Understanding seed dormancy and germination aids conservation of rainforest species from tropical montane cloud forest: a case study confirming morphophysiological dormancy in the genus Tasmannia
Ganesha S. Liyanage A * , Catherine A. Offord A , Darren M. Crayn B , Lydia K. Guja C , Stuart Worboys
A * , Catherine A. Offord A , Darren M. Crayn B , Lydia K. Guja C , Stuart Worboys  B and Karen D. Sommerville A
B and Karen D. Sommerville A
A The Australian PlantBank, Australian Institute of Botanical Science, Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia.
B Australian Tropical Herbarium, James Cook University Nguma-Bada Campus, McGregor Road, Smithfield, Qld 4878, Australia.
C National Seed Bank, Australian National Botanic Gardens, Parks Australia, Clunies Ross Street, Acton, ACT 2601, Australia.
Australian Journal of Botany 70(6) 399-408 https://doi.org/10.1071/BT22011
Submitted: 4 February 2022 Accepted: 3 August 2022 Published: 1 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Seed dormancy is one issue hindering implementation of conservation actions for rainforest species.
Aims: We studied dormancy and germination in Tasmannia sp. Mt Bellenden Ker and Tasmannia membranea, two tropical montane rainforest species threatened by climate change, to develop a better understanding of dormancy in the species and the genus.
Methods: Dormancy was classified for T. sp. Mt Bellenden Ker on the basis of an imbibition test, analysis of embryo to seed length (E:S) ratios and germination in response to the following four dormancy-breaking treatments: (1) scarification of the seedcoat near the micropylar end; (2) removal of the seedcoat; (3) application of 100 mg L−1 or (4) 500 mg L−1 gibberellic acid. The most effective treatment was then tested on T. membranea. The requirement for light for germination was also assessed.
Key results: Both scarified and intact seeds imbibed water. Initial E:S ratios were <0.22 for both species and increased up to 0.74 after 40 days, just before radicle emergence, for T. sp. Mt Bellenden Ker. Germination proportions were significantly higher in Treatments 1 and 2 than the remaining treatments for T. sp. Mt Bellenden Ker; T. membranea responded similarly well to Treatment 1. Germination under alternating light/dark conditions was slightly, but not significantly, greater than germination in the dark alone.
Conclusions: Both species have morphophysiological dormancy and treatments that remove seedcoat resistance to embryo growth facilitate germination. These treatments may improve germination in other species from the genus Tasmannia.
Implications: This knowledge will aid the germination of seeds to implement conservation strategies for Tasmannia spp.
Keywords: endemic species, E:S ratio, morphophysiological dormancy, plant conservation, seed dormancy, seed germination, seed storage, Tasmannia, tropical rainforests.
Introduction
Rapid loss of rainforests is causing ongoing environmental, social and economic problems that have drawn attention to the need for integrated ex situ and in situ conservation strategies to safeguard plant species for the future (Eaton 1996; Sommerville et al. 2018). Seed-banking can play a key role in reducing the loss of rainforest diversity by conserving species outside their natural habitats (Sommerville et al. 2018). This is a cost-effective and efficient ex situ conservation method for seeds that tolerate drying and storage at subzero temperatures (Martyn Yenson et al. 2021). Testing the suitability of seeds for storage usually entails a series of tests comparing the germination of seeds when fresh, after drying to low moisture content, and after freezing at subzero temperatures (Hong and Ellis 1996; Pritchard et al. 2004). A major impediment to performing these germination tests is seed dormancy. For example, Sommerville et al. (2021) reported that, of 313 Australian rainforest species tested for storage behaviour, 48% showed low germination (and so could not be assigned to a storage response) owing to dormancy or unknown germination requirements. Difficulty propagating plants from seed because of dormancy is also a critical problem in restoration and reintroduction of plant species (Cochrane et al. 2002). For seed-banking purposes, the issue of poor germination can now be avoided by applying a key to storage behaviour that is based on seed characteristics rather than germination (Sommerville et al. 2021). However, the ultimate use of banked seeds usually involves germination, whether the seeds are used for propagation, research, or long-term monitoring. Moreover, when time is critical, as is often the case for grant-funded restoration projects, knowledge of how to break seed dormancy is essential to being able to produce plants in a reasonable time-frame. In these cases, knowledge of how to overcome dormancy and germinate the seed efficiently is essential (Jaganathan 2020).
Seed dormancy is an innate trait preventing germination in environments that are unsuitable for seedling establishment (Cohen 1966; Philippi 1993; Baskin and Baskin 2004; Fenner and Thompson 2005). Baskin and Baskin (2004) described the following five broad classes of seed dormancy on the basis of seed characteristics and the environmental conditions required to initiate germination: (1) physical dormancy (PY), in which an impermeable seedcoat prevents the influx of water and gases needed for germination; (2) physiological dormancy (PD), in which one of a variety of mechanisms inhibits embryo growth and emergence of the radicle; (3) morphological dormancy (MD), in which the embryo is underdeveloped and must grow to a certain size before the radicle can emerge; (4) morphophysiological dormancy (MPD), in which the seed has both physiological dormancy and an underdeveloped embryo; and (5) combinational dormancy (CD), in which the seed has both physical and physiological dormancy. The small, underdeveloped embryo of seeds with MD or MPD is considered to be an ancestral angiosperm characteristic that is present in most basal angiosperm groups including Amborella trichopoda (Forbis et al. 2002; Willis et al. 2014; Fogliani et al. 2017). Categorisation as either MD or MPD depends on the time required for radicle protrusion after embryo growth; seeds that take fewer than 30 days for the radicle to protrude are categorised as MD; seeds that take more than 30 days are categorised as MPD (Baskin and Baskin 2004).
