grey Vertosol, very fine (Isbell 2002) (fine, thermic, smectitic, Typic Haplustert; Soil Survey Staff 2010). Mean particle size distribution in the 0–1.2 m depth was: clay, 64 g 100 g–1; silt, 11 g 100 g–1; and sand, 25 g 100 g–1. The electrochemical stability index, ESI [=electrical conductivity (EC1:5)/exchangeable sodium percentage (ESP)], during September 2002 in the 0.6–1.2 m depth was 0.02 and ESP was 12. In contrast, for the surface 0.6 m ESI averaged 0.10 and ESP 4. Average drainage out of the root-zone was ~25 mm per cotton season (October–May), approximating <3% of total water inputs (Hulugalle et al. 2013). Average drainage out of the root-zone was ~25 mm per cotton season (October–May), approximating <3% of total water inputs (Hulugalle et al. 2013). Between 2002 and 2010, soil quality declined in this site, with greatest falls occurring with CV (see below) and least with the cropping systems that included a wheat crop. These differences were related to changes in exchangeable potassium (K) and sodium (Na) concentrations, sodicity, and pH (Hulugalle et al. 2012a), which in turn were related to variations in drainage and leaching under the individual cropping systems and changes in irrigation water quality (Hulugalle et al. 2013).
Experimental layout
The treatments, sown on permanent beds from 2002 to 2011, were: cotton–cotton (summer cotton–winter fallow–summer cotton) (CC); cotton–vetch (Vicia spp.) (summer cotton–winter vetch–summer cotton) (CV); cotton–wheat (Triticum aestivum L.) (summer cotton–winter wheat–summer and winter fallow–summer cotton), where wheat stubble was incorporated into the beds with one or two passes of a disc-hiller (CW); and cotton–wheat–vetch (summer cotton–winter wheat–summer fallow–autumn and winter vetch–summer cotton), where wheat stubble was retained as an in-situ mulch into which the following vetch crop was sown (CWV). Vetch was killed during or just before flowering through a combination of mowing and contact herbicides, and the residues retained as in-situ mulch into which the following cotton was sown (Hulugalle et al. 2012b). The experiment was laid out as a randomised complete block with three replications and designed such that both cotton and rotation crop phases in CW and CWV sequences were sown every year. Individual plots were 165 m long and 20 rows wide. The rows (beds) were spaced at 1-m intervals, with vehicular traffic being restricted to the furrows. Details of the experiment and its management have been reported previously (Hulugalle et al. 2012a, 2013).
Crop management
Roundup Ready® cotton (Monsanto Co., St Louis, MO) was sown during October from 2002 to 2005, and Bollgard II® with Roundup Ready® Flex cotton (Monsanto Co.) thereafter. Namoi woolly pod vetch (Vicia villosa Roth.) was sown in the experiment from 2002 to 2006 and purple or Popany vetch (Vicia benghalensis L.) thereafter. Cotton in rotations that did not include a vetch component (CC and CW) received N as anhydrous ammonia injected before sowing cotton until the 2008–09 season and thereafter as urea broadcast after sowing cotton. Cotton in rotations that included vetch was not fertilised before sowing but received supplementary N broadcast as urea in December or January. Application rates were dependent on N content of the vetch biomass and estimated losses. All crops were furrow-irrigated at a rate of 1 ML ha–1 (=100 mm) of water when rainfall was insufficient to meet evaporative demand. Cotton was picked during late April or early May with a 2-row picker, after defoliation in early April. After cotton picking, the cotton was slashed and incorporated into the beds with a disc-hiller (to facilitate destruction of Helicoverpa spp. pupae). Average depth of incorporation was ~0.10 m. Wheat was sown during late May or early June and harvested during late November or early December. Vetch in CWV was sown into wheat stubble during autumn, following summer rains (any time between late February and early May), and vetch in CV was sown after cotton picking and pupae busting during May or early June. Vetch in CWV was slashed and killed with a contact herbicide usually during July or August and that in CV during September (Hulugalle et al. 2012b). Vegetative dry matter production of cotton before defoliation, wheat before harvest, and vetch before termination from 2007 to 2011 was estimated by subsampling from three locations (1 m2) in each plot.
