Evaluating the risk to Australia’s flora from Phytophthora cinnamomi
Keith L. McDougall A * , Sarah Barrett B , Renate Velzeboer C , David M. Cahill D and Tim Rudman E
A * , Sarah Barrett B , Renate Velzeboer C , David M. Cahill D and Tim Rudman E
A
B
C
D
E
Abstract
Phytophthora cinnamomi Rands is a destructive pathogen of Australian native vegetation, often causing permanent damage to ecosystems and threatening the survival of rare, susceptible species. Despite that, much information about the effects of P. cinnamomi on plant species remains unpublished and the risk of extinction to most species is unknown.
We aimed to classify the risk of extinction from P. cinnamomi to Australian native plants.
We used available data and personal knowledge about P. cinnamomi effects on plants, spatial data on plant species distribution and habitat suitability of P. cinnamomi to assign an extinction-risk category of low, moderate, high or very high.
There are currently 65 plant species at a very high risk of extinction in Australia as a result of P. cinnamomi infection. The genera Andersonia, Banksia, Darwinia, Daviesia, Epacris, Gastrolobium, Grevillea, Hibbertia, Isopogon, Lambertia, Latrobea, Leucopogon, Phebalium and Styphelia have multiple species at a very high risk of extinction, most of which occur in south-western Western Australia.
The available data confirmed the high risk to the Australian flora from P. cinnamomi and identified species in plant families not previously known to be affected, highlighting data gaps (e.g. lack of knowledge about effects and risk in orchids and grasses).
Much more work is required to fully understand the risk from P. cinnamomi (and other Phytophthora species) to the Australian flora.
Keywords: Banksia, Ericaceae, extinction risk, host response, Oomycetes, pathogens, Phytophthora susceptibility, threatened species.
Introduction
Phytophthora cinnamomi Rands is a water mould (Class: Oomycetes) with a large host range. Zentmyer and Thorn (1967) produced one of the first host lists for P. cinnamomi, containing 207 mostly horticultural plant species. Lists of Australian host plants have been developed since, and the number of species known to be hosts has greatly increased (e.g. Shivas (1989) for Western Australia, Weste (2001) for Victoria, O’Gara et al. (2006) for Australia). Shearer et al. (2004) estimated that more than 2000 species are susceptible in south-western Western Australia alone.
Since its arrival in Australia, P. cinnamomi has had an immense detrimental impact on agriculture (Irwin et al. 1995) and on native plants and faunal habitat, especially on the southern coastal fringe (Cahill et al. 2008). Infection of native plants by this pathogen is a key threatening process under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) and it is regarded as a threat or potential threat to 120 plant species, 32 ecological communities and 14 fauna species listed under the EPBC Act (Commonwealth of Australia 2018).
For species management, setting conservation priorities and the allocation of resources, knowledge about which plant species are at risk from P. cinnamomi is vital. However, determining the risk to native plant hosts has been difficult because of
A lack of information: the effects of P. cinnamomi on flora have been documented for few native plant species
Obscurity of information: much information about the effects of P. cinnamomi has not been published
Inherent difficulties in assigning risk: the response of species to the presence of the pathogen can be idiosyncratic, e.g. symptoms may only occur under some climatic conditions or in some habitats that favour the pathogen (e.g. Tippett et al. 1989; Cahill et al. 2008). Indirect effects are also known, where a change in habitat following the death of one species affects the survival of other species, including fauna (e.g. McDougall et al. 2005; Petit and Dickson 2005; Dundas et al. 2016). That is, some species may be affected even if they are not susceptible to infection
Confusing terminology: numerous studies have investigated host–P. cinnamomi interactions, but there has been no standard approach to describing the outcomes of an infection. Hosts have typically been placed subjectively into categories of susceptibility such as Resistant and Resistant Host (Barker and Wardlaw 1995), Field Resistant (e.g. Weste 2001; O’Gara et al. 2006), Slightly Susceptible (Barker and Wardlaw 1995; Weste 2001), Low Susceptibility, Susceptible, Susceptible but Persistent (O’Gara et al. 2006), Moderately Susceptible and Highly Susceptible (e.g. Barker and Wardlaw 1995; Weste 2001; O’Gara et al. 2006; Wan et al. 2019) and Q (referring to inferred susceptibility, O’Gara et al. 2006). Shearer and Dillon (1996) used numbered categories from field observations to rank susceptibility. However, susceptibility, defined in the Oxford English Dictionary as ‘capable of taking, receiving, being affected by, or undergoing something’, could refer to the likelihood of infection (receiving) or the likelihood of harm (being affected by), and both senses have been used in the literature, often interchangeably. A species that is highly susceptible to infection need not be highly susceptible to harm. For instance, Crone et al. (2013) found that P. cinnamomi could be commonly recovered from roots of the annual herb Trachymene pilosa Sm. but no symptoms were recorded for infected plants. Similarly, the root tips of the alpine shrub Podocarpus lawrencei Hook.f. were commonly infected by P. cinnamomi in glasshouse trials, but the disease progressed no further and plants exhibited no above-ground symptoms (Rigg et al. 2018). Species regarded as being resistant (inferred from the lack of symptoms in the field or absence of infection in glasshouse studies) may still be susceptible to infection but not or rarely susceptible to disease (at least under the conditions of observation).
The current classification of susceptibility is inconsistent and ambiguous, and of questionable value to land managers who are likely to want to know whether the presence of the pathogen will lead to an ongoing decline in plant health, i.e. the risk that P. cinnamomi poses to species conservation. Extinction-risk assessment tools for threatened species potentially affected by P. cinnamomi have previously been developed (Reiter et al. 2004; Velzeboer et al. 2005; Barrett et al. 2008). For instance, Barrett et al. (2008) assessed risk for threatened species according to direct impact, number of populations affected and unaffected, proximity to P. cinnamomi and tracks, and the presence of other threatening processes. However, for most plant species known to be susceptible to infection by P. cinnamomi, population-level risk variables such as those used by Barrett et al. (2008) are unavailable; risk can therefore be evaluated only for a few species using current risk assessment tools.
Assessing risk to vascular plants from any plant pathogen relies on knowing (1) whether a plant species is capable of being infected (susceptibility), (2) whether the infection can result in poor health or mortality (host response) and (3) whether the pathogen and host may co-occur (likelihood). Likelihood is scale- and time-dependent. For instance, a rare plant species potentially affected by P. cinnamomi and growing in vulnerable habitat is at greater, immediate risk of a negative impact on its population than is a population of a widespread and common plant species that can be affected by P. cinnamomi, but which has a higher probability of surviving in disease-free refugia. A simple measure of risk in the case of P. cinnamomi can, therefore, be attained by combining susceptibility/species-response data with data on the extent of host occurrence and climatic suitability for P. cinnamomi (which are available for all species from data lodged in the Atlas of Living Australia (ALA, https://www.ala.org.au)).
In this paper, we overcome some of the difficulties listed above in assessing the effects of P. cinnamomi on Australian native plant species. We update the list of O’Gara et al. (2006) with new published and unpublished data and expert observations about species responses to P. cinnamomi for Australian native plants. We then assess risk to species in the updated list from P. cinnamomi, on the basis of species response and extent of occurrence. The expanded list also provides an opportunity to explore phylogenetic patterns of species response to the pathogen and risk to survival.
Methods
P. cinnamomi risk assessment
The list of O’Gara et al. (2006) was checked and updated with recent taxonomic changes and more recently published and unpublished data on species responses were added. These data included records from the Western Australian Department of Biodiversity, Conservation and Attractions Vegetation Health Services Phytophthora database (accessed 14 January 2022), which has over 20,000 records of isolations from plants and soil in Western Australia, dating from 1982. These records are primarily derived from single baiting of soil and plant root material for the presence or absence of Phytophthora pathogens, but some samples may have been double-baited or sent to the Centre for Phytophthora Science and Management (Murdoch University) for DNA sequencing; approximately 85% of isolations in the database are for P. cinnamomi. Data for glasshouse inoculation trials on more than 400, mostly Western Australian, native plant species were utilised (C. E. Crane and B. L. Shearer, unpubl. data). Pots containing test species were inoculated with P. cinnamomi as described by Shearer et al. (2007). The species tested included 170 threatened and 217 priority flora from the WA ex situ conservation program, which were provided for research on Phytophthora susceptibility. The full list of data sources is in Supplementary material S1. We aimed to gather all available data but do not include all data sources where a subsequent review included preceding data (e.g. Weste 2001). In addition, data from a few sources cited in O’Gara et al. (2006), which could not be checked, were omitted from the list.
