Pre-dispersal seed-predation affects fruit crop and seed fitness in a highly fragmented savanna tree
S. D. Heyes A B * , J. W. Morgan A B , S. J. Sinclair
A B * , J. W. Morgan A B , S. J. Sinclair  C , Z. C. Walker D and S. E. Hoebee
C , Z. C. Walker D and S. E. Hoebee  A B
A B
A Department of Environment and Genetics, La Trobe University, Bundoora, Vic. 3086, Australia.
B Research Centre for Future Landscapes, La Trobe University, Bundoora, Vic. 3086, Australia.
C Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, Heidelberg, Vic. 3084, Australia.
D School of BioSciences, University of Melbourne, Parkville, Vic. 3010, Australia.
Australian Journal of Botany - https://doi.org/10.1071/BT23011
Submitted: 2 February 2023 Accepted: 2 August 2023 Published online: 28 August 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Silver banksia (Banksia marginata) savannas of south-eastern Australia were formerly widespread but are now largely cleared and fragmented, with small populations often showing recruitment limitation. Which may, in part, be due to seed predation by yellow-tailed black cockatoos (Zanda funerea), which have been observed foraging for seed.
Aims: The study aimed to investigate the impact of pre-dispersal seed predation by yellow-tailed black cockatoos on fruit crop between early development and seed release. Additionally, we wanted to assess seed fitness from partially damaged and discarded cones.
Methods: The study quantified silver banksia infructescence removal at four populations between early December and seed release in March. We also quantified seed mass and germinability of seeds from cones removed in December, February and March.
Key results: All populations experienced significant seed predation (>50%), with almost complete removal of cones from some populations. Early removal limits seed release from cones (only 18% of early removed follicles released seed). Further, early removal also significantly reduced seed mass and germination.
Conclusions: Pre-dispersal seed predation by cockatoos is likely to act as a strong filter for recruitment limitation in silver banksia, by negatively affecting annual seed crop size, seed fitness and germinability.
Implications: Our study showed the potential impact fragmentation can have on antagonistic plant–animal interactions. Small, isolated B. marginata populations are likely to continue to decline without active strategies to remedy seed predation such as large-scale restoration.
Keywords: Banksia marginata, fragmentation, germination, granivory, parrots, recruitment limitation, seed, seed predation, Zanda funerea.
Introduction
Seed limitation affects the ability of plants to recruit new individuals (Eriksson and Ehrlén 1992), with subsequent impacts on population viability and plant community assembly (Grubb 1977; Foster and Tilman 2003). Plant population size is an important determinant of seed set (Morgan 1995; Severns 2003; Broadhurst et al. 2008), with smaller populations often producing fewer seeds (per individual) than larger populations because of Allee effects (Forsyth 2003; Xia et al. 2013), defined as the relationship between fitness (e.g. seed set) and population size or density (Stephens et al. 1999). The causes for smaller populations producing fewer seeds can be varied and may reflect low pollinator visitation (Dauber et al. 2010; Ballarin et al. 2022a), loss of compatible mates (Young et al. 2000; Pickup and Young 2008), inbreeding outcomes (Miller et al. 2020) and high levels of seed predation (Ballarin et al. 2022b). Therefore, to understand the causes of population-level recruitment limitation, it is important to first understand the processes that limit seed production and availability.
Seed loss owing to animals has long been recognised in the ecological (Brannon 1927; Janzen 1971) and agricultural literature (Brown et al. 2007; Tschumi et al. 2018). Some plant species have evolved strategies to minimise seed loss to seed predators, such as hiding fruit and seed by use of a decoy (Groom et al. 1994), protective structures (e.g. spines and thorns; Coffey et al. 1999), production of plant-defence compounds (Veldman et al. 2007) or seed-masting events (Wright et al. 2014). Given the evolution of such responses to prevent seed loss, there is clearly a reproductive cost to the removal of fruit and seed to justify the selection and evolution of these types of traits.
Seed predators might be particularly important for small plant populations. Kurkjian et al. (2017), for example, found that in north-western California the rate of seed predation by small mammals of a threatened legume (Lupinus constancei) would likely lead to its extinction. In neotropical Brazilian savannas, seed predation has been recorded to be as high as 60% in some woody species, e.g. Schefflera macrocarpa (Araliaceae) (Salazar et al. 2012). If one also considers that the exclusion of seed predators results in increases in seedling establishment (Vaz Ferreira et al. 2011), there is clear demonstration that seed predators can be important drivers of recruitment-limitation in plant populations.
Parrots are thought to be significant pre-dispersal seed predators that consume large quantities of seed crops (Coates-Estrada et al. 1993; Francisco et al. 2008; Villaseñor-Sánchez et al. 2010; Seburanga 2014). In Australia, many parrots are considered to be generalist granivores consuming the seeds of a wide range of plant species (Long 1984; Lee et al. 2013; McNab and Sanders 2014), including the yellow-tailed black cockatoo (Zanda funerea) (Mcinnes and Carne 1978; Possingham 1986; Stock et al. 2013). The role of parrots in seed limitation might be especially important where habitat fragmentation has occurred, partly because seed predators may focus their feeding activity on few remaining patches (Francisco et al. 2002; Pizo and Vieira 2004) rather than spreading foraging activity across a larger area and may act as an important Allee effect. However, this has been poorly documented in Australia, despite the many granivorous parrots found there.
