Sabah’s hidden giant: Nepenthes pongoides (Nepenthaceae), a micro-endemic tropical pitcher plant from northern Borneo
Alviana Damit A * , Nur Adillah Mohd Yusof A , Jemson Jumian A , Charles Clarke B and Alastair S. Robinson C *
C *
A Forest Research Centre,
B
C
Handling Editor: Noushka Reiter
Abstract
A poorly characterised carnivorous tropical pitcher plant (Nepenthes) was identified from old reports of a rarely visited low-elevation ultramafic peak in central Sabah, Malaysian Borneo. Potentially apomorphic characters in the limited photographic evidence available led the authors to hypothesise that the taxon represented an undescribed species.
To locate and document the unknown taxon in situ and, if appropriate, gather sufficient data and voucher material to enable its formal description and associated conservation status assessment.
An expedition was made to the type locality to make field observations of the taxon, record habitat, population and ecological data such as infauna, prey spectra and numbers of individuals, and to collect representative vouchers, minimising negative impacts on the small population by taking material in the form of terminal cuttings to allow for the in situ regeneration of sampled individuals from axillary nodes.
Nepenthes pongoides is described and illustrated as new to science. The species is characterised by its large size, peltate tendril exsertion, absence of upper pitchers and extremely well-developed, persistent indumentum of long, coarse, dark reddish trichomes, the extent of which is unsurpassed in any other known Bornean Nepenthes species.
One of the largest species of Nepenthes described in recent years, N. pongoides is endemic to the relatively low-elevation ultramafic Meliau Range of central Sabah. Only 39 mature individuals have been observed across two subpopulations, therefore the species is here assessed as CR (Critically Endangered) under the IUCN Red List criteria owing to its extremely small population size, limited area of occurrence and very high threat of unsustainable poaching for the horticultural trade. As is the case for many microendemic species, the taxon is extremely vulnerable to stochastic events such as fire which, on sufficient scale, could represent extinction level events.
The documentation of such remarkable new species in comparatively well-explored rainforest regions such as those of northern Borneo highlights the importance of targeted exploration in remaining wilderness areas to uncover hidden biodiversity. Doing so closes gaps in scientific knowledge, and specifically increases the critical taxonomic and ecological knowledge necessary to support the development and implementation of conservation measures required to reduce the risk of species extinction and concomitant loss of biodiversity.
Keywords: biodiversity, Borneo, carnivorous, plants, Malesia, Nepenthes, new species, non-core Caryophyllales, Taxonomy, threatened species.
Introduction
Nepenthes (Caryophyllales, Nepenthaceae) is a genus of carnivorous tropical pitcher plants comprising over 160 species (Clarke et al. 2018). Nepenthes occur as terrestrial, lithophytic and epiphytic vines or subscandent shrubs that produce a characteristic leaf structure comprising a leaf-like phyllode (modified leaf base) from the apex of which a tendril (the true petiole) emerges, giving rise to an epiascidiate lamina or ‘pitcher’ (representing the true leaf), evolved primarily as a means of heterotrophic nutrition to attract, capture and digest prey (Kubitzki 2002; Bauer and Federle 2009; Moran and Clarke 2010).
Nepenthes occur throughout the Palaeotropics, primarily within the Malesian and Papuasian biogeographic regions but range from Madagascar to New Caledonia and several outlying western Pacific islands (Jebb and Cheek 1997). The genus has recognised centres of species diversity in Borneo, Sumatra and Mindanao (Cheek and Jebb 2001; Clarke 2001; Robinson et al. 2019a) though the functional diversity of evolved trapping mechanisms is unequivocally highest on Borneo (Cheek et al. 2019; Cross et al. 2022; Dančák et al. 2022). Among these, the Nepenthes of Sabah, northern Borneo, include some of the most iconic and well characterised species in the genus, and have been comparatively well-studied since the mid-19th Century (Hooker 1859; Clarke 1997).
Seven of the 24 Nepenthes species known by the authors to occur in Sabah are endemic, namely N. villosa Hook.f. (Hooker and Hooker 1851: tab. 888), N. edwardsiana Low ex Hook.f. (Hooker 1859: 420), N. rajah Hook.f. (Hooker 1859: 421), N. burbidgeae Hook.f. ex Burb. (Burbidge 1882: 56), N. macrovulgaris J.R.Turnbull & A.T.Middleton (Turnbull and Middleton 1988: 352), N. macrophylla (Marabini) Jebb & Cheek (Jebb and Cheek 1997: 58) and N. zakriana (J.H.Adam & Wilcock) J.H.Adam & Hafiza (Adam and Hamid 2006: 434). Only N. macrophylla and N. zakriana are not restricted to ultramafic substrates. The latter taxon was regarded as N. fusca Danser (Danser 1928: 288) s.lat. for decades and therefore the epithet ‘zakriana’ was not accepted widely until N. fusca was relocated, for the first time since its description, at the Kalimantan type locality in 2018, whereupon the species was found to be distinct from all taxa previously regarded as N. fusca from Sabah and Sarawak (Robinson et al. 2019b). Following the descriptions of Nepenthes zakriana and the non-endemic N. chaniana C.Clarke, Chi.C.Lee & S.McPherson (Clarke et al. 2006: 56), the only additions to Sabah’s Nepenthes flora have been new records of taxa previously described from elsewhere in Borneo, for example N. dactylifera A.S.Rob., Golos, S.McPherson & Barer (Robinson et al. 2019b: 106), N. muluensis M.Hotta (Hotta 1966: 7) and N. vogelii Schuit. & de Vogel (Schuiteman and De Vogel 2002: 537) (Phillipps et al. 2008; Bourke 2010; A. Robinson, pers. obs.).
Among several unnamed Bornean Nepenthes known to the authors prior to this work was a poorly characterised taxon from a peak located within the 123,385 Ha commercial Ulu Tungud Forest Reserve in central Sabah. This peak is situated in the Meliau Range, a relatively low-elevation ultramafic extrusion within the present day Beluran District (formerly Labuk Sugut). In 2016, the range itself was afforded separate protected forest status within the Ulu Tungud Forest Reserve as the Meliau Range Forest Reserve (Sabah Forestry Department 2017; Pirin 2018). The range has a diverse flora that includes several endemics (Andi and Suleiman 2005; Sugau 2005; Chung 2006).
The Nepenthes taxon from the Meliau Range was known to have been previously documented on only two verifiable occasions, where it was assumed to represent either a known species or a hybrid derived thereof. However, recognising potentially apomorphic characters in the scant photographic material available, we hypothesised that this plant was likely to represent an undescribed species.
In May 2023, certain authors of this work led an expedition to the Meliau Range to formally document the taxon and test our new species hypothesis. This work was supported by examination of herbarium materials held in herbaria worldwide, with a particular focus on the superficially similar nothospecies Nepenthes × alisaputrana J.H.Adam & Wilcock (1992: 37) and studies of the parent species, N. burbidgeae and N. rajah, each of which shares several distinctive characters in common with the taxon.
Materials and methods
Permits
The main research expedition was carried out under Access Licence JKM/MBS.1000-2/2 JLD. 16 (32) issued to A. Robinson by the Sabah Biodiversity Council (SaBC). Herbarium material was authorised for transfer to MEL under Transfer Licence JKM/MBS.1000-2/3 JLD.5 (29) issued to A. Robinson by SaBC, with permission to export from Sabah under Permit Number JHL(PB)600-3/18/1/1 JLD.35 and CITES Permit No. 1222, issued to A. Damit by the Sabah Wildlife Department and CITES Management Authority Sabah (Malaysia) respectively.
Field work
Field observations and collections of Nepenthes pongoides in the Meliau Range were made by authors A. Damit, N. Yusof, Jem. Jumian and A. Robinson on 16 May 2023 at a locality designated ‘Site 1’ (precise locality details withheld for conservation purposes). During a Sabah Forestry Department follow-up expedition, observations and collections were made by A. Damit, N. Yusof, Jem. Jumian and Jei. Jumian on 7 February 2024 at a second subpopulation designated ‘Site 2’ (see Author contributions). Specimen counts were made over the course of several hours exhaustively exploring all accessible terrain within the elevational range of the two subpopulations discovered.
To minimise impacts on the naturally small population of plants encountered, vouchers were collected in the form of representative cuttings of terminal growths rather than whole plants, allowing for the in situ regeneration of sampled individuals from axillary nodes. Two specimens were sampled during the first expedition (Site 1) and three specimens were sampled during the follow-up expedition (Site 2).
