Molecular electronics: an Australian perspective
Jeffrey R. Reimers A B * and Paul J. Low
A B * and Paul J. Low  C *
C *
A International Centre for Quantum and Molecular Structures and Department of Physics, Shanghai University, Shanghai, 200444, PR China.
B School of Mathematical and Physical Sciences, University of Technology Sydney, NSW 2007, Australia.
C School of Molecular Sciences, University of Western Australia, Crawley, WA 6009, Australia.

Jeffrey Reimers studied spectroscopy at ANU with Ian Ross and Gad Fischer (1978–79) before studying simulations with Robert Watts (1980–82), and then semiclassical quantum science with Kent Wilson and Eric Heller at the University of California—San Diego during 1983–85. This led to a career in molecular electronics working alongside Noel Hush and Maxwell Crossley at Sydney University from 1985 to 2013, followed by Shanghai University and University of Technology Sydney thenceforth. He is best known for his works in understanding electric-field control of biological photochemical charge separation and the development of computational strategies for modelling single-molecule conductivity and spectroscopy. He is a Fellow of the Royal Australian Chemical Institute, Royal Society of NSW and the Australian Academy of Science. |

Paul Low developed his interests in organometallic chemistry and mixed-valence complexes at the University of Adelaide under the tutelage of Michael I. Bruce. Following postdoctoral work at the Steacie Institute of Molecular Sciences with Arthur J. Carty, Paul was appointed to the Department of Chemistry at Durham University (UK) (1999 Lecturer; 2006 Reader; 2010 Professor). In the UK, Paul developed research themes in molecular electronics, with colleagues including Martin Bryce, Richard Nichols and Colin Lambert. Paul returned to Australia and the University of Western Australia in 2013. For his work, he has been awarded EPSRC Leadership (2009) and ARC Future Fellowships (2012), an Alexander von Humboldt Foundation Friedrich Wilhelm Bessel Research Award (2016) and the Royal Australian Chemical Institute’s H. G. Smith Memorial Medal (2020). |
Australian Journal of Chemistry 76(9) 559-580 https://doi.org/10.1071/CH23008
Submitted: 10 January 2023 Accepted: 15 June 2023 Published: 12 July 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Molecular electronics is a scientific endeavour that, for 60 years, has offered the promise of new technologies in which molecules integrate with, if not entirely replace, semiconductor electronics. En route to the attainment of these ambitious goals, central aspects underpinning the pursuit of this science have proven critical to the development of related technologies, including organic photovoltaics (OPV) and organic light-emitting diodes (OLEDs). Looking ahead, new opportunities in the field abound, from the study of molecular charge transport and the elucidation of molecular reaction mechanisms, to the development of biocompatible and degradable electronics, and the construction of novel chemical sensors with exquisite sensitivity and specificity. This article reviews historical developments in molecular electronics, with a particular focus on Australia’s contributions to the area. Australia’s current activity in molecular electronics research is also summarised, highlighting the capacity to both advance fundamental knowledge and develop new technologies. Scientific aspects considered include capabilities in: single molecule and molecular–monolayer junction measurement; spectroscopic analysis of molecular components and materials; synthetic chemistry; computational analysis of molecular materials and junctions; and the development of theoretical concepts that describe the electrical characteristics of molecular components, materials and putative device structures. Technological aspects considered include various aspects of molecular material design and implementation, such as: OPV and OLED construction, sensing technologies and applications, and power generation from heat gradients or friction. Missing capabilities are identified, and a future pathway for Australian scientific and technological development envisaged.
Keywords: electron transfer, electron transport, molecular devices, molecular electronics, molecular switches, nanotechnology, organic electronics, technology relevance levels.
Molecular electronics and its community of allied endeavour
Molecular electronics, at its most basic level, involves processes in which electrical current is passed between two macroscopic electrodes through single molecules. The core concepts and ideas apply also to small collections of molecules, as well as to very many molecules arranged in well-ordered monolayers, contacted between the electrode surfaces. The molecular components in these electrode–molecule–electrode junctions, although often synthetic and designed to feature or test some specific structure–property relationship, can be drawn from across the chemical and biological landscape. In essence, molecular electronics is concerned with, and driven by, the charge injection processes at the molecule–electrode interface and associated details of the molecule–electrode coupling, linked with intramolecular charge transport properties (Fig. 1a). In film-based devices, intermolecular charge transfer may also play a role in the overall electrical response of the ‘large area’ junction (Fig. 1b). As such, the science of molecular electronics underpins studies of biological processes involving electron transport, as well as charge injection and transport in conducting polymers and organic semiconductors.
Cartoon sketches illustrating the key features and characteristic functions of the principle components of: (a) single molecule; and (b) ‘large area’ molecular junctions.

Many of the concepts involved in molecular electronics research also strongly overlap with exciton science, as the motion of excitons through molecular materials can be considered as the coupled transport of opposite charges; consequently, much of the fundamental science is in common across both fields. Given that exciton transport is critically important in photosynthesis and underpins organic light emitting diode (OLED) and organic photovoltaic (OPV) function, the consequences of results from molecular electronics extend far beyond the mimicry of the function of semiconductor electronic components. Consequently, many new applications of processes based on the science of molecular electronics are being envisaged, from nanoscale biological and mechanical detectors and sensors to molecular thermoelectric materials and logic circuits.
This article stems from discussions at the ‘Molecular Electronics’ Symposium at the RACI2022 National Congress held in Brisbane, Qld, Australia, in July 2022. This was the first meeting in which a large cross-section of the molecular electronics community in Australia came together to present and propose diverse ways of advancing both the fundamental science and technological applications of molecular electronics. The concept of this meeting, chaired by Jeffrey Reimers and Paul Low, originated from an informal gathering of some members of the Australian community at the 2017 RACI Centennial Conference in Melbourne, including Andrew Abell, Thomas Becker, Simone Ciampi, Maxwell Crossley, Nadim Darwish, Deanna D’Alessandro, Daniel Kosov, George Koutsantonis, Matthew Piggott, Koushik Venkatesan and Jingxian Yu. The 2022 Symposium featured 21 speakers, covering numerous chemistry sub-disciplines and allied areas including: organic chemistry, inorganic chemistry, physical chemistry, polymer chemistry, materials chemistry, computational chemistry, device physics, nanotechnology and electrical engineering.
Origins
Landauer–Büttiker theory
The origins of molecular electronics stem from some critical pioneering works drawn from diverse areas of study, and a summary of a select few of these fundamental aspects is useful before considering current research and technology directions. In 1957, while at IBM, Rolf Landauer constructed a theory that showed the relationship between electrical conductance and the scattering properties of a single-channel quantum conductor.[1] This was subsequently extended through work with Markus Büttiker to a more generalised multi-channel case linking the scattering amplitudes of electrons passing through a quantum junction and the conductance of the junction, which was reported almost 30 years after the description of the original model.[2]
The resulting Landauer and Landauer–Büttiker models employ the concept of the transmission coefficient (T(E)) which describes the probability of an electron wave of energy E propagating between probes connected to the channel (i.e. the source and drain electrodes connected to a molecule in a two-terminal molecular junction, such as that illustrated in Fig. 1a). At low temperature, the electrical conductance (G) of a molecule or nanoscale object can be described by the Landauer equation (Eqn 1).
where G0 is referred to as the quantum of conductance (G0 = 2e2 / h = 77 μS; e is the electronic charge, h is Planck’s constant). The transmission coefficient (T(E)) describes the passage of de Broglie-like electron waves of energy E (eV) through the junction from the source to the drain. By evaluating T(EF), the transmission coefficient at the Fermi energy (EF) and the conductance of the junction (G) can be determined at zero voltage. In these scattering models, the transmission coefficient is a function of the molecular structure and molecule–electrode coupling, and it follows that, through molecular design, it is possible to control the wave patterns entering and exiting the molecule at the points of contact within a molecular junction, and hence the electrical conductance. Variations of this allow the conductance at high voltage to be determined, demonstrating features such as reactivation. A range of other physical properties can also be derived from the T(E) function, such as the Seebeck coefficient, S, which is related to the slope of T(E) at EF (Eqn 2),
where S0 = αT (α is the Lorentz number, T is temperature, K, and E is energy, eV), and the notion of electron wave transmission through multiple channels (molecular energy levels) in a junction leads to the identification of quantum interference effects.[3]
Jaklevic–Lambe experiments and IETS
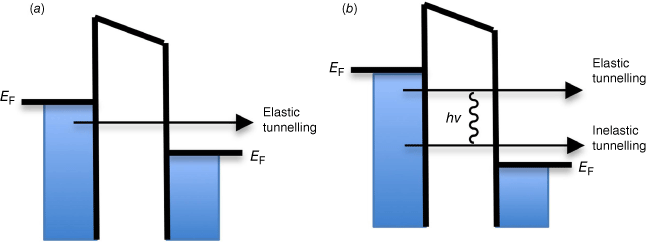
Early experimental advances in the identification and study of molecular junctions arose from the work of Jaklevic and Lambe, while exploring the properties of lubricants in bearings, in 1966 at the Ford Motor Company.[4] Large area junctions were fabricated on a vacuum deposited 2000-Å-thick Al film, on which was grown a 30-Å-thick layer of native oxide. Exposure of the substrate to vapour of a target molecule (acetic or propionic acid in the original report) resulted in self-assembly of a molecular monolayer, which was subsequently top-coated with a 1-μm-thick Pb electrode. The junction conductance (G) was found to increase at specific voltages (V) corresponding to characteristic vibrational frequencies of the molecular structures (ν) within the junction (i.e. eV = hν).
The observed conductance increases can be attributed to additional tunnelling channels arising from inelastic electron–molecule interactions (Fig. 2). The resulting phenomena give rise to a technique now known as inelastic electron tunnelling spectroscopy (IETS), and in principle the vibrational spectrum of a molecule trapped within a junction can be deduced from the interface conductivity.[4]
An energy band diagram of a molecular junction illustrating the principles of IETS. (a) Under a small negative bias at the left electrode, an electron can elastically tunnel through an idealised wire-like molecule in the gap to the right electrode giving rise to an Ohmic-like current that increases linearly with increasing bias. (b) As the bias is increased, when the tunnelling electron has sufficient energy to excite a molecular vibration (eV ≥ hν), the tunnelling electron can lose energy to this mode, opening a new inelastic tunnelling channel, and increase the tunnelling probability, leading to increased conductance. The change in tunnelling current results in a deviation of the linear I–V response, which is more readily detected as a step in the dI/dV plot or a peak in the d2I/dV2 plot.
Further early experiments carried out by Mann and Kuhn in 1971 demonstrated Simmons-like sigmoidal current–voltage responses and an exponential decay of junction current with the length in a homologous series of fatty acid salts in large area Al–monolayer–M (M═Hg, Al, Pb, Au) junctions. Together these observations are consistent with predominant contributions from electron tunnelling through the monolayer to the junction current on these structures and length scales, and provide a basis for further interpretations of the length dependent conductance of related single molecule junctions.[5]
The Aviram–Ratner rectifier proposal and Hush’s electron-transfer theory
Beyond the behaviour of molecules as simple tunnel barriers, the introduction of chemical functionality enables a more complex electrical response in molecular junctions. Most significantly, in 1974, Arieh Aviram and Mark Ratner, while at IBM and New York University, described how electrochemical processes at the molecular termini of a donor–accepter compound, coupled with an intramolecular charge transfer step, could result in current rectification across a molecular junction, and hence emulate the properties of semiconductor physics on the angstrom-molecular-length scale.[6] The Aviram–Ratner rectifier presented concepts in a form familiar to the synthetic chemical community (Fig. 3), and inspired further efforts to rationally design and synthesise molecules with some specific electrical function.
The Aviram–Ratner rectifier. Under forward bias, reduction of the acceptor (A – process i) and oxidation of the donor (D – process ii) at the respective molecule–electrode contacts is followed by intramolecular charge transfer across the saturated bridge (process iii). A substantially greater reverse bias is required to drive current in the opposite direction, giving rise to the proposed rectification behaviour.

From a chemical perspective, the behaviour of the Aviram–Ratner rectifier has usually been interpreted using Hush’s adiabatic theory of electron transfer[7] and its quantum-mechanical description of electron density. Central to this was Hush’s understanding of mixed-valence compounds and associated processes of intramolecular electron transfer,[8,9] concepts that remain important in molecular electronics to this day.[10] In 2003, Hush presented ‘An overview of the first half-century of molecular electronics’, capturing many of these ideas in that discussion.[11]
Beginnings of molecular electronics research in Australia
Research in the field of molecular electronics in Australia can perhaps be considered as commencing in the 1970s when Hush returned home from the UK, where he had commenced his academic career, rising through the ranks from Assistant Lecturer at the University of Manchester, to Lecturer and Reader in the Department of Chemistry at the University of Bristol, to found the Department of Theoretical Chemistry at the University of Sydney. With George Bacskay, Hush pioneered the application of electric fields within ab initio calculations,[12,13] inventing techniques widely applied to this day using modern computational approaches such as density-functional theory (DFT). Indeed, understanding how a molecule responds to an imposed field is one of the critical elements of molecular electronics, being only slightly perturbed at low fields while being oxidised or reduced at high ones. This theory allows these processes to be computationally modelled, permitting understanding of electric-field based measurement methods and electric-field induced chemical reactions.[14] Extended to non-equilibrium situations embodying the Landauer–Büttiker approach,[1] it leads to the non-equilibrium Green’s function (NEGF) methods[15] used universally today to study molecular devices.[15–19]
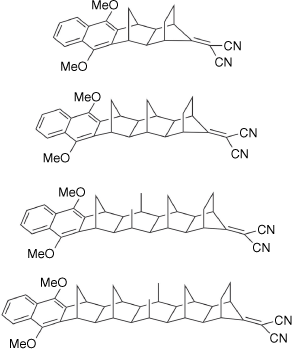
Noel Hush directly influenced a large part of the growing Australian research community, and through the 1980s he began collaborations with others that led to significant research programmes. Collaborators included Michael Paddon-Row, whose long and rigid alkanes provided the means to understand many processes in natural and artificial light-harvesting systems (Fig. 4).[20] In turn, Michael Paddon-Row went on to influence the careers of new generations of researchers with research interests at the interfaces of electrochemistry and electron transfer at interfaces and in junctions, including Justin Gooding and, through him, Nadim Darwish and Simone Ciampi.[21–24]
Examples of the Paddon-Row norbornylogous-bridged donor–acceptor compounds used in early studies of long-range charge transfer.
