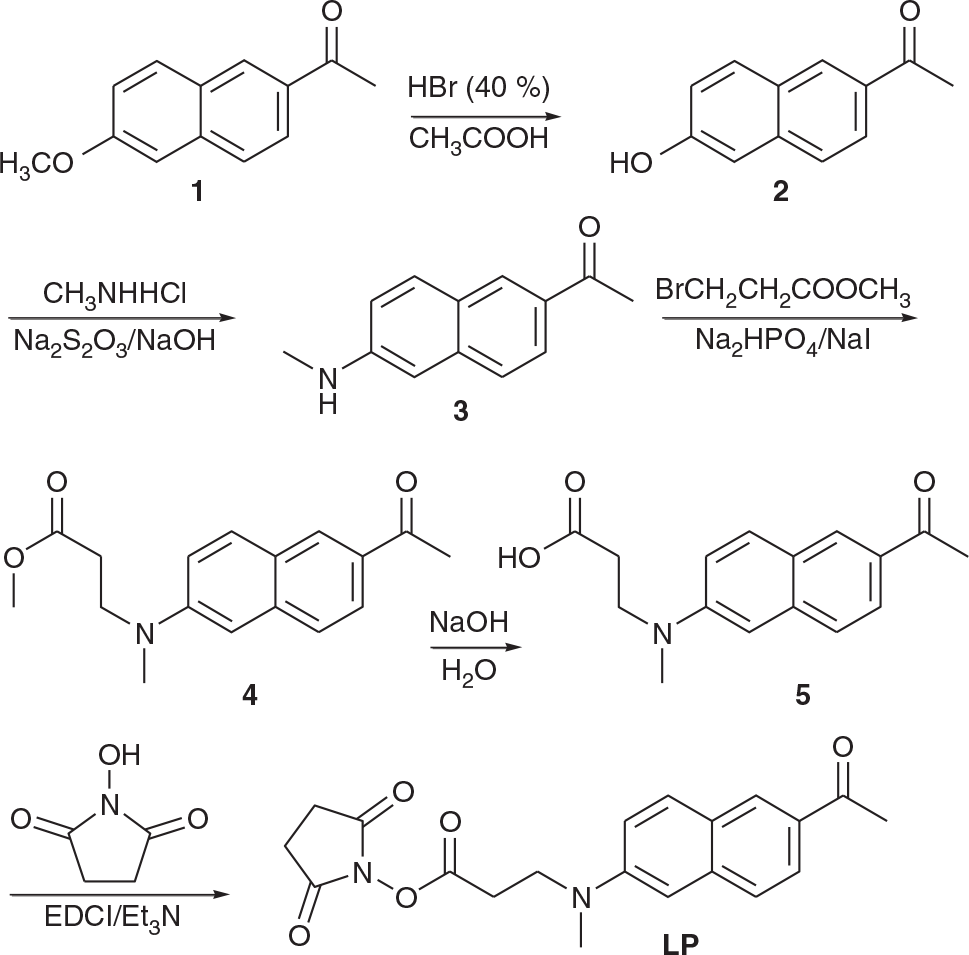
The Synthesis of a Two-Photon Fluorescence Labelling Probe and its Immunochromatographic Strip for Rapid Diagnosis of COVID-19
Chibao Huang A D E , Shuai Kang A , Fuxun Yu B and Zairong Wei C
A D E , Shuai Kang A , Fuxun Yu B and Zairong Wei C
A School of Physics and Electrical Engineering, Zunyi Normal University, Zunyi 563002, China.
B Centre Laboratory, Guizhou Provincial People’s Hospital, Guiyang 550002, China.
C Affiliated Hospital of Zunyi Medical University, Zunyi Medical University, Zunyi 563000, China.
D Henry Fok School of Biology and Agriculture, Shaoguan University, Shaoguan 512005, China.
E Corresponding author. Email: huangchibao@163.com
Australian Journal of Chemistry 74(7) 522-528 https://doi.org/10.1071/CH20344
Submitted: 26 November 2020 Accepted: 25 January 2021 Published: 25 February 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
A two-photon fluorescence labelling probe (LP) was synthesised, and LP-Ag was obtained by LP labelling the N-protein antigen (Ag) of COVID-19. LP-Ag was made into an immunochromatographic strip. When a blood sample was added to the sample hole of the test card, it would move forward along the nitrocellulose (NC) film. If the sample contained IgM, the IgM bound to LP-Ag and formed an M line with the coated mouse anti-human IgM antibody, giving a positive response to the presence of IgM of COVID-19. The sensitivity, specificity, and accuracy of the immunochromatographic strip based on the LP was compared with those of the nucleic acid detection method and the colloidal gold method, proving it to be much simpler than the nucleic acid detection method, which can greatly shorten the detection period, and to be much more stable than the colloidal gold method, which can overcome uncertainty. LP-Ag can be used to image lung tissue with COVID-19 by two-photon fluorescence microscopy (TFM).