Species in the updated list were assigned to one of the following three categories of response:
None: species are apparently unaffected when P. cinnamomi is present; this included the Resistant and Field Resistant susceptibility categories of previous authors; although such species may be susceptible to infection, as discussed above, they are likely to rarely, if ever, experience disease;
Mild: symptoms are mild and/or infrequently severe; this included the Susceptible, Slightly Susceptible and Low Susceptibility categories of previous authors;
Severe: species will usually display severe symptoms, often resulting in mortality; this included the Moderate Susceptibility and High Susceptibility categories of previous authors.
For glasshouse susceptibility trials where susceptibility had not been assigned as a category, we regarded the response of species with no mortality and no re-isolation of the pathogen as None, <25% mortality and re-isolation as Mild and greater than or equal to 25% and re-isolation as Severe.
We used ALA spatial data (accessed between 14 October 2022 and 13 March 2023) and ALA data tools to calculate the extent of occurrence (EOO) and climatic suitability for P. cinnamomi. Disjunct records were first checked in local floras and removed if not supported for their distribution; these were typically records of cultivated plants in capital city botanic gardens or data-entry errors. Accepted point locations for a species were screened for climatic habitat suitability for P. cinnamomi by using the Scatterplot tool; a species was deemed to occur in suitable habitat if >25% of records had an annual rainfall of >400 mm and a mean annual temperature of >10°C (climatic conditions considered to be thresholds for P. cinnamomi activity; O’Gara et al. 2006). For species in the updated list of O’Gara et al. (2006), we recorded conservation status (Vulnerable, Endangered or Critically Endangered) under the EPBC Act (http://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted=flora, accessed 5 May 2023). For species not listed under the EPBC Act, EOO (as an alpha hull) was calculated using the Calculate AOO and EOO tool in the ALA. Species were identified that could be regarded as threatened under IUCN criteria if other criteria relating to threat or population decline were met (IUCN Standards and Petitions Committee 2022), as follows: Vulnerable (alpha hull between 5000 and 20,000 km2), Endangered (alpha hull between 500 and 5000 km2) or Critically Endangered (alpha hull <500 km2) or as Not Threatened (alpha hull >20,000 km2). Given potential inflation of EOO because of spatial errors of species occurrence data in ALA, we included species as Vulnerable if their EOO was greater than 20,000 km2 and less than 50,000 km2, and all occurrences were in suitable habitat for P. cinnamomi.
Risk was then assigned using the criteria in Table 1. We define risk as the likelihood of a species becoming extinct at some time in the future because of disease caused by P. cinnamomi. The categories of risk (very high, high, moderate and low) are not meant to be prescriptive nor can we define the time it will take for extinction to occur under any category. The time to extinction will vary with the life history of the species potentially affected and the current proximity of the pathogen. In most cases, extinction for species at very high risk will be within the next few decades if the pathogen is present in all populations and no remedial management is undertaken. Where plant species had been assigned to different response categories by different authors, we accepted the more severe response category for assigning risk. We assumed that species in the updated response table known to be susceptible to infection but not categorised for response, will have a severe response. In a few cases, where the resulting risk assignment was inconsistent with our expert knowledge of on-ground effects, risk was assigned manually and the reasons for the change are presented in Supplementary material S1. The reasons include the population ecology of hosts, imminence of the threat from P. cinnamomi, and inflated EOO because of data denaturing in ALA. For instance, a species that is rarely killed by P. cinnamomi might still be at high risk of an enduring impact if recruitment is not replacing mortalities. Conversely, a species that is frequently killed by P. cinnamomi might be at low risk if it recruits rapidly. This seems to be the case for Banksia sessilis (Knight) A.R.Mast & K.R.Thiele in Western Australia, which can take as little as 3 years to produce copious amounts of seed after germination (McDougall 1997). In some cases, and especially for threatened species occurring in Western Australia, the simplification or deliberate denaturing of location data in ALA has resulted in inflated EOO calculations, so that, for example, species that might be regarded as Critically Endangered (EOO of <500 km2) are assessed as being endangered or vulnerable (EOO of >500 km2). Assessing risk for species affected by a change in habitat but not infected by P. cinnamomi is more challenging and we do not attempt to do that in this paper.
| Conservation status | Species response | Habitat suitability | Risk | |
|---|---|---|---|---|
| Critically Endangered | Severe | Suitable | Very high | |
| Mild | Suitable | High | ||
| None | Suitable | Moderate | ||
| Any | Unsuitable | Low | ||
| Endangered | Severe | Suitable | High | |
| Mild | Suitable | Moderate | ||
| None | Suitable | Low | ||
| Any | Unsuitable | Low | ||
| Vulnerable | Severe | Suitable | Moderate | |
| Mild | Suitable | Low | ||
| None | Suitable | Low | ||
| Any | Unsuitable | Low | ||
| Not Threatened | Any | Any | Low |
Conservation status is either that given for a species under the EPBC Act or a putative status on the basis of the extent of occurrence. Species response is based on previous assessments of susceptibility or expert review. Habitat suitability relates to the likelihood of P. cinnamomi surviving and/or being active; a species was deemed to occur in suitable habitat if >25% of records had an annual rainfall of >400 m and a mean annual temperature of >10°C.
Results
There were 1773 native plant species with data relating to P. cinnamomi (Supplementary material S1 provides the source of each data record). This included 218 plant species from which the pathogen had been isolated but no assessment of response was reported in the literature.
P. cinnamomi responses and risk
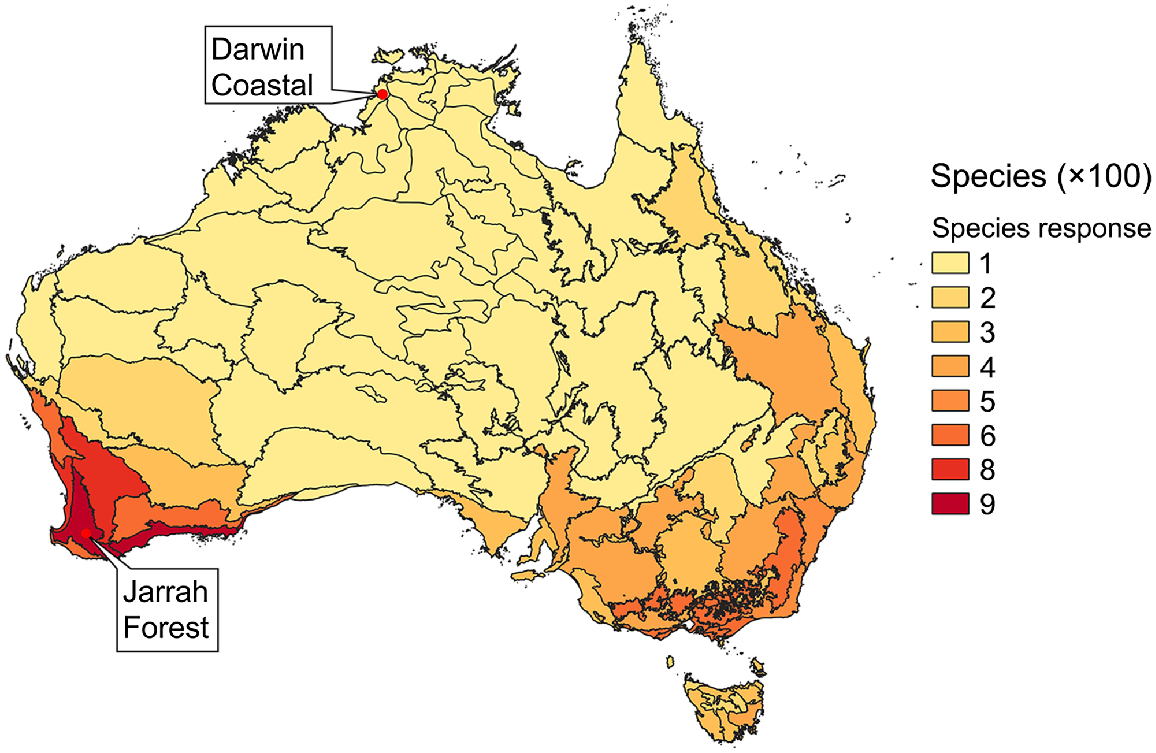
Most species assessed for susceptibility and response occur in southern Australia and especially south-western Western Australia. For example, 879 species had been assessed from the Jarrah Forest Interim Biogeographic Regionalisation for Australia (IBRA) 7 region, and by contrast only 23 species had been assessed from the Darwin Coastal region (Fig. 1).
Numbers of plant species assessed for susceptibility and response by IBRA 7 region, classified by 100s of species (with the lightest colour being 1–100 species).