Woodlands and savannas in south-eastern Australia have experienced extensive land clearing since European settlement and what remains is now highly fragmented (Yates and Hobbs 1997). Trees in this landscape were once common and widespread, with species such as Banksia marginata (Proteaceae) functionally important, supporting a diverse community of vertebrate and invertebrate pollinators (Lunt 1997; Sinclair and Atchison 2012). These Banksia trees are now largely limited to small linear remnant savanna along road and rail reserves (Sinclair and Atchison 2012; Heyes et al. 2020) and many of these populations show evidence of recruitment limitation and seed predation (Heyes et al. 2020), with cone destruction being consistent with damage recorded in banksias by Zanda spp. elsewhere (Scott and Black 1981; Witkowski et al. 1991). Many B. marginata populations are anecdotally reported by seed collectors to have lost entire crops over multiple years (D. Frost, pers. comm.). During foraging, Z. funerea has been observed removing Banksia cones to extract seed (S. Heyes, pers. obs., 2017), with many of the discarded cones containing intact follicles. This does have the potential to be a source of seed for recruitment, but such foraging is also likely to incur a cost to seed fitness if cones are removed before seeds have fully developed. The loss of such cones and seed in small and highly fragmented populations may contribute to recruitment limitation if large numbers of fruit and seed are removed from the seed pool prior to dispersal.
Despite these reports and anecdotal observations of seed predation by Z. funerea, and given that B. marginata occurs in small patches of fragmented savannas, we wanted to evaluate how seed predation affects the availability of seed for recruitment because this may be a potential driver of decline in B. marginata (Heyes et al. 2020). In this study, we wanted to understand whether seed predation is an important driver of recruitment failure and whether timing of seed predation had any impact on the fitness of seed that may escape predation; therefore, we hypothesise that (1) seed predation by Z. funerea is contributing to high rates (>50%) of B. marginata cone removal in fragmented savanna, and (2) early cone-removal events lead to reduced fitness of seeds not directly affected by predation.
Methods
Study area
The study occurred in western Victoria, Australia, on a range of quaternary basalts, granite, and sandstone soils. Mean annual rainfall ranges between 558 and 702 mm and mean maximum annual temperature is 17.4–19.2°C (Bureau of Meteorology 2018). Historically, the area has supported non-Eucalyptus grassy woodlands and savanna dominated by B. marginata and Allocasuarina verticillata, but land-use intensification (stock grazing, cropping) has reduced the original ecosystem to small, isolated remnants where grazing has been excluded. Four study sites were selected (Table 1); all had B. marginata, with populations ranging in size from 56 to >500 trees. All occur along roadsides as fragments surrounded by agricultural landscapes.
| Site name | Abbreviation | Location | Rainfall (mm) | Population size |
|---|---|---|---|---|
| Illabarook | ILA | 37°48′S, 143°40′E | 558 | 56 |
| Caramut | CAR | 37°56′S, 142°30′E | 628 | 127 |
| Pittong | PIT | 37°41′S, 143°27′E | 617 | 143 |
| Minhamite | MIN | 37°59′S, 142°20′E | 702 | >500 |
Study species
B. marginata (Proteaceae) is a widely distributed, highly variable shrub to small tree from south-eastern Australia (Taylor 1988) with both fire-sensitive and fire-tolerant variants (Collins 2009). Flowering occurs from March to April (Jury 2023), with inflorescences forming on old nodes and forming an aggregate of dry woody follicles in an infructescence (cone) approximately 12 months later. Cones can contain as many as 150 follicles, with each follicle containing two papery, winged seeds that are separated by a woody septum. Seed is dehisced annually in early autumn, approximately 12 months after flowering (N. Kerr, S.D. Heyes, J.W. Morgan, unpubl. data). Seed is shortly winged, allowing for some degree of wind dispersal.
Seed predation
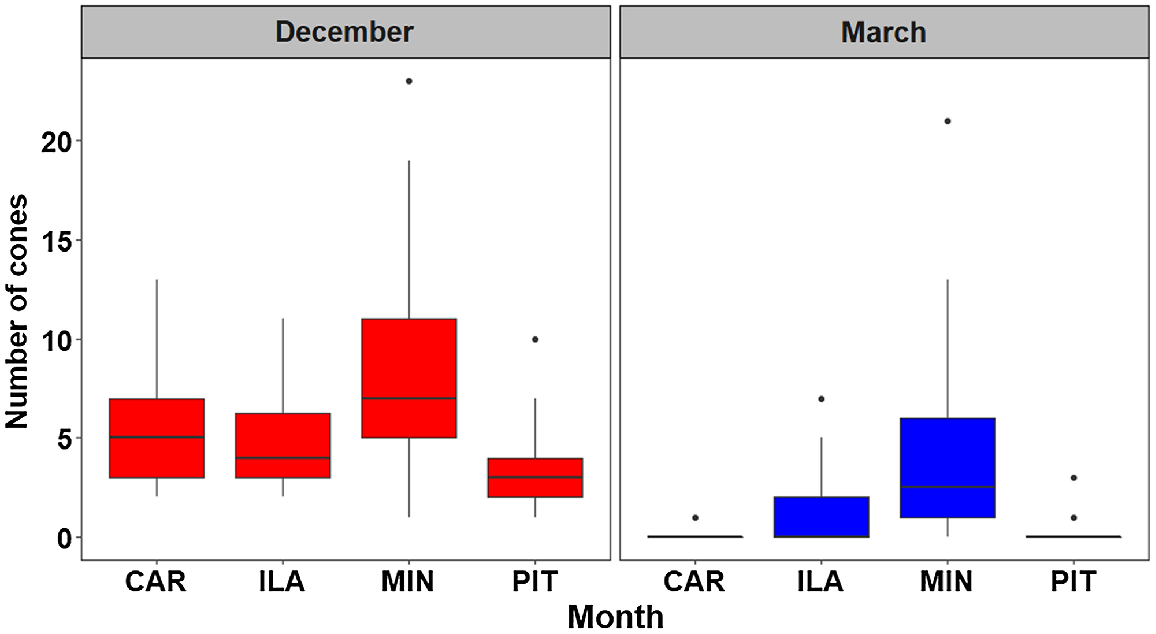
In December 2017, six branches were randomly sampled throughout the canopy of 10 randomly sampled trees at each site (n = 60 × 4 = 240), with each branch being tagged and numbered. Many Banksia species retain dead florets on cones (He et al. 2011) that obscure individual follicles from visual inspection; each cone was therefore inspected by hand to confirm the presence of developing follicles. There was some evidence that seed predation had already begun at the time of initial sampling, with Z. funerea observed foraging in December at one of our study sites (CAR), and freshly discarded cones below trees at all sites (Fig. 1b), such that the data presented here is likely to have under-estimated predation. The sites were revisited in March 2018, coinciding with seed release, and all remaining cones on tagged branches were counted.
