First records of Myxomycetes associated with members of the Cactaceae in Australia
Steven L. Stephenson A and Todd F. Elliott B *
B *
A Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA.
B Ecosystem Management, University of New England, Armidale, NSW 2351, Australia.
Australian Journal of Botany 70(8) 531-538 https://doi.org/10.1071/BT22032
Submitted: 22 March 2022 Accepted: 2 December 2022 Published: 21 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Cacti are endemic to North and South America, but numerous species have been spread all over the world after Europeans visited the Americas. Their global spread has impacted various aspects of terrestrial ecosystems, including the distributions of succulenticolous myxomycetes.
Aims: In this study, we examine the association of myxomycetes (also known as plasmodial slime moulds or myxogastrids) with three introduced species of cacti in Australia.
Methods: Using the moist chamber culturing technique we prepared 33 cultures from dead portions of three species of cacti collected in New South Wales and Queensland.
Key results: Eighty-eight percent of moist chamber cultures yielded evidence of myxomycetes (either plasmodia or fruiting bodies). A total of 12 species representing six genera were recorded. Physarum compressum was the most abundant species (appearing in 20 of the 33 cultures), with species such as Perichaena depressa also relatively common. We also report the first records of Perichaena luteola in Australia.
Conclusions: This is the first study to investigate the assemblages of myxomycetes associated with members of the Cactaceae in Australia, and we highlight the occurrence of succulenticolous myxomycetes on the continent.
Implications: This study leads to a better understanding of the biogeography, distribution and ecology of succulenticolous myxomycetes. We also add a new species record for Australia.
Keywords: Amoebozoans, biogeography, Harrisia, microbial ecology, moist chamber cultures, Opuntia, slime moulds, succulenticolous myxomycetes.
Introduction
There are no native cacti in Australia; but captain Arthur Phillip brought the first prickly pear (Opuntia sp.) to Australia from Brazil in 1787, and various species continued to be imported well into the 1800s (Frawley 2007). The British military required a red textile dye called carmine to make their signature red coats, and this dye was obtained from the cochineal insect (Dactylopius coccus) (Frawley 2007) that feeds on species of prickly pear. The British were hopeful that the new colony could capitalise on carmine as a lucrative export; however, they had very limited success with farming this scale insect.
As colonists invaded further inland and away from the more humid coastal regions, they brought various species of cacti with them. The plants were better adapted to the drier, more inland portion of the continent, and they began to become more successful and prolific. By the early 20th century, several species of prickly pear cacti had grown out of control and were becoming a serious weed species, often making regions uninhabitable for farmers (Frawley 2007). In some areas of Queensland and New South Wales, there were as many as 12 500 plants per hectare, and they were estimated to be expanding by more than 400 000 ha per year; they covered areas larger than some European countries (Osmond et al. 1979; Domico 2018). The spread of these cacti was a serious ecological and agricultural problem that led to research and the eventual introduction of the cactus moth (Cactoblastis cactorum) from South America in the 1920s and 1930s (Nature 1933; Frawley 2007). Introduction of the cactus moth was successful in killing off most of the large tracts of nearly monodominant populations of prickly pear cacti. Certain species of cacti remain relatively common in some regions of Australia, but they are no longer nearly as problematic a weed as they once were.
Research has been dedicated to the biology and taxonomy of these introduced cacti in Australia and their impact on ecosystems, but there appear to be no previous studies of the myxomycetes associated with these plants. In North and South America, studies have demonstrated that there are distinct assemblages of myxomycetes associated with native cacti (Blackwell and Gilbertson 1980; Lado et al. 1999, 2007a, 2007b; Wrigley de Basanta et al. 2008, 2009; Estrada-Torres et al. 2009; Ndiritu et al. 2009; Ferreira and Cavalcani 2011). Some of the same species of myxomycetes have been reported from cacti in other regions of the world where they were introduced, including the Hawaiian Islands (Eliasson 1991), the Canary Islands (Mosquera et al. 2003) and Ascension Island (Stephenson 2009). Lado et al. (2007b) listed 10 species of myxomycetes that were described as new to science from specimens associated with the Cactaceae and other succulent plants (e.g. certain members of the Euphorbiaceae). However, a number of other species (e.g. Estrada-Torres et al. 2009; Lado et al. 2009, 2013, 2019) have been described since then. Some of these succulenticolous myxomycetes are not yet known from any other substrate.
