Optimising conservation translocations of threatened Caladenia (Orchidaceae) by identifying adult microsite and germination niche
Noushka Reiter A B * and Myles H. M. Menz
A B * and Myles H. M. Menz  C D E F
C D E F
A Royal Botanic Gardens Victoria, Science Division, Corner of Ballarto Road and Botanic Drive, Cranbourne, Vic. 3977, Australia.
B Ecology and Evolution, Research School of Biology, ANU College of Science, The Australian National University, RN Robertson Building, 46 Sullivans Creek Road, Canberra, ACT 2600, Australia.
C College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
D Department of Migration, Max Planck Institute of Animal Behavior, D-78315 Radolfzell, Germany.
E Department of Biology, University of Konstanz, D-78547 Konstanz, Germany.
F School of Biological Sciences, The University of Western Australia, Crawley, WA 6009, Australia.
Australian Journal of Botany 70(3) 231-247 https://doi.org/10.1071/BT21132
Submitted: 9 November 2021 Accepted: 30 March 2022 Published: 6 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Conservation translocations are increasingly being used in the management of rare plants, yet have low success in maintaining populations through recruitment.
Aims: We investigated whether the survival of translocated plants, recruitment and, therefore, cost effectiveness, can be improved by selecting optimal microsites for both adults and seedlings.
Methods: Caladenia colorata plants propagated symbiotically with Serendipita australiana (n = 735) were introduced to four sites where the pollinator was present and vegetation matched wild populations. Plant demography was monitored over 6 years. The relationship between microsite variables and measures of orchid survival, re-emergence, flowering and recruitment were analysed with generalised linear mixed-effects models. We then estimated potential improvement in emergence and recruitment, if microsite selection was optimised.
Key results: A total of 77% of plants survived translocation, and populations grew by 84% through recruitment (n = 615). Survival was positively associated with cover of leaf litter, graminoids and cryptogams. Recruitment was positively correlated with soil moisture. The majority of recruitment was within 5 cm of adult C. colorata plants. The potential improvement by selecting favourable microsites increased adult survival by up to 8% and recruitment by 10–40%.
Conclusions: Incorporating both the germination niche and adult plant niche within plant translocations more broadly could significantly improve long-term population persistence and the utilisation of conservation funding.
Implications: Our results are directly applicable to 58 endangered Caladenia species in the subgenus Calonema, owing to their shared mycorrhizal association with S. australiana. Furthermore, our results are applicable to all plant translocations as understanding germination niche and microhabitat requirements is likely to improve success overall.
Keywords: Caladenia colorata, conservation, endangered species, microsite, mycorrhiza, Orchidaceae, recruitment, regenerative niche, Serendipita, translocation.
Introduction
In total, 39% of all plant species are at risk of extinction (Nic Lughadha et al. 2020). Conservation translocation, sometimes known as rewilding or reintroduction, is increasingly being used as a key tool to aid the recovery of threatened plants (Silcock et al. 2019). Conservation translocation is an intensive conservation action, typically undertaken when a species faces substantial extinction risk (Seddon et al. 2014), or if the original site becomes unsuitable through habitat degradation (Maunder 1992) or climate change (Thomas 2011). Translocations typically involve reinforcement (bolstering existing populations), reintroduction (establishing new populations within a species current range) or assisted colonisation (outside their known range; Seddon 2010). Unfortunately, despite some noted success stories (Colas et al. 2008; Draper Munt et al. 2016; Reiter et al. 2016), conservation translocations of plants are generally characterised by low success rates and little to no recruitment (see reviews by Godefroid et al. 2011 and Silcock et al. 2019). However, given the vast number of conservation translocations being conducted, improved implementation could allow this method to play a substantial role in the preservation of numerous species of threatened plants. Clearly, conservation translocations are a well-recognised part of the plant conservation toolkit, the challenge that remains is how to implement them to maximum effect.
Plant conservation translocations and associated habitat management actions require a significant time and financial investment (Zimmer et al. 2019). Reviews of the literature suggest that rather than conservation translocation being an inappropriate management action, translocations could be improved by increasing the founding population size (Albrecht and Maschinski 2012; Silcock et al. 2019), and by improving understanding of and utilising biological knowledge of a species to inform translocation site selection (Godefroid et al. 2011; Reiter et al. 2016, 2017). Critical for plant translocation success is understanding the regenerative niche of a species, which is the niche where the adult plant survives, disperses, and recruits, i.e. all that is required to produce the next generation (Grubb 1977). However, only a small proportion of translocations assess the habitat niche (or microsite; Grubb 1977) of the translocated propagules (i.e. the chemical and physical limits of the habitat, tolerated by the adults). For example, the review by Maschinski et al. (2012) found that only 11% of 200 translocations tested the microsite suitability for translocated individuals. Indeed, inferring the microsite requirements needed for recruitment on the basis of observations of senile, long-lived plants can be misleading (Rasmussen et al. 2015), often owing to threatened species in remnant populations not recruiting and being at their extinction threshold (Hanski and Ovaskainen 2002). Very few plant translocations have assessed the regenerative niche requirements at the microsite scale (for exceptions, see Maschinski et al. 2004; Wendelberger and Maschinski 2016). Not considering the regenerative niche of a species could provide a partial explanation for the low survival rates and recruitment that characterise many translocations (Godefroid et al. 2011; Silcock et al. 2019).
With over 28 484 species (WCSP 2021), the Orchidaceae is the second-largest plant family and features prominently on lists of threatened species in many countries (e.g. Dodson and Gentry 1991; Qin et al. 2017; Australian Government 2021). Typically, orchids are reliant on one or a few species of pollinator to successfully achieve pollination and, therefore, seed set (Phillips et al. 2020). In addition, all orchids are reliant on mycorrhizal fungi for germination in the wild (Leake 1994; Rasmussen 2002; Rasmussen and Rasmussen 2009) and, to varying extents, as seedlings and adult plants (Girlanda et al. 2011; Selosse and Martos 2014; Gebauer et al. 2016). Consequently, for many species, consideration of both the pollinator and mycorrhizal fungi is essential when selecting translocation sites for orchids (Reiter et al. 2017, 2019). In orchids, the suitability of microsites for germination is likely to be determined by both the spatial distribution of the fungi, and the physiological requirements of both orchid and fungus.
Studies of natural or translocated populations of orchids have often assessed habitat suitability at the population level and for suitability of adult persistence (Janes et al. 2010; Duncan and Moloney 2018; Janissen et al. 2021), or if comparing microsites, examined the persistence of flowering individuals (Moisan and Pellerin 2013), rather than seedlings. Investigation of microsites suitable for germination of terrestrial orchids (regenerative niche) has been undertaken using seed-burial trials, which is when seed is put in packets and buried near plants or across transects in existing populations (Rasmussen et al. 2015).
Seed-burial trials may not be appropriate for threatened species, because seed sources may be limited, and orchid recruitment is sporadic and often weather dependent (Phillips et al. 2020). Furthermore, this method does not resolve if microsite conditions can support adult plants (Phillips et al. 2020). Similarly, changes in rainfall patterns between years could see orchids germinate that may not survive (Jersáková and Malinová 2007). In the case of many threatened species, sites may be degraded, within the urban landscape (Duncan and Moloney 2018; Janissen et al. 2021) and unrepresentative of the broader habitat requirements of the species. To optimise conservation translocation plantings and funding to the greatest effect, and ensure successful recruitment and sustainable populations, we need to know both the sites that are suitable for adult plants and the sites that are suitable for recruitment, and where these intersect.
In Australia, the orchid flora is characterised by high endemism (Major 1988) and many threatened species, with 16% of all nationally threatened plants being orchids (Australian Government 2021). Australian orchids have recently been characterised by rapid declines, making conservation translocation a key conservation action for many species (Australian Government 2021). Caladenia, with an Australasian distribution (WCSP 2021), contains 382 species (Backhouse et al. 2019) and is the most threatened genus of Australian plants. Caladenia has 71 endangered taxa, of which 58 belong to the subgenus Calonema. Our study focuses on the subgenus Calonema, because understanding microsite preference will inform the largest proportion of proposed conservation translocations. We used Caladenia colorata D.L.Jones as a model species to examine whether a better understanding of the regenerative niche can (1) improve survival and flowering of adults, (2) increase recruitment in translocated populations, and (3) reduce translocation cost.
Materials and methods
Study species
Caladenia colorata is a terrestrial orchid that is nationally endangered under the Environment Protection Biodiversity and Conservation Act 1999 and endemic to south-eastern Australia (Reiter et al. 2018). The total number of wild plants across Victoria and South Australia is fewer than 900 individuals, known from 11 sites (Obst 2005), with many plants not having been observed for many years. Threats to the species include habitat destruction, controlled burns at inappropriate times of year, removal of roadside populations with machinery, grazing, and weed invasion (Obst 2005; Reiter et al. 2018).