Comparative visual observations of Nepenthes × alisaputrana were made without material collections at its type locality, Bukit Babi (Pig Hill) on Mt Kinabalu, with the support of Sabah Parks staff. Three living individuals were photographed at this locality and the remainder of observations of this taxon were made from herborised material held at herbaria worldwide. Herbarium acronyms follow Thiers (2024).
Genetic sampling
Phyllode tissue was cut in situ from the two voucher specimens sampled on 16 May 2023 at Site 1 for future genetic analysis. In each case, the sample was taken from a newly unfurled, unblemished phyllode with a new, sterile razor blade and immediately inserted into a coffee filter that was folded, labelled with the respective voucher number and placed inside a zip-lock bag containing an excess of self-indicating, 2–4 mm dia. silica drying gel (Silica Gel Direct, Qld, Australia). Each sample on silica gel was double-bagged and stored in an airtight container in a cool, dry location. The silica gel was replaced with fresh dry gel after 24 h and the samples transferred to a −20°C freezer on return to base.
Analyses
The species description is based on measurements made of five living plants in situ, their associated herbarium vouchers, and photographs of three other individuals with included reference scales taken for the purposes of measurement at the time of collection. Fine measurements were made from herbarium materials with a Zeiss Stemi 2000 stereo microscope with table stand (Carl Zeiss AG, Oberkochen, Germany) and a vernier calliper (Mitutoyo Corporation, Japan). Nepenthes collections were pressed as per genus-specific recommendations made in Clarke and Moran (2011).
Scanning electron microscopy (SEM) was used to accurately visualise microscopic details of the herbarium material deposited at MEL, particularly the form and distribution of glands and indumentum. SEM was performed with a TM4000Plus II low-vacuum SEM (Hitachi Co. Ltd, Tokyo, Japan) with an accelerating voltage of 15 kV in mix mode (secondary electron + backscattered electron detection) and a 30 Pa vacuum.
Pitcher fluid pH was tested in triplicate from pitchers of three different ages at Site 1 using Universal Test Paper Strips pH 0–14 (Livingstone International, Australia) and assessed against the included colour chart. Temperature and humidity were recorded with a Thermopro TP50 Thermometer Hygrometer (iTronics Co. Ltd, China).
Studies of pitcher infauna were limited to five pitchers from collected voucher specimens and achieved by tipping the pitcher fluid into transparent zip-lock bags, allowing for direct visual examination and photography of fauna. Fly larvae ensconced in the pitcher bases were exposed and photographed when sectioning the pitchers in readiness for specimen pressing.
Results
Field studies, examinations of material collections made in situ and comparisons of the target Nepenthes with its nearest putative congeners (N. × alisaputrana, N. burbidgeae, N. rajah) unequivocally corroborate its unique characteristics. The data, outlined herein, confirm our hypothesis that the taxon represents a hitherto overlooked addition to Sabah’s significant assemblage of giant-pitchered montane Nepenthes species and the first from a relatively low-elevation peak. The follow-up expedition made to the Site 2 subpopulation provided further valuable data, including additional population and ecological observations, allowing for a more accurate conservation assessment and further insights into the spectra of trapped prey.
Given the very strong support for the delimitation of the taxon at species rank, Nepenthes pongoides is described here as new to science.
Taxonomy
Nepenthes pongoides Damit, Yusof, Jumian & A.S.Rob., sp. nov. (Figs 1, 2, 3, 4 and 5a–c)
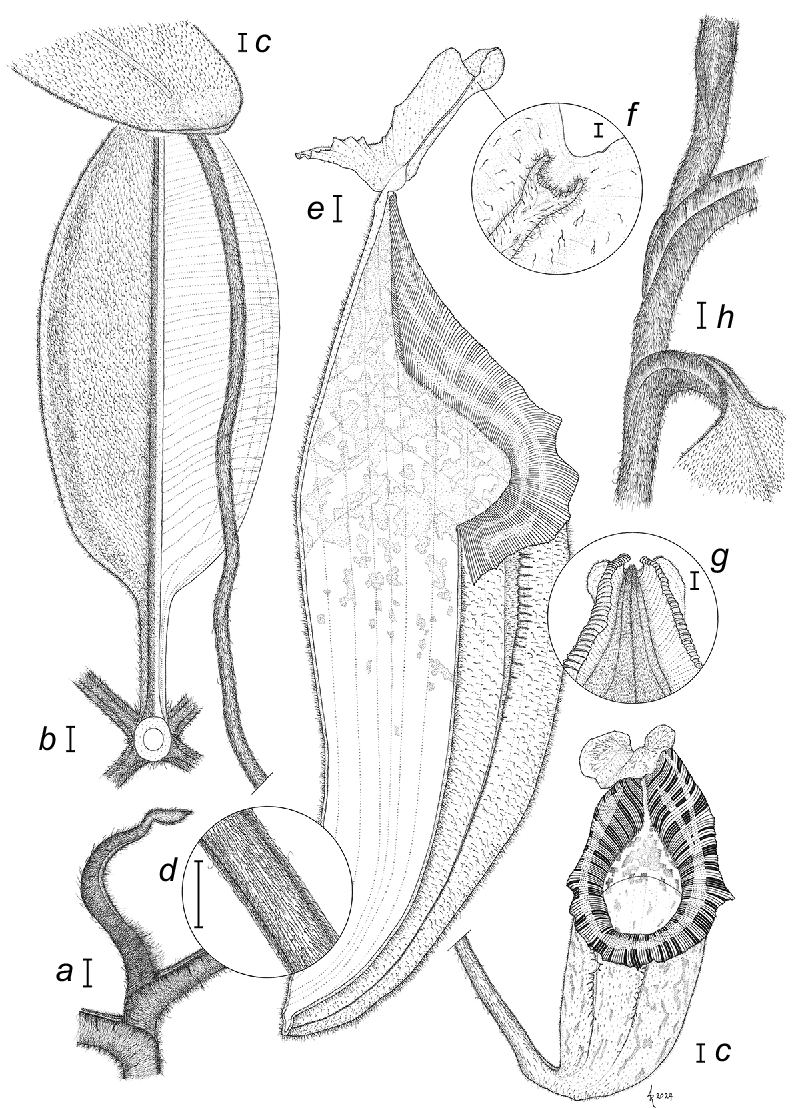
Nepenthes pongoides Damit, Yusof, Jumian & A.S.Rob. (a) Emergent phyllode of rosetted growth with clasping, shortly decurrent phyllode bases. (b) Abaxial view of attached phyllode showing (left) general distribution of indumentum with greater density between longitudinal veins in outer 1/4 and (right) typical form of transverse and longitudinal veins. (c) Apex of phyllode with detached pitcher, showing apical concavity, peltate tendril exsertion and typical pattern of pitcher and peristome pigmentation. (d) Detail of tendril showing strongly retrorse hairs. (e) Partial section of pitcher showing generalised venation, pigment distribution and form of peristome, lid and ventral wings. (f) Magnification of lid apex showing tip of midline rib with large, recessed terminal gland. Note presence of dendritic hairs. (g) Junction between peristome, pitcher and lid as seen from reverse, noting reduction of spur to a pubescent mound. (h) Section of scrambling stem showing canaliculate petioles and markedly decurrent bases. Scale bars: a, b, c, d, e, g, h = 1 cm, f = 1 mm. Based on Alviana D., Nur Adillah M.Y., Jemson J. and Robinson A.S. 161454 and 161456, and on photographs and measurements made in situ. Illustrated by A Robinson.
