Soils and climate change: potential impacts on carbon stocks and greenhouse gas emissions, and future research for Australian agriculture
J. A. Baldock A D , I. Wheeler C , N. McKenzie B and A. McBrateny CA CSIRO Land and Water/Sustainable Agriculture Flagship, PMB 2, Glen Osmond, SA 5064, Australia.
B CSIRO Land and Water, Canberra, ACT 2601, Australia.
C University of Sydney, Faculty of Agriculture Food and Natural Resources, Sydney, NSW 2006, Australia.
D Corresponding author. Email: jeff.baldock@csiro.au
Crop and Pasture Science 63(3) 269-283 https://doi.org/10.1071/CP11170
Submitted: 12 December 2011 Accepted: 20 March 2012 Published: 28 May 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Organic carbon and nitrogen found in soils are subject to a range of biological processes capable of generating or consuming greenhouse gases (CO2, N2O and CH4). In response to the strong impact that agricultural management can have on the amount of organic carbon and nitrogen stored in soil and their rates of biological cycling, soils have the potential to reduce or enhance concentrations of greenhouse gases in the atmosphere. Concern also exists over the potential positive feedback that a changing climate may have on rates of greenhouse gas emission from soil. Climate projections for most of the agricultural regions of Australia suggest a warmer and drier future with greater extremes relative to current climate. Since emissions of greenhouse gases from soil derive from biological processes that are sensitive to soil temperature and water content, climate change may impact significantly on future emissions. In this paper, the potential effects of climate change and options for adaptation and mitigations will be considered, followed by an assessment of future research requirements. The paper concludes by suggesting that the diversity of climate, soil types, and agricultural practices in place across Australia will make it difficult to define generic scenarios for greenhouse gas emissions. Development of a robust modelling capability will be required to construct regional and national emission assessments and to define the potential outcomes of on-farm management decisions and policy decisions. This model development will require comprehensive field datasets to calibrate the models and validate model outputs. Additionally, improved spatial layers of model input variables collected on a regular basis will be required to optimise accounting at regional to national scales.
Introduction
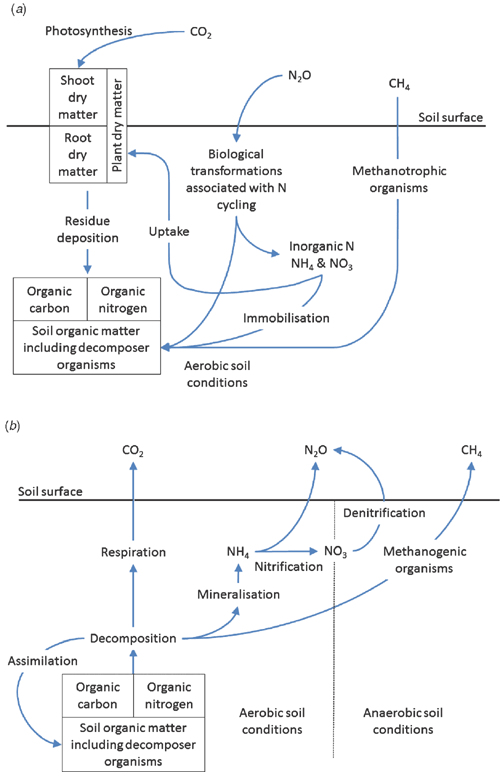
Soils contain large stores of organic carbon and nitrogen: ~1500–2400 Pg C (Eswaran et al. 1995; Houghton 2005; Jobbágy and Jackson 2000; Lal 2004; Powlson 2005) and 190 Pg N (Mackenzie 1998), depending on the soil depth over which the stores have been calculated. These stores are continuously exposed to decomposition and a range of additional biologically mediated transformations that generate or consume all three major greenhouse gases (CO2, N2O, and CH4) (Fig. 1). Globally, soils and their management therefore have the potential to either enhance or reduce atmospheric concentrations of greenhouse gases and the magnitude of any associated climate change. Using respective estimates of 1500 and 720 Pg for carbon contained in soil and the atmosphere and an atmospheric concentration of 390 ppm for CO2 (Mauna Loa Observatory, December 2010), a 1% change in the amount of carbon stored in soils would equate approximately to an 8 ppm change in atmospheric CO2 concentration, provided all other components of the carbon cycle remained constant. However, given the potential mediating responses provided by photosynthesis and oceanic exchange, it is likely that the net change brought about by a 1% change in carbon stored in soil would be somewhat less than 8 ppm. When this calculation is coupled with the observation that initiating agricultural production results in a 20–70% reduction in the amount of carbon stored in soils (Luo et al. 2010b; Sanderman et al. 2010), the way in which agricultural soils are managed has the potential to either enhance or reduce atmospheric concentrations of CO2. Additionally, the impact of agricultural management practices on emissions of N2O and CH4 must be considered, given their high levels of radiative forcing relative to CO2 (310 and 21 times that of CO2 over a 100-year time frame, respectively). Concern also exists over the potential positive feedbacks that a changing climate may have on rates of greenhouse gas emission from soil in view of the strong impact that climate can have on the biological processes leading to production and consumption/storage of CO2, N2O, and CH4.
|
This paper will examine the potential implications of climate change on greenhouse gas emissions from Australian agricultural soils. It will not examine soils under native ecosystems or managed native forests or plantations. A short description of the projected changes in climate will be presented. This will be followed by an examination of the processes responsible for emission and consumption/storage of each of the greenhouse gases (CO2, N2O, and CH4) in soil and how the predicted climate changes are likely to impact. Options for adapting to and mitigating climate change from a soils point of view will then be discussed, followed by identification of knowledge gaps and future research priorities.
Climate change predictions for Australia
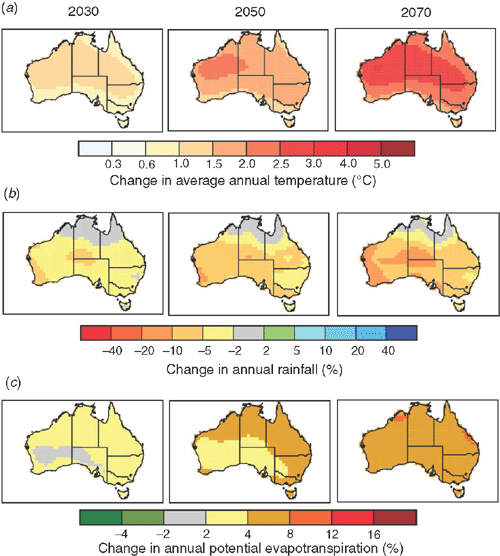
Climate projections for most of the agricultural regions of Australia suggest a warmer and drier future, with alterations to the seasonality and greater extremes relative to 1980–1999 average annual climatic conditions (Fig. 2). Although the north-eastern portion of Australia, and particularly central New South Wales, is predicted to have higher or unchanged rainfall in summer, this is accompanied by projected increases in potential evapotranspiration. Such conditions are likely to result in an overall pattern of drying with respect to the amount of water available to grow crops and pastures. The projected climatic changes will undoubtedly affect the balance between emission and storage/consumption of CO2, N2O, and CH4 in Australia’s agricultural soils. The direction and magnitude of changes will be defined by the integrated effects across all processes involved in emission and consumption or storage of each greenhouse gas.
|
Greenhouse gas emissions from soil and potential implications of climate change and agricultural management practices
Emissions of greenhouse gases from soil fluctuate both temporally and spatially due to variations in environmental factors and soil properties, and quantification of multiple processes is often required to define net fluxes. For example, net CO2 emissions result from the balance between CO2 uptake through photosynthesis and CO2 release from respiration by plants, animals, and microbes. Although measurements of net CO2 fluxes are possible, they require sophisticated equipment and data analysis and do not define the magnitude of individual contributing processes. An alternative is to quantify the changes in biological stocks of carbon, including vegetation and soil across space through time. Given the diversity of vegetation types, soil properties, and climatic conditions existing across Australia, the stock change approach has been adopted to measure and predict changes in net CO2 emissions from Australian soils. For N2O and CH4 emissions from soil, no stock change measurement is available; thus, measurement and prediction of fluxes provides the only approach to quantify emissions of these gases.
In this section, processes influencing soil carbon stock changes and net emissions of N2O and CH4 from agricultural soils will be identified, potential impacts of climate change will be discussed, and options for mitigation of emissions will be considered. Although each greenhouse gas will be examined individually, dynamic links often exist with respect to emission of these gases, and the impact of any mitigation strategies on all three gases must be considered. For example, if soil carbon stocks increase due to enhanced plant growth associated with additional fertiliser nitrogen application, the impact of fertiliser nitrogen on net N2O and CH4 emissions must be considered in any within-farm-enterprise greenhouse gas account. Additionally, it will be important to define and quantify any induced deviations in off-farm emissions associated with management change to delineate the net benefit to atmospheric concentrations of greenhouse gases. In the example just considered (application of additional fertiliser nitrogen), any CO2 emissions associated with the production and transport of the additional fertiliser nitrogen will need to be considered.
Soil organic carbon/carbon dioxide
The amount of organic carbon contained in a soil is the direct result of the balance between carbon inputs and losses. Organic carbon is added to soil via deposition in and on the soil of organic materials created by capturing CO2 through the process of photosynthesis (Fig. 1a). Soil organic carbon (SOC) is returned to the atmosphere as CO2 via respiration that occurs as soil organisms use organic materials as a source of energy and nutrients (Fig. 1b). Any agricultural practices that alter rates of carbon input to, or loss from, the soil will result in a change in the stock of SOC.