Zanda funerea. (a) Foraging in Banksia marginata at Clarence, NSW (photo by Carol Probets); (b) the litter of broken cones and follicles from Clarence, NSW, is typical of foraging by Z. funerea and was found at all our study sites (photo by Carol Probets); (c) Z. funerea foraging in B. marginata at Dunkeld, Victoria (photo by Neil Scott). All photos are used with permission.

Seed fitness
We assessed the effects of pre-dispersal cone removal on the seed fitness of unconsumed seeds in prematurely removed and discarded cones. To do this, we investigated how the timing of granivore foraging affects seed mass and germination of seed from cones removed from the parent plant. To mimic cockatoo removal, 10 trees were selected in November 2018, and six cones were caged with fine mesh bags to prevent removal by cockatoos (totalling 60 cones). Bagging occurred after flowering, so that pollination was not affected. Seed predation was already heavy in November at CAR with the entire crop of some trees being removed; therefore, 60 cones were randomly sampled across the entire population and caged using the same method. Caged cones were then removed from trees in each of December 2018 (n = 20, removed while seeds are still developing), February 2019 (n = 20) and March 2019 (n = 20, removed at a time when seeds would be naturally beginning to start dispersing). These were placed in paper bags to dry at room temperature to allow follicles to open and release seed. The number of follicles opened and closed were counted for each of the three removal times for the Pittong population, only to see whether early removal affected the proportion of follicles that opened.
Once released, seeds (n = 100) were randomly sampled for each removal time at each population. A subset of 40 of the 100 seeds was weighed to obtain the seed mass to determine whether the timing of removal affected mean seed mass. All 100 seed were then assessed for germination across a 9-week period.
To test germination, seeds were surface-sterilised with sodium hypochlorite solution (1% NaOCl) and rinsed using distilled water. Ten replicates of 10 seeds were placed on Whatman grade 1 filter paper, moistened with 5 mL distilled water, and sealed with Parafilm to prevent water loss. Petri dishes were then randomly positioned in a Thermoline controlled-temperature cabinet (18°C constant temperature; 12 h light and dark cycling). Germinants were counted every 7 days for 9 weeks, with germination defined as the emergence of the radical. If Petri dishes were drying, a supplementary 1 mL of distilled water was added, and the dishes resealed with Parafilm.
Data analysis
Analyses were performed using R ver. 4.1.2 (R Core Team 2021), with all data visualisation produced using the ‘ggplot2’ package (Wickham 2016). The reporting of P-values follows that outlined by Muff et al. (2022).
To determine how cone loss to cockatoos varied across the fruiting season, generalised linear models (GLM) with a Poisson distribution were fit to cone loss data. To determine the most important variables affecting cone loss, we fit four models each with the number of cones as the response variable and site, treatment (i.e. month) and their interaction term as main effects. The four models included main effects of (1) site only, (2) month only, (3) month + site, and (4) month + site and their interaction term. These models were compared using Akaike’s information criterion (AIC) scores, which determined the fourth model, including the interaction term was the most parsimonious.
To assess how early removal of cones by cockatoos affects seed mass, we assessed our cone-removal (simulated seed predator) data by using a two-way ANOVA (assuming a normal distribution). This included seed mass as the response variable and site and treatment (month) as the explanatory variables. Pairwise differences between terms were then investigated using Tukey’s honest significance difference tests.
To determine how early cone removal influences germination, we produced cumulative germination curves for the December, February and March cohorts by using parametric time-to-event models, as described by Onofri et al. (2018), using the ‘drcte’ package in R (Onofri 2022). A time-to-event model was used because of the uncertainty associated with when each germination event occurred, which leads to unreliable standard errors (Onofri et al. 2019). All models included the number of germinated seed as the response variable and the number of seeds prior to and after counting as the explanatory variables (as per Onofri et al. 2019). To parametrise the model, we produced six time-to-event models by using different non-linear functions, including three log-logistic and three Weibull link functions (Onofri et al. 2018). We compared these models to determine the most parsimonious model (and therefore link function) by using AIC scores, which found that a Weibull Type 1 distribution provided the best fit and was used for the final model. To test for differences between the germination curves, we used a likelihood ratio test by using the ‘compCDF’ function in R. The resulting germination curves were then plotted with 95% confidence intervals.
Results
Seed predation
Seed predation was apparent at all four sites (Fig. 2). Large numbers of cones were removed by foraging birds, namely, 56% at MIN, 77% at ILA, 96% at PIT and 99% at CAR (Fig. 2). Evidence for removal of cones at each site included direct observation, scars where cones had been removed from the branch at the point of attachment, as well as a litter of discarded cones (both damaged and intact) under trees. There was very strong evidence of cone loss observed between December and March (P < 0.001). There was moderate (PIT, P = 0.01) to very strong evidence (P < 0.001) of an interaction between cone removal and sites, suggesting that seed predation is higher at some sites.