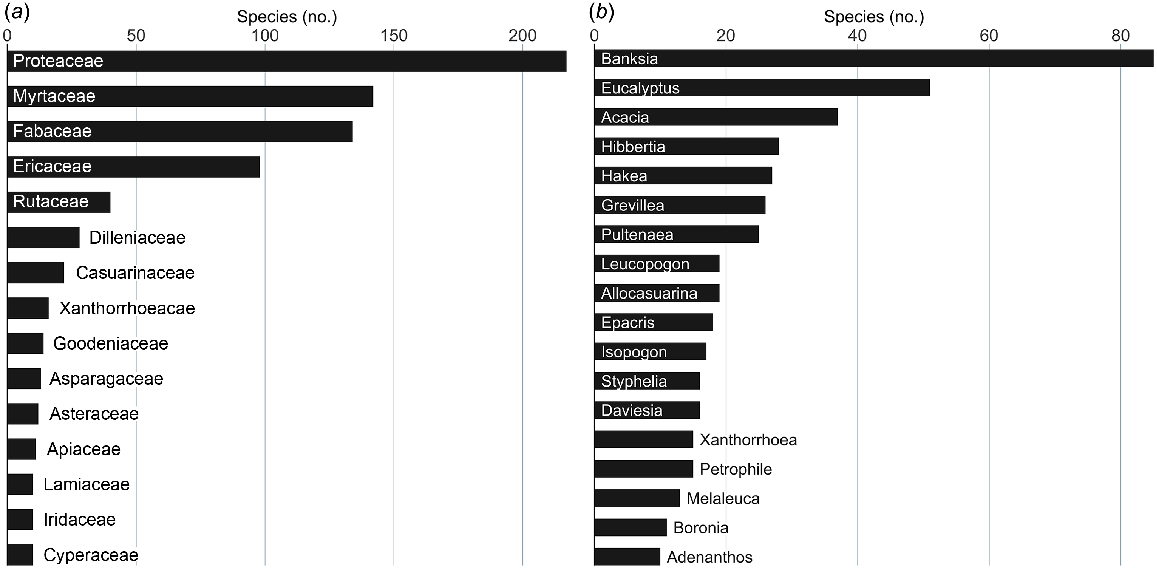
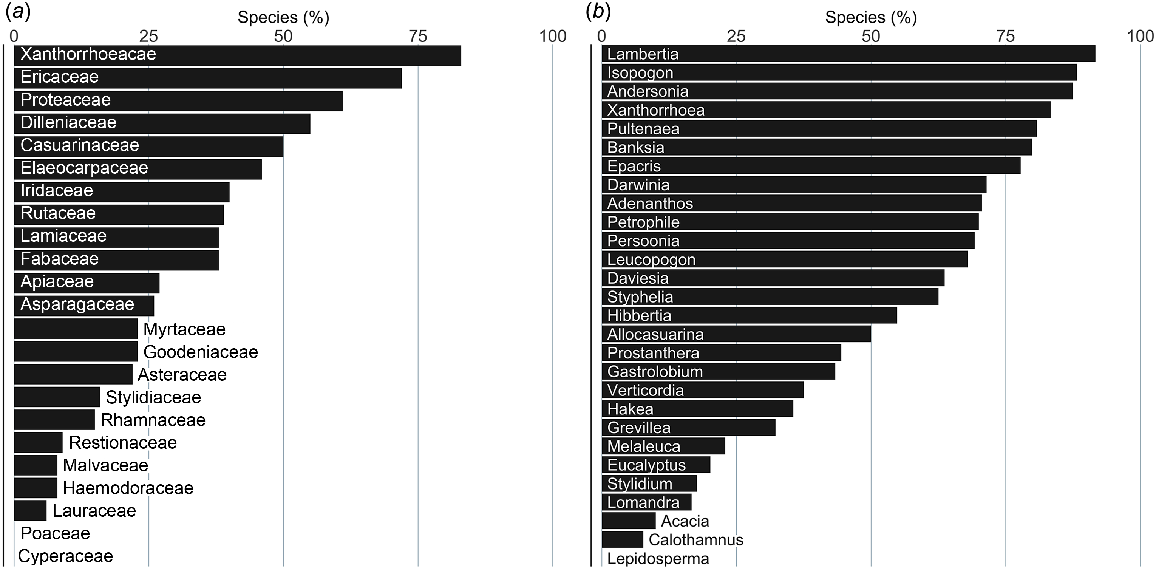
P. cinnamomi has been isolated from the roots of 908 native Australian vascular plant species, representing 69 plant families and 233 genera. Most isolations have been from plants in the families Proteaceae, Myrtaceae, Fabaceae and Ericaceae (Fig. 2a), accounting for 65% of all isolations. Most isolations have been from species in the genera Banksia, Eucalyptus, Acacia, Hibbertia, Grevillea, Hakea and Pultenaea (Fig. 2b), accounting for 31% of all isolations.
Number of species by family and genus from which P. cinnamomi has been isolated; (a) family, (b) genus. Families and genera that had 10 or more species with isolations are shown.
Of the approximately 22,000 native plant species in Australia (https://biodiversity.org.au/nsl/, accessed 7 July 2023), 1555 (7%) have been assessed for their response to P. cinnamomi (Supplementary material S1). Some plant families have been disproportionately assessed, for example, 40% of species in the family Xanthorrhoeaceae, 35% of Casuarinaceae and almost 30% of Proteaceae. There have been no assessments of response for species of four families with more than 100 native species (i.e. Boraginaceae, Brassicaceae, Convolvulaceae, Solanaceae) and there are few assessments for two of the most species-rich families in Australia (Asteraceae and Poaceae, both having 2% of species assessed).
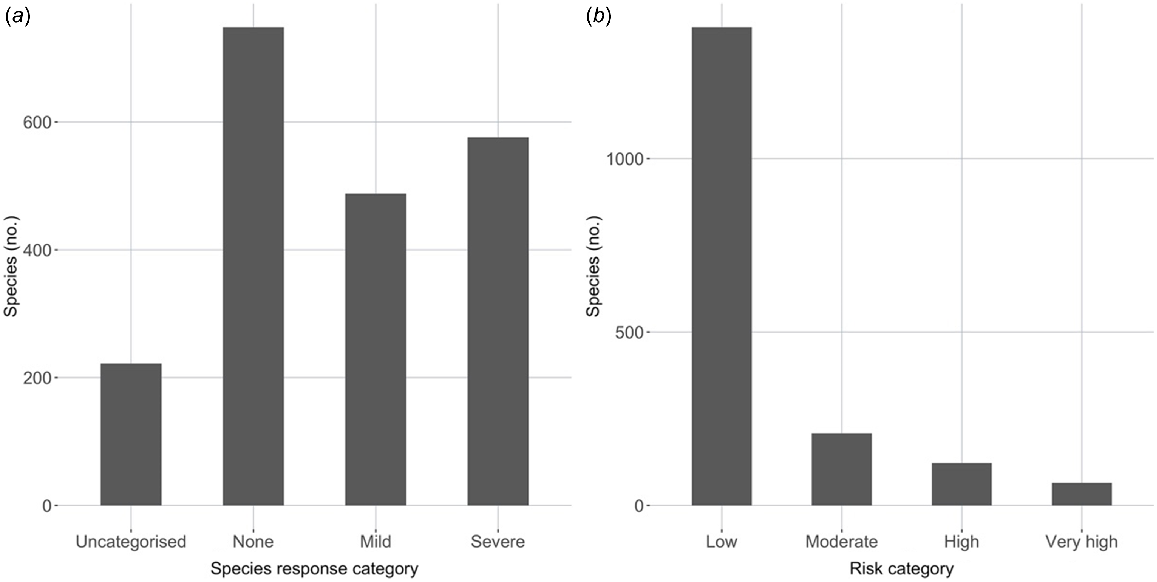
There were roughly comparable numbers of species assigned to the three categories of species response (none, mild and severe; Fig. 3a), with 218 species being uncategorised for response; 210 species were assigned to two categories by different authors, whereas 20 species were assigned to all three categories. In contrast, the risk categories were very uneven in numbers of species; of the 1773 species categorised for risk, 1378 species (78%) were found to be at low risk from P. cinnamomi infection, 208 species (12%) were at moderate risk, 122 species (7%) were at high risk and 65 species (4%) were at very high risk (Fig. 3b).
Numbers of species that were categorised for response and risk; (a) species response (none, mild or severe), (b) risk (low, moderate, high or very high) on the basis of species response, habitat suitability and extent of occurrence.