There are more than 1000 described morphospecies of myxomycetes (Lado 2005–2022). Although long thought to be related to fungi, myxomycetes belong to a totally different group, the Amoebozoans. Their life cycle consists of two trophic (feeding) stages and a reproductive stage. One of the trophic stages (the plasmodium) can achieve a size large enough to be seen with the naked eye. In their other trophic stage (which is microscopic), myxomycetes perform an important role in most terrestrial ecosystems through their feeding activities. This second trophic stage can take the form of an amoeba or be flagellated, and the term ‘amoeboflagellate’ is used to refer to both morphological expressions.
During this amoeboflagellate stage, myxomycetes feed primarily on bacteria; during their plasmodial stage, they utilise other food resources, including algae, spores, fungal fruiting bodies and possibly even lichens (Stephenson 1988; Stephenson and Stempen 1994; Rollins and Stephenson 2011). Their consumption of bacteria releases the nutrients contained within these prey organisms, thus making these nutrients available to other organisms and trophic levels (e.g. plants and animals) and aiding in the transfer of nutrients back into the surrounding environment (Crotty et al. 2012). The amoeboflagellate stage eventually gives rise to the plasmodial stage, from which the reproductive (fruiting body) stage is produced. The fruiting bodies of myxomycetes resemble those of certain fungi but are much smaller (usually no more than 1–2 mm tall) and totally different in structure. Fruiting bodies of myxomycetes can develop under natural conditions in the field, but most studies of the species associated with such microhabitats as decaying cacti use moist chamber cultures to obtain fruiting bodies in the laboratory (Stephenson and Stempen 1994). This was the approach we used in this study.
The composition of myxomycete communities associated with the large populations of cacti in Australia during the late 19th and early 20th centuries before the introduction of the cactus moth is unknown, but the smaller remaining populations are still available for study. The objectives of the project described herein were first to characterise the assemblage of species associated with some cacti populations present in Australia and then determine the extent to which succulenticolous (living or growing on succulent plants) myxomycetes make up this assemblage.
Materials and methods
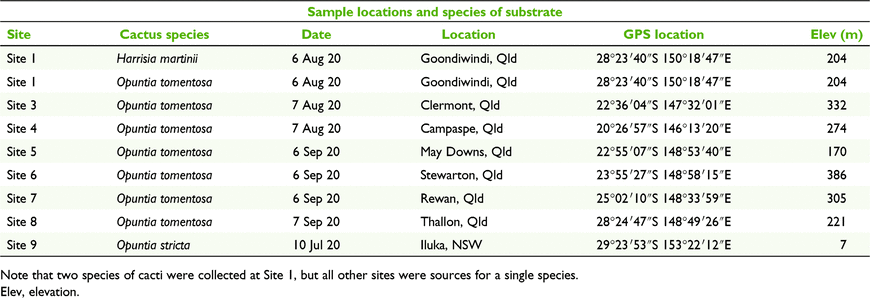
Collections were made from dead portions of cacti – especially the pads (cladodes) of prickly pear cacti – and any other dead cactus material. Collections were made only from material that had already fallen to the ground. We had eight collection sites: one in New South Wales and seven in Queensland (Table 1). Samples were collected from three species of cacti in two genera. Seven sites contained Opuntia tomentosa, one site had Opuntia stricta and one had Harrisia martinii (Fig. 1, Table 1). Most of the dead material collected in this study appeared to have been produced by the feeding activities of the cactus moth (Fig. 1). There were frequently large numbers of pads on the ground around the bases of larger/older cacti.
|
Samples were placed in paper bags, air dried, and sent to the Eumycetozoan Laboratory at the University of Arkansas, where they were processed in the manner described by Stephenson and Stempen (1994). Small pieces of cacti from each sample were placed in disposable plastic Petri dishes lined with filter paper, with enough material placed in each dish to cover most of the bottom. Water was then added to each dish, the top placed on the dish, and these moist chamber cultures set aside for approximately 24 h. After this time, pH was determined for each dish with the use of a portable pH meter and excess water in the dish was then poured off. Two to four moist chamber cultures were prepared from each sample, for a total of 33 cultures. These cultures were examined with a stereomicroscope on a weekly basis for a period of 3 months. Specimens of myxomycetes appearing in the cultures were removed along with a small portion of the substrate upon which they occurred, allowed to air dry, and then placed in small pasteboard boxes for permanent storage. All specimens cited herein are now deposited in the mycological herbarium (UARK) at the University of Arkansas.