Introduction
The new type of coronavirus (COVID-19) was the culprit of the viral pneumonia in 2019, and belongs to the family of RNA viruses. COVID-19 is subject to the same β coronavirus as the ‘SARS’ coronavirus[1] and the ‘Middle East Respiratory Syndrome’ coronavirus[2] in 2003. There are three major challenges of COVID-19: first is the contagion to wild animals, pets, herds, and humans; second is the one-step replication of the coronavirus RNA, which is similar to human mRNA, so it can be translated and synthesised directly, eliminating the RNA-DNA-mRNA transcription process; third, during the process of replication, the mutation of the virus RNA is fast, and its activity to repair the wrong enzyme is very low, so it is easy for the virus to mutate, whereas a vaccine is to be developed according to the fixed gene or protein of a virus, and therefore an RNA virus vaccine is more difficult to develop. This is also the key to the widespread circulation of viral pneumonia that is difficult to cope with.
The prevention and treatment of viral pneumonia focuses on early diagnosis and early detection. However, the detection of COVID-19 is limited to nucleic acid detection (RT-PCR, reverse transcription–polymerase chain reaction), and the steps are so many (five steps each for nucleic acid synthesis and PCR amplification), the operation is cumbersome, and the detection period long; and the detection results are also disturbed by many factors. Given the severity of coronavirus and the severe situation of viral pneumonia worldwide at present, it is urgent to develop a new method to diagnose COVID-19 accurately, specifically, and rapidly. The N-protein is the core protein of COVID-19 and its most important marker.[3] Hence, an immuno two-photon fluorescent probe based on the N-protein antigen was developed for rapid virus detection, thus providing a technical guarantee for early diagnosis, early isolation, and early treatment of latent virus.
The two-photon fluorescence probes which have been developed in the most recent 10 years, compared with the one-photon fluorescence probes, have significant advantages, such as high resolution, high clarity, high sensitivity, no photobleaching and no photodamage, fixed target excitation, high transverse and longitudinal resolution, small absorption coefficient of light in tissue, lower tissue auto-fluorescence, etc. The two-photon fluorophore 2-acetyl-6-(dimethylamino)naphthalene (acedan) is widely used in all kinds of two-photon fluorescent probes such as for Mg2+,[4] Na+,[5] Ni2+,[6] Zn2+,[7–9] H2O2,[10] H2S,[11] NO,[12] SO2/SO3,[13] RSH[14] and HClO,[15] and so on. Consequently, acedan is a ideal two-photon fluorophore.
In this study, a novel two-photon fluorescence labelling probe (LP) was developed, which contained acedan as the two-photon fluorophore and N-hydroxysuccinimide (NHS) as the activation group (Fig. 1). LP was employed to label the N-protein antigen of COVID-19 and made into an immunochromatographic strip and test kit for the rapid detection and diagnosis of COVID-19.
|
Experimental
Materials and Methods
NMR spectra were recorded on a VARIAN INOVA 400 MHz NMR spectrometer. Mass spectrometric determinations were made on an ESI-Q-TOF mass spectrometer (Micromass, UK). High resolution mass spectra measurements were performed on a GC-TOF mass spectrometer (Micromass, UK). Fluorescence measurements were performed on a PTI-C-700 Felix and Time-Master system. Fluorescence quantum yields were measured using standard methods[16] on air-equilibrated samples at room temperature. Quinine bisulfate in 0.05 mol L−1 H2SO4 (Φ = 0.546) was used as a reference.[16] The two-photon-excited fluorescence (TPEF) action cross-section spectra were measured according to the experimental protocol established by Xu and Webb,[17] using a mode-locked Ti/sapphire laser that delivers ~80 fs pulses at 76 MHz. Fluorescein (10−4 mol L−1 in 0.1 mol L−1 NaOH), whose TPEF action cross-sections are well known,[17] served as the reference. The quadratic dependence of the fluorescence intensity on the excitation intensity was verified for each data point, indicating that the measurements were carried out in intensity regimes in which saturation or photodegradation does not occur. The measurements were performed at room temperature on air-equilibrated solutions (10−5 mol L−1). The experimental uncertainty on the absolute action cross-sections determined by this method has been estimated to be ± 20 %.[17] Absorption spectra were measured on a HP-8453 spectrophotometer. Solvents were generally dried and distilled before use. Reactions were monitored by thin-layer chromatography on Merck silica gel 60 F254 precoated aluminium sheets. Column chromatography used Merck silica gel Si 60 (40–63 μm, 230–400 mesh). The pH-dependent fluorescence studies were performed according to the literature.[18]
Synthesis
The synthetic route of the LP is shown in Scheme 1.
