Development and patent synthesis of nirmatrelvir – the main component of the first oral drug against SARS-CoV-2 Paxlovid®
Bruno A. Cotrim A and José C. Barros
A and José C. Barros  B *
B *
A Instituto Federal de Educação, Ciência e Tecnologia do Rio de Janeiro, Unidade Rio de Janeiro. Rua Senador Furtado, 121, Laboratório 316, Rio de Janeiro, RJ 20270-021, Brazil.
B Instituto de Química, Universidade Federal do Rio de Janeiro, CT Bloco A, Rio de Janeiro, RJ 21941-909, Brazil.
Australian Journal of Chemistry 75(7) 487-491 https://doi.org/10.1071/CH22104
Submitted: 11 May 2022 Accepted: 6 July 2022 Published: 18 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Nirmatrelvir is the main component of the first oral drug against SARS-CoV-2 called Paxlovid®. Its development from an orally unavailable predecessor through hydrogen bond donors (HBD) replacement is highlighted, followed by an examination of the synthetic routes described in the original PCT application WO2021/250648. Based on its attributes, nirmatrelvir shows the potential to be a game changer in SARS-CoV-2 treatment.
Keywords: covid-19, drug development, medicinal chemistry, nirmatrelvir, Paxlovid®, organic synthesis, patent, process chemistry, SARS-CoV-2.
Introduction
On April 22nd 2022, the World Health Organization – WHO made a ‘strong recommendation for nirmatrelvir and ritonavir, sold under the name Paxlovid®, calling it the best therapeutic choice for high-risk patients’ of COVID-19.[1]
Additionally, 35 generic manufacturers signed agreements with the Medicines Patent Pool – MPP, a United Nations-backed public health organization working to improve access to affordable and appropriate essential medicines in low- and middle-income countries. These agreements formed with a range of stakeholders are expected to allow for voluntary licensing of patents and patent pooling (basically a consortium for cross-licensing) to produce low-cost, generic versions of their active pharmaceutical ingredients (API) of Paxlovid® against COVID-19 for supply in 95 low- and middle-income countries.[2]
Based on the game changer characteristic of a first oral treatment, which is cheaper and easier for large-scale production without the need for refrigerated shipping, its development and patented synthesis are highlighted here.
Drug development
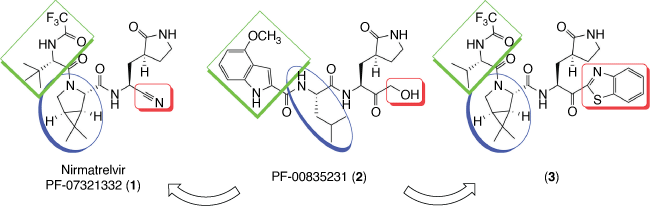
Nirmatrelvir (PF-07321332, 1) is an inhibitor of SARS-CoV-2 main protease also known as 3-chymotrypsin-like protease (Mpro or 3CLpro), a cysteine protease responsible for cleaving polyproteins in the virus replication cycle. This drug development strategy was inspired by other protease inhibitors against HIV, HCV and notably SARS-CoV-1 inhibitor PF-00835231 (2).[3–6]
In 2020, at the beginning of the COVID-19 pandemic, different research groups made screens testing diverse APIs against the SARS-COV-2 virus, in a drug repositioning strategy. The screens showed that boceprevir, a well-known hepatitis C drug, inhibits the SARS-COV-2 main protease (Mpro) and was a good candidate for the COVID-19 treatment or could be used as a scaffold for further drug development.[3,7]
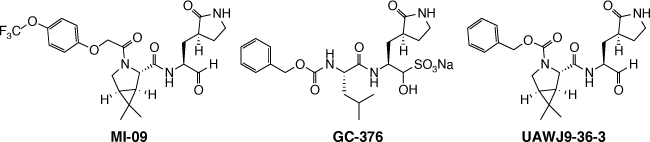