With Jeffrey Reimers, Noel Hush also worked on general methods for calculating molecular conductivity,[25] observable effects of applied and intrinsic electric fields,[26–29] and showed how protein electric fields controlled primary charge separation in purple bacteria.[30–32] Their student Gemma Solomon then rationalised modern IETS measurements.[33] Later, she was to depict the basis for the modern field of modulating quantum interference effects to control molecular conductivity.[34–36] Gemma travelled to Brisbane from her current position as Professor of Chemistry at The University of Copenhagen to give the opening Keynote Lecture of the RACI2022 Molecular Electronics Symposium.
A further synthetic initiative focusing on molecules for molecular electronics in Australia was commenced by Maxwell Crossley in 1987, making rigid fused-porphyrins from diones and tetraones and diamines[37,38] on a precisely controlled molecular architecture that provide extended-conjugated π-systems that may model the idealised properties of ‘molecular wires’ (Fig. 5).[39–41] Examples of linear architectures were synthesised with then student Paul Burn,[32,41] and synthetic strategies for the preparation of systems with more elaborate geometries developed,[42] allowing junction components with up to four attachment points based on a cruciform arrangement to be prepared.[40,41] Molecular switches were incorporated into the ‘wires’ to control electronic coupling,[43] as were ‘crocodile clips’ or anchor groups for attachment to gold and related electrodes.[42] Linked, linear multi-component molecular compounds with a charge-transfer gradient, designed to mimic photosynthetic reaction centres in plants, were also synthesised and show through-component uni-directional electron transfer, consistent with rectification.[44–48] Incorporation of a unidirectional barrier, in the form of a molecular switch, to through-molecule conduction was also explored giving rise to a new motif for chemically controlled rectification.[49] Molecular structures for the directed self-assembled molecular n-bit shift register memory devices were also designed,[50] although realisation of such ambitions were hampered by the technological state of molecular junctions at the time.
Early model systems from the Crossley group and collaborators, designed, synthesised and studied to explore: (a) wire-like properties; (b) connection to external sites’; (c) molecular switching; (d–f) directional electron-transfer, rectification and fundamental aspects of the photosynthetic electron-transfer cascade.

The synthetic efforts linked back through collaborative efforts with Hush, to establish structure–property relationships influencing valence orbital ordering in these porphyrinic systems,[51] while with Hush and Reimers the Crossley team extended their studies to explore the potential molecular electronic applications of these components in terms of the electronic coupling mediated by these structures.[38,52,53] To aid these explorations, different inter-porphyrin linking units were explored, and a number of linked donor–acceptor compounds synthesised and studied extensively.[45,48,54] From these fundamental studies of through molecule electronic coupling and donor–acceptor interactions, artificial light-harvesting systems were also synthesised and studied[47,48,55] that broke contemporary records for long-lived photochemical charge separation. The tetra-extended porphyrins proved very useful in developing up-converters,[56] as Timothy Schmidt and colleagues demonstrated, breaking previously perceived efficiency limitations (60 v. 11%)[57] and optimising conditions.[58] In work with Reimers, linked inter-porphyrin units also proved to be very useful for exposing fundamental shortcomings with DFT computational methods,[59] which led to new generations of approaches that today are widely successful through chemistry and physics applications involving electron transfer.[60–62]
The next generation(s)
Organic and polymer technologies
Following his PhD with Maxwell Crossley, Paul Burn went to the UK where he worked closely with physicist Richard Friend and Andrew Holmes to establish a distinguished career that developed many of the chemical structures that underpin plastic electronics and OLED emitters.[63] Subsequently, he returned to Australia to found one of Australia’s leading groups developing organic OLED,[64,65] OPV[66,67] and sensing[68,69] technologies, and spearhead the University of Queensland’s Centre for Organic Polymer Electronics (COPE). Andrew Holmes also returned to Australia, founding a parallel leading group with strong connections to CSIRO’s flexible electronics group.[70–73] These and other researchers are now significantly funded through The Australian Centre for Advanced Photovoltaics[74] and other funding provided by the Australian Renewable Energy Agency (ARENA).
Organometallic science development
The 1990s and beyond saw initiatives in studies of electron transfer chemistry from within the inorganic and organometallic communities flourish. A diverse range of bi- and poly-metallic structures were pursued to further studies of mixed-valence complexes and intramolecular electron transfer. These works are exemplified by the studies of teams including Dennis Arnold and Graeme Heath, who demonstrated the curious effects of the interplay of electronic structure and thermodynamic stability of the various members of redox products derived from carbon-rich ligand-bridged metalloporphyrins,[75,76] including highly delocalised mixed-valence derivatives with unusually small comproportionation constants (Fig. 6).[77]
Through the 2000s, Richard Keene and Deanna D’Alessandro used polypyridyl ruthenium and osmium complexes to demonstrate the influence of a wide range of fundamental structural, electronic and environmental factors on the intervalence charge transfer transition in mixed valence complexes,[78,79] including stereochemical effects,[80] ion-pairing leading to thermochromicity,[81] and spin-orbit coupling,[82,83] as well as exploring intervalence exchange processes taking place between more than two sites (Fig. 7).[84,85]
‘Wire-like’ bimetallic organometallic mixed-valence systems with carbon-rich bridging ligands were explored by Michael Bruce and Paul Low with collaborators Karine Costuas and Jean-Francois Halet at the University of Rennes, building on the Australian expertise in UV-Vis-NIR and IR spectroelectrochemical methods offered by Graeme Heath and Stephen Best to gain access to the critical spectroscopic information that underpinned those studies.[86] Later, work between Paul Low and Martin Kaupp used a combination of spectroelectrochemical measurements and DFT calculations with Hartree–Fock (HF) rich global or local-hybrid functionals to explore the role of molecular conformation and dynamics in the underlying electronic structures of these species, adding nuanced interpretations of the inter-valence charge transfer (IVCT) band envelope beyond the Hush two-state model.[87–89] In turn, these have led to a broader sphere of Australian interest in the electrical properties of molecular junctions containing metal complexes,[90] and mixed-valence solids based on metal–organic and coordination frameworks.[91]
West Australian focused initiative
A wide range of basic skills underpinning molecular electronics have now been developed, including fabrication of nanoscale gap electrodes using electromigration,[92] electrochemical[93] or lithographic[94] methods, as well as the formation of similar structures using mechanically controlled break junction (MCBJ),[95] scanning tunnelling microscopy-based break-junction (STM-BJ),[96] current distance spectroscopy (I(s))[97] or current time (telegraphic noise or blinking, I(t)) methods.[98] A focus hub for this type of technology in Australia was needed, and this was achieved through concerted efforts at UWA and Curtin. This included the acquisition of dedicated STM facilities at UWA in 2015 to underpin activities in the general area of molecular electronics, led by Paul Low, George Koutsantonis and Tom Becker, through collaboration with the team of Richard Nichols, Simon Higgins and Andrea Vezzoli at the University of Liverpool. At Curtin, the arrival of Nadim Darwish in 2016 to spearhead a concerted STM, atomic force microscopy (AFM) and STM-BJ initiative added additional capacity to the measurement teams in the West, and was further augmented by Simone Ciampi’s initiatives in single-molecule electrochemistry, molecular and semiconductor electrochemistry research, and more recently research into AFM-based triboelectric generators. This hub has produced Australian-based research addressing critical modern needs such as the integration of molecular and silicon electronics,[99,100] and the exploration of quantum circuit laws in molecular junctions.[101,102]
Latest Australian research at RACI2022
The work presented at the symposium mostly focused on basic science development, with some burgeoning industry connections aimed at new technology development, but then often mention of established technologies that embody molecular electronics such as OLED and OPV manufacture. Indeed, transferring basic science into commercial products was a thread that ran throughout the meeting. Information concerning basic scientific development, and impending or actual commercialisation, is often the easiest to obtain, as researchers in intermediary levels are often constrained by issues such as intellectual property protection and company advantage. Overall, the latest Australian research reflects significant collaboration of Australian researchers between themselves, often in different institutions, collaboration with overseas researchers, and new initiatives with Australian and international companies.
The maturity of research from basic science towards commercialisation is commonly discussed in terms of technology relevance levels (TRLs), such as those specified by the ISO 16290:2013 standard.[103] This approach presents nine levels representing the maturity of a commercalisable concept:
TRL 1 – basic principles observed
TRL 2 – technology concept formulated
TRL 3 – experimental proof of concept
TRL 4 – technology validated in laboratory
TRL 5 – technology validated in relevant environment (industrially relevant environment in the case of key enabling technologies)
TRL 6 – technology demonstrated in relevant environment (industrially relevant environment in the case of key enabling technologies)
TRL 7 – system prototype demonstration in operational environment
TRL 8 – system complete and qualified
TRL 9 – actual system proven in operational environment (competitive manufacturing in the case of key enabling technologies or in space)
In Australia, basic science spanning TRLS 1–2 is often funded by e.g. Australian Research Council (ARC) Discovery Project (DP) grants or National Medical Health and Research Council (NHMRC) Ideas Grants (IGs). Moving on to TRLs 3–5, industry partners are typically required, with funding coming from ARC Linkage Program (LP) or NHMRC Development Grants. CSIRO is often involved in projects at this level and beyond. Going beyond often involves state or federal funding directed at achievable commercial outcomes on the time scale of a few years. We consider the research presented at RACI2022 in terms of its type and connectedness, considering also apparent TRLs.
Theory
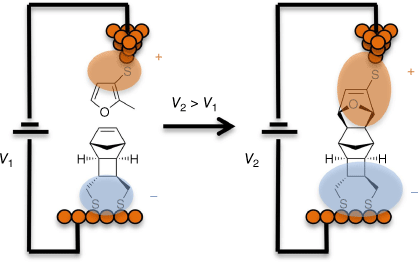
Although many recent advances in molecular electronics are based on new experimental science, modern theory also forms a key platform underpinning the science and technologies being developed in Australia. In general, theory is used to assist in developments from TRL-1 to TRL-6, ranging from developments of the fundamental concepts upon which new technologies can be created to helping with fine-tuning of properties needed to make stable, economically practical, products. Starting with TRL-1 basics, the second Keynote Speaker at the RACI2022 Molecular Electronics symposium, Michelle Coote, described advances led by chemical modelling in which complex single-molecule chemical reactions are controlled within the extraordinary electric fields generated across a molecule within a molecular junction under bias. Beyond the theoretical concept at TRL-2 to TRL-3, the phenomena of electric-field induced chemical reactions on the single-molecule level have been demonstrated through collaborative experimental and theoretical work performed by Nadim Darwish, Simone Ciampi, Gordon Wallance, Michelle Coote and colleagues (Fig. 8).[104]
An illustration of the electrostatic catalysis of a Diels–Alder reaction within a molecular junction.
At the level of basic science TRL-1 to TRL-2, similar electrostatic effects on bonding and reactivity have since then been scaled up to larger metallic electrodes,[105] demonstrated in semiconductors,[106] and even harnessed on the surface of statically charged plastics[107] and of gas bubbles.[108] An essential aspect of theoretical development is the understanding of the device physics of the assembled devices. In this way, chemical modelling becomes connected with fundamental understanding of the underlying device physics, both for scientific and technological purposes. Aiding this, a discussion of the influence of dynamic solvent–molecule interactions within a molecular junction and the influence on electrical properties were described by Daniel Kosov.[109,110] Other results from computational modelling presented by Martin Peeks at the meeting, supported by experiment, showed magnetic control of ring currents, aromaticity and persistent quantum coherence in large multi-porphyrin nanoring structures (Fig. 9).[111–113] This research also included a survey of the correlations between aromaticity, delocalisation and molecular conductance.
An example of the Peeks and Anderson butadiyne-linked porphyrin cyclic oligomers, designed to probe (anti)aromatic ring currents in molecular rings.

The computational cost of first-principles calculations often used at TRL-1 to TRL-6 may be limiting for the development of the basic science and applications of molecular electronics. Fast approaches using the DFTB electronic-structure package combined with NEGF calculations were pioneered by Gemma Solomon.[114] Improved methods for electronic-structure calculations have recently been developed by Alister Page.[115–117] These approaches have been applied e.g. to the understanding of carbon nanotubes[118] and boron nitride[116] nanotubes, practical molecules with properties underpinned by the principles of molecular electronics.
