Nickel distribution in Stackhousia tryonii shown by synchrotron X-ray fluorescence micro-computed tomography
Antony van der Ent A * , Kathryn M. Spiers
A * , Kathryn M. Spiers  B , Dennis Brueckner B C D and Peter D. Erskine A
B , Dennis Brueckner B C D and Peter D. Erskine A
A Centre for Mined Land Rehabilitation, Sustainable Minerals Institute, The University of Queensland, Saint Lucia, Qld 4072, Australia.
B Deutsches Elektronen-Synchrotron DESY, 22607 Hamburg, Germany.
C Department of Physics, Universität Hamburg, 20355 Hamburg, Germany.
D Faculty of Chemistry and Biochemistry, Ruhr-Universität Bochum, 44801 Bochum, Germany.
Australian Journal of Botany 70(4) 304-310 https://doi.org/10.1071/BT22012
Submitted: 4 February 2022 Accepted: 30 May 2022 Published: 13 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Hyperaccumulator plants are of considerable interest for their extreme physiology. Stackhousia tryonii is a nickel (Ni) hyperaccumulator plant endemic to ultramafic outcrops in Queensland (Australia) capable of attaining up to 41 300 μg g−1 foliar Ni.
Aims: This study sought to elucidate the distribution of Ni in S. tryonii by using synchrotron X-ray fluorescence micro-computed tomography (XFM-CT), complemented with elemental maps acquired from physically sectioned plant organs. Its Ni-enriched cylindrical photosynthetic stems make them particularly well suited samples for synchrotron XFM-CT.
Methods: XFM-CT enables ‘virtual sectioning’ of a sample, avoiding artefacts arising from physical sample preparation. The method can be used on fresh samples that are frozen during the analysis, which preserves ‘life-like’ conditions by limiting radiation damage. It also prevents/minimises other artefacts.
Key results: The results showed that Ni is mainly concentrated in the apoplastic space surrounding epidermal cells, and in some epidermal cell vacuoles. This finding is significant because this ‘free’ solute Ni is likely to be lost during physical sectioning.
Conclusions and implications: This case study has highlighted the utility of the XFM-CT approach for visualising metals within intact plant organs, which may be used across the plant sciences.
Keywords: apoplastic space, artefact, hyperaccumulator, nickel, Queensland, sectioning, synchrotron, X-ray fluorescence micro-computed tomography.
Introduction
Hyperaccumulators are unusual plants that accumulate metals or metalloids in their living tissues to orders of magnitude greater concentrations than other plants growing in similar soils (van der Ent et al. 2013). Trace element hyperaccumulation is rather rare, with only ∼700 hyperaccumulator species identified globally to date (Reeves 2003; Reeves et al. 2018). Stackhousia tryonii (Celastraceae) is an herbaceous nickel (Ni) hyperaccumulator endemic to ultramafic outcrops in Queensland, Australia (Batianoff et al. 1990; Bhatia et al. 2005b). It was discovered from a test of field specimens using dimethylglyoxime-impregnated paper (Fig. 1). It is the only species in the genus Stackhousia known to hyperaccumulate and can attain up to ∼40 000 μg g−1 Ni (i.e. 4 wt%) in its leaves (Batianoff et al. 1990; Burge and Barker 2010; van der Ent et al. 2015). In S. tryonii, the dominant ligands in the aqueous shoot extract is a mixture of carboxylic acids, especially malate (Bidwell 2001; Bhatia et al. 2005a), which aligns with many other (tropical) Ni-hyperaccumulator species in which Ni-citrate is the main complex (for example, van der Ent et al. 2017). Particle-induced X-ray emission (PIXE) analysis has shown that Ni is primarily localised in the epidermal cells and also in vascular tissue in the leaves and stems of S. tryonii (Bhatia et al. 2003, 2004).