Results
Of the 33 moist chamber cultures, 29 (88%) yielded some evidence (plasmodia or fruiting bodies) of myxomycetes. A total of 12 species representing six genera were recorded. Physarum compressum was the most common species (appearing in 20 of the 33 cultures). This species sometimes produced more than 100 fruiting bodies in a single moist chamber culture. Other species represented by five or more specimens were Licea succulenticola (seven specimens), Perichaena depressa (seven specimens) and Badhamia melanospora (six specimens). Five of the species recorded were represented by a single specimen. The mean pH determined for the 33 moist chamber cultures was 8.2, with a maximum of 8.6 and a minimum of 7.1.
Myxomycetes were recorded on all three of the species of cacti included in our study and for all but one site. The two cultures prepared with samples from Site 4 produced no evidence of myxomycetes.
Species recorded from moist chamber cultures
In the species list below, all taxa of myxomycetes recorded in the present study are listed alphabetically by genus and then species. The nomenclature used follows Lado (2005–2022). Information relating to the typical habitat (or substrate) given for each species is taken from Martin and Alexopoulos (1969). Details about the collection sites and comments on the distribution and ecology of each species is also provided in the list.
Badhamia melanospora Speg. (Fig. 2a)
Substrate
Dead stems of Yucca and cacti, also occasionally on bark.
Specimens
Six specimens (including 34 448, 34 452 and 34 476) recorded from Sites 1, 2, 6 and 8. These specimens were associated with H. martinii and O. tomentosa.
Comments
The association of B. melanospora with cacti and other succulent plants is well documented. This species was reported to be the most common myxomycete in the Sonoran Desert of the southwestern United States (Blackwell and Gilbertson 1980), in arid and semi-arid areas of the Tehuacán-Cuicatlán Valley Biosphere Reserve in Mexico (Estrada-Torres et al. 2009), and the Monte Desert of Argentina (Lado et al. 2011). Although typically associated with various species of cacti, B. melanospora also frequently occurs on other succulent plants such as Agave, Yucca and Puya (Lado et al. 2013). Puya is a succulent bromeliad that is widespread in the Andes Mountains in western South America. However, B. melanospora is not considered to be a ‘true’ succulenticolous myxomycete because it also occurs on other non-succulent substrates, including dung (Blackwell and Gilbertson 1980). The species appears to have a limited distribution in Australia, with only two previous records (Stephenson 2021). It should be noted that in earlier taxonomic treatments, this species is listed as B. gracilis.
Didymium anellus Morgan
Substrate
Dead leaves, herbaceous stems and twigs, rarely on wood.
Specimen
One specimen (34 451) recorded from Site 8. This specimen was associated with O. tomentosa.
Comments
Although there are reports of D. anellus from desert habitats (e.g. Lado et al. 2011; Wrigley de Basanta et al. 2013; Stephenson et al. 2019), the substrates upon which fruiting occurred include only a few succulent plants [Puya as reported by Lado et al. (2011)]. It has been reported from scattered localities within Australia (Stephenson 2021; Stephenson et al. 2022).
Didymium iridis (Ditmar) Fr.
Substrate
Dead leaves, mosses, twigs and dead wood; occasionally on old dung of herbivores.
Specimen
One (34 497) specimen recorded from Site 1. This specimen was associated with O. tomentosa.
Comments
Didymium iridis, like the preceding species, has been reported from surveys of deserts (e.g. Estrada-Torres et al. 2009; Wrigley de Basanta et al. 2013; Stephenson et al. 2019) but most of the substrates on which it occurred were derived from non-succulent plants. The species has been reported from a range of habitats in Australia (White et al. 2020; Stephenson 2021).
Didymium ochroideum G. Lister
Substrate
Dead leaves and herbaceous stems, mosses and dung of herbivores.
Specimen
One specimen (34 449) recorded from Site 5. This specimen was associated with O. tomentosa.