A potential upper limit for carbon inputs to soil can be defined for any given location by five factors:
-
amount of photosynthetically active radiation (PAR) available, which is defined by global position and cloud cover;
-
fraction of PAR that can be absorbed by plants (typically a function of leaf area and architecture);
-
efficiency with which carbon is captured by photosynthesis per unit of PAR absorbed;
-
proportion of captured carbon lost to autotrophic respiration;
-
proportion of captured carbon deposited on or in the soil.
The summation of the first four factors provides an estimate of the potential net primary productivity (NPP) of an agricultural system. Other factors such as low availabilities of water and nutrients, changes in temperature, or low soil pH may constrain potential NPP to lower values by limiting the efficiency of carbon capture (factors 2 and 3) or enhancing respiratory losses (factor 4). Agricultural breeding programs have been designed to reduce the magnitude of such limitations through genetic manipulation to allow crops and pastures to maximise NPP at any given location. Factor 5 also needs to be considered, since agricultural systems are designed typically to maximise the allocation of captured carbon to products harvested and transported off-farm. As a result, enhanced productivity, as defined by estimates of agricultural yield, does not necessarily translate to increased inputs of carbon to soils, and in fact may be occurring at the expense of carbon inputs to soils if harvest indices are increasing (for a summary of harvest indices for Australian crops see Unkovich et al. 2010).
In most Australian agricultural systems, the availability of water or nutrients represents a major limitation to achieving potential NPP. Therefore, efforts to enhance inputs of carbon to soil should first focus on identifying soils where carbon capture per unit of available resource (water and nutrients) is not maximised under the agricultural systems in use. Then, an assessment should be made as to whether alterations to current management practices can enhance resource use efficiency. For example, if water-use efficiency is being held back due to a lack of fertility or low pH, application of appropriate levels of fertiliser or agricultural lime may enhance productivity and inputs of carbon to the soil. However, if low water-use efficiency is resulting from the presence of high subsoil concentrations of salt and boron, it may not be possible to enhance productivity by simply altering management within the operating production system. Under such conditions, significant changes to the production system may be required to achieve increased inputs of carbon to the soil (e.g. reduced extent or frequency of cropping, reduced grazing intensity, and introduction of plant species with a greater tolerance to adverse soil conditions).
Plant residues enter the soil system through deposition on the soil surface (shoot residues) or within the soil matrix (root residues, exudates, and root-associated mycorrhizal fungi). The majority of residue carbon will be decomposed and respired back to the atmosphere as CO2, with the balance resisting decomposition as a result of its chemical recalcitrance, assimilation into decomposer tissues, or interactions with soil minerals. However, even the more biologically stable forms of SOC are gradually decomposed and their carbon is returned to the atmosphere. Thus, the retention of a small fraction of residue carbon as stabilised SOC is essential to replace the SOC continuously being lost at low rates by decomposition.
The diversity of biological transformations and interactions with soil minerals that occur as residue carbon enters soil means that SOC has a diverse composition and a range of susceptibilities to decomposition, as demonstrated by Δ14C measurements (e.g. Ladd et al. 1981; Anderson and Paul 1984; Swanston et al. 2005). To gain further insight into SOC composition and cycling, various methodologies based on variations in chemical and physical properties have been developed to allocate SOC to a series of ‘biologically significant’ fractions (Blair et al. 1995; Golchin et al. 1997; Paul et al. 2001; Six et al. 2002; Skjemstad et al. 2004). Skjemstad et al. (2004) used a combination of physical and chemical properties to allocate SOC to the following three fractions:
-
particulate organic carbon (POC), organic carbon associated with particles >50 µm (excluding charcoal carbon);
-
humus organic carbon (HUM), organic carbon associated with particles <50 µm (excluding charcoal carbon);
-
resistant organic carbon (ROC), organic carbon found in the <2 mm soil and having a poly-aromatic chemical structure consistent with the structure of charcoal.
Skjemstad et al. (2004) went on to demonstrate that the RothC soil carbon model (Jenkinson et al. 1987) could be re-parameterised using these measureable fractions by substituting the RPM, HUM, and IOM model pools with the measured POC, HUM, and ROC fractions, respectively, and altering the decomposition rate constant assigned to the POC fraction. Based on these findings, the National Carbon Accounting System has adopted the use of a version of RothC modified to simulate the dynamics of these measureable fractions. This approach allows the model to be initialised using measured values for the amounts of SOC and fractions rather than estimated values as typically used in other SOC models based on conceptual pools of carbon.
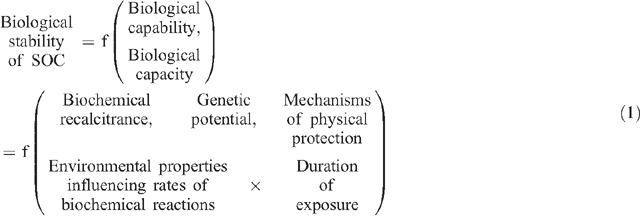
Although estimates of SOC composition are not essential to allow carbon accounting, an understanding of SOC composition will provide an assessment of the vulnerability of SOC stocks to subsequent changes in management practice. Vulnerability will increase as the proportion of energy-rich and decomposable POC increases and will decrease as the proportions of the more stable HUM and ROC fractions of SOC increase. Accurate prediction of the impact of climate change on SOC stocks will require an understanding of SOC composition and climatic responses to a series of factors that influence the biological stability of different forms of SOC, and thus the magnitude of SOC loss (see Eqn 1 below) (modified from Baldock 2007). Factors defining the biological stability of SOC can be divided into two types: those responsible for defining whether or not a particular form of SOC can be decomposed (biological capability), and those defining the rate of decomposition (biological capacity). Of primary importance to biological capability are:
-
chemical nature of SOC, which defines the inherent biochemical recalcitrance of the carbon-containing compounds present;
-
genetic potential of decomposer organisms to create the enzymes required to degrade both plant residues and the various forms of SOC present;
-
mechanisms of physical protection that can alter molecular conformation through adsorption reactions, isolate organic molecules from enzyme attack through the creation of a physical barrier, or encapsulate pieces of plant residue creating an environment less conducive to decomposition.
Of importance to biological capacity are:
-
environmental properties that govern rates of biochemical reactions, including temperature, and the availability of oxygen, water, and nutrients;
-
duration of exposure to conditions conducive to decomposition.
By crossing the two factors governing biological capacity (environmental properties × duration of exposure), an index of microbially active days can be constructed and used to provide an indication of the potential for SOC to decompose, provided the capability exists.
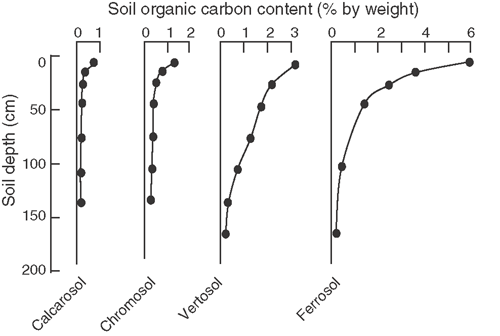
Most of the factors described in Eqn 1, particularly mechanisms of physical protection, environmental properties, and duration of exposure, show strong spatial variability across the Australian agricultural regions due to differences in soil clay content and mineralogy and climate. When such variability is combined with spatial differences in plant productivity and the amount of residue deposited in and on soils, it becomes apparent that the potential for soils to capture and retain carbon will also vary spatially. Such variability is exemplified by the large differences noted in depth profiles of soil carbon for several indicative Australian soils (Fig. 3). The practical implication of these observations is that the magnitude of SOC change induced by agricultural management practices will vary across Australia’s agricultural regions. Particular practices may work well in some areas but not others. For example, where carbon storage results from a strong dependence on mechanisms of physical protection, it is unlikely that the same magnitudes of SOC change will be noted on a sandy Calcarosol, a medium-textured Chromosol, or a high clay content Vertosol.
|
Projected increases in temperature and reductions in the availability of water will undoubtedly influence stocks of SOC in Australia because of the controls that these parameters exert on rates of carbon capture and addition to soil as well as on rates of decomposition. However, the direction and magnitude of changes and the potential for feedbacks that may accentuate climate change are still under debate. Progressing the understanding of climate change effects on SOC requires an assessment of the potential impacts that climate change will have on relative magnitude of carbon inputs and losses from soil.
Under dryland agriculture where water availability is the first dictator of potential carbon capture by plants, a drier and warmer climate is likely to reduce potential plant growth and the inputs of carbon to soil. Identification and implementation of agricultural management strategies that allow plants to access and transpire a greater fraction of the rainfall received, and development of new genetic material (species and varieties) with greater water-use efficiency (carbon capture per mm of available water), will be required to ensure that inputs of carbon to soils are maintained. Enhanced carbon capture by plants due to ‘CO2 fertilisation’ associated with increased atmospheric CO2 concentrations (Grace and Rayment 2000) may help to alleviate potential negative impacts of lower water availability on SOC levels. Under irrigated agriculture, where water requirements to maximise plant productivity can be met, the likely outcome of climate change would be enhanced productivity unless temperatures increase to levels beyond that at which plant growth is optimised. Under both dryland conditions and irrigation, the greenhouse gas costs associated with maintaining or enhancing productivity (e.g. petrol for irrigation pumps, additional nitrogen fertiliser, etc.) will need to be quantified to define net benefits.