Caladenia colorata typically grows up to 30 cm in height when flowering, with a single hairy leaf to 10 cm and one to three flowers on a solitary scape per year, with flowers in colour from pale yellow to pink or yellow with a red lip, and having a subtle sweet smell (Fig. 1a). Caladenia colorata flowers in spring, from mid-September to October, and undergoes annual dormancy as an underground tuber over the summer and autumn months (December–April). Caladenia colorata occurs in sandy soils in open Eucalyptus-dominated woodland (Obst 2005; Reiter et al. 2018). The majority of Caladenia in subgenus Calonema are pollinated by thynnid wasps (Hymenoptera, Thynnidae; Phillips et al. 2009), with C. colorata being primarily pollinated by a single species of nectar-foraging thynnid wasp (Reiter et al. 2018).
|
|
Caladenia and related genera are known to form mycorrhizal associations with members of the genus Serendipita (Serendipitaceae) in the order Sebacinales (Weiß et al. 2016; Whitehead et al. 2017). Serendipita forms mycorrhizal associations with orchids world-wide, along with liverworts, herbaceous angiosperms and other ectomycorrhizal plants (Weiß et al. 2016). In Caladenia subgenus Calonema, mycorrhizal specificity is high, with most orchid species using a single species of mycorrhiza (Reiter et al. 2020), Serendipita australiana, including C. colorata (Reiter et al. 2020; Oktalira et al. 2021).
Propagation
Sixty plants from across the remaining Victorian populations of C. colorata were hand-pollinated between flowers greater than 10 m apart. Seed was collected from pods, 4–6 weeks after pollination. The seed was cleaned and dried at 15% relative humidity for 10 days, before being stored at −20°C until further use.
Mycorrhiza were isolated from the collar region of six orchids. Collars were collected and taken back to the laboratory where they were washed thoroughly to remove dirt. Samples were then rinsed for 15 min under running water in a tea strainer. Samples were sterilised using 0.05% NaOCl for 3 min under a laminar flow and then rinsed three times in sterile distilled water. Glass knives were used to slice open the collars and individual pelotons were pipetted out and serially rinsed in seven droplets of sterile distilled water, and plated onto fungal isolation medium (FIM; Clements et al. 1986) containing 0.05 g L−1 streptomycin. Cultures were grown for 2 months, before being used in germination trials. The identification of the mycorrhizal fungi used in the germination trial as S. australiana was published in Reiter et al. (2020).
Plants were grown from seed to mature flowering individuals by using the techniques described in Reiter et al. (2016). Seeds were surface-sterilised with 0.5% NaOCl (10% Domestos) for 3 min, drained by vacuum onto a 3-μm pore filter, rinsed with sterile water and plated onto sterile filter paper on plates of oatmeal agar (OMA; Clements and Ellyard 1979) with 0.1 g L−1 yeast extract, in a laminar flow cabinet. A 1-cm OMA agar block colonised with S. australiana was placed on two ends of the filter paper. Plates were sealed with Parafilm and incubated in the dark at a temperature cycle of 16°C for 8 h and 22°C for 16 h, for 3 months. Once protocorms germinated, they were transferred to growth racks, with the above temperature cycle and a light period of 16 h and dark period of 8 h. Seedlings were aseptically transferred to plastic food containers, containing a 3-cm layer of OMA with a 3-cm layer of symbiotic replating media, consisting of 2.5 g finely ground oats L−1, 0.1 g yeast extract L−1, 1000 mL vermiculite L−1 and 100 mL de-ionised water L−1 over the top of the agar base. Seedlings were grown for a further 8–12 weeks before de-flasking. Plants were de-flasked into Royal Botanic Gardens Victoria terrestrial orchid potting mix (Bio Gro) in the shade house. De-flasked plants had their container upturned, covering the pot for 2 weeks after de-flasking, to maintain humidity and minimise transplant shock. Pots were watered as required. After plants had been through two summer dormancies in the nursery, they were considered ready for translocation (Fig. 1a).
Selection of the conservation translocation sites
Four translocation sites were selected on the basis of the following requirements: the bushland containing the sites was greater than 100 ha, the sites had vegetation and soil matching the extant sites of C. colorata (Fig. 1b, including having sandy loam soil and the presenence of Eucalyptus costata subsp. murrayana), the land is permanently protected (covenanted private property) and the pollinator was confirmed to be present prior to translocation (Reiter et al. 2016, 2018; Reiter 2021). In addition, each site was fenced with rabbit proof fencing, and individual plants were caged to prevent herbivory. The translocation sites chosen for C. colorata were located inside a large (>100 ha), intact, privately owned property under a Trust for Nature conservation covenant, adjacent to the Little Desert National Park in south-eastern Australia (Fig. 2). The closest wild population was 6 km away from the translocation sites (Fig. 2). The four translocation sites within the overall property were in open woodland that occurs in swales within the landscape and are dominated by Eucalyptus leucoxylon and Eucalyptus incrassata (Fig. 1b). The rainfall at the translocation sites is typically seasonal, with winter rainfall being dominant (Supplementary Fig. S1), and hot, dry summers (Bureau of Meteorology 2020). Within the study time frame, rainfall in 2015 was considered below average and a year of drought, whereas all other years were near average rainfall (Bureau of Meteorology 2020).
|
|
Layout of the translocated populations
One population of C. colorata was planted at each of the four translocation sites. Orchids were planted in three 30 × 30-m populations (Sites A, C and D) and one 15 × 15-m population (Site B). The translocation sites were separated by at least 200 m. Orchids within an introduced population were planted in a grid design, where each unit within the grid was 1 m2, with a plant in each corner of the square and one in the middle. Each unit was separated from another by 4 m (Fig. S2). This ensured that each plant in a population had the microsite selected by the plot design rather than any predefined vegetation characteristics. Plants were introduced in 2015, 2016, 2017 and 2018. In total, 735 plants were included in this study, with the following numbers planted each year: n = 320 (2015), n = 187 (2016), n = 177 (2017) and n = 51 (2018). Random plants in 2016–2018 were planted as doubles (two orchids approximately 5 cm apart from each other) and the majority of 2015 plants were planted as doubles (as we had assumed in 2015, there would be a higher mortality due to translocation stress). Orchids that were unable to be traced through time as individuals were excluded from this study.
Orchids were planted in July, with a numbered permanent marker placed 10 cm to the north of each plant, to aid in re-emergence monitoring and identification of the plants in subsequent years (Fig. 1c). The location of each plant was triangulated in the field to aid future detection. Each plant was caged and watered (up to the monthly average rainfall, only if rainfall fell below average) for the first season only, until plants entered their first summer dormancy in the wild.
Microsite variable collection
In 2018, we assessed the microsite characteristics of each of the 735 introduced plants and that of the recruits from the 2015 to 2016 plantings. Six plants were excluded from the analysis due to incomplete emergence data (leaving a total of n = 729). To help estimate the cover of microsite variables, we used a 10 × 10-cm wire square and placed this around the plant, with the focal plant at the centre of the square (Fig. 1c). We used a 10 × 10-cm square because we considered that this would be appropriate to record microsite charactieristics at a scale relevant to orchid emergence and recruitment. For each square, we recorded percentage cover of leaf litter, bare ground, cryptogams (mosses, liverworts, hornworts and lichens), shrubs, and graminoid monocots (grasses, sedges and rushes). The orchid was not included in the estimation of vegetation cover. In each square, we measured moisture and temperature by using a WET-2 Sensor (Delta-T Devices Ltd) attached to a HH2 Moisture Meter (Delta-T Devices Ltd) calibrated to sandy soil (with a maximum error of 4%), by inserting the probe three times randomly into different areas within the 10 × 10-cm square. All sites were sampled for moisture on the same day, during the active growing time of C. colorata, in late winter. We used the mean of the three soil moisture measurements for the analysis. Because moisture varies throughout the year, these measurements serve as an indicative qualitative comparison among microsites.
Monitoring of the translocated orchids
Plants were monitored each year after planting for emergence (July), flowering (September–October) and seed set (November–December). In addition, any recruits were recorded (Fig. 1d). Substantial numbers (hundreds) of recruits were first seen in the introduced populations in July–September of 2018. The translocated populations were then surveyed by walking transects 1 m apart within and outside the 30 × 30-m fences, to record the number and location of the recruits. This resulted in 615 recruits being located, that were subsequently tagged, with their distance from the closest adult plant measured (cm). Of these recruits, 416 were within the 10 × 10-cm squares that had microsite variables recorded and, thus, were used in the analysis of the regenerative niche (Fig. 1d).