Seed fitness
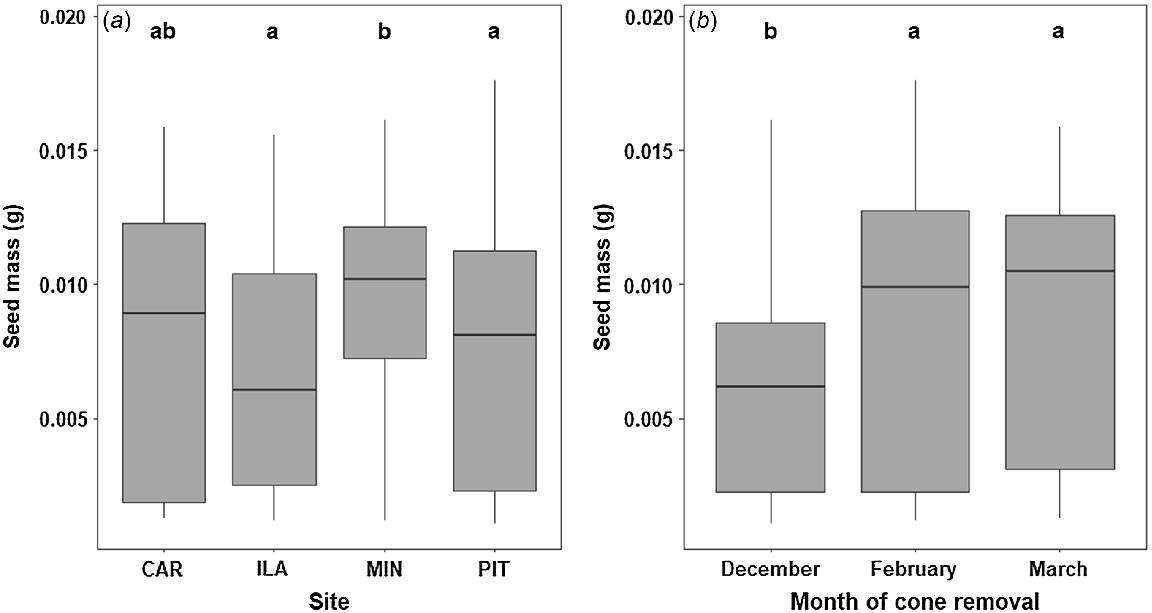
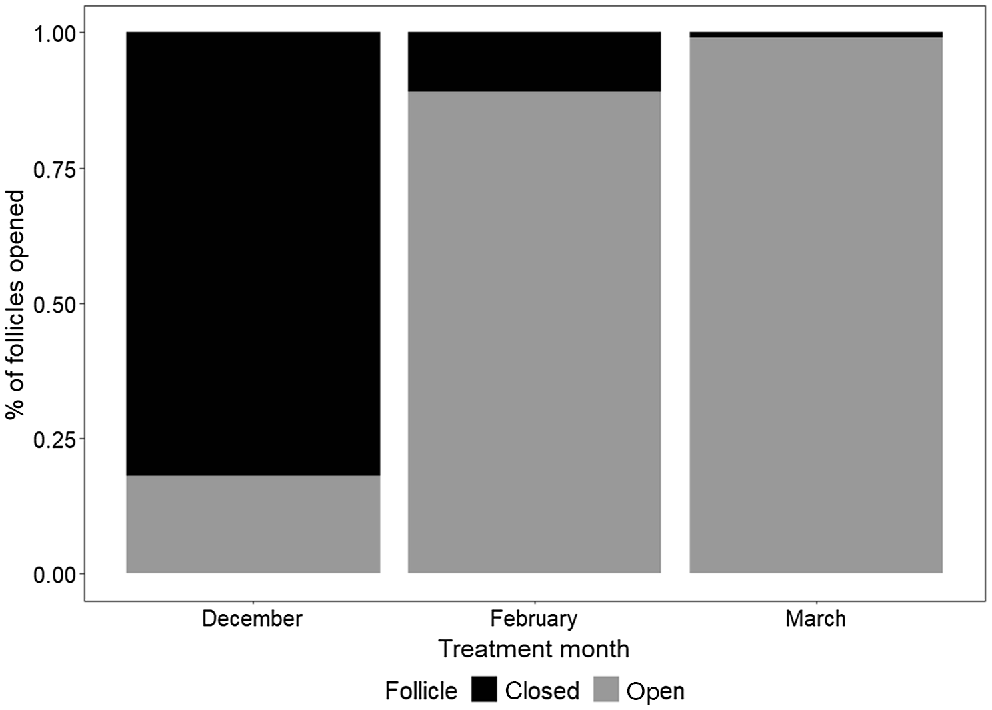
The timing of cone removal had a significant effect on seed mass (F = 18.269, P < 0.001), with removal of cones in December resulting in lower mean seed mass (Fig. 3, 0.0062 g; Tukey’s post hoc test, P < 0.001) compared with removal in February (0.0086 g) and March (0.0089 g). There was weak to moderate evidence (F = 2.306, P = 0.03) of a significant interaction between the timing of cone removal and site, suggesting that some sites are experiencing heavier foraging than others. The removal of cones also affected the ability of follicles to naturally open when dried under ambient conditions. Far fewer follicles opened when removed in December (18%) than in February (89%) and March (99%) (Fig. 4).
Boxplots comparing (a) the seed mass across four sites and (b) seed mass at each cone removal time (December, February and March). The results of the Tukey HSD post hoc test represented by compact letter display above each boxplot showing a shared letter where the means are not significantly different.
Stacked bar plot showing the cohort (December, February, March) effects of removal of Banksia marginata cones on the percentage of follicles that were able to open and release seed.