Understanding the class of dormancy a seed possesses is helpful for determining the treatments that can relieve it. For example, PY can be relieved by creating an opening in the seedcoat with a scalpel or by rubbing the seed on sandpaper to render the seedcoat water permeable (Baskin and Baskin 1998). In rainforests, this type of dormancy can be relieved when direct sunlight penetrates through a canopy gap to the forest floor, increasing the soil temperature and leading to rupture of the seedcoat (Vázquez-Yanes and Smith 1982). Techniques to relieve PD or MPD depend on the mechanism inhibiting embryo growth but can include a period of dry after-ripening, mimicking conditions that occur (for example) during the dry season in monsoon rainforest, or a period of stratification (holding seeds at warm/cool temperatures in moist conditions), mimicking conditions that can occur (for example) in wet tropical rainforest in summer or winter (Singh Ramesh et al. 2022).
Although dormancy and germination of seeds with underdeveloped embryos have been studied for a few basal plant families (Hydatellaceae, Tuckett et al. 2010; Magnoliaceae, Baskin and Baskin 1998; Amborellaceae, Fogliani et al. 2017; Nymphaeaceae, Dalziell et al. 2016), very limited work on seed dormancy has been published for Winteraceae, one of the most basal plant families in the early diverging clade, Magnoliidae (Van der Ham and van Heuven 2002; Offord et al. 2004; Campbell et al. 2012). This family is commonly represented in tropical and southern temperate rainforests (Massoni et al. 2014; APG IV (Angiosperm Phylogeny Group IV) 2016) and has been a focus of palynological, phylogenetic and physiological studies (e.g. Doyle 2000; Van der Ham and van Heuven 2002; Brea et al. 2021). However, few seed dormancy and germination studies, which are critical to establishing conservation strategies as well as to understanding population dynamics, have been published for genera in this family.
Globally, the Winteraceae family contains five genera and 93 accepted species (POWO 2021). Only two genera, namely Tasmannia R.Br. ex DC. and Bubbia Tiegh. (considered a synonym of Zygogynum Baill. in POWO 2021), occur in Australia and the majority of species in those two genera are restricted to mesic habitats such as rainforest (Guymer 2007). Of nine Australian species in the genus Tasmannia, two are endemic to tropical rainforests in far-northern Queensland; Tasmannia sp. Mt Bellenden Ker (J.R.Clarkson 6571) Qld Herbarium is known only from cloud forest at elevations of 1500–1600 m on Mount Bellenden Ker; Tasmannia membranea (F.Muell.) A.C.Sm. has a wider distribution, typically occurring at elevations from 300 to 1550 m (Zich et al. 2020). Both species are components of rainforest understorey but appear to favour disturbance and are common along tracks or around infrastructure. The mountain habitat of T. sp. Mt Bellenden Ker receives a mean annual rainfall of 8072 mm, with 50% falling in the summer monsoon months of December–March (Bureau of Meteorology 2022a). Cloud interception adds significantly to the rainfall total (McJannet et al. 2008). Under the closed forest canopy, soil-surface temperatures range from 5.25°C to 28.5°C (Singh Ramesh et al. 2022). In contrast, T. membranea occurs in a wider range of habitats, from lowland rainforest to high mountain ridges. It often occurs in areas with lower rainfall and more marked wet/dry seasonality; populations near the Millaa Millaa weather station, for example, receive a mean annual rainfall of 2226 mm, 59% of which falls in summer (Bureau of Meteorology 2022b). Neither of these species has previously been studied to determine dormancy class or the conditions required to break dormancy. The ability to germinate seed of T. sp. Mt Bellenden Ker is particularly important because the habitat of this species is considered at great risk from climate change (Costion et al. 2015); this species is now an important target of a multi-organisation project to conserve at-risk flora endemic to tropical montane cloud forest habitat.
Related studies of other species in the genus Tasmannia, including laboratory studies of Tasmannia purpurascens (Vickery) A.C.Sm. (Offord et al. 2004) and Tasmannia lanceolata (Poir.) A.C.Sm. (RTBG 2021), and in situ burial studies of Tasmannia stipitata (Vickery) A.C.Sm. (Campbell et al. 2012), have demonstrated that Tasmannia species produce dormant seeds that can be difficult to germinate. However, none of those studies was able to identify effective dormancy-breaking cues or techniques for the genus. Studies at the Australian PlantBank on dried-seed collections of T. purpurascens (collected from two locations in New South Wales, Australia) showed germination ranging from 0 to 48% when incubated at 20°C with gibberellic acid (Royal Botanic Gardens and Domain Trust, unpubl. data). These results suggest that there may be a physiological component contributing to dormancy in Tasmannia spp; however, the use of dried seeds in those experiments limits the ability to draw firm conclusions about dormancy class. Baskin and Baskin (2014) inferred from Offord et al. (2004) that dormancy in T. purpurascens may be classed as MPD but this classification has not yet been confirmed by experimental work. In this study, therefore, we explored dormancy class and the conditions required to break dormancy for T. sp. Mt Bellenden Ker and T. membranea, with a view to developing a better understanding of dormancy in these and other species in the genus. Specifically, we addressed the following questions: (1) are Tasmannia seeds permeable to water; (2) do Tasmannia seeds have underdeveloped embryos that need to grow before radicle emergence; (3) do Tasmannia species exhibit morphophysiological dormancy; and (4) which dormancy-breaking techniques and germination conditions are suitable for germinating Tasmannia seeds?
Materials and methods
Fresh mature fruits of T. sp. Mt Bellenden Ker were collected in June 2019 from Mount Bellenden Ker, in far-northern Queensland, Australia. Fruits of T. membranea were collected in December 2019 from Mount Windsor National Park in the same region. Fruits of both species were held at ambient temperatures for less than 1 week during transport to the Australian PlantBank, followed by temporary storage at 15°C for up to 1 week. Seeds were then extracted by gently crushing the fruit in a sieve and washing the flesh off under running water. The extracted seeds were blotted dry with paper towelling before use in the experiments described below. The quantity of seeds available for use was limited because of the difficulties associated with collecting seeds in tropical montane cloud forests, and the need to balance seed use with long-term conservation by seed banking. The number of seeds per treatment was therefore carefully determined to facilitate statistical analysis while minimising seed use.
