Unusual, human-mediated prevalence of epiphytes in semi-arid New South Wales, Australia
J. L. Silcock A B * , J. Pye C , A. Tighe D , P. Reid-Loynes E , R. Ashby F and R. J. Fairfax G
A B * , J. Pye C , A. Tighe D , P. Reid-Loynes E , R. Ashby F and R. J. Fairfax G
A
B
C
D
E
F
G Present address:
Abstract
Epiphytes are typically associated with wet forests and are seldom documented in drylands. This absence is presumed to reflect moisture limitations to their establishment and survival.
In response to a large body of epiphyte observations made by a pastoralist in collaboration with local Indigenous people, we investigated and documented an unusually high concentration of woody epiphytes from semi-arid eastern Australia and describe this in relation to ecological and cultural factors.
We searched for, recorded and measured epiphytic trees and shrubs in semi-arid eucalypt woodlands of northern New South Wales and southern Queensland. Factors influencing their distribution were examined.
Eucalypts growing along the Barwon River palaeochannels host 21 species of shrubs and trees. Over 95% of the 712 woody epiphytes documented were alive, and some appeared decades old; 70% were growing in trees that had been modified by humans, and at least half of the host sites were directly anthropogenically created. Epiphytes are widely, but typically sparsely, distributed in other semi-arid eucalypt woodlands, with a further 311 found during regional surveys.
The large trees of the Barwon palaeochannels, their extensive human modification to create favourable sites for epiphyte establishment, and the diversity of understorey shrubs providing a propagule source have combined to create this epiphyte-rich woodland. Their association with Culturally Modified Trees and the relatively low density of epiphytes elsewhere suggest that Aboriginal people have played a direct role in creating this landscape, to which they remain deeply connected.
Epiphytes may be more widespread in drylands than previously recognised. We hope that this study stimulates further research on their distribution, characteristics, and ecological and cultural associations.
Keywords: accidental epiphytes, Culturally Modified Trees, drylands, Ehretia saligna, Eucalyptus coolabah, Eucalyptus largiflorens, Eucalyptus populnea, Geijera parviflora, New South Wales, Queensland, ringbarking, woodland.
Introduction
Epiphytes, defined as species that grow non-parasitically on other species, have fascinated botanists and gardeners since Europeans first ventured into the tropics, where they are a common and conspicuous element of the flora. No doubt they have held cultural significance for peoples over millennia. Epiphytes, as opposed to mistletoes, which are parasitic and directly derive nutrients from their host plants, rely on their hosts for support and indirect provision of nutrients and water through accumulated soil, litter and ant waste in tree hollows and nooks. They are prominent components of wet forest ecosystems worldwide and comprise up to 10% of the world’s total flora (Benzing 2004). Vascular epiphytism is often seen as a subtropical and tropical phenomenon (Madison 1977; Freiberg and Freiberg 2000; Hsu et al. 2002), but it is also widespread in some temperate forests (Oliver 1930; Burns and Dawson 2005; Zotz 2005).
Epiphytes can be divided into three groups: (i) obligate epiphytes (also termed typical or true epiphytes), which grow on trees >95% of the time; (ii) accidental epiphytes (also termed ephemeral or pseudo), which occur on trees <5% of the time; and (iii) facultative or casual epiphytes, which have a proportion of arboreal to terrestrial growth between the other two classes (Benzing 2004). Facultative or casual epiphytes are similar to accidental epiphytes but tend to be better adapted to a deficiency of water or nutrients and thus survive and mature more often, and are more common as epiphytes (Wallace 1981). Obligate epiphytes normally pass their whole life cycle growing on another plant and are strongly adapted to the epiphytic way of life. They are the most speciose and well-known type of epiphytes and include species of ferns, bromeliads and orchids. There are also hemi-epiphytes, which germinate and establish as epiphytes but send roots to the ground (e.g. strangler figs) or which germinate and establish terrestrially but climb up other plants, eventually losing connection with the ground but continuing to grow epiphytically (Wallace 1981).
There has been little published work on accidental epiphytes, which are individuals of species that are normally terrestrial but have a dispersal method that enables propagules to be deposited in trees that afford at least temporary conditions suitable for germination and establishment. Most epiphyte studies disregard or exclude accidental epiphytes, meaning that there is little understanding of their distribution, prevalence in different ecosystems or characteristics (Zotz 2005; Machado et al. 2022). Recent studies have revealed them to be common components of some temperate forests in Europe (Hoeber et al. 2019) and Tasmania (Winoto-Lewin and Kirkpatrick 2020), as well as in tropical forests (Machado et al. 2022) and even urban street trees (Izuddin and Webb 2015). In extensive surveys through Australia’s wet forests, Wallace (1981) observed just 17 ‘accidental’ epiphytes (Wallace 1981, pp. 46–47), as well as numerous examples of two species he considered ‘casual’ epiphytes (Pittosporum bicolor and Pittosporum undulatum). In temperate south-eastern Australia, Maiden (1904) recorded two eucalypts and an Angophora that had become established inside hollows of other Eucalyptus species and ultimately fused or ‘grafted’ with the host tree.
Water limitations and availability of suitable host trees are considered the major environmental factors that limit accidental epiphytism of vascular plants. Studies note the small size and high turnover of accidental epiphytes in temperate and tropical forests (Hoeber et al. 2019; Winoto-Lewin and Kirkpatrick 2020). Accidental epiphytes most often die before maturity owing to their lack of adaptation to epiphytic conditions, especially in regard to water relations, meaning that most of those observed are seedlings and juveniles (Wallace 1981; Brock and Burns 2021). A small proportion live long enough to attain a relatively large size and reproduce in the epiphytic habit (Wallace 1981; Hoeber et al. 2019).
Epiphyte diversity and density appear to rely most critically on water availability, which is influenced by precipitation and water-holding capacity of host sites (Winoto-Lewin and Kirkpatrick 2020). Epiphyte diversity has also been shown to increase with the size and age of host trees (Hirata et al. 2009). In essence, greater tree surface area means a higher likelihood of propagules landing on the host, while age increases the chance of this having occurred and of the tree having developed soil-holding nooks or branch architecture to support epiphytes. Accidental epiphytes are mostly restricted to host trees that provide water-storing substrates such as extensive moss pads or arboreal soil accumulated in crotches (Hoeber et al. 2019). Winoto-Lewin and Kirkpatrick (2020) found that most epiphytes were observed on eucalypts, occurring on built-up litter and soil on basal duffs or in branch crooks.
Very few studies document any types of vascular epiphytism in dryland environments globally. These mostly focus on Tillandsia species (Bromeliaceae), obligate epiphytes which occur in arid and semi-arid America (Bernal et al. 2005; Abril and Bucher 2009; Stanton et al. 2014; Pérez-Noyola et al. 2020). In the driest areas, they follow relatively humid ‘fog corridors’ along coastlines (Moreno-Chacón and Saldaña 2019). The only true epiphyte that occurs in semi-arid Australia is the orchid Cymbidium canaliculatum, which occurs patchily in areas receiving 450–600 mm average annual rainfall (AVH 2023). Its leaves and roots have considerable water storage capacity, and it grows in hollow limbs and knot-holes, with roots penetrating the rotten centres of branches and trunks that remain moist for long periods after rainfall (Wallace 1981). We were unable to find any published studies that refer to accidental or casual epiphytism in semi-arid or arid environments globally.
Here we document an unusual concentration of epiphytes growing on the Barwon River palaeochannels in north-western New South Wales, and place this in the context of broader epiphyte surveys in semi-arid eastern Australia. We examine tree and hollow characteristics that influence epiphyte occurrence and discuss their distribution in relation to the ecological and cultural landscape. Possible explanations for the density of epiphytes recorded are discussed, along with suggestions for further research. We hope that this largely descriptive but highly novel paper will stimulate further documentation and collaborative research of epiphytes in drylands to improve our understanding of their prevalence, distribution and characteristics.