Data analysis
All analyses were conducted in R (ver. 4.0.2, R Foundation for Statistical Computing, Vienna, Austria, see https://cran.r-project.org/bin/windows/base/). We used generalised linear mixed-effects models (GLMM) with a binomial error distribution, to assess the relationship among microsite variables (soil moisture (%), soil temperature (°C), percentage cover of leaf litter, bare ground, cryptogams, shrubs or graminoids) and measures of orchid survival (emergence after 1 year and after 2 or more years), and flowering 1 and 2 years after planting. Modelling was undertaken using the package ‘lme4’ (ver. 1.1.28, see https://cran.r-project.org/web/packages/lme4/; Bates et al. 2015).
Predictor variables (soil moisture, soil temperature, percentage cover of leaf litter, bare ground, cryptogams, shrub or graminoid) were checked for pairwise correlations by using Spearman’s rank correlation coefficient. Leaf litter cover and bare ground were correlated (Spearman’s coefficient = −0.79), so we retained leaf litter cover for the models. All other variables were not strongly correlated (Spearman’s coefficient < 0.7). Multicollinearity between the predictor variables in the models was assessed using variance inflation factors (VIF) in the package ‘car’ (ver. 3.0.12, see https://CRAN.R-project.org/package=car; Fox and Weisberg 2019). In all cases, VIF was <2, indicating no issues with multicollinearity.
Models were run separately for C. colorata planted in 2015 and those planted in 2016–2018, because these years differed in the number of double plantings. In 2015, 309 of 319 plants were doubles, whereas there were only 69 of 410 planted as doubles in the following years (2016–2018). The 10 singles from 2015 and the 69 doubles from 2016 to 2018 were excluded from the analysis. Each of the double plants (planted within 10 cm of each other) from 2015 were assigned to a unique pair number.
Models for 2015 included pair number nested within site as a random factor, whereas models for 2016–2018 included only site as a random factor. In all cases, model selection was conducted using backwards stepwise regression, starting with a model containing all the predictors and removing those that were not significant. Significance of predictors (P < 0.05) was determined by likelihood-ratio tests (X2) comparing a model with and without the variable of interest, using ANOVA. Only significant predictors were retained for the final models. Model assumptions were assessed using the simulateResiduals function in the package ‘DHARMa’ (ver. 0.4.1, see https://cran.r-project.org/package1/4DHARMa). We also tested for a possible interaction between leaf litter cover and mean soil moisture, for all measures of orchid survival, flowering and recruitment.
We used a GLMM with a negative binomial error distribution to investigate the relationship between microsite variables and recruitment (number of seedlings). A negative binomial distribution was used to account for overdispersion. When modelling recruitment, we included only recruitment data from the sites that were planted in 2015 and 2016 and those plants that had flowered at least once during the study period (because these had likely set seed at least once during the study period, average pollination rate for C. colorata is 50.70 ± 2.20% (s.e.) for an individual flower, with plants having up to four flowers, Reiter et al. 2018). Data from the 2017 planting year was excluded from the analysis, because only 14 microsites had recruits, whereas 62 microsites in 2015 and 69 microsites in 2016 plantings had recruits. Our analysis of recruitment also excluded all microsites that did not have a surviving adult plant (that had flowered and set seed). There were only 16 occurrences of recruitment that were excluded, with the other 153 excluded microsites having no recruitment.
Cost analysis
We calculated the potential improvement in survival and recruitment, if we were to select favourable microsites for translocation of plants, on the basis of the results of our models. Initially, we calculated the base rates (proportion) of emergence in the first year, after 2 or more years (for orchids planted in 2015 and 2016–2018), and recruitment (orchids planted in 2015 and 2016). Base rates were calculated as the proportion of orchids that emerged for each planting year, or the proportion of microsites with at least one recruit.
We then used the models to predict the value of each of the significant explanatory variables where the 95% confidence intervals for emergence or recruitment exceeded the base rate. On the basis of this, we then selected the microsites with these values for the explanatory variables and calculated the probability of emergence, and there being at least one recruit, for these sites. Finally, we compared the difference between the emergence and recruitment rates for the ‘optimal’ sites and the base rates for the whole sample, to determine a percentage improvement, if optimal microsites were selected for translocations.
The percentage improvement in survival was then converted into a cost saving, on the basis of published estimates of the costs of conservation translocations. The average price in Australian dollars (A$) for introduction of a single population was estimated by Zimmer et al. (2019) as A$102 941 ± $49 089 (s.e.). For a population of approximately 200 individuals, the cost of translocation would equate to approximately A$514 ± $245 (s.e.) per plant. However, this is likely to represent only a small proportion of the total cost of translocation, once considerations such as preliminary ecological studies on mycorrhiza, pollinators and genetic diversity are included. In addition, this is likely to underestimate cost because the majority of translocations that have occurred in Australia were fewer than 200 individuals (Silcock et al. 2019).
Results
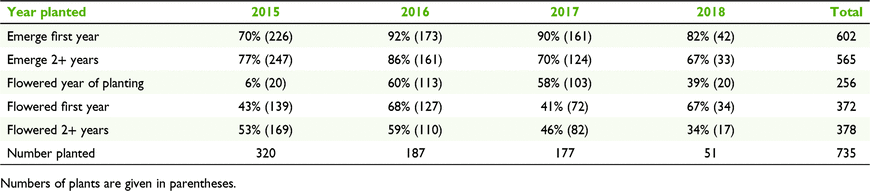
Between 70 and 90% of the 735 adult C. colorata plants translocated between 2015 and 2018 re-emerged 12 months after planting, depending on the year of translocation (Table 1). Emergence 2 or more years post-translocation (monitoring 2015–2020) ranged between 67 and 86% of plants (Table 1), depending on year of translocation. Flowering ranged from 41 to 68% in the first year post-planting and between 34 and 59% 2 or more years post-planting (Table 1). Emergence after 2 or more years did not differ between those plants planted as doubles (78%, 297 of 379) and those planted as singles (77%, 274 of 355).
|
Is emergence and flowering of translocated orchids affected by microsite conditions?
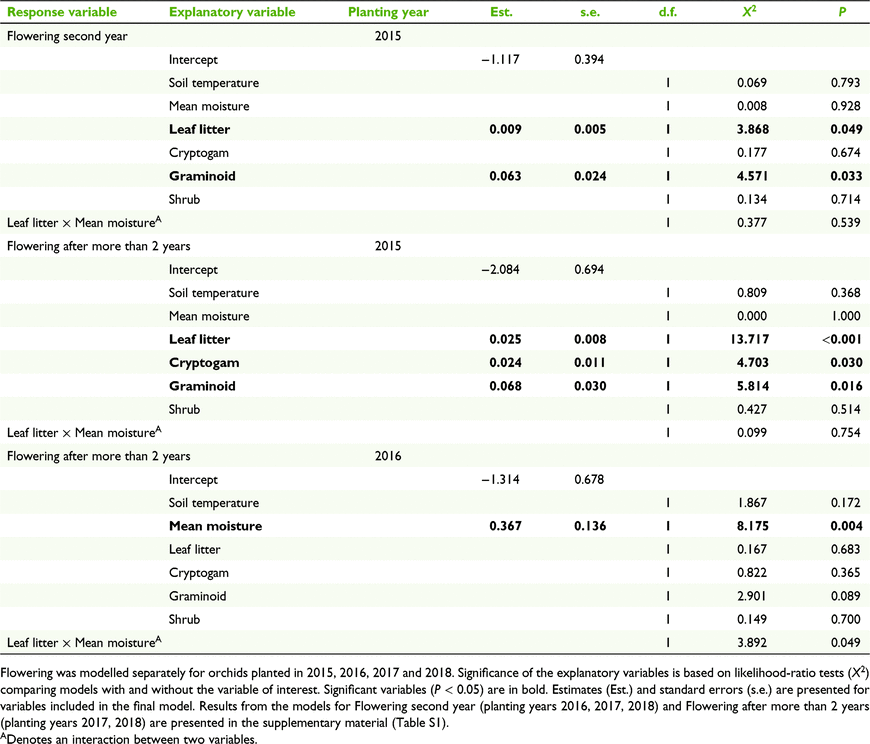
For those orchids planted in 2015, emergence 1 year after introduction was positively related to cryptogam cover (P = 0.004) and negatively related to shrub cover (P = 0.039, Fig. 3a). For those orchids planted in 2016–2018, emergence was positively related to leaf litter cover (P = 0.012, Table 2, Fig. 3b).
Emergence 2 or more years after planting was positively correlated with leaf litter cover, for all planting years (P < 0.001; P = 0.001, for 2015 and 2016–2018, respectively; Fig. 3c, f). In addition, emergence was also positively correlated with cryptogam cover (P = 0.005, Fig. 3e) and graminoid cover (P = 0.007, Table 2, Fig. 3d), for those plants planted in 2015.