Comments
Estrada-Torres et al. (2009) recorded this species on dead cacti, while Wrigley de Basanta et al. (2013) reported it on the litter of Agave and Euphorbia, both of which are succulent plants. However, there appear to be few records of this relatively uncommon species from deserts, and there is no indication that it has any affinity for succulent plants. Didymium ochroideum appears to have a limited distribution within Australia, with only three previous reports (Stephenson 2021).
Didymium squamulosum (Alb. & Schwein.) Fr. & Palmquist
Substrate
Dead plant remains of all sorts and on herbivore dung.
Specimens
One specimen (34 546) from Site 9. This specimen was associated with O. stricta.
Comments
There are a number of reports of D. squamulosum on dead portions of succulent plants (e.g. Estrada-Torres et al. 2009; Lado et al. 2011, 2019), but this species also commonly occurs on non-succulent substrates (e.g. Lado et al. 2013) in desert habitats. Its distribution within Australia is scattered but widespread (Stephenson 2021).
Licea succulenticola Mosquera, Lado, Estrada & Beltrán-Tej. (Fig. 2b)
Substrate
Decaying succulent plant parts from species of Agave, Euphorbia and Opuntia along with the stems of plants such as Yucca.
Specimens
Seven specimens (including 34 487 and 34 521) recorded from Sites 1, 7 and 8. These specimens were associated with H. martinii and O. tomentosa.
Comments
Licea succulenticola was described as new to science by Mosquera et al. (2003) based on material collected on the Canary Islands. It has since been reported from a number of other desert localities where it is often abundant, including Mexico (Estarda-Toress et al. 2009), Argentina (Lado et al. 2011) and Peru (Lado et al. 2019). Although the most common substrates for this species are dead portions of succulent plants, it also occurs on bark, as was the case for the first report from mainland Africa (Stephenson et al. 2019). Licea succulenticola was first reported from Australia by Stephenson et al. (2020), where it was collected from the dead outer bark of Eucalypus coolabah. Interestingly, four of the seven specimens recorded in the present study were all collected at the same site (Site 8, located near the town of Thallon in Queensland).
Perichaena depressa Lib. (Fig. 2c)
Substrate
Dead bark and wood, occasionally also on leaves and other plant debris, and on herbivore dung.
Specimens
Seven specimens (including 34 528 and 34 530) recorded from Sites 5, 7, 8 and 9. These specimens were associated with O. stricta and O. tomentosa.
Comments
The occurrence of P. depressa on dead portions of succulent plants has been reported in several studies (Estrada-Torres et al. 2009; Lado et al. 2011, 2019; Wrigley de Basanta et al. 2013). However, the species has been recorded from a wide range of substrates and does not appear to have a preference for succulent substrates. The species is widely distributed in Australia (Stephenson 2021; Stephenson et al. 2022).
Perichaena luteola (Kowalski) Gilert
Substrate
Indicated as cow dung in the original description but since reported from dead portions of succulent plants.
Specimens
Two specimens (34 547 and 34 639) recorded from Sites 5 and 6. This specimen was associated with O. tomentosa.
Comments
This species was originally described as Calonema luteolum by Kowalski (1969) from material collected on cow dung. Gilert (1995) moved the species to the genus Perichaena based on features of the capillitium. Estrada-Torres et al. (2009) found it to be relatively common on dead Opuntia, and Wrigley de Basanta et al. (2013) recorded the species from an Opuntia sp. in Madagascar. In the original description, Kowalski gave the spore size as 12–13 µm, but Estrada-Torres et al. (2009) indicated that spores were 10–12.5 µm in their specimens. This was also the size in the collection recorded in the present study. Perichaena luteola is a new record for Australia.
Physarum compressum Alb. & Schwein. (Fig. 2d)
Substrate
Dead leaves and other plant debris.
Specimens
Twenty specimens (including 34 454, 34 458 and 34 465) recorded from Sites 1, 3, 5, 6 and 8. These specimens were associated with H. martinii and O. tomentosa.
Comments
Estrada-Torres et al. (2009), Lado et al. (2011) and Lado et al. (2013) reported P. compressum from dead portions of succulents, but in these and other studies (e.g. Stephenson et al. 2019), this species also has been commonly associated with non-succulent substrates. Its distribution within Australia is scattered but widespread (Stephenson 2021; Stephenson et al. 2022).