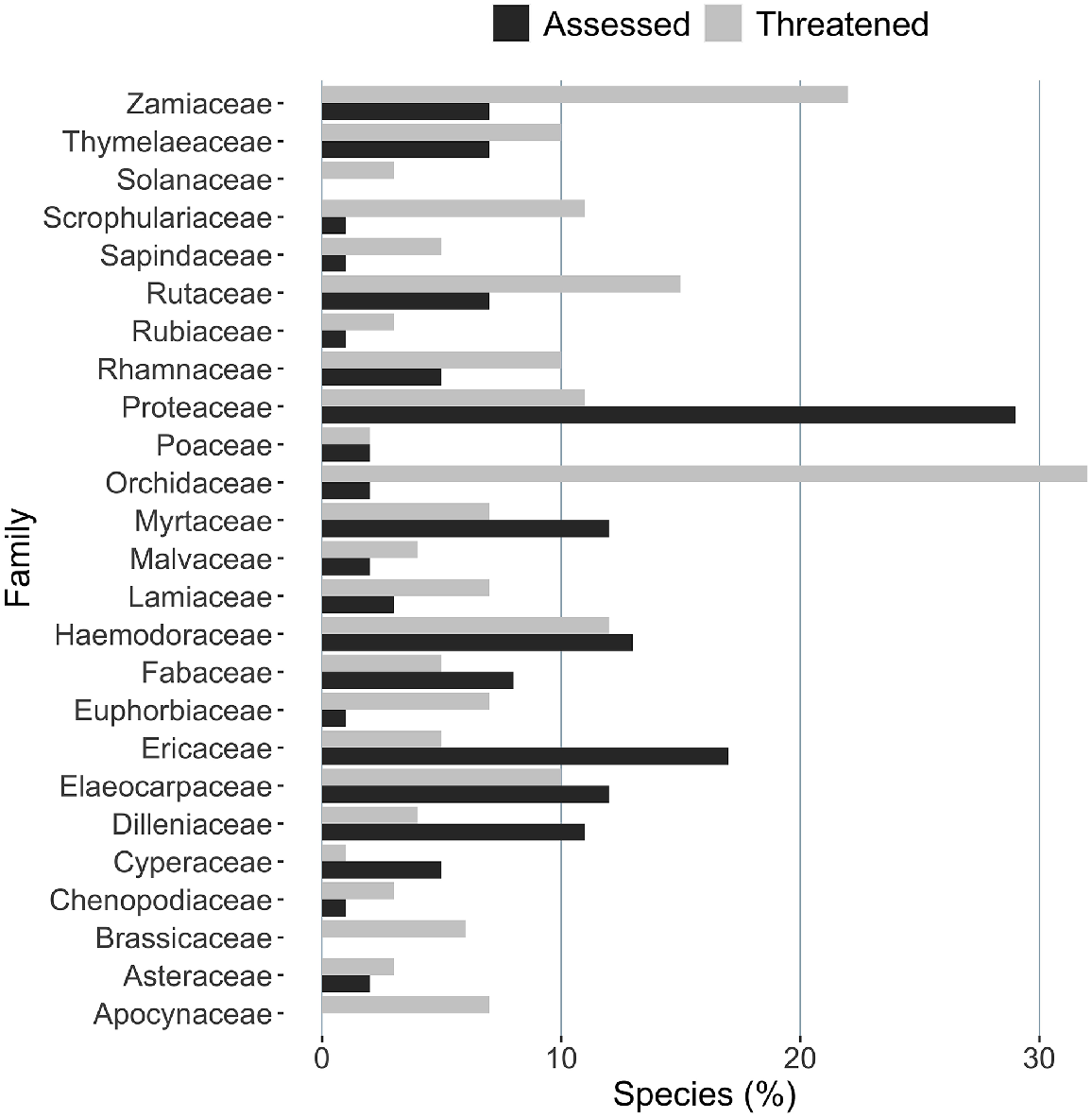
Despite the large number of isolations from species in the family Myrtaceae (Fig. 2a), only 23% of the species assessed for susceptibility in that family are reported as displaying a severe response (Fig. 4a) and most of those are in the genera Darwinia and Verticordia rather than Eucalyptus and Melaleuca, which are more species-numerous (Fig. 4b). Similarly, although more than 40% of species in the family Fabaceae were reported as having a severe response (Fig. 4a), few of those were Acacia species, whereas many were in the genera Pultenaea and Daviesia (Fig. 4b). Families with more than half of the species assessed as having a severe response were Xanthorrhoaeaceae (Xanthorrhoea species), Ericaceae (mostly species of Andersonia, Epacris, Leucopogon and Styphelia), Proteaceae (mostly species of Adenanthos, Banksia, Isopogon, Lambertia, Persoonia and Petrophile) and Dilleniaceae (Hibbertia species; Fig. 4a, b). No species from the large families of sedges (Cyperaceae) and grasses (Poaceae) were reported as having a severe response to infection.
Species by family or genus reported as having a severe response to P. cinnamomi infection (as a % of species assessed); (a) species by family, (b) species by genus. Families and genera are shown for which 12 or more species had been assessed for response.
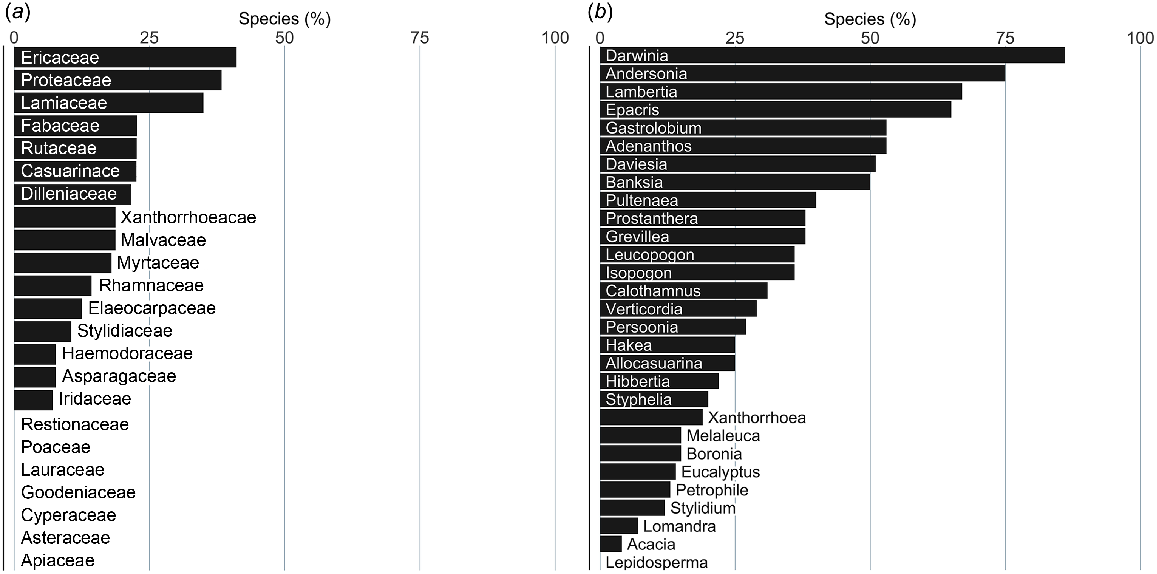
Although Xanthorrhoea species had a very high proportion of species reported as having a severe response to infection by P. cinnamomi (Fig. 4), relatively few species were found to be at a moderate to high risk of extinction (Fig. 5a, b). More than 30% of species assessed in the families Ericaceae, Lamiaceae and Proteaceae were placed in the moderate- to very high-risk categories (Fig. 5a). In contrast, no species in the families Apiaceae, Asteraceae, Goodeniaceae, Lauraceae or Restionaceae had species determined to be at moderate to very high risk of extinction. More than half the species assessed in the genera Andersonia, Darwinia, Epacris and Lambertia were placed in the moderate to very high-risk categories (Fig. 5b).
Species by family and genus that are at moderate to very high risk of extinction from P. cinnamomi; expressed as a % of species assessed; (a) species by family, (b) species by genus. Families and genera are shown for which 12 or more species were assessed for risk.