For plants translocated in 2015, flowering in the second year after planting (i.e. 16 months post-planting) and 2 or more years after planting (28, 40 months plus after planting) was positively associated with leaf litter cover (P = 0.049 and P < 0.001 respectively, Table 3) and graminoid cover (P = 0.033 and P = 0.016 respectively, Table 3). Furthermore, for the 2015 planting year, flowering 2 or more years after planting was positively associated with cryptogam cover (P = 0.030, Table 3). For the 2016 planting year, flowering 2 or more years post planting was positively associated with soil moisture (P = 0.004, Tables 3, S1). No other microsite variables had significant associations with flowering (Tables 3, S1)
What affects the germination niche of translocated orchids?
Of the 615 recruits, 73.8% (n = 454) were within 5 cm of an adult plant and 4% (n = 24) were within 5–10 cm from an adult plant, with recruitment sharply decreasing the longer the distance from an adult plant, to a maximum distance of 440 cm from any adult (Fig. 4). Recruitment occurred patchily across the four introduction sites.
|
|
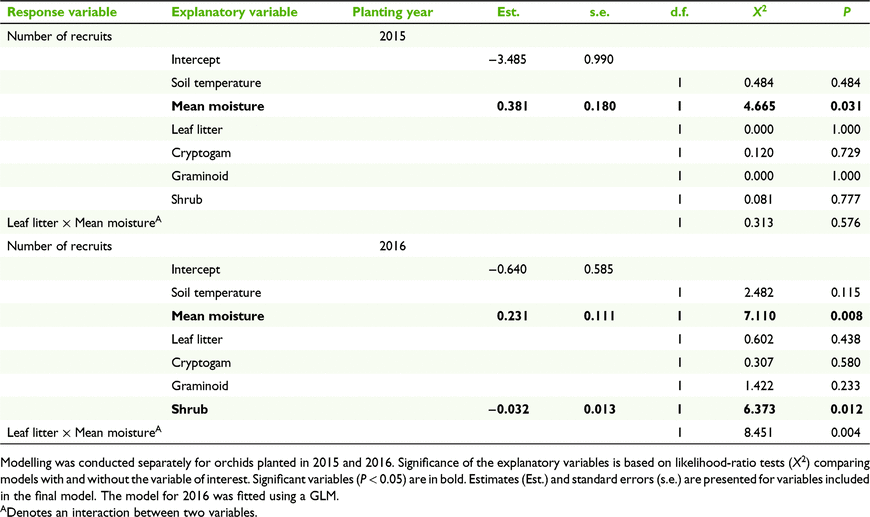
Number of recruits was positively correlated with soil moisture in both the 2015 and 2016 planting years (P = 0.031 and P = 0.008 respectively, Table 4, Fig. 5a, b). Number of recruits was also negatively correlated with shrub cover for the 2016 planting year (P = 0.012, Table 4, Fig. 5c).
|
|
|
Cost analysis
Emergence in the first year for orchids planted in 2015 was 72.2% (n = 223 of 309), whereas emergence of plants at sites with low shrub cover (0% cover) was 73.6% (n = 198 of 269), resulting in a change of 1.4%. Emergence of orchids planted in 2016–2018 was 85.0% (n = 290 of 341), with a 5.2% improvement to 90.2% if only sites with 100% litter cover were selected (n = 175 of 194).
To maintain consistency and keep results comparable among years, we used leaf litter cover to calculate the improvement in emergence after more than 2 years, only for those orchids planted in 2015. Emergence after 2 years was 77.4% (n = 247 of 319) and increased to 85.4% when only microsites with greater than 90% leaf litter cover were selected (n = 135 of 158), an increase of 8%. For those orchids planted in 2016–2018, emergence after 2 years was 76.3% (n = 258 of 338), and increased to 83.0%, an improvement of 6.7%, for microsites with 100% leaf litter cover (n = 195 of 235).
For those orchids planted in 2015, the base level of recruitment (at least one recruit) was 30.3% (n = 61 of 201 microsites). For microsites that had 4% soil moisture or greater, the percentage of sites with recruits only marginally increased to 32.1% (n = 53 of 165). Recruitment rate was higher for those orchids planted in 2016, with at least one recruit at 46.8% (n = 66 of 141) of microsites. When considering microsites with less than 5% shrub cover and greater than or equal to 4% moisture, the percentage of microsites with at least one recruit increased by 10.9%, to 57.7% (n = 30 of 52).
In this study, which included 735 translocated plants, an increased survival of 8% of adult plants would equate to an equivalent cost saving of A$30 223 ± $14 406 (s.e.), on the basis of cost estimates in Zimmer et al. (2019). In addition, an improved recruitment rate of 10% in our study (based on 615 recruits; at least one recruit per microsite) would translate to a cost saving of A$31 611 ± 15 067 (s.e.) to $179 900 ± $85 750 (s.e.) in an increased number of plants that would not otherwise need to be introduced into the population.
Discussion
Using Caladenia colorata as our model species, we were able to show that through a better understanding of the regenerative niche, microsites for translocation could be tailored to improve both survival of adult plants and recruitment. By choosing sites with high leaf litter cover and soil moisture, and low shrub cover, on the basis of our modelled data, we could achieve an 8% increase in adult survival and a 10.9% increase in recruitment, in translocated populations. The improvement in survival of adults and increased recruitment through selecting favourable microsites will lead to reduced translocation costs, by reducing the number of individuals needed to establish a viable population and a reduction in death of translocated plants. Furthermore, this is the first study to examine the distribution of recruits from adult plants in an introduced orchid population, showing that most recruits are within 5 cm of adult plants, with limited and sporadic recruitment up to 4 m.
Our conservation translocations of Caladenia colorata, which had plants propagated with their mycorrhiza and that had the pollinator present at the translocation site and suitable vegetation, had an average survival across the four sites of 77%, for plants 2 or more years after planting, and a growth in the populations of 84% through recruitment. When compared with the majority of plant translocations, which have typically had limited success (a global average of survival in translocations using mature plants of 30.6 ± 5.8% (s.e.), and recruitment or seed set 16.6 ± 2.3% (s.e.), Godefroid et al. 2011; Dalrymple et al. 2012; Reiter et al. 2016; Silcock et al. 2019), our approach that maximises the survival of the founder population and recruitment of the next generation, will provide significant financial savings and conservation outcomes for threatened plants.
Is survival of translocated orchids affected by microsite conditions?
The positive relationship between emergence and leaf litter cover strengthened when orchids had been planted for 2 or more years, regardless of year of planting or whether orchids had been planted in doubles. The link with leaf litter may be due to the relationship between C. colorata and its mycorrhizal partner, S. australiana. Sites in our study with higher litter may be acting as a carbon source for S. australiana, thus benefitting C. colorata. In laboratory trials, Mehra et al. (2017) demonstrated that orchid mycorrhizal fungi (OMF) of Caladenia, including S. australiana, grew well on litter, the complex carbon sources in litter and their metabolites, and hypothesised that litter in the wild may support the growth of orchids.
Orchid mycorrhizal fungi can be highly patchy within orchid populations (Waud et al. 2016; Rock-Blake et al. 2017; Voyron et al. 2017). Furthermore, the presence of OMF can be correlated with the presence of orchids and microsite conditions (McCormick et al. 2009; Waud et al. 2016; Rock-Blake et al. 2017). The extent to which OMF supports mature photosynthetic orchids is an area of active research and varies between species and habitats (McCormick et al. 2018). However, green orchids can be highly dependent on their OMF for a source of carbon and nitrogen (Hynson et al. 2013). Caladenia do not have true roots, and are partly dependent on OMF for nutrition (Smith 1966; Harley and Smith 1983). Achlorophyllous mutants are regularly seen in symbiotic laboratory cultivation (N. Reiter, pers. obs), showing that as seedlings they can be completely reliant on their symbiotic fungi for nutrition.
Like many Australian terrestrial orchids, C. colorata is annually summer dormant, and is capable of prolonged dormancy of 1–3 years (Tremblay et al. 2009). Increased mycorrhizal density in and surrounding adults in the temperate North American orchid Isotria medeoloides is correlated with re-emergence of adult orchids from dormancy (Rock-Blake et al. 2017). Introduction sites with higher litter cover at the microsite scale thus could increase the density of mycorrhiza and therefore aid emergence post-dormancy, as was seen in our study. We found no consistent association with microsite characters and flowering, among years or sites (Table 3). A longer monitoring period may be required to see the effects of microsite on flowering, as only the oldest introductions (2015) had any significant correlation with microsites, with significant positive effects of leaf litter on flowering 2 or more years post-planting.