Physarum leucophaeum Fr. & Palmquist
Substrate
Dead wood and leaves.
Specimens
One specimen (34 466) recorded from Site 6. This specimen was associated with O. tomentosa.
Comments
Blackwell and Gilbertson (1980) recorded P. leucophaeum from cacti, but specimens of this species were associated with non-succulent substrates in other surveys that included at least some desert areas (e.g. Lado et al. 2013; Wrigley de Basanta et al. 2013; Stephenson et al. 2019). Its distribution within Australia is scattered but widespread (Stephenson 2021).
Physarum pusillum (Berk. and M.A. Curtis) G. Lister
Substrate
Dead leaves and herbaceous litter, often on compost.
Specimens
Two specimens (34 470 and 34 473) recorded from Sites 6 and 8. This species was associated with O. tomentosa.
Comments
Several studies (e.g. Estrada-Torres et al. 2009; Lado et al. 2011; Stephenson et al. 2019) have recorded P. pusillum on dead portions of succulent plants; in others (e.g. Lado et al. 2013; Wrigley de Basanta et al. 2013) this species occurred only on non-succulent substrates. The species is widely distributed in Australia (Stephenson 2021).
Stemonitis flavogenita E. Jahn
Substrate
Dead wood and plant debris.
Specimens
Two specimens (34 527 and 34 536) recorded from Sites 5 and 9. These specimens were associated with O. stricta and O. tomentosa.
Comments
Wrigley de Basanta et al. (2013) recorded S. flavogenita on the litter of Agave and Aloe, both of which are succulents. However, there appear to be no other reports of this species from succulent plants. Its distribution within Australia is scattered but widespread (Stephenson 2021; Stephenson et al. 2022).
Discussion
The data obtained in the present study suggest that the species richness of myxomycetes associated with cacti in Australia is somewhat lower than reported for other regions of the world (Blackwell and Gilbertson 1980). However, our study covers a smaller geographic area to which cacti are not native, and we did not have teams of three or more investigators (as was the case in the following studies: Lado et al. 2007a, 2019; Estrada-Torres et al. 2009; Wrigley de Basanta et al. 2013; Stephenson et al. 2019).
All species recorded in the present study have been reported from succulent plant substrates in other regions of the world, but most of these species can also be associated with other non-succulent substrates. The only exception is L. succulenticola, which is a ‘true’ succulenticolous myxomycete, although P. compressum and especially B. melanospora are also commonly associated with succulent plants in deserts. For all other species reported herein, the association with succulents appears to be incidental.
The occurrence data we obtained were derived solely from moist chamber cultures, and it is possible that a field survey could increase the number of myxomycete species recorded. Some species of myxomycetes do not necessarily appear in moist chamber cultures (Martin and Alexopoulos 1969; Stephenson and Stempen 1994). Our study indicates that succulenticolous myxomycetes are poorly represented in Australia. This may be due to the low diversity of native succulent plants. However, more extensive survey efforts that include more populations of cacti and encompass a larger geographic area may uncover other regions of Australia where succulenticolous myxomycetes are present and/or more abundant and diverse (Stephenson 2021).
Data availability
Any additional data are available upon reasonable request from the authors.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
TFE received funding for the fieldwork of this project from a Holsworth Wildlife Research Endowment Grant from the Ecological Society of Australia. The School of Environmental and Rural Science at the University of New England provided TFE with an international post graduate research fellowship and a 4WD vehicle to travel to the field sites as part of a larger project.
Acknowledgements
We appreciate field assistance provided by Kelsey Elliott and Karl Vernes. Kelsey Elliott provided helpful editorial comments on this manuscript, and Arturo Estrada-Torres confirmed the identification of P. luteola. Thanks are extended to Yuri Novozhilov and Alison Pollack for contributing the images used in Fig. 2.
