How important is fire-induced disturbance in the maintenance of a threatened perennial forb, Solanum papaverifolium?
Omofomwan Kingsley Osazuwa A * , John Matthew Dwyer
A * , John Matthew Dwyer  A and Roderick John Fensham
A and Roderick John Fensham  A B
A B
A Department of Biological Sciences, University of Queensland, St Lucia, Qld 4072, Australia.
B Queensland Herbarium, Mt Coot-tha Road, Toowong, Qld 4066, Australia.
Australian Journal of Botany 70(5) 335-343 https://doi.org/10.1071/BT21146
Submitted: 28 November 2021 Accepted: 21 June 2022 Published: 26 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Many grassland forb populations have greatly diminished because their habitat coincides with prime agricultural land and remnants lack appropriate disturbance regimes.
Aim: The aim of the current study is to examine the survivorship, vegetative recruitment, biomass and sexual reproduction of S. papaverifolium in response to burning in one of a few natural surviving populations in Queensland.
Methods: We monitored the survivorship, vegetative recruitment, and reproduction of a rare native forb, Solanum papaverifolium, within a randomised replicated experiment by using 14 circular plots measuring 12.56 m2 (2 m radius).
Key results: Plant survival rate after 5 months was greater in the burnt plots (56%) than the unburnt plots (44%). Vegetative recruitment from suckers in the burnt plots was almost twice that in the unburnt plots. The probability of flowering was also enhanced by burning. Fruit set in new recruits was higher in the burnt plots (20%) than in the unburnt plots (5%). Whereas fruiting did not occur in unburnt plots for surviving plants that flowered, only 1% fruited in the burnt plots. Seedling production was not observed.
Conclusion: Previous studies have demonstrated that the subtropical grassland flora in the study region is unaffected by burning, whereas the present study suggests that some grassland species respond positively to burning.
Implications: Although fire increased flowering in this species, suggesting that fire may be a useful tool to stimulate sexual reproduction, ongoing research is required to understand the precise factors that promote flowering after burning and the barriers to seedling reproduction.
Keywords: competition and inter-tussock species, disturbance, fire, plant survival, Solanum papaverifolium, subtropical grassland, vegetative recruitment and reproduction.
Introduction
Grasslands in arable landscapes are imperilled by conversion to crop production (Helkowski and Norman 1997; Hoekstra et al. 2005; Valkó et al. 2012; Williams et al. 2015; Cronk 2016; Bernardo et al. 2020). Subtropical grasslands in Australia have largely been cultivated and remaining remnant grassland patches are threatened by invasive plant species (Fensham 1997, 1998; Lewis et al. 2008; Fensham et al. 2016). Unsurprisingly, many grassland species persist only in small populations in isolated remnant grassland (Morgan 1999b; Kéry et al. 2000; Matthies et al. 2004). In addition, a large proportion of rare and threatened grassland plants are inter-tussock species (Pavlovic 1994; Lunt 1995; Morgan 1997; Price et al. 2019), which are less competitive than dominant tussock grasses (Munk et al. 2002; Valkó et al. 2012). For their persistence, these species depend on disturbances to reduce competition from dominant grasses (Lunt 1995; Munk et al. 2002; Valkó et al. 2012).
Diversity is commonly maintained at moderate levels of disturbance (Vujnovic et al. 2002; Roxburgh et al. 2004). However, grassland communities comprise species whose response to disturbances may be idiosyncratic (McIntyre et al. 1999). Fire is an example of a natural disturbance that is commonly used to maintain grassland diversity, particularly the inter-tussock flora (Pavlik et al. 1993; Menges and Gordon 1996; Lesica 1999; Menges and Quintana-Ascencio 2004). Although other methods such as grazing and mowing can increase canopy gaps (Williams et al. 2007; Araújo et al. 2013), fire eliminates dead plant material and aboveground biomass and modifies resource availability (MacDougall and Turkington 2007). Furthermore, heat and smoke generated by fire also break seed dormancy of fire-dependent species (Whelan 1995; Clarke and French 2005; White et al. 2020) and fire can enhance light availability and nutrients for seedlings (Burke and Grime 1996; Morgan 1998).
Lunt and Morgan (2002) reported increased germination of some herbaceous perennial species following prescribed burning in grassland plant communities in Australia. Also, fire enhances seedling recruitment of the endangered perennial forb Silene spaldingii in a Montana prairie reserve, USA (Lesica 1999). Conversely, fire has a modest negative impact on seedling recruitment of a threatened perennial herb Trioncinia retroflexa in a subtropical grassland in Australia (Fensham et al. 2002).