Survival of adult plants 2 years or more after introduction was positively associated with the presence of cryptogams (mosses, liverworts, hornworts and lichens) and graminoids (grasses and sedges), for C. colorata planted in a drought year (2015). Furthermore, flowering in the oldest introductions (2015) was positively associated with graminoids and cryptogams (Table 2). The association of C. colorata with the presence of graminoids and cryptogams may be due to these plants forming an endophytic or mycorrhizal relationship with Serendipita (which Caladenia are mycorrhizal with). There are no examples that we are aware of where Australian orchid mycorrhizal Serendipita has been found associating with Australian native graminoids. However, Riess et al. (2014) found that Sebacinales, including 13 operational taxonomic units of Serendipita, occurred across agricultural and native grasslands. Serendipita have also been flagged as playing a potential role in facilitating decomposition of organic matter to crop species (Craven and Ray 2019). In addition, members of the Serendipitaceae have been found to be the main symbiont of leafy liverworts (Bidartondo and Duckett 2010; Newsham and Bridge 2010) and thalloid liverworts (Kottke et al. 2003). The positive association of emergence with graminoids and cryptogams, along with the wide distribution of Serendipita australiana, suggests that further research is warranted into the mycorrhizal and endophytic association of Serendipita in Australia, and whether Australian native grasses or cryptogams form symbioses with Serendipita.
For orchids planted in 2015, survival of adult plants in the year after planting was negatively correlated with shrub cover. The negative association with shrub cover may be due to restrictions in light, water and general competition with these species (Wilson 1988; Casper and Jackson 1997) that had been exacerbated due to the drought in 2015, with less than 127 mL of rain during the non-dormant period of C. colorata (May–October), compared with a long-term average of 252 mL (Bureau of Meteorology 2020, Fig. S1). To our knowledge, there are few studies that assess survival of orchids with their co-occurring plant species. However, Reiter et al. (2018) found no benefit of 10 commonly co-occurring plants on the growth of the Australian terrestrial orchid, Thelymitra epipactoides (Orchidaceae), and a negative effect of five co-occurring plant species, suggesting that competition is occurring either between the plants or their mycobionts.
What affects the germination niche of translocated orchids?
Recruitment is key to ensuring self-sustaining populations following the implementation of conservation translocations. However, a review of orchid translocations globally found that only 2% (n = 74) had any level of recruitment (Reiter et al. 2016). The majority of all recruits in this study were within 5 cm of a translocated adult plant, with sporadic recruitment found to a distance of 4 m from adult plants. This suggests that the area within close proximity to the adults and the selection of favourable microsites for the adults is crucial for subsequent population growth.
Given the proximity of recruits to introduced adult plants, site management of threatened Caladenia introductions will benefit from protection of adult plants from weeds and herbivory in the immediate vicinity of the introduced individuals (and thus potential recruits). This is the first study to examine recruitment location relative to adult plants and the microhabitat of recruits at a novel translocation site. In studies of orchids at wild sites, it is often difficult to distinguish parent plants from offspring without genetic studies. For example, in Jacquemyn et al. (2007), recruits of Orchis purpurea were found to cluster within 4–5 m from adult plants. Our study of natural recruitment in an introduced population concurs with seed packet studies (i.e. Goodyera pubescens, Diez 2007; Caladenia arenicola, Batty et al. 2001; Monotropa hypopitys, Leake et al. 2004) that have generally found decreasing seed germination the further the packets were placed from adult plants (for exceptions, see Spiranthes sinensis, Masuhara and Katsuya 1994; Corallorhiza trifida, McKendrick et al. 2000).
Germination and subsequent growth of seedlings are influenced profoundly by microsite (Grubb 1977; Veblen 1992; Wendelberger and Maschinski 2009). For example, seedling survival has been shown to correlate with litter and soil moisture at scales of less than 1 m2 (in rainforest trees, Molofsky and Augspurger 1992; Fabaceae, Wendelberger and Maschinski 2009). We found recruitment of C. colorata seedlings to be positively correlated with soil moisture. In vesicular arbuscular mycorrhiza, distribution and abundance of the fungus was affected by soil moisture (Jacobson 1997). There are few studies on the abiotic factors affecting the distribution of orchid mycorrhiza; however, in seed packet trials of Goodyera pubescences and two common American orchid species that form mycorrhizal associations with Tulasnella (Tullasnellaceae), protocorm germination increased with higher soil moisture and organic content (Scott and Carey 2002; Diez 2007). Furthermore, in a recent study of the distribution of Ceratobasidium associated with orchids across the Australian continent, MaxEnt modelling found that orchid-associating Ceratobasidium taxa were correlated with both temperature and moisture (Freestone et al. 2021). Microsite conditions that support germination may differ from those conditions that support adult plants and are often influenced by the existing plants in the microsite (Tilman 2004). However, although we found no correlation with recruitment and leaf litter in this study (although increased leaf litter was associated with survival of adult plants), recruits were generally found at sites that were also favourable to adult plants.
Orchids require mycorrhizal fungi for germination and establishment, and therefore recruitment is highly dependent on the density (McCormick et al. 2012) and distribution of their mycorrhizal fungi (Rasmussen 1995). Some adult orchids can remain heavily colonised by their mycorrhizal fungi (Selosse et al. 2002; Gebauer and Meyer 2003; Bidartondo et al. 2004), receiving nutrition to some extent from the mycorrhizal fungi. All Caladenia species in subgenus Calonema examined are heavily colonised by Serendipita, which both germinates seedlings and maintains symbiosis with the adult plant (Reiter et al. 2020). The subsequent cluster of seedlings in the nearby vicinity of adult plants as seen with the majority of recruitment in this study may be due to a commensal relationship of the seedlings with the mycorrhizal fungi of the adult plants. Common mycorrhizal networks in other plants can transport minerals (Finlay and Read 1986) and carbon (Simard et al. 1997) between co-occurring plants. Our study suggests that initiation of translocation sites with adult plants to facilitate subsequent seedlings (either recruited or translocated), may be beneficial.
Financial implications
With limited financial resources currently available globally for conservation and translocation of threatened plants (Dalrymple et al. 2021) and increased pressure to prioritise species for conservation (see papers reviewed in Cullen 2012), maximising what limited resources are currently available for plant conservation is critical. Given that anthropogenic climate change (Ciavarella et al. 2020) will result in assisted migration being the only option for translocation of many species (Dalrymple et al. 2021), understanding the niche that will sustain both adults and seedlings becomes increasingly important when choosing sites outside of their known habitat (Bellis et al. 2020). Given that 70% of translocations are unsuccessful (average of results provided in reviews by Godefroid et al. 2011, Dalrymple et al. 2021, Reiter et al. 2016 and Silcock et al. 2019), large financial savings could be made by modest improvements. On the basis of the assumptions of costs of translocating a population in Zimmer et al. (2019), broken down into a cost per propagule, we showed that combining the optimal microsite niche for both adult survival and recruitment can lead to a cost saving of A$31 611 ± $15 067 to $179 900 ± $85 750 for a single threatened species. If these microsite considerations were applied globally, financial savings could be large.
Conservation implications
Conservation translocations are on the rise (Silcock et al. 2019). We demonstrated that by considering habitat matching, along with obligate symbiotic relationships with pollinators and mycorrhizal fungi, plant translocations can be successful. Orchids with Serendipita australiana OMF are continentally widespread in Australia (Reiter et al. 2020) and orchids that associate with other Serendipita are globally widespread, with many species such as Caladenia subgenus Calonema being a high priority for large-scale conservation translocation programs (Reiter et al. 2017, 2019; Reiter and Thomson 2018; Waudby et al. 2018). We showed that not only can orchids be introduced successfully at novel sites, but that their survival and recruitment can be enhanced by selecting the microsite habitat for both the adult plant and seedling recruitment. There are four key conservation implications arising from this research: (1) plant conservation translocations can be highly successful when symbiotic interactions and habitat are considered, (2) re-emergence of adult plants and recruitment around adult plants are associated with different microsite factors, (3) selecting microsites that are beneficial for both adult survival and seedling recruitment is possible within the same microsite and would further improve translocation outcomes, and (4) seedling recruitment in Caladenia introductions is typically close to adult plants, the majority within 5 cm, thus protection from threats in the immediate vicinity of introduced plants is likely to be beneficial for population growth.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was supported by federal National Landcare Program funding through the Wimmera Catchment Management Authority, Department of Land Water and Primary Industry funding through Victorian threatened species initiatives, and the Australian Research Council (LP200200264 to N. Reiter).
Author contributions
NR contributed towards the design, data collection, analysis and writing of the paper. MM contributed towards the design, analysis and writing of the paper.
Acknowledgements
We thank the following people and organisations for assistance in the field and with the translocation process, or as landholders; Richard Dimon, Ilham Kurnia Abywijaya, Len and Josie Carrigan, Sam and Sari Cuce, Mary Argall, Wendy Bedggood, Australasian Native Orchid Society (Vic Branch) volunteers, Trust for Nature, Australian Network for Plant Conservation. We thank the Department of Environment, Land, Water and Planning and Parks Victoria for permits and access to wild study sites. Ryan Phillips provided comments on earlier versions of the manuscript.