3-(N-Methyl-N-(6-acetyl-2-naphthyl)amino) Methyl Propionate (4)
2-Acetyl-6-methylaminaphthalene (3) (5.53 g, 27.79 mmol), methyl 3-bromopropionate (7.00 g, 42.17 mmol), NaH2PO4 (5.90 g, 41.55 mmol), and NaI (1.72 g, 11.47 mmol) were dissolved in acetonitrile (190 mL), and the mixture was then refluxed for 38 h with stirring under N2, followed by the vacuum removal of MeCN. The resulting mixture was dissolved in CH2Cl2 (100 mL), and then washed with water, dried with anhydrous MgSO4, filtered, and the filtrate was concentrated by evaporating the solvent to get a viscous liquid. The crude product was purified by column chromatography using CH2Cl2 to afford 1.48 g (5.19 mmol, 30 %) of a yellow powder. Further purification could be achieved by recrystallisation from methanol to give needle-like crystals (mp 247−248°C). νmax (KBr)/cm−1 1731 (COOCH3), 1665 (CO). HRMS (EI) m/z: 285.8953, calcd for C17H19NO3: 285.3377. δH (DMSO-d6) 8.47 (s, 1H), 7.94 (d, J 8.0, 1H), 7.84 (d, J 8.0, 1H), 7.70 (d, J 8.0, 1H), 7.31 (d, J 8.0, 1H), 6.99 (s, 1H), 3.81 (t, J1 8.0, J2 4.0, 2H, NCH2), 3.61 (s, 3H, NCH3), 3.05 (s, 3H, OCH3), 2.64 (s, 3H, COCH3), 2.65 (t, J1 = J2 8.0, 2H, COCH2). δC (CDCl3, 100 MHz) 197.86, 172.44, 148.47, 137.69, 131.00, 130.98, 130.35, 126.26, 125.23, 124.53, 116.07, 105.63, 51.88, 48.46, 38.49, 31.84, 26.45. Anal. Calc. for C17H19NO3 (MW 285.34): C 71.56, H 6.71, N 4.91, O 16.82 %. Found: C 71.72, H 6.83, N 4.95, O 16.50.
3-(N-Methyl-N-(6-acetyl-2-naphthyl)amino) Propionic Acid (5)
3-(N-Methyl-N-(6- acetyl-2-naphthyl)amino) methyl propionate (4) (1.48 g, 5.19 mmol), KOH (0.62 g, 11.07 mmol), ethanol (50 mL), and water (10 mL) were placed into a 100 mL flask, the mixture was stirred for 20 h at room temperature and poured into 100 mL of ice water. Concentrated hydrochloric acid was then added dropwise while stirring. The resultant precipitate was filtered off and recrystallised from ethanol to give 1.40 g (5.17 mmol, 99.6 %) of a yellow powder (mp 285−284°C). νmax (KBr)/cm−1 3387 (OH), 1699 (COOH), 1645 (CO). HRMS (EI) m/z 271.9996; calcd for C16H17NO3: 271.1208. δH (DMSO-d6, 400 MHz) 8.46 (s, 1H), 7.94 (d, J 8.0, 1H), 7.84 (d, J 12.0, 1H), 7.70 (d, J 8.0, 1H), 7.32 (d, J 12.0, 1H), 6.99 (s, 1H), 3.77 (t, J1 = J2 8.0, 2H, NCH2), 3.06 (s, 3H, NCH3), 2.64 (s, 3H, COCH3), 2.55 (t, J1 8.0, J2 4.0, 2H, CH2CO). δC (DMSO-d6, 100 MHz) 197.55, 173.72, 149.13, 137.79, 131.25, 130.93, 130.56, 126.38, 125.02, 124.44, 116.80, 105.31, 48.24, 38.47, 32.00, 26.85. Anal. Calc. for C16H17NO3 (MW 271.31): C 70.83, H 6.32, N 5.16, O 17.69 %. Found: C 70.92, H 6.43, N 5.31, O 17.34.