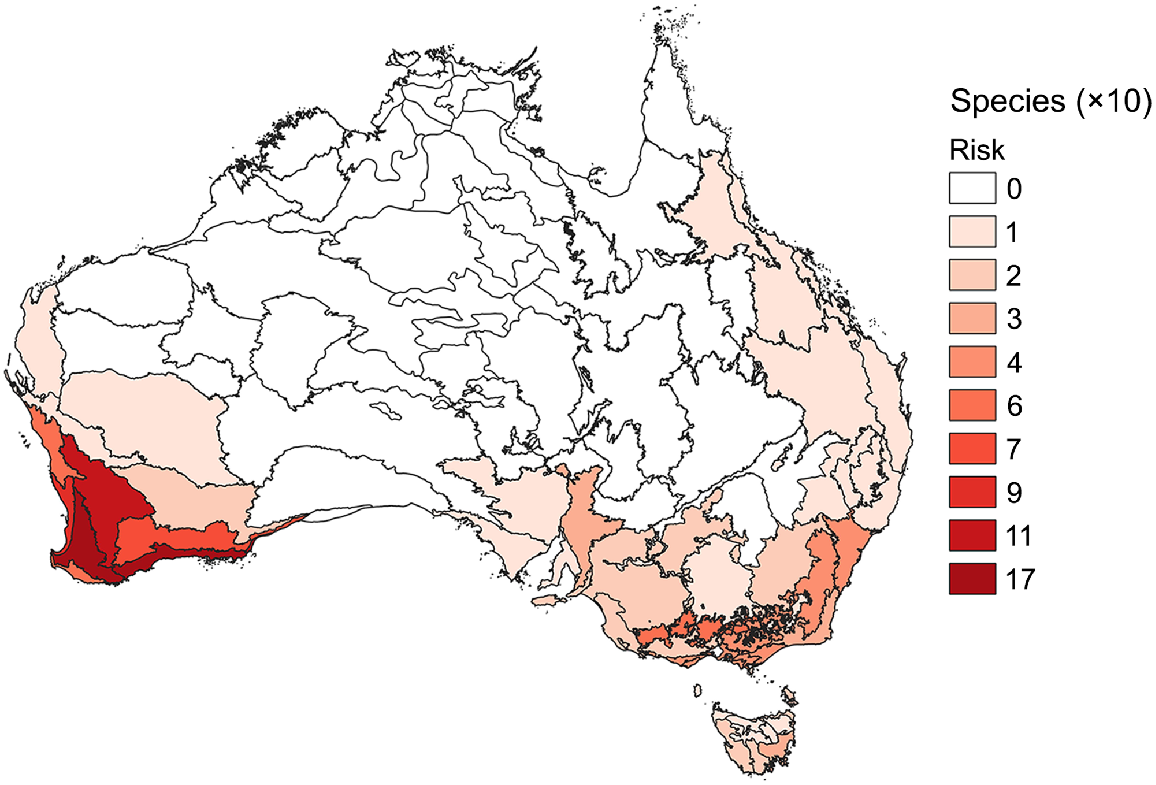
Most species at moderate to very high risk of extinction from P. cinnamomi occur in south-western Western Australia (Fig. 6). Indeed, the Jarrah Forest and Esperance Plain IBRA 7 regions had more than twice the number of species at moderate to very high risk of any region outside Western Australia. Most IBRA 7 regions had no species at moderate to very high risk of extinction.
Numbers of plant species assessed at moderate to very high risk of extinction from P. cinnamomi infection by IBRA 7 region, classified by tens of species (with white being no species).
Of the 65 species assessed as being at very high risk of extinction from P. cinnamomi, 48 (74%) were in the families Ericaceae, Fabaceae and Proteaceae, and 10 were in the genus Banksia (Table 2). The genera Andersonia, Darwinia, Daviesia, Epacris, Gastrolobium, Grevillea, Hibbertia, Isopogon, Lambertia, Latrobea, Leucopogon, Phebalium and Styphelia also had multiple species at a very high risk of extinction. Most species at very high risk (49 species, 75%) occur in Western Australia, whereas eight species occur in New South Wales, seven species occur in Tasmania, two species occur in Victoria and no species occur in the Australian Capital Territory, Northern Territory or South Australia. In total, 18 of the 65 species at very high risk (28%) are not listed as threatened under the EPBC Act 1999 and P. cinnamomi is not recognised as a threat to 17 (26%) of the species (Table 2).
| Family | Species | State | Status | Recognition | |
|---|---|---|---|---|---|
| Araucariaceae | Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen | NSW | CE | CA | |
| Boryaceae | Borya mirabilis Churchill | Vic. | E | CA | |
| Dilleniaceae | Hibbertia circinata K.L. McDougall & G.T. Wright | NSW | CE | CA | |
| Dilleniaceae | Hibbertia porongurupensis J.R.Wheeler & Hoogland | WA | – | – | |
| Dilleniaceae | Hibbertia wheelerae K.R.Thiele | WA | CE | CA | |
| Elaeocarpaceae | Tetratheca gunnii Hook.f. | Tas. | CE | CA | |
| Ericaceae | Andersonia axilliflora (Stschegl.) Druce | WA | CE | CA | |
| Ericaceae | Andersonia pinaster Lemson | WA | V | CA | |
| Ericaceae | Andersonia sp. Virolens (G.J. Keighery 12000) | WA | – | – | |
| Ericaceae | Dielsiodoxa tamariscina (F.Muell.) Albr. | WA | – | – | |
| Ericaceae | Epacris apsleyensis Crowden | Tas. | E | CA | |
| Ericaceae | Epacris barbata Melville | Tas. | E | CA | |
| Ericaceae | Epacris grandis Crowden | Tas. | E | CA | |
| Ericaceae | Epacris limbata K.J.Williams & F.Duncan | Tas. | CE | CA | |
| Ericaceae | Epacris stuartii Stapf | Tas. | CE | CA | |
| Ericaceae | Leucopogon acicularis Benth. | WA | – | UMI | |
| Ericaceae | Leucopogon atherolepis Stschegl. | WA | – | UMI | |
| Ericaceae | Leucopogon gnaphalioides Stschegl. | WA | CE | CA | |
| Ericaceae | Leucopogon lasiophyllus Stschegl. | WA | – | – | |
| Ericaceae | Styphelia exilis Hislop | WA | – | – | |
| Ericaceae | Styphelia psilopus (Stschegl.) Hislop, Crayn & Puente-Lel. | WA | – | – | |
| Fabaceae | Daviesia euphorbioides Benth. | WA | E | – | |
| Fabaceae | Daviesia glossosema Crisp | WA | CE | CA | |
| Fabaceae | Daviesia megacalyx Crisp | WA | E | CA | |
| Fabaceae | Daviesia ovata Benth. | WA | CE | CA | |
| Fabaceae | Daviesia pseudaphylla Crisp | WA | CE | CA | |
| Fabaceae | Gastrolobium leakeanum J.Drumm. | WA | – | – | |
| Fabaceae | Gastrolobium luteifolium (Domin) G.Chandler & Crisp | WA | CE | CA | |
| Fabaceae | Gastrolobium mondurup G.Chandler & Crisp | WA | – | – | |
| Fabaceae | Gastrolobium papilio | WA | E | CA | |
| Fabaceae | Gastrolobium pulchellum (Crisp) G.Chandler & Crisp | WA | – | – | |
| Fabaceae | Gastrolobium vestitum (Domin) G.Chandler & Crisp | WA | CE | CA | |
| Fabaceae | Latrobea colophon Chappill & C.F.Wilkins | WA | CE | CA | |
| Fabaceae | Latrobea pinnaculum Chappill & C.F.Wilkins | WA | – | – | |
| Fabaceae | Pultenaea sp. Genowlan Point (NSW 417813) | NSW | CE | CA | |
| Lamiaceae | Prostanthera marifolia R.Br. | NSW | CE | CA | |
| Myrtaceae | Chamelaucium sp. Cataby (G.J.Keighery 11009) | WA | V | CA | |
| Myrtaceae | Darwinia collina C.A.Gardner | WA | CE | CA | |
| Myrtaceae | Darwinia nubigena Keighery | WA | V | CA | |
| Myrtaceae | Darwinia oxylepis (Turcz.) N.G.Marchant & Keighery | WA | E | CA | |
| Myrtaceae | Darwinia wittwerorum N.G.Marchant & Keighery | WA | E | CA | |
| Proteaceae | Banksia anatona (A.S.George) A.R.Mast & K.R.Thiele | WA | E | CA | |
| Proteaceae | Banksia brownii Baxter ex R.Br. | WA | CE | CA | |
| Proteaceae | Banksia concinna (R.Br.) A.R.Mast & K.R.Thiele | WA | – | CA | |
| Proteaceae | Banksia foliolata (R.Br.) A.R.Mast & K.R.Thiele | WA | – | – | |
| Proteaceae | Banksia fuscobractea (A.S.George) A.R.Mast & K.R.Thiele | WA | CE | – | |
| Proteaceae | Banksia ionthocarpa (A.S.George) A.R.Mast & K.R.Thiele | WA | E | CA | |
| Proteaceae | Banksia montana (C.A.Gardner ex A.S.George) A.R.Mast & K.R.Thiele | WA | CE | CA | |
| Proteaceae | Banksia rufa subsp. pumila (A.S.George) A.R.Mast & K.R.Thiele | WA | E | CA | |
| Proteaceae | Banksia serratuloides subsp. perissa (A.S.George) A.R.Mast & K.R.Thiele | WA | CE | CA | |
| Proteaceae | Banksia solandri R.Br. | WA | – | UMI | |
| Proteaceae | Grevillea maxwellii McGill. | WA | E | CA | |
| Proteaceae | Grevillea pieroniae Olde | WA | – | – | |
| Proteaceae | Isopogon buxifolius R.Br. var. buxifolius | WA | – | – | |
| Proteaceae | Isopogon latifolius R.Br. | WA | – | UMI | |
| Proteaceae | Isopogon uncinatus R.Br. | WA | E | CA | |
| Proteaceae | Lambertia echinata R.Br. | WA | E | CA | |
| Proteaceae | Lambertia fairallii Keighery | WA | E | CA | |
| Proteaceae | Lambertia orbifolia C.A.Gardner subsp. orbifolia | WA | E | CA | |
| Proteaceae | Persoonia micranthera P.H.Weston | WA | E | CA | |
| Rhamnaceae | Pomaderris delicata N.G.Walsh & Coates | NSW | CE | A | |
| Rutaceae | Correa lawrenceana var. genoensis Paul G.Wilson | NSW, Vic. | E | – | |
| Rutaceae | Nematolepis rhytidophylla (Albr. & N.G.Walsh) Paul G.Wilson | NSW | V | UMI | |
| Rutaceae | Phebalium daviesii Hook.f. | Tas. | CE | CA | |
| Rutaceae | Phebalium speciosum I.Telford | NSW | CE | – |
State: NSW, New South Wales; Tas., Tasmania; Vic., Victoria; WA, Western Australia. Status under the EPBC Act 1999: V, Vulnerable; E, Endangered; CE, Critically Endangered. Recognition (of P. cinnamomi as a threat requiring management): CA, reported in conservation advice or approved recovery plan for a species or community (https://www.dcceew.gov.au/environment/biodiversity/threatened); UMI, identified as requiring urgent management intervention following fires of 2019/20 (https://www.dcceew.gov.au/environment/biodiversity/bushfire-recovery/bushfire-impacts/priority-plants).
