A high level of outcrossing in the vulnerable species Prosopis rubriflora in a Chaco remnant
Fábio M. Alves A B , Ângela L. B. Sartori C , Maria I. Zucchi D , Ana M. G. Azevedo-Tozzi A , Evandro V. Tambarussi E and Anete P. de Souza A B FA Department of Plant Biology, Institute of Biology, PO Box: 6109, University of Campinas – UNICAMP, 13083-970, Campinas, SP, Brazil.
B Center for Molecular Biology and Genetic Engineering (CBMEG), University of Campinas – UNICAMP, CEP 13083-875, Campinas, SP, Brazil.
C Center for Biological and Health Sciences (CCBS), Federal University of Mato Grosso do Sul – UFMS, Cidade Universitária s/n, PO Box 549, 79070-900, Campo Grande, MS, Brazil.
D São Paulo Agency of Technology and Agro-Business, Pólo Apta Centro Sul, SP 127, km 30, 13400-970, Piracicaba, SP, Brazil.
E Department of Forestry Engineering, State University of the Central-West, PR 153, km 7, Irati, PR, 84500-000, Brazil.
F Corresponding author. Email: anete@unicamp.br
Australian Journal of Botany 66(4) 360-368 https://doi.org/10.1071/BT17195
Submitted: 11 October 2017 Accepted: 05 July 2018 Published: 6 August 2018
Abstract
Prosopis rubriflora Hassl. is a tree species typically found in chaquenian areas, mainly with an arborised phytophysiognomy in the southern region of the Pantanal wetland. This species has become vulnerable in recent decades as a result of considerable increases in anthropogenic activities such as cattle breeding, and this vulnerability has also been observed in several other native species. The goal of this study was to estimate the mating system of P. rubriflora in a Chaco remnant by analysing 10 microsatellite markers. Samples were collected over 2 years (2010–213 seedlings and 2011–180 seedlings), and the results suggest that the mating system of P. rubriflora is preferably allogamous. A progeny array was predominantly composed of half-sibs (from 76 to 79%), full-sibs (from 15%) and self-half-sibs (from 6 to 9%). The outcrossing rate between related individuals was significant in 2011 but not in 2010. The average co-ancestry coefficient (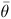 ) ranged from 0.158 to 0.162, and the variance effective size (Ne) ranged from 3.05 to 3.13. The number of seed trees required for seed collection (m) to retain an effective size of 150 in progeny array samples was 48–49. The high levels of outcrossing of P. rubriflora appear to be related to several mechanisms that avoid selfing and due to the behaviour of native pollinators, which clearly contribute to the gene flow of the species.
) ranged from 0.158 to 0.162, and the variance effective size (Ne) ranged from 3.05 to 3.13. The number of seed trees required for seed collection (m) to retain an effective size of 150 in progeny array samples was 48–49. The high levels of outcrossing of P. rubriflora appear to be related to several mechanisms that avoid selfing and due to the behaviour of native pollinators, which clearly contribute to the gene flow of the species.
Additional keywords: conservation, mating system, neotropics, pantanal, stepic savanna.
References
Aizen MA, Feinsinger P (1994) Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 75, 330–351.| Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina.Crossref | GoogleScholarGoogle Scholar |
Alves FM, Zucchi MI, Azevedo-Tozzi AMG, Sartori ALB, Souza AP (2014) Characterization of microsatellite markers developed from Prosopis rubriflora and Prosopis ruscifolia (Leguminosae – Mimosoideae), legume species that are used as models for genetic diversity studies in Chaquenian areas under anthropization in South America. BMC Research Notes 7, 375
| Characterization of microsatellite markers developed from Prosopis rubriflora and Prosopis ruscifolia (Leguminosae – Mimosoideae), legume species that are used as models for genetic diversity studies in Chaquenian areas under anthropization in South America.Crossref | GoogleScholarGoogle Scholar |
Alves FM, Sartori ALB, Zucchi MI, Azevedo-Tozzi AMG, Tambarussi EV, Alves-Pereira A, Souza AP (2018) Genetic structure of two Prosopis species in Chaco areas: a lack of allelic diversity diagnosis and insights into the allelic conservation of the affected species. Ecology and Evolution
| Genetic structure of two Prosopis species in Chaco areas: a lack of allelic diversity diagnosis and insights into the allelic conservation of the affected species.Crossref | GoogleScholarGoogle Scholar |
Bessega C, Ferreyra L, Julio N, Montoya S, Saidman B, Vilardi J (2000) Mating system parameters in species of genus Prosopis (Leguminosae). Hereditas 132, 19–27.