3-(N-Methyl-N-(6-acetyl-2-naphthyl)amino) Propionic Acid N-Hydroxysuccinimide Ester (LP)
Compound 5 (1.40 g, 5.17 mmol), N(C2H5)3 (1.58 g, 15.64 mmol), and MeCN (55 mL) were added into a 100 mL flask and cooled to 0°C. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 1.20 g, 6.26 mmol) and N-hydroxysuccinimide (NHS, 0.66 g, 5.74 mmol) were added to the mixture. The reaction mixture was stirred for 4 h at 50°C, followed by the vacuum removal of solvent. The resulting mixture was dissolved in CH2Cl2 (150 mL), washed in turn with dilute hydrochloric acid (10 %), saturated sodium bicarbonate solution, saturated sodium chloride solution, and then dried with anhydrous MgSO4, filtered, and the filtrate was concentrated by evaporating the solvent to get a viscous solid. The crude product was purified by column chromatography (eluent: CH2Cl2/ethyl acetate, 10:1, v/v) to afford a yellow powder (1.00 g, 2.72 mmol) (mp 263−265°C) in 52 % yield. νmax (KBr)/cm−1 1621–1743 (CO). δH (CDCl3, 400 MHz) 8.34 (s, 1H), 7.95 (d, J 8.0, 1H), 7.85 (d, J 4.0, 1H), 7.67 (d, J 8.0, 1H), 7.18 (d, J 8.0, 1H), 6.93 (s, 1H), 3.94 (t, J1 8.0, J2 4.0, 2H, NCH2), 3.14 (s, 3H, NCH3), 2.95 (t, J1 4.0, J2 8.0, 2H, COCH2), 2.86 (s, 4H, 2CH2CO), 2.68 (s, 3H, COCH3). δC (CDCl3, 100 MHz) 197.77, 168.96, 167.16, 147.87, 137.58, 131.32, 131.18, 130.30, 126.41, 125.54, 124.79, 115.99, 106.16, 60.42, 48.18, 38.85, 28.95, 26.49, 25.60, 21.08. HRMS (EI) m/z 368.9676; calcd for C20H20N2O5: 368.1372. Anal. Calc. for C20H20N2O5 (MW 368.38): C 65.21, H 5.47, N 7.60, O 21.72. Found: C 65.36, H 5.53, N 7.30, O 21.81.
LP Labelling of N-Protein Antigen (Ag) for COVID-19
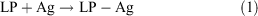
Antigen Labelling
To 50 μL of a N-protein antigen solution (5 mg mL−1), 5 μL carbonate buffer solution (pH 9.3, Na2CO3 8.6 g, NaHCO3 17.3 g, H2O 1 L), and 15 μL of LP (2 mg mL−1, DMSO) were added. The mixture was shaken for 2 h at 25°C on an orbital shaker at 200 rpm.
Ultrafiltration Purification
The above solution was added to an ultrafiltration tube, and PBS (phosphate buffer solution) (KH2PO4 2 mM, Na2HPO4 8 mM, NaCl 136 mM, KCl 2.6 mM, pH 7.2) was added to 80 % of the total volume, and then the mixture was centrifuged (7100–11100 g, 4°C) for 5–20 min. Centrifugal separation time was determined according to the remaining liquid in the ultrafiltration tube. The concentrate was centrifuged and washed 3–5 times with PBS. (If the amount of solution in the ultrafiltration tube was reduced in less than 20 min of centrifugation, the rotational speed was increased appropriately). HR-MS-ESI m/z 45719.62 ([M + H]+), 45741.60 ([M + Na]+); Calcd. 45719.64, 45741.63.
Preparation of an Immunochromatographic Strip
Principle and Structure of the Immunochromatographic Strip
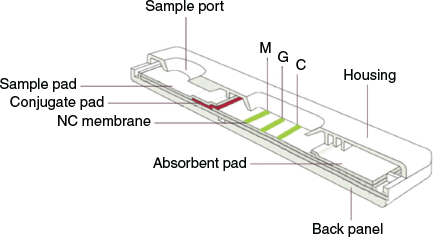
The immunochromatographic strip (ICS) had two test lines (M and G) and one quality control line (C). Its structure is shown in Fig. 2 and Fig. S1 (Supplementary Material).
|
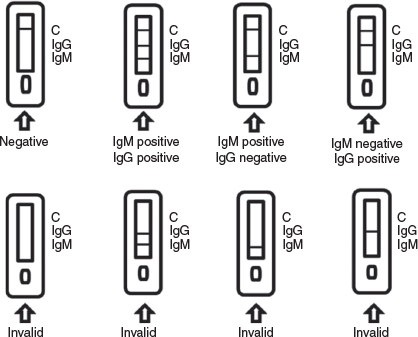
When an appropriate amount of test sample (50 μL of a dilute blood sample, which comprised 40 μL of whole blood sample and 160 μL of diluent. The diluent comprised 5 % sucrose, 5 % glucose, 0.5 % tween 20, 30 mmol L−1 3-morpholinopropanesulfonic acid (MOPS), 100 mmol L−1 KCl, 10 mmol L−1 ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA), pH 7.2) was added to the sample port of the detection card, the sample moved forward along the nitrocellulose (NC) membrane. When the sample contains a IgM antibody, the antibody will bind to the two-photon fluorescent probe-labelled antigen (COVID-19 N/S protein antigen) LP-Ag. The resulting immune complex formed an M line (Fig. 3) with the coated mouse anti-human monoclonal antibody (Anti-human IgM second antibody), indicating a positive response to the presence of the COVID-19 IgM antibody.
|
If the sample contained IgG antibodies, the IgG antibody also bound to the LP-Ag, and the resulting immune complex formed a G line (Fig. 3) with the coated mouse anti-human monoclonal antibody (Anti-human IgG second antibody), indicating a positive response to the presence of the COVID-19 IgG antibody.
If the test did not form G and M lines, a negative result was produced.