Basic science of switching and rectification, TRL-1 to TRL-3
The ability to switch molecules between different conductance states, and to emulate electrical functions such as rectification, will be central to new technological applications of molecular electronics. Many investigators at RACI2022 provided descriptions of molecular components and systems with classical electronic functions, including presentations by George Koutsantonis on the development of molecular photoswitches (Fig. 10a),[90,119] and further aspects of molecular switching phenomena based on valence tautomerisation in metal complexes by Colette Boskovic,[120] work now being done also in collaboration with Paul Low.[121] Other aspects of molecular switching within single-molecule junctions were discussed by Andrew Abell and Jingxian Yu who presented the construction of nanoscale piezoresistors based on the mechanical compression and extension of 1,1′-diethynyl-functionalised ferrocene cores within an STM-BJ (Fig. 10b).[122]
Examples of molecular switches based on: (a) photochemical processes of dihydropyrenes; and (b) mechanical stretching and compression of a 1,1′-diethynyl ferrocene.

Further examples of ferrocene-based molecular electronics, using self-assembled monolayer large-area devices, were presented by Max Roemer who described results of studies of molecular diodes carried out in the lab of Christian Nijhus from The Netherlands. These components, based on remarkably simple ferrocene complexes including mixed-valence derivatives, use remarkably precise control over molecule–electrode coupling and sequential versus direct tunnelling pathways to achieve very high ON/OFF conductance ratios (Fig. 11).[123,124] In addition, key synthetic methodologies to the ferrocene-based diodes[125] and related long-alkanoyl organics[126,127] were presented.
An example of a ferrocene-based molecular rectifier. A self-assembled film of FcCCFcC15H30SH on a Pt electrode top-contacted by eutectic gallium–indium alloy (eGaIn) to which the HOMO and HOMO-1 of the bis(ferrocenyl) headgroup are pinned. At negative (forward) bias the charge tunnels from the bis(ferrocenyl) headgroup through the alkyl tail to the Pt drain electrode. The resulting mixed-valence complex is stabilised by the proximity to the negatively charged eGaIn electrode, from which a second tunnelling step completes the circuit. Under reverse bias, as a result of orbital pinning, there are no readily accessible molecule energy levels within the bias window, and current can only flow by direct tunnelling through the entire molecular junction. The different mechanisms lead to rectification ratios of 6.3 × 105.

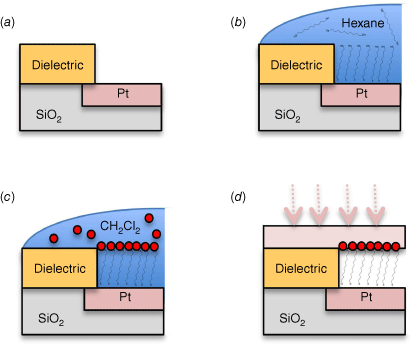
Chemical control of device design, through directed synthesis, is always paramount in molecular electronics. Insights concerning structure–property relationships governing the electrical characteristics of single-molecule junctions formed with metal complexes were presented by Masnun Naher from the Low group.[128] Koushik Venkatesan demonstrated the retention of intrinsic molecular function within large-area nanofabricated devices (Fig. 12).[129] These devices were assembled by taking advantage of a conformal layer of metallic nanoparticles on top of a self-assembled molecular monolayer to form a top-electrode contact. Subsequently, these were reinforced by direct metal evaporation, providing impetus to discussions of strategies to progress from studies of single molecule junctions towards true device applications.
The steps in Venktaesan’s hybrid device fabrication strategy: (a) Pores are etched into a dielectric film on a Pt electrode; (b) selective self-assembly of an alkanedithiol monolayer on the exposed Pt surface; (c) solution deposition of metallic nanoparticles on the exposed self-assembled monolayer (SAM) top-surface; (d) thermal evaporation of the top-contact electrode.
New applications foci: to TRL-3 and beyond
Modern scientific advances have driven a considerable resurgence in molecular materials science, indicating the critical role that molecular electronics plays as an enabling science underpinning modern technologies. Indeed, much of the discussion at RACI2022 focused on molecular materials and their applications.
Prashant Sonar provided a summary of self-organised arrays of carbon-dot emitters (derived from human hair as a rather unexpected source of bio-waste!) for OLED applications.[130] For sensing applications, he also described organic field-effect transistor design strategies.[131]
Andrew Abell presented means of establishing peptide-based biomolecular electronics (Fig. 13).[132] He also described the use of modified electrodes to permit electrochemical transduction of peptide–protein interactions to demonstrate the power of hybrid systems for biosensing.[133] In a similar vein, Matthew Griffith described techniques for biocompatible electronics.[134]
Molecular junctions containing a thiol-modified peptide; calculated conductance values increase significantly upon coordination by zinc ions as a result of the increased electron density brought by the metal ion into the molecular backbone.

Agustin Schiffrin described the use of optical waves to induce rapid switch-on (~1 fs) of conductance properties in a dielectric insulator, which holds promise for high speed data processing.[135] His current research within the ARC Centre of Excellence in Future Low-Energy Electronics Technologies deals with the synthesis of low-dimensional (i.e. 1-D, 2-D) organic and metal–organic nanostructures and nanometarials with tailored electronic and optoelectronic properties (e.g. 1-D spin-crossover networks,[136] 2-D correlated-electron metal–organic frameworks, field-induced chemical switches[137]). For this purpose, he employs methods of supramolecular chemistry on solid surfaces, with atomic-scale structural and electronic characterisation by low-temperature scanning probe microscopy techniques.
Finding new ways to extract usable energy from waste is at the forefront of modern research, with applications ranging from local energy generation, to nanoscale on-site energy production, to applications in the burgeoning space industry. Integrating electronic and materials design concepts for energy applications therefore becomes critical. Building on the fundamental aspects of the molecule–electrode interface in molecular electronic science, Matthew Griffith described design strategies to improve charge generation by controlling molecular composition[138] and then morphology of organic semiconductor nanoparticles for solar cell applications (Fig. 14),[139] while Zhigang Chen described work on the detection and utilisation of thermal gradients in thermoelectric materials.[140] This includes the possibility of using flexible materials,[141] as well as high-efficiency devices.[142]
Griffith’s (a) polymer donors and (b) molecular and (c) polymer non-fullerene acceptors (NFAs) used in the construction of organic semiconductor nanoparticles of controlled composition and morphology.
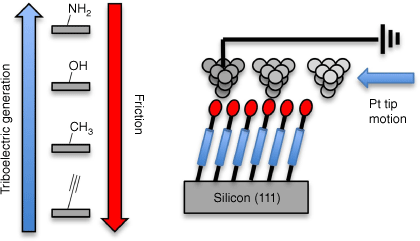
Simone Ciampi presented the use of molecularly modified Si surfaces for triboelectric power generation at the nanoscale (Fig. 15).[143] Substrate doping, monolayer surface chemistry and substrate surface shapes were found to be important for efficient power generation,[144] with many observations defying initial intuition, such as the greater influence of substrate doping on the generated triboelectric currents than chemically induced changes in adhesive forces between the monolayer and the dragging tip, thus providing significant challenges for basic science.
The motion of a Pt tip over an alkyl monolayer modified silicon substrate generates a triboelectric current, the magnitude of which is sensitive to both the monolayer chemistry and the substrate doping.
Koushik Venkatesan presented new conceptual design approaches (Fig. 16) to the design of deep-blue emitting n-heterocyclic carbene (NHC) based platinum(II) bisalkyne systems and the first proof of concept OLED device based on NHC gold(III) cyclometalated molecular systems. These new design principles provide new avenues to solve the significant challenges in OLED technology.[145–149]
Venkatesan’s AuIII-based emitters. The strongly s-donating carbene is introduced to raise low-lying dd states and the LUMO, in order to reduce non-radiative decay and blue-shift the emission, whereas the chelating dianionic ancillary ligand increases rigidity of the molecular structure to further reduce non-radiative decay rates and improve the thermal stability of the resulting complexes.

Australian work in communities underpinned by molecular electronics also often takes a strong applications focus. In particular, New South Wales already has a Smart Sensing Network (see https://www.nssn.org.au/) that involves researchers in molecular electronics including Matthew Griffith and Justin Gooding. There also exists a national Centre of Excellence in Exciton Science (see https://excitonscience.com/), including Jared Cole, Alison Funston, Ken Ghiggino, Christopher Hall, James Hutchison, Jacek Jasieniak, Girish Lakhwani, Dane McCamey, Paul Mulvaney, Salvy Russo, Timothy Schmidt, Trevor Smith, Asaph Widmar-Cooper and Wallace Wong, making solar cells,[70,150–152] upconverters,[57,58,153] solar concentrators,[154] optically switchable conductors,[155] etc. Spin physics is an area that can also be closely related to molecular electronics, with overlap with the exciton community and OPV community coming from Murad Tayebjee and Dane McCamey’s use of magnetic resonance approaches to be the first to observe spin quintets in closed shell molecular dyads which are designed to boost solar cell currents (Fig. 17).[156–158]
Multidisciplinary research
Multidisciplinary work at TRL-1 to TRL-2 has always been at the forefront in molecular electronics, and as indicated in parts above, many recent Australian examples arise from well-developed and productive collaborative teams that bring diverse skills and methods to bear on significant problems. These partnerships include:
The development of technologies for embedding single-molecule aspects into standard silicon-chip manufacture, achieved by Nadim Darwish, Simone Ciampi, Jeffrey Reimers and Daniel Kosov.[159,160]
Paul Low and George Koutsantonis with the leading experimental and computational groups of Richard Nichols, Simon Higgins and Andrea Vezzoli at the University of Liverpool, UK, and Colin Lambert at Lancaster University, UK.[119,128]
Teams formed between the Low group and Wenjing Hong’s team at Xiamen University, China, and Martin Kaupp’s group at Technische Universitat Berlin to explore the effects of the position and conformation of pendant groups on interference effects in molecular junctions[161] and mixed-valence complexes[162] respectively.
Collaborative studies of methods for the assembly of ‘top-contact’ electrodes and electrical characteristics of large area junctions by the Low group and the Cea and Martin groups at the University of Zaragoza.[163]
Colette Boskovic with leading computational chemist Lars Goerigk.[164]
Max Roemer and George Koutsantonis with world-renowned nanotechnologist Christian Nijhuis at the University of Twente,[123,124] including the additional Australian collaborators Nadim Darwish and Simone Ciampi.[165,166]
Extensive national and international collaborations involving Agustin Schiffrin, including Udo Bach, Michael Fuhrer,[136] Jennifer MacLeod and Chris Ritchie, concerning synthesis, as well as Jared Cole, Nikhil Medhekar,[136,137,167] Ben Powell and Muhammad Usman[168,169] concerning theory and computation.