Methods
Study area
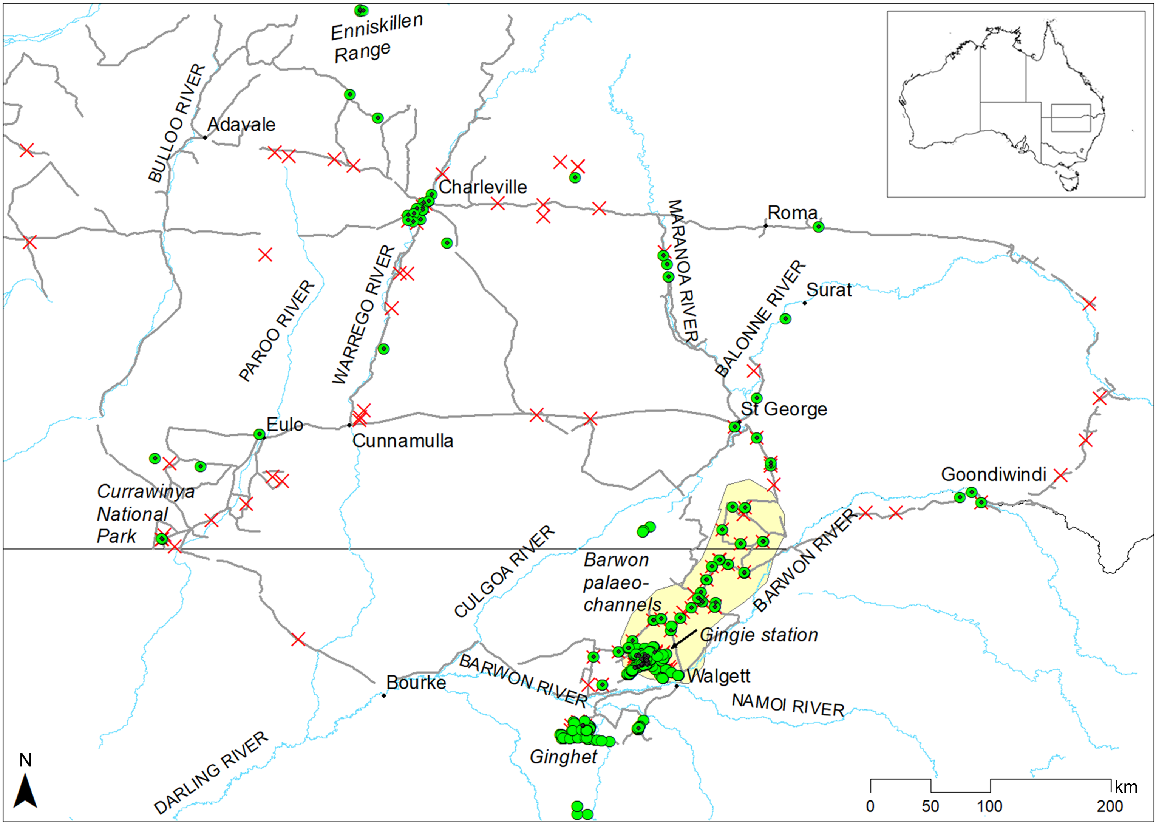
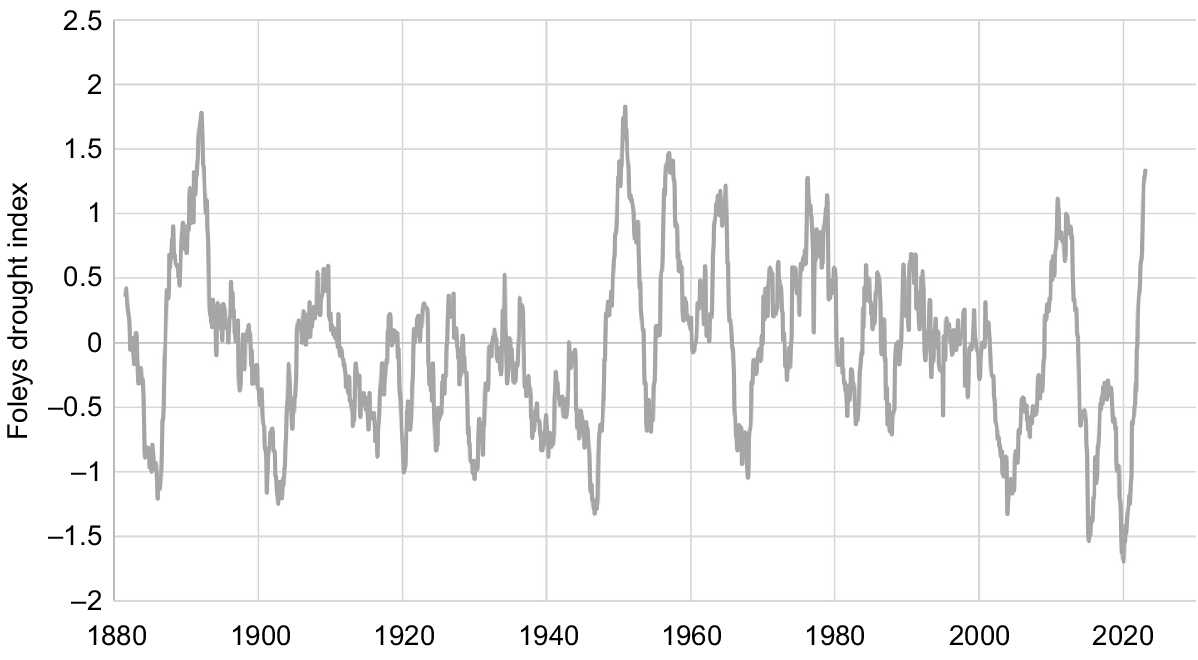
The study area is focused on Gingie station, ~30 km north-west of Walgett, in semi-arid north-western New South Wales (Fig. 1). Average annual rainfall for Walgett is 470 mm, with ~60% of rainfall falling in the summer months from October to March inclusive (data for Walgett Airport and Council Depot combined, 1879–2021; Bureau of Meteorology, http://www.bom.gov.au/climate/data/, accessed March 2022). Rainfall is variable among years, with 16% of years receiving <300 mm annual rainfall and 10% of years receiving >700 mm. Extended dry periods are common, with Foley’s drought index (Foley 1957) falling below −1 (two-thirds of expected rainfall in the 3 years prior to any given month) nine times since records began in 1879 (Fig. 2). Summer temperatures are hot, with average maximum temperatures of >34°C from December to February. Winters are mild (average daily maximums 18–21°C from June to August) with cool nights (average daily minimums <5°C from June to August).
Study area showing major towns and rivers, places mentioned in the text, epiphytes recorded (green circles; epiphytes that were measured are marked with a black dot in the centre of the green circle), and areas (red crosses) and tracks (grey lines) searched in southern Queensland and northern New South Wales. The area containing the Barwon River palaeochannels is shaded yellow.
Foleys drought index, showing expected rainfall in the 3 years prior to any given month, for Walgett, New South Wales, 1883–2023 (data sourced from Bureau of Meteorology, http://www.bom.gov.au/climate/data/, accessed March 2022).
The landscape of Gingie and surrounding properties is dominated by low sandhills and swamps that mark a palaeodrainage system of the Barwon River (Fig. 1), where water still runs underground. These palaeochannels are clearly visible on satellite imagery and extend from the present Barwon River, an upper tributary of the Darling River, 160 km north into southern Queensland. This area is here termed the ‘Barwon palaeochannels’. There is no permanent surface water in the palaeochannels, although there are Aboriginal and European wells accessing shallow groundwater, and many ephemeral swamps. Most of the earth tanks in the area were built on semi-permanent swamps. The Big Warrambool, which heads in southern Queensland, runs through the western part of the palaeochannels and into the Barwon River, but does not contain permanent water. The closest permanent surface water is at Cumborah Springs, 15 km north-west of Gingie.
Aboriginal people have inhabited inland New South Wales for at least 45 000 years (Hiscock 2008). The Barwon palaeochannels is known as a ‘border’ region between Gamilaraay/Gomeroi and Yuwaalaraay territories, and is bisected by a major Aboriginal pathway (now roughly followed by the Stock Route along the Cumborah–Walgett road) between the Barwon River and Cumborah Springs to the north-west and the ephemerally flooded Narran Lake to the west. After being driven away from the rivers, Aboriginal people lived in camps around Gingie and at surrounding stations from the 1850s until the 1940s (Fields 2001). Their descendants mostly live in the nearby towns of Walgett and Lightning Ridge and maintain strong spiritual, cultural and familial connections with Country. In the words of Gamilaroi/Yuwaalaraay women and co-authors of this study Priscilla Reid-Loynes and Rhonda Ashby:
Ngali winangaylanha dhawunda Gamilaraaygu nhalay.
[We acknowledge that this is Gamilaraay land.]
Yilaambiyali dhawun nhalay, maran.gu ngiyaningu galumay.
[From the beginning, our ancestors have cared for this land.]
Dhawun nhalay ngiyani dhirral, nhama ngarranmaldanha bigan wilay, winangali, galumali.
[This land is our teacher, it shows/guides us the lore to live, to listen to connect.]
Galumay ngiyani galligu, dhawun.gu, dhulugu dhawunda Gamilaraaygu. Ganunga ngiyaningu dhiiyaan.
[We have always cared for our waterways, country, plants and trees on Gamilaroi country. They are our family.]
Ngiyani Wayamaa winangaya, ganungawu.
[Let us respect the Elders, all of them.]
Nguwa yanayindaay, nguwa yanaylandaay, nguwa yanangayiylandaay.
[Who walked here long ago, now and in the future.]
A feature of the Barwon palaeochannels is the high density of Culturally Modified Trees including hundreds of scar trees where bark has been removed by Aboriginal people for constructing shelters, watercraft and containers (Long 2005), and numerous ring trees whose growing limbs were manipulated by Aboriginal people to form circles or rings (Power 2013), as documented and photographed by Jane Pye on the ‘Scar Trees’ website (https://scartrees.com.au). Where possible throughout this manuscript, we provide Gamilaraay/Yuwaalaraay as well as modern common names for plants mentioned, as compiled by McKerney and White (2011) and Menkins (2012).
Gingie was one of the first pastoral stations in the region, being taken up around 1843. In 1902, Gingie encompassed 73 650 ha, but over time blocks have been split off and today it is ~22 250 ha. The Pye family have held this station since 1916. Clay plains have largely been cleared for agriculture, but the palaeochannel woodlands on sandy rises remain largely intact apart from patchy but widespread ringbarking, wherein a strip was cut around the circumference of the base of the trunk to kill the central trunk and any higher limbs, practised from the 1880s (many of these trees subsequently resprouted) and poisoning of eucalypt trees in the 1980s. There are areas of smaller outer palaeochannels to the east of the main system, within predominantly cleared cropping lands.