| Mating system parameters in species of genus Prosopis (Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Bessega C, Pometti CL, Ewens M, Saidman BO, Vilardi JC (2012) Strategies for conservation for disturbed Prosopis alba (Leguminosae, Mimosoideae) forests based on mating system and pollen dispersal parameters. Tree Genetics & Genomes 8, 277–288.
| Strategies for conservation for disturbed Prosopis alba (Leguminosae, Mimosoideae) forests based on mating system and pollen dispersal parameters.Crossref | GoogleScholarGoogle Scholar |
Burkart A (1976) A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae) (Part 2.). Catalogue of the recognized species of Prosopis. Journal of the Arnold Arboretum 57, 450–525.
Bush JK, van Auken OW (1990) Growth and survival of Prosopis glandulosa seedlings associated with shade and herbaceous competition. Botanical Gazette 151, 234–239.
| Growth and survival of Prosopis glandulosa seedlings associated with shade and herbaceous competition.Crossref | GoogleScholarGoogle Scholar |
Campos CM, Campos VE, Miguel F, Cona MI (2016) Management of protected areas and its effect as ecosystem function: removal of Prosopis flexuosa seeds by mammals in Argentinian drylands. PLoS One 11,
| Management of protected areas and its effect as ecosystem function: removal of Prosopis flexuosa seeds by mammals in Argentinian drylands.Crossref | GoogleScholarGoogle Scholar |
Carreras R, Bessega C, López CR, Saidman BO, Vilardi JC (2017) Developing a breeding strategy for multiple trait selection in Prosopis alba Griseb., a native forest species of the Chaco Region in Argentina. Forestry: An International Journal of Forest Research 90, 199–210.
Catalano SA, Vilardi JC, Tosto D, Saidman BO (2008) Molecular phylogeny and diversification history of Prosopis (Fabaceae: Mimosoideae). Biological Journal of the Linnean Society. Linnean Society of London 93, 621–640.
| Molecular phylogeny and diversification history of Prosopis (Fabaceae: Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Molecular Biology and Evolution 24, 621–631.
| Microsatellite null alleles and estimation of population differentiation.Crossref | GoogleScholarGoogle Scholar |
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nature Reviews. Genetics 10, 783–796.
| The genetics of inbreeding depression.Crossref | GoogleScholarGoogle Scholar |
Doolittle DP (1987) ‘Population genetics: basic principles.’ (Springer-Verlag: New York)
Eduardo A, De Lacerda B, Kanashiro M, Sebbenn AM (2008) Long-pollen movement and deviation of random mating in a low-density continuous population of a tropical tree Hymenaea courbaril in the Brazilian Amazon. Biotropica 40, 462–470.
| Long-pollen movement and deviation of random mating in a low-density continuous population of a tropical tree Hymenaea courbaril in the Brazilian Amazon.Crossref | GoogleScholarGoogle Scholar |
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annual Review of Ecology and Systematics 24, 217–242.
| Population genetic consequences of small population size: implications for plant conservation.Crossref | GoogleScholarGoogle Scholar |
Freitas TG, Souza CS, Aoki C, Arakaki LMM, Stefanello TH, Sartori ALB, Sigrist MR (2013) Flora of Brazilian humid Chaco: composition and reproductive phenology. Check List 9, 973–979.
| Flora of Brazilian humid Chaco: composition and reproductive phenology.Crossref | GoogleScholarGoogle Scholar |
Fterich A, Mahdhi M, Caviedes MA, Pajuelo E, Rivas R, Rodriguez-Llorente ID, Mars M (2011) Characterization of root-nodulating bacteria associated to Prosopis farcta growing in the arid regions of Tunisia. Archives of Microbiology 93, 385–397.
| Characterization of root-nodulating bacteria associated to Prosopis farcta growing in the arid regions of Tunisia.Crossref | GoogleScholarGoogle Scholar |
Furlan E, Stoklosa J, Griffiths J, Gust N, Ellis R, Huggins RM, Weeks AR (2012) Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchus anatinus. Ecology and Evolution 2, 844–857.
| Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchus anatinus.Crossref | GoogleScholarGoogle Scholar |
Genise J (1990) Observaciones sobre la biologia floral de Prosopis (Leguminosae, Mimosoideae). II. Fases florales y visitantes en el distrito chaqueño serrano. Darwiniana 30, 71–85.