The detection card also contained a quality control line C (Fig. 3), for the fixed mouse anti-human IgG monoclonal antibody (Anti-human IgG first antibody). As the sample flowed, it drove the LP-Ag forward, and when the excess LP-Ag reached the C line, its coupled antigen bound to the antibody on the line. After a large amount of accumulation, the C line turned green (the C line must display colour to allow visual detection). Whether or not the test lines appear, there should be a quality control line C (which proves that the probe did pass through the whole test strip); if the quality control line C did not appear, it indicated that the test result was invalid and the sample needed to be re-tested.
Preparation Process of Immunochromatographic Strip
The preparation of test lines and quality control line. Using a fully automatic continuous dispenser and sprayer (Bio-Dot XYZ-3050), 1 mg mL−1 of purified mouse anti-human IgM second antibody, mouse anti-human IgG second antibody, and mouse anti-human IgG first antibody were sprayed and coated the positions of the test lines (M and G) and the quality control line C on the NC membrane at a speed of 0.80 μL cm−1. The NC membrane was then dried at 35°C for 5 h, and sealed and stored at 4°C.
The coating of fluorescent antigen LP-Ag on the conjugate pad. Fluorescent antigen LP-Ag was resuspended in the storage solution (800 μL) (5 % sucrose, 5 % glucose, 0.5 % tween 20, 30 mmol L−1 MOPS, 100 mmol L−1 KCl, 10 mmol L−1 EGTA, pH 7.2) to be transformed into a 2 mg mL−1 fluorescent antigen solution and stored at 1–4°C.
The conjugate pad was immersed in buffer solution (5 % sucrose, 5 % glucose, 0.5 % tween 20, 30 mmol L−1 MOPS, 100 mmol L−1 KCl, 10 mmol L−1 EGTA, pH 7.2) for 15 min, and then dried in air at room temperature. The dried conjugate pad was transferred to a thermostatic air-blast dryer, and dried at 35°C for 2 days. The conjugate pad was then sprayed and coated with LP-Ag at a speed of 0.20 μL mm−1, and dried at 35°C for 1 day.
The pretreatment of the sample pad. The sample pad was immersed in buffer solution (5 % sucrose, 5 % glucose, 0.5 % tween 20, 30 mmol L−1 MOPS, 100 mmol L−1 KCl, 10 mmol L−1 EGTA, pH 7.2) for 15 min, and then dried in air at room temperature. The dried sample pad was transferred to a thermostatic air-blast dryer, and dried at 35°C for 1 day.
The assembly of the test strip. The test strip assembly is shown in Fig. 2. The NC membrane, the absorbent pad, the conjugate pad, and the sample pad were sequentially pasted on a PVC bottom plate (back panel), and the distance between adjacent pads was 2 mm. The NC membrane was attached to the PVC bottom plate, the conjugate pad and the absorbent pad were lapped at both ends of the NC membrane, and the sample pad was lapped at one end of the conjugate pad.
Clinical Application Statistical Evaluation
Clinical validation of the COVID-19 two-photon fluorescence detection kit (fluorescence immunochromatography) was carried out according to the requirements of ‘the vitro diagnostic reagent registration management method (Trial)’, to evaluate the clinical application, effectiveness and applicability of the kit.
We collected 320 serum, plasma, and whole blood samples in this clinical study, and analysed samples using the COVID-19 two-photon fluorescence detection kit (fluorescence immunochromatography) developed here, the COVID-19 nucleic acid detection kit (fluorescence PCR method) produced by Shanghai Zhijiang Biotechnology Co., Ltd, and the 2019-nCoV antibody detection kit (colloidal gold method) produced by Guangzhou Wanfu Biotechnology Co., Ltd. The presence of COVID-19 in human whole blood, plasma, and serum was determined. By comparing the results of the detection systems, it was verified that the COVID-19 two-photon fluorescence detection kit (fluorescence immunochromatography) was equivalent to the COVID-19 nucleic acid detection kit (fluorescence PCR method) produced by Shanghai Zhijiang Biotechnology Co., Ltd and the COVID-19 antibody detection kit (colloidal gold method) produced by Guangzhou Wanfu Biotechnology Co., Ltd.
Collection and Storage of Clinical Specimens
-
This kit was suitable for serum, EDTA anticoagulant plasma, and EDTA anticoagulant whole blood samples. Fresh samples are recommended.
-
Serum and plasma samples were only stored for 7 days at 2–8°C, whole blood samples only for 2 days at 2–8°C, and serum, plasma, and whole blood samples were only frozen for 3 months at –20 ± 5°C. Avoid repetitively freezing and thawing. Transport time of samples was not able to exceed 7 days under 20 ± 5°C.
-
Samples must be restored to room temperature before testing. The frozen and preserved samples need to be completely melted, reheated, and mixed evenly before use. Avoid repeated freeze-thaw.
-
Preservatives (e.g. 1 % proclin 300) may be added to the sample and will not interfere with the experimental results.