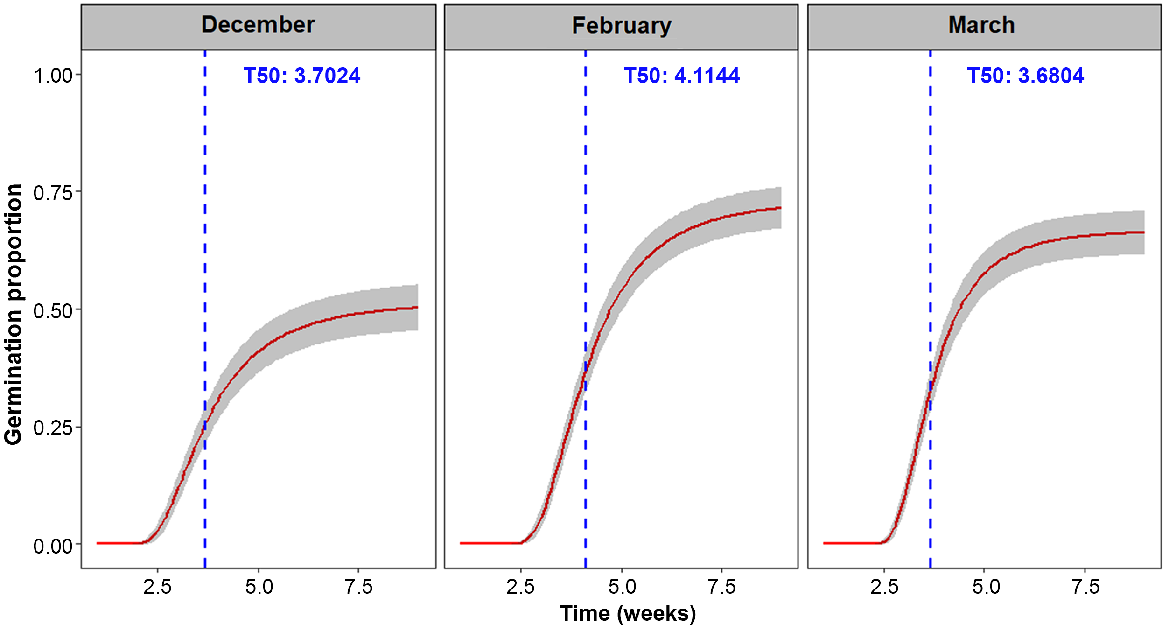
Early removal of cones had a very strong negative effect on the germination of seed that was released from removed cones, with significant differences among germination curves (P < 0.005). The mean percentage germination of seeds removed in December was low (50%) when compared with cones removed in February (71%) and March (66%) (Fig. 5). However, cone removal did not appear to have much effect on median germination time (T50), with estimated scores for seeds from cones removed in March (3.7 weeks) being the same as for seeds germinated from cones removed in December (3.7 weeks) and February (4.1 weeks) (Table 2). The seeds germinated from cones removed in December also noticeably lacked a dark seed coat and appeared to be susceptible to moulds during germination.
Germination curves showing the effect of cone removal on germination response for seed extracted from Banksia marginata cones removed in December, February and March. Red line indicates mean germination proportion and the grey bands indicate 95% CI. Time to 50% of total germinated seed is marked by the blue dashed line.
Discussion
Pre-dispersal seed predators are capable of removing large proportions of the cone crop in fragmented populations of B. marginata. We found high removal rates (>50%) in all populations and rates exceeding 90% of all cones at two sites. These rates are particularly high when compared with those in similar studies on other Banksia spp. Work on Banksia attenuata by Scott and Black (1981) found the highest removal to be 35% of cones, whereas Witkowski et al. (1991) found cone removal by parrots for Banksia baxteri and Banksia speciosa to be 20% and 10%, respectively. It is unclear from our study whether the substantial proportion of cones we observed being removed occurs across years, but anecdotal observations by seed collectors suggest that this could be the case (D. Frost, pers. comm.) and Z. funerea is known to exhibit habitual feeding behaviour, repeatedly visiting favoured food plants over multiple years (Way and van Weenen 2008; Moore 2022). Invertebrate seed predation can also contribute significantly to seed losses in Banksia (Cowling and Lamont 1987; Vaughton 1990) and, coupled with the exceptionally high rates of seed predation seen here, may well be a leading cause of recruitment failure observed previously in B. marginata in our study area (Heyes et al. 2020).
High rates of seed predation by parrots have been reported in other fragmented habitats such as the cerrado (Francisco et al. 2002; Pizo and Vieira 2004), but the effects of fragmentation on seed predation are less clear when we examine the broader literature, with some studies showing an easing of seed-predation pressure in fragmented plant populations (Chacoff et al. 2004; Pizo and Vieira 2004; Fleury and Galetti 2006; García and Chacoff 2007; Herrerías-Diego et al. 2008; Mendes et al. 2016; Chen et al. 2017). Seed predation has also been shown to be density dependent, with seed predator abundance increasing with stand density in tropical legumes (Jones and Comita 2010; Ballarin et al. 2022b); however, this was not found in our study, with lower cone loss at the largest and densest stand (MIN) and the second smallest and one of the least dense (ILA). Fragmentation of original savanna habitats in western Victoria has reduced most B. marginata populations to small, highly isolated fragments, and isolation may play a role in the high rates of seed predation these populations are experiencing. Although Z. funerea is highly mobile and can readily access isolated B. marginata populations, the isolated nature of many populations (Miller et al. 2020; Heyes et al. 2020) may mean that birds are spending more time within a population, satiating themselves on the entire crop, rather than moving among populations as might happen in a contiguous landscape with many trees. Indeed, in our study both populations that experienced most cone removal were both highly isolated stands, being located over 10 km from their nearest fragmented Banksia population, whereas two sites (MIN and ILA) have numerous populations in the neighbourhood and may act to buffer over-exploitation by the foraging Z. funerea. Way and van Weenen (2008) suggested that Z. funerea nest attendance rates decrease as the birds travel further between foraging sites, which increases the risk of losing offspring. Given this risk, it is likely that birds would spend more time at a known B. marginata populations rather than searching the neighbourhood for more populations.