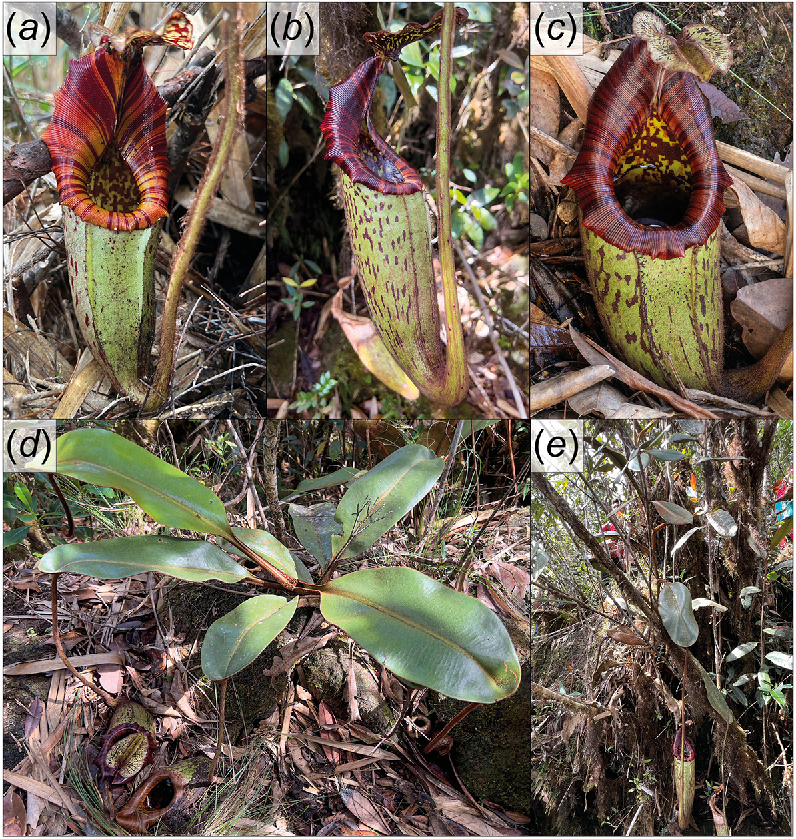
Pitchers and habit of Nepenthes pongoides. (a) Rosette pitcher of immature plant showing highly developed peristome column. (b) Pendent pitcher of a scrambling plant. Note ventral attachment of tendril and scattered large nectar glands of same. (c) Large terrestrial pitcher. (d) Mature rosette emergent from humus-filled fissure between ultramafic boulders; a cutting from this rosette was sampled as voucher 161456. (e) An individual demonstrating occasionally observed scrambling habit. Photographs (a), (e) by A. Damit; (b), (c), (d) by A.S. Robinson.
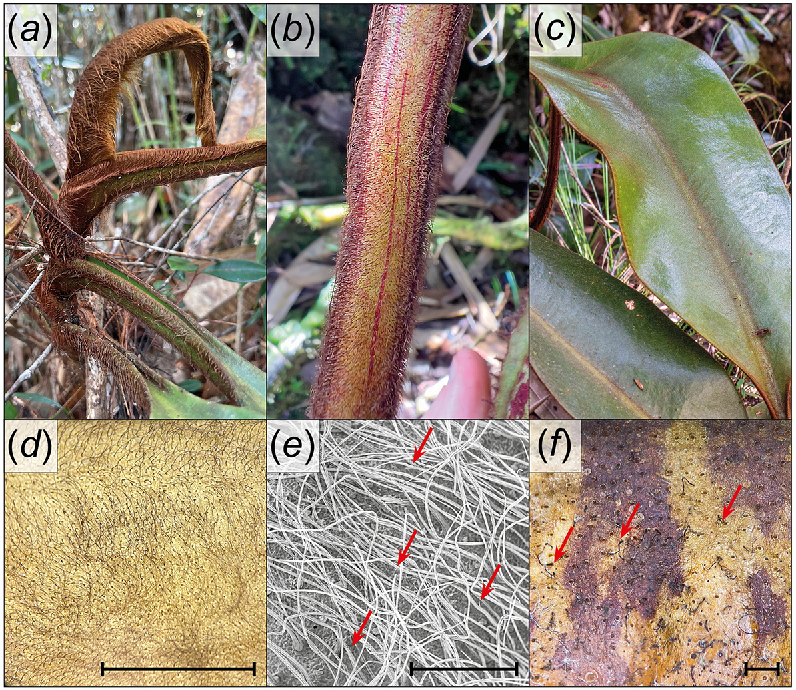
Indumentum of Nepenthes pongoides. (a) Emerging foliage and phyllode bases densely covered with long, rufous to reddish-gold hairs. (b) Tendril bearing long, pilose to hirsute and uniformly retrorse hairs. (c) Adaxial surface of phyllode with reddish indumentum of pilose hairs, particularly apparent along midrib. (d) Pubescent hairs of phyllode abaxial surface. (e) Scanning electron micrograph of phyllode abaxial surface hairs with bristle-like processes indicated (red arrows). Note scattered sessile glands between hairs. (f) Simple and variably dendritic (red arrows) hairs on lower surface of lid. Scales: (d) = 1 cm; (e), (f) = 1 mm. Photographs (a) by A. Damit; (b)–(f) by A.S. Robinson.
Various characters of Nepenthes pongoides at Site 1. (a) A large, pendent pitcher showing extreme development of peristome column, a character reminiscent of N. mollisDanser (1928: 338), a species found primarily in northern Sarawak, north-western Kalimantan and only south-westernmost Sabah. (b) Upper surface of lid. Note absence of a spur at junction with peristome column. (c) Phyllode bases clasp stem for entirety of its circumference. These become markedly decurrent in scrambling stems. (d) Phyllode apex is obtuse and tendril exsertion peltate, appearing deeply concave from above. (e) Even in seedlings, dense indumentum is apparent on emergent foliage and surface of phyllodes (see e.g. top right). Photographs (a), (b), (d) by A.S. Robinson; (c), (e) by A. Damit.

Nepenthes pongoides at Site 2. (a, b) Particularly robust pitchers encountered along a summit ridgeline. (c) A subscandent stem demonstrating increasing internode length, with concomitant transition in length of the decurrent phyllode wings and the loss of the peltate apex over the course of three leaves giving rise to non-peltate phyllode tips (red arrow and inset). Photographs (a)–(c) by A. Damit.

Type: – MALAYSIA. Borneo, Sabah, Beluran District: Meliau Range, [~1000 m elevation] summit of ascending ridge, climbing [=subscandent, scrambling without tendril support] to 1.5 m, very large pitchers on basal rosette leaves containing giant millipedes, Alviana D., Nur Adillah M.Y., Jemson J. & Jeisin J. 161148, 7 February 2024 (holotype SAN! [three sheets; one carpol.]; isotype MEL! [three sheets]; isotype KEP! distribuendis [two sheets]) [scrambling stem fragment bearing two phyllodes and detached stem apex (SAN 1/3); detached phyllode with large, dissected pitcher showing internal surface (SAN 2/3); rosette stem fragment with phyllode and detached, dissected lower pitcher showing external surface (SAN 3/3); 51 cm tall intact pitcher of 1.6 L volume (SAN carpol.); rosette stem fragment bearing large phyllode (MEL 1/3: 2541028A); dissected pitcher half, showing internal surface (MEL 2/3: 2541029A); large, detached phyllode with remaining dissected pitcher half, showing external surface (MEL 3/3: 2541030A); detached phyllode with large, dissected pitcher showing internal surface (KEP 1/2); remainder of dissected pitcher showing external surface (KEP 2/2);].
– Nepenthes stenophylla in Chung (2006: 37, right-hand figure only) and in Sabah Forestry Department (2010: front cover and ‘Noteworthy Plants’ panel) pamphlet.
– Nepenthes burbidgeae hybrid or ‘potentially undescribed species’ in Pirin (2018: 2).
– Nepenthes sp. ‘Sabah’ in McPherson (2023: 1984–1987, figs 2390–2394).
Diagnosis
Nepenthes pongoides superficially resembles the nothospecies N. × alisaputrana (Fig. 6a, b) but differs in the production of (differences in parentheses): stems, phyllodes and pitchers covered with long, rufous hairs (stems, phyllodes and pitchers minutely pubescent to glabrescent); phyllodes elliptic in shape with obtuse, deeply and uniformly peltate phyllode apices in the rosette stage, becoming non-peltate in phyllodes of long, scrambling stems (oblong to lanceolate with apices more or less acute to narrowly retuse, in some individuals sometimes slightly peltate — see Additional specimens examined); petiole bases that clasp the stem for the entirety of its circumference at rosette stage, and 4/5 its circumference and decurrent for 1/3–1/2 of internode length in scrambling stems (clasping stem for 2/3 its circumference, only shortly decurrent even in climbing stems); spurs often reduced to swellings, appearing absent, or relatively squat and subconic, ≤6 mm long, ≤4 mm wide (spur ±filiform, 7–15 mm long, 1.5–2 mm wide); lower pitchers only, ventrally to laterally attached, with a greatly expanded peristome column orientated from vertical to overarching (true upper pitchers with dorsal attachment commonly produced post rosette phase, peristome column relatively short, slightly wider than remainder of peristome, suberect to erect).
(a) The pitchers of the nothospecies Nepenthes × alisaputrana (=N. burbidgeae × N. rajah) are superficially similar to those of N. pongoides but typically minutely pubescent to glabrescent, usually bear pronounced spurs and do not have a strongly developed peristome column. (b) The phyllode of N. × alisaputrana is oblong to lanceolate and frequently terminates in an acute tip (bottom left) without peltate tendril exsertion. (c) Captured giant millipede prey in specimen 161148. (d) An array of captured prey remains in specimen 161147, including centipede and crab (inset) material, and living fly larvae on the left side of the image. (e) Natural hybrid N. pongoides × N. stenophylla. (f) Natural hybrid N. pongoides × N. veitchii. Photographs (a), (b) by A.S. Robinson; (c)–(f) by A. Damit.