Current Australian capabilities
The significant body of Australian research described above has resulted in a critical mass of expertise in experimental synthesis and measurement capacity, computational capability and theoretical modelling directed towards studies of intramolecular charge transfer and charge transport in molecular junctions, light absorption and emission, exciton transport, etc. Embracing TRL-1 to TRL-6, Australia therefore has significant capacity in terms of both the basic science underpinning molecular electronics and in the creation of new devices based on this science. These capabilities are summarised in Tables 1–7 listing the Australian institution, contact personnel, capabilities, sources of external funding and key references. Beyond these, lies a wider body of the Australian national research effort that draws on the concepts of molecular electronics and associated areas of science and technology, exemplified by the substantial programs in organic polymer electronics[64–69] and molecular materials with large non-linear optical response.[170]
| Location and contact | Capability | External funding sources | References |
|---|---|---|---|
| Curtin, Simone Ciampi | Conductive AFM and macroscopic junctions | ARC | [143,144,171] |
| Curtin, Simone Ciampi | Carriers life-time semiconductor AFM measurements | ARC | [143] |
| Curtin, Nadim Darwish | STM break junction and electrochemical STM break junction | ARC | [24,100,159,166,172] |
| Monash, Agustin Schiffrin | Low-temperature (4 K) STM, STS, ncAFM | [137,167–169,173,174] | |
| UWA, Paul Low | STM break-junction | ARC | [101,102,128,175] |
| UWA, Paul Low | Top-contact metallation strategies | ARC | [163,176–178] |
| Location and contact | Capability | External Funding sources | References |
|---|---|---|---|
| Curtin, Simone Ciampi | Electrochemistry coupled to single photon Counting and fluorescence microscopy | ARC | [108,179] |
| Macquarie, Koushik Venkatesan | Absorption and photoluminescence spectroscopy | [146–149] | |
| Curtin, Nadim Darwish | Magnetic force microscopy | ||
| Melbourne, Trevor Smith, Ken Ghiggino, Christopher Hall | Femtosecond transient spectroscopy (UV/Vis, NIR, IR) | ARC | [180,181] |
| Melbourne, Trevor Smith | Time-correlated Single photon counting (TCSPC) | ARC | [182,183] |
| Melbourne, Trevor Smith | Fluorescence lifetime imaging microscopy (FLIM) | ARC | [184,185] |
| Melbourne, Trevor Smith | Photocurrent microspectrosopy | ARC | [186] |
| Melbourne, Trevor Smith, Ken Ghiggino | Nanosecond-gated spectroscopy | ARC | [187] |
| Melbourne, Trevor Smith | Single molecule fluorescence spectroscopy, polarised, total internal reflection, super-resolution | ARC | [188–190] |
| Monash, Agustin Schiffrin | Wavelength-tunable (650–15 000 nm) femtosecond laser for pump-probe spectroscopy and THz time-domain spectroscopy | ARC | |
| UNSW, Timothy Schmidt | Femtosecond and nanosecond transient absorption and photoluminescence spectroscopy | ARC | [58,191–195] |
| ARENA | |||
| UNSW, Timothy Schmidt | Time-correlated single-photon counting | ARC | [191] |
| ARENA | |||
| UNSW, Timothy Schmidt | Magnetic field effects on photoluminescence | ARC | [58,192] |
| ARENA | |||
| UNSW, Murad Tayebjee | Femtosecond stimulated Raman and transient absorption spectroscopies, time-resolved THz spectroscopy and magnetic-field variants of the above | ARC | |
| ARENA | |||
| UWA, Paul Low | UV-Vis-NIR/IR spectroelectrochemistry | ARC | [89,162,196–198] |
| Location and contact | Capability | External funding sources | References |
|---|---|---|---|
| Adelaide, Andrew Abell, Jingxian Yu | Linear peptides, constrained peptides and photoswitchable peptides | ARC | [199,200] |
| Curtin, Nadim Darwish, Simone Ciampi | Nanomaterials, including 2-D, electrosynthesis | ARC | [108,201–204] |
| Macquarie, Koushik Venkatesan | Small molecules and conjugated polymer synthesis with redox and luminescence properties, biosensors, large area device fabrication, sensor and OLED device fabrication | ARC | [146–149] |
| SNSF | |||
| Melbourne, Colette Boskovic | Switchable metal complexes, redox-active ligands | ARC | [121,205–209] |
| Melbourne, David Jones, Wallace Wong | Organic semiconductors including dyes and conjugated polymers | ARC | [70,71,153,154,210–214] |
| ARENA | |||
| Monash, Agustin Schiffrin | Nanomaterial synthesis (including organic and metal-organic) by molecular beam epitaxy | [137,167–169,173,174] | |
| Sydney, Maxwell Crossley | Small molecule synthesis, particularly porphyrins, designed for functionality as molecular switches, connectors, light harvesting, upconversion, photonics and sensing | ARC | [37–43,45,47,48,51–59] |
| [215] | |||
| Sydney, Matthew Griffith | Nanomaterial synthesis (organic and composite blends) by miniemulsion ultrasonification | ARC | [139,216–218] |
| NHMRC | |||
| UWA, Paul Low, George Koutsantonis | Small molecule synthesis, conjugated carbon-rich molecules and materials, organic and organometallic compounds and complexes, anchor groups and strategies | ARC | [102,219–222] |
| Various |
| Location and contact | Capability | External funding sources | References |
|---|---|---|---|
| Adelaide, Jingxian Yu | Computational modelling of the conductivity and spectroscopy of molecules and single-molecule junctions | NCI | [122,199,200] |
| Adelaide, Jingxian Yu | Electron transfer kinetics and dynamics in peptides | [199,200,223] | |
| Curtin, Simone Ciampi | Digital simulations of electrochemical systems | ARC | [107] |
| Flinders, Michelle Coote | Design of molecules with switchable spin states, conductivity and spectroscopy. Computational modelling of the reactivity, conductivity, aromaticity and spectroscopy of molecules and polymers | ARC NCI | [104,224,225–228] |
| James Cook, Daniel Kosov | Modelling of noise in nanoscopic devices | [229–238] | |
| James Cook, Daniel Kosov | Alternating currents in nanoscopic devices | [239–241] | |
| James Cook, Daniel Kosov | Effects of current on the natures of molecular junctions, including non-adiabatic effects | [242–247] | |
| James Cook, Daniel Kosov | Current-induced chemical reactions in junctions and solvent control | [110,248–250] | |
| James Cook, Daniel Kosov | First principles NEGF calculations of conductivity in nanoscopic devices | [160,251–253] | |
| Newcastle, Alister Page | Computational modelling of structure and growth of nanomaterials, CVD processes, condensed phases and interfaces | ARC | [115–118,254] |
| Machine learning and computational method development | NCI | ||
| Newcastle, Alister Page | Machine learning and computational method development | ARC | [255,256] |
| NCI | |||
| UTS, Jeffrey Reimers | Computational modelling of the conductivity and spectroscopy of molecules, self-assembled monolayers, materials and molecular junctions | NCI | [14,31,48,159,160,251,257–259] |
| ARC | |||
| UTS, Jeffrey Reimers | Theory development for molecular devices | NCI | [14,25,33,114,260–263] |
| ARC |
| Location and contact | Capability | External funding sources | References |
|---|---|---|---|
| Adelaide, Jingxian Yu | Electrochemical impedance spectroscopy | ARC | [133] |
| Curtin, Simone Ciampi | Attoamper current, dielectric, lock-in techniques, charge measurements (down to picocoulomb), life-time semiconductor charge carriers measurements | ARC | [107,143,264,265] |
| Curtin, Nadim Darwish, Simone Ciampi | Electrochemical impedance spectroscopy | ARC | [203,266] |
| Macquarie, Koushik Venkatesan | Luminescent biosensors | SNSF | [267] |
| Sydney, Maxwell Crossley | Molecular biosensors | [268–271] | |
| Sydney, Matthew Griffith | Patch clamp electrophysiology, electrochemical impedance spectroscopy | ARC, NHMRC | [134,272–274] |
| Location and contact | Capability | External funding sources | References |
|---|---|---|---|
| Curtin, Nadim Darwish | Silicon passivation | ARC | [275,276] |
| Melbourne, Colette Boskovic | SQUID magnetometer equipped with UV-Vis photomagnetism capabilities | ARC LIEF | |
| UniMelb, David Jones, Wallace Wong | Solution processed solar cell fabrication and characterisation | ARC, ARENA | [70,71,153,154,210–214] |
| Sydney, Matthew Griffith | Device fabrication (nitrogen glovebox, spin coater, thermal evaporator, SonoPlot inkjet printer, roll-to-toll printing) | ARC, NHMRC | [276–281] |
| Sydney, Matthew Griffith | Steady state characterisation (current–voltage under calibrated solar simulator, external quantum efficiency measurements) | ARC, NHMRC | [138,282–284] |
| Sydney, Matthew Griffith | Transient electrical characterisation (charge carrier lifetime (transient photovoltage), charge carrier mobility (transient photocurrent, photoCELIV), carrier density measurement, capacitance–voltage | ARC, NHMRC | [73,285–288] |
Tables 1–4 focus on general issues such as the experimental and conceptual capabilities that, in principle, could be put to use in manifold applications. Table 1 pertains to single-molecule device and large-area junction fabrication and measurement capability, for which significant capabilities exist at Curtin, Monash and UWA.
Table 2 pertains to capabilities in spectroscopic measurements. These are always relevant in terms of characterising molecular devices, but can take on greater significance if the device of interest involves absorption or emission of light. Thus this table includes overlap between molecular electronics capabilities and related ones pertinent to OPV, OLEDs, exciton science, etc., with the table entries not fully reflecting the full scope of these broader communities. Available capabilities include a wide range of picosecond or femtosecond time-resolved measurements, single-photon measurements, and various microscopies and imaging methods. Significant capabilities of particular relevance to aspects of molecular electronics research are identified in Curtin, Macquarie, Melbourne, Monash, UNSW and UWA.
As George Koutsantonis has been known to observe, without molecules there cannot be molecular electronics, and so at the heart of the field is the ability to synthesise pertinent molecules; identified relevant synthetic capabilities are listed in Table 3. Particular expertise includes the synthetic chemistry of peptides, nanomaterials, metal complexes, organic functional units including switches and connectors, and light absorbers, emitters and upconverters. Capabilities are identified at: Adelaide, Curtin, Macquarie, Melbourne, Monash, Sydney and UWA.
Table 4 pertains to computational and theoretical capabilities identified as prioritising molecular electronics outcomes. Capabilities range from basic research into the theory of molecular junctions, to their detailed simulation, to properties simulations, to design of new devices and experiments. Capabilities are identified at: Adelaide, Curtin, Flinders, James Cook, Newcastle and UTS.
The final three tables consider molecular electronics applications being developed into new technologies. Table 5 is for sensing technologies that apply to both single-molecule detection and detection of small molecular layers. This can include detection of currents down to the attoampere level (tens of electrons per second), capacitive charging down to the picocoulomb level, electrochemical impedance spectroscopy, patch-clamp electrophysiology and molecular biosensor construction. Applications are being developed at: Adelaide, Curtin, Macquarie and Sydney.
Use of molecular electronics concepts is also being applied to the development of PV and OLED technologies, as exampled in Table 6. Applications include silicon passivation, SQUID magnetometry, device fabrication by inkjet printing and device testing, with development happening at Curtin, Melbourne and Sydney.
Finally, Table 7 pertains to novel methods of power generation at the nanometre level, possibly for powering nanoscale electronic circuitry. This is currently only being developed at Curtin, but many possibilities pertain for the future.
Desired new capabilities
Device-oriented research, up to TRL-6, generally requires access to a range of techniques, often preferably located in the one facility. Some conceivable products, and the needed technologies, are listed in Table 8. To empower these are other technologies, based on Tables 1–7, and missing capabilities needed to further advance molecular electronics development in Australia are identified to be:
There is no mechanically controlled break-junction (MCBJ) capacity in Australia. This is an alternate route to single-molecule measurements that would add to, or else complement, existing STM-based single-molecule break-junction facilities in Western Australia.
Similarly, there is no electromigration capacity in Australia that could be used to fabricate nanogaps and place molecules within them.
A nanofabrication platform in which new molecular electronic devices could be developed is needed. This would involve ‘large area’ junctions and top electrode deposition, and electromigration–lithography nanogap formation. This would complement existing facilities and any new MCBJ or electromigration capacity. Applications could include: thermoelectrics, triboelectrics, single molecule sensing to open up applications in security threat detection and mitigation, personalised health, omics, single molecule reactions, biodegradable or green electronics, biosensors, etc.
A dedicated electron-beam lithography facility for characterisation of fabricated devices, as well as for device fabrication. For fabrication, a unit running at 150 keV intended to burn line of size <4 nm would be appropriate.
A cryogenic-temperature fabrication and characterisation facility. There are several low-temperature STMs in Australia, but these are for dedicated purposes. To focus on molecular electronics requires an ultra-high vacuum (UHV) chamber with: (i) tools of molecular beam epitaxy for deposition and material growth from the gas phase, (ii) electrospray ionisation source for compounds such as large molecules that cannot be sublimated, (iii) load-lock and UHV sample transfer suitcase for loading ex situ grown materials coupled to another UHV chamber for characterisation (e.g. low-temperature STM–STS–ncAFM–electron transport including applied magnetic field) could open the door to a large variety of samples and avoid compatibility or contamination issues.
| Desired products | Fabrication technology | Gate control | Chip integration |
|---|---|---|---|
| Molecular transistor, integrated circuit, sensors | Mechanically controllable break junction | Electrostatic | Yes |
| Electromigration nanogap | Electrostatic | Yes | |
| Electrochemical deposition | Electrostatic | Yes | |
| Nanoimprint lithographyA | Electrostatic | Yes | |
| Self-aligned lithographyA | Electrostatic | Yes | |
| On-wire lithographyA | Electrostatic | Yes | |
| Molecular memory | Crosswire or crossbar | Not possible | Yes |
| General devices | Scanning probe microscopy | Electrochemical | No |
| Surface-diffusion-mediated deposition | Not possible | No | |
| Lift-and-float approach | Not possible | No | |
| Nanopore or nanowell | Not possible | Yes | |
| Buffer interlayer-based junction | Not possible | Yes | |
| On-edge molecular junction | Not possible | No |
AEspecially suited for sensor applications.
Outlook
Molecular electronics has advanced a long way from its inception and the early dreams that it could provide an alternative to silicon semiconductor electronics. The control of charge between two electrodes mediated by a molecular structure is intrinsically a phenomenon that occurs on the nanoscale or smaller, and hence represents the ultimate in miniaturisation. Most possibilities would still appear yet to be explored. Current work explores sensors, biosensors, power generation, light emission, molecular-scale control logic, but much more can be envisaged.
The expanding horizons of Australian molecular electronics are highlighted by the appointment of Nobel Laureate Professor Sir Fraser Stoddart to a position in the School of Chemistry at UNSW. Already engaged in battery research with UNSW’s Dong-Jun Kim,[289,290] he brings access to his current world-leading advances in molecular electronics.[291–293]
More broadly, the transformation of molecular electronics from scientific curiosity to present reality is witnessed by the rise of companies such as Roswell Biotech, merging proteomics and sensing technologies with molecular electronics to achieve unprecedented results (see https://www.roswellbiotech.com/). Australia is ideally suited for the development of this type of game-changing industry.