In addition to consuming seeds, Z. funerea and other cockatoos may potentially act as seed dispersers by carrying and dropping cones containing viable seed, and thus contribute to gene flow and long-distance dispersal (Tella et al. 2019). Anecdotal observations of cones dropped long distances from parent trees support this idea. However, we found that cone removal by cockatoos affected the subsequent release of seed from discarded cones and reduced the fitness of the remaining seed that was released, particularly for the seed from cones that were removed early. Earlier removal had a noticeable (negative) effect on seed mass and overall germination. An unexpected outcome was the observation that seeds from follicles removed early (December) failed to develop a dark seed coat; many of these became affected by mould in the germination cabinet study. Seed coats may function to protect the embryo from pathogens such as moulds (Hurd 1921; Warr et al. 1992) and, given that so few seeds are naturally released from cones when removed early and that such seed becomes mouldy and exhibits low germinability, any factor that leads to early cone removal appears likely to affect annual seed crops negatively. As such, the negative effects of early cone removal becomes crucial in small populations where reduced seed rain potentially already limits recruitment success. So, although it is possible that cockatoos occasionally facilitate long-distance gene flow, their involvement as a dispersal agent generally has a cost to the fitness of the parent plants.
Seed predation can act as a strong filter on regeneration, compounding the effects of small population size (Allee effects), habitat isolation (inbreeding) and habitat quality (Krushelnycky 2014; Kurkjian et al. 2017). Managing small populations of B. marginata, so as to maintain (or grow) populations, will be challenging in the current landscape setting where cockatoos target small, isolated patches. Novel approaches, such as netting and caging plants, have been used by community groups to prevent seed predation, but this can be applied only at small scales. Increasing the number of populations across the landscape, and the number of plants in each population, via targeted re-introduction programs may alleviate seed limitation in the smaller populations by spreading predation ‘risk’ across larger and less isolated populations. Such activities have a lag period between planting and cone production, but these activities may also have added benefits by improving gene flow and reducing inbreeding risk to existing populations (Miller et al. 2020).
Acknowledgements
We thank Lisa McIntyre, Hayley Sime and Nina Kerr for their valuable assistance and Dan Frost from the Ballarat Region Seed Bank for his initial observations and conversations. We also thank Carol Probets and Neil Scott for kindly allowing us to use their photographs. We would especially like to thank the invaluable input from the anonymous reviewers who have taken the time to review this paper.
References
Ballarin CS, Hachuy-Filho L, Doria MJW, Giffu MM, Polizello DS, Oliveira PH, Lacerda-Barbosa PA, Amorim FW (2022a) Intra-seasonal and daily variations in nectar availability affect bee assemblage in a monodominant afforested Brazilian Cerrado. Austral Ecology 47(6), 1315-1328.
| Crossref | Google Scholar |
Ballarin CS, Hachuy-Filho L, Fontúrbel FE, Amorim FW (2022b) Density-dependent effects on the reproductive outcome of a native tree at tropical restored habitats. Forest Ecology and Management 520, 120391.
| Crossref | Google Scholar |
Brannon CH (1927) Life history of the plum curculio (Conotrachelus nenuphar Herbst.). Journal of the Elisha Mitchell Scientific Society 43, 79-83 Available at https://www.jstor.org/stable/24332758.
| Google Scholar |
Broadhurst LM, Young AG, Forrester R (2008) Genetic and demographic responses of fragmented Acacia dealbata (Mimosaceae) populations in southeastern Australia. Biological Conservation 141, 2843-2856.
| Crossref | Google Scholar |
Brown PR, Huth NI, Banks PB, Singleton GR (2007) Relationship between abundance of rodents and damage to agricultural crops. Agriculture, Ecosystems & Environment 120, 405-415.
| Crossref | Google Scholar |
Bureau of Meteorology (2018) Monthly climate statistics. Available at http://www.bom.gov.au
Chacoff NP, Morales JM, Vaquera MdP (2004) Efectos de la Fragmentatión Sobre la Aborción y Depredación de Semillas en el Chaco Serrano. Biotropica 36, 109-117.
| Crossref | Google Scholar |
Chen Q, Tomlinson KW, Cao L, Wang B (2017) Effects of fragmentation on the seed predation and dispersal by rodents differ among species with different seed size. Integrative Zoology 12, 468-476.
| Crossref | Google Scholar | PubMed |
Coates-Estrada R, Estrada A, Meritt D, Jr (1993) Foraging by parrots (Amazona autumnalis) on fruits of Stemmadenia donnell-smithii (Apocynaceae) in the tropical rain forest of Los Tuxtlas, Mexico. Journal of Tropical Ecology 9, 121-124.
| Crossref | Google Scholar |
Coffey K, Benkman CW, Milligan BG (1999) The adaptive significance of spines on pine cones. Ecology 80, 1221-1229.
| Crossref | Google Scholar |
Cowling RM, Lamont BB (1987) Post-fire recruitment of four co-occurring Banksia species. Journal of Applied Ecology 24(2), 645-658.
| Crossref | Google Scholar |
Dauber J, Biesmeijer JC, Gabriel D, Kunin WE, Lamborn E, Meyer B, Nielsen A, Potts SG, Roberts SPM, Sõber V, Settele J, Steffan-Dewenter I, Stout JC, Teder T, Tscheulin T, Vivarelli D, Petanidou T (2010) Effects of patch size and density on flower visitation and seed set of wild plants: a pan-European approach. Journal of Ecology 98, 188-196.
| Crossref | Google Scholar |
Eriksson O, Ehrlén J (1992) Seed and microsite limitation of recruitment in plant populations. Oecologia 91, 360-364.
| Crossref | Google Scholar | PubMed |
Fleury M, Galetti M (2006) Forest fragment size and microhabitat effects on palm seed predation. Biological Conservation 131, 1-13.