-
Sample: Bilirubin ≤ 50 mg dL−1, hemoglobin ≤ 1000 mg dL−1, and chylus ≤ 1000 mg dL−1 will not interfere with the test.
Results and Discussion
Design and Synthesis of 3-(N-Methyl-N-(6-acetyl-2-naphthyl)amino) Propionic Acid N-Hydroxysuccinimide Ester (LP)
6-Acyl-2-hydroxynaphthalene (2)[4] and 6-Acyl-N-methyl-2-naphthylamine (3),[4] were synthesised according to literature procedures. The acedan derivative LP was obtained in 52 % yield by the reaction of N-hydroxysuccinimide (NHS) with 5 in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (Scheme 1).
Absorption and One-Photon Excited Fluorescence Spectra of LP and LP-Ag in PBS
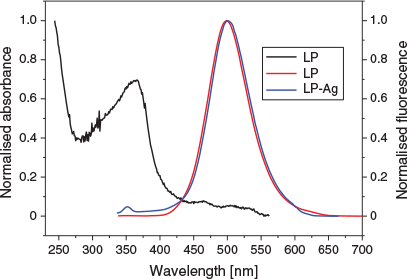
To obtain insight into the emission properties of LP and LP-Ag in PBS, one-photon (OP) excited fluorescence spectra changes were investigated upon their addition to PBS solvent (Fig. 4). One-photon emission spectra of LP and LP-Ag were almost identical in PBS, and similar to those of other acedan fluorophore-derived dyes. Their maximum absorption and emission wavelengths were 365 and 500 nm, respectively.
|
Two-Photon Absorption Cross-Section of LP and LP-Ag versus Two-Photon Excited Wavelength in PBS
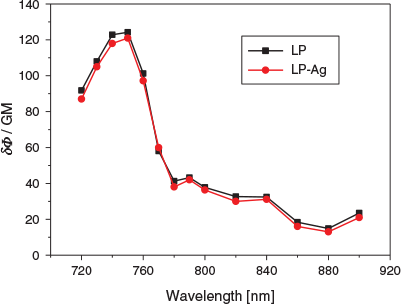
The two-photon (TP) action spectra of LP and LP-Ag were similar and did not differ much, and they had a TP excitation maximum at 750 nm, and the corresponding two-photon action cross-sections (δΦ) were 124 (LP) and 121 GM (LP-Ag) (1 GM ≡ 10−50 cm4 s photon−1), respectively (Fig. 5). When the two-photon excitation wavelength was less than 750 nm, the two-photon emission cross-section augmented with the increase of wavelength, while when the two-photon excitation wavelength was more than 750 nm, it decreased significantly with the increase of wavelength.
|
Clinical Findings and Analysis
Comparison with the COVID-19 Nucleic Acid Detection Kit (PCR Method) (Table 1)

|
(1) Negative and Positive Coincidence Rate, Total Coincidence Rate, and its 95 % Confidence Interval
(i) Positive coincidence rate: 98/(98 + 1) × 100 % = 98.98 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [98.98 %, 98.98 %]
(ii) Negative coincidence rate: 220/(1 + 220) × 100 % = 99.55 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [99.55 %, 99.55 %]
(iii) Total coincidence rate: (98 + 220)/(98 + 1 + 1 + 220) × 100 % = 99.38 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [99.38 %, 99.38 %]
In the above equations, CI is the confidence interval; p is the positive coincidence rate, negative coincidence rate, or total coincidence rate, if the p value is 100 %, its recommended value is 99.99 % for analysis.
(2) Consistency Coefficient Kappa Value (K)
Kappa = 0.972 (K > 0.75) (SPSS software)
The detection results of test reagent and reference reagent are consistent (K = 0.972 > 0.75), and there is no statistical difference between the detection results of the test reagent and reference reagent. Therefore, the detection results of the two reagents are highly consistent.
(3) Sensitivity (SE), Specificity (SP), and Accuracy (AC)
SE = 98/(98 + 1) × 100 % = 98.99 %
SP = 220/(1 + 220) × 100 % = 99.55 %
AC = (98 + 220)/320 × 100 % = 99.38 %
Comparison with the COVID-19 Antibody Detection Kit (Colloidal Gold Method) (Table 2)

|
(1) Negative and Positive Coincidence Rate, Total Coincidence Rate and its 95 % Confidence Interval
(i) Positive coincidence rate: 103/(103 + 2) × 100 % = 98.10 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [95.87 %, 100.00 %]
(ii) Negative coincidence rate: 124/(1 + 124) × 100 % = 99.20 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [97.64 %, 100.00 %]
(iii) Total coincidence rate: (113 + 124)/(113 + 1 + 2 + 124) × 100 % = 98.75 %
95 % CI: p ± 1.96 × [p (1 – p)/n]1/2 = [97.34 %, 100.00 %]
(2) Consistency Coefficient Kappa Value (K)
Kappa = 0.975 (K > 0.75)
The detection results of the test reagent and reference reagent are consistent (K = 0.975 > 0.75), and there is no statistical difference between the detection results of the test reagent and reference reagent. Therefore, the detection results of the two reagents are highly consistent.