Extrapolating within genus by the number of species assessed for risk of extinction and the total number of species in a genus, an additional 204 native Australian species may be at a very high risk of extinction because of P. cinnamomi (i.e. 269 species in total may be at very high risk). Most of the potentially very high-risk species are in the genera Hibbertia (27 species), Darwinia (17), Gastrolobium (17), Pomaderris (16), Styphelia (16), Leucopogon (15), Phebalium (14) and Daviesia (13), reflecting the large proportion of species of unknown response in those genera. In total, 509 species may be at high risk and 809 species may be at moderate risk, giving a projected total of 1587 species at moderate to very high risk of extinction from disease caused by P. cinnamomi in Australia.
Only 138 of the 395 species at moderate to very high risk (35%) in this study are listed as threatened under the EPBC Act; i.e. 257 species might be eligible for listing on the basis of their extent of occurrence and level of threat from P. cinnamomi. More than 10% of species in the family Proteaceae are listed as threatened under the EPBC Act (http://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted=flora, accessed 14 February 2023) and, so, it is not surprising that almost 30% of species in the family Proteaceae have been assessed for response to P. cinnamomi (Fig. 7). However, some families have had few assessments of response despite a large number of species being listed as Threatened. Most notable of these are species in the Orchidaceae family, where more than 30% of recognised species are listed as threatened but the response of only 2% to P. cinnamomi has been assessed (and none of that 2% is a Threatened species). Other families with more than 10 threatened species to have few assessments of response are Apocynaceae, Brassicaceae, Euphorbiaceae, Lamiaceae, Rhamnaceae, Rutaceae, Sapindaceae, Scrophulariaceae and Zamiaceae.
Discussion
Evaluating the risk of P. cinnamomi
In the Introduction, we identified the follwowing four impediments to evaluating the risk of Australia’s flora to infection by P. cinnamomi: (1) a lack of information; (2) obscurity of information; (3) inherent difficulties in assigning risk; and (4) confusing terminology. We have overcome the second impediment by compiling available published and unpublished information and interpreting it with our expert knowledge. However, the compiled list (Supplementary material S1) may quickly become obsolete and it should be maintained and updated when new information becomes available. The third impediment is difficult to address; we have annotated the list with idiosyncrasies that we are aware of, and these can be updated as new information becomes available, but secondary effects resulting from changes in habitat are easily overlooked or difficult to quantify. Improving knowledge about species reliant on habitat that can be adversely affected by P. cinnamomi is recommended to prevent or limit secondary effects. For example, the importance of Xanthorrhoea species as shelter for several small mammal species and an orchid is recognised (e.g. Caladenia behrii Schltdl., Petit and Dickson 2005; various small mammals, Laidlaw and Wilson 2006; mardo, Swinburn et al. 2007), but Xanthorrhoea plants are also habitat for endemic insects (e.g. weevils; Hsiao and Oberprieler 2021) and are likely to have cultural importance (Rots et al. 2020). Invertebrate species dependent on threatened plants such as Banksia montana and Banksia brownii (assessed by us as being at very high risk of extinction) may be at a very high risk of co-extinction (Moir et al. 2016; Moir 2021). In addition, Banksia species at risk from P. cinnamomi are known to be important food sources for a small mammal (Dundas et al. 2016) and avifauna (Davis et al. 2014) in Western Australia. Future local, yet unknown, secondary effects are likely because many Banksia and Xanthorrhoea species show a severe response when infected.
To address confusing terminology, we suggest that previous susceptibility categories be replaced by categories reflecting response of species to the presence of P. cinnamomi, whether through direct infection or indirect effects; no response, mild response and severe response are used in this paper to reflect the degree or likelihood of a detrimental response over time. The susceptibility categories of the past could lead to unnecessary management costs. We identified several species in the current study that display severe disease under glasshouse conditions, but which grow in habitats unfavourable to P. cinnamomi (e.g. Avicennia marina (Forssk.) Vierh. (grey mangrove) from saline environments; Phebalium squamulosum subsp. alpinum (Benth.) Paul G.Wilson from cold environments; and Acacia sciophanes Maslin from semi-arid environments). Similarly, Pultenaea daphnoides J.C.Wendl. has been regarded as highly susceptible to disease (McDougall and Summerell 2003; Schahinger et al. 2003; Kueh 2011), but it is widespread and its risk of extinction will be low in the foreseeable future. That does not mean that local conservation measures are not necessary to protect widespread species such as this, but these species are likely to require much less immediate management intervention than are species of limited extent with severe responses to infection.
There are alternative ways of defining susceptibility and host response. Shearer et al. (2007), for instance, used mortality curves in glasshouse trials as an objective way of describing susceptibility and disease severity; the curves show the rate of symptom development and whether full mortality is reached under the conditions of the trials. Allardyce et al. (2012) recommend a standardised approach to identifying resistance to P. cinnamomi in test plants, which may partly overcome temporal issues of symptom development. However, there are likely to be idiosyncratic responses of hosts to P. cinnamomi regardless of the methods used to explore host–pathogen interactions; results obtained from glasshouse experiments will often differ from those obtained from field sampling and field results may differ in time and space. Although our response categories are as arbitrary as previous classifications of susceptibility, we have used response only as a variable in assessing risk of extinction, which we believe is more useful to land managers. The consequence of assigning a species to the wrong response category is important only for species of high conservation significance, and land managers might choose a precautionary approach to this. The response category can be changed if new evidence suggests that it was incorrect, and the resulting risk category can be ignored by land managers if there are other reasons to manage or not manage a P. cinnamomi risk. The risk categories provide guidance to managers about the urgency of remedial action.
The lack of information about P. cinnamomi effects and risk is less easy to rectify. Our projection of 1587 species (7% of the Australian flora) being at moderate, high or very high risk of extinction because of disease caused by P. cinnamomi reinforces the magnitude of the threat to Australia’s native plants and vegetation from this pathogen reported by many authors (see Cahill et al. 2008). However, that projection is based on knowledge about species response in a few plant families and genera. There have been few studies of response to P. cinnamomi in orchids and none of the 234 orchid species currently listed as threatened under the EPBC Act has been tested for its response in glasshouse studies. Similarly, more testing of species response is required in the families Apocynaceae, Brassicaceae, Euphorbiaceae, Lamiaceae, Rhamnaceae, Rutaceae, Sapindaceae, Scrophulariaceae and Zamiaceae, which we found to be under-represented for species listed as Threatened. Taxonomic revisions are also likely to increase the number of species at risk. Many new species of Hibbertia have been named in recent decades (e.g. Toelken and Miller 2012) and several have very limited distributions. One of these, Hibbertia circinata, is regarded as being at a very high risk of extinction because it is usually killed in the presence of P. cinnamomi and its single population is infested (McDougall et al. 2023). Prior to its recent recognition as a species, it was part of Hibbertia linearis R.Br. ex DC., which we identified as being at low risk of extinction. Glasshouse testing of rare, newly described Hibbertia species, occurring in habitat suitable to P. cinnamomi, is recommended. Indeed, glasshouse testing of all species of restricted extent occurring in habitat suitable to P. cinnamomi is recommended because they will potentially be species at the highest immediate risk from the pathogen.
The predominance in southern Australia of species assessed for susceptibility and response to P. cinnamomi reflects the areas where P. cinnamomi has caused most damage to vegetation and where that pathogen is most likely to occur on the basis of climatic suitability (Burgess et al. 2017). However, large areas in northern Australia may also be suitable for P. cinnamomi survival (O’Gara et al. 2006), but pathogen distribution and species response in these areas are largely unknown. Future glasshouse inoculation and field studies should also focus on localised plant species from parts of northern Australia where P. cinnamomi is most likely to be dispersed, such as mining developments and popular remote tourist destinations.