References
Albrecht MA, Maschinski J (2012) Influence of founder population size, propagule stages, and life history on the survival of reintroduced plant populations. In ‘Plant reintroduction in a changing climate’. (Eds J Maschinski, KE Haskins) pp. 171–188. (Island Press: Washington, DC, USA)Australian Government (2021) EPBC Act of threatened flora. https://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted=flora [Verified 12 March 2021]
Backhouse GN, Bates RJ, Brown AP, Copeland LM (2019) ‘A checklist of the orchids of Australia including its island territories.’ (Gary Backhouse: Melbourne, Vic., Australia)
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Batty AL, Dixon KW, Brundrett M, Sivasithamparam K (2001) Constraints to symbiotic germination of terrestrial orchid seed in a Mediterranean bushland. New Phytologist 152, 511–520.
| Constraints to symbiotic germination of terrestrial orchid seed in a Mediterranean bushland.Crossref | GoogleScholarGoogle Scholar |
Bellis J, Bourke D, Maschinski J, Heineman K, Dalrymple S (2020) Climate suitability as a predictor of conservation translocation failure. Conservation Biology 34, 1473–1481.
| Climate suitability as a predictor of conservation translocation failure.Crossref | GoogleScholarGoogle Scholar | 32304113PubMed |
Bidartondo MI, Duckett JG (2010) Conservative ecological and evolutionary patterns in liverwort–fungal symbioses. Proceedings of the Royal Society B: Biological Sciences 277, 485–92.
| Conservative ecological and evolutionary patterns in liverwort–fungal symbioses.Crossref | GoogleScholarGoogle Scholar | 19812075PubMed |
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings of the Royal Society London B: Biological Sciences 271, 1799–1806.
| Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2020) Average annual, seasonal and monthly rainfall. Available at http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall/index.jsp
Casper BB, Jackson RB (1997) Plant competition underground. Annual Review of Ecology and Systematics 28, 545–570.
| Plant competition underground.Crossref | GoogleScholarGoogle Scholar |
Ciavarella A, Cotterill D, Stott P, Kew S, Sjoukje P, Van Oldenborgh, GJ, Skålevåg A, Lorenz P, Robin Y, Otto F, Hauser M, Seneviratne SI, Lehner F, Zolina O (2020) Prolonged Siberian heat of 2020. Availablet at https://www.worldweatherattribution.org/siberian-heatwave-of-2020-almost-impossible-without-climatechange
Clements MA, Ellyard RK (1979) The symbiotic germination of Australian terrestrial orchids. American Orchid Society Bulletin 48, 810–816.
Clements MA, Muir H, Cribb PJ (1986) A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bulletin 1, 437–45.
Colas B, Kirchner F, Riba M, Olivieri I, Mignot A, Imbert E, Beltrame C, Carbonell D, Fréville H (2008) Restoration demography: a 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa (Asteraceae). Journal of Applied Ecology 45, 1468–1476.
| Restoration demography: a 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa (Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Craven KD, Ray P (2019) More than serendipity: the potential to manage soil carbon and emissions while promoting low-input agriculture with serendipitoid mycorrhizae. Phytobiomes Journal 3, 161–164.
| More than serendipity: the potential to manage soil carbon and emissions while promoting low-input agriculture with serendipitoid mycorrhizae.Crossref | GoogleScholarGoogle Scholar |
Cullen R (2012) Biodiversity protection prioritisation: a 25-year review. Wildlife Research 40, 108–116.
| Biodiversity protection prioritisation: a 25-year review.Crossref | GoogleScholarGoogle Scholar |
Dalrymple SE, Banks E, Stewart GB, Pullin AS (2012) A meta-analysis of threatened plant reintroductions from across the globe. In ‘Plant reintroduction in a changing climate’. pp. 31–50. (Island Press: Washington, DC, USA)
Dalrymple SE, Winder R, Campbell EM (2021) Exploring the potential for plant translocations to adapt to a warming world. Journal of Ecology 109, 2264–2270.
| Exploring the potential for plant translocations to adapt to a warming world.Crossref | GoogleScholarGoogle Scholar |
Diez JM (2007) Hierarchical patterns of symbiotic orchid germination linked to adult proximity and environmental gradients. Journal of Ecology 95, 159–170.
| Hierarchical patterns of symbiotic orchid germination linked to adult proximity and environmental gradients.Crossref | GoogleScholarGoogle Scholar |
Dodson CH, Gentry AH (1991) Biological extinction in Western Ecuador. Annals of the Missouri Botanical Garden 78, 273–295.
| Biological extinction in Western Ecuador.Crossref | GoogleScholarGoogle Scholar |
Draper Munt D, Marques I, Iriondo JM (2016) Acquiring baseline information for successful plant translocations when there is no time to lose: the case of the neglected Critically Endangered Narcissus cavanillesii (Amaryllidaceae). Plant Ecology 217, 193–206.
| Acquiring baseline information for successful plant translocations when there is no time to lose: the case of the neglected Critically Endangered Narcissus cavanillesii (Amaryllidaceae).Crossref | GoogleScholarGoogle Scholar |
Duncan M, Moloney PD (2018) Comparing wild and reintroduced populations of the threatened orchid Diuris fragrantissima (Orchidaceae) in south-eastern Australia. Australian Journal of Botany 66, 459–467.
| Comparing wild and reintroduced populations of the threatened orchid Diuris fragrantissima (Orchidaceae) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Finlay RD, Read DJ (1986) The structure and function of the vegetative mycelium of ectomycorrhizal plants: II. The uptake and distribution of phosphorus by mycelial strands interconnecting host plants. New Phytologist 103, 157–165.
| The structure and function of the vegetative mycelium of ectomycorrhizal plants: II. The uptake and distribution of phosphorus by mycelial strands interconnecting host plants.Crossref | GoogleScholarGoogle Scholar |
Fox J, Weisberg S (2019) ‘An R Companion to Applied Regression’, 3rd edn. (Sage: Thousand Oaks CA, USA)
Freestone MW, Swarts ND, Reiter N, Tomlinson S, Sussmilch FC, Wright MM, Holmes GD, Phillips RD, Linde CC (2021) Continental-scale distribution and diversity of Ceratobasidium orchid mycorrhizal fungi in Australia. Annals of Botany 128, 329–343.
| Continental-scale distribution and diversity of Ceratobasidium orchid mycorrhizal fungi in Australia.Crossref | GoogleScholarGoogle Scholar | 34077492PubMed |
Gebauer G, Meyer M (2003) 15N and 13C natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytologist 160, 209–223.
| 15N and 13C natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association.Crossref | GoogleScholarGoogle Scholar |
Gebauer G, Preiss K, Gebauer AC (2016) Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytologist 211, 11–15.
| Partial mycoheterotrophy is more widespread among orchids than previously assumed.Crossref | GoogleScholarGoogle Scholar |
Girlanda M, Segreto R, Cafasso D, Liebel HT, Rodda M, Ercole E, Cozzolino S, Gebauer G, Perotto S (2011) Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. American Journal of Botany 98, 1148–1163.
| Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations.Crossref | GoogleScholarGoogle Scholar | 21712419PubMed |
Godefroid S, Piazza C, Rossi G, et al. (2011) How successful are plant species reintroductions? Biological Conservation 144, 672–682.
| How successful are plant species reintroductions?Crossref | GoogleScholarGoogle Scholar |
Grubb PJ (1977) The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52, 107–145.
| The maintenance of species-richness in plant communities: the importance of the regeneration niche.Crossref | GoogleScholarGoogle Scholar |
Hanski I, Ovaskainen O (2002) Extinction debt at extinction threshold. Conservation Biology 16, 666–673.
| Extinction debt at extinction threshold.Crossref | GoogleScholarGoogle Scholar |
Harley JL, Smith SE (1983) ‘Mycorrhizal symbiosis.’ (Academic Press: London, UK)
Hynson NA, Madsen TP, Selosse M-A, Adam IKU, Ogura-Tsujita Y, Roy M, Gebauer G (2013) The physiological ecology of mycoheterotrophy. In ‘Mycoheterotrophy: the biology of plants living on fungi’. (Ed. VSFT Merckx) pp. 297–342. (Springer: New York, NY, USA)
Jacobson KM (1997) Moisture and substrate stability determine VA-mycorrhizal fungal community distribution and structure in an arid grassland. Journal of Arid Environments 35, 59–75.
| Moisture and substrate stability determine VA-mycorrhizal fungal community distribution and structure in an arid grassland.Crossref | GoogleScholarGoogle Scholar |
Jacquemyn H, Brys R, Vandepitte K, Honnay O, Roldán-Ruiz I, Wiegand T (2007) A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea. New Phytologist 176, 448–459.