Goudet J (1995) Fstat (version 2.9.3.2.): a computer program to calculate F-statistics. Journal of Heredity 86, 485–486.
| Fstat (version 2.9.3.2.): a computer program to calculate F-statistics.Crossref | GoogleScholarGoogle Scholar |
Hueck K (1972) ‘As florestas da América do Sul: ecologia, composição e importância econômica.’ (Polígono: São Paulo, Brazil)
IBGE (2012) ‘Manual técnico da vegetação Brasileira.’ (IBGE: Rio de Janeiro)
Kageyama PY, Gandara FB (1998) Consequências genéticas da fragmentação sobre populações de espécies arbóreas. Série Técnica IPEF 32, 65–70.
Lewis GP, Schrire B, Mackinder B, Lock M (2005) ‘Legumes of the world.’ (Royal Botanic Gardens, Kew, UK)
Lima TE (2012) Perturbação ambiental em remanescentes de Chaco e mecanismos de defesa em leguminosas. MSc thesis. Universidade Federal de Mato Grosso do Sul, Brazil.
Lorenzi H (2002) ‘Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. Vol. 2.’ (2nd edn) (Instituto Plantarum: Nova Odessa-SP, Brazil)
Manoel RO, Freitas MLM, Furlani-Júniro E, Alves PF, Morales MLT, Sebbenn AM (2015) Individual, fruit, and annual variation in correlated mating in Genipa americana population. Silvae Genetica 64, 108–116.
Miranda RQ, Oliveira MTP, Correia RM, Almeida-Cortez JS, Pompelli MF (2011) Germination of Prosopis juliflora (Sw) DC seeds after scarification treatments. Plant Species Biology 26, 186–192.
| Germination of Prosopis juliflora (Sw) DC seeds after scarification treatments.Crossref | GoogleScholarGoogle Scholar |
MMA-IBAMA (2010) ‘Projeto de monitoramento do desmatamento dos biomas brasileiros por satélite. Monitoramento do bioma Pantanal 2002 a 2008.’ (MMA: Brasília)
Mori ES, Sebbenn AM, Tambarussi EV, Guries RP (2013) Sistema de reprodução em populações naturais de Peltophorum dubium. Scientia Forestalis 41, 307–317.
Mottura MC (2006) Development of microsatellites in Prosopis spp. and their application to study the reproduction system. PhD thesis. TheUniversity of Göttingen, Göttingen, Germany.
Mottura MC, Finkeldey R, Verga AR, Gailing O (2005) Development and characterization of microsatellite markers for Prosopis chilensis and Prosopis flexuosa and cross-species amplification. Molecular Ecology Resources 5, 487–489.
Pometti CL, Vilardi JC, Saidman BO (2011) Mating system parameters and genetic structure in Argentinean populations of Acacia caven (Leguminosae, Mimosoideae). Plant Systematics and Evolution 292, 25–32.
| Mating system parameters and genetic structure in Argentinean populations of Acacia caven (Leguminosae, Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Pott A, Pott VJ (2003) ‘Espécies de Fragmentos Florestais em Mato Grosso do Sul. In ’Fragmentação florestal e Alternativas de Desenvolvimento rural na Região Centro-Oeste.’ (Ed. RB Costa). pp. 26–52. (UCDB: Campo Grande, Brazil)
Ribeiro RA, Lovato MB (2004) Mating system in a Neotropical tree species, Senna multijuga (Fabaceae). Genetics and Molecular Biology 27, 418–424.
| Mating system in a Neotropical tree species, Senna multijuga (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Ritland K (1989) Correlated matings in the partial selfer Mimulus guttatus. Evolution 43, 848–859.
| Correlated matings in the partial selfer Mimulus guttatus.Crossref | GoogleScholarGoogle Scholar |
Ritland K (2002) Extensions of models for the estimation of mating systems using n independent loci. Heredity 88, 221–228.
| Extensions of models for the estimation of mating systems using n independent loci.Crossref | GoogleScholarGoogle Scholar |
Ritland K, Jain S (1981) A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 47, 35–52.
| A model for the estimation of outcrossing rate and gene frequencies using n independent loci.Crossref | GoogleScholarGoogle Scholar |
Rocha BN, Martins CR, Missio EL (2009) Superação de dormência e germinação de sementes de Inhanduvá (Prosopis affinis) Sprenger. Revista da FZVA 16, 278–287.