References
Blackwell M, Gilbertson RL (1980) Sonoran desert myxomycetes. Mycotaxon 11, 139–149.Crotty FV, Adl SM, Blackshaw RP, Murray PJ (2012) Using stable isotopes to differentiate trophic feeding channels within soil food webs. Journal of Eukaryotic Microbiology 59, 520–526.
| Using stable isotopes to differentiate trophic feeding channels within soil food webs.Crossref | GoogleScholarGoogle Scholar |
Domico T (2018) ‘The Great Cactus War: true story of the greatest plant invasion in history.’ (Turtleback Books: Sydney, NSW)
Eliasson UH (1991) The myxomycete biota of the Hawaiian Islands. Mycological Research 95, 257–267.
| The myxomycete biota of the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Estrada-Torres A, Wrigley de Basanta D, Conde E, Lado C (2009) Myxomycetes associated with dryland ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico. Fungal Diversity 36, 17–56.
Ferreira IN Ferreira IN (2011) Myxomycetes associados a cactáceas no agreste e sertão de Pernambuco, Brasil. Biotemas 24, 1–11.
| Myxomycetes associados a cactáceas no agreste e sertão de Pernambuco, Brasil.Crossref | GoogleScholarGoogle Scholar |
Frawley J (2007) Prickly pear land: transnational networks in settler Australia. Australian Historical Studies 38, 323–338.
| Prickly pear land: transnational networks in settler Australia.Crossref | GoogleScholarGoogle Scholar |
Gilert E (1995) Taxonomic evaluation of the myxomycete Calonema luteolum. Mycological Research 99, 311–316.
| Taxonomic evaluation of the myxomycete Calonema luteolum.Crossref | GoogleScholarGoogle Scholar |
Kowalski DT (1969) A new coprophilous species of Calonema (Myxomycetes). Madrono 20, 229–231.
Lado C (2005–2022) An on-line nomenclatural information system of Eumycetozoa. Available at http://www.eumycetozoa.com [Accessed 20 December 2021]
Lado C, Mosquera J, Beltrán-Tejera E (1999) Cribraria zonatispora, development of a new myxomycete with unique spores. Mycologia 91, 157–165.
| Cribraria zonatispora, development of a new myxomycete with unique spores.Crossref | GoogleScholarGoogle Scholar |
Lado C, Estrada-Torres A, Stephenson SL (2007a) Myxomycetes collected in the first phase of a north-south transect of Chile. Fungal Diversity 25, 81–101.
Lado C, Mosquera J, Estrada-Torres A, Beltrán-Tejera E, Wrigley de Basanta D (2007b) Description and culture of a new succulenticolous Didymium (Myxomycetes). Mycologia 99, 602–611.
| Description and culture of a new succulenticolous Didymium (Myxomycetes).Crossref | GoogleScholarGoogle Scholar |
Lado C, Wrigley de Basanta D, Estrada-Torres A, García Carvajal E, Aguilar M, Hernández-Crespo JC (2009) Description of a new species of Perichaena (Myxomycetes) from arid areas of Argentina. Anales del Jardín Botánico de Madrid 66, 63–70.
| Description of a new species of Perichaena (Myxomycetes) from arid areas of Argentina.Crossref | GoogleScholarGoogle Scholar |
Lado C, Wrigley de Basanta D, Estrada-Torres A (2011) Biodiversity of Myxomycetes from the Monte Desert of Argentina. Anales del Jardín Botánico de Madrid 68, 61–95.
| Biodiversity of Myxomycetes from the Monte Desert of Argentina.Crossref | GoogleScholarGoogle Scholar |
Lado C, Wrigley de Basanta D, Estrada-Torres A, Stephenson SL (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59, 3–32.
| The biodiversity of myxomycetes in central Chile.Crossref | GoogleScholarGoogle Scholar |
Lado C, Wrigley de Basanta D, Estrada-Torres A, Stephenson SL, Treviño I (2019) Diversity of Myxomycetes in arid zones of Peru part II: the cactus belt and transition zones. Anales del Jardín Botánico de Madrid 76, e083
| Diversity of Myxomycetes in arid zones of Peru part II: the cactus belt and transition zones.Crossref | GoogleScholarGoogle Scholar |
Martin GW, Alexopoulos CJ (1969) ‘The Myxomycetes.’ (University of Iowa Press: Iowa City, USA)
Mosquera J, Lado C, Estrada-Torres A, Beltrán Tejera E, Wrigley de Basanta D (2003) Description and culture of a new myxomycete, Licea succulenticola. Anales del Jardín Botánico de Madrid 60, 3–10.