Seed weight and dimensions
Fresh seed weight for T. sp Mt Bellenden Ker was determined by weighing 20 seeds individually on a digital balance (±0.0001 g) and calculating the mean. Seed length and width (at the widest part of the seed) were measured on the same seeds by using a stereo microscope (M60; Leica Microsystems, Macquarie Park, NSW, Australia) and associated image-processing software (Leica Application Suite v4.3). Seed weight and dimensions were not determined for T. membranea.
Imbibition test
An imbibition test was performed to investigate the permeability of the seedcoat and determine whether there is a physical component to dormancy in T. sp. Mt Bellenden Ker. Twenty fresh and healthy-appearing seeds were selected for this test; 10 seeds were left intact and 10 were scarified by removing a portion of the seedcoat opposite the micropylar end with a scalpel. Intact and scarified seeds were weighed individually before placing them on two separate Petri dishes containing 0.8% (w/v) water agar. The Petri dishes were sealed using cling wrap to minimise water loss and incubated at 25°C/10°C in 12 h light (9.05 μmol m−2 s−1)/12 h dark; these conditions had previously been used successfully to germinate several other rainforest species from the same habitat. The seeds were reweighed at intervals of 24 h until no further weight increments were observed for scarified seeds. Seeds were blotted dry before each measurement to avoid apparent increases in weight owing to moisture adhering to the seedcoat.
Embryo to seed length (E:S) ratio
The embryo to seed length ratio of fresh T. sp. Mt Bellenden Ker seeds was measured at five time points to study embryo growth during germination. Twenty-four intact seeds were placed on Petri dishes containing 0.8% (w/v) water agar and incubated as described above. Four seeds were extracted at intervals of 1, 4, 14, 29 and 43 days until splitting of the seedcoat for radicle protrusion was observed. Extracted seeds were dissected longitudinally and embryo and seed lengths were measured using a stereo microscope (M60; Leica Microsystems) and associated image-processing software (Leica Application Suite v4.3). The initial E:S ratio was also measured for T. membranea seeds after 24 h of incubation on agar.
Determination of effective dormancy-breaking technique for Tasmannia spp.
Germination of intact seeds of T. sp. Mt Bellenden Ker was compared to germination in response to the following four different dormancy-breaking treatments: (1) manual scarification of the seedcoat near the micropylar end (precision nicking); (2) full removal of the seedcoat; (3) incubation of seeds with 100 mg L−1 gibberellic acid (GA3); and (4) incubation of seeds with 500 mg L−1 GA3. Three replicates of 15–20 seeds were used for each treatment and seeds were incubated on 0.8% (w/v) water agar in glass Petri dishes. For Treatments 3 and 4, ∼1 mL of GA3 solution was added as a thin layer to cover the whole surface of the agar just before placing intact seeds on the surface. Prior to the seeds being placed in agar dishes in Treatments 1 and 2, a scalpel was used to scarify or remove the seedcoat. These procedures were performed under a stereo microscope (A60; Leica Microsystems) to avoid damaging the internal tissues of the seeds. All Petri dishes were sealed and incubated as described in section ‘Imbibition test’. Germination was recorded every second day for 3 months and terminated when no further germination was detected during two consecutive inspections. At the end of the experiment, seeds that did not germinate were gently pressed using forceps to check whether they were empty or filled. Firm seeds were cut in half and observed under the microscope; the presence of firm white endosperm and embryo was considered an indication that the seed remained viable. Empty seeds were subtracted from the initial number of viable seeds sown. Because the viability of seeds from control replicates was ∼100% (98 ± 2.1% and 97 ± 3.3% for T. sp. Mt Bellenden Ker and T. membranea respectively), mushy seeds found in dormancy-breaking treatments at end of the experiment were considered to have been viable at the beginning of the experiment and may have deteriorated because of the effect of the treatment. The number of germinated, viable but not germinated, and mushy seeds were combined to get the total initial viable seed number per replicate.
Tasmannia membranea was used as an example to investigate the germination response of other species from the genus Tasmannia to the dormancy-breaking treatment that was most effective on T. sp. Mt Bellenden Ker. Three replicates of 15–20 seeds of T. membranea were scarified near the micropylar end and incubated as described above, along with three control replicates containing intact seeds. Germination was recorded for almost 3 months as for T. sp. Mt Bellenden Ker and finalised with a cut test.
Effect of light on germination of T. sp. Mt Bellenden Ker
The requirement of light for germination was determined for T. sp. Mt Bellenden Ker by comparing germination under alternating light/dark conditions with germination in the dark. Two sets of three replicates of 15–20 seeds were scarified near the micropylar end and placed on Petri dishes filled with 0.8% (w/v) water agar. One set of Petri dishes was completely covered with aluminium foil to exclude light, whereas the other set was left uncovered. Both sets of Petri dishes were incubated in the same cabinet at 25°C/10°C and 12 h/12 h light/dark. Replicates subjected to the dark treatment were observed after 35 days in a dark room and then again after 56 days. Both dark and light/dark treatments were finalised by performing a cut test to determine the total number of viable seeds.
Data analysis
Data from the imbibition test were analysed using Welch’s t-test. Mean seed weights of intact and manually scarified seeds were compared to determine seedcoat permeability. One-factor generalised linear models (GLMs) were used to compare the effects of (1) dormancy-breaking treatments, and (2) light/dark treatments, on germination. A binomial error structure and logit link function were used for each model where final germination and treatment were considered as the response and predictor variables respectively. GLMs with binomial data distribution provide a better fit for germination data than analyses based on the normal distribution (Carvalho et al 2018). When the final germination proportions were significantly different among dormancy-breaking treatments, Tukey’s test was used for multiple comparisons (Hothorn et al. 2008). Germination data were analysed after 1 month and again after 2 months for T. sp. Mt Bellenden Ker and after 2 and 3 months for T. membranea. The time taken to initiate germination and achieve maximum germination was analysed using GLM with Gamma data distribution. All analyses were performed using R statistical software ver. 3.5.0 (R Core Team 2018).