| Crossref | Google Scholar |
Forsyth SA (2003) Density-dependent seed set in the Haleakala silversword: evidence for an Allee effect. Oecologia 136, 551-557.
| Crossref | Google Scholar | PubMed |
Foster BL, Tilman D (2003) Seed limitation and the regulation of community structure in oak savanna grassland. Journal of Ecology 91, 999-1007.
| Crossref | Google Scholar |
Francisco MR, de Oliveira Lunardi V, Galetti M (2002) Massive seed predation of Pseudobombax grandiflorum (Bombacaceae) by parakeets Brotogeris versicolurus (Psittacidae) in a forest fragment in Brazil. Biotropica 34, 613-615.
| Crossref | Google Scholar |
Francisco MR, Lunardi VO, Guimarães PR, Jr., Galetti M (2008) Factors affecting seed predation of Eriotheca gracilipes (Bombacaceae) by parakeets in a cerrado fragment. Acta Oecologica 33, 240-245.
| Crossref | Google Scholar |
García D, Chacoff NP (2007) Scale-dependent effects of habitat fragmentation on hawthorn pollination, frugivory, and seed predation. Conservation Biology 21, 400-411.
| Crossref | Google Scholar | PubMed |
Groom PK, Lamont BB, Duff HC (1994) Self-crypsis in Hakea trifurcata as an avian granivore deterrent. Functional Ecology 8, 110-117.
| Crossref | Google Scholar |
Grubb PJ (1977) The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52, 107-145.
| Crossref | Google Scholar |
He T, Lamont BB, Downes KS (2011) Banksia born to burn. New Phytologist 191(1), 184-196.
| Crossref | Google Scholar |
Herrerías-Diego Y, Quesada M, Stoner KE, Lobo JA, Hernández-Flores Y, Sanchez Montoya G (2008) Effect of forest fragmentation on fruit and seed predation of the tropical dry forest tree Ceiba aesculifolia. Biological Conservation 141, 241-248.
| Crossref | Google Scholar |
Heyes SD, Sinclair SJ, Hoebee SE, Morgan JW (2020) How widespread are recruitment bottlenecks in fragmented populations of the savanna tree Banksia marginata (Proteaceae)? Plant Ecology 221, 545-557.
| Crossref | Google Scholar |
Hurd AM (1921) Seed-coat injury and viability of seeds of wheat and barley as factors in susceptibility to molds and fungicides. Journal of Agricultural Research 21, 99-122.
| Google Scholar |
Janzen DH (1971) Seed predation by animals. Annual Review of Ecology and Systematics 2(1), 465-492.
| Crossref | Google Scholar |
Jones FA, Comita LS (2010) Density-dependent pre-dispersal seed predation and fruit set in a tropical tree. Oikos 119(11), 1841-1847.
| Crossref | Google Scholar |
Krushelnycky PD (2014) Evaluating the interacting influences of pollination, seed predation, invasive species and isolation on reproductive success in a threatened alpine plant. PLoS ONE 9(2), e88948.
| Crossref | Google Scholar | PubMed |
Kurkjian HM, Carothers SK, Jules ES (2017) Seed predation has the potential to drive a rare plant to extinction. Journal of Applied Ecology 54, 862-871.
| Crossref | Google Scholar |
Lee J, Finn H, Calver M (2013) Feeding activity of threatened black cockatoos in mine-site rehabilitation in the jarrah forest of south-western Australia. Australian Journal of Zoology 61, 119-131.
| Crossref | Google Scholar |
Long JL (1984) The diets of three species of parrots in the South of Western Australia. Wildlife Research 11, 357-371.
| Crossref | Google Scholar |
Lunt ID (1997) Tree densities last century on the lowland Gippsland plain, Victoria. Australian Geographical Studies 35, 342-348.
| Crossref | Google Scholar |
Mcinnes RS, Carne PB (1978) Predation of cossid moth larvae by Yellow-tailed Black Cockatoos causing losses in plantations of Eucalyptus grandis in north coastal New South Wales. Wildlife Research 5, 101-121.
| Crossref | Google Scholar |
McNab A, Sanders MG (2014) Consumption of exotic grass seeds (Poaceae: Cynodon dactylon) by the Eastern Ground Parrot (Pezoporous wallicus). Queensland Naturalist 52, 82-84 Available at https://search.informit.org/doi/10.3316/informit.863219475618836.
| Google Scholar |
Mendes CP, Ribeiro MC, Galetti M (2016) Patch size, shape and edge distance influence seed predation on a palm species in the Atlantic forest. Ecography 39, 465-475.
| Crossref | Google Scholar |
Miller AD, Nitschke C, Weeks AR, Weatherly WL, Heyes SD, Sinclair SJ, Holland OJ, Stevenson A, Broadhurst L, Hoebee SE, Sherman CDH, Morgan JW (2020) Genetic data and climate niche suitability models highlight the vulnerability of a functionally important plant species from south-eastern Australia. Evolutionary Applications 13(8), 2014-2029.
| Crossref | Google Scholar | PubMed |
Moore G (2022) The impact of Yellow-tailed Black-Cockatoo ‘Calyptorhynchus funereus’ (Shaw, 1794) feeding on a Monterey Pine Pinus radiata D.Don. The Victorian Naturalist 139(1), 13-20 Available at https://search.informit.org/doi/10.3316/informit.334288469779684.
| Google Scholar |
Morgan JW (1995) Ecological studies of the endangered Rutidosis leptorrhynchoides. I. Seed production, soil seed bank dynamics, population density and their effects on recruitment. Australian Journal of Botany 43, 1-11.
| Crossref | Google Scholar |
Muff S, Nilsen EB, O’Hara RB, Nater CR (2022) Rewriting results sections in the language of evidence. Trends in Ecology & Evolution 37(3), 203-210.