Soil sampling and analyses
Soil was sampled from beds before planting cotton each year from September 2002 to October 2011, except during 2003 and 2004. This was done to enable at least one cropping cycle to be completed in all treatments. Soil was also sampled from the wheat phases of CW and CWV but results were excluded from subsequent analyses to avoid confounding. Soil cores (50 mm diameter) were extracted from 0–0.1, 0.1–0.3, 0.3–0.6, and 0.6–1.2 m depths using a stratified randomised sampling design from four locations in each plot with a tractor-mounted soil corer. Due to inadequate soil volume in the cores sampled from the surface 0.1 m, additional soil was sampled from the same depth in each location with a spade. A composite sample was made up for each depth in each plot and transported back to the laboratory and air-dried.
Air-dried soil was passed through a 0.5-mm sieve and total soil organic carbon (SOC) concentration determined by the wet oxidation method of Walkley and Black (Rayment and Lyons 2011). Soil clods extracted from the cores taken from 0–0.1, 0.1–0.3, 0.3–0.6, and 0.6–1.2 m depths were oven-dried for 48 h at 110°C and weighed, and volume was determined by coating in paraffin wax and displacement in water (Cresswell and Hamilton 2002). Bulk density was estimated by dividing oven-dried clod weight by its volume. In the 0–0.1 m depth, the volume of air-dried aggregates (1–10 mm diameter) was determined with the kerosene saturation method (McIntyre and Stirk 1954). Aggregate weights were converted to an oven-dried equivalent using an air-dry water content determined on subsamples. Bulk density of aggregates was determined by dividing the oven-dried equivalent of aggregate weight by its air-dry volume, as soil shrinkage curves had indicated that there was no significant difference in volume between air-dried and oven-dried soil (Hulugalle and Entwistle 1997). Bulk density for the 0–0.1 m depth was expressed as a weighted mean of the bulk densities of aggregates and clods (2 : 1 aggregates : clods) (Hulugalle and Entwistle 1997).
Storage of SOC (‘stocks’) in any one depth was estimated as the product of bulk density, sampling depth interval, and SOC concentration. The SOC storage was reported as that in the 0–0.3 m depth (sum of storage in the 0–0.10 and 0.10–0.30 m depths) and that in the 0–1.2 m depth (sum of storage in all depths sampled).
A potential source of error when evaluating storage and sequestration of soil carbon is the use of a fixed depth in the calculation rather than an equivalent soil mass, as the former does not account for possible changes in bulk density either over time or between treatments and when the entire profile is not sampled. Thus, it is preferable that carbon storage be reported on an equal mass of soil between the times being compared, as described by Ellert and Bettany (1995). Carbon storage was estimated with the fixed depth method (FD) and the method of Ellert and Bettany (1995) (ESM) for a subset of the results (2002–09) to ascertain the magnitude of differences, if any, between the methods, and values were compared using linear regression analysis.
Data analyses
Analyses were restricted to samples taken before sowing cotton; that is, results from the wheat phase of CW and CWV that at the time of sampling had an actively growing wheat crop were excluded to avoid confounding. Results of carbon concentration in soil in individual depths and carbon storage in the 0–0.3 and 0–1.2 m depths at each time of sampling were analysed using analysis of variance for a randomised complete block design. Means and standard errors of the means were calculated. The rates of change in carbon concentration for soil layers and carbon storage (SOC sequestration rates) in the 0–0.3 and 0–1.2 m depths between 2002 and 2011 among cropping systems were estimated and compared with linear regression analysis.