Results
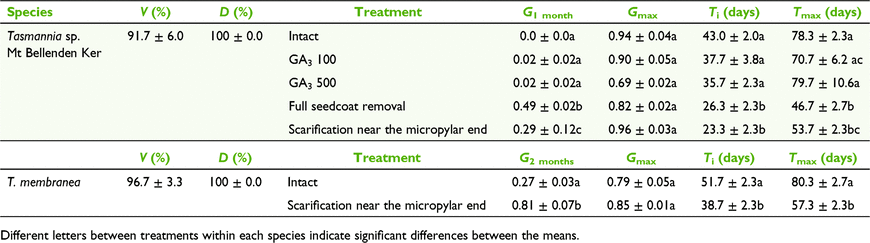
Initial mean seed weight, length and width of T. sp. Mt Bellenden Ker seeds were 7.4 ± 0.6 mg, 3.9 ± 0.1 mm and 1.9 ± 0.1 mm respectively (Fig. 1a). Both intact and scarified seeds of T. sp. Mt Bellenden Ker imbibed water readily over the first 6 days, with no considerable increase in weight observed after that point. Mean seed weight increments were significantly higher in scarified than in non-scarified seeds (Welch’s t-test, d.f. = 117.15, t = 2.5, P = 0.01); however, overall weight increments were less than 27% of the initial seed weights for both scarified and intact seeds.
After 24 h of imbibition, both T. sp. Mt Bellenden Ker and T. membranea had small undifferentiated, globular embryos (Fig. 1b). The E:S ratio of T. sp. Mt Bellenden Ker increased from 0.14 ± 0.03 after 24 h to 0.74 ± 0.05 after 40 days at the point of radicle emergence (Figs 1c, 2). Embryo growth was evident by Day 29 and germination commenced after Day 43 (Fig. 2). Mean embryo length increased by about 390%, compared with the initial embryo size, prior to radicle emergence. The initial E:S ratio of T. membranea was 0.21 ± 0.07 after 24 h of imbibition.
|
|
Removal or scarification of the seedcoat significantly decreased the time to initiate germination of T. sp. Mt Bellenden Ker seeds compared with intact and GA3-treated seeds (GLM, d.f.=4, χ2 = 0.2, P < 0.01). After 1 month of incubation, the number of germinated seeds also differed significantly in response to different dormancy-breaking treatments (GLM, d.f. = 4, χ2 = 48.2, P < 0.01; Table 1). Full seedcoat removal and scarification resulted in a germination proportion of 0.25–0.50 within 1 month, whereas the germination proportions of the remaining treatments were less than 0.03. However, after 2 months of incubation, germination proportions increased significantly to more than 0.75 in all treatments except 500 mg L−1 GA3, which showed a germination proportion of 0.69 ± 0.02 (GLM, d.f. = 4, χ2 = 5.0, P < 0.01). The germination proportion of seeds treated with 500 mg L−1 GA3 was significantly lower than the 0.94 ± 0.04 and 0.96 ± 0.03 germination proportion of seeds in the control (Tukey’s test, d.f. = 4, Z = 2.74, P = 0.04) and scarified (Tukey’s test, d.f. = 4, Z = 3.76, P < 0.01) treatments respectively.
Scarified seeds took longer to initiate germination in T. membranea (38.7 ± 2.3 days) than in T. sp. Mt Bellenden Ker (23.3 ± 2.3 days; Table 1). Therefore, the germination proportions of T. membranea seeds in response to treatments were compared after 2 and 3 months. As with T. sp. Mt Bellenden Ker, germination of T. membranea seeds commenced significantly earlier in scarified than in intact seeds (GLM, d.f. = 1, χ2 = 0.03, P < 0.01) and the germination proportion of scarified seeds of T. membranea (0.81 ± 0.07) was significantly higher than that of the intact seeds of control replicates (0.27 ± 0.02) after 2 months (GLM, d.f. = 1, χ2 = 4.12, P < 0.01; Table 1). However, the germination proportions were not significantly different after 3 months (0.85 ± 0.01 and 0.79 ± 0.05 for scarified and control replicates respectively; GLM, d.f. = 1, χ2 = 0.3, P = 0.56).
The seeds of T. sp. Mt Bellenden Ker germinated in both alternating light/dark and dark conditions with an enhanced germination (∼25% greater) under alternating light/dark treatment (Fig. 3). However, the germination proportion was not significantly higher in the light/dark treatment (0.76 ± 0.04) than in the dark-only treatment (0.51 ± 0.11) after 56 days (GLM, d.f. = 1, χ2 = 1.5, P = 0.06).
|
|
Discussion
The primary aims of this study were to explore dormancy type, and conditions required for dormancy break and germination, for species in the mesic forest genus Tasmannia. Two endemic Australian rainforest species, namely T. sp. Mt Bellenden Ker and T. membranea, were used as representatives of the genus. Information on dormancy and germination requirements is critical when implementing ex situ conservation protocols because seed germination plays an integral part in long-term monitoring of seeds conserved in conventional seedbanks (Hong and Ellis 1996; Pritchard et al. 2004). Such information also helps overcome difficulties associated with germination in situ for reintroduction and restoration (Cochrane et al. 2002). Developing an understanding of the dormancy and germination requirements of individual species can therefore be very valuable to efforts to conserve highly threatened ecosystems such as tropical montane cloud forests.
A first step to understanding dormancy in a species is to check the permeability of the seedcoat. An impermeable seed or fruitcoat prevents the uptake of water necessary for germination in seeds with a physical dormancy component (PY and CD). Water uptake (imbibition) and germination following scarification therefore confirms the presence of PY. Imbibition following scarification has been shown to increase seed weight by more than 140% of initial seed weight in the physically dormant species Prosopis juliflora (Sw.) DC. (Sanjeewani et al. 2010) and by more than 175% in Cuscuta australis R.Br. (Jayasuriya et al. 2008). The increase in weight of both intact and scarified seeds of T. sp. Mt Bellenden Ker indicated the seedcoat is permeable without scarification. This result confirmed seedcoat permeability and therefore ruled out physical and combinational dormancy in Tasmannia spp. The slight but significantly greater weight increment in scarified seeds (+25%) was likely to be due to the creation of an opening larger than the naturally occurring water passages in the seedcoat, enabling water to be absorbed more quickly.