| A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea.Crossref | GoogleScholarGoogle Scholar |
Janes JK, Steane DA, Vaillancourt RE (2010) An investigation into the ecological requirements and niche partitioning of Pterostylidinae (Orchidaceae) species. Australian Journal of Botany 58, 335–341.
| An investigation into the ecological requirements and niche partitioning of Pterostylidinae (Orchidaceae) species.Crossref | GoogleScholarGoogle Scholar |
Janissen B, French G, Selby-Pham J, Lawrie AC, Huynh T (2021) Differences in emergence and flowering in wild, re-introduced and translocated populations of an endangered terrestrial orchid and the influences of climate and orchid mycorrhizal abundance. Australian Journal of Botany 69, 9–20.
| Differences in emergence and flowering in wild, re-introduced and translocated populations of an endangered terrestrial orchid and the influences of climate and orchid mycorrhizal abundance.Crossref | GoogleScholarGoogle Scholar |
Jersáková J, Malinová T (2007) Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytologist 176, 237–241.
| Spatial aspects of seed dispersal and seedling recruitment in orchids.Crossref | GoogleScholarGoogle Scholar |
Kottke I, Beiter A, Weiss M, Haug I, Oberwinkler F, Nebel M (2003) Heterobasidiomycetes form symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while Aneura pinguis (Metzgeriales) is associated with a Tulasnella species. Mycological Research 107, 957–968.
| Heterobasidiomycetes form symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while Aneura pinguis (Metzgeriales) is associated with a Tulasnella species.Crossref | GoogleScholarGoogle Scholar | 14531618PubMed |
Leake JR (1994) The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist 127, 171–216.
| The biology of myco-heterotrophic (‘saprophytic’) plants.Crossref | GoogleScholarGoogle Scholar |
Leake JR, McKendrick SL, Bidartondo M, Read DJ (2004) Symbiotic germination and development of the myco-heterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp. New Phytologist 163, 405–423.
| Symbiotic germination and development of the myco-heterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp.Crossref | GoogleScholarGoogle Scholar |
Major J (1988) Endemism: a botanical perspective. In ‘Analytical biogeography’. (Eds AA Myers, PS Giller) pp. 117–146. (Springer: Dordrecht, Netherlands)
Maschinski J, Baggs JE, Sacchi CF (2004) Seedling recruitment and survival of an endangered limestone endemic in its natural habitat and experimental reintroduction sites. American Journal of Botany 91, 689–698.
| Seedling recruitment and survival of an endangered limestone endemic in its natural habitat and experimental reintroduction sites.Crossref | GoogleScholarGoogle Scholar | 21653424PubMed |
Maschinski J, Falk DA, Wright SJ, Possley J, Roncal J, Wendelberger KS (2012) Optimal locations for plant reintroductions in a changing world. In ‘Plant reintroduction in a changing climate’. (Eds J Maschinski, KE Haskins) pp. 109–129. (Island Press: Washington, DC, USA)
Masuhara G, Katsuya K (1994) In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon) Ames, var. amoena (M. Bieberstein) Hara (Orchidaceae). New Phytologist 127, 711–718.
| In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon) Ames, var. amoena (M. Bieberstein) Hara (Orchidaceae).Crossref | GoogleScholarGoogle Scholar |
Maunder M (1992) Plant reintroduction: an overview. Biodiversity and Conservation 1, 51–61.
| Plant reintroduction: an overview.Crossref | GoogleScholarGoogle Scholar |
McCormick MK, Lee Taylor D, Juhaszova K, Burnett RK, Whigham DF, O’Neill JP (2012) Limitations on orchid recruitment: not a simple picture. Molecular Ecology 21, 1511–1523.
| Limitations on orchid recruitment: not a simple picture.Crossref | GoogleScholarGoogle Scholar | 22272942PubMed |
McCormick MK, Whigham DF, Canchani-Viruet A (2018) Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytologist 219, 1207–1215.
| Mycorrhizal fungi affect orchid distribution and population dynamics.Crossref | GoogleScholarGoogle Scholar |
McCormick MK, Whigham DF, O’Neill JP, Becker JJ, Werner S, Rasmussen HN, Bruns TD, Taylor DL (2009) Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecological Monographs 79, 619–635.
| Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae.Crossref | GoogleScholarGoogle Scholar |
McKendrick SL, Leake JR, Taylor DL, Read DJ (2000) Symbiotic germination and development of myco-heterotrophic plants in nature: ontogeny of Corallorhiza trifida and characterization of its mycorrhizal fungi. New Phytologist 145, 523–537.
| Symbiotic germination and development of myco-heterotrophic plants in nature: ontogeny of Corallorhiza trifida and characterization of its mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar |
Mehra S, Morrison PD, Coates F, Lawrie AC (2017) Differences in carbon source utilisation by orchid mycorrhizal fungi from common and endangered species of Caladenia (Orchidaceae). Mycorrhiza 27, 95–108.
| Differences in carbon source utilisation by orchid mycorrhizal fungi from common and endangered species of Caladenia (Orchidaceae).Crossref | GoogleScholarGoogle Scholar | 27639577PubMed |
Moisan C, Pellerin S (2013) Factors associated with the presence of flowering individuals of Arethusa bulbosa (Orchidaceae) in peatlands of southern Quebec. Écoscience 20, 1–8.
| Factors associated with the presence of flowering individuals of Arethusa bulbosa (Orchidaceae) in peatlands of southern Quebec.Crossref | GoogleScholarGoogle Scholar |
Molofsky J, Augspurger CK (1992) The effect of leaf litter on early seedling establishment in a tropical forest. Ecology 73, 68–77.
| The effect of leaf litter on early seedling establishment in a tropical forest.Crossref | GoogleScholarGoogle Scholar |
Newsham KK, Bridge PD (2010) Sebacinales are associates of the leafy liverwort Lophozia excisa in the southern maritime Antarctic. Mycorrhiza 20, 307–313.
| Sebacinales are associates of the leafy liverwort Lophozia excisa in the southern maritime Antarctic.Crossref | GoogleScholarGoogle Scholar | 19921285PubMed |
Nic Lughadha E, Bachman SP, Leão TC, et al. (2020) Extinction risk and threats to plants and fungi. Plants, People, Planet 2, 389–408.
| Extinction risk and threats to plants and fungi.Crossref | GoogleScholarGoogle Scholar |
Obst C (2005) South Australian Murray–Darling Basin threatened flora recovery plan. Report to the Threatened Species and Communities Section, Australian Government Department of the Environment and Heritage, Canberra, ACT, Australia.
Oktalira F, May TW, Dearnaley JDW, Linde CC (2021) Seven new Serendipita species associated with Australian terrestrial orchids. Mycologia 113, 968–987.
| Seven new Serendipita species associated with Australian terrestrial orchids.Crossref | GoogleScholarGoogle Scholar | 34338610PubMed |
Phillips RD, Faast R, Bower CC, Brown GR, Peakall R (2009) Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae). Australian Journal of Botany 57, 287–306.
| Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae).Crossref | GoogleScholarGoogle Scholar |
Phillips RD, Reiter N, Peakall R (2020) Orchid conservation: from theory to practice. Annals of Botany 126, 345–362.
| Orchid conservation: from theory to practice.Crossref | GoogleScholarGoogle Scholar | 32407498PubMed |
Qin H, Yang Y, Dong S, et al. (2017) Threatened species list of China’s higher plants. Biodiversity Science 25, 696–744.
| Threatened species list of China’s higher plants.Crossref | GoogleScholarGoogle Scholar |
Rasmussen HN (1995) ‘Terrestrial orchids: from seed to mycotrophic plant.’ (Cambridge University Press: Cambridge, UK)
Rasmussen HN (2002) Recent developments in the study of orchid mycorrhiza. Plant and Soil 244, 149–163.
| Recent developments in the study of orchid mycorrhiza.Crossref | GoogleScholarGoogle Scholar |
Rasmussen HN, Dixon KW, Jersáková J, Těšitelová T (2015) Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany 116, 391–402.
| Germination and seedling establishment in orchids: a complex of requirements.Crossref | GoogleScholarGoogle Scholar | 26271118PubMed |
Rasmussen HN, Rasmussen FN (2009) Orchid mycorrhiza: implications of a mycophagous life style. Oikos 118, 334–345.
| Orchid mycorrhiza: implications of a mycophagous life style.Crossref | GoogleScholarGoogle Scholar |
Reiter N (2021) Conservation translocation of the endangered colourful spider-orchid (Caladenia colorata). Australian Plant Conservation 29, 13–15.