Results and discussion
Dry matter production and carbon inputs to soil
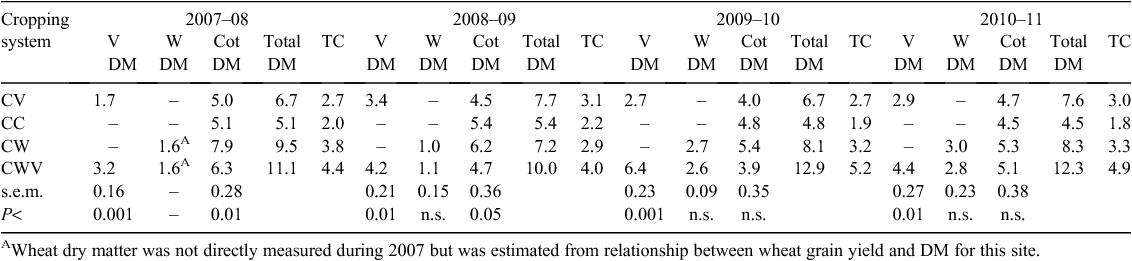
Dry matter production during the vetch phase of the CWV cropping system was always greater than that of CV, being on average 75% more than that of CV (Table 1). This may be due to differences in the length of the growing season (5–6 months for vetch in CWV and 3–4 months for that in CV), in-crop rainfall, and soil water storage (Hulugalle et al. 2013). Wheat dry matter did not differ significantly between CW and CWV. Cotton dry matter yields were significantly higher in CW than in other cropping systems, except during the 2010–11 season. This may be associated with better soil quality in CW relative to the other cropping systems (Hulugalle et al. 2012a). When all crops in the cropping systems were accounted for, however, aboveground dry matter production and carbon inputs to soil were generally in the order CWV > CW > CV > CC. The 2008–09 cotton season was an exception, in that total dry matter produced by CV was greater than by CW.
The literature proposes that 2–15% of plant inputs from fertilised crop residues may be sequestered in soil (Follett et al. 2005; Johnson et al. 2006; Grace et al. 2010), although assessment of Australian data (Grace et al. 2010) suggests a range of 2–10%. Based on these values and assuming that soil carbon sequestration rate in our study was ~5%, carbon sequestered by aboveground dry matter inputs was estimated to be (in Mg C ha–1 year–1): 0.10 with CC, 0.14 with CV, 0.17 with CW, and 0.23 with CWV. With respect to inputs by roots (to a depth of 1.0 m), average carbon inputs to soil by cotton roots in this site were (Mg C ha–1 year–1): 1.41 with CC, 1.26 with CV, 1.68 with CW, and 1.37 with CWV (Hulugalle et al. 2009). Using the same assumptions, carbon sequestered on average by cotton roots would have been of the order of (Mg C ha–1 year–1): 0.07 with CC, 0.06 with CV, 0.08 with CW, and 0.07 with CWV. Hulugalle et al. (2012c) also estimated that average carbon sequestration rates by roots of rotation crops were (Mg C ha–1 year–1): 0.10 with vetch in CV, 0.08 with wheat in CW, and 0.34 with wheat and vetch in CWV. Estimated average carbon sequestration rates from both above- and below-ground inputs (cotton and rotation crops) were, therefore, of the order of (Mg C ha–1 year–1): 0.17 with CC, 0.30 with CV, 0.33 with CW, and 0.64 with CW. The values in CC, CV, and CW are comparable to those reported by Potter (2010) for no-tilled row crops in Vertosols and that for CWV comparable to pasture systems in the same soil type.
Soil organic carbon concentration
Cropping system only influenced the concentration of carbon in the surface 0.1 m of soil during 2005, 2009, and 2010 (Fig. 1). During 2005, rotations that included wheat had lower carbon concentrations in the surface 0.1 m that those that did not, and during 2009 those that included vetch had lower carbon concentrations than those that did, whereas during 2010 the reverse occurred. The differences among treatments were, however, small. Taking into account the abovementioned inconsistent trends and small differences, we suggest that these ‘differences’ were not real but statistical aberrations. Averaged among cropping systems, carbon concentration in the 0–0.1 m depth was of the order of (g 100 g–1): 1.06 with CV, 1.05 with CWV, 1.01 with CW, and 1.00 with CC. Soil carbon concentration varied significantly among years (P < 0.001) in all depths (Fig. 1). Across the experiment as a whole, annual fluctuations in soil carbon concentrations were several times greater than observed with cropping systems. A detailed regional analysis of climatic and management variables in cotton-based farming systems has suggested that depth of soil disturbance, N fertiliser inputs, annual temperatures, and winter rainfall are major driving forces of changes in soil carbon, with crop rotations playing a secondary role (Hulugalle et al. 2011).