Synchrotron X-ray fluorescence microscopy (XFM)-based techniques are capable of elucidating the distribution of metals and metalloids from the whole plant down to tissue and cellular level (Kopittke et al. 2018, 2020). However, one of the major challenges with XFM is sample preparation. Appropriate sample preparation is difficult to achieve without causing artefacts that can modify internal structures and elemental composition (van der Ent et al. 2018). Typically, physical sectioning is performed to visualise the internal distribution of metals/metalloids in tissues, cells and plant organs, such as roots or leaves. However, this has the potential to cause elemental deportment and redistribution. Synchrotron X-ray fluorescence microscopy computed tomography (XFM-CT) avoids the need for making physical thin sections and eliminates these sectioning artefacts by producing ‘virtual’ sections of a specimen from much larger plant organs (de Jonge and Vogt 2010; van der Ent et al. 2018). XFM-CT can be used on frozen-hydrated samples to preserve ‘life-like’ conditions and avoid radiation damage (Jones et al. 2020). In hyperaccumulator plants, for example, XFM-CT has been applied to show Ni in Odontarrhena chalcidica (formerly Alyssum murale) (McNear et al. 2005), thallium in Iberis linifolia (formerly I. intermedia) (Scheckel et al. 2007) and arsenic in Pteris vittata (van der Ent et al. 2020). There remains much potential for this method to be used on both native and cultured plants to show the cellular-level distribution of a wide range of trace elements. Stackhousia tryonii has reduced leaves and photosynthetic stems, and the spherical nature and small diameter (1–3 mm) of these organs make them well suited for XFM-CT. This study aimed to acquire XFM-CT data of S. tryonii and compare this with physically sectioned specimens and published data on elemental distribution in this species.
Materials and methods
Plant material and specimen preparation for XFM analysis
Plant stock, in the form of fresh stems, and soil were collected from a roadside close to the Bruce Highway (near the Eden Bann turnoff at −23.093298, 150.275231) north of Rockhampton, Queensland, Australia (Fig. 1). The stems were rooted as cuttings with rooting hormone gel (Clonex Red, Yates, contains 8 g L−1 indole-3-butryic acid) in perlite–vermiculite mix, and subsequently planted in the natural ultramafic soil collected from the habitat. Plants were grown to mature size over 6 months in the glasshouse in Brisbane, and fresh plant material was brought to DESY in Germany for the XFM analysis described below. Sections were prepared from the root and the stem by hand-cutting with a stainless-steel razor blade (utilizing the ‘dry knife method’) and the sections were immediately mounted between two sheets of 4 μm Ultralene thin film in a tight sandwich to limit evaporation. They were analysed (at room temperature) within 20 min. after excision. An intact leaflet was excised and similarly mounted and analysed. The stem specimen for XFM-CT was mounted inside a Kapton capillary tube and analysed in frozen-hydrated state under a liquid nitrogen cryostream (operated at −140°C).
X-ray fluorescence microscopy (XFM) and data analysis
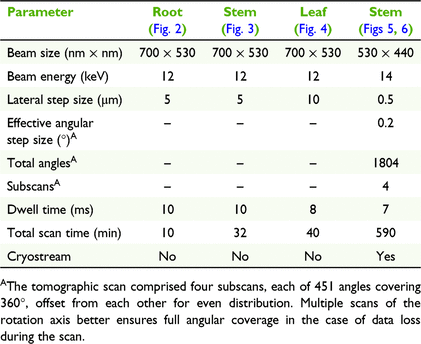
The X-ray fluorescence microscopy experiments were undertaken at PETRA III (Deutsches Elektronen-Synchrotron; DESY), a 6 GeV synchrotron radiation source, specifically at the hard X-ray microprobe experiment at the undulator beamline P06. P06 is equipped with a cryogenically cooled double-crystal monochromator with Si(111) crystals. For these experiments, a KB mirror pair was used to focus the X-ray beam to 700 × 530 nm or 530 × 440 nm, resulting in flux on the sample in the order of 1010 photon s−1. An incident X-ray energy of 12 or 14 keV was used for these experiments (full details on scan parameters are given in Table 1). The modalities utilised were standard 2D (x–y) planar and 2D (x–θ) single-slice tomography (CT) scans. Located upstream of the sample position was the 384-element Maia detector array system (Model C) in backscattering geometry, for fast and efficient acquisition of X-ray fluorescence data (Kirkham et al. 2010; Siddons et al. 2014). The XRF event stream was analysed using the Dynamic Analysis method (Ryan and Jamieson 1993; Ryan 2000) as implemented in GeoPIXE (Ryan et al. 1990). The tomographic data were aligned using consistency and cross-correlation methods to correct for unwanted sample movement. The abundant angular sampling and statistics of the tomographic data allowed the use of the time-efficient filtered back-projection (FBP) method for reconstruction (Bruyant 2002) without suffering from too low signal-to-noise ratio.