A second step to understanding seed dormancy is to investigate embryo morphology. Seeds possessing an embryo that is (1) differentiated (i.e. has a clearly defined radicle and cotyledon) but small, and needs to grow inside the seed before germination, or (2) undifferentiated (i.e. has no clearly defined structure), at the dispersal stage are considered to be underdeveloped and morphologically dormant (Baskin and Baskin 2014). There have been some studies on embryology of the plant family Winteraceae and the embryos of genus Tasmannia have been defined as globular and undifferentiated at the time of dispersal (Doyle 2000; Tobe and Sampson 2000; Brea et al. 2021). However, embryo differentiation or growth during germination has not previously been addressed and such information is important to defining dormancy class (Baskin and Baskin 2014). Studies on post-dispersal embryo growth have shown that the increase in embryo size before radicle emergence can be highly variable among species. Erickson et al. (2016), for example, reported that the final embryo size increase can be as little as 27.5% in comparison to the initial embryo size in Wahlenbergia tumidifructa P.J.Sm., whereas Chien et al. (2011) reported that it can be as great as 360% in Schisandra arisanensis Hayata. Both Tasmannia species in this study had embryos that occupied less than 25% of their seed length at dispersal and the embryo of T. sp. Mt Bellenden Ker grew to ∼75% of its seed length before radicle emergence, an increase in embryo size of 390% of its initial size. These observations indicated the presence of a morphological component to dormancy in seeds of Tasmannia species.
According to the dormancy classification system of Baskin and Baskin (2004), seeds with underdeveloped embryos that need more than 30 days for embryo growth and germination may be classified as morphophysiologically dormant (MPD). Because germination was not observed for control replicates for more than 30 days of incubation, and because the embryo size increased significantly before radicle emergence, we can confirm the presence of MPD in both T. sp. Mt Bellenden Ker and T. membranea. Both test species showed considerable germination within 30 days in response to treatments that cleared the radicle emergence point (seedcoat removal and scarification near the micropylar end). Such treatments may be effective techniques for overcoming MPD in other species from the genus Tasmannia.
Both full seedcoat removal and scarification near the micropylar end led to greater than 50% germination within 30 and 50 days in T. sp. Mt Bellenden Ker and T. membranea respectively. Although the initiation of germination took longer in T. membranea seeds than in T. sp. Mt Bellenden Ker seeds, scarified seeds achieved high germination faster than did intact seeds in both species. Faster germination indicated faster growth in the underdeveloped embryo, once the seedcoat was removed from the micropylar end, which may have been due to the removal of physical resistance to embryo expansion, faster uptake of water through the enlarged seedcoat opening, or a combination of both. By using seeds of Chaerophyllum tainturieri Hook. & Arn., Baskin and Baskin (1990) demonstrated that, once seeds have lost physiological dormancy (PD) and are placed under favourable germination conditions, underdeveloped embryos can grow rapidly, in that case doubling their length within 6 days. The improvement in germination following scarification near the micropylar end or seedcoat removal confirmed the presence of a PD component in the dormancy of seeds of Tasmannia spp. Precision nicking or seedcoat clearance around the micropylar end is a technique that removes the resistance of the seedcoat against radicle protrusion and has been used to overcome PD in species such as Acronychia imperforata F.Muell., A. laevis J.R.Forst. & G.Forst., A. oblongifolia (A.Cunn. ex Hook.) Endl. ex Heynh. (Liyanage et al. 2020) and Grevillea juniperina R.Br. (Briggs et al. 2016). Scarification to clear the micropylar end and remove resistance to radicle emergence can therefore be suggested as an effective laboratory technique to achieve faster and more consistent germination for species with MPD. Moreover, this precision nicking technique helps achieve consistent germination within a shorter time period than for the irregular pattern of germination observed in control replicates. This is helpful in performing consecutive germination tests on fresh, dried and frozen seeds to assess seed storage behaviour, and monitoring viability in storage over long time periods. The decreased time to final germination helps complete storage behaviour tests more quickly, enabling rapid identification of suitable storage conditions and ensuring that important collections are banked under those conditions as soon as possible to achieve maximum longevity in storage.
Seed germination was also observed in intact seeds (i.e. control replicates) of both tested species after 43 days, which raises the question as to how PD was broken in those seeds. High temperatures in summer (such as 25°C/15°C, 30°C/15°C and 35°C/20°C) have been shown to break PD in the natural environment (Baskin and Baskin 2014); therefore, the relatively high day-time temperature (25°C) used in this study may have helped overcome PD in the species tested. In rainforest environments, habitat disturbance can lead to greater sunlight penetration and increased soil temperatures, which may explain the tendency of both species to grow in disturbed habitats. For the more widely distributed T. membranea, the change from relatively cool, dry conditions during winter to wet warm conditions during summer may also play a role in relieving PD.
A light requirement for germination has been associated with two ecological roles, namely (1) as a mechanism to prevent the germination of buried seeds until the soil surface is detected, and (2) to prevent germination in seeds that are dispersed to shade until a gap in the canopy is formed (Vázquez-Yanes and Orozco-Segovia 1993). In this study, germination of T. sp. Mt Bellenden Ker did not differ significantly between alternating light/dark and dark treatments, although germination was slightly enhanced (∼25% greater) by exposure to alternating light/dark. This result contrasted with several earlier studies that showed light-dependent germination for a number of rainforest species (e.g. Orozco-Segovia and Vázquez-Yanes 1989; Raich and Khoon 1990; Vázquez-Yanes et al. 1990; Goulding 2001). The response of rainforest seeds to light can be an indication of successional stage, with seeds of climax species able to germinate and establish in the dim light of the rainforest floor, whereas pioneer species require high light levels for germination and establishment (Baskin and Baskin 2014). However, Pons et al. (2005) found that many rainforest species fall somewhere between the two extremes of climax and pioneer. Tasmannia sp. Mt Bellenden Ker grows in relatively high-light environments in cloud forest on exposed mountain ridges, suggesting that light should not be a factor limiting germination. However, the seeds of this species are quite small and likely to be quickly buried in thick leaf litter on dispersal. The observed capacity to germinate in the dark would allow seeds of this species to germinate under litter; however, whether seedling growth and establishment would occur in the same environment remains to be tested.