| Crossref | Google Scholar | PubMed |
Onofri A (2022) drcte: statistical approaches for time-to-event data in agriculture. R package version 1.0.6. Available at https://www.statforbiology.com
Onofri A, Benincasa P, Mesgaran MB, Ritz C (2018) Hydrothermal-time-to-event models for seed germination. European Journal of Agronomy 101, 129-139.
| Crossref | Google Scholar |
Onofri A, Piepho H-P, Kozak M (2019) Analysing censored data in agricultural research: a review with examples and software tips. Annals of Applied Biology 174(1), 3-13.
| Crossref | Google Scholar |
Pickup M, Young AG (2008) Population size, self-incompatibility and genetic rescue in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Heredity 100, 268-274.
| Crossref | Google Scholar | PubMed |
Pizo MA, Vieira EM (2004) Granivorous birds as potentially important post-dispersal seed predators in a Brazilian forest fragment. Biotropica 36, 417-423.
| Crossref | Google Scholar |
Possingham HP (1986) The Funereal Cockatoo on Eyre Peninsula. South Australian Ornithologist 30, 1-4.
| Google Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org/
Salazar A, Goldstein G, Franco AC, Miralles-Wilhelm F (2012) Seed limitation of woody plants in Neotropical savannas. Plant Ecology 213, 273-287.
| Crossref | Google Scholar |
Scott JK, Black R (1981) Selective predation by White-tailed Black Cockatoos on fruit of Banksia attenuata containing the seed-eating weevil Alphitopis nivea. Australian Wildlife Research 8, 421-430.
| Crossref | Google Scholar |
Seburanga JL (2014) Evidence for pre-dispersal predation of seeds of Tithonia diversifolia by the black-faced canary (Serinus capistratus). Journal of Plant Interactions 9, 832-837.
| Crossref | Google Scholar |
Severns P (2003) Inbreeding and small population size reduce seed set in a threatened and fragmented plant species, Lupinus sulphureus ssp. kincaidii (Fabaceae). Biological Conservation 110, 221-229.
| Crossref | Google Scholar |
Sinclair SJ, Atchison K (2012) The pre-colonial distribution of grasslands, woodlands and forests on the Werribee plains, Victoria. Cunninghamia 12, 213-227.
| Crossref | Google Scholar |
Stephens PA, Sutherland WJ, Freckleton RP (1999) What is the Allee effect? Oikos 87(1), 185-190.
| Crossref | Google Scholar |
Stock WD, Finn H, Parker J, Dods K (2013) Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape. PLoS ONE 8, e61145.
| Crossref | Google Scholar | PubMed |
Tella JL, Blanco G, Dénes FV, Hiraldo F (2019) Overlooked parrot seed dispersal in Australia and South America: insights on the evolution of dispersal syndromes and seed size in Araucaria trees. Frontiers in Ecology and Evolution 7, 82.
| Crossref | Google Scholar |
Tschumi M, Ekroos J, Hjort C, Smith HG, Birkhofer K (2018) Rodents, not birds, dominate predation-related ecosystem services and disservices in vertebrate communities of agricultural landscapes. Oecologia 188, 863-873.
| Crossref | Google Scholar | PubMed |
Vaughton G (1990) Predation by insects limits seed production in Banksia spinulosa var. neoanglica (Proteaceae). Australian Journal of Botany 38(4), 335-340.
| Crossref | Google Scholar |
Vaz Ferreira A, Bruna EM, Vasconcelos HL (2011) Seed predators limit plant recruitment in Neotropical savannas. Oikos 120, 1013-1022.
| Crossref | Google Scholar |
Veldman JW, Greg Murray K, Hull AL, Mauricio Garcia-C J, Mungall WS, Rotman GB, Plosz MP, Mcnamara LK (2007) Chemical defense and the persistence of pioneer plant seeds in the soil of a tropical cloud forest. Biotropica 39, 87-93.
| Crossref | Google Scholar |
Villaseñor-Sánchez EI, Dirzo R, Renton K (2010) Importance of the lilac-crowned parrot in pre-dispersal seed predation of Astronium graveolens in a Mexican tropical dry forest. Journal of Tropical Ecology 26, 227-236.
| Crossref | Google Scholar |
Warr SJ, Thompson K, Kent M (1992) Antifungal activity in seed coat extracts of woodland plants. Oecologia 92, 296-298.
| Crossref | Google Scholar | PubMed |
Wickham H (2016) ‘ggplot2: elegant graphics for data analysis.’ (Springer-Verlag: New York, NY, USA). Available at https://ggplot2.tidyverse.org
Witkowski ETF, Lamont BB, Connell SJ (1991) Seed bank dynamics of three co-occurring Banksias in south coastal Western Australia: the role of plant age, cockatoos, senescence and interfire establishment. Australian Journal of Botany 39, 385-397.
| Crossref | Google Scholar |
Wright BR, Zuur AF, Chan GCK (2014) Proximate causes and possible adaptive functions of mast seeding and barren flower shows in spinifex grasses (Triodia spp.) in arid regions of Australia. The Rangeland Journal 36, 297-308.
| Crossref | Google Scholar |
Xia J, Sun S, Liu G (2013) Evidence of a component Allee effect driven by predispersal seed predation in a plant (Pedicularis rex, Orobanchaceae). Biology Letters 9, 20130387.
| Crossref | Google Scholar | PubMed |
Yates CJ, Hobbs RJ (1997) Temperate Eucalypt woodlands: a review of their status, processes threatening their persistence and techniques for restoration. Australian Journal of Botany 45, 949-973.
| Crossref | Google Scholar |
Young A, Miller C, Gregory E, Langston A (2000) Sporophytic self-incompatibility in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Australian Journal of Botany 48(5), 667-672.
| Crossref | Google Scholar |