Changes in climate will also affect the magnitude of SOC loss from agricultural systems. It is widely accepted that drying will reduce rates of SOC decomposition; however, the effect of increasing temperature has been debated. The majority of evidence available suggests that increasing temperature will increase rates of SOC decomposition (Powlson 2005). However, by quantifying the turnover time of SOC at locations exhibiting a 5−30°C range in annual temperature, Giardina and Ryan (2000) suggested that rates of SOC decomposition were not sensitive to temperature. In that work, Giardina and Ryan (2000) expressed SOC as a single component with a single turnover time, which was a significant oversimplification considering the diversity of different materials contained in SOC and the range of locations from which soils were collected. Subsequent work completed using the same data (Knorr et al. 2005) established that, when SOC was divided into several fractions having different rates of decay, a positive sensitivity of SOC decomposition to variations in temperature existed. Knorr et al. (2005) also showed that the relative temperature sensitivity of SOC fractions increased as the stability of the SOC fraction against biological attack increased. The implication of the analysis by Knorr et al. (2005) is that an enhanced decomposition of SOC in a warming environment may have a positive feedback on atmospheric CO2 values. An accurate manifestation of the influence of temperature alone on SOC decomposition is only possible if all other factors controlling accessibility of organic carbon to decomposer enzymes remain non-limiting. In a review, Davidson and Janssens (2006) suggested that environmental constraints (e.g. availability of water or nutrients) may result in low ‘apparent’ temperature sensitivity of SOC decomposition by obscuring the intrinsic temperature sensitivity and that the environmental constraints themselves may also be sensitive to temperature. Additional research is required to more accurately delineate the temperature response of the decomposition of SOC and its component fractions.
Many variations in land use and agricultural management practices exist across Australian agricultural regions. Reviews by Hutchinson et al. (2007), Luo et al. (2010b), and Sanderman et al. (2010) have defined the magnitude of potential soil carbon changes under a variety of management practices and regimes. Going forward, options will undoubtedly be available to enhance the input and/or reduce the emission of carbon to most soils, even under projected climate change scenarios. The guiding principal when designing management regimes to maximise SOC stocks will be to maximise the capture of carbon given the resources available at any particular location. The challenge will be to design appropriate agricultural systems that meet the financial requirements of the farm business (converting carbon captured by photosynthesis into a product that can be sold) and enhance the input and retention of organic carbon in soils.
Innovations such as the introduction of tropical perennial species into previously annual pastures, implementation of rotational grazing, and adoption of pasture–cropping systems are being reported to increase soil carbon values in some agricultural regions of Australia (Sanderman et al. 2010). Further definition of the mechanisms and quantification of rates of SOC change under these innovative systems are required. Additionally, the extent of soil carbon decline induced by agricultural production prior to the introduction of alternative ‘SOC friendly’ management practices requires consideration. Evidence is emerging to suggest that the magnitude of measured SOC gains induced by the introduction of perennial pasture species into previously annual pastures increases as the extent of SOC decline under previous management regimes increases (J. Sanderman, unpubl. data).
The influence that increased SOC can have on a range of soil properties should also be considered. Increases in SOC can benefit a range of biological, chemical, and physical properties. Among the more important, in terms of maintaining productivity in a changing climate, is the enhancement that can occur to soil water-holding capacity. Availability of water will remain the major factor limiting plant productivity across many of Australia’s agricultural regions under the predicted climate change scenarios. Enhancing the water-holding capacity of soil by increasing SOC may help maintain productivity as drying occurs.
Nitrous oxide
Nitrous oxide is generated in soils as a byproduct of two natural biological processes involved in transformations of inorganic nitrogen: nitrification (conversion of ammonium to nitrate), and denitrification (conversion of nitrate to N2O and N2 gases) (Fig. 1a). Although microorganisms can reduce N2O to N2 under anaerobic conditions, rates of atmospheric N2O consumption by soil are suggested to be small (Freney et al. 1978).
Because nitrification and denitrification are biochemical processes, process rates will increase with increasing temperature provided the appropriate form of inorganic nitrogen, and other required materials (e.g. biologically available carbon for denitrification), are present and the soil is sufficiently wet. Chen et al. (2010b) obtained near-exponential increases in N2O emission from soils as temperature increased from 5 to 25°C but also noted reduced rates of emission as the soil dried from 60 to 40% water-filled pore space. Dalal et al. (2003) presented a generalised relationship showing that relative rates of N2O emission were negligible at water-filled pore space values <40%, were maximised between 60 and 70%, and were again negligible at values >90%. Increasing temperature has also been shown to enhance the ratio of N2O/nitrate from nitrification (Goodroad and Keeney 1984) and reduce N2O/N2 ratios from denitrification (Keeney et al. 1979; Castaldi 2000). Under dryland agriculture, the influence of proposed climate changes will depend on the relative responses to temperature and drying. It is suggested that, for the current warmer and wet tropical and subtropical regions, N2O emissions may increase, while for the cooler and drier regions, N2O emissions may decrease (all other factors being constant). However, under irrigated systems, where water content limitations are removed, the higher temperatures associated with projected climate change would enhance the emission of N2O unless soil inorganic nitrogen levels are tightly controlled.
The key to mitigating emissions of N2O from agricultural soils is to minimise concentrations of inorganic nitrogen, particularly given the non-linear increases in N2O emissions from soils in response to incremental fertiliser nitrogen additions (McSwiney and Robertson 2005). By limiting the amount of inorganic N available, process rates of nitrification and denitrification and emissions of N2O will be reduced. Options available for reducing the concentration of inorganic nitrogen in soil, and thus N2O emission, include:
-
Better matching of fertiliser nitrogen applications to plant demand as defined by growing season conditions. Australia uses significant quantities of ammonium-based nitrogen fertilisers in agriculture. Through the use of flexible nitrogen fertiliser systems based on multiple small applications consistent with plant growth stage and growing season conditions, a better matching between rates of nitrogen supply and plant demand can be achieved. Adoption of such application strategies will reduce the potential for generating high inorganic nitrogen status and N2O emissions. Under irrigated agriculture, where high rates of nitrogen fertiliser addition are common, more frequent irrigation or fertigation events that reduce the extent of soil saturation and inorganic nitrogen concentrations will help mitigate N2O emissions. Such processes would be expected to have the added benefit of enhancing nitrogen-use efficiency.
-
Increased reliance on biological nitrogen fixation to enhance soil nitrogen status. Building soil organic N status through the incorporation of legumes into pastures and crop rotations can be used to enhance the availability of nitrogen to subsequent crops (Chalk 1998; Peoples and Baldock 2001). Given that nitrogen mineralisation and plant growth are controlled by the same environmental factors, the provision of plant-available nitrogen through mineralisation of organic nitrogen is likely to be well matched temporally with plant demand. However, it is unlikely that provision of nitrogen through organic nitrogen mineralisation will optimise productivity. Peak demands for nitrogen by well-managed crops with no water limitations will exceed the capacity of the soil to supply nitrogen from mineralisation, so additional nitrogen is required to meet the shortfall (Angus 2001). Judicious use of nitrogen fertilisers will be required to ensure that excess inorganic nitrogen levels do not result and to optimise fertiliser nitrogen use efficiency and productivity.
-
Alteration of animal diets to avoid an intake of excess nitrogen and excretion of high nitrogen content urine and faeces. Pastures often contain an excess of protein relative to animal requirements (Whitehead 1995). Production of pastures with a balanced legume/non-legume mixture, applications of appropriate fertiliser nitrogen rates to maintain optimal nitrogen status of non-legume pasture biomass, and supplementation of high nitrogen content diets with a low nitrogen content material (e.g. crop residues) offer mechanisms by which the provision of food with appropriate nitrogen content to reduce the nitrogen content of urine and faeces can be achieved. Such strategies can increase nitrogen-use efficiency (Kebreab et al. 2001) and thereby reduce urine nitrogen concentrations and subsequent emissions of N2O (Mulligan et al. 2004; Nielsen et al. 2003).
-
Application of inhibitors to reduce rates of formation and transformation of soil ammonium. Urease activity and nitrification inhibitors are available commercially to alter nitrogen transformations in soil. Urease inhibitors slow the hydrolysis of urea in fertilisers or animal urine, limiting the accumulation of ammonium (Watson 2000), whereas nitrification inhibitors reduce the transformation of ammonium to nitrate (Luo et al. 2010a). Reductions of up to 70% in N2O emissions have been noted due to the application of these inhibitors, particularly through the application of nitrification inhibitors to urine-affected pastures (Di and Cameron 2003, 2006; Di et al. 2007; Smith et al. 2008; Chen et al. 2010b).