Gibberellic acid has been widely used to overcome non-deep physiological dormancy in many species under laboratory conditions (Baskin and Baskin 2014). However, in this study, the use of gibberellic acid at a concentration of 100 mg L−1 did not have a significant effect on germination, whereas a high concentration of GA3 (500 mg L−1) significantly reduced the germination of T. sp. Mt Bellenden Ker. High concentrations of GA3 have been shown to reduce germination in Australian daisies when the concentration was greater than 500 mg L−1 and in Rhodanthe humboldtiana (Gaudich.) Paul G.Wilson germination was inhibited even by concentrations as low as 100 mg L−1 (Plummer and Bell 1995). All together, these results may indicate that the level of MPD present in the Tasmannia species tested is non-deep; however, stratification with and without GA3 would be needed to confirm this. Future studies on Tasmannia species could also include a range of incubation temperatures to identify the exact cues needed to break dormancy in their natural habitat.
The results of this study clearly confirmed the presence of morphophysiological dormancy in T. sp. Mt Bellenden Ker and T. membranea because of the presence of underdeveloped embryos that take more than 30 days to germinate. Treatments that removed seedcoat resistance against embryo growth allowed germination within 30 days by allowing the underdeveloped embryo to grow. In natural environments, increased soil temperatures or a change from cool to warm temperatures after disturbance may provide a suitable environment for overcoming PD, favouring the regeneration of Tasmannia species following disturbance. The suitability of precision nicking as an effective dormancy-breaking technique for both T. sp. Mt Bellenden Ker and T. membranea suggests that these treatments may help improve germination in other species from the same genus. To our knowledge, no studies have identified dormancy class and a suitable dormancy-breaking technique for Tasmannia spp. apart from the inference of MPD based on embryo size and observations of germination difficulty (Baskin and Baskin 2014). This knowledge will greatly aid the germination of seeds to implement ex situ and in situ conservation strategies. Further studies on stratification conditions, and incubation in different combinations of light levels and temperature regimes may improve our understanding of dormancy-breaking cues in the species’ natural habitat.
Data availability
The dataset used during the current work are available from the corresponding author upon request.
Conflicts of interest
Authors declared that they have no conflicts of interest.
Declaration of funding
We thank the supporters of the Rainforest Seed Conservation Project, including HSBC Bank Australia, TransGrid, Klorane Botanical Foundation, The Ian Potter Foundation and several generous individuals, for funding the work of GL and KS. We thank The Ian Potter Foundation for funding seed collection as part of a larger project to preserve the flora of Australian tropical montane cloud forest.
Acknowledgements
Plant material was collected under Permit PTU18-001474 issued by the Queensland Government to the Australian Tropical Herbarium.
References
APG IV (Angiosperm Phylogeny Group IV) (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181, 1–20.| An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV.Crossref | GoogleScholarGoogle Scholar |
Baskin JM, Baskin CC (1990) Germination ecophysiology of seeds of the winter annual Chaerophyllum tainturieri: a new type of morphophysiological dormancy. Journal of Ecology 78, 993–1004.
| Germination ecophysiology of seeds of the winter annual Chaerophyllum tainturieri: a new type of morphophysiological dormancy.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (1998) ‘Seeds: ecology, biogeography, and evolution of dormancy and germination.’ (Academic Press: San Diego, CA, USA)
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Science Research 14, 1–16.
| A classification system for seed dormancy.Crossref | GoogleScholarGoogle Scholar |
Baskin CC, Baskin JM (2014) ‘Seeds: ecology, biogeography, and evolution of dormancy and germination.’ 2nd edn. (Academic Press: New York, NY, USA)
Brea M, Iglesias A, Wilf P, Moya E, Gandolfo MA (2021) First South American record of Winteroxylon, Eocene of Laguna del Hunco (Chubut, Patagonia, Argentina): new link to Australasia and Malesia. International Journal of Plant Sciences 182, 185–197.
| First South American record of Winteroxylon, Eocene of Laguna del Hunco (Chubut, Patagonia, Argentina): new link to Australasia and Malesia.Crossref | GoogleScholarGoogle Scholar |
Briggs CL, Morris EC, Stone G (2016) Micropylar seed coat restraint and embryonic response to heat shock and smoke control seed dormancy in Grevillea juniperina. Seed Science Research 26, 111–123.
| Micropylar seed coat restraint and embryonic response to heat shock and smoke control seed dormancy in Grevillea juniperina.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2022a) Monthly rainfall (Bellenden Ker Top Station). Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_stn_num=031141 [Accessed 25 May 2022]
Bureau of Meteorology (2022b) Monthly rainfall (Millaa Millaa Alert). Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_stn_num=031185 [Accessed 25 May 2022]
Campbell ML, Clarke PJ, Keith DA (2012) Seed traits and seed bank longevity of wet sclerophyll forest shrubs. Australian Journal of Botany 60, 96–103.
| Seed traits and seed bank longevity of wet sclerophyll forest shrubs.Crossref | GoogleScholarGoogle Scholar |
Carvalho FJ, de Santana DG, de Araújo LB (2018) Why analyze germination experiments using generalized linear models? Journal of Seed Science 40, 281–287.
| Why analyze germination experiments using generalized linear models?Crossref | GoogleScholarGoogle Scholar |
Chien C-T, Chen S-Y, Baskin JM, Baskin CC (2011) Morphophysiological dormancy in seeds of the ANA grade angiosperm Schisandra arisanensis (Schisandraceae). Plant Species Biology 26, 99–104.
| Morphophysiological dormancy in seeds of the ANA grade angiosperm Schisandra arisanensis (Schisandraceae).Crossref | GoogleScholarGoogle Scholar |
Cochrane A, Kelly A, Brown K, Cunneen S (2002) Relationships between seed germination requirements and ecophysiological characteristics aid the recovery of threatened native plant species in Western Australia. Ecological Management & Restoration 3, 47–60.
| Relationships between seed germination requirements and ecophysiological characteristics aid the recovery of threatened native plant species in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cohen D (1966) Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12, 119–129.
| Optimizing reproduction in a randomly varying environment.Crossref | GoogleScholarGoogle Scholar |
Costion CM, Simpson L, Pert PL, Carlsen MM, John Kress W, Crayn D (2015) Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biological Conservation 191, 322–330.
| Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia.Crossref | GoogleScholarGoogle Scholar |
Dalziell EL, Baskin CC, Baskin JM, Young RE, Dixon KW, Merritt DJ (2019) Morphophysiological dormancy in the basal angiosperm order Nymphaeales. Annals of Botany 123, 95–106.