P. cinnamomi responses and risk
From our review of susceptibility and species response publications, the health of many Australian species of a few plant families (e.g. Proteaceae, Ericaceae, Fabaceae, Xanthorrhoeaceae, Dilleniaceae) and some genera (e.g. Adenanthos, Andersonia, Banksia, Lambertia, Xanthorrhoea) is usually severely affected following infection with P. cinnamomi, as has been noted before (e.g. Cahill et al. 2008). However, since the list of O’Gara et al. (2006) was published, species that display severe symptoms when infected have been added in genera and families not noted for their negative host response, namely, Astelia (Asteliaceae), Haloragodendron (Haloragaceae), Pimelea (Thymelaeaceae) and Pomaderris (Rhamnaceae). This perhaps highlights a bias in previous glasshouse assessments of susceptibility and host response to genera and families that have been regarded as containing species typically affected. A comprehensive evaluation of phylogenetic patterns is hampered by the lack of response evaluation of species in some families, including the largest families of Asteraceae and Poaceae.
On the basis of our assessment of risk, 257 plant species may be eligible for listing as Threatened under the EPBC Act, because of their limited distribution and threat from P. cinnamomi. Although listing does not guarantee protection, it does increase awareness of risk and the likelihood that resources will be allocated for conservation. Some species considered to be at low risk nationally may be at high risk in some jurisdictions. Dillwynia cinerascens R.Br., for instance, is a widespread species in south-eastern Australia, which often has severe symptoms when infected. We found that it was at low risk as a species nationally, but it is listed as endangered at the edge of its range in South Australia (National Parks and Wildlife Act 1972); conservation measures there to protect the species from a detrimental effect from P. cinnamomi may be warranted. In addition, some species assessed for susceptibility and risk comprise subtaxa, some of which may be at much greater risk than the species overall. Some of the 63 species we identified at very high risk of extinction because of P. cinnamomi were not listed as threatened nor recognised as being at threat because of the pathogen. In at least one case, namely Nematolepis rhytidophylla, the lack of recognition is likely because P. cinnamomi does not yet co-occur with this shrub, which grows in very remote country, rarely visited by humans. Strict hygiene will be required for its long-term protection, as may be essential for other very high-risk species not currently regarded as threatened by P. cinnamomi.
While some native plant species are clearly at risk from P. cinnamomi, the plant communities in which they occur may be equally at risk, with flow-on effects to other biota and ecosystem services. Of the 32 communities listed under the EPBC Act, which are regarded as threatened by P. cinnamomi, six (Banksia Woodlands of the Swan Coastal Plain Ecological Community, Eastern Stirling Range Montane Heath and Thicket, Proteaceae Dominated Kwongkan Shrublands of the South-east Coastal Floristic Province of Western Australia, Scott River Ironstone Association, Shrublands and Woodlands of the Eastern Swan Coastal Plain, Shrublands on Southern Swan Coastal Plain Ironstones) are typically dominated by species known to be severely affected by the pathogen. The loss of structural dominants in these communities could ultimately lead to irreversible floristic change.
We found that 233 plant species were assigned to different categories of response by different cited sources and that 20 species of those were assigned to all three categories; i.e. no response, mild response and severe response. There may be many reasons for the apparent anomalies, including genetic or taxonomic differences in the populations assessed or habitat differences affecting pathogenicity. Banksia serrata L.f. for instance has been regarded as severely affected in the field by P. cinnamomi (e.g. Weste 2001), but has displayed ‘field resistance’ or ‘low susceptibility’ in glasshouse studies (Wan et al. 2019; C. E. Crane and B. L. Shearer, unpubl. data). Glasshouse conditions exclude factors present under field conditions that may either favour or disfavour the pathogen and host. For example, other micro-organisms, insect herbivores or climate extremes such as drought may influence the survival of wild plant populations, which could make them more or less vulnerable to infection by P. cinnamomi than under ideal glasshouse conditions (e.g. Malajczuk 1988; Corcobado et al. 2014). Similarly, Xanthorrhoea preissii Endl. can display a mild response to infection on long-affected sites in its natural habitat in the jarrah forest (McDougall 1997), but can have a severe response in recently affected sites in the same forest (Shearer and Dillon 1995). This difference in response may relate to differences in inoculum levels of the pathogen in the soil or suppressive soil properties rather than to an attribute of the species overall. For Oxylobium ellipticum (Vent.) R.Br., which was apparently unaffected in a glasshouse study (Rigg et al. 2018), rarely symptomatic in one wild population (McDougall et al. 2023), yet usually symptomatic in another wild population (McDougall et al. 2003), the differences in response may be taxonomic; i.e. there may be more than one species in the current concept of O. ellipticum.
Responses to other Phytophthora species
Whereas knowledge about the effects of P. cinnamomi on Australian plant species has grown over many decades, knowledge about susceptibility and risk from other Phytophthora species is poor, partly because many of these species have only recently been described (Burgess et al. 2021). However, some other Phytophthora species do pose a threat. Phytophthora gregata has recently been found to significantly affect Pimelea bracteata in subalpine wetlands of Kosciuszko National Park (McDougall et al. 2018). This once common shrub has become locally rare and is now listed as Critically Endangered as a result of widespread infestation of its habitat by P. gregata. In south-western Western Australia, P. multivora is emerging as a significant pathogen with a wide host range and a wider geographical distribution than for P. cinnamomi (Migliorini et al. 2019). Further research into the pathogenicity of all Phytophthora species in Australia is urgently required.
Recommendations
On the basis of our review of responses of Australian native plants to P. cinnamomi, we make the following recommendations:
Our compiled list (Supplementary material S1) should be maintained and updated when new information becomes available. Finding an ongoing data manager, and maintaining data integrity and version control will require further investigation.
Research on secondary effects is needed. The profound changes in habitat caused by P. cinnamomi are likely to affect a range of fauna and these effects have received little attention. The research might focus at first on rare plant communities threatened by Phytophthora species (e.g. the critically endangered Eastern Stirling Range Montane Heath and Thicket) and habitat containing severely affected keystone species such as Banksia and Xanthorrhoea.
We suggest that the vast array of susceptibility categories be replaced by simple categories reflecting response of plants of species to the presence of pathogens: no response, mild response and severe response.
Glasshouse testing of all plant species of restricted extent occurring in habitat suitable to P. cinnamomi is recommended because such species will often be at highest immediate risk from the pathogen. Where possible, the effect of other Phytophthora species should be included in the same trials.
Knowledge about P. cinnamomi distribution and plant species response in northern Australia is extremely poor. Field sampling there of roots of symptomatic plants and soil from vegetation in poor health, and glasshouse inoculation trials of rare species, is urgently required.
Plant species identified as being at risk of extinction from P. cinnamomi but not yet listed as threatened should be assessed and nominated if they meet IUCN criteria for listing.
Data availability
The data from this paper are available online, associated with the paper as Supplementary material S1.
Declaration of funding
No funding was obtained for the preparation of this paper. Support for previously published work cited in Supplementary material S1 is reported in the relevant acknowledgement sections.
Author contributions
KM, SB and RV collated data used in the paper (Supplementary material S1). All authors reviewed the collated data and contributed to the preparation of the final paper.
Acknowledgements
We are indebted to Colin Crane and Bryan Shearer for supplying their unpublished data, and to Juanita Ciampini (WA Department of Biodiversity, Conservation and Attractions) for compiling, checking and providing the Vegetation Health Service Phytophthora database. Our thanks go to Greg Freebury (WA Department of Biodiversity, Conservation and Attractions) and Louise Shuey (Horticulture and Forestry Science, Department of Agriculture and Fisheries, Dutton Park, Queensland, Australia) for contributing their unpublished data to our list, to Ian Moore (WA Department of Biodiversity, Conservation and Attractions) for comments and suggestions on an early draft, and to those who provided data to the list of O’Gara et al. (2006). Emer O’Gara kindly located and supplied unpublished data from WA, which were invaluable to our study.