Methane
Methane can be produced by methanogens under anaerobic soil conditions and consumed through oxidation by methanotrophs under aerobic soil conditions Fig. 1. Significant CH4 production can occur in soils once redox potentials become more negative than –100 mV (Hou et al. 2000), and 10-fold increases in CH4 emission have been noted for each –50 mV decrease in redox potential over the range –150 to –250 mV (Masscheleyn et al. 1993). Such conditions are typically associated with soils exposed to prolonged flooding (e.g. under rice cultivation) or saturated conditions. Rates of CH4 emission from flooded soils are generally ≤10 µg CH4 m–2 h–1 (Dalal et al. 2008). Given the high dependence on redox potential, soil properties influencing rates of oxygen diffusion (e.g. soil bulk density and pore size distribution) and oxygen consumption (e.g. presence of decomposable C substrates) exert primary control over rates of CH4 production (Conrad 2005). Bossio et al. (1999) found 5-fold increases in CH4 emissions where rice straw was incorporated rather than being burnt over a 4-year period (92 v. 19 kg CH4 ha–1). Management practices that enhance the presence of electron acceptors such as Fe3+ (creation of transient oxic conditions through temporary drainage) or SO42– (application of gypsum or ammonium sulfate) can reduce CH4 emissions when soils are exposed to saturated conditions such as occur under rice production (Corton et al. 2000; Wassmann et al. 2000; Kumaraswamy et al. 2001; Le Mer and Roger 2001; Mosier et al. 2004; Conrad 2005). Methane production increases with increasing temperature, with an average optimum around 35°C and an average Q10 temperature coefficient of 4.0, depending on a range of environmental factors and amount of biologically available substrate (Dalal et al. 2008).
Dryland agricultural soils provide a net sink for CH4 due to their predominant oxidative condition, with mean consumption rates generally ≤100 µg CH4 m–2 h–1 (Dalal et al. 2008). Temperature effects on CH4 consumption are much smaller than those associated with CH4 production (Dunfield et al. 1993); however, soil water content or water-filled pore space exerts strong control (Kessavalou et al. 1998; Veldkamp et al. 2001). At low soil water contents, CH4 consumption can be limited, as sufficient water is required to initiate methanotrophic activity. Dalal et al. (2008) presented a generalised relationship showing enhanced rates of methane consumption in progressing from 80 to 30% water-filled pore space. Observations of enhanced CH4 consumption with increasing headspace CH4 concentration suggest that rates of consumption are likely CH4-limited under favourable environment conditions, possibly due to limits on rates of CH4 diffusion into soil. Consumption of CH4 in arable soils is generally lower than that in pasture or forest soils under similar environmental conditions and decreases in progression from temperate to tropical regions (Dalal et al. 2008).
Increased temperatures and drier conditions associated with projected climate changes for Australia would be expected to affect CH4 emissions from irrigated and dryland agriculture differently. Under irrigated agriculture, prolonged periods of flooding associated with increased temperature will have the potential to enhance CH4 emissions, particularly where crop residues are retained. Adequate water management strategies to allow temporary oxic soil conditions will be essential to minimise CH4 emissions under such conditions. The greatest potential to consume CH4 is likely to exist where non-flood irrigation is practiced. Through judicious control of soil water content, rates of CH4 consumption can be optimised; however, whether this can be achieved at the same time as optimising plant productivity will require assessment. Under dryland agricultural systems, the lack of strong temperature effects on CH4 consumption suggests that projected reductions in the availability of water will be most influential. For soils that currently experience prolonged wet periods, enhanced CH4 consumption is likely to be associated with future climate scenarios, provided that the soils do not dry to the extent that the activity of methanotrophs is limited. Where methanotrophic activity becomes limiting due to reduced water availability, rates of CH4 consumption may decline.
As a concluding note to this section on soil carbon storage and greenhouse gas emissions, it is important to recognise that when a change in management practice is applied to increase soil carbon storage or reduce N2O and CH4 emissions, the net effect of the practice change on the total greenhouse gas emission will require consideration. This consideration may also require a quantification of off-farm emissions. As an example, if soil carbon values are increased by enhanced crop productivity through the application of additional nitrogen fertiliser, emissions of CO2 during the production and transport of the fertiliser and potential enhanced emissions of N2O will have to be considered to define the net benefit associated with increasing soil carbon.
Current research priorities
In this section, current research priorities required to define the role of soils in the mitigation of greenhouse gas emissions and removal of CO2 from the atmosphere will be identified and discussed. Priorities common to all three greenhouse gases will be identified first, followed by those specific to soil carbon and then N2O and CH4.
All greenhouse gases
The diversity of climate, soil types, and agricultural practices in place across Australia will make it difficult to define and apply generic greenhouse gas emissions factors with confidence, particularly where accurate estimates are desired for the diverse range of agricultural enterprises. Research will need to focus on the development and implementation of measurement technologies, as well as the improvement of current simulation modelling capabilities. Debate exists as to whether the focus should be on measurement or modelling; however, both will be needed, and with appropriate coordination the two approaches can be used to inform and enhance the value of each other. While appropriate measurement technologies can be used to retrospectively define carbon stock changes or alterations to net greenhouse gas emissions, they cannot be used to guide changes in land use and management practice through forecasting potential outcomes. Definition of potential outcomes of on-farm management and policy decisions on local, regional, and national greenhouse gas emissions will require a robust modelling capability. However, appropriate model development will require comprehensive field datasets to define baseline soil conditions and to calibrate and validate model outputs. Establishing efficient (accurate, rapid, cost-effective) measurement technologies will be essential to facilitate the acquisition of appropriate datasets. Such measurement technologies need be designed to provide data that are consistent with those required by the simulation models and to allow temporal measurements of carbon stocks or N2O and CH4 emissions for calibration and validation of models.
As Australia enters into carbon-trading systems, simply measuring or predicting a carbon stock change or changes in net greenhouse gas emissions will not be good enough. The uncertainty associated with such values will be required and will need to include that associated with the measurement technology applied, model predictions (if used), and the spatial variability associated with the variable of interest (e.g. soil carbon content) and all other variables required to complete calculations (e.g. bulk density in the case of soil carbon stocks). Where possible, it is recommended that this uncertainty be expressed as a cumulative probability distribution (Snedecor and Cochran 1989) to allow land managers, potential buyers of carbon credits, and financial institutions to assess the level of risk associated with carbon transactions.
As an example, consider the situation where an estimate is desired for the potential outcome on soil carbon of adopting a 10% increase in the proportion of pastures grown in rotation with crops across an agricultural region. A measurement campaign would be used to define the current frequency distribution of soil carbon in farm paddocks across the region (Fig. 4a). This distribution would form the input to a soil carbon simulation model set up to model both the current business-as-usual situation as well as the changed management regime into the future. Through repeated sampling of the initial baseline soil carbon frequency distribution, a cumulative probability distribution (Fig. 4b) or a relative frequency distribution (Fig. 4c) of the estimated change in soil carbon stocks could be derived. The example presented suggests that 60% of the time, a net change in soil carbon of 10 Mg C ha–1 would be expected after 10 years, or an average value of 8 ± 3 Mg C ha–1. Subsequent measurements at some point in the future should be conducted to assess the predictive capabilities of the model and used to facilitate model alteration where poor agreement between model predictions and future measurements are obtained. Under such a scheme, the combination of measurement and modelling allows current assessment, predictions of future outcomes, assessment of these outcomes, and possible recalibration of models if required. Although Fig. 4 is based on soil carbon change, a similar approach could be applied to estimate the effect of management regimes on net emissions of N2O or CH4. A possible exception to this would be the use of an initial frequency distribution, which would much more difficult and expensive to collect for N2O and CH4 emissions.
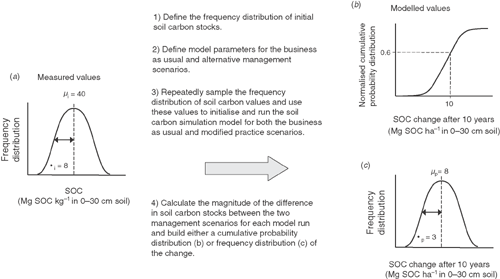
|
Soil organic carbon/carbon dioxide
Future research directions for SOC will need to be structured around optimising the efficiency of soil carbon stock measurements and improving the capability of soil carbon models to accurately predict the impact of changes in land use and management practice. Approaches to optimise soil carbon sampling will first be examined, followed by a consideration of the duration required between measurements and an assessment of progress against development of rapid and cost-effective methods for measuring soil carbon and its composition.
Measuring soil carbon stocks can be costly, particularly where substantial variations in soil, landscape, and environmental properties exist. Approaches that use existing spatial datasets to optimise sample placement and minimise the number of sampling locations are required. Stratified simple random sampling introduces a non-random element into sampling design by portioning the sample space into more homogeneous subgroups before the application of a random sample to each stratum. This approach can reduce sampling error by decreasing the variance of sample estimates within each stratum and therefore yield SOC data with smaller uncertainties (see for example de Gruijter et al. 2006).
In order to obtain information on the spatial distribution of SOC required to optimise soil sampling, numerous spatial data layers for variables that exhibit some degree of relation to SOC levels (i.e. soil type, land use, normalised difference vegetation index, terrain attributes) can be used. However, in the case of SOC, these relationships can be non-linear as well as subject to threshold conditions, indicating that variables alter in importance depending on the complex, non-linear interplay of several factors at the site of interest (e.g. see Bui et al. 2009). As a result, monothetic division, where included variables are treated as both necessary and sufficient to classify SOC distribution, may not be particularly advantageous when used for stratification. For instance, stratification of a single attribute (such as soil type) may not reflect enough of the controlling factors on SOC levels to produce statistically worthwhile groupings. As more variables are introduced to the classification, such as terrain attributes or vegetation cover, groupings may become less disparate and increasingly disjointed, thereby reducing stratification effectiveness. This is, in part, due to the decreasing ratio of signal to noise, which may outweigh the benefits of including additional attributes in this manner in the first place.