References
1 Landauer R. IBM J Res Dev 1957; 1: 223-231.
| Crossref |
2 Büttiker M, Imry Y, Landauer R, Pinhas S. Phys Rev B 1985; 31: 6207-6215.
| Crossref |
4 Jaklevic RC, Lambe J. Phys Rev Lett 1966; 17: 1139-1140.
| Crossref |
5 Mann B, Kuhn H, Szentpály L. Chem Phys Lett 1971; 8: 82-84.
| Crossref |
6 Aviram A, Ratner MA. Chem Phys Lett 1974; 29: 277-283.
| Crossref |
7 Hush NS. J Chem Phys 1958; 28: 962-972.
| Crossref |
9 Hush NS. Chem Phys 1975; 10: 361-366.
| Crossref |
11 Hush NS. Ann N Y Acad Sci 2003; 1006: 1-20.
| Crossref |
12 Bacskay GB, Hush NS. Theor Chim Acta 1974; 32: 311-320.
| Crossref |
13 Gready JE, Bacskay GB, Hush NS. Chem Phys 1977; 22: 141-150.
| Crossref |
14 Hush NS, Wong AT, Bacskay GB, Reimers JR. J Am Chem Soc 1990; 112: 4192-4197.
| Crossref |
15 Taylor J, Guo H, Wang J. Phys Rev B 2001; 63: 245407.
| Crossref |
16 Rocha AR, García-Suárez VM, Bailey S, Lambert C, Ferrer J, Sanvito S. Phys Rev B 2006; 73: 085414.
| Crossref |
17 Ferrer J, Lambert CJ, García-Suárez VM, Manrique DZ, Visontai D, Oroszlany L, Rodríguez-Ferradás R, Grace I, Bailey SWD, Gillemot K, Sadeghi H, Algharagholy LA. New J Phys 2014; 16: 093029.
| Crossref |
18 Michaud-Rioux V, Zhang L, Guo H. J Comput Phys 2016; 307: 593-613.
| Crossref |
19 Brandbyge M, Mozos J-L, Ordejón P, Taylor J, Stokbro K. Phys Rev B 2002; 65: 165401.
| Crossref |
20 Warman JM, De Haas MP, Paddon-Row MN, Cotsaris E, Hush NS, Oevering H, Verhoeven JW. Nature 1986; 320: 615-616.
| Crossref |
21 Gooding JJ, Ciampi S. Chem Soc Rev 2011; 40: 2704-2718.
| Crossref |
22 Darwish N, Eggers PK, Ciampi S, Tong Y, Ye S, Paddon-Row MN, Gooding JJ. J Am Chem Soc 2012; 134: 18401-18409.
| Crossref |
23 Darwish N, Díez-Pérez I, Da Silva P, Tao N, Gooding JJ, Paddon-Row MN. Angew Chem Int Ed 2012; 51: 3203-3206.
| Crossref |
24 Darwish N, Díez-Pérez I, Guo S, Tao N, Gooding JJ, Paddon-Row MN. J Phys Chem C 2012; 116: 21093-21097.
| Crossref |
25 Hall LE, Reimers JR, Hush NS, Silverbrook K. J Chem Phys 2000; 112: 1510-1521.
| Crossref |
26 Reimers JR, Hush NS. Electric field perturbation of electronic (vibronic) absorption envelopes: application to characterization of mixed-valence states. In: Prassides K, editor. Mixed Valency Systems: Applications in Chemistry, Physics and Biology. Dordrecht, Netherlands: Kluwer Acad. Publishers; 1991. pp. 29–50. 10.1007/978-94-011-3606-8
27 Hush NS, Reimers JR. J Phys Chem 1995; 99: 15798-15805.
| Crossref |
28 Reimers JR, Zeng J, Hush NS. J Phys Chem 1996; 100: 1498-1504.
| Crossref |
29 Reimers JR, Hush NS. J Phys Chem A 1999; 103: 10580-10587.
| Crossref |
30 Reimers JR, Hughes JM, Hush NS. Biochemistry 2000; 39: 16185-16189.
| Crossref |
31 Reimers JR, Hush NS. J Am Chem Soc 2004; 126: 4132-4144.
| Crossref |
32 Kanchanawong P, Dahlbom MG, Treynor TP, Reimers JR, Hush NS, Boxer SG. J Phys Chem B 2006; 110: 18688-18702.
| Crossref |
33 Solomon GC, Gagliardi A, Pecchia A, Frauenheim T, Di Carlo A, Reimers JR, Hush NS. J Chem Phys 2006; 124: 094704.
| Crossref |
34 Solomon GC, Andrews DQ, Goldsmith RH, Hansen T, Wasielewski MR, Van Duyne RP, Ratner MA. J Am Chem Soc 2008; 130: 17301-17308.
| Crossref |
35 Solomon GC, Andrews DQ, Hansen T, Goldsmith RH, Wasielewski MR, Van Duyne RP, Ratner MA. J Chem Phys 2008; 129: 054701.
| Crossref |
36 Solomon GC, Herrmann C, Hansen T, Mujica V, Ratner MA. Nat Chem 2010; 2: 223-228.
| Crossref |
37 Crossley MJ, Burn PL. J Chem Soc Chem Commun 1987; 1987(1): 39-40.
| Crossref |
38 Reimers JR, Lü TX, Crossley MJ, Hush NS. Chem Phys Lett 1996; 256: 353-359.
| Crossref |
39 Crossley MJ, Burn PL. J Chem Soc Chem Commun 1991; 1991(21): 1569-1571.
| Crossref |
40 Khoury T, Crossley MJ. Chem Commun 2007; 2007(46): 4851-4853.
| Crossref |
41 Crossley MJ, Burn PL, Langford SJ, Prashar JK. J Chem Soc Chem Commun 1995; 1995(18): 1921-1923.
| Crossref |
42 Crossley MJ, Govenlock LJ, Prashar JK. J Chem Soc Chem Commun 1995; 1995(23): 2379-2380.
| Crossref |
43 Crossley MJ, Johnston LA. Chem Commun 2002; 2002(10): 1122-1123.
| Crossref |
44 Crossley MJ, Sintic PJ, Walton R, Reimers JR. Org Biomol Chem 2003; 1: 2777-2787.
| Crossref |
45 Crossley MJ, Sintic PJ, Hutchison JA, Ghiggino KP. Org Biomol Chem 2005; 3: 852-865.
| Crossref |
46 Ohkubo K, Sintic PJ, Tkachenko NV, Lemmetyinen H, Wenbo E, Ou Z, Shao J, Kadish KM, Crossley MJ, Fukuzumi S. Chem Phys 2006; 326: 3-14.
| Crossref |
47 Lee S-H, Blake IM, Larsen AG, McDonald JA, Ohkubo K, Fukuzumi S, Reimers JR, Crossley MJ. Chem Sci 2016; 7: 6534-6550.
| Crossref |
48 Lee S-H, Larsen AG, Ohkubo K, Cai Z-L, Reimers JR, Fukuzumi S, Crossley MJ. Chem Sci 2012; 3: 257-269.
| Crossref |
49 Wendo E, Kadish KM, Sintic PJ, Khoury T, Govenlock LJ, Ou Z, Shao J, Ohkubo K, Reimers JR, Fukuzumi S, Crossley MJ. J Phys Chem A 2008; 112: 556-570.
| Crossref |
50 Lambropoulos NA, Reimers JR, Crossley MJ, Hush NS, Silverbrook K. Nanotechnology 2013; 24: 505202.
| Crossref |
51 Binstead RA, Crossley MJ, Hush NS. Inorg Chem 1991; 30: 1259-1264.
| Crossref |
52 Reimers JR, Hall LE, Crossley MJ, Hush NS. J Phys Chem A 1999; 103: 4385-4397.
| Crossref |
53 Lü TX, Reimers JR, Crossley MJ, Hush NS. J Phys Chem 1994; 98: 11878-11884.
| Crossref |
54 Wong WWH, Khoury T, Vak D, Yan C, Jones DJ, Crossley MJ, Holmes AB. J Mater Chem 2010; 20: 7005-7014.
| Crossref |
55 Larsen J, Brüggemann B, Polívka T, Sundström V, Åkesson E, Sly J, Crossley MJ. J Phys Chem A 2005; 109: 10654-10662.
| Crossref |
56 Cheng YY, Fückel B, MacQueen RW, Khoury T, Clady RGCR, Schulze TF, Ekins-Daukes NJ, Crossley MJ, Stannowski B, Lips K, Schmidt TW. Energy Environ Sci 2012; 5: 6953-6959.
| Crossref |
57 Cheng YY, Khoury T, Clady RGCR, Tayebjee MJY, Ekins-Daukes NJ, Crossley MJ, Schmidt TW. Phys Chem Chem Phys 2010; 12: 66-71.
| Crossref |
58 Dover CB, Gallaher JK, Frazer L, Tapping PC, Petty AJ, Crossley MJ, Anthony JE, Kee TW, Schmidt TW. Nat Chem 2018; 10: 305-310.
| Crossref |
59 Sendt K, Johnston LA, Hough WA, Crossley MJ, Hush NS, Reimers JR. J Am Chem Soc 2002; 124: 9299-9309.
| Crossref |
60 Li M, Kobayashi R, Amos RD, Ford MJ, Reimers JR. Chem Sci 2022; 13: 1492-1503.
| Crossref |
61 Reimers JR, Cai Z-L, Kobayashi R, Rätsep M, Freiberg A, Krausz E. Sci Rep 2013; 3: 2761.
| Crossref |
62 Cai Z-L, Crossley MJ, Reimers JR, Kobayashi R, Amos RD. J Phys Chem B 2006; 110: 15624-15632.
| Crossref |
63 Burn PL, Holmes AB, Kraft A, Bradley DDC, Brown AR, Friend RH, Gymer RW. Nature 1992; 356: 47-49.
| Crossref |
64 Lo SC, Burn PL. Chem Rev 2007; 107: 1097-1116.
| Crossref |
65 Burn PL, Lo SC, Samuel IDW. Adv Mater 2007; 19: 1675-1688.
| Crossref |
66 Lin Q, Armin A, Nagiri RCR, Burn PL, Meredith P. Nat Photon 2015; 9: 106-112.
| Crossref |
67 Stolterfoht M, Wolff CM, Márquez JA, Zhang S, Hages CJ, Rothhardt D, Albrecht S, Burn PL, Meredith P, Unold T, Neher D. Nat Energy 2018; 3: 847-854.
| Crossref |
68 Jansen-van Vuuren RD, Armin A, Pandey AK, Burn PL, Meredith P. Adv Mater 2016; 28: 4766-4802.
| Crossref |
69 Lin Q, Armin A, Burn PL, Meredith P. Nat Photon 2015; 9: 687-694.
| Crossref |
70 Sun K, Xiao Z, Lu S, Zajaczkowski W, Pisula W, Hanssen E, White JM, Williamson RM, Subbiah J, Ouyang J, Holmes AB, Wong WWH, Jones DJ. Nat Commun 2015; 6: 6013.
| Crossref |
71 Wong WWH, Banal JL, Geraghty PB, Hong Q, Zhang B, Holmes AB, Jones DJ. Chem Rec 2015; 15: 1006-1020.
| Crossref |
72 Nicolaidis NC, Hollott PV, Stanwell B, Gill IA, Bull JE, Bentsen S, Iredale J, Pappenfus TM, Dastoor PC, Feron K, Griffith MJ, Holmes NP. J Chem Educ 2020; 97: 3751-3757.
| Crossref |
73 Posar JA, Davis J, Alnaghy S, Wilkinson D, Cottam S, Lee DM, Thompson KL, Holmes NP, Barr M, Fahy A, Nicolaidis NC, Louie F, Fraboni B, Sellin PJ, Lerch MLF, Rosenfeld AB, Petasecca M, Griffith MJ. Adv Mater Technol 2021; 6: 2001298.
| Crossref |
74 Corkish R, Green MA, Blakers AW, Burn PL, Cheng Y-B, Egan R, Ghiggino KP, Meredith P, Scholes FH, Wilson G. Mater Res Soc Symp Proc 2015; 1771: 33-44.
| Crossref |
75 Arnold DP, Heath GA. J Am Chem Soc 1993; 115: 12197-12198.
| Crossref |
76 Arnold DP, Hartnell RD, Heath GA, Newby L, Webster RD. Chem Commun 2002; 2: 754-755.
| Crossref |
77 Arnold DP, Heath GA, James DA. J Porphyr Phthalocyanines 1999; 3: 5-31.
| Crossref |
78 D’Alessandro DM, Keene FR. Chem Rev 2006; 106: 2270-2298.
| Crossref |
79 D’Alessandro DM, Keene FR. Chem Soc Rev 2006; 35: 424-440.
| Crossref |
80 D’Alessandro DM, Keene FR. Pure Appl Chem 2008; 80: 1-16.
| Crossref |
81 D’Alessandro DM, Junk PC, Richard Keene F. Supramol Chem 2005; 17: 529-542.
| Crossref |
82 D’Alessandro DM, Dinolfo PH, Davies MS, Hupp JT, Keene FR. Inorg Chem 2006; 45: 3261-3274.
| Crossref |
83 D’Alessandro DM, Dinolfo PH, Davies MS, Hupp JT, Keene FR. Inorg Chem 2006; 45: 4576.
| Crossref |
84 D’Alessandro DM, Richard Keene F. Dalton Trans 2006; 2006(8): 1060-1072.
| Crossref |
85 D’Alessandro DM, Davies MS, Keene FR. Inorg Chem 2006; 45: 1656-1666.
| Crossref |
86 Bruce MI, Low PJ, Costuas K, Halet JF, Best SP, Heath GA. J Am Chem Soc 2000; 122: 1949-1962.
| Crossref |
87 Parthey M, Gluyas JBG, Schauer PA, Yufit DS, Howard JAK, Kaupp M, Low PJ. Chem Eur J 2013; 19: 9780-9784.
| Crossref |
88 Parthey M, Gluyas JBG, Fox MA, Low PJ, Kaupp M. Chem Eur J 2014; 20: 6895-6908.
| Crossref |
89 Gluyas JBG, Gückel S, Kaupp M, Low PJ. Chem Eur J 2016; 22: 16138-16146.
| Crossref |
90 Naher M, Roemer M, Koutsantonis GA, Low PJ. 9.03 – Metal complexes for molecular electronics. In: Constable EC, Parkin G, Que Jr L, editors. Comprehensive Coordination Chemistry III. Elsevier; 2021. pp. 38–80. 10.1016/B978-0-12-409547-2.14952-2
91 Murase R, Leong CF, D’Alessandro DM. Inorg Chem 2017; 56: 14373-14382.
| Crossref |
92 Park H, Lim AKL, Alivisatos AP, Park J, McEuen PL. Appl Phys Lett 1999; 75: 301-303.
| Crossref |
93 He HX, Boussaad S, Xu BQ, Li CZ, Tao NJ. J Electroanal Chem 2002; 522: 167-172.
| Crossref |
94 Chen X, Jeon YM, Jang JW, Qin L, Huo F, Wei W, Mirkin CA. J Am Chem Soc 2008; 130: 8166-8168.
| Crossref |
95 Reed MA, Zhou C, Muller CJ, Burgin TP, Tour JM. Science 1997; 278: 252-254.
| Crossref |
96 Xu B, Tao NJ. Science 2003; 301: 1221-1223.
| Crossref |
97 Haiss W, Van Zalinge H, Higgins SJ, Bethell D, Höbenreich H, Schiffrin DJ, Nichols RJ. J Am Chem Soc 2003; 125: 15294-15295.