|
|
|
|
|
|
|
|
|
Results and discussion
The physical cross-section of a fresh-hydrated root shows that potassium (K) is enriched in the cortex, and the distribution of Ni has a similar distribution (Fig. 2). Physical cross-sections of fresh-hydrated stems shows K concentrations in the epidermal cell layer and in the cortex surrounding the pith, whereas calcium (Ca) occurs in the epidermal area and Ni is located in the epidermal cells (Fig. 3). Planar 2D elemental maps of a leaf blade show K enrichment towards the apex, Ca concentration to be very low in the leaf blade and indicate that Ni concentration is lowest towards the apex and highest in the central part of the blade, with distinct enrichment along the margins (Fig. 4). The XFM-CT reconstructions through the stem (Fig. 5) showed that Ni is mainly concentrated in the apoplastic space surrounding epidermal cells, and in the vacuoles of some epidermal cells. The Ni localisation in the apoplastic space would likely have been disturbed (because of losses of Ni from sectioning) if commonly used physical sample-sectioning techniques had been employed; however, this situation has been avoided here by using the XFM-CT approach. The Compton scatter and Absorption maps clearly show the sclerenchyma rays that provide structural support, epidermal cells surrounding the spongy mesophyll and the vacuolar bundles and xylem in the pith. These structures are all extremely delicate and would likely not survive physical sectioning (compare with the 2D physical section in Fig. 3, in which this detail is lost). It should be noted that the 2D physical stem section was scanned with a step-size of 5 μm, which, although significantly larger than the beamsize, enables fast scans of fresh samples. In contrast, the XFM-CT single-slice image of the frozen-hydrated stem piece has an order of magnitude higher resolution, having been performed with a lateral step-size of 0.5 μm (closer to the beamsize) and angular spacing of 0.2° in four interleaved subscans with 0.8° step size each.
Comparing the elemental maps of the physical stem cross-sections (Fig. 2) with the XFM-CT reconstructions (Fig. 5) shows that, overall, the distribution of Ni is similar, with distinct enrichment in the epidermal apoplastic space. However, in the 2D physical section, it cannot be seen whether Ni is present in the apoplast (as is clear from the XFM-CT data) or in the vacuoles of the epidermal cells. Even though great care was undertaken to limit elemental losses by dry-cutting and immediate cryo-fixation of the stem sections (followed by freeze-drying), the Ni elemental maps obtained by Bhatia et al. (2004) were very different from the 2D and XFM-CT data shown here. The earlier micro-PIXE-based study showed Ni enrichment in the broader epidermal area and around vascular tissues. In part this can be attributed to the ostensibly different sensitivity of the methods used (although this is not necessarily a limitation of PIXE, but rather of the experimental setup, detectors used, etc.), with relatively poor pixel statistics compared with the synchrotron XFM data. The XFM data presented here has megapixel definition and the use of the Maia detector-array results in exceptional detection sensitivity. There is also a distinct possibility that there are differences in the distribution of Ni within cell types depending on the age of the stem and the prevailing level of Ni accumulation. It is conceivable that in younger stems, or at lower or higher Ni concentrations, distribution differs among storage sites, with allocation in the apoplastic space or epidermal vacuoles. This might also (partly) explain the differences in the results of this study and those of Bhatia et al. (2004).
Of interest is that Bhatia et al. (2004) tested sections that were frozen and freeze–dried after sectioning, and sections that were soaked in water. The latter lost nearly all K and 88% of Ni, pointing to the great risks involved with sample preparation to introduce catastrophic artefacts. It is therefore highly likely that sectioning under liquid (water), as is common when using a vibratome, would have led to very substantial losses of Ni and other elements from our specimens. This will confound the data obtained from subsequent elemental mapping of these tissues, because those elements would have been lost from the specimen before the analysis even takes place. Of course, a fully cryogenic pathway in which specimens are (flash) frozen, cryo-microtomed and subsequently analysed with XFM in this frozen state, would minimise or eliminate such problems. However, it is extremely challenging to prepare and cryo-transfer specimens for XFM analysis, and the required equipment for doing so at synchrotron facilities, for samples of millimetre dimensions and larger, while of significant interest, is not currently widely available. The main limitations of the XFM-CT technique are its highly restrictive availability at synchrotron facilities. Furthermore, the method is limited by the size of the specimen that can be examined, as absorption of the escaping fluorescence X-rays is dependent on their energy and, hence, chemical element in question. Elements with a higher K-absorption edge energy (>7 keV), such as Ni, Zn, As, Se, are therefore best suited for tomographic analysis of plant organs up to ∼2 mm in diameter, such as roots, stems, and seeds (van der Ent et al. 2018).