| Description and culture of a new myxomycete, Licea succulenticola.Crossref | GoogleScholarGoogle Scholar |
Nature (1933) Eradication of prickly pear in Australia. Nature 131, 613
| Eradication of prickly pear in Australia.Crossref | GoogleScholarGoogle Scholar |
Ndiritu GG, Spiegel FW, Stephenson SL (2009) Distribution and ecology of the assemblages of myxomycetes associated with major vegetation types in Big Bend National Park, USA. Fungal Ecology 2, 168–183.
| Distribution and ecology of the assemblages of myxomycetes associated with major vegetation types in Big Bend National Park, USA.Crossref | GoogleScholarGoogle Scholar |
Osmond CB, Nott DL, Firth PM (1979) Carbon assimilation patterns and growth of the introduced CAM plant Opuntia inermis in Eastern Australia. Oecologia 40, 331–350.
| Carbon assimilation patterns and growth of the introduced CAM plant Opuntia inermis in Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Rollins AW, Stephenson SL (2011) Global distribution and ecology of myxomycetes. Current Topics in Plant Biology 12, 1–14.
Stephenson SL (1988) Distribution and ecology of Myxomycetes in temperate forests. I. Patterns of occurrence in the upland forests of southwestern Virginia. Canadian Journal of Botany 66, 2187–2207.
| Distribution and ecology of Myxomycetes in temperate forests. I. Patterns of occurrence in the upland forests of southwestern Virginia.Crossref | GoogleScholarGoogle Scholar |
Stephenson SL (2009) First records of myxomycetes from Ascension Island. Sydowia 61, 105–115.
Stephenson SL (2021) ‘Secretive slime moulds: Myxomycetes of Australia.’ (CSIRO Publishing: Australia)
Stephenson SL, Stempen H (1994) ‘Myxomycetes: a handbook of slime molds.’ (Timber Press: Portland, Oregon, USA)
Stephenson SL, Wrigley de Basanta D, Lado C, Estrada-Torres A, Darrah R (2019) Myxomycete biodiversity revealed in the Namib desert. South African Journal of Botany 124, 402–413.
| Myxomycete biodiversity revealed in the Namib desert.Crossref | GoogleScholarGoogle Scholar |
Stephenson SL, Kaur G, Payal N, Elliott TF, Vernes K (2020) Myxomycetes associated with arid habitats in northeastern South Australia. Transactions of the Royal Society of South Australia 144, 139–153.
| Myxomycetes associated with arid habitats in northeastern South Australia.Crossref | GoogleScholarGoogle Scholar |
Stephenson SL, Elliott TF, Elliott K, Vernes K (2022) Myxomycetes associated with Australian vertebrate dung. Australian Zoologist
| Myxomycetes associated with Australian vertebrate dung.Crossref | GoogleScholarGoogle Scholar |
White MA, Elliott TF, Kennedy BPA, Stephenson SL (2020) First records of myxomycetes from Bathurst Island (one of the Tiwi Islands) in the Northern Territory, Australia. Austral Ecology 45, 1183–1187.
| First records of myxomycetes from Bathurst Island (one of the Tiwi Islands) in the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Wrigley de Basanta D, Lado C, Estrada-Torres A (2008) Morphology and life cycle of a new species of Didymium (Myxomycetes) from arid areas of Mexico. Mycologia 100, 921–929.
| Morphology and life cycle of a new species of Didymium (Myxomycetes) from arid areas of Mexico.Crossref | GoogleScholarGoogle Scholar |
Wrigley de Basanta D, Lado C, Estrada-Torres A, Stephenson SL (2009) Description and life cycle of a new Didymium (Myxomycetes) from arid areas of Argentina and Chile. Mycologia 101, 707–716.
| Description and life cycle of a new Didymium (Myxomycetes) from arid areas of Argentina and Chile.Crossref | GoogleScholarGoogle Scholar |
Wrigley de Basanta D, Lado C, Estrada-Torres A, Stephenson SL (2013) Biodiversity studies of myxomycetes in Madagascar. Fungal Diversity 59, 55–83.
| Biodiversity studies of myxomycetes in Madagascar.Crossref | GoogleScholarGoogle Scholar |