|
Cropping system did not affect soil carbon concentration in the 0.1–0.3, 0.3–0.6, and 0.6–1.2 m depths, and averaged 0.59, 0.49, and 0.46 g 100 g–1, respectively. This suggests that carbon concentrations in the subsoils of annual agricultural systems in semi-arid zones are unlikely to be modified or will be difficult to modify by changes to farming practices such as crop rotations and stubble management. Similar views have been expressed in several recent reviews of soil carbon management in agricultural soils (Luo et al. 2010; Sanderman et al. 2010; Powlson et al. 2011). The annual fluctuations in soil carbon concentrations in the 0.1–1.2 m depth were, however, far less than that observed in the surface 0.1 m. This may be because carbon inputs by roots to individual depths in the subsoil were less that that by aboveground biomass and roots to the surface 0.1 m, and the subsoil was sheltered from extremes in soil water content and temperature fluctuations.
The rates of change in SOC concentration with years in all depths did not differ significantly among cropping systems. The rate of change in the 0–0.1 m depth was positive, i.e. a net increase occurred (0.02 g C 100 g soil–1 year–1, R2 = 0.10**, n = 96), whereas the rate of change in other depths was negative, i.e. a net loss occurred: 0.02 g C 100 g soil–1 year–1 in the 0.1–0.3 m depth (R2 = 0.18***, n = 96); 0.01 g C 100 g soil–1 year–1 in the 0.3–0.6 m (R2 = 0.08**, n = 96) and 0.6–1.2 m depths (R2 = 0.09**, n = 96). The low R2 values suggest that time was a poor explanatory variable for variations in SOC concentration, whereas the previously noted management and climatic variables may be better choices.
Comparing soil carbon storage with FD and ESM methods
Storage of SOC estimated with the ESM and FD methods for the 0–0.3 and 0–1.2 m depths indicated that differences were very small (Fig. 2). Values estimated with the ESM method differed from those estimated by the FD method by an average of 0% (range–4% to +2%) in the 0–0.3 m depth, and 2% (range 1–3%) in the 0–1.2 m depth. The small differences may be due to the fact that all treatments were sown on permanent beds with relatively little soil disturbance and no inversion. Use of the FD method is, therefore, permissible in the present study.

|
Soil carbon storage and sequestration
Soil carbon storage in the 0–0.3 m depth was significantly affected by cropping system only during 2006 and 2010, and in the 0–1.2 m depth only during 2006 (Fig. 3). Significant variations in carbon storage in both depths occurred among years (P < 0.001). Mean carbon storage in CV, CC, CW, and CWV was 37, 35, 35, and 37 Mg C ha–1, respectively, in the 0–0.3 m depth, and 119, 111, 116, and 118 Mg C ha–1, respectively, in the 0–1.2 m depth.