One solution to this problem is to employ a polythetic approach to conceptual division. Polythetic approaches to classifying dissimilarity use a broad set of criteria (that are neither necessarily explicit nor sufficient) to provide the information to be stratified. An example of this approach is to predict the spatial distribution of SOC from a combination of legacy SOC data and continuous environmental variables. This allows stratification of the sampling space in a way that incorporates the best available information while minimising the introduction of variable noise. Once a continuous prediction of SOC has been obtained, spatial stratification can be applied either proportionally or optimally. Proportional allocation uses arbitrary incremental steps in predicted SOC levels to determine strata, whereas optimal allocation can stratify on the basis of the frequency distribution. As optimal allocation allows the statistical structure of the (predicted) target variable to be taken into account, it is considered an advantageous approach. Proportional or optimal allocation can also be applied at the random sampling step rather than the stratification step.
In general, stratified sampling schemes can provide better estimates of the mean with fewer samples compared with a simple random design for a single survey. When multiple surveys are conducted through time (i.e. for monitoring purposes), stratification has the added benefit of allowing the incorporation of new information/observations into the design as it becomes available, and therefore increases in efficacy with time.
An example of the polythetic approach to obtaining exhaustive spatial information of SOC distribution for an area of ~5000 km2 is given in Fig. 5a. This spatial prediction was constructed at a resolution of 250 m by combining depth-harmonised legacy profile information with environmental attributes using a machine learning approach (I. Wheeler, unpubl. data). This layer can then be stratified using optimal allocation to account for the variability of the predicted distribution into six strata showing internal similarity (Fig. 5b), from which random samples can then be drawn.
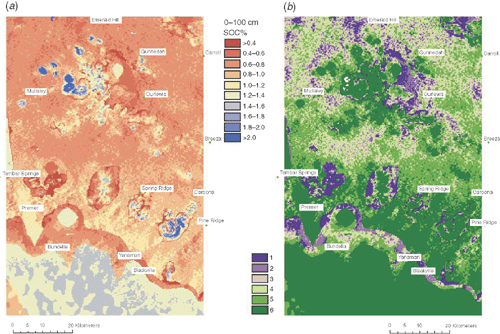
|
Where an assessment of management impacts on SOC values is desired, it is important to consider the duration over which a defined management strategy has been implemented and what the implications of prior management may have been. In a recent survey of Australian peer-reviewed journal papers, Sanderman et al. (2010) noted that changes in soil carbon obtained through the adoption of ‘carbon friendly’ management relative to more intensive management strategies ranged from 0.1 to 0.5 Mg C ha–1 year–1 over the 0–15 cm soil layer. At a rate of change of 0.5 Mg C ha–1 year–1, it would take 3–4.5 years to detect a change in soil carbon content equivalent to 0.1% of the total 0–15 cm soil mass, depending on bulk density (Fig. 6). If higher rates of carbon accumulation can be achieved, this time will decrease (e.g. for an increase of 2.0 Mg C ha–1 year–1, 0.75–1.13 years would be required). The implication of this observation is that, unless a management practice induces significant increases in soil carbon (>0.3 Mg C ha–1 year–1 for the 0–15 cm layer or >0.6 Mg C ha–1 year–1 for the 0–30 cm layer), it will take >5 years to detect changes equivalent to 0.1% of the soil mass. Another implication is that the potential for detecting a change in soil carbon will increase as the thickness of the soil layer decreases. Given that carbon tends to accumulate to a greater extent near the soil surface, where an assessment of changes in SOC stocks for the 0–30 cm layer are required, a greater capability to detect change would exist where this layer is broken up into 0–10, 10–20, and 20–30 cm layers.
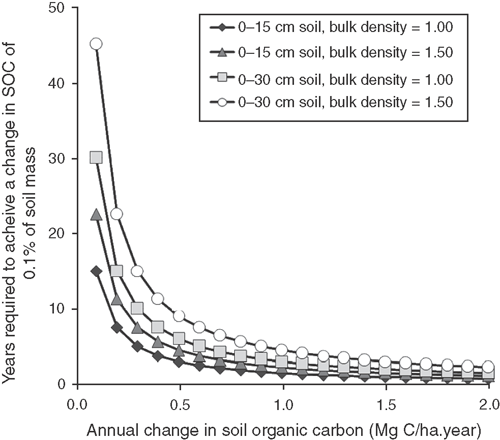
|
Given the annual variations that exist in climate and other factors controlling crop and pasture productivity (e.g. disease, late sowing, inadequate nutrition, etc.), inter-annual variability in rates of soil carbon capture will undoubtedly exist. To deal with these issues effectively, time-averaged trends calculated by performing multiple measurements of soil carbon stocks over appropriate time scales will be required. One approach to deal with this issue at a national level and build Australia’s capability to both document and predict future impacts of land use and management on soil carbon storage would be to establish a national soil carbon monitoring system. The monitoring system would require sampling locations positioned throughout Australia’s agricultural regions. Soil at each monitoring location would have to be repeatedly sampled through time. Such data, if combined with accurate documentation of the agricultural management practices employed by the land owner, would allow the impact of land management to be defined at regional levels and will provide a robust national soil carbon dataset for optimisation of the model included in the national carbon accounting system through validation of model predictions and recalibration where required.
A related issue that requires consideration is how to sample new and innovative farming systems in a statistically robust manner. One approach would be to compare measurements of soil carbon stocks obtained from such systems to frequency distributions obtained for soil under the dominant practices in the same agricultural region. Where new systems produce soil carbon stocks that are >95th percentile of these distributions, strong evidence would exist to suggest an enhanced accumulation of soil carbon. However, care must be taken to ensure that the measured values of soil carbon are reflective of the imposed management and not dominated by prior practices.
Given the variable run-down of soil carbon that has occurred due to the implementation of agriculture across Australia (Luo et al. 2010b; Sanderman et al. 2010) and the length of time required for soil carbon to attain a value indicative of any new management strategy, the sampling of soils under new and innovative practices too early is more likely to result in the acquisition of soil carbon data that are reflective of prior rather than current management practices. Application of both repeated sampling through time and modelling to define potential trajectories and final outcomes would provide the best approach, but would also require time to complete the assessment. Development of alternative approaches would help to identify practices with the potential to enhance soil carbon and mitigate emissions.
Measurements of SOC have traditionally focussed on quantification of the total amount of organic carbon present in soil through sample collection followed by laboratory analysis. The costs of this exercise, compounded with spatial and temporal variations in soil carbon, often mean that the number of samples collected and analysed limits the usefulness of acquired results. The quest should continue for rapid and cost-effective analytical methodologies for both laboratory and field situations. Analytical technologies have recently emerged that extend the nature of data generated beyond that provided by conventional dry combustion analyses including: laser-induced spectroscopy (Herrmann et al. 2005; Bai and Houlton 2009), inelastic neutron scattering (Rawluk et al. 2001; Li et al. 2005), and near- and mid-infrared spectroscopies (Gerber et al. 2010; Ma and Ryan 2010). Research should be directed towards the possible extension of these technologies to field-based approaches capable of accounting for spatial variability. In Australia, the use of mid-infrared spectroscopy combined with partial least-squares analysis (MIR/PLS) has been shown to allow rapid and cost-effective predictions of not only the total organic carbon content of soils, but also the allocation of this carbon to component fractions with a defined confidence (Janik et al. 2007). It has also been demonstrated that these fractions could be substituted for the conceptual pools to produce a variant of the RothC soil carbon simulation model capable of modelling the dynamics of both total soil organic carbon and its component fractions (Skjemstad et al. 2004). Extension of these measurement and modelling capabilities across a greater range of soils and agricultural systems is warranted. The definition of upper limits of soil carbon capture and storage will be critical to allow estimates of soil carbon sequestration potential to be realised.
Nitrous oxide and methane
Quantification of N2O and CH4 emissions from soil relies completely on the measurement of net gas fluxes because no stock change measurements, such as those applicable to CO2 emissions, are possible. Various field-based methodologies exist to estimate fluxes of non-CO2 greenhouse gases (Denmead et al. 2010). Measurements of net fluxes of N2O and CH4 integrated over time are obtained using either micrometeorological techniques (e.g. Phillips et al. 2007; Macdonald et al. 2011) or ground-based monitoring chambers that collect continuous data (e.g. Barton et al. 2008, 2010, 2011; Scheer et al. 2011; Wang et al. 2011). Although both of these continuous monitoring approaches can provide estimates of fluxes integrated over time, they each have relative strengths and weaknesses. Micrometeorological techniques do not disturb the soil or cause alterations to soil or plant processes and they provide an estimated flux integrated over relatively large distances (100 times the height) compared with the typical size of continuous monitoring chambers. However, where relationships between the magnitude of N2O or CH4 flux and soil properties (e.g. water) are sought, obtaining appropriate spatially averaged values applicable to the entire fetch area of micrometeorological equipment will be difficult. Use of datalogging equipment to monitor soil conditions directly under the chambers may provide a more appropriate approach for such studies.