Recruitment of new individuals arises either from genets(seeds) or ramets (vegetative resprouts from stolons, rhizomes or roots) and in most species where both modes of reproduction exist, reproduction from ramets is dominant (Pausas 2001; Pausas et al. 2004; Clarke et al. 2013; Zhao et al. 2013). Vegetative resprouters are commonly associated with frequent burning, whereas regeneration from seed (reseeders) occurs under less frequent fire (Hoffmann 1999). In fire-prone ecosystems, resprouters allocate resources to protective structures and the remobilisation of reserves of non-structural carbohydrate in the roots and unburnt stems aid in enhancing their resilience and survival after fire (Clarke et al. 2013; Simpson et al. 2021). Re-seeders germinate from the soil seed bank or newly dispersed seeds (Whelan 1995; Pausas et al. 2004).
Solanum papaverifolium is an endangered perennial forb found in subtropical grassland (Fensham 1997). This rhizomatous forb of short stature (<50 cm high) is capable of both vegetative and sexual reproduction (Bean 2004). Although there are no ecological studies of S. papaverifolium, previous studies have shown that fire promotes production of S. centrale (Walsh and Douglas 2011) and flowering, fruit set and germination of S. prinophyllum (Kubiak 2009). However, these congeners are not grassland species and the response of S. papaverifolium to fire is uncertain, especially as experimental burning, applied twice over 3 years in the same grassland system, did not affect species richness and composition significantly (Fensham et al. 2017). Nevertheless, it is possible that the rarity and/or decline of some grassland species, such as S. papaverifolium has been exacerbated by the lack of appropriate disturbance (Silcock and Fensham 2018; Silcock et al. 2021). The aim of the current study was to examine the survivorship, vegetative recruitment, biomass and sexual reproduction of S. papaverifolium in response to burning in one of a few natural surviving populations in Queensland.
Materials and methods
Study area
The study took place in grassland on the alluvial floodplain of the Darling Downs, 16 km north-west of Dalby, Queensland (lat. −27.1345, long. 151.1256). The climate is subtropical, with mean maximum and minimum temperatures of 27°C and 12°C respectively. The mean annual rainfall was 584 mm for the period of 1992–2021. The grasslands have been extensively converted to croplands and remnants are confined to roadsides, railway lines and travelling stock routes. Subtropical grassland in this region are now classified as threatened ecosystems (Fensham 1997). The soil at the study site is a heavy clay classified as a vertosol. Generally, fire was an important management tool in natural grassland in Queensland until the 1960s, after which the use of fire drastically declined with the expansion of cropping on pastoral land (Pressland 1982). The area does not appear to have been mechanically disturbed in recent times.
Description, distribution and habitat of S. papverifolium
Solanum papaverifolium (Solanaceae) is a weak-stemmed, rhizomatous perennial forb that grows to a height of 0.6 m (Fig. 1). The leaves are broadly ovate, deeply lobed and spiny. Adult plants typically commence flowering in October, producing three to five solitary flowers and fruiting occurs in April–May. The aboveground portion of the plant dies each year but the rhizome is persistent. S. papaverifolium grows on clay-rich soil in grassland and open eucalypt woodland in Queensland and New South Wales in Australia (Bean 2004) and the loss of populations over the past two decades in the Darling Downs (R. J. Fensham 2018, unpubl. data) indicates that the species is threatened with extinction. The natural habitat of S. papaverifolium has declined with agriculture and roadsides are being degraded by exotic invasive species and road construction and there are no records from conservation reserves (Bean 2004). S. papaverifolium is considered one of the most threatened plants in grassland and it is listed as ‘Critically Endangered’ under the Queensland Nature Conservation Act, 1992.
Experimental design
In late March 2020, 14 circular plots measuring 12.56 m2 (2 m radius) were established within a population of Solanum papaverifolium that occupied an area of about 1200 m2. A permanent marked peg was placed in the centre of each plot and the location was recorded using a GPS. Plot locations were selected to represent a relatively moderate and uniform density of S. papaverifolium plants. The location of each S. papaverifolium plant from the centre peg was ascertained by measuring the distance and bearing from the centre peg with the aid of a measuring tape and a compass respectively. The height of each stem was also measured at this time. Seven of the circular plots were randomly selected and a 1 m wide strip was mowed around each plot as a fire break. On 6 October 2020, these seven plots were burnt and the other seven plots were left unburnt. The dry fuel weight was not measured but similar grassland sites have between 1.5 and 4.3 t ha−1 (Fensham et al. 2017). The wind speed at the time of the burn was 19 km h−1 and the fires were lit down-wind of the plots such that the fire crept slowly against the wind. The precipitation for the 12-month periods prior to March 2020 was below average (340.1 mm) and prior to March 2021, it was about average (575.3 mm). All stems in burned plots were removed by fire, and all aboveground stems in the unburnt plot died back to below the ground surface.