| Crossref |
98 Haiss W, Nichols RJ, Van Zalinge H, Higgins SJ, Bethell D, Schiffrin DJ. Phys Chem Chem Phys 2004; 6: 4330-4337.
| Crossref |
99 Aragonès AC, Darwish N, Ciampi S, Sanz F, Gooding JJ, Díez-Pérez I. Nat Commun 2017; 8: 15056.
| Crossref |
100 Peiris CR, Vogel YB, Le Brun AP, Aragonès AC, Coote ML, Díez-Pérez I, Ciampi S, Darwish N. J Am Chem Soc 2019; 141: 14788-14797.
| Crossref |
101 Gorenskaia E, Naher M, Daukiya L, Moggach SA, Costa Milan D, Vezzoli A, Lambert CJ, Nichols RJ, Becker T, Low PJ. Aust J Chem 2021; 74: 806-818.
| Crossref |
102 Naher M, Gorenskaia E, Moggach SA, Becker T, Nichols RJ, Lambert CJ, Low PJ. Aust J Chem 2022; 75: 506-522.
| Crossref |
104 Aragonès AC, Haworth NL, Darwish N, Ciampi S, Bloomfield NJ, Wallace GG, Diez-Perez I, Coote ML. Nature 2016; 531: 88-91.
| Crossref |
105 Zhang L, Laborda E, Darwish N, Noble BB, Tyrell JH, Pluczyk S, Le Brun AP, Wallace GG, Gonzalez J, Coote ML, Ciampi S. J Am Chem Soc 2018; 140: 766-774.
| Crossref |
106 Vogel YB, Zhang L, Darwish N, Gonçales VR, Le Brun A, Gooding JJ, Molina A, Wallace GG, Coote ML, Gonzalez J, Ciampi S. Nat Commun 2017; 8: 2066.
| Crossref |
107 Zhang J, Rogers FJM, Darwish N, Gonçales VR, Vogel YB, Wang F, Gooding JJ, Peiris MCR, Jia G, Veder J-P, Coote ML, Ciampi S. J Am Chem Soc 2019; 141: 5863-5870.
| Crossref |
108 Vogel YB, Evans CW, Belotti M, Xu L, Russell IC, Yu LJ, Fung AKK, Hill NS, Darwish N, Gonçales VR, Coote ML, Swaminathan Iyer K, Ciampi S. Nat Commun 2020; 11: 6323.
| Crossref |
109 Dzhioev AA, Kosov DS. J Chem Phys 2011; 134: 044121.
| Crossref |
110 Gelin MF, Kosov DS. J Chem Phys 2021; 154: 044107.
| Crossref |
111 Peeks MD, Claridge TDW, Anderson HL. Nature 2017; 541: 200-203.
| Crossref |
112 Rickhaus M, Jirasek M, Tejerina L, Gotfredsen H, Peeks MD, Haver R, Jiang HW, Claridge TDW, Anderson HL. Nat Chem 2020; 12: 236-241.
| Crossref |
113 Bradley D, Branley CP, Peeks MD. Phys Chem Chem Phys 2022; 24: 11486-11490.
| Crossref |
114 Reimers JR, Solomon GC, Gagliardi A, Bilić A, Hush NS, Frauenheim T, Di Carlo A, Pecchia A. J Phys Chem A 2007; 111: 5692-5702.
| Crossref |
115 Hourahine B, Aradi B, Blum V, Bonafé F, Buccheri A, Camacho C, Cevallos C, Deshaye MY, Dumitrică T, Dominguez A, Ehlert S, Elstner M, Van Der Heide T, Hermann J, Irle S, Kranz JJ, Köhler C, Kowalczyk T, Kubař T, Lee IS, Lutsker V, Maurer RJ, Min SK, Mitchell I, Negre C, Niehaus TA, Niklasson AMN, Page AJ, Pecchia A, Penazzi G, Persson MP, Řezáč J, Sánchez CG, Sternberg M, Stöhr M, Stuckenberg F, Tkatchenko A, Yu VW-z, Frauenheim T. J Chem Phys 2020; 152: 124101.
| Crossref |
116 Mitchell I, Aradi B, Page AJ. J Comput Chem 2018; 39: 2452-2458.
| Crossref |
117 McLean B, Webber GB, Page AJ. J Am Chem Soc 2019; 141: 13385-13393.
| Crossref |
118 Ding LP, McLean B, Xu Z, Kong X, Hedman D, Qiu L, Page AJ, Ding F. J Am Chem Soc 2022; 144: 5606-5613.
| Crossref |
119 Roemer M, Gillespie A, Jago D, Costa-Milan D, Alqahtani J, Hurtado-Gallego J, Sadeghi H, Lambert CJ, Spackman PR, Sobolev AN, Skelton BW, Grosjean A, Walkey M, Kampmann S, Vezzoli A, Simpson PV, Massi M, Planje I, Rubio-Bollinger G, Agraït N, Higgins SJ, Sangtarash S, Piggott MJ, Nichols RJ, Koutsantonis GA. J Am Chem Soc 2022; 144: 12698-12714.
| Crossref |
120 Tezgerevska T, Alley KG, Boskovic C. Coord Chem Rev 2014; 268: 23-40.
| Crossref |
121 Hay MA, Janetzki JT, Kumar VJ, Gable RW, Clérac R, Starikova AA, Low PJ, Boskovic C. Inorg Chem 2022; 61: 17609-17622.
| Crossref |
122 Pei LQ, Horsley JR, Seng JW, Liu X, Yeoh YQ, Yu MX, Wu XH, Abell AD, Zheng JF, Zhou XS, Yu J, Jin S. ACS Appl Mater Interfaces 2021; 13: 57646-57653.
| Crossref |
123 Chen X, Roemer M, Yuan L, Du W, Thompson D, Del Barco E, Nijhuis CA. Nat Nanotechnol 2017; 12: 797-803.
| Crossref |
124 Song P, Guerin S, Tan SJR, Annadata HV, Yu X, Scully M, Han YM, Roemer M, Loh KP, Thompson D, Nijhuis CA. Adv Mater 2018; 30: 1706322.
| Crossref |
125 Roemer M, Donnadieu B, Nijhuis CA. Eur J Inorg Chem 2016; 2016: 1314-1318.
| Crossref |
126 Roemer M, Keaveney ST, Proschogo N. J Org Chem 2021; 86: 9007-9022.
| Crossref |
127 Roemer M, Luck I, Proschogo N. Adv Synth Catal 2022; 364: 2957-2971.
| Crossref |
128 Naher M, Milan DC, Al-Owaedi OA, Planje IJ, Bock S, Hurtado-Gallego J, Bastante P, Abd Dawood ZM, Rincón-García L, Rubio-Bollinger G, Higgins SJ, Agraït N, Lambert CJ, Nichols RJ, Low PJ. J Am Chem Soc 2021; 143: 3817-3829.
| Crossref |
129 Puebla-Hellmann G, Venkatesan K, Mayor M, Lörtscher E. Nature 2018; 559: 232-235.
| Crossref |
130 Singh A, Wolff A, Yambem SD, Esmaeili M, Riches JD, Shahbazi M, Feron K, Eftekhari E, Ostrikov K, Li Q, Sonar P. Adv Mater 2020; 32: 1906176.
| Crossref |
131 Yuvaraja S, Nawaz A, Liu Q, Dubal D, Surya SG, Salama KN, Sonar P. Chem Soc Rev 2020; 49: 3423-3460.
| Crossref |
132 Yu J, Horsley JR, Abell AD. Phys Chem Chem Phys 2020; 22: 8409-8417.
| Crossref |
133 Horsley JR, Yu J, Wegener KL, Hoppmann C, Rück-Braun K, Abell AD. Biosens Bioelectron 2018; 118: 188-194.
| Crossref |
134 Griffith MJ, Holmes NP, Elkington DC, Cottam S, Stamenkovic J, Kilcoyne ALD, Andersen TR. Nanotechnology 2020; 31: 092002.
| Crossref |
135 Schiffrin A, Paasch-Colberg T, Karpowicz N, Apalkov V, Gerster D, Mühlbrandt S, Korbman M, Reichert J, Schultze M, Holzner S, Barth JV, Kienberger R, Ernstorfer R, Yakovlev VS, Stockman MI, Krausz F. Nature 2013; 493: 70-74.
| Crossref |
136 Schiffrin A, Capsoni M, Farahi G, Wang CG, Krull C, Castelli M, Roussy T, Cochrane KA, Yin Y, Medhekar NV, Fuhrer M, Shaw AQ, Ji W, Burke SA. ACS Nano 2018; 12: 6545-6553.
| Crossref |
137 Kumar D, Krull C, Yin Y, Medhekar NV, Schiffrin A. ACS Nano 2019; 13: 11882-11890.
| Crossref |
138 Griffith MJ, Sunahara K, Wagner P, Wagner K, Wallace GG, Officer DL, Furube A, Katoh R, Mori S, Mozer AJ. Chem Commun 2012; 48: 4145-4162.
| Crossref |
139 Barr MG, Chambon S, Fahy A, Jones TW, Marcus MA, Kilcoyne ALD, Dastoor PC, Griffith MJ, Holmes NP. Mater Chem Front 2021; 5: 2218-2233.
| Crossref |
140 Shi XL, Zou J, Chen ZG. Chem Rev 2020; 120: 7399-7515.
| Crossref |
141 Cao T, Shi XL, Chen ZG. Prog Mater Sci 2023; 131: 101003.
| Crossref |
142 Zhang L, Xia B, Shi XL, Liu WD, Yang Y, Hou X, Ye X, Suo G, Chen ZG. Carbon 2022; 196: 718-726.
| Crossref |
143 Lyu X, Ferrie S, Pivrikas A, MacGregor M, Ciampi S. Nano Energy 2022; 102: 107658.
| Crossref |
144 Ferrie S, Darwish N, Gooding JJ, Ciampi S. Nano Energy 2020; 78: 105210.
| Crossref |
145 Maganti T, Venkatesan K. ChemPlusChem 2022; 87: e202200014.
| Crossref |
146 Malmberg R, Venkatesan K. Eur J Inorg Chem 2021; 2021: 4890-4902.
| Crossref |
147 Malmberg R, Venkatesan K. Coord Chem Rev 2021; 449: 214182.
| Crossref |
148 Malmberg R, von Arx T, Hasan M, Blacque O, Shukla A, McGregor SKM, Lo SC, Namdas EB, Venkatesan K. Chem Eur J 2021; 27: 7265-7274.
| Crossref |
149 Zhang Y, Blacque O, Venkatesan K. Chem Eur J 2013; 19: 15689-15701.
| Crossref |
150 Gan Z, Wen X, Chen W, Zhou C, Yang S, Cao G, Ghiggino KP, Zhang H, Jia B. Adv Energy Mater 2019; 9: 1900185.
| Crossref |
151 Zheng F, Hall CR, Angmo D, Zuo C, Rubanov S, Wen Z, Bradley SJ, Hao X-T, Gao M, Smith TA, Ghiggino KP. J Mater Chem C 2021; 9: 5362-5372.
| Crossref |
152 Mao W, Hall CR, Bernardi S, Cheng Y-B, Widmer-Cooper A, Smith TA, Bach U. Nat Mater 2021; 20: 55-61.
| Crossref |
153 Gao C, Prasad SKK, Zhang B, Dvořák M, Tayebjee MJY, McCamey DR, Schmidt TW, Smith TA, Wong WWH. J Phys Chem C 2019; 123: 20181-20187.
| Crossref |
154 Banal JL, Zhang B, Jones DJ, Ghiggino KP, Wong WWH. Acc Chem Res 2017; 50: 49-57.
| Crossref |
155 El Gemayel M, Börjesson K, Herder M, Duong DT, Hutchison JA, Ruzié C, Schweicher G, Salleo A, Geerts Y, Hecht S, Orgiu E, Samorì P. Nat Commun 2015; 6: 6330.