References
Allardyce JA, Rookes JE, Cahill DM (2012) Defining plant resistance to Phytophthora cinnamomi: a standardized approach to assessment. Journal of Phytopathology 160, 269-276.
| Crossref | Google Scholar |
Barker PCJ, Wardlaw TJ (1995) Susceptibility of selected Tasmanian rare plants to Phytophthora cinnamomi. Australian Journal of Botany 43, 379-386.
| Crossref | Google Scholar |
Barrett S, Shearer BL, Crane CE, Cochrane A (2008) An extinction-risk assessment tool for flora threatened by Phytophthora cinnamomi. Australian Journal of Botany 56, 477-486.
| Crossref | Google Scholar |
Burgess TI, Scott JK, McDougall KL, Stukely MJC, Crane C, Dunstan WA, Brigg F, Andjic V, White D, Rudman T, Arentz F, Ota N, Hardy GESJ (2017) Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Global Change Biology 23, 1661-1674.
| Crossref | Google Scholar | PubMed |
Burgess TI, Edwards J, Drenth A, Massenbauer T, Cunnington J, Mostowfizadeh-Ghalamfarsa R, Dinh Q, Liew ECY, White D, Scott P, Barber PA, O’Gara E, Ciampini J, McDougall KL, Tan YP (2021) Current status of Phytophthora in Australia. Persoonia 47, 151-177.
| Crossref | Google Scholar | PubMed |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279-310.
| Crossref | Google Scholar |
Corcobado T, Cubera E, Juárez E, Moreno G, Solla A (2014) Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agricultural and Forest Meteorology 192–193, 1-8.
| Crossref | Google Scholar |
Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013) Annual and herbaceous perennial native Australian plant species are symptomless hosts of Phytophthora cinnamomi in the Eucalyptus marginata (jarrah) forest of Western Australia. Plant Pathology 62, 1057-1062.
| Crossref | Google Scholar |
Davis RA, Valentine LE, Craig MD, Wilson B, Bancroft WJ, Mallie M (2014) Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia. Biological Conservation 171, 136-144.
| Crossref | Google Scholar |
Dundas SJ, Hardy GESJ, Fleming PA (2016) The plant pathogen Phytophthora cinnamomi influences habitat use by the obligate nectarivore honey possum (Tarsipes rostratus). Australian Journal of Zoology 64, 122-131.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG (2021) A review of Paratranes Zimmerman, 1994, Xanthorrhoea-associated weevils of the Tranes group (Coleoptera, Curculionidae, Molytinae), with description of a new species. European Journal of Taxonomy 767, 117-141.
| Crossref | Google Scholar |
Irwin JAG, Cahill DM, Drenth A (1995) Phytophthora in Australia. Australian Journal of Agricultural Research 46, 1311-1337.
| Crossref | Google Scholar |
IUCN Standards and Petitions Committee (2022) Guidelines for using the IUCN red list categories and criteria. Version 15. Prepared by the Standards and Petitions Committee. Available at https://www.iucnredlist.org/documents/RedListGuidelines.pdf
Laidlaw WS, Wilson BA (2006) Habitat utilisation by small mammals in a coastal heathland exhibiting symptoms of Phytophthora cinnamomi infestation. Wildlife Research 33, 639-649.
| Crossref | Google Scholar |
Malajczuk N (1988) Interaction between Phytophthora cinnamomi zoospores and micro-organisms on non-mycorrhizal and ectomycorrhizal roots of Eucalyptus marginata. Transactions of the British Mycological Society 90, 375-382.
| Crossref | Google Scholar |
McDougall KL, Summerell BA (2003) The impact of Phytophthora cinnamomi on the flora and vegetation of New South Wales – a re-appraisal. In ‘Phytophthora in forests and natural ecosystems. 2nd International IUFRO Working Party 7.02.09 Meeting’, Albany, Western Australia, October 2001. (Eds JA McComb, GESJ Hardy, IC Tommerup) pp. 49–56. (Murdoch University Print: Murdoch, WA, Australia)
McDougall KL, Wright GT, Burgess TI, Farrow R, Khaliq I, Laurence MH, Wallenius T, Liew ECY (2018) Plant, invertebrate and pathogen interactions in Kosciuszko National Park. Proceedings of the Royal Society of New South Wales 140, 295-312.
| Google Scholar |
McDougall KL, Hobbs RJ, Hardy GESJ (2005) Distribution of understorey species in forest affected by Phytophthora cinnamomi in south-western Western Australia. Australian Journal of Botany 53, 813-819.
| Crossref | Google Scholar |
McDougall KL, Wright GT, Bredell PM, James EA, Simmons L (2023) Mount Imlay – an island of floristic significance on the brink. Cunninghamia 23, 1-9.
| Google Scholar |
Migliorini D, Khdiar MY, Padrón CR, Vivas M, Barber PA, Hardy GESJ, Burgess TI (2019) Extending the host range of Phytophthora multivora, a pathogen of woody plants in horticulture, nurseries, urban environments and natural ecosystems. Urban Forestry & Urban Greening 46, 126460.
| Crossref | Google Scholar |
Moir ML (2021) Coextinction of Pseudococcus markharveyi (Hemiptera: Pseudococcidae): a case study in the modern insect extinction crisis. Austral Entomology 60, 89-97.
| Crossref | Google Scholar |
Moir ML, Coates DJ, Kensington WJ, Barrett S, Taylor GS (2016) Concordance in evolutionary history of threatened plant and insect populations warrant unified conservation management approaches. Biological Conservation 198, 135-144.
| Crossref | Google Scholar |
O’Gara E, Howard K, Wilson B, Hardy GESJ (2006) The responses of native Australian plant species to Phytophthora cinnamomi. Appendix 4. In ‘Management of Phytophthora cinnamomi for biodiversity conservation in Australia: Part 2. National best practice.’ (Department of the Environment and Heritage: Canberra, ACT, Australia)
Petit S, Dickson CR (2005) Grass-tree (Xanthorrhoea semiplana, Liliaceae) facilitation of the endangered pink-lipped spider orchid (Caladenia syn. Arachnorchis behrii, Orchidaceae) varies in South Australia. Australian Journal of Botany 53, 455-464.
| Crossref | Google Scholar |
Reiter N, Weste G, Guest D (2004) The risk of extinction resulting from disease caused by Phytophthora cinnamomi to endangered, vulnerable or rare plant species endemic to the Grampians, Western Victoria. Australian Journal of Botany 52, 425-433.
| Crossref | Google Scholar |
Rigg JL, McDougall KL, Liew ECY (2018) Susceptibility of nine alpine species to the root rot pathogens Phytophthora cinnamomi and P. cambivora. Australasian Plant Pathology 47, 351-356.
| Crossref | Google Scholar |
Rots V, Hayes E, Akerman K, Green P, Clarkson C, Lepers C, Bordes L, McAdams C, Foley E, Fullagar R (2020) Hafted tool-use experiments with Australian aboriginal plant adhesives: Triodia Spinifex, Xanthorrhoea Grass Tree and Lechenaultia divaricata Mindrie. Exarc. Available at https://exarc.net/ark:/88735/10487
Shearer BL, Dillon M (1995) Susceptibility of plant species in Eucalyptus marginata forest to infection by Phytophthora cinnamomi. Australian Journal of Botany 43, 113-134.
| Crossref | Google Scholar |
Shearer BL, Dillon M (1996) Susceptibility of plant species in Banksia Woodlands on the Swan Coastal Plain, Western Australia, to infection by Phytophthora cinnamomi. Australian Journal of Botany 44, 433-445.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435-443.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Assessment of threatened flora susceptibility to Phytophthora cinnamomi by analysis of disease progress curves in shadehouse and natural environments. Australasian Plant Pathology 36, 609-620.
| Crossref | Google Scholar |
Shivas RG (1989) Fungal and bacterial diseases of plants in Western Australia. Journal of the Royal Society of Western Australia 72, 1-62.
| Google Scholar |
Swinburn ML, Fleming PA, Craig MD, Grigg AH, Garkaklis MJ, Hobbs RJ, Hardy GESJ (2007) The importance of grasstrees (Xanthorrhoea preissii) as habitat for mardo (Antechinus flavipes leucogaster) during post-fire recovery. Wildlife Research 34, 640-651.
| Crossref | Google Scholar |
Tippett JT, McGrath JF, Hill TC (1989) Site and seasonal effects on susceptibility of Eucalyptus marginata to Phytophthora cinnamomi. Australian Journal of Botany 37, 481-490.
| Crossref | Google Scholar |
Toelken HR, Miller RT (2012) Notes on Hibbertia (Dilleniaceae) 8. Seven new species, a new combination and four new subspecies from subgen. Hemistemma, mainly from the central coast of New South Wales. Journal of the Adelaide Botanic Gardens 25, 71-96.
| Google Scholar |
Wan JSH, McDougall KL, Liew ECY (2019) The susceptibility of rare and threatened NSW species to the root-rot pathogen Phytophthora cinnamomi: 1. Initial testing and identification of key research questions. Australian Journal of Botany 67, 510-516.
| Crossref | Google Scholar |
Weste G (2001) Interaction between Phytophthora cinnamomi and Victorian native plant species growing in the wild. Australasian Mycologist 20, 64-72.
| Google Scholar |
Zentmyer GA, Thorn WA (1967) Hosts of Phytophthora cinnamomi. California Avocado Society Yearbook 51, 177-178.
| Google Scholar |