Although non-continuous measurements using static chambers have been used to examine relative differences in N2O and CH4 emission between treatments (Allen et al. 2009, 2010; Huang et al. 2011) and spatial variations in N2O emissions (Turner et al. 2008), integrating such values to provide accurate estimates of emissions through time and space is not possible given the magnitude of diurnal fluctuations (Macdonald et al. 2011), the high dependence of emission rates on soil properties that vary significantly through time (Dalal et al. 2003), and the potential episodic nature of emission events (Chen et al. 2010a).
Given the expense and technical requirements to maintain continuous monitoring systems, routine estimation of N2O and CH4 emissions from individual landholdings and the formation of a national emission accounting system cannot be based on measurement alone. Satisfying the requirements for accounting systems will rely on the use of emissions factors and/or the development of modelling capabilities. However, derivation of appropriate emission factors and models will require continued use of monitoring technologies to quantify emissions at representative locations and provide data to construct, calibrate, and validate simulation models.
The current Australian national Nitrous Oxide Research Program (NORP) is collecting valuable data that will be critical to model development; however, the number of sites where monitoring of N2O and CH4 emissions is occurring is limited given the diversity of Australia’s climate, soils, and agricultural systems. Such experimental measurement programs will need to be continued and extended to additional agricultural regions and as new agricultural management practices are developed. Questions that require consideration to help guide the acquisition of N2O and CH4 emissions data and model development include:
-
How can the limited number of continuous monitoring systems be optimally located within the diverse mix of climates, soil types, and agricultural practices across Australia?
-
What are the relative responses of N2O and CH4 emissions to soil temperature and water content, do the responses interact, and do they vary significantly between soil types?
-
How will changes in soil bulk density and pore size distribution that are induced by compaction or reduced tillage impact on net fluxes of N2O and CH4?
-
Will calibration of current models be adequate to deal with potential climate change?
-
At present, a much greater emphasis is being placed on monitoring the net emissions of N2O; should more emphasis be placed on the additional acquisition of net CH4 emission data for soils?
Summary
Soils contain significant stocks of carbon and nitrogen. Biochemical processes associated with carbon and nitrogen cycling can lead to both the consumption and emission of the three major greenhouse gases (CO2, N2O, and CH4). These processes are strongly influenced by temperature and soil water content (balance between rainfall and potential evapotranspiration). Thus, the predicted hotter and drier climatic conditions associated with climate change projections will impact on net greenhouse gas emissions from soil; however, the direction and extent of influence require further consideration. Guiding principles exist to help develop agricultural management strategies capable of enhancing soil carbon stocks or reducing net N2O and CH4 emissions.
The guiding principal to enhance carbon capture in soils under any climate-change scenario will be to maximise carbon inputs. Where the ability of a soil to protect organic carbon against decomposition is not saturated and/or where inefficiencies in resource use (water and nutrients) can be improved by altered management to allow plants to capture additional atmospheric CO2, the potential exists to increase SOC and enhance soil resilience and productivity. This potential will vary from location to location as a function of soil type (clay content, depth, bulk density, etc.), environmental conditions (amount of available water and nutrients, temperature, etc.), and past management regimes (how much carbon has been lost due to past management). Tailoring management solutions that optimise SOC at a defined location will be required.
To reduce N2O emissions, the guiding principal is to reduce concentrations of the inorganic nitrogen. Inorganic nitrogen serves as the substrate for the two main processes responsible for N2O emissions—nitrification and denitrification. However, in developing practices to reduce inorganic nitrogen concentrations in soil, it is important to ensure that crop/pasture nitrogen requirements are still met. Development of flexible nitrogen-application strategies is required to allow a better matching of fertiliser additions to crop demand as defined by seasonal climatic conditions. Methane emissions from soils can be minimised by maintaining the soil in an oxic state for as long as possible.
Going forward, the delivery of cost-effective measurement technologies coupled to adequately developed computer simulation models will be required. Such a combination will produce the required capabilities to develop management practices that can accumulate soil carbon and minimise the emissions of N2O and CH4 and underpin a national carbon accounting system. Development and support of a soil-monitoring program into the future is essential to ensure adequate coverage of all of Australia’s agricultural regions and allow an assessment of the impacts of variations in climate, soil, and agricultural production systems.
Acknowledgments
Dr Baldock acknowledges financial support from the Department of Climate Change and Energy Efficiency, the Department of Agriculture, Fisheries and Forestry and the Grains Research and Development Corporation who have all funded projects that have led to the development of the ideas presented within this paper.
References
Allen DE, Mendham DS, Bhupinderpal S, Cowie A, Wang W, Dalal RC, Raison RJ (2009) Nitrous oxide and methane emissions from soil are reduced following afforestation of pasture lands in three contrasting climatic zones. Australian Journal of Soil Research 47, 443–458.Allen DE, Kingston G, Rennenberg H, Dalal RC, Schmidt S (2010) Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agriculture, Ecosystems & Environment 136, 209–217.
| Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFentb8%3D&md5=369487e29288b8d884b620fab0ee5228CAS |
Anderson DW, Paul EA (1984) Organo-mineral complexes and their study by radiocarbon dating. Soil Science Society of America Journal 48, 298–301.
| Organo-mineral complexes and their study by radiocarbon dating.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXitVyns7g%3D&md5=053ce1d190eff8c25a407e35ba005397CAS |
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277–288.
| Nitrogen supply and demand in Australian agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1CrsbY%3D&md5=6bc2f63475c9af917c6e06b78f8e746dCAS |
Bai E, Houlton BZ (2009) Coupled isotopic and process-based modeling of gaseous nitrogen losses from tropical rain forests. Global Biogeochemical Cycles 23, Art No. GB2011
| Coupled isotopic and process-based modeling of gaseous nitrogen losses from tropical rain forests.Crossref | GoogleScholarGoogle Scholar |
Baldock JA (2007) Composition and cycling of organic carbon in soil. In ‘Soil biology. Volume 10. Nutrient cycling in terrestrial ecosystems’. (Eds P Marschner, Z Rengel) (Springer-Verlag: Berlin)
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
Barton L, Murphy DV, Kiese R, Butterbach-Bahl K (2010) Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate. Global Change Biology – Bioenergy 2, 1–15.
| Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1antb0%3D&md5=2174b14e02cecf0ceeb467216cfa3674CAS |
Barton L, Butterbach-Bahl K, Kiese R, Murphy DV (2011) Nitrous oxide fluxes from a grain-legume crop (narrow-leafed lupin) grown in a semiarid climate. Global Change Biology 17, 1153–1166.
| Nitrous oxide fluxes from a grain-legume crop (narrow-leafed lupin) grown in a semiarid climate.Crossref | GoogleScholarGoogle Scholar |
Blair GJ, Lefory RDB, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Australian Journal of Agricultural Research 46, 1459–1466.
| Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems.Crossref | GoogleScholarGoogle Scholar |
Bossio DA, Horwath WR, Mutters RG, Van Kessel C (1999) Methane pool and flux dynamics in a rice field following straw incorporation. Soil Biology & Biochemistry 31, 1313–1322.
| Methane pool and flux dynamics in a rice field following straw incorporation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktlyju7o%3D&md5=0fa1724d86b96045270e4aa55f17a2fbCAS |
Bui E, Henderson B, Viergever K (2009) Using knowledge discovery with data mining from the Australian Soil Resource Information System database to inform soil carbon mapping in Australia. Global Biogeochemical Cycles 23, Art. No. GB2011
| Using knowledge discovery with data mining from the Australian Soil Resource Information System database to inform soil carbon mapping in Australia.Crossref | GoogleScholarGoogle Scholar |
Castaldi S (2000) Responses of nitrous oxide, dinitrogen and carbon dioxide production and oxygen consumption to temperature in forest and agricutlural light-textured soils determined by model experiment. Biology and Fertility of Soils 32, 67–72.
| Responses of nitrous oxide, dinitrogen and carbon dioxide production and oxygen consumption to temperature in forest and agricutlural light-textured soils determined by model experiment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmvFWrtrw%3D&md5=80f1db6be19496be3bce85e31a020646CAS |
Chalk PM (1998) Dynamics of biologically fixed N in legume-cereal rotations: a review. Australian Journal of Agricultural Research 49, 303–316.
| Dynamics of biologically fixed N in legume-cereal rotations: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFGqsbs%3D&md5=23a823672fd60bfd6c93900151eddfcbCAS |
Chen D, Li Y, Kelly K, Eckard R (2010a) Simulation of N2O emissions from an irrigated dairy pasture treated with urea and urine in Southeastern Australia. Agriculture, Ecosystems & Environment 136, 333–342.
| Simulation of N2O emissions from an irrigated dairy pasture treated with urea and urine in Southeastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFenurs%3D&md5=aea5d708fb4a23800370c81685fc0087CAS |
Chen D, Suter HC, Islam A, Edis R (2010b) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biology & Biochemistry 42, 660–664.
| Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Sgtrw%3D&md5=6c789e0bf535a24d13955858d280e046CAS |
Conrad R (2005) Quantification of methanogenic pathways using stable carbon isotopic signatures, a review and a proposal. Organic Geochemistry 36, 739–752.