All plots were monitored in mid-March 2021. Minor excavation and the appearance of the stems indicated that all plants were ramets regenerating from rhizomes. As such, stems re-emerging from the same ramet location as original stems were categorised as surviving and where no stems re-emerged they were considered to have died. Stems emerging from different locations from those originally mapped were treated as recruits. We also examined the fruits to assess whether viable seeds were formed after fruit development.
During monitoring we measured the height of each stem, as well as which stems were flowering or not and the number of flowers per individual plant. We returned in May 2021 to see whether the plants that had flowered during our March 2021 visit had produced any fruits.
Data analysis
All statistical analyses were performed with R version 4.0.2 (R Development Core Team 2020). Analyses were undertaken at both the stem and plot scales. At the stem scale, height of individual stems (including surviving stems and recruits) was log-transformed and modelled as a function of burning treatment in a linear mixed-effects model by using the lmer() function in the lme4 package (Bates et al. 2015) with plot ID included as a random effect. Survival, flowering and fruiting (of those plants that flowered) were coded as binary response variables. Each of these variables was modelled as a function of burning treatment in generalised linear mixed effects models (GLMMs) with a binomial error distribution and logit-link function using the glmer() function, again with plot ID being included as a random effect. For the plants that flowered, the number of flowers was modelled as a function of burning treatment using a GLMM with Poisson error distribution and log-link function. The flowering and fruiting response variables were analysed separately for surviving and recruiting plants. In the models of flowering probability and flower number, plant height (log-transformed) and its interaction with burning treatment were included to account for size effects. Non-significant variables (as shown by model estimates and P-values) were sequentially removed to improve model parsimony, starting with interactions and then main terms. The interaction was included to test for differences in flowering–height relationships between the burnt and unburnt treatments. In the fruiting model, only stems that flowered were included and we did not account for height effects. Even though these analyses were undertaken at the stem scale, most are graphically represented as plot-scale proportions in the results figures.
At the plot scale, we calculated the change in the total number of stems (no. stems before / no. stems after × 100) and counted the number of new recruits (excluding surviving stems). To estimate the change in plot-scale biomass before versus after the treatments were applied, we first summed the height of all stems in each plot before and after the treatments. We then calculated log response ratios for each plot as:
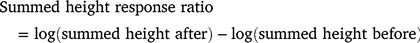
Positive values of this response ratio indicate an increase in summed plant height, zero indicates no change and negative values indicate a decline in summed plant height. These plot-scale response variables were modelled as a function of burning by using a linear model.
Results
Effects of fire on survival, recruitment rates and biomass
Prior to treatment, the number of individual stems for the unburnt and burnt plots was 115 and 130 respectively. On average, burnt plots had more stems in 2021 than in 2020 (average increase of 62%), whereas unburnt plots had fewer stems in 2021 than in 2020 (average decrease of 38%), and this difference was significant (P = 0.027, Fig. 2a).
We recorded 215 new S. papaverifolium recruits for existing ramets, of which 136 were in the burned plots and 79 in the unburnt plots and the mean number of recruits per plot was significantly greater in burnt than unburnt plots (P = 0.012, Fig. 2b).
The probability of survival was higher (0.56) in the burnt plots than the unburnt plots (0.44, Z = −1.975, P = 0.048). Individual stems in burnt plots were 19 cm tall, on average, compared with 36 cm tall in unburnt plots, and this difference was significant (t = 11.00, P = <0.001; Fig. 2c). On average, the summed height response ratio in the unburnt plots decreased from the original value after treatment (depicted as zero) and in the burnt plots it slightly increased from zero after treatment and this difference was significant (Fig. 2d; P = 0.042). These results indicate clear differences in biomass allocation between the burning treatments. Burning resulted in the emergence of many shorter stems the heights of which summed to pre-burning levels, whereas the summed heights in unburnt plots declined despite stems in these plots being taller on average.
Effect of treatment on flower and fruit production in surviving and recruiting plants
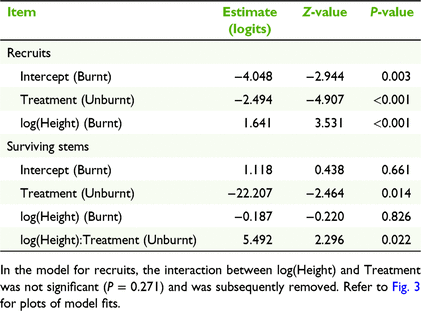
In the model of flowering probability for recruits, the interaction between treatment and height was not significant and was removed. In the resulting model, the effect of height was positive and significant (Table 1, Fig. 3a) and the effect of treatment (unburnt compared with burnt) was negative and strongly significant (Table 1). For a 30 cm tall recruit, the probability of flowering in a burnt plot was 0.82 (95% confidence intervals = 0.70–0.90) compared with 0.23 (0.17–0.42) in an unburnt plot (Fig. 3a). For survivors, the interaction between treatment and height was significant (Table 1) and indicated that flowering probability in burnt plots was unrelated to height (probabilities were ∼0.6 regardless of height), whereas flowering increased strongly with height in the unburnt plots (Fig. 3b). For a 30 cm tall survivor, the probability of flowering was 0.62 (0.40–0.80) in a burnt plot compared with 0.05 (0.006–0.28) in an unburnt plot (Fig. 3a). The number of flowers per plant was not significantly related to plant, burning treatment or their interaction (Supplementary Table S1).