Locating and recording epiphytes
Epiphytes were intensively searched for and recorded on Gingie and surrounding properties by Jane Pye in collaboration with local Indigenous people, particularly Gamilaraay/Yuwaalaraay Elder Allan Tighe, between 2018 and 2023. The core area searched intensively, here termed the ‘Gingie palaeochannels’, is ~30 km long and 15 km across. Much of this area is not accessible via vehicle tracks, and an all-terrain vehicle was primarily used. Remnant woodland on the outer Barwon palaeochannels was also searched for epiphytes. Some vines and climbing shrubs are relatively common as epiphytes in semi-arid Australia, notably gargaloo (Parsonsia eucalyptophylla), nepine/guwiibir (Capparis lasiantha) and native jasmine (Jasminum didymium), as are annual and perennial grasses, forbs and sub-shrubs. Only non-climbing woody shrubs and trees are considered here.
For each epiphyte found, host and epiphytic species were recorded, and whether the host and epiphyte were alive or dead. Approximately 70% of epiphytes found during these surveys were noted as ‘big’, ‘medium’, ‘small’ or ‘tiny’; the others were located opportunistically, and this information was not systematically recorded. We measured the diameters of a subset of these, and size classes were approximately defined as: ‘big’, largest stem >15 cm diameter at 10 cm height; ‘medium’, main stem 5–15 cm diameter at 10 cm; ‘small’, main stem 1.5–4 cm diameter; and ‘tiny’, saplings or seedlings, main stem <1 cm diameter. Obvious human modification to the host tree, including to create the host site (trunk or branch hollows, central crotch or scar), was often noted but not recorded systematically.
In April 2021 and April 2022 we visited 189 epiphytes throughout the Barwon palaeochannels, encompassing all species represented by at least five individuals (Table 1) and aiming for as broad a geographic spread across the palaeochannel area as possible (Fig. 1). We recorded GPS location, broad habitat, host species and diameter at breast height (DBH) of host (where there were multiple stems at 1.5 m, each stem was measured separately); whether the tree had been modified by humans through removal of bark (Long 2005), cutting of limbs, ringbarking or poisoning (deduced from growth habit, presence of old ringbarking cuts or poison holes and local knowledge); and hollow characteristics including size (measured as surface area of hollow where it abutted trunk or branch), height above ground, architecture and whether it appeared to be created by anthropogenic activity (either pre- or post-contact). For each epiphyte, we identified the species and recorded its stem diameter at 10 cm height and estimated its height. We also recorded the distance to the nearest human-modified tree. Where anthropogenic modification involved cutting of branches, we recorded whether the cut was apparently made by a stone (rough, uneven cut) or steel (smooth, clean cut) axe, although in some cases this was impossible to discern, and these were noted as being of ambiguous origin.
| Species (common name, Kamilaroi name; family) | Total recorded (measured) | Av. diameter of largest stem (range) (cm) | Notes | |
|---|---|---|---|---|
| Acacia oswaldii (miljee, midjirr; Fabaceae) | 2 (0) | |||
| Alectryon oleifolius (boonaree, bunbarr; Sapindaceae) | 45 (12) | 14.1 (1–45) | Some very large epiphytes but also medium-sized tree and saplings | |
| Alstonia constricta (quinine, gadibundu; Apocynaceae) | 25 (9) | 4.9 (0.5–12) | Mostly tiny to medium | |
| Apophyllum anomalum (currant/warrior bush, wayaara; Capparaceae) | 70 (14) | 8.9 (0.5–20) | Range from tiny to big; about half multi-stemmed | |
| Atalaya hemiglauca (whitewood, birraa; Sapindaceae) | 32 (13 | 7.5 (0.5–31) | Range from very large trees to seedlings | |
| Callitris glaucophylla (white cypress, guraay; Cupressaceae) | 7 (0) | |||
| Capparis mitchellii (native orange, bambul; Capparaceae) | 39 (10) | 17.4 (2–31) | Mostly in anthropogenic hollows; mostly large old trees | |
| Casuarina cristata (belah, murrgu; Casuarinaceae) | 1 (0) | |||
| Ehretia saligna (peach bush; Boraginaceae) | 170 (32) | 6.1 (0.3–25) | Majority small, although some large trees | |
| Eremophila longifolia (emu bush, ngawil; Scrophulariaceae) | 1 (0) | |||
| Eremophila mitchellii (budda/false sandalwood, badha; Scrophulariaceae) | 29 (9) | 6.5 (0.3–18) | Three big ones, but mostly small to medium; 24% dead | |
| Eucalyptus seedling (E. coolabah/E. populnea; Myrtaceae) | 3 (0) | One small coolibah sapling growing in poplar box tree + two unidentified | ||
| Exocarpos aphyllus (leafless ballart; Santalaceae) | 3 (0) | Typically a root parasite | ||
| Flindersia maculosa (leopardwood, balgala; Rutaceae) | 3 (2) | Two seedlings and one sapling | ||
| Geijera parviflora (wilga, dhiil; Rutaceae) | 166 (52) | 10.8 (0.6–44) | Seedlings to big trees; only one dead, all others healthy and often fertile | |
| Myoporum montanum (boobialla, buubiyala; Scrophulariaceae) | 19 (7) | 5.0 (0.5–14) | Mostly tiny to medium, tend to occur higher in hosts than other epiphytes | |
| Pimelea microcephala (shrubby riceflower; Thymeleaceae) | 17 (6) | 3.3 (1–6) | Mostly smaller shrubs | |
| Pittosporum angustifolium (gumby gumby, guwiirra/miyaymiyaay; Pittosporaceae) | 18 (3) | 10.3 (5–16) | Range from tiny seedlings to big trees | |
| Psydrax oleifolium (wild lemon, dharriwa; Rubiaceae) | 2 (1) | |||
| Senna artemisioides subsp. artemisioides (punty bush, bandi; Caesalpiniaceae) | 1 (1) | One occurrence in northern palaeochannels | ||
| Ventilago viminalis (supplejack/vinetree, ganayanay; Rhamnaceae) | 41 (5) | 1.5 (0.5–2) | Mostly seedlings or saplings, still with vine-like stems |
Diameters were measured 10 cm from base of plant. Seedlings <10 cm tall were recorded for Alectryon oleifolius, Atalaya hemiglauca and Flindersia maculosa; DBH was not measurable and these are not included in average diameters. Averages calculated only where at least three epiphytes were measured. Eighteen epiphytes that were dead or tiny seedlings were not able to be confidently identified and are not included in Table 1.
Regional surveys and analyses
We searched for epiphytic occurrences of trees and shrubs in other areas of arid and semi-arid eastern Australia through surveys in poplar box and coolabah woodlands in similar climatic zones between September 2020 and January 2024 (Fig. 1). We drove roads throughout the distribution of Eucalyptus populnea and Eucalyptus coolabah in semi-arid southern Queensland and northern New South Wales, including along the Barwon palaeochannels to the north of Gingie (here termed ‘Palaeochannels north’), and situated survey sites where we saw an epiphyte from a slow-moving vehicle or in other areas of potential habitat (i.e. remnant woodland dominated by E. populnea, Eucalyptus largiflorens or E. coolabah). Such ‘windshield surveys’ have some drawbacks; however, they are able to cover large areas where roads pass through representative examples of widespread target habitat (Elzinga et al. 2001). For each survey site, we recorded broad habitat, approximate area searched, time spent searching, total number of potential host hollows seen, density of hollows per hectare, abundance of shrubs (abundant, common, scattered, rare), total number of potentially epiphytic species (defined as those documented occurring as epiphytes in this study) occurring at the site, and presence and density of anthropogenically modified trees (Aboriginal or European).
All data were entered into Excel spreadsheets and mapped in ArcGIS and Google Earth. The factors influencing presence of epiphytes at search sites were investigated using Generalised Linear Models in the R package (R Core Team 2022). To complement the field variables described above, the average relative humidity at minimum temperature, average relative humidity at maximum temperature, average annual rainfall and average evaporation were extracted for each site from SILO, a modelled surface informed by a network of weather stations (Jeffrey et al. 2001). Multiple models were attempted using a combination of variables. Non-significant variables were excluded, and models were chosen based on the lowest Akaike information criterion (AIC). A log transformation was used to normalise variables when required and data were checked for heteroscedasticity. The random effect of site was tested for and removed after being found non-significant. Two models were retained: one using the densities of anthropogenically modified trees and hollows to account for the area searched, and one based on the presence of anthropogenically modified trees and hollows to eliminate any area-based bias.
We mapped the distribution of all species with >20 epiphytic occurrences from the Australasian Virtual Herbarium (AVH 2023) and overlaid this with vegetation mapping of ecosystems dominated or co-dominated by E. populnea, E. coolabah and/or E. largiflorens, the most common host species. We also included communities dominated by Eucalyptus brownii, which is closely related to, and replaces, E. populnea in the north-east of its range and has a similar growth habit. In Queensland, all Regional Ecosystems (REs) with E. populnea, E. coolibah, E. brownii or E. largiflorens described as dominant or codominant were included (Queensland Herbarium 2021). REs dominated by mulga but with emergent or scattered poplar box were not included. In New South Wales, Plant Community Types dominated by one or more of these eucalypt species were extracted (Department of Planning and Environment 2022; Roff et al. 2022). This allowed us to identify areas with potential epiphyte occurrence in eastern Australia.
We also questioned 15 ecologists and land managers who live and work in inland eastern Australia about whether they had observed trees or shrubs growing in other trees. Together these informants had >400 years of experience working in these environments. If the respondents answered affirmatively, we requested GPS points or location descriptions, photos, identification of host and epiphyte species, and any other observations.