| Quantification of methanogenic pathways using stable carbon isotopic signatures, a review and a proposal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtl2rtLk%3D&md5=0e3591e022248d5857058cc955b310cbCAS |
Corton TM, Bajita JB, Grospe FS, Pamplona RR, Asis CAJ, Wassmann R, Lantin RS, Buendia LV (2000) Methane emission from irrigated and intensively managed rice fields in Central Luzon (Philippines). Nutrient Cycling in Agroecosystems 58, 37–53.
| Methane emission from irrigated and intensively managed rice fields in Central Luzon (Philippines).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtVeisL4%3D&md5=5fff77bee7febe4ab90de5ab9d3c81cfCAS |
CSIRO (2007) Climate Change in Australia. Technical Report. Available at: www.climatechangeinaustralia.gov.au/technical_report.php
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=0247e07ef53d3b67dedf89daa649a534CAS |
Dalal RC, Allen DE, Livesley SJ, Richards G (2008) Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant and Soil 309, 43–76.
| Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVyhsrg%3D&md5=8f4e7b629bdb98c448f0268be198aa74CAS |
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173.
| Temperature sensitivity of soil carbon decomposition and feedbacks to climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitFGitLo%3D&md5=90569b10944b265dacc4b570ecfe8240CAS |
de Gruijter J, Brus D, Bierkens M, Knotters M (2006) ‘Sampling for natural resource monitoring.’ (Springer: Berlin)
Denmead OT, Macdonald BCT, Bryant G, Naylor T, Wilson S, Griffith DWT, Wang WJ, Salter B, White I, Moody PW (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agricultural and Forest Meteorology 150, 748–756.
| Emissions of methane and nitrous oxide from Australian sugarcane soils.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC (2003) Mitigation of nitrous oxide emissions in spray-irrigated grazed grassland by treating the soil with dicyandiamide, a nitrification inhibitor. Soil Use and Management 19, 284–290.
| Mitigation of nitrous oxide emissions in spray-irrigated grazed grassland by treating the soil with dicyandiamide, a nitrification inhibitor.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC (2006) Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biology and Fertility of Soils 42, 472–480.
| Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntlGhtrw%3D&md5=5600421ac0e6ab1a18e2a6ccd4f7079eCAS |
Di HJ, Cameron KC, Sherlock RR (2007) Comparison of the effectiveness of a nitrification inhibitor dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use and Management 23, 1–9.
| Comparison of the effectiveness of a nitrification inhibitor dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions.Crossref | GoogleScholarGoogle Scholar |
Dunfield P, Knowles R, Dumont R, Moore TR (1993) Methane production and consumption in temperate and subarctic peat soils, response to temperature and pH. Soil Biology & Biochemistry 25, 321–326.
| Methane production and consumption in temperate and subarctic peat soils, response to temperature and pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeqtL8%3D&md5=075017cc4334ce63f62152fae038092eCAS |
Eswaran H, Van den Berg E, Reich P, Kimble JM (1995) Global soil C resources. In ‘Soils and global change’. (Eds R Lal, JM Kimble, E Levine, BA Stewart) pp. 27–43. (Lewis Publishers: Boca Raton, FL)
Freney JR, Denmead OT, Simpson JR (1978) Soil as a source or sink for atmospheric nitrous oxide. Nature 273, 530–532.
| Soil as a source or sink for atmospheric nitrous oxide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXmtVSktrk%3D&md5=e36118f9b34353b51e4b2c7d10be3ea9CAS |
Gerber S, Hedin LO, Oppenheimer M, Pacala SW, Shevliakova E (2010) Nitrogen cycling and feedbacks in a global dynamic land model. Global Biogeochemical Cycles 24, Art. No. GB1001
| Nitrogen cycling and feedbacks in a global dynamic land model.Crossref | GoogleScholarGoogle Scholar |
Giardina CP, Ryan MG (2000) Evidence that decomposition rates of organic matter in mineral soil do not vary with temperature. Nature 404, 858–861.
| Evidence that decomposition rates of organic matter in mineral soil do not vary with temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVCrsL4%3D&md5=4721505fc18109fbdc375b5253945a4bCAS |
Golchin A, Baldock JA, Oades JM (1997) A model linking organic matter decomposition, chemistry and aggregate dynamics. In ‘Soil processes and the carbon cycle’. (Eds R Lal, JM Kimble, RF Follett, BA Stewart) pp. 245–266. (CRC Press: Boca Raton, FL)
Goodroad LL, Keeney DR (1984) Nitrous oxide proudction in aerobic soils under varying pH, temperature and water content. Soil Biology & Biochemistry 16, 39–43.
| Nitrous oxide proudction in aerobic soils under varying pH, temperature and water content.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXksFeqs7s%3D&md5=ee9f4edbe77cb7b684839ea97239c8fcCAS |
Grace J, Rayment M (2000) Respiration in the balance. Nature 404, 819–820.
| Respiration in the balance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVCmtbo%3D&md5=638f3c8786d92381db570bbc2658b1d6CAS |
Herrmann A, Witter E, Katterer T (2005) A method to assess whether ‘preferential use’ occurs after 15N ammonium addition; implication for the 15N isotope dilution technique. Soil Biology & Biochemistry 37, 183–186.
| A method to assess whether ‘preferential use’ occurs after 15N ammonium addition; implication for the 15N isotope dilution technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVKntb4%3D&md5=b91278c38d9b92e74534e9b9f95780f9CAS |
Hou AX, Chen GX, Wang ZP, Van Cleempu TO, Patrick WHJ (2000) Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Science Society of America Journal 64, 2180–2186.
| Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFSrtQ%3D%3D&md5=fdddb7bc3f07a102fdcf2cf859d55ac0CAS |
Houghton RA (2005) The contemporary carbon cycle. In ‘Biogeochemistry’. (Ed. WH Schlesinger) pp. 473–513. (Elsevier Science: Amsterdam)
Huang X, Grace P, Mengersen K, Weier K (2011) Spatio-temporal variation in soil derived nitrous oxide emissions under sugarcane. The Science of the Total Environment 409, 4572–4578.
| Spatio-temporal variation in soil derived nitrous oxide emissions under sugarcane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCrtbrJ&md5=7c894066696007dc49aca4ab74552eabCAS |
Hutchinson JJ, Campbell CA, Desjardins RL (2007) Some perspectives on carbon sequestration in agriculture. Agricultural and Forest Meteorology 142, 288–302.
| Some perspectives on carbon sequestration in agriculture.Crossref | GoogleScholarGoogle Scholar |
Janik LJ, Skjemstad JO, Shepherd KD, Spouncer LR (2007) The prediction of soil carbon fractions using mid-infrared-partial least square analysis. Australian Journal of Soil Research 45, 73–81.
| The prediction of soil carbon fractions using mid-infrared-partial least square analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsFygurk%3D&md5=63ae64f9cdd032480e23410b3b99a8dcCAS |
Jenkinson DS, Hart PBS, Rayner JH, Parry LC (1987) Modelling the turnover of organic matter in long-term experiments at Rothamsted. Intecol Bulletin 15, 1–8.
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10, 423–436.
| The vertical distribution of soil organic carbon and its relation to climate and vegetation.Crossref | GoogleScholarGoogle Scholar |
Kebreab E, France J, Beever DE, Castillo AR (2001) Nitrogen pollution by dairy cows and its mitigation by dietary manipulation. Nutrient Cycling in Agroecosystems 60, 275–285.
| Nitrogen pollution by dairy cows and its mitigation by dietary manipulation.Crossref | GoogleScholarGoogle Scholar |
Keeney DR, Fillery IR, Marx GP (1979) Effect of temperature on the gaseous nitrogen products of denitrification in a silt loam soil. Soil Science Society of America Journal 43, 1124–1128.
| Effect of temperature on the gaseous nitrogen products of denitrification in a silt loam soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhs1aksro%3D&md5=b765f7b87139e1be177b10cb71e755f9CAS |
Kessavalou A, Mosier AR, Doran JW, Drijber RA, Lyon DJ, Heinemeyer O (1998) Fluxes of carbon dioxide, nitrous oxide, and methane in grass sod and winter wheat–fallow tillage management. Journal of Environmental Quality 27, 1094–1104.
| Fluxes of carbon dioxide, nitrous oxide, and methane in grass sod and winter wheat–fallow tillage management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtl2js7Y%3D&md5=98a2c92ffd16c34435e2bb9d97cbc506CAS |
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433, 298–301.
| Long-term sensitivity of soil carbon turnover to warming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1KksA%3D%3D&md5=82974d2ebc209f0024097c72f8c6bbabCAS |
Kumaraswamy S, Ramakrishnan B, Sethunathan N (2001) Methane production and oxidation in an anoxic rice soil as influenced by inorganic redox species. Journal of Environmental Quality 30, 2195–2201.
| Methane production and oxidation in an anoxic rice soil as influenced by inorganic redox species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1Slt7s%3D&md5=37805f51b7b2f249d9851eae0c539a42CAS |
Ladd JN, Oades JM, Amato M (1981) Microbial biomass formed from 14C, 15N-labelled plant material decomposing in soils in the field. Soil Biology & Biochemistry 13, 119–126.
| Microbial biomass formed from 14C, 15N-labelled plant material decomposing in soils in the field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkt1entLY%3D&md5=5c725596ea4ab339ac5a229ee60a1dceCAS |
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123, 1–22.
| Soil carbon sequestration to mitigate climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXoslSmsLY%3D&md5=2ebebc7c26ad7be1ad95dccf27605f6aCAS |
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. European Journal of Soil Biology 37, 25–50.