|
For the new recruits that flowered, burning significantly increased the probability of fruiting (P = 0.012, Fig. 4). For the surviving stems that flowered, only burnt stems produced fruit (Fig. 4). In addition, our examination of the fruit showed that seeds were absent in fruits of S. papaverifolium plants.
Discussion
The single application of fire promoted the asexual recruitment of S. papaverifolium in subtropical grassland. Overall, the number of stems increased in burnt and remained constant in the unburnt plots (Fig. 2a). The number of recruits was significantly greater in burnt than unburnt plots (Fig. 2b). Some studies have shown that climatic conditions influence demographic responses to disturbance (Fensham et al. 2002; Moore et al. 2019; Griffith and Rutherford 2020). In the present study, rainfall in the preceding year was below average and about average during the experiment. During wetter conditions, perennial grasses are known to have a competitive advantage over smaller-statured forb species for resources such as water, light and nutrients (Bond and Parr 2010; Coller et al. 2013). The competitive effects of increased grass growth with average rainfall is a potential explanation for the decline in the number of ramets in unburnt plots at the start of the experiment compared with the previous year.
The greatly increased number of stems in burnt plots was partly offset by their smaller size than in unburnt plots. This suggests that belowground resources provide a partial limitation to aboveground biomass. However, the net result of burning overall was a greater height-response ratio (proxy for biomass) in the burnt plots (Fig. 2d), suggesting that fire does promote aboveground growth. Other studies have suggested that rhizomatous resprouters require fire or grazing to stimulate regrowth by axillary buds (Fidelis et al. 2010, 2014; Clarke et al. 2013; Zhao et al. 2013; Vidaller et al. 2019). Consistent with our findings, on semi-arid steppe in China, fire increased the density of recruits emerging from suckers, rhizomes, tillers and stolons (Zhao et al. 2013).
Stems of S. papaverifolium die back to below the ground during the subtropical dry season, even in the absence of fire. Thus, it was not the loss of aboveground parts in the burnt plots that triggered the positive response in the burnt plots. The positive response was either to smoke of the fire, or to nutrients from ash (Saulnier and Reekie 1995) or was a response to the temporary release from grass competition (Milton and Dean 2000). Future research could separate the influence of fire and competition experimentally by using burning and clipping surrounding perennial grasses (to remove biomass without fire).
The probability of flowering increased with plant height except for burnt survivors (Fig. 3b). Fire had a positive effect on flowering regardless of plant height, although this effect was complicated by an interaction between height and burning for survivors (Fig. 3b). Sixty-two per cent of plants flowered in the burnt plots compared with 30% in the unburnt plots, despite the burnt plants being shorter. This result is consistent with the documented positive effects of fire on flowering in grassland species (Table S2, Cummings et al. 2007; Pavlovic et al. 2011; Fidelis and Blanco 2014; Valkó et al. 2018; Griffith and Rutherford 2020). For instance, there were flushes of flower production after fire for the perennial herb Blandfordia grandiflora, particularly in the second post-fire year in Australian heathland (Griffith and Rutherford 2020). Although the reason for the increase in flower flush in S. papaverifolium after fire remains unknown, other studies have identified increased light availability and large stores of carbohydrate reserves as the triggers for increased flowering among fire-adapted plants (Abrahamson 1984; Saulnier and Reekie 1995; Bellingham and Sparrow 2000; Huffman and Werner 2000; Wyka and Galen 2000; Fidelis and Blanco 2014; Simpson et al. 2021).
Despite the positive effects of fire on S. papaverifolium flowering, it promoted fruiting only in 20% of the flowering stems among the new recruits in the burnt plots. In contrast, only 5% of the flowering stems in the unburnt plots were able to form fruits (in new recruits only). Limited fruit production and the rarity of recruitment from seeds appears to be offset by prolific vegetative reproduction in S. papaverifolium, a persistence strategy not uncommon for perennial species in grasslands (Morgan 1999a; Zhao et al. 2013). Increased probability of flowering after fire suggests that the resprouting recovery of aboveground biomass did not have a significant drain on root reserves, although this was not investigated in this study. We do not know whether repeated burning would sustain the positive response associated with flowering in these species. Future research would investigate whether pollination is limited after a fire.