Knowing that boceprevir acts as a SARS-COV-2 Mpro inhibitor and knowing the gem-dimethylcyclopropylproline is a rigid hydrophobic moiety that can fit S2 subsite of the Mpro, new prototypes were developed having this molecular moiety.[4,5] Qiao and co-workers developed and tested the compound MI-09, which showed promising in vitro and preclinical assays in mice.[4] Noteworthily, this prototype showed in vivo activity also when orally administrated. In parallel, Xia and co-workers developed and tested the compound UAWJ9-36-3, which was developed through a molecular hybridization strategy between boceprevir and GC-376 (an active compound against a feline coronavirus species).[5] Compound UAWJ9-36-3 showed a better in vitro activity when compared to GC-376[5] as indicated by IC50 of 3.40 μM in Flip-GFP Mpro Assay, a new developed assay to quantify the cellular protease inhibitory activity. The fact that MI-09 and UAWJ9-36-3 possess an aldehyde group as a warhead raised concern, as this could lead to a lack of selectivity against human cysteine proteases (Fig. 1).[4,5]
PF-00835231 (2) developed by Pfizer in 2002 against SARS-CoV-1 as well as its prodrug PF-07304814, which has the primary hydroxyl group phosphorylated and is more water-soluble, were tested against SARS-CoV-2 and presented good results, being considered promissing drug prototypes.[8] Nevertheless, 2 was not orally available due to the presence of several hydrogen bond donors (HBD), so project leader Dafydd Owen and co-workers systematically modified the molecule to remove HBD without a decrease in activity.[6,9]
In a first approach, recognizing that the α-hydroxymethyl ketone in 2 covalently interacts with the thiol group from the cysteine protease, it was substituted by benzothiazole-2-yl ketone or a nitrile to afford 3 and 1 respectively. Then, the leucine moiety of 2 was substituted by a cyclic amino acid to eliminate an N–H bond. The isopropyl group of leucine moiety in 2 was substituted by dimethyl substituent in the gem-dimethylcyclopropylproline guided by computational studies and inspired by use of these fused rings in the development of the HCV protease inhibitor boceprevir. The third replacement was based on the substitution of indole by a trifluoroacetamide to maintain interaction with glutamine of the Mpro and improve bioavailability. Finally, the choice for the drug candidate based on nitrile (1) instead of that containing benzothiazole-2-yl ketone (3) was based on its higher solubility, scalability and less risk of epimerization of 1 (Scheme 1).[6,9]
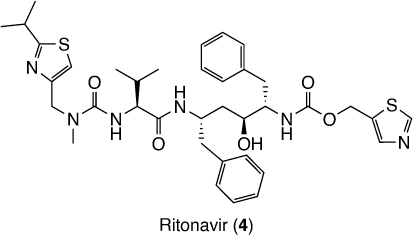
Besides being active against SARS-CoV-2, nirmatrelvir showed only moderate metabolic stability, measured by intrinsic clearance against oxidative metabolism in human liver microsomes, which was significantly inhibited by the CYP3A4/5 inhibitor ketoconazole.[6] Then to improve the therapeutic concentrations of the compound in the clinic, it is co-administered with the potent inhibitor of CYP34A ritonavir (4).[6] Ritonavir (Fig. 2) is an antiretroviral rarely used as a protease inhibitor against HIV, however, it is widely used as a booster of other antiretrovirals.[9] Its combination with nirmatrelvir is marketed as Paxlovid®.[9]
|
WHO recommended Paxlovid® based on two randomized controlled trials involving 3078 patients, the results indicated a risk of hospitalization reduced by 85% following this treatment, and in a high-risk group (over 10% risk of hospitalization), it means 84 fewer hospitalizations per 1000 patients.[1] WHO suggests against its use in patients at lower risk, as the benefits were found to be negligible.[1,10]
Patent synthesis
Nirmatrelvir synthesis is described in patent US11,351, 149[11] having Pfizer as its assignee. The document was filled on 5 August 2021. This document was also published as the PCT application WO2021/250648.[12] This document discloses the nirmatrelvir molecule and several other analogs. Nirmatrelvir synthesis is described in Example 13 of the US patent, where different options for nirmatrelvir synthesis are described.