| Crossref |
156 Tayebjee MJY, Sanders SN, Kumarasamy E, Campos LM, Sfeir MY, McCamey DR. Nat Phys 2017; 13: 182-188.
| Crossref |
157 Kumarasamy E, Sanders SN, Tayebjee MJY, Asadpoordarvish A, Hele TJH, Fuemmeler EG, Pun AB, Yablon LM, Low JZ, Paley DW, Dean JC, Choi B, Scholes GD, Steigerwald ML, Ananth N, McCamey DR, Sfeir MY, Campos LM. J Am Chem Soc 2017; 139: 12488-12494.
| Crossref |
158 Pun AB, Asadpoordarvish A, Kumarasamy E, Tayebjee MJY, Niesner D, McCamey DR, Sanders SN, Campos LM, Sfeir MY. Nat Chem 2019; 11: 821-828.
| Crossref |
159 Peiris CR, Ciampi S, Dief EM, Zhang J, Canfield PJ, Le Brun AP, Kosov DS, Reimers JR, Darwish N. Chem Sci 2020; 11: 5246-5256.
| Crossref |
160 Reimers JR, Yang J, Darwish N, Kosov DS. Chem Sci 2021; 12: 15870-15881.
| Crossref |
161 Jiang F, Trupp DI, Algethami N, Zheng H, He W, Alqorashi A, Zhu C, Tang C, Li R, Liu J, Sadeghi H, Shi J, Davidson R, Korb M, Sobolev AN, Naher M, Sangtarash S, Low PJ, Hong W, Lambert CJ. Angew Chem Int Ed 2019; 58: 18987-18993.
| Crossref |
162 Harrison DP, Grotjahn R, Naher M, Ghazvini SMBH, Mazzucato DM, Korb M, Moggach SA, Lambert C, Kaupp M, Low PJ. Angew Chem Int Ed 2022; 61: e202211000.
| Crossref |
163 Gorenskaia E, Turner KL, Martín S, Cea P, Low PJ. Nanoscale 2021; 13: 9055-9074.
| Crossref |
164 Janetzki JT, Zahir FZM, Gable RW, Phonsri W, Murray KS, Goerigk L, Boskovic C. Inorg Chem 2021; 60: 14475-14487.
| Crossref |
165 Aragonès AC, Darwish N, Ciampi S, Jiang L, Roesch R, Ruiz E, Nijhuis CA, Díez-Pérez I. J Am Chem Soc 2019; 141: 240-250.
| Crossref |
166 Walkey MC, Peiris CR, Ciampi S, Aragonès AC, Domínguez-Espíndola RB, Jago D, Pulbrook T, Skelton BW, Sobolev AN, Díez Pérez I, Piggott MJ, Koutsantonis GA, Darwish N. ACS Appl Mater Interfaces 2019; 11: 36886-36894.
| Crossref |
167 Kumar D, Hellerstedt J, Field B, Lowe B, Yin Y, Medhekar NV, Schiffrin A. Adv Funct Mater 2021; 31: 2106474.
| Crossref |
168 Hellerstedt J, Castelli M, Tadich A, Grubišić-Čabo A, Kumar D, Lowe B, Gicev S, Potamianos D, Schnitzenbaumer M, Scigalla P, Ghan S, Kienberger R, Usman M, Schiffrin A. Nanoscale Adv 2022; 4: 3845-3854.
| Crossref |
169 Castelli M, Hellerstedt J, Krull C, Gicev S, Hollenberg LCL, Usman M, Schiffrin A. Small 2021; 17: 2005974.
| Crossref |
170 Zhang L, Humphrey MG. Coord Chem Rev 2022; 473: 214820.
| Crossref |
171 Ferrie S, Le Brun AP, Krishnan G, Andersson GG, Darwish N, Ciampi S. Nano Energy 2022; 93: 106861.
| Crossref |
172 Dief EM, Darwish N. ACS Sens 2021; 6: 573-580.
| Crossref |
173 Krull A, Hirsch P, Rother C, Schiffrin A, Krull C. Commun Phys 2020; 3: 54.
| Crossref |
174 Krull C, Castelli M, Hapala P, Kumar D, Tadich A, Capsoni M, Edmonds MT, Hellerstedt J, Burke SA, Jelinek P, Schiffrin A. Nat Commun 2018; 9: 3211.
| Crossref |
175 Naghibi S, Sangtarash S, Kumar VJ, Wu JZ, Judd MM, Qiao X, Gorenskaia E, Higgins SJ, Cox N, Nichols RJ, Sadeghi H, Low PJ, Vezzoli A. Angew Chem Int Ed 2022; 61: e202116985.
| Crossref |
176 Martín-Barreiro A, Soto R, Chiodini S, García-Serrano A, Martín S, Herrer L, Pérez-Murano F, Low PJ, Serrano JL, de Marcos S, Galban J, Cea P. Adv Mater Interfaces 2021; 8: 2100876.
| Crossref |
177 Ezquerra R, Eaves SG, Bock S, Skelton BW, Pérez-Murano F, Cea P, Martín S, Low PJ. J Mater Chem C 2019; 7: 6630-6640.
| Crossref |
178 Sangiao S, Martín S, González-Orive A, Magén C, Low PJ, De Teresa JM, Cea P. Small 2017; 13: 1603207.
| Crossref |
179 Belotti M, El-Tahawy MMT, Yu L-J, Russell IC, Darwish N, Coote ML, Garavelli M, Ciampi S. Angew Chem Int Ed 2022; 61: e202209670.
| Crossref |
180 Bradley SJ, Chi M, White JM, Hall CR, Goerigk L, Smith TA, Ghiggino KP. Phys Chem Chem Phys 2021; 23: 9357-9364.
| Crossref |
181 Schwarz KN, Geraghty PB, Mitchell VD, Khan SUZ, Sandberg OJ, Zarrabi N, Kudisch B, Subbiah J, Smith TA, Rand BP, Armin A, Scholes GD, Jones DJ, Ghiggino KP. J Am Chem Soc 2020; 142: 2562-2571.
| Crossref |
182 Smith DA, McKenzie G, Jones AC, Smith TA. Methods Appl Fluoresc 2017; 5: 042001.
| Crossref |
183 Smith TA, Ghiggino KP. Methods Appl Fluoresc 2015; 3: 022001.
| Crossref |
184 Hao XT, McKimmie LJ, Smith TA. J Phys Chem Lett 2011; 2: 1520-1525.
| Crossref |
185 Mao W, Hall CR, Chesman ASR, Forsyth C, Cheng YB, Duffy NW, Smith TA, Bach U. Angew Chem Int Ed 2019; 58: 2893-2898.
| Crossref |
186 Laird JS, Ravishankar S, Rietwyk KJ, Mao W, Bach U, Smith TA. Small Methods 2022; 6: 2200493.
| Crossref |
187 Stevens AL, Novakovic S, White JM, Wong WWH, Smith TA, Ghiggino KP, Paige MF, Steer RP. J Phys Chem A 2018; 122: 9605-9614.
| Crossref |
188 Bo YF, Liu YY, Soleimaninejad H, Yu MN, Xie LH, Smith TA, Ghiggino KP, Huang W. J Phys Chem A 2019; 123: 2789-2795.
| Crossref |
189 Hooley EN, Tilley AJ, White JM, Ghiggino KP, Bell TDM. Phys Chem Chem Phys 2014; 16: 7108-7114.
| Crossref |
190 Yu MN, Soleimaninejad H, Lin JY, Zuo ZY, Liu B, Bo YF, Bai LB, Han YM, Smith TA, Xu M, Wu XP, Dunstan DE, Xia RD, Xie LH, Bradley DDC, Huang W. J Phys Chem Lett 2018; 9: 364-372.
| Crossref |
191 Dvořák M, Prasad SKK, Dover CB, Forest CR, Kaleem A, Macqueen RW, Petty AJ, Forecast R, Beves JE, Anthony JE, Tayebjee MJY, Widmer-Cooper A, Thordarson P, Schmidt TW. J Am Chem Soc 2021; 143: 13749-13758.
| Crossref |
192 Gholizadeh EM, Prasad SKK, Teh ZL, Ishwara T, Norman S, Petty II AJ, Cole JH, Cheong S, Tilley RD, Anthony JE, Huang S, Schmidt TW. Nat Photon 2020; 14: 585-590.
| Crossref |
193 Gholizadeh EM, Prasad SKK, Gillan LV, Nielsen MP, Ekins-Daukes NJ, McCamey DR, Tayebjee MJY, Schmidt TW. J Phys Chem C 2021; 125: 22464-22471.
| Crossref |
194 Tayebjee MJY, Clady RGCR, Schmidt TW. Phys Chem Chem Phys 2013; 15: 14797-14805.
| Crossref |
195 Wimberger L, Prasad SKK, Peeks MD, Andréasson J, Schmidt TW, Beves JE. J Am Chem Soc 2021; 143: 20758-20768.
| Crossref |
196 Gückel S, Safari P, Bagher Hosseini Ghazvini SM, Hall MR, Gluyas JBG, Kaupp M, Low PJ. Organometallics 2021; 40: 346-357.
| Crossref |
197 Gückel S, Gluyas JBG, Eaves SG, Safari P, Yufit DS, Sobolev AN, Kaupp M, Low PJ. Chem Eur J 2019; 25: 8837-8853.
| Crossref |
198 Gückel S, Gluyas JBG, El-Tarhuni S, Sobolev AN, Whiteley MW, Halet JF, Lapinte C, Kaupp M, Low PJ. Organometallics 2018; 37: 1432-1445.
| Crossref |
199 Yu J, Horsley JR, Abell AD. Acc Chem Res 2018; 51: 2237-2246.
| Crossref |
200 Chen X, Yeoh YQ, He Y, Zhou C, Horsley JR, Abell AD, Yu J, Guo X. Angew Chem Int Ed 2020; 59: 22554-22562.
| Crossref |
201 Vogel YB, Gonçales VR, Al-Obaidi L, Gooding JJ, Darwish N, Ciampi S. Adv Funct Mater 2018; 28: 1804791.
| Crossref |
202 Peiris CR, Ferrie S, Ciampi S, Rickard WDA, Darwish N. ACS Appl Nano Mater 2022; 5: 6609-6617.
| Crossref |
203 Li T, Dief EM, Kalužná Z, MacGregor M, Foroutan-Nejad C, Darwish N. Langmuir 2022; 38: 5532-5541.
| Crossref |
204 Li T, Ciampi S, Darwish N. ChemElectroChem 2022; 9: e202200255.
| Crossref |
205 Nadurata VL, Hay MA, Janetzki JT, Gransbury GK, Boskovic C. Dalton Trans 2021; 50: 16631-16646.
| Crossref |
206 Gransbury GK, Livesay BN, Janetzki JT, Hay MA, Gable RW, Shores MP, Starikova A, Boskovic C. J Am Chem Soc 2020; 142: 10692-10704.
| Crossref |
207 Gransbury GK, Boulon ME, Mole RA, Gable RW, Moubaraki B, Murray KS, Sorace L, Soncini A, Boskovic C. Chem Sci 2019; 10: 8855-8871.
| Crossref |
208 Tezgerevska T, Rousset E, Gable RW, Jameson GNL, Sañudo EC, Starikova A, Boskovic C. Dalton Trans 2019; 48: 11674-11689.
| Crossref |
209 Gransbury GK, Boulon ME, Petrie S, Gable RW, Mulder RJ, Sorace L, Stranger R, Boskovic C. Inorg Chem 2019; 58: 4230-4243.
| Crossref |
210 Subbiah J, Purushothaman B, Chen M, Qin T, Gao M, Vak D, Scholes FH, Chen X, Watkins SE, Wilson GJ, Holmes AB, Wong WWH, Jones DJ. Adv Mater 2015; 27: 702-705.
| Crossref |
211 Masoomi-Godarzi S, Liu M, Tachibana Y, Goerigk L, Ghiggino KP, Smith TA, Jones DJ. Adv Energy Mater 2018; 8: 1801720.
| Crossref |
212 Zhang B, Zhao P, Wilson LJ, Subbiah J, Yang H, Mulvaney P, Jones DJ, Ghiggino KP, Wong WWH. ACS Energy Lett 2019; 4: 1839-1844.
| Crossref |
213 O’Shea R, Gao C, Bradley S, Owyong TC, Wu N, White JM, Ghiggino KP, Wong WWH. Mater Adv 2021; 2: 7751-7758.
| Crossref |
214 Saxena S, Marlow P, Subbiah J, Colsmann A, Wong WWH, Jones DJ. ACS Appl Mater Interfaces 2021; 13: 36044-36052.
| Crossref |
215 Kafi AKM, Crossley MJ. Biosens Bioelectron 2013; 42: 273-279.
| Crossref |
216 Anderson D, Cottam S, Heim H, Zhang H, Holmes NP, Griffith MJ. MRS Commun 2019; 9: 1206-1213.
| Crossref |
217 Al-Mudhaffer MF, Holmes NP, Kumar P, Barr MG, Cottam S, Crovador R, Jones TW, Lim R, Zhou X, Holdsworth J, Belcher WJ, Dastoor PC, Griffith MJ. MRS Commun 2020; 10: 600-608.