(3) Sensitivity (SE), Specificity (SP), and Accuracy (AC)
SE = 103/(103 + 2) × 100 % = 98.10 %
SP = 214/(1 + 214) × 100 % = 99.53 %
AC = (103 + 214)/320 × 100 % = 99.06 %
The clinical validation of our COVID-19 TP fluorescence immunochromatographic kit (TPICK) (fluorescence immunochromatography) was carried out according to the requirements of ‘the vitro diagnostic reagent registration management method (Trial)’, to evaluate the clinical application, effectiveness, and applicability of the kit. By comparing the results of the two detection systems, it was verified that the TPICK was equivalent to the COVID-19 nucleic acid detection kit (PCR method) produced by Shanghai Zhijiang Biotechnology Co., Ltd and the COVID-19 antibody detection kit (colloidal gold method) produced by Guangzhou Wanfu Biotechnology Co., Ltd.
In comparison with the COVID-19 PCR method and the COVID-19 colloidal gold method, the positive coincidence rate (PCR), negative coincidence rate (NCR), and total coincidence rate (TCR) of TPICK were 98.98 and 98.10, 99.55 and 99.20, and 99.38 and 98.75 %, respectively, and its consistency coefficient kappa value (K) was 0.972 and 0.975 (K > 0.75) (SPSS software). The sensitivity (SE), specificity (SP), and accuracy (AC) of TPICK were 98.99 and 98.10, 99.55 and 99.53, and 99.38 and 99.06 %, respectively. The detection results of the test reagent and reference reagents were consistent (K > 0.75), and there is no statistical difference between the detection results of the test reagent and reference reagents. Therefore, the detection results of the two reagents are highly consistent.
In addition, the detection time of the two-photon fluorescence immunochromatographic strip (TPICS) is very short, generally the detection of a sample takes not more than 10 min, and the amount of the sample is very small, 5–10 μL, which brings great convenience to the detection.
Two-Photon Scanning Microscopy (TPM) Imaging
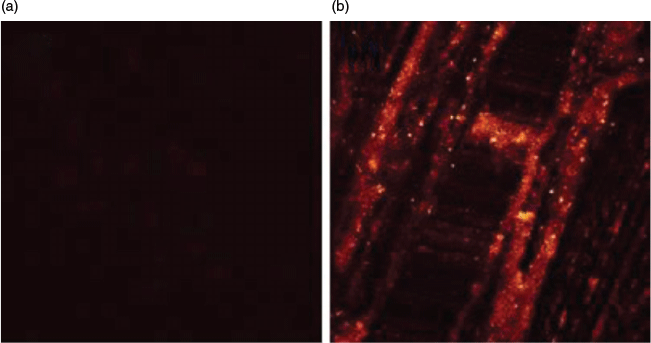
To further investigate the utility of this fluorescent antigen LP-Ag in deep tissue imaging, TPM images were obtained from a part of a mouse lung tissue slice incubated with 1 µM LP-Ag for 30 min at 37°C. TPM images were obtained in the same plane at a depth of ~120 µm. For normal mouse lung tissue, the TPM image appeared dark and the fluorescence was invisible because there were no antibodies in the tissue. The fluorescence antigen LP-Ag was not able to be adsorbed into the tissue cells, so it did not show fluorescence when excited, and a negative result was produced (Fig. 6a). While, for mouse lung tissue infected with COVID-19, the fluorescence emission was clearly visible (Fig. 6b). This result demonstrates that LP-Ag is capable of detecting COVID-19 at a depth of 120 µm in living tissues by using TPM.
Conclusion
In conclusion, a two-photon fluorescence immunochromatographic strip/kit for rapid diagnosis of COVID-19 (TPICS or TPICK) was developed. By means of TPICS, which uses only 5–10 μL of blood sample, a participant can be quickly diagnosed (generally less than 10 min) as positive or negative for COVID-19, which allows for large-scale routine physical and outpatient screening. The sensitivity, specificity, and accuracy of TPICK can be compared with those of the nucleic acid detection and colloidal gold methods. It is much simpler than the nucleic acid detection method, which can greatly shorten the detection period, and it is much more stable, and which overcomes greater uncertainty in the detection results because of the loss of functional groups adsorbed by colloidal gold in colloidal gold method.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Chibao Huang completed the main research work and Shuai Kang, Fuxun Yu, and Zairong Wei participated in part of the research work.