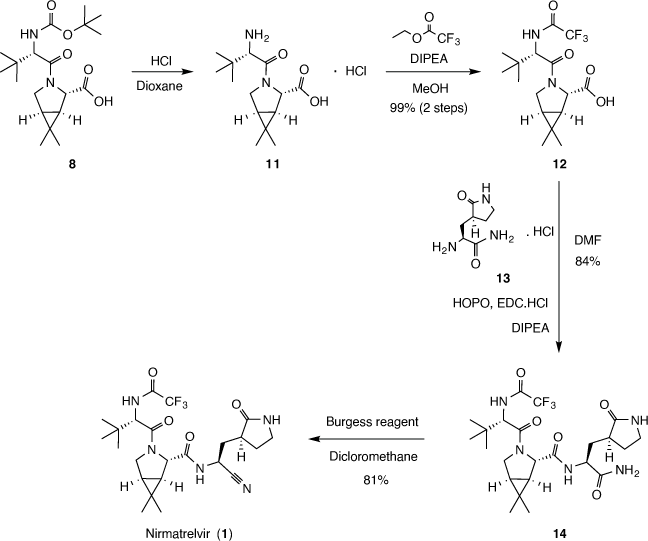
The first of the synthetic routes (Scheme 2) start with the pyrrolidine derivative 5, which is coupled with 6 in the presence of peptide coupling reagent hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) and N,N-diisopropylethylamine (DIPEA) to afford 7 with a 50% yield after preparative chromatography purification. Basic hydrolysis of compound 7 with aqueous LiOH solution furnished the carboxylic acid derivative 8 in 89% yield. The intermediate 8 then is coupled with 9 in the presence of HATU, DIPEA and N-methylmorpholine (NMM) to furnish 10, which was not purified, and the raw product was further reacted with methanesulfonic acid (MsOH) in 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP), and then with trifluoroacetic anhydride (TFAA) and NMM to afford nirmatrelvir (1) with 17% yield over the two last steps. This first route was suitable for the medicinal chemistry studies, however, gave low yields and resulted in 7.5 mg of nirmatrelvir, so a second synthetic route was developed.
Another route (Scheme 3) is presented as Alternate Synthesis of Example 13 in the patent US11,351,149.[5] The document describes the hydrolyzation of 8 followed by the reaction of 11 with ethyl trifluoroacetate in the presence of DIPEA giving intermediate 12 with a 99% yield. Finally, compound 12 is coupled with the hydrochloric salt 13 in the presence of DIPEA, 2-hydroxypyridine 1-oxide (HOPO) and 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDC.HCl) to furnish 12 in 84% yield. Finally, dehydration of 12 employing N-(triethylammoniumsulfonyl)carbamate (Burgess reagent) afforded nirmatrelvir (1) in 81% yield. This second route was conducted at a multigram scale, represents several improvements over the first one, showed higher yields, and finally was suitable to produce batches for subsequent tests.
Noteworthily, 5 is a key intermediate for nirmatrelvir synthesis. Compound 5 is a bicyclic proline methyl ester analog which presents three defined chiral centers. Different synthetic routes were described for 5, usually having as starting material either a proline scaffold or a cyclopropyl group-containing compound.[13]
Conclusion
The development and patent synthesis of nirmatrelvir – the main component of Paxlovid® was highlighted. As the first oral drug against SARS-CoV-2, this drug has the potential to be a game-changer to combat COVID-19 and accelerate the end of the pandemic.