Results
Barwon palaeochannel survey
In total, 712 woody epiphytes were documented in the Barwon palaeochannel system, including 33 ‘doubles’, where two epiphytes were living in the same host tree, and three ‘triples’. The core area of epiphyte occurrence, the Gingie palaeochannels (Fig. 1), contains 88% of those documented and covers ~40 km2, within which there is a density of >10 epiphytes km−2. Fifty-six epiphytes were found in the same palaeochannel system to the north-east of Gingie, and 34 were found in outer palaeochannels to the east of the main system in country that has mostly been cleared for cropping. All of these epiphytes are hereafter referred to as the ‘Barwon palaeochannel’ epiphytes.
The majority of the 673 host trees (accounting for double and triple epiphytes in a single tree) were poplar box/bibil (E. populnea, 90%), and 6% were coolibah/gulabaa (E. coolabah). Five dead host trees may have been either E. coolabah or E. populnea, and one E. coolabah or E. largiflorens. There were nine host cypress pines/guraay (Callitris glaucophylla), five black box/guburuu (E. largiflorens), four ironwood (Acacia excelsa), and one each of whitewood (Atalaya hemiglauca), belah/murrgu (Casuarina cristata), river red gum/yaraan (Eucalyptus camaldulensis), leopardwood/bagala (Flindersia maculosa), needlewood (Hakea leucoptera), and supplejack/ganyanay (Ventilago viminalis). Over 80% of host trees were alive; the remainder had died naturally, had been poisoned in the 1980s or were cut stumps. Numerous host trees had been ring-barked or cut for timber historically (Fig. 3d), but the majority of these were resprouting.
Examples of epiphytes documented on the Barwon River palaeochannels, north-western New South Wales: (a) large Ehretia saligna growing in hollow created by falling branch; (b) Capparis mitchellii growing in apparent stone axe cut in Indigenous-created scar on Eucalyptus populnea; (c) Apophyllum anomalum in central crotch of multi-stemmed Eucalyptus coolabah on outer Barwon palaeochannels; (d) large Geijera parviflora in mid-trunk of multi-stemmed Eucalyptus populnea that has likely resprouted after ringbarking in the 1920s; (e) multi-trunked Eucalyptus populnea with four Indigenous-created scars (two visible in image) and Capparis mitchellii epiphyte; (f) hollow in a multi-trunked Eucalyptus largiflorens on the Ginghet south-west of the Barwon palaeochannels, showing soil accumulation. An image archive of epiphytes on the Barwon palaeochannels is available at www.scartrees.com.au/galleries.

Twenty-one tree and shrub species were recorded growing as epiphytes in the Barwon palaeochannels. Peach bush (Ehretia saligna) and wilga/dhiil (Geijera parviflora) were the most common epiphytes, comprising 24% and 23% of all epiphytes recorded, respectively (Fig. 3a, d). Both species were strongly associated with E. populnea and were not recorded in E. coolabah hosts with the exception of a single E. saligna. Currant bush/wayaara (Apophyllum anomalum, n = 70; Fig. 3c), boonaree/bunbarr (Alectryon oleifolius, n = 45), supplejack/ganayanay (Ventilago viminalis, n = 41), native orange/bambul (Capparis mitchellii, n = 39; Fig. 3b, e), whitewood/birraa (Atalaya hemiglauca, n = 32), budda/badha (Eremophila mitchellii, n = 29), quinine/gadibundu (Alstonia constricta, n = 245), boobialla/buubiyala (Myoporum montanum, n = 19), gumby gumby/guwiirra/miyaymiyaay (Pittosporum angustifolium, n = 18), and shrubby riceflower (Pimelea microcephala, n = 17) were all well represented as epiphytes. The remaining eight species are rare epiphytes in the study area, with between one and three examples of each, except white cypress/guraay (C. glaucophylla) with seven epiphytic occurrences recorded (Table 1). Sixteen epiphytes were dead with no bark remaining and were unable to be identified, and two epiphytes were tiny seedlings or resprouts that could have been A. constricta, E. saligna, P. angustifolium or G. parviflora.
Of the epiphytes documented, 95% were alive, and most of these were healthy with no dead branches and many were flowering and/or fruiting. The only species of epiphyte with a relatively high proportion (24%) dead was E. mitchellii. A range of size classes was recorded for all epiphyte species, indicating a range of plant ages. Where size class was recorded (n = 304) or diameter measured (n = 169), 25% were classified big, 21% medium, 32% small and 22% tiny (see Methods for definitions of size classes). A small number of live epiphytes had old, dead stems, indicating that they had died back, probably during dry times, and then resprouted. The Gingie and outer palaeochannels had a wide range of epiphyte sizes but were the only areas to contain ‘big’ epiphytes; all epiphytes found in the northern palaeochannels were of <15 cm diameter and 79% were of <5 cm diameter.
Detailed measurements
In total, 176 epiphytes and their 159 host trees were measured in the Barwon palaeochannels. The majority of host trees (n = 149) were E. populnea, with seven E. coolabah, two F. maculosa and one C. glaucophylla. Most host eucalypts were large, and the majority (82%) were multi-stemmed; 57% had at least three stems and 23% five or more stems. Average DBH of the largest stem was 46 cm (range 16–120 cm, excluding the two leopardwoods that had DBHs of 21 cm and 8 cm). On all but six host trees, DBH of the largest stem was at least 20 cm.
Almost 70% of host trees had been modified by humans in some ways (mostly with cut branches or stumps, from both stone and steel axe cuts, scar trees or ringbarked; Fig. 3b, d, e). Others may have been modified (e.g. branches cut or tree burnt historically, resulting in a multi-stemmed habit; Burrows 2013; Rayner et al. 2014), but unless this was obvious, these were not classified as anthropogenically modified. Those host trees that were not obviously modified themselves were typically <100 m from an unambiguous Culturally Modified Tree, usually a scar tree, which are common throughout the Barwon palaeochannels.
At least 93 of the 170 hollows that hosted epiphytes (55%) were directly anthropogenically created (encompassing 46 apparently Indigenous-created and 47 created post-contact through cutting branches or historical ringbarking), with a further seven potentially of anthropogenic (Indigenous) origin. Thirty hollows were natural (obviously created by fallen branches or in the central crotch of an apparently unmodified multi-stemmed tree); the remaining 40 are considered probably of natural origin but occurred in modified trees and their formation may have been assisted by human modification of the tree.
Fifty-seven host hollows were created by cutting of a branch or stump; 12 of these cuts appeared to have been created by stone axes (characterised by rough edges, as though made with a blunt object), 23 were unambiguously steel axe (including one chainsaw); the origins of the remainder were ambiguous. Nineteen host hollows were directly created by Indigenous scars where bark had been removed for materials. Fifty-three hollows occurred in the central ‘bowl’ of a trunk created by multiple stems, which in many cases probably resulted from cutting or burning of a central stem.
Hollows hosting epiphytes were, on average, 0.85 m above the ground (range 0–4 m). Average hollow size was 1550 cm2 (range 12–56 000 cm2); however, this was highly variable, skewed by a few very large hollows, with a median hollow size of 431 cm2. Over 80% of hollows were horizontal; aspect where it existed was highly variable and displayed no consistent pattern.
Of the 176 epiphytes measured (Table 1), 170 were alive. Average diameter at 10 cm of the largest stem was 10.4 cm (0.3–45 cm) range. One-quarter were multi-stemmed. Average height of epiphytes was 3 m (0.1–15 m). C. mitchellii, Alectryon oleifolium and G. parviflora tended to be the largest epiphytes, whereas the shrubs P. microcephala and M. montanum were the smallest. E. saligna and A. anomalum both occasionally grew into large trees, but were mostly represented by smaller specimens <10 cm in diameter.
Regional epiphyte surveys
Over 5000 km of gazetted roads and property tracks through woodlands dominated by E. populnea, E. coolabah and E. largiflorens in southern Queensland and northern New South Wales were driven at 40–70 km h−1 with a passenger observer scanning for epiphytes, and 130 areas of 0.25–100 ha were searched on foot; 312 individual woody epiphytes were documented during these surveys and 82 of these were measured (Fig. 1, Table 2).
Region | No. of epiphytes recorded (no. measured) | Host trees (no. recorded) | Epiphytic species (no. recorded) | |
|---|---|---|---|---|
Ginghet, north-western NSW | 213 (12) | Eucalyptus populnea (150) Eucalyptus largiflorens (56) Casuarina cristata (4) Eucalyptus coolabah (3) | Geijera parviflora (109) Ehretia saligna (32) Alectryon oleifolius (17) Atalaya hemiglauca (11) Pimelea microcephala (9) Apophyllum anomalum (6) Pittosporum angustifolium (6) Ventilago viminalis (4) Eremophila longifolia (3) Flindersia maculosa (3) Capparis mitchellii (2) Casuarina cristata (2) Callitris glaucophylla (1) Eremophila deserti (1) Eremophila mitchellii (1) Myoporum montanum (1) Pittosporum angustifolium (1) Scaevola spinescens (1) | |
Barwon palaeochannel surrounds, north-west NSW and southern Qld | 27 (15) | Eucalyptus populnea (26) | Geijera parvifolia (13) Ehretia saligna (10) Eremophila mitchellii (1) Eucalyptus sp. (1) | |
Alluvial levees and plains, north-west NSW and southern Qld | 67 (48) | Eucalyptus populnea (54) Eucalyptus coolabah (4) Eucalyptus ochrophloia (2) Eucalyptus camaldulensis (1) Eucalyptus largiflorens (1) | Geijera parviflora (19) Acacia salicina (15) Alectryon oleifolius (6) Atalaya hemiglauca (4) Eremophila mitchellii (4) Eremophila longifolia (3) Brachychiton populneus (3) Myoporum montanum (2) Senna artemisioides subsp. filifolia (2) Apophyllum anomalum (1) Brachychiton rupestris (1) Denhamia cunninghamii (1) Denhamia oleaster (1) Duma florulenta (1) Eremophila glabra (1) Santalum lanceolatum (1) | |
Other (mulga tableland and sand dune) | 5 (5) | Eucalyptus populnea (4) Eucalyptus melanophloia (1) | Brachychiton rupestris (2) Archidendropsis basaltica (1) Eremophila longifolia (1) Geijera parviflora (1) |
Three epiphytes in the Ginghet area and two surrounding the Barwon palaeochannels were dead and unable to be identified. Regions are shown in Fig. 1.