Description
Stout upright to subscandent shrub. Stems 40–70(−200) cm long, terete to subovate, 1.5–2.5 cm dia., internodes 2–4 cm in rosettes, 3–7(−16) cm in scrambling stems. Phyllodes coriaceous, somewhat brittle, marcescent, petiolate, petiole of phyllodes 5–8(−10) cm long, 0.8–1.3 cm wide, deeply canaliculate, subcylindric, opposing margins parallel and often nearly appressed, basally sheathing, and clasping stem for its entire circumference and shortly decurrent in rosettes, becoming decurrent 1/3(−1/2) internode length in scrambling stems and clasping for 4/5 its circumference. Lamina elliptic, 25–37(−42) cm long, 10–15(−20) cm wide at midpoint, 3–4 longitudinal veins either side of midrib in outer 1/4 of lamina, transverse veins adaxially prominent, numerous, ~40–60 pairs per phyllode, base attenuate, apex obtuse, in rosette phyllodes adaxially deeply concave owing to peltate tendril exsertion, tendril emergent ~1.8–3.6 cm from apex, in phyllodes of scrambling stem not peltate, tendrils 42–46(−52) cm long, 6–10 mm dia., uncoiled in both rosette and scrambling stems. Pitchers basally ellipsoid and cylindric-subinfundibular above, wholly prolate spheroid or broadly infundibular throughout, ventrally to rarely laterally attached to tendril, 15–40(−51) cm tall including column, 6–17 cm wide, to 39 cm in circumference at the widest point, to ~1.6 litres volume, pitcher walls thick, up to 4 mm at base and 6 mm at column, interior surface entirely glandular, gland density ~2800 cm−2 at base of pitcher, decreasing to ~1600 cm−2 slightly below peristome, exterior surface with two fringed, ventral wings 4–7 mm wide running from base of pitcher to mouth, fringe elements 8–14 mm long, 4–8 mm apart; peristome broad, oblique throughout but strongly raised at rear, becoming vertical to overarching, narrowest at front of pitcher between wings where 8–17 mm wide, and 3–5 times broader either side of column below lid, to 8.5 cm wide prior to reflexion, ribs pronounced, fine, ~1 mm apart, ≤0.3 mm tall at front of pitcher, and 1.5 mm apart and ≤0.5 mm tall on column, teeth narrowly acuminate, 0.8–1.8 mm long. Lid ovate, base cordate, apex obtuse to retuse, ±complanate but typically furrowed along the midline, typically between horizontal and 30° from horizontal, 5–13 cm long, 3.5–12 cm wide, with a large basal keel on lower surface, ~15–20 mm long, 5–8 mm high, median rib in large pitchers often terminating in an arched, concave, glandular recess, lower surface of lid with crateriform glands, 0.2–0.3 mm dia., ~400 glands cm−2, minute and less dense on keel, ~0.1 mm dia. Spur often absent, reduced to a mound or, if present, relatively squat and subconic, ≤6 mm long, ≤4 mm wide. Upper pitchers absent, suspended pitchers of scrambling stems ±ventrally or occasionally laterally attached, never dorsally, and produced from uncoiled tendrils. Inflorescences unknown. Seeds unknown. Indumentum abundant, persistent on all parts of plant, primarily comprising long, dark, rufous red to reddish-gold hairs, drying to very dark brown, on the stems hirsute, 0.8–1.8 cm long, mainly antrorse, dense, ~2800 cm−2 along internodes, and becoming villous to silky and extremely dense, ~10,000 cm−2, at phyllode axils, adaxial surface of phyllode with ±pilose hairs, 1.5–3 mm long, 120 cm−2, abaxial surface pubescent, hairs 3–5 mm long, 280 cm−2 but increasing in density between longitudinal veins in outer 1/4 of phyllode, tendrils with pilose to hirsute hairs 8–14 mm long, retrorse and often reflexed, external surface of pitchers with pilose hairs 0.8–2.5 mm long, comprising several segments each terminating in a short, bristle-like process but not dendritic, ~450 cm−2, increasing in density towards junction with tendril, indumentum on lower surface of lid a mix of simple or dendritic trichomes, absent around keel but increasing in density towards margins, to ~180 hairs cm−2, 0.6–1.4 mm long. Colour stems, midrib of phyllodes and abaxial lamina surface of same pale green, adaxial lamina surface of phyllodes becoming dark green with age, pitchers initially whitish- or yellowish-green, becoming pale green with age, external surface blotched throughout with bright to deep red, pitcher interior green in the lower half, speckled above with red, peristome base colour golden to reddish-yellow, typically becoming suffused with red towards margins and heavily striped with deep red, purplish-red, mahogany and reddish-black to appear almost entirely deep red or brown with age.
Etymology
The specific epithet pongoides is derived from the primate genus name Pongo (the orangutans) and the Greek suffix –oides (resembling). This name was chosen in light of the highly developed, persistent reddish indumentum covering the stems, phyllodes, tendrils and pitchers; the long, dark, rufous hairs of living plants are similar in colour to those of this critically endangered great ape, a population of which persists within the area of the Ulu Tungud Forest Reserve, as evidenced by a fleeting encounter with a single individual during the expedition.
Phenology
No evidence of flowering was found at the time of survey. Since Nepenthes inflorescences are terminal, sympodial branching was sought as evidence of past flowering events but no candidate individuals were located. However, some recruitment was clearly taking place, because 12 of the 37 plants observed at Site 1 were seedlings, estimated at 2–3 years of age based on horticultural observations of typical size progression in ultramafic-growing intermediate to highland Nepenthes germinants.
Distribution and ecology
Nepenthes pongoides is endemic to the Meliau Range, where the species has been recorded from ~900 m elevation and above on ridges leading to low elevation summits.
At Site 1, plants were observed to occur overwhelmingly in pockets of humus either on or between ultramafic boulders amongst bamboo thickets or edaphically stunted lower montane summit trees of 3–6 m in height. Twelve plants were found growing lithophytically in small hummocks or thin veneers of moss adherent to ultramafic rock, however, these plants were predominantly very young, potentially indicating that such occurrences are not viable in the longer term. Co-occurring Nepenthes species at Site 1 include N. stenophylla, which was recorded in similar habitat from ~780 m elevation to the summit, N. tentaculata Hooker (Hooker 1873: 101), which slightly overlaps with N. pongoides mainly in more closed forest at ~1000 m elevation, and N. macrovulgaris, which was restricted to mid-montane (500–900 m) closed forest. A similar summit habitat niche for N. pongoides was observed at Site 2 but thin soils and increased sun and wind exposure on this rocky summit has limited the vegetation height to only 3 m. Here, N. macrovulgaris, N. stenophylla and N. veitchii Hook.f. (Hooker 1859: 421) were observed as co-occurring species.
The temperature at the Site 1 voucher elevation was recorded on 16 May 2023 as 23.7°C at 07:30 and 28°C at 13:00, humidity was ~65% at both times of day. Pitcher fluid was recorded as pH 5 from a sample of three pitchers, one of which was freshly opened with peristome incompletely expanded; one close to senescence (Fig. 2c) based on examination of the condition of preceding pitchers; and one within weeks of having fully matured (Fig. 4a). Note that pH 5 is no more acidic than the equilibrium pH of air-exposed water at standard conditions, which typically ranges in pH from 4.5–5.6 in pristine natural environments (Charlson and Rodhe 1982).
Qualitative observations of macroscopic pitcher fauna of Nepenthes pongoides suggest that the metazoan community is species-poor and structurally simple with no evidence of the presence of predatory genera such as Toxorhynchites (Diptera: Culicidae) or Corethrella (Diptera: Corethrelliidae). Two larvae of an unidentified species of fly were present in the dissected pitcher illustrated in Fig. 6c, d. These could belong to Lestodiplosis (Diptera: Cecidomyiidae), Nepenthosyrphus (Diptera: Syrphidae) or one of the nepenthebiont phorids (Diptera: Phoridae, Calliphoridae), some of which may be predatory but are normally associated with pitchers that trap large volumes of arthropod prey that frequently become putrid (Clarke and Kitching 1993). Larvae of Armigeres sp. (Diptera: Culicidae) and Uranotaenia (Diptera: Culicidae) sp. were observed, both of which are widespread in Bornean Nepenthes (particularly species from low elevations) and general consumers of captured prey in Nepenthes. Given the apparent small size of the N. pongoides population, most metazoan species that colonise the pitchers likely do so opportunistically, despite being nepenthebionts, as they are common components of the invertebrate fauna of widespread species such as N. stenophylla and N. tentaculata that also occur in the Meliau Range.