| Production, oxidation, emission and consumption of methane by soils: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvFOnsbs%3D&md5=d2b5576333bd01d55b5c8662fe8dd120CAS |
Li CS, Frolking S, Butterbach-Bahl K (2005) Carbon sequestration in arable soils is likely to increase nitrous oxide emissions, offsetting reductions in climate radiative forcing. Climatic Change 72, 321–338.
| Carbon sequestration in arable soils is likely to increase nitrous oxide emissions, offsetting reductions in climate radiative forcing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKmsLvP&md5=d8e50257b0d66f4deb26b09265529a6aCAS |
Luo J, de Klein CAM, Ledgard SF, Saggar S (2010a) Management options to reduce nitrous oxide emissions from intensively grazed pastures: a review. Agriculture, Ecosystems & Environment 136, 282–291.
| Management options to reduce nitrous oxide emissions from intensively grazed pastures: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFentbc%3D&md5=ac59d1808229c73ff3789253679c036eCAS |
Luo Z, Wang E, Sun OJ (2010b) Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: a review and synthesis. Geoderma 155, 211–223.
| Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: a review and synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlWgtb0%3D&md5=b7a89e32cfe8955b3737e3e8f9b12231CAS |
Ma JF, Ryan PR (2010) Understanding how plants cope with acid soils. Functional Plant Biology 37, iii–vi.
| Understanding how plants cope with acid soils.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Denmead OT, White I, Byrant G (2011) Gaseous nitrogen losses from coastal acid sulfate soils: a short-term study. Pedosphere 21, 197–206.
| Gaseous nitrogen losses from coastal acid sulfate soils: a short-term study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVyrsLw%3D&md5=5653ebbdc357fc210259c47245c12976CAS |
Mackenzie FT (1998) ‘Our changing planet: an introduction to earth system science and global environmental change.’ 2nd edn (Prentice Hall: Upper Saddle River, NJ)
Masscheleyn PH, DeLaune RD, Patrick WHJ (1993) Methane and nitrous oxide emissions from laboratory measurements of rice soil suspension: effect of soil oxidation-reduction status. Chemosphere 26, 251–260.
| Methane and nitrous oxide emissions from laboratory measurements of rice soil suspension: effect of soil oxidation-reduction status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXitlaqs7Y%3D&md5=bb0d3ee0932a9def7393ed978fb50880CAS |
McSwiney CP, Robertson GP (2005) Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Global Change Biology 11, 1712–1719.
| Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system.Crossref | GoogleScholarGoogle Scholar |
Mosier A, Wassmann R, Verchot L, King J, Palm C (2004) Methane and nitrogen oxide fluxes in tropical agricultural soils, sources, sinks and mechanisms. Environment, Development and Sustainability 6, 11–49.
| Methane and nitrogen oxide fluxes in tropical agricultural soils, sources, sinks and mechanisms.Crossref | GoogleScholarGoogle Scholar |
Mulligan FJ, Dillon P, Callan JJ, Rath M, O’Mara FP (2004) Supplementary concentrate type affects nitrogen excretion of grazing dairy cows. Journal of Dairy Science 87, 3451–3460.
| Supplementary concentrate type affects nitrogen excretion of grazing dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotFykurs%3D&md5=f23d9afc51a31e8979748daa667ab260CAS |
Nielsen NM, Kristensen T, Nørgaard P, Hansen H (2003) The effect of low protein supplementation to dairy cows grazing clover grass during half of the day. Livestock Production Science 81, 293–306.
| The effect of low protein supplementation to dairy cows grazing clover grass during half of the day.Crossref | GoogleScholarGoogle Scholar |
Paul EA, Collins HP, Leavitt SW (2001) Dynamics of resistant soil carbon of Midwestern agricultural soils measured by naturally occurring 14C abundance. Geoderma 104, 239–256.
| Dynamics of resistant soil carbon of Midwestern agricultural soils measured by naturally occurring 14C abundance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnsFGht7c%3D&md5=7732f366d2a4a62a27db541e8232cf25CAS |
Peoples MB, Baldock JA (2001) Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems. Australian Journal of Experimental Agriculture 41, 327–346.
| Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1Crtrw%3D&md5=0ff51c42b388da7654025c9d4c32eea8CAS |
Phillips FA, Leuning R, Baigenta R, Kelly KB, Denmead OT (2007) Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques. Agricultural and Forest Meteorology 143, 92–105.
| Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques.Crossref | GoogleScholarGoogle Scholar |
Powlson D (2005) Climatology: will soil amplify climate change? Nature 433, 204–205.
| Climatology: will soil amplify climate change?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1Khsw%3D%3D&md5=ccbdfaa9ac7f4f2475ad89e174d96464CAS |
Rawluk CDL, Grant CA, Racz GJ (2001) Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Canadian Journal of Soil Science 81, 239–246.
| Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVGqsrc%3D&md5=3ba4272db089ff188b139fb9da795a13CAS |
Sanderman J, Farquharson R, Baldock JA (2010) Soil Carbon Sequestration Potential: A review for Australian agriculture. CSIRO Sustainable Agriculture Flagship Report, prepared for Department of Climate Change and Energy Efficiency. Available at: www.csiro.au/resources/Soil-Carbon-Sequestration-Potential-Report.html
Scheer C, Grace PR, Rowlings DW, Kimber S, Van Zwieten L (2011) Effect of biochar amendment on the soil–atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant and Soil 345, 47–58.
| Effect of biochar amendment on the soil–atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFymt7o%3D&md5=3ebf4faf0d5dc0e612132825d8d92f00CAS |
Six J, Callewaert P, Lenders S, De Gryze S, Morris SJ, Gregorich EG, Paul EA, Paustian K (2002) Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Science Society of America Journal 66, 1981–1987.
| Measuring and understanding carbon storage in afforested soils by physical fractionation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XoslKhtr0%3D&md5=645a4455cd79b208349a8365a746cf88CAS |
Skjemstad JO, Spouncer LR, Cowie B, Swift RS (2004) Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Australian Journal of Soil Research 42, 79–88.
| Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXht1ahsbo%3D&md5=8ca7a9b18f84ee4593bfad5ae1a917dbCAS |
Smith LC, de Klein CAM, Catto WD (2008) Effect of dicyandiamide applied in a granular form on nitrous oxide emissions from a grazed dairy pasture in Southland, New Zealand. New Zealand Journal of Agricultural Research 51, 387–396.
| Effect of dicyandiamide applied in a granular form on nitrous oxide emissions from a grazed dairy pasture in Southland, New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFersbk%3D&md5=28d171ad771db2a6274f13404f45bf50CAS |
Snedecor GW, Cochran WG (1989) ‘Statistical methods.’ 8th edn (Iowa State University Press: Ames, IA)
Spain AV, Isbell RF, Probert ME (1983) Soil organic matter. In ‘Soils: an Australian viewpoint’. pp. 551–563. (CSIRO: Melbourne/Academic Press: London)
Swanston CW, Torn MS, Hanson PJ, Southon JR, Garten CT, Hanlon EM, Ganio L (2005) Initial characterization of processes of soil carbon stabilization using forest stand-level radiocarbon enrichment. Geoderma 128, 52–62.
| Initial characterization of processes of soil carbon stabilization using forest stand-level radiocarbon enrichment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvV2ks7c%3D&md5=804befc6da9a3acbf1c51d5e9ab7e9b6CAS |
Turner DA, Chen D, Galbally IE, Leuning R, Edis RB, Li Y, Kelly K, Phillips F (2008) Spatial variability of nitrous oxide emissions from an Australian irrigated dairy pasture. Plant and Soil 309, 77–88.
| Spatial variability of nitrous oxide emissions from an Australian irrigated dairy pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVyhsrs%3D&md5=97917e83281ec2a8a9f69e871b91ab0eCAS |
Unkovich M, Baldock J, Forbes M (2010) Variability in harvest index of grain crops and potential significance for carbon accounting: examples from Australian agriculture. Advances in Agronomy 105, 173–219.
| Variability in harvest index of grain crops and potential significance for carbon accounting: examples from Australian agriculture.Crossref | GoogleScholarGoogle Scholar |
Veldkamp E, Weitz AM, Keller M (2001) Management effects on methane fluxes in humid tropical pasture soils. Soil Biology & Biochemistry 33, 1493–1499.
| Management effects on methane fluxes in humid tropical pasture soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFSls70%3D&md5=da65ddbaee48734d71f8978b65d0ad5eCAS |
Wang W, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Wassmann R, Neue HU, Lantin RS, Makarim K, Chareonsilp N, Buendia LV, Rennenberg H (2000) Characterization of methane emissions from rice fields in Asia. II differences among irrigated, rainfed, and deepwater rice. Nutrient Cycling in Agroecosystems 58, 13–22.
| Characterization of methane emissions from rice fields in Asia. II differences among irrigated, rainfed, and deepwater rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtVeis7Y%3D&md5=494294ee1731eb8355bc3cbb431ec164CAS |
Watson CJ (2000) Urease activity and inhibition—principles and practice. In ‘Proceedings of the International Fertilizer Society No. 454’. London, UK. (Ed. CJ Watson) p. 40. (International Fertiliser Society)
Whitehead DC (1995) ‘Grassland nitrogen.’ (CAB International: Wallingford, UK)