Doyle JA (2000) Paleobotany, relationships, and geographic history of Winteraceae. Annals of the Missouri Botanical Garden Journal 87, 303–316.
| Paleobotany, relationships, and geographic history of Winteraceae.Crossref | GoogleScholarGoogle Scholar |
Eaton P (1996) Socio-economic values and tropical rain forest conservation. In ‘Tropical rainforest research – current issues’. (Eds DS Edwards, WE Booth, SC Choy) pp. 417–423. (Springer: Dordrecht, Netherlands)
| Crossref |
Erickson TE, Merritt DJ, Turner SR (2016) Seed dormancy and germination of arid zone species. In ‘Pilbara seed atlas and field guide: plant restoration in Australia’s Arid Northwest’. (Eds TE Erickson, RL Barrett, DJ Merritt, KW Dixon) pp. 17–34. (CSIRO Publishing: Melbourne, Vic., Australia)
Fenner M, Thompson K (2005) ‘The ecology of seeds.’ (Cambridge University Press: Cambridge, UK)
Fogliani B, Gâteblé G, Villegente M, Villegente M, Fabre I, Klein N, Anger N, Baskin CC, Scutt CP (2017) The morphophysiological dormancy in Amborella trichopoda seeds is a pleisiomorphic trait in angiosperms. Annals of Botany 119, 581–590.
| The morphophysiological dormancy in Amborella trichopoda seeds is a pleisiomorphic trait in angiosperms.Crossref | GoogleScholarGoogle Scholar |
Forbis TA, Floyd SK, Queiroz Ad (2002) The evolution of embryo size in angiosperms and other seed plants: implications for the evolution of seed dormancy. Evolution 56, 2112–2125.
| The evolution of embryo size in angiosperms and other seed plants: implications for the evolution of seed dormancy.Crossref | GoogleScholarGoogle Scholar |
Goulding JA (2001) Germination of seeds of tropical rainforest species: responses to time and light quality. Master of Science Thesis, James Cook University, Australia. Available at https://researchonline.jcu.edu.au/62619/ [Accessed 11 October 2021]
Guymer GP (2007) Winteraceae. In ‘Flora of Australia. Vol. 2: Winteraceae to Platanaceae’. (Ed. AJG Wilson) pp. 1–10. (CSIRO Publishing: Melbourne, Vic., Australia)
Hong TD, Ellis RH (1996) ‘A protocol to determine seed storage behaviour. IPGRI Technical Bulletin No. 1.’ (International Plant Genetic Resources Institute: Rome, Italy) Available at https://cropgenebank.sgrp.cgiar.org/images/file/learning_space/technicalbulletin1.pdf [Accessed 15 October 2021]
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363.
| Simultaneous inference in general parametric models.Crossref | GoogleScholarGoogle Scholar |
Jaganathan GK (2020) Defining correct dormancy class matters: morphological and morphophysiological dormancy in Arecaceae. Annals of Forest Science 77, 100
| Defining correct dormancy class matters: morphological and morphophysiological dormancy in Arecaceae.Crossref | GoogleScholarGoogle Scholar |
Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC, Chien C-T (2008) Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling. Annals of Botany 102, 39–48.
| Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling.Crossref | GoogleScholarGoogle Scholar |
Liyanage GS, Offord CA, Sommerville KD (2020) Techniques for breaking seed dormancy of rainforest species from genus Acronychia. Seed Science and Technology 48, 159–165.
| Techniques for breaking seed dormancy of rainforest species from genus Acronychia.Crossref | GoogleScholarGoogle Scholar |
Martyn Yenson AJ, Offord CA, Meagher PF, Auld T, Bush D, Coates DJ, Commander LE, Guja LK, Norton SL, Makinson RO, Stanley R, Walsh N, Wrigley D, Broadhurst L (2021) ‘Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections.’ 3rd edn. (Australian Network for Plant Conservation: Canberra, ACT, Australia) Available at https://www.anpc.asn.au/wp-content/uploads/2021/09/GermplasmGuidelinesThirdEdition_FINAL_210902.pdf [Accessed 27 January 2022]
Massoni J, Forest F, Sauquet H (2014) Increased sampling of both genes and taxa improves resolution of phylogenetic relationships within Magnoliidae, a large and early-diverging clade of angiosperms. Molecular Phylogenetics and Evolution 70, 84–93.
| Increased sampling of both genes and taxa improves resolution of phylogenetic relationships within Magnoliidae, a large and early-diverging clade of angiosperms.Crossref | GoogleScholarGoogle Scholar |
McJannet D, Wallace J, Fitch P, Disher M, Reddell P (2008) Hydrological processes in the tropical rainforests of Australia. In ‘Living in a dynamic tropical forest landscape’. (Eds NE Stork, SM Turton). (Blackwell Publishing: Melbourne, Vic., Australia)
Offord CA, McKensy ML, Cuneo PV (2004) Critical review of threatened species collections in the New South Wales Seedbank: implications for ex situ conservation of biodiversity. Pacific Conservation Biology 10, 221–236.
| Critical review of threatened species collections in the New South Wales Seedbank: implications for ex situ conservation of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Orozco-Segovia A, Vázquez-Yanes C (1989) Light effect on seed germination in Piper L. Acta Oecologica Oecologia Plantarum 10, 123–146.