This study on S. tryonii has provided an example of how the XFM-CT approach can be used to visualise metals within intact plant organs while minimising sample preparation artefacts. The methodology described here is applicable to plant species and plant organs for a wide range of metals and metalloids. We hope that more plant scientists will use this technology, which is available at many synchrotron facilities, including at the Australian Synchrotron, to visualise metals and metalloids. This technology is not limited to hyperaccumulators, but could equally be used to probe elements of interest in a wide range of wild and crop plants.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest relevant to the content of this manuscript.
Declaration of funding
The research leading to this result has been supported by the project CALIPSOplus under the Grant Agreement 730872 from the EU Framework Programme for Research and Innovation HORIZON 2020.
Author contributions
PDE collected the plant material and AVDE cultivated the plants and prepared the samples for analysis. KMS, DB and AVDE conducted the synchrotron experiment. DB and KMS processed the synchrotron data. All authors contributed to writing the manuscript.
Acknowledgements
We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were conducted at PETRA III and we thank Gerald Falkenberg and Jan Garrevoet for assistance in using P06. Beamtime was allocated for proposal I-20190028, and as part of inhouse research (K.M.S).
References
Batianoff GN, Specht RL (1992) Queensland (Australia) serpentinite vegetation. In ‘The vegetation of ultramafic (serpentine) soils’. (Eds J Proctor, AJM Baker, RD Reeves) pp. 109–128. (Intercept Ltd: Andover, UK)Batianoff GN, Reeves RD, Specht RL (1990) Stackhousia tryonii Bailey: a nickel-accumulating serpentine-endemic species of Central Queensland. Australian Journal of Botany 38, 121–130.
| Stackhousia tryonii Bailey: a nickel-accumulating serpentine-endemic species of Central Queensland.Crossref | GoogleScholarGoogle Scholar |
Bhatia NP, Orlic I, Siegele R, Ashwath N, Baker AJM, Walsh KB (2003) Elemental mapping using PIXE shows the main pathway of nickel movement is principally symplastic within the fruit of the hyperaccumulator Stackhousia tryonii. New Phytologist 160, 479–488.
| Elemental mapping using PIXE shows the main pathway of nickel movement is principally symplastic within the fruit of the hyperaccumulator Stackhousia tryonii.Crossref | GoogleScholarGoogle Scholar | 33873657PubMed |
Bhatia NP, Walsh KB, Orlic I, Siegele R, Ashwath N, Baker AJM (2004) Studies on spatial distribution of nickel in leaves and stems of the metal hyperaccumulator Stackhousia tryonii Bailey using nuclear microprobe (micro-PIXE) and EDXS techniques. Functional Plant Biology 31, 1061–1074.
| Studies on spatial distribution of nickel in leaves and stems of the metal hyperaccumulator Stackhousia tryonii Bailey using nuclear microprobe (micro-PIXE) and EDXS techniques.Crossref | GoogleScholarGoogle Scholar | 32688974PubMed |
Bhatia NP, Walsh KB, Baker AJM (2005a) Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey. Journal of Experimental Botany 56, 1343–1349.
| Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey.Crossref | GoogleScholarGoogle Scholar | 15767321PubMed |
Bhatia NP, Baker AJM, Walsh KB, Midmore DJ (2005b) A role for nickel in osmotic adjustment in drought-stressed plants of the nickel hyperaccumulator Stackhousia tryonii Bailey. Planta 223, 134–139.
| A role for nickel in osmotic adjustment in drought-stressed plants of the nickel hyperaccumulator Stackhousia tryonii Bailey.Crossref | GoogleScholarGoogle Scholar | 16200406PubMed |
Bidwell SD (2001) Hyperaccumulation of metals in Australian native plants. PhD Thesis, University of Melbourne, Vic., Australia.
Bruyant PP (2002) Analytic and iterative reconstruction algorithms in SPECT. Journal of Nuclear Medicine 43, 1343–1358.
Burge D, Barker WR (2010) Evolution of nickel hyperaccumulation by Stackhousia tryonii (Celastraceae), a serpentinite-endemic plant from Queensland, Australia. Australian Systematic Botany 23, 415–430.
de Jonge MD, Vogt S (2010) Hard X-ray fluorescence tomography-an emerging tool for structural visualization. Current Opinion in Structural Biology 20, 606–614.