|
Net carbon sequestration rates in the 0–0.3 and 0–1.2 m depths did not differ significantly among cropping systems. Results were therefore pooled among treatments. Pooled results for the 0–0.3 m depth indicated that net carbon sequestration rate did not change significantly with time and was of the order of 0.004 ± 0.21 Mg C ha–1 year–1, whereas it decreased (P < 0.05) at a rate of 1.60 ± 0.69 Mg C ha–1 year–1 in the 0–1.2 m depth (Fig. 4). The low R2 values indicate, however, that time was a poor predictor of variations in soil carbon storage. Hulugalle et al. (2011) suggested that N fertiliser inputs, depth of soil disturbance, annual temperatures, and winter rainfall were more closely related to carbon gains and losses than was time. Although some authors have claimed carbon sequestration rates of ~2 Mg C ha–1 year–1 or more in the surface 0.3 m of semi-arid irrigated Vertosols, our results do not support this view but concur with most of the studies conducted in irrigated and dryland row-cropped farming systems under semi-arid conditions (Hulugalle 2000; Luo et al. 2010; Sanderman et al. 2010; Chan et al. 2011; Powlson et al. 2011; White 2012).

|
These values do not correspond to biomass inputs (and estimated sequestration rates), which were in the order CWV >> CW = CV > CC. The previously estimated values of carbon sequestered from biomass inputs suggest, however, that a net increase should have occurred with time in this study. It may be that the value of 5% used to estimate carbon sequestration from biomass inputs was an overestimate for this site or that post-sequestration losses, as either dissolved carbon or transported sediments in runoff and erosion, and deep drainage were high (King et al. 2009). Although there are no data on soil carbon losses through erosion processes in furrow-irrigated Australian Vertosols, results from the United States suggest that losses could be ~0.02–0.05 Mg C ha–1 year–1 (King et al. 2009). If similar rates of loss were to occur in Australian Vertosols, then the proportions of sequestered carbon removed by erosion and runoff (based on biomass inputs in this study) could range between 5 and 20%.
Conclusions
Estimates of carbon sequestered by above- and below-ground biomass inputs were in the order CWV >> CW = CV > CC, but carbon concentrations in the 0–1.2 m depth and carbon storage in the 0–0.3 and 0–1.2 m depths were similar among all cropping systems. Net carbon sequestration rates did not differ among cropping systems and did not change significantly with time in the 0–0.3 m depth, whereas net losses occurred in the 0–1.2 m depth. These results do not correspond to estimated carbon sequestration rates based on biomass quantity. The discrepancy between measured and estimated values of sequestered carbon suggests that either the value of 5% used to estimate carbon sequestration from biomass inputs was an overestimate for this site, or that post-sequestration losses may have been high. The latter has rarely been investigated in Australian Vertosols. Future research efforts should aim to quantify the magnitude of these losses.
Acknowledgments
Funding for this study was provided by the Cotton Co-operative Research Centre, and the Cotton Research and Development Corporation of Australia.
References
Chan KY, Conyers MK, Li GD, Helyar KR, Poile G, Oates A, Barchia IM (2011) Soil carbon dynamics under different cropping and pasture management in temperate Australia: Results of three long-term experiments. Soil Research 49, 320–328.| Soil carbon dynamics under different cropping and pasture management in temperate Australia: Results of three long-term experiments.Crossref | GoogleScholarGoogle Scholar |
Cooper JL (1999) A grower survey of rotations used in the New South Wales cotton industry. Australian Journal of Experimental Agriculture 39, 743–755.
| A grower survey of rotations used in the New South Wales cotton industry.Crossref | GoogleScholarGoogle Scholar |
Cresswell HP, Hamilton G (2002). Bulk density and pore space relations. In ‘Soil physical measurement and interpretation for land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 35–58. (CSIRO Publishing: Melbourne)
Ellert BH, Bettany JR (1995) Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Canadian Journal of Soil Science 75, 529–538.
| Calculation of organic matter and nutrients stored in soils under contrasting management regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhslKlsbo%3D&md5=c9bf81c924aff885d59d4a4a3ee31e70CAS |
Follett RF, Castellanos JZ, Buenger ED (2005) Carbon dynamics and sequestration in an irrigated Vertisol in Central Mexico. Soil & Tillage Research 83, 148–158.
| Carbon dynamics and sequestration in an irrigated Vertisol in Central Mexico.Crossref | GoogleScholarGoogle Scholar |
Grace PR, Antle J, Ogle S, Paustian K, Basso B (2010) Soil carbon sequestration rates and associated economic costs for farming systems of south-eastern Australia. Soil Research 48, 720–729.