Supplementary Material
Nuclear magnetic resonance spectra (1H NMR and 13C NMR) for intermediates and target compound are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Guizhou Province High-level Innovative Talents Training Project – Hundred Talents Program (No. [2016]5683), Science and Technology Project in Guizhou Province (Nos. [2020]4Y001, [2020]4Y004, [2018]2785), the Natural Science Research Project of Guizhou Department of Education (Nos. KY[2019]068, KY[2018]028), the National Natural Science Foundation of China (No. 21562050), the Special Fund Project of the Construction of the Eighth Batch of Scientific and Technological Innovation Talent Team in Guizhou Province [No. (2015)4007], Guizhou Science and Technology Fund Project (No. J[2015]2146), the Key Project of Education Department of Guizhou Province (No. KY[2014]296), and Science and Technology Support Project of Guizhou Province: 2020–5012.
References
[1] Q. Zhuang, S. Wang, M. Wu, S. Liu, W. Jiang, G. Hou, J. Li, K. Wang, J. Yu, J. Chen, J. Chen, Chin. Sci. Bull. 2013, 58, 3183.| Crossref | GoogleScholarGoogle Scholar | 32214742PubMed |
[2] H. Geng, W. Tan, Sci. China Life Sci. 2013, 56, 683.
| Crossref | GoogleScholarGoogle Scholar | 23917839PubMed |
[3] J. W. Wang, Y. B. Wang, Z. R. Chang, K. X. Yan, W. J. Tan, J. G. Qu, N. S. Xia, L. Ruan, T. Hung, Virol. Sin. 2006, 21, 207.
[4] H. M. Kim, C. Jung, B. R. Kim, S.-Y. Jung, J. H. Hong, Y.-G. Ko, K. J. Lee, B. R. Cho, Angew. Chem. Int. Ed. 2007, 46, 3460.
| Crossref | GoogleScholarGoogle Scholar |
[5] M. K. Kim, C. S. Lim, J. T. Hong, J. H. Han, H.-Y. Jang, H. M. Kim, B. R. Cho, Angew. Chem. Int. Ed. 2010, 49, 364.
| Crossref | GoogleScholarGoogle Scholar |
[6] M. Y. Kang, C. S. Lim, H. S. Kim, E. W. Seo, H. M. Kim, O. Kwon, B. R. Cho, Chem. – Eur. J. 2012, 18, 1953.
| Crossref | GoogleScholarGoogle Scholar | 22241648PubMed |
[7] H. M. Kim, M. S. Seo, M. J. An, J. H. Hong, Y. S. Tian, J. H. Choi, O. Kwon, K. J. Lee, B. R. Cho, Angew. Chem. Int. Ed. 2008, 47, 5167.
| Crossref | GoogleScholarGoogle Scholar |
[8] L. Xue, Z. J. Fang, G. Li, H. H. Wang, H. Jiang, Sens. Actuators B Chem. 2011, 156, 410.
| Crossref | GoogleScholarGoogle Scholar |
[9] A. S. Rao, D. Kim, H. Nam, H. Jo, K. H. Kim, C. Ban, K. H. Ahn, Chem. Commun. 2012, 48, 3206.
| Crossref | GoogleScholarGoogle Scholar |
[10] C. Chung, D. Srikun, C. S. Lim, C. J. Chang, B. R. Cho, Chem. Commun. 2011, 47, 9618.
| Crossref | GoogleScholarGoogle Scholar |
[11] L. Yuan, F. Jin, Z. Zeng, C. Liu, S. Luo, J. Wu, Chem. Sci. 2015, 6, 2360.
| Crossref | GoogleScholarGoogle Scholar | 28706654PubMed |
[12] E. W. Seo, J. H. Han, C. H. Heo, J. H. Shin, H. M. Kim, B. R. Cho, Chemistry 2012, 18, 12388.
| Crossref | GoogleScholarGoogle Scholar | 22907767PubMed |
[13] X. Yang, Y. Zhou, X. Zhang, S. Yang, Y. Chen, J. Guo, X. Li, Z. Qing, R. Yang, Chem. Commun. 2016, 52, 10289.
| Crossref | GoogleScholarGoogle Scholar |
[14] J. H. Lee, C. S. Lim, Y. S. Tian, J. H. Han, B. R. Cho, J. Am. Chem. Soc. 2010, 132, 1216.
| Crossref | GoogleScholarGoogle Scholar | 20052975PubMed |
[15] H. D. Xiao, J. H. Li, J. Zhao, G. Yin, Y. W. Quan, J. Wang, R. Y. Wang, J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 1633.
| Crossref | GoogleScholarGoogle Scholar |
[16] D. F. Eaton, J. Photochem. Photobiol. B 1988, 2, 523.
| Crossref | GoogleScholarGoogle Scholar | 3150004PubMed |
[17] C. Xu, W. W. Webb, J. Opt. Soc. Am. B 1996, 13, 481.
| Crossref | GoogleScholarGoogle Scholar |
[18] S. C. Burdette, G. K. Walkup, B. Spingler, R. Y. Tsien, S. J. Lippard, J. Am. Chem. Soc. 2001, 123, 7831.
| Crossref | GoogleScholarGoogle Scholar | 11493056PubMed |