The largest cluster of epiphytes (n = 213) found during regional surveys centred on Ginghet and Marra Creeks, ~50 km south-west of the Barwon palaeochannels. These were spread over ~500 km2 and were recorded during on-foot surveys between May 2020 and January 2024. This area lies between the Macquarie River and Marra Creek and is drained by Ginghet and Marra Creeks and smaller channels, which bisect palaeochannels, sandplains and swamps dominated by E. largiflorens and E. populnea. Almost 65% of host trees were E. populnea, and the remainder mostly E. largiflorens, with three E. coolabah and four C. cristata hosts. Most epiphytes were G. parviflora (51%), followed by E. saligna (15%); the other 16 species had between one and 17 representatives (Table 2). With the exception of spiny fan-flower (Scaevola spinescens) and Ellangowan poison bush (Eremophila deserti), all were also recorded as epiphytes in the Barwon palaeochannels.
Twenty-seven epiphytes were found in the area immediately surrounding but outside of the Barwon palaeochannels, roughly bounded by Thallon in Queensland, Lightning Ridge and Narran Lake, all growing in E. populnea (Fig. 1; Table 2). Clusters were found around the permanent Cumborah Springs to the north-west of Gingie (17 small–medium epiphytes in an area of ~8 km2) and on a low, stony rise above the Moonie River just north-east of the Barwon palaeochannels (eight small–medium epiphytes in an area of 0.25 km2). Both areas were characterised by multi-trunked E. populnea with extensive anthropogenic modification (both pre- and post-contact, including cut branches and historical ringbarking).
In other areas, epiphytes were found as isolated specimens or in localised clusters, either seen from a vehicle or found during targeted foot searches. All of the remaining 67 epiphytes were found on alluvial levees, plains or swamps associated with major drainage systems including the Moonie, Balonne, Castlereagh, Maranoa, Warrego, Paroo and McIntyre Rivers, except for a small Eremophila longifolia growing in a silver-leaf ironbark (Eucalyptus melanophloia) on a low, spinifex-dominated sand dune and a G. parviflora growing in an E. populnea hollow on a red earth plain, both near Charleville, and three epiphytes (two Brachychiton rupestris and an Archidendropsis basaltica) growing in E. populnea hollows on a mulga-dominated tableland of the Enniskillen Range south-west of Blackall (Fig. 1). The largest concentrations were found in an area of 2.9 km2 south of the Castlereagh River on the Cumbadoon Way, where 18 epiphytes (mostly wilgas) were found in poplar boxes, and in an area of 0.04 km2 south of St George adjacent to the Balonne River, where nine sally wattles (Acacia salicina) were found growing in E. populnea trees that had been ringbarked and resprouted, creating a central crotch for the seedlings to establish and grow. Nine additional species (i.e. not documented in the Barwon palaeochannels) were recorded as epiphytes (Table 2). All except nine host trees were E. populnea, and 54% of host hollows were anthropogenically created. Most epiphytes measured were small or medium, with just seven having a DBH >15 cm including two huge A. salicina near Dunkeld on the Maranoa River and three A. oleifolius, two near Charleville and one on the Moonie River at Nindigully.
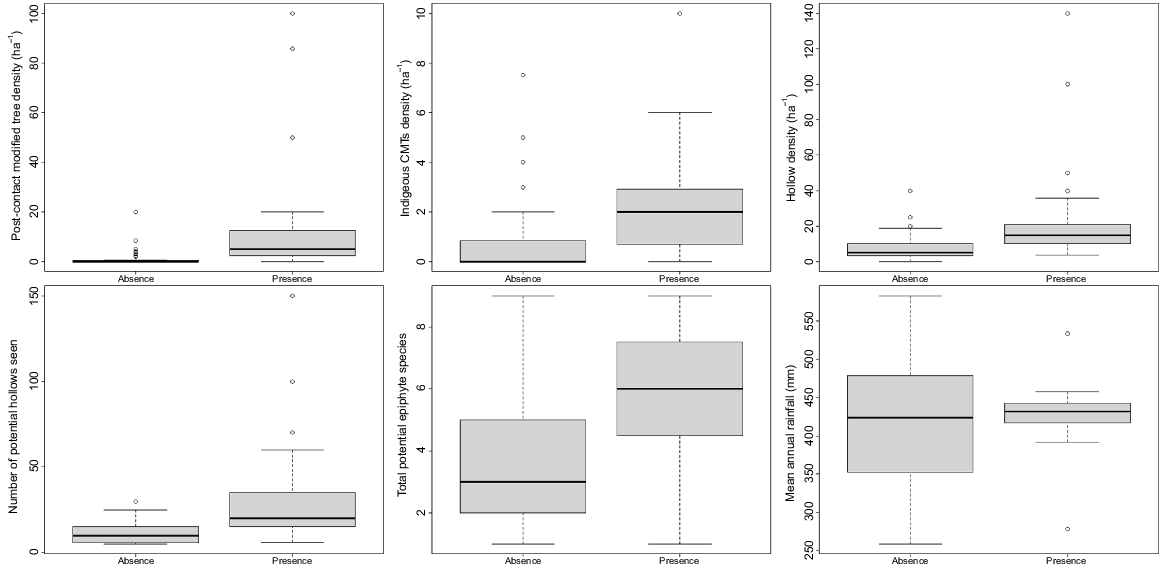
Epiphyte presence at search sites where habitat variables were recorded (n = 79) was positively correlated with density of Indigenous Culturally Modified Trees (P = 0.013), density of post-colonial (European) modified trees (P = 0.007) and total number of potential hollows seen (P = 0.002). The two former variables were also positively correlated with each other (P = 0.008). Mean annual rainfall was weakly negatively correlated with epiphyte presence (P = 0.027), probably reflecting the fact that most epiphytes were found in the Barwon palaeochannels area, where average rainfall is 420–440 mm per annum, and were rarely found in the lower and higher rainfall areas to the north-west and north-east of this area (Figs 1 and 4). The number of potential epiphytes present at a site was higher where epiphytes were found (Fig. 4), but this was only significant on its own and was not a good predictor within the model based on the AIC.
Boxplots showing habitat variables as related to epiphyte presence and absence at survey sites in northern New South Wales and southern Queensland (n = 79). CMTs, Culturally Modified Trees. ‘Potential hollows’ describes sites within trees that could potentially host epiphytes. Potential epiphyte species include all those recorded growing epiphytically in the study area.
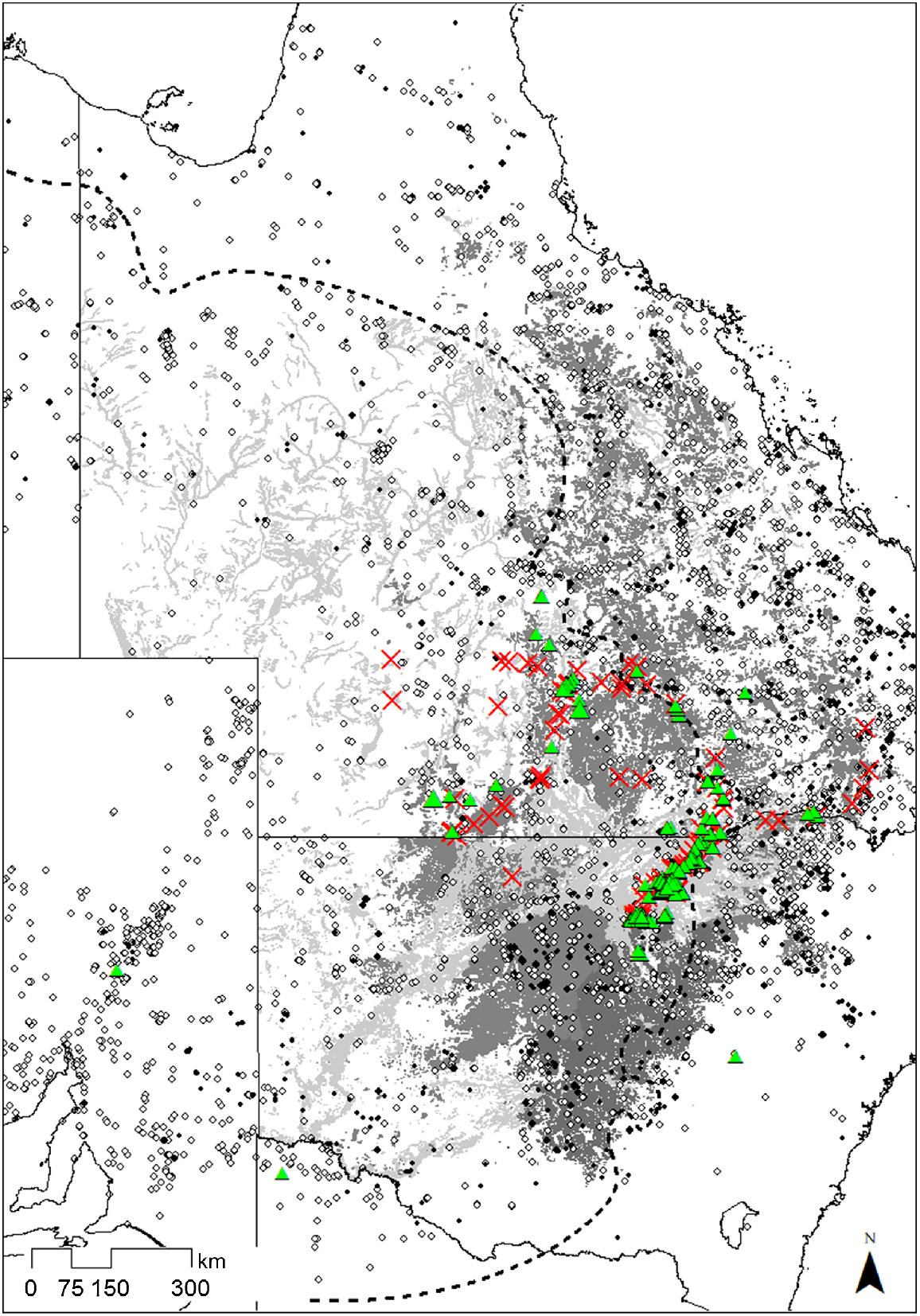
The eight species commonly recorded as epiphytes occur widely across inland eastern Australia, and many extend into other areas of arid and semi-arid Australia (Fig. 5). In total, 30 mapped REs in Queensland and 14 Plant Community Types (PCT) in New South Wales are dominated by E. populnea, and five by the closely related E. brownii. Twenty-nine REs and five PCTs are dominated by E. coolabah, and two REs and eight PCTs by E. largiflorens. Most of the extensive E. coolabah-dominated ecosystems occur in more arid regions and do not overlap with high concentrations of potential epiphytic species. Large areas of E. populnea and E. largiflorens occur in central and southern Queensland and central-western New South Wales and overlap with numerous potential epiphyte species (Fig. 5).