Philippi T (1993) Bet-hedging germination of desert annuals: variation among populations and maternal effects in Lepidium lasiocarpum. The American Naturalist 142, 488–507.
| Bet-hedging germination of desert annuals: variation among populations and maternal effects in Lepidium lasiocarpum.Crossref | GoogleScholarGoogle Scholar |
Plummer JA, Bell DT (1995) The effect of temperature, light and gibberellic acid (GA3) on the germination of Australian everlasting daisies (Asteraceae, Tribe Inuleae). Australian Journal of Botany 43, 93–102.
| The effect of temperature, light and gibberellic acid (GA3) on the germination of Australian everlasting daisies (Asteraceae, Tribe Inuleae).Crossref | GoogleScholarGoogle Scholar |
Pons TL, Alexander EE, Houter NC, Rose SA, Rijkers T (2005) Ecophysiological patterns in Guianan forest plants. In ‘Tropical forests of the Guiana Shield: ancient forests in a modern world’. (Ed. DS Hammond) pp. 195–231. (CABI Publishing: Wallingford, UK)
POWO (2021) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available at http://www.plantsoftheworldonline.org/ [Accessed 20 December 2021]
Pritchard HW, Wood CB, Hodges S, Vautier HJ (2004) 100-seed test for desiccation tolerance and germination: a case study on eight tropical palm species. Seed Science and Technology 32, 393–403.
| 100-seed test for desiccation tolerance and germination: a case study on eight tropical palm species.Crossref | GoogleScholarGoogle Scholar |
Raich JW, Khoon GW (1990) Effects of canopy openings on tree seed germination in a Malaysian dipterocarp forest. Journal of Tropical Ecology 6, 203–217.
| Effects of canopy openings on tree seed germination in a Malaysian dipterocarp forest.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2018) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria). Available at www.R-project.org [Accessed 15 October 2021]
RTBG (2021) Germination details report: all tests for genus Tasmannia. (Royal Tasmanian Botanical Gardens) Available at https://gardens.rtbg.tas.gov.au/rtbgtsccgermdata/ [Accessed 16 December 2021]
Sanjeewani BLG, Jayasuriya KMGG, Jayasinghe JHLDHC (2010) Seed dormancy and hydrotime model for seed populations in two habitats of an invasive Fabaceae species, Prosopis juliflora. In ‘IUFRO Tree Seed Symposium: recent advances in seed research and ex situ conservation’. Taipei, Taiwan., pp. 153–163. (Taiwan Forestry Research Institute: Taiwan)
Singh Ramesh A, Cheesman A, Cernusak L (2022) ‘Far North Queensland microclimate data. Version 1.0.0.’ (Terrestrial Ecosystem Research Network. (Mt Bellendenker_understory_20191201_20211105.csv)). Available at https://portal.tern.org.au/far-north-queensland-microclimate/23482 [Accessed 25 May 2022]
Sommerville KD, Clarke B, Keppel G, McGill C, Newby Z-J, Wyse SV, James SA, Offord CA (2018) Saving rainforests in the South Pacific: challenges in ex situ conservation. Australian Journal of Botany 65, 609–624.
| Saving rainforests in the South Pacific: challenges in ex situ conservation.Crossref | GoogleScholarGoogle Scholar |
Sommerville KD, Errington G, Newby Z-J, Liyanage GS, Offord CA (2021) Assessing the storage potential of Australian rainforest seeds: a decision-making key to aid rapid conservation. Biodiversity and Conservation 30, 3185–3218.
| Assessing the storage potential of Australian rainforest seeds: a decision-making key to aid rapid conservation.Crossref | GoogleScholarGoogle Scholar |
Tobe H, Sampson B (2000) Embryology of Takhtajania (Winteraceae) and a summary statement of embryological features for the family. Annals of the Missouri Botanical Garden 87, 389–397.
| Embryology of Takhtajania (Winteraceae) and a summary statement of embryological features for the family.Crossref | GoogleScholarGoogle Scholar |
Tuckett RE, Merritt DJ, Rudall PJ, Hay F, Hopper SD, Baskin CC, Baskin JM, Tratt J, Dixon KW (2010) A new type of specialized morphophysiological dormancy and seed storage behaviour in Hydatellaceae, an early-divergent angiosperm family. Annals of Botany 105, 1053–1061.
| A new type of specialized morphophysiological dormancy and seed storage behaviour in Hydatellaceae, an early-divergent angiosperm family.Crossref | GoogleScholarGoogle Scholar |
Van der Ham R, van Heuven BJ (2002) Evolutionary trends in Winteraceae pollen. Grana 41, 4–9.
| Evolutionary trends in Winteraceae pollen.Crossref | GoogleScholarGoogle Scholar |
Vázquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical rainforest. Annual Review of Ecology and Systematics 24, 69–87.
| Patterns of seed longevity and germination in the tropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Vázquez-Yanes C, Smith H (1982) Phytochrome control of seed germination in the tropical rain forest pioneer trees Cecropia obtusifolia and Piper auritum and its ecological significance. New Phytologist 92, 477–485.
| Phytochrome control of seed germination in the tropical rain forest pioneer trees Cecropia obtusifolia and Piper auritum and its ecological significance.Crossref | GoogleScholarGoogle Scholar |
Vázquez-Yanes C, Orozco-Segovia A, Rincón E, Sánchez-Coronado ME, Huante P, Toledo JR, Barradas VL (1990) Light beneath the litter in a tropical forest: effect on seed germination. Ecology 71, 1952–1958.
| Light beneath the litter in a tropical forest: effect on seed germination.Crossref | GoogleScholarGoogle Scholar |
Willis CG, Baskin CC, Baskin JM, Auld JR, Venable DL, Cavender-Bares J, Donohue K, de Casas RR, The NESCent Germination Working Group (2014) The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203, 300–309.
| The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants.Crossref | GoogleScholarGoogle Scholar |
Zich FA, Hyland BPM, Whiffin T, Kerrigan RA (2020) ‘Australian tropical rainforest plants.’ 8th edn. Available at https://apps.lucidcentral.org/rainforest/ [Accessed 1 December 2021]