Conclusions
Burning increased the number of ramets, total biomass and reproductive effort in S. papaverifolium. However, the importance of fire to the persistence of this threatened plant species remains uncertain. The proliferation of rhizomatous stems is contained within the pre-existing population of the species, and with the small-scale burns of this experimental trial, S. papaverifolium did not spread beyond where it originally occurred. Recruitment from seed and the constraints on the dispersal and distribution of this species need further investigation, especially because this study showed that even though fire promotes vegetative reproduction and flowering of this threatened herb, it may not be essential for population persistence. Ongoing research is required to understand the precise factors that promote flowering after burning and the barriers to seedling reproduction.
Supplementary material
Supplementary material is available online.
Data availability
Data are available at https://cloudstor.aarnet.edu.au/plus/s/ed3LrVdB4NuzRab.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported by The University of Queensland RTP scholarship to O. K.O.
Acknowledgements
We acknowledge the Traditional Custodians of the land on which this study was conducted.
References
Abrahamson WG (1984) Species responses to fire on the Florida Lake Wales Ridge. American Journal of Botany 71, 35–43.| Species responses to fire on the Florida Lake Wales Ridge.Crossref | GoogleScholarGoogle Scholar |
Araújo GM, Amaral AF, Bruna EM, Vasconcelos HL (2013) Fire drives the reproductive responses of herbaceous plants in a Neotropical swamp. Plant Ecology 214, 1479–1484.
| Fire drives the reproductive responses of herbaceous plants in a Neotropical swamp.Crossref | GoogleScholarGoogle Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bean AR (2004) The taxonomy and ecology of Solanum subg. Leptostemonum (Dunal) Bitter (Solanaceae) in Queensland and far north-eastern New South Wales, Australia. Austrobaileya 6, 639–816.
Bellingham PJ, Sparrow AD (2000) Resprouting as a life history strategy in woody plant communities. Oikos 89, 409–416.
| Resprouting as a life history strategy in woody plant communities.Crossref | GoogleScholarGoogle Scholar |
Bernardo HL, Goad R, Vitt P, Knight TM (2020) Nonadditive effects among threats on rare plant species. Conservation Biology 34, 1029–1034.
| Nonadditive effects among threats on rare plant species.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Parr CL (2010) Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biological Conservation 143, 2395–2404.
| Beyond the forest edge: ecology, diversity and conservation of the grassy biomes.Crossref | GoogleScholarGoogle Scholar |
Burke MJW, Grime J (1996) An experimental study of plant community invasibility. Ecology 77, 776–790.
| An experimental study of plant community invasibility.Crossref | GoogleScholarGoogle Scholar |
Clarke S, French K (2005) Germination response to heat and smoke of 22 Poaceae species from grassy woodlands. Australian Journal of Botany 53, 445–454.
| Germination response to heat and smoke of 22 Poaceae species from grassy woodlands.Crossref | GoogleScholarGoogle Scholar |
Clarke PJ, Lawes MJ, Midgley JJ, Lamont BB, Ojeda F, Burrows GE, Enright NJ, Knox KJE (2013) Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197, 19–35.
| Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire.Crossref | GoogleScholarGoogle Scholar |
Coller HV, Siebert F, Siebert SJ (2013) Herbaceous species diversity patterns across various treatments of herbivory and fire along the sodic zone of the Nkuhlu exclosures, Kruger National Park: original article. Koedoe: African Protected Area Conservation and Science 55, 1–6. https://hdl.handle.net/10520/EJC132975
Cronk Q (2016) Plant extinctions take time. Science 353, 446–447.
| Plant extinctions take time.Crossref | GoogleScholarGoogle Scholar |
Cummings DC, Fuhlendorf SD, Engle DM (2007) Is altering grazing selectivity of invasive forage species with patch burning more effective than herbicide treatments? Rangeland Ecology & Management 60, 253–260.
| Is altering grazing selectivity of invasive forage species with patch burning more effective than herbicide treatments?Crossref | GoogleScholarGoogle Scholar |
Fensham RJ (1997) Options for conserving large vegetation remnants in the Darling Downs, south Queensland. In ‘Conservation outside nature reserves’. (Eds P Hale, D Lamb) pp. 419–423. (Centre for Conservation Biology, University of Queensland: Brisbane, Qld, Australia)
Fensham RJ (1998) The grassy vegetation of the Darling Downs, south-eastern Queensland, Australia. Floristics and grazing effects. Biological Conservation 84, 301–310.
| The grassy vegetation of the Darling Downs, south-eastern Queensland, Australia. Floristics and grazing effects.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Fairfax RJ, Holman JE (2002) Response of a rare herb (Trioncinia retroflexa) from semi-arid tropical grassland to occasional fire and grazing. Austral Ecology 27, 284–290.