| Soil carbon sequestration rates and associated economic costs for farming systems of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR (2000) Carbon sequestration in irrigated Vertosols under cotton-based farming systems. Communications in Soil Science and Plant Analysis 31, 645–654.
| Carbon sequestration in irrigated Vertosols under cotton-based farming systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsFemsbs%3D&md5=9591673e081fba7dfe80785e38686ad6CAS |
Hulugalle NR, Entwistle P (1997) Soil properties, nutrient uptake and crop growth in an irrigated Vertosol after nine years of minimum tillage. Soil & Tillage Research 42, 15–32.
| Soil properties, nutrient uptake and crop growth in an irrigated Vertosol after nine years of minimum tillage.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR, Scott F (2008) A review of the changes in soil quality and profitability accomplished by sowing rotation crops after cotton in Australian Vertosols from 1970 to 2006. Australian Journal of Soil Research 46, 173–190.
| A review of the changes in soil quality and profitability accomplished by sowing rotation crops after cotton in Australian Vertosols from 1970 to 2006.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR, Weaver TB, Finlay LA, Luelf NW, Tan DKY (2009) Potential contribution by cotton roots to soil carbon stocks in irrigated Vertosols. Australian Journal of Soil Research 47, 243–252.
| Potential contribution by cotton roots to soil carbon stocks in irrigated Vertosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlWrurk%3D&md5=c372ac02c77f60a471344d09c9eedeadCAS |
Hulugalle NR, Weaver TB, Kimber S, Powell J, Scott F (2011) Maintaining profitability and soil quality in cotton farming systems III. Final Report to Cotton Catchment Communities Co-operative Research Centre on CRC Project 1.04.16. Cotton CRC, Narrabri, NSW. Available at: www.cottoncrc.org.au/general/Research/Projects/1_04_16
Hulugalle NR, Weaver TB, Finlay LA, Lonergan P (2012a) Soil properties, black root-rot incidence, yield and greenhouse gas emissions in irrigated cotton cropping systems sown in a Vertosol with subsoil sodicity. Soil Research 50, 278–292.
| Soil properties, black root-rot incidence, yield and greenhouse gas emissions in irrigated cotton cropping systems sown in a Vertosol with subsoil sodicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFKmsrg%3D&md5=c9d38dc2188b8e7f177234371bbaff7cCAS |
Hulugalle NR, Finlay LA, Weaver TB (2012b) An integrated mechanical and chemical method for managing prostrate cover crops on permanent beds. Renewable Agriculture and Food Systems 27, 148–156.
| An integrated mechanical and chemical method for managing prostrate cover crops on permanent beds.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR, Weaver TB, Finlay LA (2012c) Carbon inputs by wheat and vetch roots to an irrigated Vertosol. Soil Research 50, 177–187.
| Carbon inputs by wheat and vetch roots to an irrigated Vertosol.Crossref | GoogleScholarGoogle Scholar |
Hulugalle NR, Weaver TB, Finlay LA (2013) Soil water storage, drainage, and leaching in four irrigated cotton-based cropping systems sown in a Vertosol with subsoil sodicity. Soil Research 51, 652–663.
Isbell RF (2002) ‘The Australian Soil Classification.’ 2nd edn. (CSIRO Publishing: Melbourne)
Johnson JMF, Allmaras RR, Reicosky DC (2006) Estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database. Agronomy Journal 98, 622–636.
| Estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlsFamtb0%3D&md5=1bd91675b760154407eea28989695286CAS |
King AP, Evatt KJ, Six J, Poch RM, Rolston DE, Hopmans JW (2009) Annual carbon and nitrogen loadings for a furrow-irrigated field. Agricultural Water Management 96, 925–930.
| Annual carbon and nitrogen loadings for a furrow-irrigated field.Crossref | GoogleScholarGoogle Scholar |
Knowles TA, Singh B (2003) Carbon storage in cotton soils of northern New South Wales. Australian Journal of Soil Research 41, 889–903.