Data availability
Data sharing is not applicable as no new data were generated or analyzed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] World Health Organization – WHO. WHO recommends highly successful COVID-19 therapy and calls for wide geographical distribution and transparency from the originator. Statement. 22 April. 2022. Available at https://www.who.int/news/item/22-04-2022-who-recommends-highly-successful-covid-19-therapy-and-calls-for-wide-geographical-distribution-and-transparency-from-originator[2] Medicines Patent Pool – MPP. 35 generic manufacturers sign agreements with MPP to produce low-cost, generic versions of Pfizer’s oral COVID-19 treatment nirmatrelvir in combination with ritonavir for supply in 95 low- and middle-income countries. Press Release. 17 March. 2022. Available at https://medicinespatentpool.org/news-publications-post/35-generic-manufacturers-sign-agreements-with-mpp-to-produce-low-cost-generic-versions-of-pfizers-oral-covid-19-treatment-nirmatrelvir-in-combination-with-ritonavir-for-supply-in-95-low-and
[3] L Fu, F Ye, Y Feng, F Yu, Q Wang, Y Wu, et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun 2020, 11, 4417.
| Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease.Crossref | GoogleScholarGoogle Scholar |
[4] J Qiao, YS Li, R Zeng, FL Liu, RH Luo, C Huang, et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374.
| SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model.Crossref | GoogleScholarGoogle Scholar |
[5] Z Xia, M Sacco, Y Hu, C Ma, X Meng, F Zhang, et al. Rational Design of Hybrid SARS-CoV-2 Main Protease Inhibitors Guided by the Superimposed Cocrystal Structures with the Peptidomimetic Inhibitors GC-376, Telaprevir, and Boceprevir. ACS Pharmacol Transl Sci 2021, 4, 1408.
| Rational Design of Hybrid SARS-CoV-2 Main Protease Inhibitors Guided by the Superimposed Cocrystal Structures with the Peptidomimetic Inhibitors GC-376, Telaprevir, and Boceprevir.Crossref | GoogleScholarGoogle Scholar |
[6] DR Owen, CMN Allerton, ALS Anderson, L Aschenbrenner, M Avery, S Berritt, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586.
| An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19.Crossref | GoogleScholarGoogle Scholar |
[7] C Ma, MD Sacco, B Hurst, JA Townsend, Y Hu, T Szeto, et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res 2020, 30, 678.
| Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease.Crossref | GoogleScholarGoogle Scholar |
[8] B Boras, RM Jones, BJ Anson, D Arenson, L Aschenbrenner, MA Bakowski, et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun 2021, 12, 6055.
| Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19.Crossref | GoogleScholarGoogle Scholar |
[9] (a) B Halford, The Path to Paxlovid. ACS Cent Sci 2022, 8, 405.
| The Path to Paxlovid.Crossref | GoogleScholarGoogle Scholar |
(b) B Halford, How Pfizer scientists transformed an old drug lead into a COVID-19 antiviral. C&EN. 14 January. 2022. Available at https://cen.acs.org/pharmaceuticals/drug-discovery/How-Pfizer-scientists-transformed-an-old-drug-lead-into-a-COVID-19-antiviral/100/i3
[10] World Health Organization – WHO. Therapeutics and COVID-19: living guideline. COVID-19: Clinical care. 22 April. 2022. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3
[11] Owen DR, Pettersson MY, Reese MR, Sammons MF, Tuttle JB, Verhoest PR et al. Nitrile‐containing compounds. US 11,351,149; 2022. Available at https://patft.uspto.gov/netacgi/nph‐Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch‐bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=%22Nitrile‐containing+antiviral+compounds%22&OS=
[12] Owen DR, Pettersson MY, Reese MR, Sammons MF, Tuttle JB, Verhoest PR et al. Nitrile‐containing antiviral compounds. WO/2021/250648; 2021. Available at https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021250648
[13] SR Kallam, VR Eda, S Sen, R Datrika, RK Rapolu, S Khobare, et al. A diastereoselective synthesis of boceprevir’s gem-dimethyl bicyclic [3.1.0] proline intermediate from an insecticide ingredient cis-cypermethric acid. Tetrahedron 2017, 73, 4285.
| A diastereoselective synthesis of boceprevir’s gem-dimethyl bicyclic [3.1.0] proline intermediate from an insecticide ingredient cis-cypermethric acid.Crossref | GoogleScholarGoogle Scholar |