In the absence of quantitative data for prey capture, determining whether Nepenthes pongoides targets any specific arthropod taxa is difficult. Observed prey included the remains of a geosesarmid crab, scolopendrids and a Trigoniulid millipede (Fig. 6c, d). No evidence for the capture of vertebrate faeces was observed, nor were remains of Formicid prey. In other montane Nepenthes studied to date, this prey spectrum corresponds to that of a montane Bornean Nepenthes that lacks adaptations for an alternative nutrient sequestration strategy but occurs in habitats where diversity and abundance in ants are low (Adam 1997; Chin et al. 2014).
The compression of elevational vegetation zones, akin to the ‘Massenerhebung effect’, is often observed on major ultramafic mountains in Sabah, including the Meliau Range (van der Ent et al. 2015), and is typically more prominent in isolated peaks than those that form part of large mountain complexes. The ridge top and summit vegetation at Sites 1 and 2, characterised by stunted trees, exhibit diminished stature, and smaller diameter and crown size, contributing to increased sun exposure compared to the vegetation found at lower elevations or at similar elevations on non-ultramafic peaks. The climbing bamboo Dinochloa scabrida (Poaceae), along with Gymnostoma sumatranum (Casuarinaceae), Tristaniopsis spp., Syzygium spp. (Myrtaceae), Timonius spp. (Rubiaceae) and Prunus spp. (Rosaceae), were found to be prevalent in this vegetation zone.
Natural hybrids
A single hybrid of Nepenthes pongoides × N. stenophylla was identified at Site 1, while the same hybrid and N. pongoides × N. veitchii were also recorded at Site 2, in each case showing characteristics intermediate between the co-occurring putative parents (Fig. 6e, f) as is typical within the genus. A mature, detached pitcher of N. pongoides × N. stenophylla was also uncovered amongst herbarium specimens collected from 900 m elevation at Site 2, along with a carpological specimen of N. pongoides (see Additional specimens examined).
Conservation status
A survey at Site 1 documented a total of 37 individual Nepenthes pongoides plants. Twelve of these were juvenile, amounting to a functional population size (IUCN 2012) of 25 observed mature plants. The species was first recorded at an elevation of ~1000 m, represented by two closely situated plants. All other plants (95% of the total observed) were found mainly in two localised clusters of several individuals situated slightly above this elevation. The area of available habitat at this elevation amounted to ~1.1 km2, and any additional plants not observed would likely have been few and patchily distributed within this optimal zone because the topography was precipitously steep, and punctuated by barren ultramafic outcrops and boulders incapable of supporting plant growth.
The 2004 collection of this species from Site 2 was made at ~900 m elevation. At that site, only 0.23 km2 of suitable habitat existed between 900 m and the summit, and observations made on the follow-up expedition conducted as part of this work confirmed that plants at Site 2 were likewise highly localised along the summit ridge, with only 14 mature individuals noted. Added to the 25 mature individuals noted from Site 1, the total observed population of mature plants for both sites was only 39.
The various peaks of the Meliau Range are not all contiguous, with certain prominences situated in relative isolation, in some cases over 5 km distant from the next. Several peaks in the range are high enough to support N. pongoides, however, intervening valleys are as low as 140 m elevation and may be sufficiently low to reduce or preclude movement of montane species between these locations, as seen with comparable microendemic Nepenthes elsewhere. There are currently no other known records of the taxon and as such, the presence elsewhere in the range cannot be inferred without direct observation. The species is certainly absent from the comparatively well explored Gunung Mantapok (1528 m, ~20 km WSW of the Meliau Range), also an ultramafic peak home to several obligate ultramafic taxa endemic to the state of Sabah.
Per the IUCN 3.1 Red List criteria (IUCN 2012), Nepenthes pongoides is assessed as CR (Critically Endangered), satisfying Criteria A3d+B1ab(ii,v)+B2ab(ii,v)+C2a(i), i.e. the species is projected to have a population size reduction of ≥80% over 10 years or three generations as a result of the severe poaching risks already posed by strong horticultural interest; has an extent of occurrence (EOO) <100 km2 and is known to exist at only a single location (comprising two subpopulations), with projected declines in the number of mature individuals owing to said poaching risk; has an area of occupancy (AOO) <10 km2 at a single location, again with projected decline in number of mature individuals as a result of poaching; and a population size estimated at well under the threshold of 250 mature individuals, again subject to declines in response to illegal collection, with no subpopulation estimated to contain more than 50 mature individuals. The very small AOO also makes the taxon extremely vulnerable to destructive stochastic events such as fire, that may present extinction-level threats. While criterion D (population size estimated to number fewer than 50 mature individuals) may well be satisfied by the cumulative total of only 39 observed mature individuals between the two subpopulations, this is not assessed as such here owing to the uncertainties resulting from insufficient exploration of the Meliau Range specifically targeting this taxon.
Additional specimens examined
Nepenthes pongoides: – MALAYSIA. Borneo, Sabah, Beluran District: Meliau Range, along ridge line atop ultramafic boulder in humus pocket, Alviana D., Nur Adillah M.Y., Jemson J. & Robinson A.S. 161456, 16 May 2023 (MEL!) [three sheets: 2529961A (stem frag. with attached phyllode and large dissected pitcher showing external surface), 2529962A (stem frag. with apical meristem, attached phyllode and developing pitcher), 2529963A (remainder of dissected pitcher showing glandular interior)]; along ridge line on soil amongst ultramafic boulders, Alviana D., Nur Adillah M.Y., Jemson J. & Robinson A.S. 161454, 16 May 2023 (SAN!) [three sheets: Sheet 1/3 (stem frag. with attached phyllode), Sheet 2/3 (detached phyllode with large, dissected pitcher), Sheet 3/3 (remainder of dissected pitcher showing glandular interior)]; terrestrial in lower montane forest with notable characteristics of upper montane forest [due to edaphic effects of ultramafic substratum causing compression of altitudinal vegetation zones], Jamirus J. & Jemson J. 161961, 12 August 2004 (SAN!) [carpological specimen plus sheet with det. label and attached image of Jamirus J. holding phyllode with attached pitcher]; ridge summit, scrambling on mossy ground with digested remains of centipedes and a small terrestrial crab, Alviana D., Nur Adillah M.Y., Jemson J. & Jeisin J. 161147, 7 February 2024 (SAN!) [two sheets: Sheet 1/2 (detached phyllode with large, dissected pitcher showing internal surface), Sheet 2/2 (detached phyllode with large, dissected pitcher showing external surface)].
Nepenthes pongoides × stenophylla: – MALAYSIA. Borneo, Sabah, Beluran District: Meliau Range, 900 m, [det. as N. stenophylla], terrestrial in lower montane forest, Jamirus J. JJ103, 12 August 2004 (SAN!) [large cylindrical pitcher with attached tendril and characteristic sub-peltate phyllode apex].
Nepenthes burbidgeae: – MALAYSIA. Borneo: Sabah, Ranau District: Kina Balu [Kinabalu], Marie Parie [Marai Parai] Spur, Burbidge F.W. s.n., 1877–78 (lecto K!, isolecto K! [two sheets], BM!) [stem frag. with five phyllodes and one upper pitcher (isolecto K 000651542); stem frag. with two phyllodes and two upper pitchers (isolecto K 000651543); stem frag. with five phyllodes and two upper pitchers (lecto K 000651544); stem frag. with seven phyllodes and four upper pitchers (BM 000040183)]; Colombon basin, Mt Kinabalu, 5500 ft [1676 m], J. Clemens & M.S. Clemens 34494, 18 August 1933 (photo GH!, L!) [stem frag. with five phyllodes and one upper pitcher (GH 01871125); stem frag. with detached phyllode bearing upper pitcher (L 0576680)]; W. Maraia Parai [Marai Parai], Mt Kinabalu, 4–5000 ft [1220–1524 m], J. Clemens & M.S. Clemens 32501, 01 April 1933 [detached phyllode bearing upper pitcher with second partial upper pitcher (GH 01871126)]; Mamut Copper Mine, Mt Kinabalu, ridge above quarry, 1350–1450 m, J.H. Beaman 10363, with R.S. Beaman and B. Sutton, 28 June 1984 (L!, photo MO!) [stem frag. with two phyllodes and one large lower pitcher (GH 01871127); two phyllodes, one with lower pitcher (L 0577250); stem frag. with two phyllodes and one upper pitcher (MO 04997581)]; Penibukan, Mt Kinabalu, 5000 ft [1524 m], J. Clemens & M.S. Clemens 30915, 10 January 1933 (photo GH!, L!) [stem frag. with detached phyllode bearing pitcher (GH 01871129); stem frag. with detached phyllode bearing upper pitcher (L 0576679)]; Mamut Hill, south of Mamut Camp, 1400–1600 m, S. Kokawa & M. Hotta 5458, 11 February 1969 (SAN!; L!) [stem frag., two phyllodes with attached upper pitchers and detached upper pitcher (SAN); two stem frags. with female infl. and upper pitcher (L 0576677)], S. Kokawa & M. Hotta 5469, 11 February 1969 [stem frag. with female and separate partial male infl. (L 0576678)]; Pig Hill, 7500 ft [2286 m], W.L. Chew & J.H. Corner 4514, 24 February 1964 (SAN!) [short stem node with single phyllode and upper pitcher]; Kiau, Mahandoi trail, 4500 ft [1370 m], W. Meijer 54009, 16 September 1965 (SAN!) [stem frag. with six phyllodes]; Mamut Copper [Mine] trail, 5200 ft [1585 m], W. Meijer 62712, 3 March 1968 (SAN!) [two detached phyllodes bearing lower pitchers].