| Response of a rare herb (Trioncinia retroflexa) from semi-arid tropical grassland to occasional fire and grazing.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Butler DW, Fairfax RJ, Quintin AR, Dwyer JM, Souza L (2016) Passive restoration of subtropical grassland after abandonment of cultivation. Journal of Applied Ecology 53, 274–283.
| Passive restoration of subtropical grassland after abandonment of cultivation.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ, Butler DW, Laffineur B, MacDermott HJ, Morgan JW, Silcock JL (2017) Subtropical native grasslands may not require fire, mowing or grazing to maintain native-plant diversity. Australian Journal of Botany 65, 95–102.
| Subtropical native grasslands may not require fire, mowing or grazing to maintain native-plant diversity.Crossref | GoogleScholarGoogle Scholar |
Fidelis A, Blanco C (2014) Does fire induce flowering in Brazilian subtropical grasslands? Applied Vegetation Science 17, 690–699.
| Does fire induce flowering in Brazilian subtropical grasslands?Crossref | GoogleScholarGoogle Scholar |
Fidelis A, Müller SC, Pillar VD, Pfadenhauer J (2010) Population biology and regeneration of forbs and shrubs after fire in Brazilian Campos grasslands. Plant Ecology 211, 107–117.
| Population biology and regeneration of forbs and shrubs after fire in Brazilian Campos grasslands.Crossref | GoogleScholarGoogle Scholar |
Fidelis A, Appezzato-da-Glória B, Pillar VD, Pfadenhauer J (2014) Does disturbance affect bud bank size and belowground structures diversity in Brazilian subtropical grasslands? Flora – Morphology, Distribution, Functional Ecology of Plants 209, 110–116.
| Does disturbance affect bud bank size and belowground structures diversity in Brazilian subtropical grasslands?Crossref | GoogleScholarGoogle Scholar |
Griffith SJ, Rutherford S (2020) Flowering of Blandfordia grandiflora (Christmas bells) in response to fire frequency and temperature. Australian Journal of Botany 68, 449–457.
| Flowering of Blandfordia grandiflora (Christmas bells) in response to fire frequency and temperature.Crossref | GoogleScholarGoogle Scholar |
Helkowski JH, Norman EM (1997) Effects of fire on an endangered florida plant, Deeringothamnus rugelii. Florida Scientist 60, 118–123.
Hoekstra JM, Boucher TM, Ricketts TH, Roberts C (2005) Confronting a biome crisis: global disparities of habitat loss and protection. Ecology Letters 8, 23–29.
| Confronting a biome crisis: global disparities of habitat loss and protection.Crossref | GoogleScholarGoogle Scholar |
Hoffmann WA (1999) Fire and population dynamics of woody plants in a neotropical savanna: matrix model projections. Ecology 80, 1354–1369.
| Fire and population dynamics of woody plants in a neotropical savanna: matrix model projections.Crossref | GoogleScholarGoogle Scholar |
Huffman JM, Werner PA (2000) Restoration of florida pine savanna: flowering response of Lilium atesbaei to fire and roller-chopping. Natural Areas Journal 20, 12–23.
Kéry M, Matthies D, Spillmann H-H (2000) Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. Journal of Ecology 88, 17–30.
| Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea.Crossref | GoogleScholarGoogle Scholar |
Kubiak PJ (2009) Fire responses of bushland plants after the January 1994 wildfires in northern Sydney. Cunninghamia 11, 131–165.
Lesica P (1999) Effects of fire on the demography of the endangered, geophytic herb Silene spaldingii (Caryophyllaceae). American Journal of Botany 86, 996–1002.
| Effects of fire on the demography of the endangered, geophytic herb Silene spaldingii (Caryophyllaceae).Crossref | GoogleScholarGoogle Scholar |
Lewis T, Clarke PJ, Reid N, Whalley RDB (2008) Perennial grassland dynamics on fertile plains: is coexistence mediated by disturbance? Austral Ecology 33, 128–139.
| Perennial grassland dynamics on fertile plains: is coexistence mediated by disturbance?Crossref | GoogleScholarGoogle Scholar |
Lunt I (1995) A flexible approach to grassland management based on plant life-forms and growth-forms. In ‘Management of relict lowland grasslands: proceedings of a workshop and public seminar’. 24 and 25 September 1993. Conservation Series no. 8. (Eds Sharp, R Rehwinkel) (ACT Parks and Conservation Service, Wildlife Research Unit: Canberra, ACT, Australia)
Lunt I, Morgan J (2002) The role of fire regimes in temperate lowland grasslands of southeastern Australia. In ‘Flammable Australia: fire regimes and biodiversity of a continent’. (Eds RA Bradstock, JE Williams, MA Gill) (Cambridge University Press: Cambridge, UK)
MacDougall AS, Turkington R (2007) Does the type of disturbance matter when restoring disturbance-dependent grasslands? Restoration Ecology 15, 263–272.