| Carbon storage in cotton soils of northern New South Wales.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnt1Crt74%3D&md5=f40acdd8a346e30dfebf01588cf09552CAS |
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15, 259–263.
| World Map of the Köppen-Geiger climate classification updated.Crossref | GoogleScholarGoogle Scholar |
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627.
| Soil carbon sequestration impacts on global climate change and food security.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXks1Cgsrk%3D&md5=c2e269b7d28289090140803d5dcb350cCAS |
Luo Z, Wang E, Sun OJ (2010) Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems, A review and synthesis. Geoderma 155, 211–223.
| Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems, A review and synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlWgtb0%3D&md5=c9602ed3e1c7be481a6c1f9d8d8726bdCAS |
McIntyre DS, Stirk GB (1954) A method for determination of apparent density of soil aggregates. Australian Journal of Agricultural Research 5, 291–296.
| A method for determination of apparent density of soil aggregates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG2cXkslKhtA%3D%3D&md5=1f60b58c1a1c1f53a994abd123827893CAS |
Potter KN (2010) Building soil carbon content of Texas Vertisols. In ‘Soil Solutions for a Changing World, Proceedings of 19th World Congress of Soil Science’. 1–6 August 2010, Brisbane, Australia. (DVD-ROM) (Eds R Gilkes, N Prakongkep) pp. 13–16. (IUSS: Brisbane, Qld)
Powell J, Scott F (2011) A representative irrigated farming system in the Lower Namoi Valley of NSW, An economic analysis. Economic Research Report No. 46, Industry and Investment NSW, Narrabri. Available at: www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/377346/ERR-46.pdf
Power B, Rodriguez D, deVoil P, Harris G, Payero J (2011) A multi-field bio-economic model of irrigated grain-cotton farming systems. Field Crops Research 124, 171–179.
| A multi-field bio-economic model of irrigated grain-cotton farming systems.Crossref | GoogleScholarGoogle Scholar |
Powlson DS, Whitmore AP, Goulding KWT (2011) Soil carbon sequestration to mitigate climate change, a critical re-examination to identify the true and the false. European Journal of Soil Science 62, 42–55.
| Soil carbon sequestration to mitigate climate change, a critical re-examination to identify the true and the false.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVGgtrk%3D&md5=2057c20e3928cbac7a6901d256e04b59CAS |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods.’ (CSIRO Publishing: Melbourne)
Rochester IJ (2011) Sequestering carbon in minimum-tilled clay soils used for irrigated cotton and grain production. Soil & Tillage Research 112, 1–7.
| Sequestering carbon in minimum-tilled clay soils used for irrigated cotton and grain production.Crossref | GoogleScholarGoogle Scholar |
Sanderman J, Farquharson R, Baldock J (2010) Soil carbon sequestration potential: A review for Australian agriculture. A report prepared for Department of Climate Change and Energy Efficiency, Canberra. CSIRO Land and Water, Canberra. Available at: www.csiro.edu.au/files/files/pwiv.pdf
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant and Soil 241, 155–176.
| Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltV2jsbo%3D&md5=bf67f556c4db6ea18ff9af912eb9f30fCAS |
Soil Survey Staff (2010) ‘Keys to Soil Taxonomy.’ 11th edn. (Natural Resources Conservation Service of the United States Department of Agriculture: Washington, DC)
White RE (2012) ‘Tis an ill wind that blows nobody any good. In ‘Soil solutions for diverse landscapes. Proceedings of the 5th Joint Australian and New Zealand Soil Science Conference’. 3–7 December 2012, Hobart, Tas. (Eds LL Burkitt, LA Sparrow) pp. 430–440. (Australian Society of Soil Science Inc.: Warragul, Vic.)


 , cotton–wheat–vetch. Vertical bars are standard error of the mean.
, cotton–wheat–vetch. Vertical bars are standard error of the mean.