Specimen collection records (AVH 2023) of the eight species with at least 20 recorded epiphytic occurrences in this study. Ehretia saligna and Geijera parviflora, the two most common epiphytes, are represented by black dots; other species (Alectryon oleifolium, Alstonia constricta, Apophyllum anomalum, Atalaya hemiglauca, Capparis mitchellii, Eremophila mitchellii, Ventilago viminalis) by white dots. Eucalyptus populnea and E. brownii-dominated Regional Ecosystems/Plant Community Types are shaded dark grey; E. coolabah and E. largiflorens are shaded light grey. The 500-mm isohyet delineating the semi-arid zone is shown as a dashed line. Green triangles indicate epiphytes recorded during this study; red crosses show areas searched with no epiphytes found.
Of the 15 ecologists and land managers interviewed, three were able to provide photos and details of woody epiphytes. One of these epiphytes (a Brachychiton populneus growing in an E. populnea) occurs near the Langlo River in south-west Queensland (Chris Crafter, pers. comm. March 2022), and is included in the results presented here. Also reported were a C. glaucophylla growing in an E. camaldulensis in the Flinders Ranges (Mike Bremers, pers. comm., June 2022), and a Banksia marginata in an E. camaldulensis in western Victoria (Attila Kapitany, pers. comm., November 2011). Two other respondents stated that they may have observed this phenomenon but could not provide a specific example with a location, whereas the remaining 10 respondents stated that they had not observed epiphytic occurrences of woody species in semi-arid or arid areas.
Discussion
Despite not being previously recorded in the literature or widely known by ecologists or land managers, our study shows that accidental epiphytes are likely to be widespread in semi-arid woodlands on alluvial substrates, with a total of 1024 documented during this study, encompassing 32 species growing in 13 host tree species. However, their density on the Barwon palaeochannels, in particular the area around Gingie station, appears to be unusual. Although the densities of accidental epiphytes recorded here are much lower than those reported in temperate forests (Winoto-Lewin and Kirkpatrick 2020; Hoeber and Zotz 2021), they are surprising given that extended dry periods are regarded as a key limitation for epiphytes (Freiberg and Turton 2007; Ticktin et al. 2016). Accidental epiphytes are likely to be particularly ill-adapted to water stress associated with living arboreally, and this is assumed to explain their rarity except in wet forests (Wallace 1981).
Most of the accidental epiphytes documented in previous studies are small in stature and apparently short-lived. Only nine accidental epiphytes in a Tasmanian study (Winoto-Lewin and Kirkpatrick 2020) had grown taller than 1 m, and Hoeber and Zotz (2021) found high turnover of epiphytes in a central European forest, as well as high mortality during establishment of young plants. This is not the case on the Barwon palaeochannels, where accidental epiphytes are both abundant and, in many cases, long-lived (although some undocumented mortality is likely during the seedling stage, and some mortality almost certainly occurs during extended dry periods, which were not experienced during this study). Standing dead trunks remain present in semi-arid environments for many years; however, <5% of epiphytes were dead during the present surveys. This is despite the area regularly experiencing extended periods of rainfall deficit (Fig. 2).
Physiological adaptions to periods of moisture stress (e.g. CAM (crassulacean acid metabolism) photosynthesis, thickened leaves) may allow the species documented here to survive and thrive as epiphytes. Many epiphytes, particularly those growing <1 m from the ground (35% of the epiphytes measured in this study), have almost certainly sent down roots through the hollow eucalypt trunks and are now accessing terrestrial water and nutrients, enabling them to grow to a similar stature and age as non-epiphytic individuals. These individuals began life as epiphytes but would now be classified as ‘hemi-epiphytes’ (Wallace 1981). Old trees have effectively provided a ‘nurse’ environment for them to establish and grow, and continue to provide a favourable environment in terms of protection from herbivores (Azuma et al. 2022), as well as shade, moisture retention and nutrient accumulation (Hoeber and Zotz 2021; Machado et al. 2022). Nevertheless, epiphytes would have to survive for multiple seasons before their roots could become established in the ground. A sequence of above-average rainfall La Niña years, as was experienced in the study area in the 1890s, 1950s, 1970s and 2010s (Fig. 2), may be required for establishment of accidental epiphytes, as is the case for shrub establishment in these areas in general (Hodgkinson 1979).
It is possible that the five epiphytic root parasites documented here (a single sandalwood Santalum lanceolatum and four leafless cherries Exocarpos aphyllus) were hemiparasitic on their eucalypt host. Baird (2014) documented an example of apparent aerial hemiparasitism by an E. aphyllus shrub growing on a hollow E. largiflorens branch on a floodplain north of St George in Queensland (Fig. 4). Hemiparasitism was not confirmed and this may have been an epiphyte living non-parasitically on its host.
The distribution of epiphytes is affected primarily by dispersal and establishment. Epiphytes rely on aerial vectors to disperse their seeds, with the majority of true epiphytes having small, wind-dispersed seeds (Madison 1977). Most of the epiphyte species, accounting for 88% of individual epiphytes recorded during this study, have bird-dispersed seeds (the exceptions being A. hemiglauca and V. viminalis, which have wind-dispersed seeds, as do the very occasional epiphytes F. maculosa, C. glaucophylla and Eucalyptus spp.). The Barwon palaeochannels are characterised by extensive swampy areas and a high diversity of seed resources, thus are likely to be a haven for birds. The diverse, shrubby understorey also means that there are many potential propagules to be eaten and dispersed by birds. Wind-dispersed seeds would also land in hollows more frequently where shrub density is higher.
Epiphytes also depend upon appropriate sites for deposition, germination and survival. This is a function of host tree traits, including the area available for establishment and architecture of the tree (Hirata et al. 2009; Izuddin and Webb 2015; Wagner et al. 2015; Hoeber and Zotz 2021). Most studies have found a positive relationship between host size and epiphyte occurrence or diversity (Hirata et al. 2009; Winoto-Lewin and Kirkpatrick 2020). Ishii et al. (2018) found that age and abundance of trunk breaks was an important predictor of epiphyte abundance, because epicormic branches had sprouted below trunk breaks, creating large surface areas upon which arboreal soil accumulated and woody plants established. Host traits affecting water availability are likely to be critical in more xeric environments (Callaway et al. 2002). E. populnea trees on the Gingie palaeochannels are among the largest in diameter observed anywhere in the range of poplar box (R. Fairfax, unpubl. data), and based on an average DBH growth rate of 0.23 cm year−1 (Ngugi et al. 2015; at 600 mm average annual rainfall, higher than the present study area), hosts are likely to range in age from >100 to >500 years old. Tree DBH is the strongest predictor of hollow occurrence in eucalypts in north-western New South Wales (Rayner et al. 2014) and other parts of the world (Bennett et al. 1994; Fox et al. 2008). E. populnea is one of the most prominent providers of hollows in western New South Wales woodlands, with a high probability of bearing large hollows and bearing hollows at relatively small tree sizes compared with other species (Rayner et al. 2014).
In addition, the Barwon palaeochannel trees have been heavily modified over a long period of time by different cultures and using different technologies, resulting in a high proportion of multi-trunked, coppiced trees and a high density of hollows. This creation of coppiced trees and hollows by Aboriginal peoples has been documented in other Australian woodlands, particularly around favoured camp sites (Bellchambers 1931; Pyne 1991; Clarke 2007). Post-colonial ringbarking of Eucalyptus trees often does not kill the trees but causes re-sprouting below the cut trunk (Long 2005). Although coppiced stems are less likely to form hollows (Rayner et al. 2014), the multi-trunked habit creates a central ‘crotch’ where seeds can fall, establish and grow. Direct cutting of stems for fenceposts and firewood has also created hollows conducive to the germination and establishment of seedlings. These factors have combined to create a high density of large hollows and crotches with deposits of suspended soil and favourable microclimates where water collects after rain and losses from evaporation would be lower than surrounding terrestrial environments. Pruning of street trees was similarly shown to increase available microhabitats for epiphytes in urban Singapore (Izuddin and Webb 2015).