Sebbenn AM (2002) Número de árvores matrizes e conceitos genéticos na coleta de sementes para reflorestamento com espécies nativas. Revista do Instituto Florestal 14, 115–132.
Sebbenn AM (2006) Sistema de reprodução em espécies arbóreas tropicais e suas implicações para a seleção de árvores matrizes para reflorestamentos ambientais. In ‘Pomares de sementes de espécies nativas’. (Eds AR Higa, LD Silva) pp. 193–198. (FUPEF: Curitiba, Brazil)
Sigrist MR, Stefanello TH, Souza CS, Vargas W, Almeida KSM, Laroca S, Mansano VF (2018) Phenology and pollination ecology of Prosopis rubriflora (Leguminosae, Mimosoideae), a species from the semi-arid Brazilian Chaco. Brazilian Journal of Botany 41, 103–115.
| Phenology and pollination ecology of Prosopis rubriflora (Leguminosae, Mimosoideae), a species from the semi-arid Brazilian Chaco.Crossref | GoogleScholarGoogle Scholar |
Sobierajski GR, Kageyama PY, Sebbenn AM (2006) Sistema de reprodução em nove populações de Mimosa scabrella Bentham (Leguminosae). Scientia Forestalis 71, 37–49.
Solbrig OT, Cantino PD (1975) Reproductive adaptations in Prosopis (Leguminosae, Mimosoideae). Journal of the Arnold Arboretum 56, 185–210.
Souza-Lima ES, Sinani RT, Pott A, Sartori ALB (2017) Mimosoideae (Leguminosae) in the Brazilian Chaco of Porto Murtinho, Mato Grosso do Sul. Rodriguésia 68, 263–290.
| Mimosoideae (Leguminosae) in the Brazilian Chaco of Porto Murtinho, Mato Grosso do Sul.Crossref | GoogleScholarGoogle Scholar |
Squillace AE (1974) Average correlations among offspring from open-pollinated forest trees. Silvae Genetica 23, 149–156.
Szczecińska M, Sramko G, Wołosz K (2016) Genetic diversity and population structure of the rare and endangered plant species Pulsatilla patens (L.) Mill in east central Europe. PLoS One 11,
| Genetic diversity and population structure of the rare and endangered plant species Pulsatilla patens (L.) Mill in east central Europe.Crossref | GoogleScholarGoogle Scholar |
Tambarussi EV, Boshier DH, Vencovsky R, Freitas MLM, Di-Dio OJ, Sebbenn AM (2016) Several small: how inbreeding affects conservation of Cariniana legalis Mart. Kuntze (Lecythidaceae) the Brazilian Atlantic Forest’s largest tree. International Forestry Review 18, 502–510.
| Several small: how inbreeding affects conservation of Cariniana legalis Mart. Kuntze (Lecythidaceae) the Brazilian Atlantic Forest’s largest tree.Crossref | GoogleScholarGoogle Scholar |
Vencovsky R, Crossa J (2003) Measurements of representativeness used in genetic resources conservation and plant breeding. Crop Science 43, 1912–1921.
| Measurements of representativeness used in genetic resources conservation and plant breeding.Crossref | GoogleScholarGoogle Scholar |
Vilela AE, Ravetta DA (2001) The effect of seed scarification and soil-media on germination, growth, storage, and survival of seedlings of five species of Prosopis L. (Mimosaceae). Journal of Arid Environments 48, 171–184.
| The effect of seed scarification and soil-media on germination, growth, storage, and survival of seedlings of five species of Prosopis L. (Mimosaceae).Crossref | GoogleScholarGoogle Scholar |
Walter KS, Gillett HJ (1998) ‘1997 IUCN Red list of threatened plants.’ (IUCN – The World Conservation Union: Gland, Switzerland)
Yanosky A (2013) Paraguay’s challenge of conserving natural habitats and biodiversity with global markets demanding for products. In ‘Conservation biology: voices from the tropics’. (Eds PH Raven, NS Sodhi, L Gibson), pp. 113–119. (John Wiley & Sons Ltd: Oxford, UK)
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends in Ecology & Evolution 11, 413–418.
| The population genetic consequences of habitat fragmentation for plants.Crossref | GoogleScholarGoogle Scholar |