| Crossref |
218 Marks M, Holmes NP, Sharma A, Pan X, Chowdhury R, Barr MG, Fenn C, Griffith MJ, Feron K, Kilcoyne ALD, Lewis DA, Andersson MR, Belcher WJ, Dastoor PC. Phys Chem Chem Phys 2019; 21: 5705-5715.
| Crossref |
219 Kumar VJ, Wu JZ, Judd M, Rousset E, Korb M, Moggach SA, Cox N, Low PJ. J Mater Chem C 2022; 10: 1896-1915.
| Crossref |
220 Naher M, Bock S, Langtry ZM, O’Malley KM, Sobolev AN, Skelton BW, Korb M, Low PJ. Organometallics 2020; 39: 4667-4687.
| Crossref |
221 Escorihuela E, Cea P, Bock S, Milan DC, Naghibi S, Osorio HM, Nichols RJ, Low PJ, Martin S. J Mater Chem C 2020; 8: 672-682.
| Crossref |
222 Bock S, Low PJ. Aust J Chem 2018; 71: 307-310.
| Crossref |
223 Yu J, Huang DM, Shapter JG, Abell AD. J Phys Chem C 2012; 116: 26608-26617.
| Crossref |
224 Gryn’ova G, Marshall DL, Blanksby SJ, Coote ML. Nat Chem 2013; 5: 474-481.
| Crossref |
225 Gryn’ova G, Barakat JM, Blinco JP, Bottle SE, Coote ML. Chem Eur J 2012; 18: 7582-7593.
| Crossref |
226 Gryn’ova G, Coote ML, Corminboeuf C. WIREs Comput Mol Sci 2015; 5: 440-459.
| Crossref |
227 Li Y, Haworth NL, Xiang L, Ciampi S, Coote ML, Tao N. J Am Chem Soc 2017; 139: 14699-14706.
| Crossref |
228 Hill NS, Coote ML. J Am Chem Soc 2018; 140: 17800-17804.
| Crossref |
229 Rudge SL, Kosov DS. Phys Rev B 2021; 104: 165307.
| Crossref |
230 Davis NS, Rudge SL, Kosov DS. Phys Rev B 2021; 103: 205408.
| Crossref |
231 Rudge SL, Kosov DS. Phys Rev B 2019; 100: 235430.
| Crossref |
232 Rudge SL, Kosov DS. Phys Rev B 2019; 99: 115426.
| Crossref |
233 Rudge SL, Kosov DS. J Chem Phys 2019; 151: 034107.
| Crossref |
234 Rudge SL, Kosov DS. Phys Rev B 2018; 98: 245402.
| Crossref |
235 Kosov DS. J Chem Phys 2018; 149: 164105.
| Crossref |
236 Kosov DS. J Chem Phys 2018; 148: 184108.
| Crossref |
237 Kosov DS. J Chem Phys 2017; 147: 104109.
| Crossref |
238 Kosov DS. J Chem Phys 2017; 146: 074102.
| Crossref |
239 Honeychurch TD, Kosov DS. Phys Rev B 2020; 102: 195409.
| Crossref |
240 Preston RJ, Honeychurch TD, Kosov DS. J Chem Phys 2020; 153: 121102.
| Crossref |
241 Honeychurch TD, Kosov DS. Phys Rev B 2019; 100: 245423.
| Crossref |
242 Preston RJ, Honeychurch TD, Kosov DS. Phys Rev B 2022; 106: 195406.
| Crossref |
243 Kershaw VF, Kosov DS. J Chem Phys 2020; 153: 154101.
| Crossref |
244 Preston RJ, Kershaw VF, Kosov DS. Phys Rev B 2020; 101: 155415.
| Crossref |
245 Kershaw VF, Kosov DS. J Chem Phys 2019; 150: 074101.
| Crossref |
246 Kershaw VF, Kosov DS. J Chem Phys 2018; 149: 044121.
| Crossref |
247 Kershaw VF, Kosov DS. J Chem Phys 2017; 147: 224109.
| Crossref |
248 Preston RJ, Gelin MF, Kosov DS. J Chem Phys 2021; 154: 114108.
| Crossref |
249 Dzhioev AA, Kosov DS, von Oppen F. J Chem Phys 2013; 138: 134103.
| Crossref |
250 Dzhioev AA, Kosov DS. J Chem Phys 2011; 135: 074701.
| Crossref |
251 Reimers JR, Wang Y, Kosov DS. J Phys Chem C 2019; 123: 15569-15574.
| Crossref |
252 Li Z, Kosov DS. Phys Rev B 2007; 76: 035415.
| Crossref |
253 Li Z, Kosov DS. J Phys Chem B 2006; 110: 9893-9898.
| Crossref |
254 Ahmad S, Mustonen K, McLean B, Jiang H, Zhang Q, Hussain A, Khan AT, Ding E-X, Liao Y, Wei N, Monazam MRA, Nasibulin AG, Kotakoski J, Page AJ, Kauppinen EI. Adv Funct Mater 2020; 30: 2005016.
| Crossref |
255 Stefanovic R, Ludwig M, Webber GB, Atkin R, Page AJ. Phys Chem Chem Phys 2017; 19: 3297-3306.
| Crossref |
256 Li X, Chiong R, Page AJ. J Phys Chem Lett 2021; 12: 5156-5162.
| Crossref |
257 Reimers JR, Ford MJ, Marcuccio SM, Ulstrup J, Hush NS. Nat Rev Chem 2017; 1: 0017.
| Crossref |
258 Reimers JR, Panduwinata D, Visser J, Chin Y, Tang C, Goerigk L, Ford MJ, Sintic M, Sum T-J, Coenen MJJ, Hendriksen BLM, Elemans JAAW, Hush NS, Crossley MJ. Proc Natl Acad Sci USA 2015; 112: E6101-E6110.
| Crossref |
259 Reimers JR, Ford MJ, Halder A, Ulstrup J, Hush NS. Proc Natl Acad Sci USA 2016; 113: E1424-E1433.
| Crossref |
260 Solomon GC, Reimers JR, Hush NS. J Chem Phys 2005; 122: 224502.
| Crossref |
261 Solomon GC, Gagliardi A, Pecchia A, Frauenheim T, Di Carlo A, Reimers JR, Hush NS. Nano Lett 2006; 6: 2431-2437.
| Crossref |
262 Gagliardi A, Solomon GC, Pecchia A, Frauenheim T, Di Carlo A, Hush NS, Reimers JR. Phys Rev B 2007; 75: 174306.
| Crossref |
263 Wohlthat S, Solomon GC, Hush NS, Reimers JR. Theor Chem Acc 2011; 130: 815-828.
| Crossref |
264 Vogel YB, Zhang J, Darwish N, Ciampi S. ACS Nano 2018; 12: 8071-8080.
| Crossref |
265 Belotti M, Lyu X, Xu L, Halat P, Darwish N, Silvester DS, Goh C, Izgorodina EI, Coote ML, Ciampi S. J Am Chem Soc 2021; 143: 17431-17440.
| Crossref |
266 Dief EM, Hoffmann N, Darwish N. Surfaces 2022; 5: 218-227.
| Crossref |
267 Rai B, Malmberg R, Srinivasan V, Ganesh KM, Kambhampati NSV, Andar A, Rao G, Sanjeevi CB, Venkatesan K, Ramamurthy SS. ACS Sens 2021; 6: 4360-4368.
| Crossref |
268 Kafi AKM, Azmi NS, Yusoff MM, Crossley MJ. J Nanosci Nanotechnol 2017; 17: 5896-5899.
| Crossref |
269 Kafi AKM, Yusoff MM, Choucair M, Crossley MJ. J Solid State Electrochem 2017; 21: 2761-2767.
| Crossref |
270 Kafi AKM, Naqshabandi M, Yusoff MM, Crossley MJ. Enzyme Microb Technol 2018; 113: 67-74.
| Crossref |
271 Kafi AKM, Azmi NS, Yusoff MM, Crossley MJ. Procedia Technol 2017; 27: 201-202.
| Crossref |
272 Sherwood CP, Elkington DC, Dickinson MR, Belcher WJ, Dastoor PC, Feron K, Brichta AM, Lim R, Griffith MJ. IEEE J Sel Top Quantum Electron 2021; 27: 1-12.
| Crossref |
273 Crovador R, Heim H, Cottam S, Feron K, Bhatia V, Louie F, Sherwood CP, Dastoor PC, Brichta AM, Lim R, Griffith MJ. ACS Appl Bio Mater 2021; 4: 6338-6350.
| Crossref |
274 Posar JA, Davis J, Brace O, Sellin P, Griffith MJ, Dhez O, Wilkinson D, Lerch MLF, Rosenfeld A, Petasecca M. Phys Imaging Radiat Oncol 2020; 14: 48-52.
| Crossref |
275 Rahpeima S, Dief EM, Peiris CR, Ferrie S, Duan A, Ciampi S, Raston CL, Darwish N. Chem Commun 2020; 56: 6209-6212.
| Crossref |
276 Rahpeima S, Le Brun A, Raston CL, Darwish N. J Colloid Interface Sci 2022; 626: 985-994.
| Crossref |
277 Griffith MJ, Cooling NA, Elkington DC, Muller E, Belcher WJ, Dastoor PC. Appl Phys Lett 2014; 105: 143301.
| Crossref |
278 Griffith MJ, Cooling NA, Elkington DC, Wasson M, Zhou X, Belcher WJ, Dastoor PC. Nanomaterials 2021; 11: 1185.
| Crossref |
279 Griffith MJ, Cottam S, Stamenkovic J, Posar JA, Petasecca M. Front Phys 2020; 8: 22.
| Crossref |
280 Al-Ahmad AY, Almayhi F, Al-Mudhaffer MF, Griffith MJ, Liu W, Li S, Sivunova K, Elkington D, Cooling NA, Feron K, Shi M, Belcher W, Chen H, Dastoor P, Andersen TR. Sustain Energy Fuels 2020; 4: 940-949.
| Crossref |
281 Andersen TR, Almyahi F, Cooling NA, Elkington D, Wiggins L, Fahy A, Feron K, Vaughan B, Griffith MJ, Mozer AJ, Sae-Kung C, Wallace GG, Belcher WJ, Dastoor PC. J Mater Chem A 2016; 4: 15986-15996.
| Crossref |
282 Griffith MJ, Mozer AJ, Tsekouras G, Dong Y, Wagner P, Wagner K, Wallace GG, Mori S, Officer DL. Appl Phys Lett 2011; 98: 163502.
| Crossref |
283 Griffith MJ, Willis MS, Kumar P, Holdsworth JL, Bezuidenhout H, Zhou X, Belcher W, Dastoor PC. ACS Appl Mater Interfaces 2016; 8: 7928-7937.
| Crossref |
284 Posar JA, Davis J, Large MJ, Basiricò L, Ciavatti A, Fraboni B, Dhez O, Wilkinson D, Sellin PJ, Griffith MJ, Lerch MLF, Rosenfeld A, Petasecca M. Med Phys 2020; 47: 3658-3668.
| Crossref |
285 Ameri M, Al-Mudhaffer MF, Almyahi F, Fardell GC, Marks M, Al-Ahmad A, Fahy A, Andersen T, Elkington DC, Feron K, Dickinson M, Samavat F, Dastoor PC, Griffith MJ. ACS Appl Mater Interfaces 2019; 11: 10074-10088.
| Crossref |
286 Al-Mudhaffer MF, Griffith MJ, Feron K, Nicolaidis NC, Cooling NA, Zhou X, Holdsworth J, Belcher WJ, Dastoor PC. Sol Energy Mater Sol Cells 2018; 175: 77-88.
| Crossref |
287 Sunahara K, Griffith MJ, Uchiyama T, Wagner P, Officer DL, Wallace GG, Mozer AJ, Mori S. ACS Appl Mater Interfaces 2013; 5: 10824-10829.
| Crossref |
288 Large MJ, Posar JA, Mozer AJ, Nattestad A, Alnaghy S, Carolan M, Sellin PJ, Davies J, Pastuovic Z, Lerch MLF, Guatelli S, Rosenfeld A, Griffith MJ, Petasecca M. ACS Appl Mater Interfaces 2021; 13: 57703-57712.
| Crossref |
289 Nam KW, Kim H, Beldjoudi Y, Kwon T-w, Kim DJ, Stoddart JF. J Am Chem Soc 2020; 142: 2541-2548.
| Crossref |
290 Feng Y, Ovalle M, Seale JSW, Lee CK, Kim DJ, Astumian RD, Stoddart JF. J Am Chem Soc 2021; 143: 5569-5591.
| Crossref |
291 Chen H, Brasiliense V, Mo J, Zhang L, Jiao Y, Chen Z, Jones LO, He G, Guo Q-H, Chen X-Y, Song B, Schatz GC, Stoddart JF. J Am Chem Soc 2021; 143: 2886-2895.
| Crossref |
292 Chen H, Jiang F, Hu C, Jiao Y, Chen S, Qiu Y, Zhou P, Zhang L, Cai K, Song B, Chen X-Y, Zhao X, Wasielewski MR, Guo H, Hong W, Stoddart JF. J Am Chem Soc 2021; 143: 8476-8487.
| Crossref |
293 Chen H, Jia C, Zhu X, Yang C, Guo X, Stoddart JF. Nat Rev Mater 2022; 8: 165-185.
| Crossref |