There is increasing evidence that Aboriginal peoples deliberately transported, planted and nurtured plant propagules throughout Australia (Rangan et al. 2015; Rossetto et al. 2017; Silcock 2018). Given the association of epiphytes with Culturally Modified Trees, and the cultural significance of all species involved (mostly as medicinal and ceremonial plants; Clarke 2007; Lassack and McCarthy 2011; McKerney and White 2011), it is possible that people played a role in seeding plants into hollows. There is some anecdotal evidence for this practice. Gamilaraay/Yuwaalaraay Elder Allan Tighe recounted that he had heard from Gamilaraay man Reggie Murray that a new tree was planted in another tree when an important person died (Murray n.d.). Ian Menkins, who has documented Aboriginal words and natural history in the Murray Darling Basin (Menkins 2012), has also been told by an Indigenous Elder of trees being planted in other trees (I Menkins, pers. comm., September 2021). Such practices are not mentioned by explorers or other early observers of Aboriginal life including Katie Langloh Parker, who closely observed semi-traditional Euahlayi life to the north of Gingie on the Narran River in the early 1900s (Langloh-Parker 1905), or RH Mathews who wrote about Aboriginal life in northern New South Wales and southern Queensland from the 1850s (Mathews 1904). However, many practices that are now known to have been common in certain areas are not recorded in the ethnographic record. Certainly complex initiation, burial and ceremonial rites involving trees and their marking or decoration have been documented throughout eastern Australia, including in and around the Barwon River (Fraser 1882; Mathews 1904; Etheridge 1918; Black 1941). On the Goulburn River, teeth that were knocked out during initiation rites were placed in the fork of a young gum tree by the initiate’s mother; this tree would be stripped of its bark at its base and burnt to kill it when the person died (Blandowski 1855).
Few data are available on the growth rate of the epiphytic species recorded here; however, the range of sizes documented – from recently germinated seedlings to large, apparently very old trees – suggests that epiphyte establishment is a relatively regular and ongoing process. The last permanent Aboriginal residents left Gingie in the early to mid-1960s, and although some epiphyte species have seeds that remain viable for decades (i.e. seeds could have been placed in hollows and not germinated until years later), more recent germinants are almost certainly ‘naturally’ occurring, as are those occurring in ring-barked trees and others modified post-contact. Collaborative research with Traditional Owners and pastoralists, who are custodians of most of the rangelands of inland eastern Australia, should investigate the spatial patterning of epiphytes, including areas with few or no epiphytes, in relation to significant Aboriginal sites including campsites and travel routes, water sources and Culturally Modified Trees.
The epiphytes documented here can also inform important ecological and conservation questions. Numerous species are palatable to domestic and feral herbivores and have limited regeneration across inland eastern Australia, particularly A. oleifolius, E. saligna, A. anomalum, C. mitchellii, P. angustifolium and Ventilago viminalis (Wilson et al. 1976; Auld 1995; Tiver and Andrew 1997; J. Silcock, unpubl. data). The fact that they are readily geminating and establishing as epiphytes indicates that recruitment is not limited by seed viability or sporadic rainfall events, but is more likely due to the impacts of grazing and trampling. There is a large body of literature on ‘nurse’ shrubs in arid ecosystems (Drezner 2006; López et al. 2007). Tree hollows have not previously been recognised as such in Australia, although an early Gundagai pastoralist advised the planting of kurrajong trees in old tree stumps to protect them from rabbits (Anon 1908). Our results shows that hollows effectively provide a ‘nurse’ environment for seedling germination and establishment, through accumulation of soil and nutrients, as well as protection from the elements and herbivory/trampling.
Introduced buffel grass (Cenchrus ciliaris) now forms dense swards across large areas of the Barwon palaeochannels, and fire following wet seasons is a substantial threat to the epiphytes and the Culturally Modified Trees (scar and ring trees) that are a feature of these woodlands. Planned burns and grazing management to reduce biomass after above-average rainfall seasons are required to conserve the trees, which provide a direct link to ancestors for Gamilaraay people and also with post-contact pastoral heritage and vegetation management. In the words of Priscilla Reid-Loyns and Rhonda Ashby:
Despite the ongoing act of colonisation and Government policies creating obstacles to our knowledge systems and our ability to access places within Country, it is the passing on stories orally and our unbroken connection to trees and plants of our traditional lands that allows us to know what the trees in trees and other species mean for us. Despite the clearing of land, for pastoral and grazing purposes, some trees in the trees still exist there. We understand that the ancestors want us to continue to see how everything and everyone is connected and related to each other in all places of Country. These places have been in our family since the Burrugu (time of creation which is ongoing) and we will continue to be custodians of the land and carry the knowledge that Country holds for our future generations.
As Gamilaroi, Ularoi women we know that trees are Country and family. Trees are one of the many knowledge holders to carry the stories and teachings of our Ancestors. The trees in trees teaches not only about relationships between one another as trees but also about people and Country. As every part of the tree has a purpose not only for its own survival but it has also been a provider for us and our survival as Gamilaroi peoples and all people. Where there are libraries full of books, we have Country with trees full of our cultural knowledge. Tree connects us from the land up into Sky Camp and to all the spaces in between. They were used for birthing, vessels to hold babies and other items, cleansing, seasonal knowledge, medicine and food, portals, shelter, canoes, sign guides, tools and weapons and other uses for animals and plants and of course fire for warmth and heat.
The mubirr (markings) on trees are to guide us on how to move through Country and to inform us of the cultural business of that place. There are sacred places across Country that are specifically for the men’s business (Buura) and women’s business (ngurrumbaa) birthing, community business along with mubirr for boundaries.
Traditionally when a birth takes place on country the baby is given a tree as a gift and that provides a child’s connection and responsibility of looking after that tree throughout life. We are gifted the tree along with other entities of Gamilaroi Country and we are raised to know and listen to the knowledge they share with us.
When we think of the trees and the relationships they hold with people and places we recognise that this brought us to Jane. She observes, listens, and understands the trees and knows their cultural story needs to be acknowledged. Jane has opened the gates for us to visit Gingie again and the trees on country so that their stories can be told and remembered.
Although our surveys suggest that the density and variety of epiphytes in the Barwon palaeochannels is unusual, the host tree species are widespread and only a few areas (the Ginghet, around Charleville and parts of Currawinya National Park; Fig. 5) have been searched as thoroughly as Gingie and surrounds. It is possible that there are other areas with similarly high concentrations of epiphytes. We predict that these areas would be characterised by large, hollow-bearing trees with a history of human modification (Indigenous and/or post-contact) and high diversity and abundance of shrub species. The area between the Barwon palaeochannels and the Ginghet/Macquarie River should be thoroughly searched. These two concentrations of epiphytes, situated 50 km apart, may prove to be part of one larger cluster. We hope that this paper raises the profile of dryland epiphytes, stimulates further research on their ecological and cultural associations, and perhaps brings to light examples from elsewhere. Our study also demonstrates another facet of human–plant interactions and their potentially far-reaching ecological effects, particularly the role of humans in creating tree hollows that provide habitat for other species.
Data availability
The data for this paper are available (with limited-precision GPS points) from the corresponding author upon request.
Acknowledgements
We acknowledge the Traditional Owners of the lands on which this study was undertaken, particularly the Gamilaraay and Yuwaalaraay people, and their long and ongoing connections with the trees and woodlands. Boris Laffineur assisted with the regional survey analysis. Chris Crafter, Tracy Wattz, Rosie Kerr and Jay Griffiths assisted with epiphyte searching in western Queensland. Rowan Fairfax was an unwitting participant in field work and is thanked for his general enthusiasm and tolerance of palaeochannel mosquitoes. The comments of two anonymous reviewers substantially improved this paper.
References
Abril AB, Bucher EH (2009) A comparison of nutrient sources of the epiphyte Tillandsia capillaris attached to trees and cables in Cordoba, Argentina. Journal of Arid Environments 73, 393-395.
| Crossref | Google Scholar |
Anon (1908) ‘Lay of the land.’ The Worker (Wagga) 3 September, p. 23. Available at https://trove.nla.gov.au/newspaper/article/145757121 (Accessed 14 February 2024)
Auld TD (1995) The impact of herbivores on regeneration in four trees from arid Australia. The Rangeland Journal 17, 213-227.
| Crossref | Google Scholar |
AVH (2023) The Australasian Virtual Herbarium. Council of Heads of Australasian Herbaria. Available at https://avh.chah.org.au [Accessed 16 February 2023]
Azuma WA, Komada N, Ogawa Y, Ishii H, Nakanishi A, Noguchi Y, Kanzaki M (2022) One large tree crown can be defined as a local hotspot for plant species diversity in a forest ecosystem: a case study in temperate old-growth forest. Plant Ecology 223, 99-112.
| Crossref | Google Scholar |
Baird IRC (2014) A novel observation of putative aerial hemiparasitism in Exocarpus aphyllus (Santalaceae). Queensland Naturalist 52, 1-3.
| Google Scholar |
Bennett AF, Lumsden LF, Nicholls AO (1994) Tree hollows as a resource for wildlife in remnant woodlands: spatial and temporal patterns across the northern plains of Victoria, Australia. Pacific Conservation Biology 1, 222-235.