Nepenthes rajah: – MALAYSIA. Borneo: Sabah, Ranau District: North side of Kina Balou [Kinabalu] 5000 ft [1524 m], plant about 4 ft [1.2 m] high, Low s.n. (holo K! [two sheets]) [detached phyllode with large lower pitcher (K 000651480); separate male and female inflorescences (holo K 000651481)]; Burbidge F.W. s.n., 1877–78 (photo GH!; photo NY!; photos US! [three sheets]) [detached phyllode with small pitcher and separate male and female infl. frags. (GH 01871265); two male inflorescences (NY 03839887); stem frag., three partial phyllodes (US 40050); infl. frags., four female one partial male (US 40051); stem frag. with three phyllodes and infl. base (US 40052)]; Marai Parai Spur, Mt Kinabalu, M.S. Clemens 11073, 01 December 1915 (photo GH!) [male infl. and small, detached lower pitcher (GH 01871266)]; Mesilau, opposite cave, W. Meijer 48104, 20 February 1965 (SAN! [two sheets]) [detached phyllode with large pitcher (sheet 1); detached phyllode with small pitcher (sheet 2)]; Marai Parai Spur, Mt Kinabalu, 5–6000 ft [1524–1828 m], A. Kanis & B. Weber 54000, 17 September 1965 (SAN!) [partial detached phyllode and pitcher]; along E. Mesilau River en route from Mesilau Camp to Mesilau Cave, 1600–2000 m, S. Kokawa & M. Hotta 4058, 21 January 1969 (photo L!) [detached phyllode with large pitcher (L 0577894)]; Marai Parai Spur, Mt Kinabalu, 5000 ft [1524 m], J. Clemens & M.S. Clemens 32224, 22 March 1933 (photos GH! [two sheets]; L! [three sheets]) [stem frag. with single phyllode and detached large pitcher and male infl. (GH 01871271); detached phyllode with male and female inflorescences (GH 01871270)]; detached stem frag. with dissected pitcher and male infl. frag. (L 0577891); detached phyllode with large pitcher and male infl. frag. (L 0577892); phyllode and male and infl. frags. (L 0577893)]; Gurualau [Gurulau] Spur, Mt Kinabalu, mossy forest crest, 7–9000 ft [2130–2740 m], J. Clemens & M.S. Clemens 51033 and 51078, 12 December 1933 (photo GH!) [phyllode and pitcher frag. with male and female infl. (GH 01871267)]; East Mesilau River near Mesilau Cave, 6°03′N, 116°36′E, 1950–2100 m, opening in oak-laurel forest, ultramafic landslide area, J.H. Beaman 9137, with R.S. Beaman, T.E. Beaman, J.T. Atwood, E. Besse & P. Decker, 26 March 1984 (photos GH! [two sheets]; L! [three sheets]; photos MO! [three sheets]; photos US! [three sheets]) [detached phyllode with mature lower pitcher (GH 01176792); female infl. (GH 01176793); detached phyllode and pitcher (L 0577308); male and female inflorescences (L 0577309); three detached phyllodes with two small pitchers (L 0577398); phyllode frag. with attached pitcher (MO 05009232); stem frag. with three phyllodes (MO 05009233); male and female inflorescences (MO 05009234); separate male and female inflorescences (US 3532512); stem frag. with male inflorescence, five phyllodes, detached phyllode with small lower pitcher] (US 3532513); single large phyllode (US 3532514)]; Mt Kinabalu, upper edge of landslide on steep slope above E. Mesilau River tributary, 2080 m, S.H. Collenette 21608, 06 September 1963 (photos A! [two sheets]; L! [four sheets]; photos WAN! [four sheets]) [stem frag. with phyllode and pitcher base (A 01871268!); stem frag. with phyllode, pitcher part and male infl. (A 01871269!)]; two male inflorescences (L 0577896); two pitcherless stem frags. (L 0577897); upper portion of pitcher (L 0577898); female infl. with seeds (L 0577899); detached phyllode with large pitcher (WAN 0013603); stem frag. with three phyllodes and one pitcher (WAN 0013604); stem frag. with phyllode and male infl. (WAN 0013605); stem frag. with phyllode and female infl. (WAN 0013606)]; H.P. Fuchs, H. Sleumer and S.H. Collenette 21146, 24 July 1963 (L!; photos WAN! [three sheets]) [two detached phyllodes, pitcher frags. and female infl. (L 0577895); detached phyllode with small pitcher and female infl. (WAN 0013850); frag. of large pitcher including peristome and lid (WAN 0013851); stem frag. with two phyllodes and female infl. (WAN 0013852)]; Mesilau, landslide above eastern tributary of Mesilau River, 2070 m, S.H. Collenette 21609 [as N. rajah × hirsuta], 06 September 1963 (photo WAN!) [mixta; young N. rajah stem frag. with four phyllodes and two pitchers, and N. zakriana rosette with several pitchers (WAN 0013607)].
Note – the specimen J. Clemens 51078 (NY 03839886 photo!) [pitcher frag. with portion of female inflorescence] labelled as N. rajah shows a rather typical example of the hybrid N. × kinabaluensis, featuring a comparatively small, ±complanate lid and enlarged peristome flanges, both characteristic of the N. villosa parent. The sheet S.H. Collenette 21609 (WAN 0013608 photo!) [two small rosettes with one pitcher each] labelled as N. rajah × hirsuta represents material of typical N. zakriana only.
Nepenthes × alisaputrana (=N. burbidgeae × N. rajah): – MALAYSIA. Borneo: Sabah, Ranau District: Mt Kinabalu, Pig Hill, 1900–1930 m, J. Adam, J. Adam & A. Mahdi 2442–1,2,3,4 [as N. × alisaputraiana], 2 February 1988 (holo UKMS n.v., iso ABD n.v., BO n.v., K!, L!, SAN n.v., SAR n.v., SNP n.v., UKMS n.v.) [detached and dissected upper pitcher with additional lid (iso K 000651453); detached lower pitcher (iso L 0597872)]; Mesilau, landslide above eastern tributary of Mesilau River, 2070 m, S.H. Collenette 21609, 06 September 1963 (L!) [two stem frags. with several phyllodes and one intact lower pitcher each (L 0578352)].
Notes – as mentioned in the protologue of N. × alisaputrana (Adam and Wilcock 1992), but at odds with the key presented in that same paper, this plant does not always produce phyllodes with peltate tendril exsertion, a character typical of the N. rajah parent. As in most hybrids, different F1 individuals exhibit different combinations of characteristics from both parents. Observations of three mature plants at the Bukit Babi type locality in May 2023 as part of this work found no individuals with peltate tendril exsertion (Fig. 5b), while the specimen S.H. Collenette 21609 (L 0578352) bears seven phyllodes, of which four clearly have subpeltate phyllode apices.
Discussion
Remarkably, Nepenthes pongoides has gone undescribed until now despite its large size and striking apomorphies. The relatively low accessibility of the Meliau Range may partly account for the species having been overlooked; the range is surrounded by privately operated oil palm plantations and commercial forest that require permits for access. The range is also of relatively low elevation and, given the positive association between elevation and rates of endemism (Steinbauer et al. 2016), might easily be dismissed as being likely to exhibit comparatively low levels of endemism, making the range a lower priority target for research. This, compounded by difficulty of safe ascent owing to spiny and dense secondary forest growth below 700 m and lack of water courses above 150 m, has ensured low visitation.