| Does the type of disturbance matter when restoring disturbance-dependent grasslands?Crossref | GoogleScholarGoogle Scholar |
Matthies D, Bräuer I, Maibom W, Tscharntke T (2004) Population size and the risk of local extinction: empirical evidence from rare plants. Oikos 105, 481–488.
| Population size and the risk of local extinction: empirical evidence from rare plants.Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Díaz S, Lavorel S, Cramer W (1999) Plant functional types and disturbance dynamics – Introduction. Journal of Vegetation Science 10, 603–608.
| Plant functional types and disturbance dynamics – Introduction.Crossref | GoogleScholarGoogle Scholar |
Menges ES, Gordon DR (1996) Three levels of monitoring intensity for rare plant species. Natural Areas Journal 16, 227–237.
Menges ES, Quintana-Ascencio PF (2004) Population viability with fire in Eryngium cuneifolium: deciphering a decade of demographic data. Ecological Monographs 74, 79–99.
| Population viability with fire in Eryngium cuneifolium: deciphering a decade of demographic data.Crossref | GoogleScholarGoogle Scholar |
Milton SJ, Dean WRJ (2000) Disturbance, drought and dynamics of desert dune grassland, South Africa. Plant Ecology 150, 37–51.
| Disturbance, drought and dynamics of desert dune grassland, South Africa.Crossref | GoogleScholarGoogle Scholar |
Moore NA, Camac JS, Morgan JW (2019) Effects of drought and fire on resprouting capacity of 52 temperate Australian perennial native grasses. New Phytologist 221, 1424–1433.
| Effects of drought and fire on resprouting capacity of 52 temperate Australian perennial native grasses.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1997) The effect of grassland gap size on establishment, growth and flowering of the endangered Rutidosis leptorrhynchoides (Asteraceae). Journal of Applied Ecology 34, 566–576.
| The effect of grassland gap size on establishment, growth and flowering of the endangered Rutidosis leptorrhynchoides (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1998) Importance of canopy gaps for recruitment of some forbs in Themeda triandra-dominated grasslands in south-eastern Australia. Australian Journal of Botany 46, 609–627.
| Importance of canopy gaps for recruitment of some forbs in Themeda triandra-dominated grasslands in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1999a) Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia. Plant Ecology 144, 127–144.
| Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Morgan JW (1999b) Effects of population size on seed production and germinability in an endangered, fragmented grassland plant. Conservation Biology 13, 266–273.
| Effects of population size on seed production and germinability in an endangered, fragmented grassland plant.Crossref | GoogleScholarGoogle Scholar |
Munk LM, Hild AL, Whitson TD (2002) Rosette recruitment of a rare endemic forb (Gaura neomexicana Subsp. coloradensis) with canopy removal of associated species. Restoration Ecology 10, 122–128.
| Rosette recruitment of a rare endemic forb (Gaura neomexicana Subsp. coloradensis) with canopy removal of associated species.Crossref | GoogleScholarGoogle Scholar |
Pausas JG (2001) Resprouting vs seeding: a Mediterranean perspective. Oikos 94, 193–194.
| Resprouting vs seeding: a Mediterranean perspective.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Bradstock RA, Keith DA, Keeley JE (2004) Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85, 1085–1100.
| Plant functional traits in relation to fire in crown-fire ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pavlik BM, Nickrent DL, Howald AM (1993) The recovery of an endangered plant. I. Creating a new population of Amsinckia grandiflora. Conservation Biology 7, 510–526.
| The recovery of an endangered plant. I. Creating a new population of Amsinckia grandiflora.Crossref | GoogleScholarGoogle Scholar |
Pavlovic NB (1994) Disturbance-dependent persistence of rare plants: anthropogenic impacts and restoration implications. In ‘Restoration of endangered species: conceptual issues, planning and implementation’. (Eds CJ Whelan, ML Bowles) pp. 159–193. (Cambridge University Press: Cambridge, UK)
Pavlovic NB, Leicht-Young SA, Grundel R (2011) Short-term effects of burn season on flowering phenology of savanna plants. Plant Ecology 212, 611–625.
| Short-term effects of burn season on flowering phenology of savanna plants.Crossref | GoogleScholarGoogle Scholar |
Pressland AT (1982) Fire in the management of grazing lands in Queensland. Tropical Grasslands 16, 104–112.