Jones MWM, Kopittke PM, Casey L, Reinhardt J, Blamey FPC, van der Ent A (2020) Assessing radiation dose limits for X-ray fluorescence microscopy analysis of plant specimens. Annals of Botany 125, 599–610.
| Assessing radiation dose limits for X-ray fluorescence microscopy analysis of plant specimens.Crossref | GoogleScholarGoogle Scholar | 31777920PubMed |
Kirkham R, Dunn PA, Kuczewski AJ, Siddons DP, Dodanwela R, Moorhead GF, Ryan CG, De Geronimo G, Beuttenmuller R, Pinelli D, Pfeffer M, Davey P, Jensen M, Paterson DJ, de Jonge MD, Howard DL, Küsel M, McKinlay J (2010) The Maia Spectroscopy Detector System: Engineering for Integrated Pulse Capture, Low-Latency Scanning and Real-Time Processing. AIP Conference Proceedings 1234, pp. 240–243.
| Crossref |
Kopittke PM, Punshon T, Paterson DJ, Tappero RV, Wang P, Blamey FPC, van der Ent A, Lombi E (2018) Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiology 178, 507–523.
Kopittke PM, Lombi E, van der Ent A, Wang P, Laird JS, Moore KL, Persson DP, Husted S (2020) Methods to visualize elements in plants. Plant Physiology 182, 1869–1882.
McNear DH, Peltier E, Everhart J, Chaney RL, Sutton S, Newville M, Rivers M, Sparks DL (2005) Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale. Environmental Science & Technology 39, 2210–2218.
| Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale.Crossref | GoogleScholarGoogle Scholar |
Reeves RD (2003) Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant and Soil 249, 57–65.
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist 218, 407–411.
| A global database for plants that hyperaccumulate metal and metalloid trace elements.Crossref | GoogleScholarGoogle Scholar | 29139134PubMed |
Ryan CG (2000) Quantitative trace element imaging using PIXE and the nuclear microprobe. International Journal of Imaging Systems and Technology 11, 219–230.
Ryan CG, Jamieson DN (1993) Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 77, 203–214.
Ryan CG, Cousens DR, Sie SH, Griffin WL (1990) Quantitative analysis of PIXE spectra in geoscience applications. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 49, 271–276.
Scheckel KG, Hamon R, Jassogne L, Rivers M, Lombi E (2007) Synchrotron X-ray absorption-edge computed microtomography imaging of thallium compartmentalization in Iberis intermedia. Plant and Soil 290, 51–60.
| Synchrotron X-ray absorption-edge computed microtomography imaging of thallium compartmentalization in Iberis intermedia.Crossref | GoogleScholarGoogle Scholar |
Siddons DP, Kirkham R, Ryan CG, De Geronimo G, Dragone A Siddons DP, Kirkham R, Ryan CG, De Geronimo G, Dragone A (2014) Maia X-ray microprobe detector array system. Journal of Physics: Conference Series 499, 012001
| Maia X-ray microprobe detector array system.Crossref | GoogleScholarGoogle Scholar |
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant and Soil 362, 319–334.
| Hyperaccumulators of metal and metalloid trace elements: facts and fiction.Crossref | GoogleScholarGoogle Scholar |
van der Ent A, Jaffré T, L’Huillier L, Gibson N, Reeves RD (2015) The flora of ultramafic soils in the Australia–Pacific Region: state of knowledge and research priorities. Australian Journal of Botany 63, 173–190.
| The flora of ultramafic soils in the Australia–Pacific Region: state of knowledge and research priorities.Crossref | GoogleScholarGoogle Scholar |
van der Ent A, Callahan DL, Noller BN, Mesjasz-Przybylowicz J, Przybyłowicz WJ, Barnabas A, Harris HH (2017) Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia). Scientific Reports 7, 41861
| Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia).Crossref | GoogleScholarGoogle Scholar | 28205587PubMed |
van der Ent A, Przybyłowicz WJ, de Jonge MD, Harris HH, Ryan CG, Tylko G, Paterson DJ, Barnabas AD, Kopittke PM, Mesjasz-Przybyłowicz J (2018) X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phytologist 218, 432–452.
| X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants.Crossref | GoogleScholarGoogle Scholar | 28994153PubMed |
van der Ent A, de Jonge MD, Spiers KM, Brueckner D, Montargès-Pelletier E, Echevarria G, Wan X-M, Lei M, Mak R, Lovett JH, Harris HH (2020) Confocal volumetric μXRF and fluorescence computed μ-tomography reveals arsenic three-dimensional distribution within intact Pteris vittata fronds. Environmental Science & Technology 54, 745–757.
| Confocal volumetric μXRF and fluorescence computed μ-tomography reveals arsenic three-dimensional distribution within intact Pteris vittata fronds.Crossref | GoogleScholarGoogle Scholar |