There have only been three formally documented ascents of the Meliau Range; the first was the 1956 British Museum North Borneo [ornithological] Expedition, when R.W. Sims and his team explored the summit of the eponymous Gunung Meliau (1336 m) between 7 and 12 May, accurately describing the character of the summit and noting no exceptional flora other than the stunted trees and its ultramafic nature (Sims 1956; Banks 1982); the second was a 2004 joint scientific research expedition to the Meliau Range by Sabah’s Forest Research Centre, Sabah Forestry Department, and Institute for Tropical Biology and Conservation (Universiti Malaysia Sabah), that saw experienced naturalist teams explore parts of the range; and the third was a joint mountaineering expedition to the range involving the North Borneo Mountaineers and Kelab Pendaki Cap Kapal Sabah in August 2018. A 2006 ornithological expedition by Sabah Museum and Louisiana State University staff explored the range but did not ascend Gn. Meliau (Sheldon 2015).
Nepenthes pongoides was first documented during the 2004 research expedition, when at least two pitchers were photographed. One of these was subsequently printed in the resulting book about the range (Chung 2006) and therein identified as N. stenophylla. After our own expedition, the same image was found to feature in a pamphlet about the distant Mt Silam, again as N. stenophylla (Sabah Forestry Department 2010), while a different pitcher was identified as featuring on an extant poster hanging at Sabah’s Rainforest Discovery Centre (Sepilok) captioned, ‘N. burbidgea? [sic] or N. stenophylla?’ This image eventually revealed the aforementioned misplaced carpological collection also from this locality, resulting in the follow-up expedition to Site 2 as part of this work that provided more complete population and ecological data.
The second reliable record of Nepenthes pongoides was made during the 2018 mountaineering expedition, when additional photographs were taken by Joseph Pirin (Sabah Forestry Department) and colleague Jastin Kiek. In the resulting trip report (Pirin 2018), also sighted only after our expedition, the taxon was more perspicaciously identified as a ‘rare giant one [pitcher plant], suggested (N. burbidgeae hybrid) – potentially new undescribed species’, the latter suspicion proving correct. Images by both individuals were shared on social media and some of those by J. Kiek were subsequently featured in a recent guide to the genus Nepenthes under the name ‘Nepenthes sp. ‘Sabah’’ (McPherson 2023 n.v., M. Golos pers. comm.).
The superficial similarities between Nepenthes pongoides and its congeners in Sabah provide an additional reason for this taxon having remained unresearched for so long. The unusual location notwithstanding, the suggestion by non-specialist observers that the taxon might represent N. burbidgeae or one of its hybrids is perfectly understandable, because lid morphology, patterns of pitcher colouration and, to a lesser extent, pitcher shape in N. pongoides can be very similar to those of N. × alisaputrana (=N. burbidgeae × N. rajah), while the abundant indumentum might, in young plants, lead to conflation with N. stenophylla. The species’ mostly low-growing habit results in most pitchers developing at ground level, often situated between boulders and out of sight, making it easy to appreciate how this taxon might have escaped notice until now.
The abundance, length, relative distribution and form of the hairs in mature Nepenthes pongoides appear to be autapomorphic within the genus and are clearly not directly derived from a parent species.
The precise role of this extraordinarily developed indumentum has yet to be investigated, however, it is reasonable to speculate that the density and length of hairs might guard against high levels of insolation and/or periodic desiccation given the habitat similarities with Nepenthes erucoides, previously considered to be the most densely hirsute Nepenthes described to date, and the remarkably hairy N. villosa. Nepenthes erucoides occupies one of the most extreme lower montane ultramafic Nepenthes habitats known, tolerating high insolation, clinker-like inorganic soils, pronounced seasonal drought and constant winds in a stunted bonsai forest with a canopy rarely more than 50 cm high (Robinson et al. 2019a), while N. villosa, which mainly occurs at 2300–3200 m on Mt Kinabalu, experiences high insolation and, though subject to frequent cloud cover, drying winds and increased rates of evaporation as a result of the high elevational range, particularly during dry weather when cloud cover is low.
Nepenthes pongoides will almost certainly be subject to poaching to satisfy strong demand for rare and morphologically distinctive Nepenthes species in the horticultural market. Poaching is the primary threat facing all threatened montane Nepenthes (Cross et al. 2020) and is a significant issue even for Nepenthes endemic to the comparatively well-funded, professionally managed and frequently patrolled Kinabalu Park, from where species such as N. edwardsiana and N. villosa continue to be illegally collected in unsustainable quantities yet are openly sold on Facebook (see, e.g. Cross et al. 2020; A. Robinson pers. obs.). Should laboratory and nursery-based propagation of wild collected seed be deemed by Malaysian authorities to be an appropriate means of guarding against extinction – a near certainty if poaching cannot be prevented — maximising representation of genetic diversity through exhaustive survey of the Meliau Range for all possible clusters of plants from which to sample seed is recommended. Removal of plants for the purposes of ex situ conservation is not recommended except as a last resort owing to both the low survival rates of mature Nepenthes transplants and the significant potential for detrimental effects of genetic bottlenecking through removal of valuable allelic diversity from nature.
The characteristic reddish hair produced by this remarkable pitcher plant has inspired a positive conservation link with the orangutan. While the latter is predominantly threatened by habitat loss rather than poaching, the survival of both critically endangered species is entirely dependent on decisive and effective conservation actions by government that safeguard both the rainforest habitat and its many threatened inhabitants.
Declaration of funding
All travel, expedition and herbarium research related costs for the initial research expedition were borne by a grant from the International Carnivorous Plant Society, Inc. (California, USA). In-kind funding was provided by the Royal Botanic Gardens Victoria. Costs for the follow-up expedition to Site 2 were covered by the ‘Conservation and Recovery of Rare, Threatened, and Endemic Plants in Sabah (2022–2024)’ project from the National Conservation Trust Fund for Natural Resources (Malaysia) under the Minister of Natural Resources and Environmental Sustainability of Malaysia.
Author contributions
The project was conceptualised by A. Robinson; applications for permits made by A. Robinson and A. Damit; expedition logistics organised by A. Damit, N. Yusof and Jem. Jumian; funding acquired, the project administered, manuscript prepared, and figures and illustration generated by A. Robinson; methodology established by A. Robinson, C. Clarke and A. Damit; in situ data and material collections made by A. Damit, N. Yusof, Jemson Jumian, Jamirus Jumian, Jeisin Jumian and A. Robinson; analyses conducted by A. Robinson, C. Clarke and A. Damit; and review and editing of the manuscript overseen by all co-authors.
Acknowledgements
The authors thank rangers Mohd Noor Mansor Abd Hadis, Mohd Noor Tajuddin Abd Hadis, Marinus Raimin and Jeffsin Supin from TSH Resources Berhad for accompanying the team on the expedition, and their manager, Johnny Vun, for facilitating access and logistical support; KLK Sawit Nusantara Berhad for use of a vehicle ferry; Joseph Pirin, Senior Planning Officer (Climate Change), Forest Sector Planning Division, Sabah Forestry Department, for critical expedition advice specific to the route of ascent, who, along with Jastin Kiek, also shared images of the pitcher plant from their 2018 climb; staff of the Forest Research Centre, Sepilok, particularly Robert Ong, former Deputy Chief Conservator of Forests, Arthur Chung, Deputy Chief Conservator of Forests (Research and Development), John Sugau, Head of Forest Biodiversity and Conservation and Joan Pereira, Head of Systematic Botany Section, Jonathan Jimmey Lucas, Research Officer, Jamirus and Jeisin Jumian, Research Assistants and Postar Miun, driver, for supporting expedition aims throughout and providing administrative and logistical assistance, area-specific expertise, herbarium access and orientation, specimen processing, collections support and extremely generous hospitality; Sukaibin Sumail, Research Officer Sabah Parks for guiding us to photograph Nepenthes × alisaputrana on Bukit Babi, Mt Kinabalu; Sabah Wildlife Department staff Mohd Soffian Bin Abu Bakar (Deputy Director II), and District Wildlife Officers Hussien Miun (Sandakan) and Xenia Surinday (Kota Kinabalu) for administrative assistance; Mike Miki, Sadib Miki, and Tham Yau Kong for providing guiding and point of contact services across Sabah; Michal R. Golos, University of Exeter (UK), for photographing and sharing specimen images that were difficult to access, and for valuable comments on the manuscript; Alison Vaughan, Manager Collections, Collections Officers Eugenia Pacitti, Helen Barnes, Catherine Gallagher, Madison Hughes and Casey Jurgens, and Nimal Karunajeewa, Digital Collections Advisor, at the Royal Botanic Gardens Victoria for facilitating specimen transfer, mounting, accessioning and imaging. Finally, the team and associated institutions are grateful to the Board and Members of the International Carnivorous Plant Society for generous financial support, a contribution critical to this endeavour.