Price JN, Good MK, Schultz NL, Guja LK, Morgan JW (2019) Multivariate drivers of diversity in temperate Australian native grasslands. Australian Journal of Botany 67, 367–380.
| Multivariate drivers of diversity in temperate Australian native grasslands.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2020) ‘R: A language and environment for statistical computing.’ (R Development Core Team: Vienna, Austria)
Roxburgh SH, Shea K, Wilson JB (2004) The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85, 359–371.
| The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence.Crossref | GoogleScholarGoogle Scholar |
Saulnier TP, Reekie EG (1995) Effect of reproduction on nitrogen allocation and carbon gain in Oenothera biennis. Journal of Ecology 83, 23–29.
| Effect of reproduction on nitrogen allocation and carbon gain in Oenothera biennis.Crossref | GoogleScholarGoogle Scholar |
Silcock JL, Fensham RJ (2018) Using evidence of decline and extinction risk to identify priority regions, habitats and threats for plant conservation in Australia. Australian Journal of Botany 66, 541–555.
| Using evidence of decline and extinction risk to identify priority regions, habitats and threats for plant conservation in Australia.Crossref | GoogleScholarGoogle Scholar |
Silcock J, Collingwood TL, Llorens T, Fensham R (2021) Action Plan for Australia’s Imperilled Plants 2021. NESP Threatened Species Recovery Hub, Brisbane, Qld, Australia.
Simpson KJ, Jardine EC, Archibald S, Forrestel EJ, Lehmann CER, Thomas GH, Osborne CP (2021) Resprouting grasses are associated with less frequent fire than seeders. New Phytologist 230, 832–844.
| Resprouting grasses are associated with less frequent fire than seeders.Crossref | GoogleScholarGoogle Scholar |
Valkó O, Török P, Matus G, Tóthmérész B (2012) Is regular mowing the most appropriate and cost-effective management maintaining diversity and biomass of target forbs in mountain hay meadows? Flora – Morphology, Distribution, Functional Ecology of Plants 207, 303–309.
Valkó O, Kelemen A, Miglécz T, Török P, Deák B, Tóth K, Tóth JP, Tóthmérész B (2018) Litter removal does not compensate detrimental fire effects on biodiversity in regularly burned semi-natural grasslands. Science of The Total Environment 622–623, 783–789.
| Litter removal does not compensate detrimental fire effects on biodiversity in regularly burned semi-natural grasslands.Crossref | GoogleScholarGoogle Scholar |
Vidaller C, Dutoit T, Ramone H, Bischoff A (2019) Fire increases the reproduction of the dominant grass Brachypodium retusum and Mediterranean steppe diversity in a combined burning and grazing experiment. Applied Vegetation Science 22, 127–137.
| Fire increases the reproduction of the dominant grass Brachypodium retusum and Mediterranean steppe diversity in a combined burning and grazing experiment.Crossref | GoogleScholarGoogle Scholar |
Vujnovic K, Wein RW, Dale MRT (2002) Predicting plant species diversity in response to disturbance magnitude in grassland remnants of central Alberta. Canadian Journal of Botany 80, 504–511.
| Predicting plant species diversity in response to disturbance magnitude in grassland remnants of central Alberta.Crossref | GoogleScholarGoogle Scholar |
Walsh F, Douglas J (2011) No bush foods without people: the essential human dimension to the sustainability of trade in native plant products from desert Australia. The Rangeland Journal 33, 395–416.
| No bush foods without people: the essential human dimension to the sustainability of trade in native plant products from desert Australia.Crossref | GoogleScholarGoogle Scholar |
Whelan RJ (1995) ‘The ecology of fire.’ (Cambridge University Press: Cambridge, UK)
White L, Catterall C, Taffs K (2020) Fire can promote germination, recruitment and seed bank accumulation of the threatened annual grass Arthraxon hispidus. Australian Journal of Botany 68, 413–424.
| Fire can promote germination, recruitment and seed bank accumulation of the threatened annual grass Arthraxon hispidus.Crossref | GoogleScholarGoogle Scholar |
Williams DW, Jackson LL, Smith DD (2007) Effects of frequent mowing on survival and persistence of forbs seeded into a species-poor grassland. Restoration Ecology 15, 24–33.
| Effects of frequent mowing on survival and persistence of forbs seeded into a species-poor grassland.Crossref | GoogleScholarGoogle Scholar |
Williams N, Marshall A, Morgan J (2015) ‘Land of sweeping plains: managing and restoring the native grasslands of south-eastern Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Wyka T, Galen C (2000) Current and future costs of reproduction in Oxytropis sericea, a perennial plant from the colorado rocky mountains, U.S.A. Arctic, Antarctic, and Alpine Research 32, 438–448.
| Current and future costs of reproduction in Oxytropis sericea, a perennial plant from the colorado rocky mountains, U.S.A.Crossref | GoogleScholarGoogle Scholar |
Zhao L-P, Wu G-L, Shi Z-H (2013) Post-fire species recruitment in a semiarid perennial steppe on the Loess Plateau. Australian Journal of Botany 61, 29–35.
| Post-fire species recruitment in a semiarid perennial steppe on the Loess Plateau.Crossref | GoogleScholarGoogle Scholar |