| Conservation translocation of the endangered colourful spider-orchid (Caladenia colorata).Crossref | GoogleScholarGoogle Scholar |
Reiter N, Thomson R (2018) Involvement in the Royal Botanic Gardens Victoria’s Orchid conservation program by volunteers from the Australasian Native Orchid Society Victoria Group. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 27, 19–22.
| Involvement in the Royal Botanic Gardens Victoria’s Orchid conservation program by volunteers from the Australasian Native Orchid Society Victoria Group.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Bohman B, Batley M, Phillips RD (2019) Pollination of an endangered Caladenia species (Orchidaceae) by nectar-foraging behaviour of a widespread species of colletid bee. Botanical Journal of the Linnean Society 189, 83–98.
| Pollination of an endangered Caladenia species (Orchidaceae) by nectar-foraging behaviour of a widespread species of colletid bee.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Whitfield J, Pollard G, Bedggood W, Argall M, Dixon K, Davis B, Swarts N (2016) Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecology 217, 81–95.
| Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Vlcek K, O’Brien N, Gibson M, Pitts D, Brown GR, Bower CC, Phillips RD (2017) Pollinator rarity limits reintroduction sites in an endangered sexually deceptive orchid (Caladenia hastata): implications for plants with specialized pollination systems. Botanical Journal of the Linnean Society 184, 122–136.
| Pollinator rarity limits reintroduction sites in an endangered sexually deceptive orchid (Caladenia hastata): implications for plants with specialized pollination systems.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Bohman B, Flematti GR, Phillips RD (2018) Pollination by nectar-foraging thynnine wasps: evidence of a new specialized pollination system for Australian orchids. Botanical Journal of the Linnean Society 188, 327–337.
| Pollination by nectar-foraging thynnine wasps: evidence of a new specialized pollination system for Australian orchids.Crossref | GoogleScholarGoogle Scholar |
Reiter N, Phillips RD, Swarts ND, Wright M, Holmes G, Sussmilch FC, Davis BJ, Whitehead MR, Linde CC (2020) Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): implications for conservation. Annals of Botany 126, 943–955.
| Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): implications for conservation.Crossref | GoogleScholarGoogle Scholar | 32574356PubMed |
Riess K, Oberwinkler F, Bauer R, Garnica S (2014) Communities of endophytic Sebacinales associated with roots of herbaceous plants in agricultural and grassland ecosystems are dominated by Serendipita herbamans sp. nov. PLoS ONE 9, e94676
| Communities of endophytic Sebacinales associated with roots of herbaceous plants in agricultural and grassland ecosystems are dominated by Serendipita herbamans sp. nov.Crossref | GoogleScholarGoogle Scholar | 24743185PubMed |
Rock-Blake R, McCormick MK, Brooks HEA, Jones CS, Whigham DF (2017) Symbiont abundance can affect host plant population dynamics. American Journal of Botany 104, 72–82.
| Symbiont abundance can affect host plant population dynamics.Crossref | GoogleScholarGoogle Scholar | 28062407PubMed |
Scott HS, Carey PD (2002) The effects of water application on seed germination and infection in Gymnadenia conopsea under field conditions. In ‘Trends and fluctuations, and underlying mechanisms in terrestrial orchid populations’. (Eds P Kindlmann, DF Ehigham, JH Willems) pp. 155–165. (Bakhuys Publishers: Leiden, Netherlands)
Seddon PJ (2010) From reintroduction to assisted colonization: moving along the conservation translocation spectrum. Restoration Ecology 18, 796–802.
| From reintroduction to assisted colonization: moving along the conservation translocation spectrum.Crossref | GoogleScholarGoogle Scholar |
Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP (2014) Reversing defaunation: restoring species in a changing world. Science 345, 406–412.
| Reversing defaunation: restoring species in a changing world.Crossref | GoogleScholarGoogle Scholar | 25061203PubMed |
Selosse M-A, Martos F (2014) Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends in Plant Science 19, 683–685.
| Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon?Crossref | GoogleScholarGoogle Scholar | 25278267PubMed |
Selosse M-A, Weiss M, Jany J-L, Tillier A (2002) Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring tree ectomycorrhizae. Molecular Ecology 11, 1831–1844.
| Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring tree ectomycorrhizae.Crossref | GoogleScholarGoogle Scholar | 12207732PubMed |
Silcock JL, Simmons CL, Monks L, Dillon R, Reiter N, Jusaitis M, Vesk PA, Byrne M, Coates DJ (2019) Threatened plant translocation in Australia: a review. Biological Conservation 236, 211–222.
| Threatened plant translocation in Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388, 579–582.
| Net transfer of carbon between ectomycorrhizal tree species in the field.Crossref | GoogleScholarGoogle Scholar |
Smith SE (1966) Physiology and ecology of orchid mycorrhizal fungi with reference to seedling nutrition. New Phytologist 65, 488–499.
Thomas CD (2011) Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends in Ecology & Evolution 26, 216–221.
| Translocation of species, climate change, and the end of trying to recreate past ecological communities.Crossref | GoogleScholarGoogle Scholar |
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proceedings National Academy of Sciences of the United States of America 101, 10854–10861.
| Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly.Crossref | GoogleScholarGoogle Scholar |
Tremblay RL, Perez M-E, Larcombe M, Brown A, Quarmby J, Bickerton D, French G, Bould A (2009) Dormancy in Caladenia: a Bayesian approach to evaluating latency. Australian Journal of Botany 57, 340–350.
| Dormancy in Caladenia: a Bayesian approach to evaluating latency.Crossref | GoogleScholarGoogle Scholar |
Veblen TT (1992) Regeneration dynamics. In ‘Plant succession: theory and prediction. Vol. 11’. (Eds D Glenn-Lewin, R Peet) pp. 152–187. (Chapman and Hall: London, UK)
Voyron S, Ercole E, Ghignone S, Perotto S, Girlanda M (2017) Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytologist 213, 1428–1439.
| Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands.Crossref | GoogleScholarGoogle Scholar |
Waud M, Busschaert P, Lievens B, Jacquemyn H (2016) Specificity and localised distribution of mycorrhizal fungi in the soil may contribute to co-existence of orchid species. Fungal Ecology 20, 155–165.
| Specificity and localised distribution of mycorrhizal fungi in the soil may contribute to co-existence of orchid species.Crossref | GoogleScholarGoogle Scholar |
Waudby HP, Cameron M, Robertson G, Caynes R, Reiter N (2018) Wild orchids: saving three endangered orchid species in southern New South Wales. In ‘Recovering Australian threatened species: a book of hope’. (Eds S Garnett, P Latch, D Lindenmeyer, J Woinarski) pp. 159–168. (CSIRO Publishing: Melbourne, Vic., Australia)
WCSP (2021) World checklist of selected plant families. (Facilitated by the Royal Botanic Gardens, Kew: London, UK) Available at http://wcsp.science.kew.org/ [Verified10 March 2021]
Weiß M, Waller F, Zuccaro A, Selosse MA (2016) Sebacinales: one thousand and one interactions with land plants. New Phytologist 211, 20–40.
| Sebacinales: one thousand and one interactions with land plants.Crossref | GoogleScholarGoogle Scholar |
Wendelberger KS, Maschinski J (2009) Linking geographical information systems and observational and experimental studies to determine optimal seedling microsites of an endangered plant in a subtropical urban fire-adapted ecosystem. Restoration Ecology 17, 845–853.
| Linking geographical information systems and observational and experimental studies to determine optimal seedling microsites of an endangered plant in a subtropical urban fire-adapted ecosystem.Crossref | GoogleScholarGoogle Scholar |
Wendelberger KS, Maschinski J (2016) Assessing microsite and regeneration niche preferences through experimental reintroduction of the rare plant Tephrosia angustissima var. corallicola. Plant Ecology 217, 155–167.
| Assessing microsite and regeneration niche preferences through experimental reintroduction of the rare plant Tephrosia angustissima var. corallicola.Crossref | GoogleScholarGoogle Scholar |
Whitehead MR, Catullo RA, Ruibal M, Dixon KW, Peakall R, Linde CC (2017) Evaluating multilocus Bayesian species delimitation for discovery of cryptic mycorrhizal diversity. Fungal Ecology 26, 74–84.
| Evaluating multilocus Bayesian species delimitation for discovery of cryptic mycorrhizal diversity.Crossref | GoogleScholarGoogle Scholar |
Wilson JB (1988) Shoot competition and root competition. Journal of Applied Ecology 25, 279–296.
| Shoot competition and root competition.Crossref | GoogleScholarGoogle Scholar |
Zimmer HC, Auld TD, Cuneo P, Offord CA, Commander LE (2019) Conservation translocation–an increasingly viable option for managing threatened plant species. Australian Journal of Botany 67, 501–509.
| Conservation translocation–an increasingly viable option for managing threatened plant species.Crossref | GoogleScholarGoogle Scholar |