References
Adam JH (1997) Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika Journal of Tropical Agricultural Science 20, 121-134.
| Google Scholar |
Adam JH, Hamid HA (2006) Pitcher plants (Nepenthes) recorded from Keningau–Kimanis Road in Sabah, Malaysia. International Journal of Botany 2(4), 431-436.
| Crossref | Google Scholar |
Adam JH, Wilcock CC (1992) A new natural hybrid of Nepenthes from Mt. Kinabalu (Sabah). Reinwardtia 11(1), 35-40 Available at https://e-journal.biologi.lipi.go.id/index.php/reinwardtia/article/viewFile/1011/887 [Verified 21 November 2023].
| Google Scholar |
Andi MA, Suleiman M (2005) Preliminary list of mosses from Meliau Range, Ulu Tungud Forest Reserve, Sabah. Sepilok Bulletin 3, 57-64.
| Google Scholar |
Banks E (1982) A short account of an expedition to Sabah (Borneo). Brunei Museum Journal 1982, 119-122.
| Google Scholar |
Bauer U, Federle W (2009) The insect-trapping rim of Nepenthes pitchers: surface structure and function. Plant Signaling & Behavior 4(11), 1019-1023.
| Crossref | Google Scholar | PubMed |
Bourke G (2010) The Climbing Pitcher Plants of the Kelabit Highlands Borneo. Captive Exotics Newsletter 1(1), 4-7.
| Google Scholar |
Burbidge FW (1882) Notes on the new Nepenthes. The Gardeners’ Chronicle, new series 17(420), 56 Available at https://www.biodiversitylibrary.org/page/25598125 [Verified 21 November 2023].
| Google Scholar |
Charlson RJ, Rodhe H (1982) Factors controlling the acidity of natural rainwater. Nature 295(5851), 683-685.
| Crossref | Google Scholar |
Cheek MR, Jebb MHP (2001) ‘Flora Malesiana. Series I – Seed plants. Volume 15: Nepenthaceae.’ (Nationaal Herbarium Nederland: Leiden) iv + 164 pp. Available at https://repository.naturalis.nl/pub/532692/FM1S2001015001001.pdf [Verified 21 November 2023]
Cheek M, Jebb M, Murphy B (2019) A classification of functional pitcher types in Nepenthes (Nepenthaceae). bioRxiv Nov 25 852137.
| Crossref | Google Scholar |
Chin L, Chung AYC, Clarke C (2014) Interspecific variation in prey capture behavior by co-occurring Nepenthes pitcher plants: evidence for resource partitioning or sampling-scheme artifacts. Plant Signaling and Behavior 9, e27930.
| Crossref | Google Scholar | PubMed |
Clarke CM, Kitching RL (1993) The metazoan food webs of six Bornean Nepenthes species. Ecological Entomology 18(1), 7-16.
| Crossref | Google Scholar |
Clarke C, Moran JA (2011) Incorporating ecological context: a revised protocol for the preservation of Nepenthes pitcher plant specimens (Nepenthaceae). Blumea 56(3), 225-228.
| Crossref | Google Scholar |
Clarke CM, Lee CC, McPherson SR (2006) Nepenthes chaniana (Nepenthaceae), a new species from north-western Borneo. Sabah Parks Nature Journal 7, 53-66.
| Google Scholar |
Clarke CM, Schlauer J, Moran JA, Robinson AS (2018) Systematics and evolution of Nepenthes. In ‘Carnivorous plants: physiology, ecology, and evolution.’ (Eds AM Ellison, L Adamec) pp. 58–69. (Oxford University Press: Oxford) 10.1093/oso/9780198779841.003.0005
Cross AT, Krueger TA, Gonella PM, Robinson AS, Fleischmann AS (2020) Conservation of carnivorous plants in the age of extinction. Global Ecology and Conservation 24, e01272.
| Crossref | Google Scholar |
Cross AT, van der Ent A, Wickmann M, Skates LM, Sumail S, Gebauer G, Robinson AS (2022) Capture of mammal excreta by Nepenthes is an effective heterotrophic nutrition strategy. Annals of Botany 130(7), 927-938.
| Crossref | Google Scholar | PubMed |
Dančák M, Majeský Ľ, Čermák V, Golos MR, Płachno BJ, Tjiasmanto W (2022) First record of functional underground traps in a pitcher plant: Nepenthes pudica (Nepenthaceae), a new species from North Kalimantan, Borneo. PhytoKeys 201, 77-97.
| Crossref | Google Scholar | PubMed |
Hooker JD (1859) On the origin and development of the pitchers of Nepenthes, with an account of some new Bornean plants of that genus. Transactions of the Linnean Society of London 22(4), 415-424.
| Crossref | Google Scholar |
Hooker JD (1851) Nepenthes villosa. tab. 888. In ‘Icones Plantarum, vol. 9.’ (Ed WJ Hooker) tabs. 801–900. (Reeve & Co.: London) Available at https://www.biodiversitylibrary.org/page/16044811 [Verified 04 December 2023]
Hotta M (1966) Notes on Bornean plants, I. Acta Phytotaxonomica et Geobotanica 22(1–2), 1-10.
| Crossref | Google Scholar |
IUCN (2012) ‘IUCN Red List Categories and Criteria Version 3.1’, 2nd edn. (IUCN: Gland, Switzerland and Cambridge, UK) Available at https://www.iucnredlist.org/resources/categories-and-criteria [Verified 01 November 2023]
Jebb MHP, Cheek MR (1997) A skeletal revision of Nepenthes (Nepenthaceae). Blumea 42(1), 1-106 Available at https://repository.naturalis.nl/pub/524717/ [Verified 17 December 2023].
| Google Scholar |
Moran JA, Clarke CM (2010) The carnivorous syndrome in Nepenthes pitcher plants: current state of knowledge and potential future directions. Plant Signaling & Behavior 5(6), 644-648.
| Crossref | Google Scholar |
Robinson AS, Zamudio SG, Caballero RB (2019a) Nepenthes erucoides (Nepenthaceae), an ultramaficolous micro-endemic from Dinagat Islands Province, northern Mindanao, Philippines. Phytotaxa 423(1), 21-32.
| Crossref | Google Scholar |
Robinson AS, Golos MR, Barer M, Sano Y, Forgie JJ, Garrido D, Gorman CN, Luick AO, McIntosh NW, McPherson SR, Palena GJ (2019b) Revisions in Nepenthes following explorations of the Kemul Massif and the surrounding region in north-central Kalimantan, Borneo. Phytotaxa 392(2), 97-126.
| Crossref | Google Scholar |
Sabah Forestry Department (2010) Mount Silam and its Natural Wonders. Available at http://www.mysabah.com/download/mount-silam-pamphlet.pdf [Verified 28 October 2023]
Schuiteman A, De Vogel EF (2002) Nepenthes vogelii (Nepenthaceae): a new species from Sarawak. Blumea: Biodiversity, Evolution and Biogeography of Plants 47(3), 537-40.
| Google Scholar |
Sheldon FH (2015) Gazetteer and site-based history of the ornithology of Sabah, Malaysian Borneo. Occasional Papers of the Museum of Natural Science, Louisiana State University 1(86), 1-91.
| Crossref | Google Scholar |
Sims RW (1956) ‘The Origin and Affinities of the Avifauna of eastern North Borneo’ (unpublished manuscript based on the British Museum (Natural History) North Borneo Expedition 1956; 117 pp plus appendices). Available at https://repository.lsu.edu/cgi/viewcontent.cgi?article=1005&context=opmns [Verified 24 December 2023]
Steinbauer MJ, Field R, Grytnes JA, Trigas P, Ah-Peng C, Attorre F, Birks HJB, Borges PAV, Cardoso P, Chou CH, De Sanctis M, et al. (2016) Topography-driven isolation, speciation and a global increase of endemism with elevation. Global Ecology and Biogeography 25(9), 1097-1107.
| Crossref | Google Scholar |
Thiers B (2024) Index Herbariorum. A global directory of public herbaria and associated staff. New York Botanical Garden’s virtual Herbarium. Available at http://sweetgum.nybg.org/science/ih [accessed 26 November 2023]
Turnbull JR, Middleton AT (1988) A new species of Nepenthes from Sabah, Malaysia. Botanical Journal of the Linnean Society 96(4), 351-358.
| Crossref | Google Scholar |
van der Ent A, Repin R, Sugau J, Wong KM (2015) Plant diversity and ecology of ultramafic outcrops in Sabah (Malaysia). Australian Journal of Botany 63(4), 204-215.
| Crossref | Google Scholar |