| Crossref | Google Scholar |
Bernal R, Valverde T, Hernandez-Rosas L (2005) Habitat preference of the epiphyte Tillandsia recurvata (Bromeliaceae) in a semi-desert environment in Central Mexico. Canadian Journal of Botany-Revue Canadienne De Botanique 83, 1238-1247.
| Crossref | Google Scholar |
Blandowski W (1855) Personal observations made in an excursion towards the central parts of Victoria, including Mount Macedon, McIvor and Black Ranges. Transactions of the Philosophical Society of Victoria 1, 50-74.
| Google Scholar |
Brock JMR, Burns BR (2021) Patterns of woody plant epiphytism on tree ferns in New Zealand. New Zealand Journal of Ecology 45, 3433.
| Crossref | Google Scholar |
Burns KC, Dawson J (2005) Patterns in the diversity and distribution of epiphytes and vines in a New Zealand forest. Austral Ecology 30, 883-891.
| Crossref | Google Scholar |
Burrows GE (2013) Buds, bushfires and resprouting in the eucalypts. Australian Journal of Botany 61, 331-349.
| Crossref | Google Scholar |
Callaway RM, Reinhart KO, Moore GW, Moore DJ, Pennings SC (2002) Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132, 221-230.
| Crossref | Google Scholar | PubMed |
Department of Planning and Environment (2022) New South Wales state vegetation type map, version C1.1.M1. Available at https://datasets.seed.nsw.gov.au/dataset/nsw-state-vegetation-type-map [accessed 13 July 2022]
Drezner TD (2006) Plant facilitation in extreme environments: the non-random distribution of saguaro cacti (Carnegiea gigantea) under their nurse associates and the relationship to nurse architecture. Journal of Arid Environments 65, 46-61.
| Crossref | Google Scholar |
Fox JC, Hamilton F, Ades PK (2008) Models of tree-level hollow incidence in Victorian State forests. Forest Ecology and Management 255, 2846-2857.
| Crossref | Google Scholar |
Fraser J (1882) The Aborigines of New South Wales. Journal and Proceedings of the Royal Society of New South Wales 16, 193-233.
| Crossref | Google Scholar |
Freiberg M, Freiberg E (2000) Epiphyte diversity and biomass in the canopy of lowland and montane forests in Ecuador. Journal of Tropical Ecology 16, 673-688.
| Crossref | Google Scholar |
Freiberg M, Turton SM (2007) Importance of drought on the distribution of the birds nest fern, Asplenium nidus, in the canopy of a lowland tropical rainforest in north-eastern Australia. Austral Ecology 32, 70-76.
| Crossref | Google Scholar |
Hirata A, Kamijo T, Saito S (2009) Host trait preferences and distribution of vascular epiphytes in a warm-temperate forest. Plant Ecology 201, 247-254.
| Crossref | Google Scholar |
Hodgkinson KC (1979) The shrubs of poplar box (Eucalyptus populnea) lands and their biology. The Australian Rangeland Journal 1, 280-293.
| Crossref | Google Scholar |
Hoeber V, Zotz G (2021) Not so stressful after all: epiphytic individuals of accidental epiphytes experience more favourable abiotic conditions than terrestrial conspecifics. Forest Ecology and Management 479, 118529.
| Crossref | Google Scholar |
Hoeber V, Weichgrebe T, Zotz G (2019) Accidental epiphytism in the Harz Mountains, Central Europe. Journal of Vegetation Science 30, 765-775.
| Crossref | Google Scholar |
Hsu C-C, Horng F-W, Kuo C-M (2002) Epiphyte biomass and nutrient capital of a moist subtropical forest in north-eastern Taiwan. Journal of Tropical Ecology 18, 659-670.
| Crossref | Google Scholar |
Ishii HR, Minamino T, Azuma W, Hotta K, Nakanishi A (2018) Large, retained trees of Cryptomeria japonica functioned as refugia for canopy woody plants after logging 350 years ago in Yakushima, Japan. Forest Ecology and Management 409, 457-467.
| Crossref | Google Scholar |
Izuddin M, Webb EL (2015) The influence of tree architecture, forest remnants, and dispersal syndrome on roadside epiphyte diversity in a highly urbanized tropical environment. Biodiversity and Conservation 24, 2063-2077.
| Crossref | Google Scholar |
Jeffrey SJ, Carter JO, Moodie KB, Beswick AR (2001) Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environmental Modelling & Software 16, 309-330.
| Crossref | Google Scholar |
López RP, Valdivia S, Sanjinés N, de la Quintana D (2007) The role of nurse plants in the establishment of shrub seedlings in the semi-arid subtropical Andes. Oecologia 152, 779-790.
| Crossref | Google Scholar | PubMed |
Machado GMO, Grittz GS, de Gasper AL (2022) Neglected epiphytism: accidental epiphytes dominate epiphytic communities on tree ferns in the Atlantic Forest. Biotropica 54, 251-261.
| Crossref | Google Scholar |
Madison M (1977) Vascular epiphytes: their systematic occurrence and salient features. Selbyana 2, 1-13.
| Google Scholar |
Maiden JH (1904) On some natural grafts between indigenous trees. Journal and Proceedings of the Royal Society of New South Wales 38, 36-40.
| Crossref | Google Scholar |
Mathews RH (1904) Ethnological notes on the Aboriginal tribes of New South Wales and Victoria. Journal and Proceedings of the Royal Society of New South Wales 38, 203-381.
| Crossref | Google Scholar |
Moreno-Chacón M, Saldaña A (2019) α, β and γ-diversity of vascular epiphytes along the climatic gradient of continental Chile. New Zealand Journal of Botany 57, 18-31.
| Crossref | Google Scholar |
Ngugi MR, Doley D, Cant M, Botkin DB (2015) Growth rates of Eucalyptus and other Australian native tree species derived from seven decades of growth monitoring. Journal of Forestry Research 26, 811-826.
| Crossref | Google Scholar |
Oliver WRB (1930) New Zealand epiphytes. Journal of Ecology 18, 1-50.
| Crossref | Google Scholar |
Pérez-Noyola FJ, Flores J, Yáñez-Espinosa L, Bautista-Redonda FE, Badano EI (2020) Effect of induced warming on survival and growth of Tillandsia recurvata seedlings: a two-year experiment. Journal of Arid Environments 179, 104177.
| Crossref | Google Scholar |
Queensland Herbarium (2021) Regional ecosystem mapping. Department of Environment and Science, Queensland Government. Available at https://www.qld.gov.au/environment/plants-animals/plants/herbarium/mapping-ecosystems [accessed 5 May 2023]
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org/
Rangan H, Bell KL, Baum DA, Fowler R, McConvell P, Saunders T, Spronck S, Kull CA, Murphy DJ (2015) Correction: New genetic and linguistic analyses show ancient human influence on baobab evolution and distribution in Australia. PLoS ONE 10, e0127582.
| Crossref | Google Scholar |
Rayner L, Ellis M, Taylor JE (2014) Hollow occurrence and abundance varies with tree characteristics and among species in temperate woodland Eucalyptus. Austral Ecology 39, 145-157.
| Crossref | Google Scholar |
Roff A, Day M, Thonell J, Denholm B (2022) NSW state vegetation type map: technical notes. NSW Department of Planning and Environment, Parramatta. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/BioNet/nsw-state-vegetation-type-map-technical-notes-220275.pdf [accessed 13 July 2022]
Rossetto M, Ens EJ, Honings T, Wilson PD, Yap J-YS, Costello O, Round ER, Bowern C (2017) From Songlines to genomes: prehistoric assisted migration of a rain forest tree by Australian Aboriginal people. PLoS ONE 12, e0186663.
| Crossref | Google Scholar |
Silcock JL (2018) Aboriginal translocations: the intentional propagation and dispersal of plants in Aboriginal Australia. Journal of Ethnobiology 38, 390-405.
| Crossref | Google Scholar |
Stanton DE, Huallpa Chávez J, Villegas L, Villasante F, Armesto J, Hedin LO, Horn H (2014) Epiphytes improve host plant water use by microenvironment modification. Functional Ecology 28, 1274-1283.
| Crossref | Google Scholar |
Ticktin T, Mondragón D, Gaoue OG (2016) Host genus and rainfall drive the population dynamics of a vascular epiphyte. Ecosphere 7, e01580.
| Crossref | Google Scholar |
Tiver F, Andrew MH (1997) Relative effects of herbivory by sheep, rabbits, goats and kangaroos on recruitment and regeneration of shrubs and trees in eastern South Australia. Journal of Applied Ecology 34, 903-914.
| Crossref | Google Scholar |
Wagner K, Mendieta-Leiva G, Zotz G (2015) Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7, plu092.
| Crossref | Google Scholar |
Wallace BJ (1981) The Australian vascular epiphytes: flora & ecology. University of New England, Armidale. Available at https://rune.une.edu.au/web/bitstream/1959.11/23348/4/open/SOURCE03.pdf [accessed 20 January 2022]
Wilson AD, Mulham WE, Leigh JH (1976) A note on the effects of browsing by feral goats on a belah (Casuarina cristata)-rosewood (Heterodendrum oleifolium) woodland. The Australian Rangeland Journal 1, 7-12.
| Crossref | Google Scholar |
Winoto-Lewin Y, Kirkpatrick JB (2020) Species of accidental woody epiphytes vary between host trees in Tasmanian wet forests. Australian Journal of Botany 68, 532-541.
| Crossref | Google Scholar |
Zotz G (2005) Vascular epiphytes in the temperate zones – a review. Plant Ecology 176, 173-183.
| Crossref | Google Scholar |


