Genetics of meat quality and carcass traits and the impact of tenderstretching in two tropical beef genotypes
M. L. Wolcott A B E , D. J. Johnston A B , S. A. Barwick A B , C. L. Iker A B , J. M. Thompson A C and H. M. Burrow A DA Cooperative Research Centre for Beef Genetic Technologies, Armidale, NSW 2351, Australia.
B Animal Genetics and Breeding Unit 1 , University of New England, Armidale, NSW 2351, Australia.
C University of New England, Armidale, NSW 2351, Australia.
D CSIRO Livestock Industries, Rockhampton, Qld 4702, Australia.
E Corresponding author. Email: mwolcott@une.edu.au
Animal Production Science 49(6) 383-398 https://doi.org/10.1071/EA08275
Submitted: 11 November 2008 Accepted: 23 February 2009 Published: 13 May 2009
Abstract
Meat quality and carcass traits were measured for 2180 feedlot finished Brahman (BRAH) and Tropical Composite (TCOMP) steers to investigate genetic and non-genetic influences on shear force, and other meat quality traits. Genetic and phenotypic correlations were estimated between carcass and meat quality traits, and with live animal measurements collected in steers from weaning to feedlot exit, and their heifer half-sibs up to their first mating, which were managed in Australia’s tropical or subtropical environments. Left sides of carcasses were tenderstretched (hung by the aitch-bone) while right sides were conventionally hung (by the Achilles tendon). Tenderstretching reduced mean shear force by 1.04 kg, and phenotypic variance by 77% of that observed in conventionally hung sides. Genotype differences existed for carcass traits, with TCOMP carcasses significantly heavier, fatter, with greater eye muscle area, and lower retail beef yield than BRAH. TCOMP had lower shear force, and higher percent intramuscular fat. Meat quality and carcass traits were moderately heritable, with estimates for shear force and compression of 0.33 and 0.19 for BRAH and 0.32 and 0.20 for TCOMP respectively. In both genotypes, estimates of heritability for carcass traits (carcass weight, P8 and rib fat depths, eye muscle area and retail beef yield) were consistently moderate to high (0.21 to 0.56). Shear force and compression were genetically correlated with percent intramuscular fat (r g = –0.26 and –0.57, respectively), and meat colour (r g = –0.41 and –0.68, respectively). For TCOMP, lower shear force was genetically related to decreased carcass P8 fat depth (r g = 0.51). For BRAH steers and heifers measured at pasture, fatness traits and growth rates were genetically correlated with shear force, although the magnitude of these relationships varied with time of measurement. Net feed intake was significantly genetically correlated with carcass rib fat depth (r g = 0.49), eye muscle area (r g = –0.42) and retail beef yield (r g = –0.61). These results demonstrate that selection to improve production and carcass traits can impact meat quality traits in tropically adapted cattle, and that genotype specific evaluations will be necessary to accommodate different genetic relationships between meat quality, carcass and live animal traits.
Additional keywords: feed efficiency, genetic correlations, growth, heritability, tenderness, tenderstretch.
Introduction
Extensive consumer evaluation in Australia has demonstrated that tenderness is the single most important characteristic in determining the level of satisfaction reported for cooked beef products (Egan et al. 2001). The Meat Standards Australia (MSA) grading system predicts meat quality as a tenderness dominated meat quality score (Thompson 2002; Watson et al. 2008), via several critical control points identified through extensive consumer taste panel assessment of meat samples from animals of diverse genetic, environmental and nutritional backgrounds. Thompson (2002) and Watson et al. (2008), in describing the development and implementation of the MSA system, demonstrated that Bos indicus content had a quantifiable, and negative impact on beef meat quality, which was consistent with the results of studies by Shackelford et al. (1995), Pringle et al. (1997), Johnston et al. (2003b) and Thompson et al. (2006). This is particularly relevant to beef production in Australia, where ~40% of cattle are B. indicus or B. indicus derived (Bindon and Jones 2001).
Studies by Reverter et al. (2003b) and Johnston et al. (2003b) examined the genetic parameters of carcass and meat quality traits in tropically adapted cattle in Australia, and reported that moderate heritabilities meant that selection to improve these traits was possible (h 2 = 0.18–0.50). Reverter et al. (2003a) estimated the genetic relationships between liveweight, liveweight gain, flight time and real time ultrasound scanned measurements of fat depth and eye muscle area measurements from live steers, and their carcass and meat quality traits assessed after slaughter. Absent from this research, however, was an examination of the genetic correlations between carcass and meat quality traits measured in finished steers, and production traits assessed in females which are required to grow and maintain body condition, and eventually reproduce under the harsh environments of Australia’s northern, tropical and semiarid environments (see Johnston et al. 2009). Evidence presented by Mackinnon et al. (1991) showed that growth performance was likely to be under separate genetic control for tropically adapted animals when managed under benign v. harsh production conditions. As producers select to improve traits such as liveweight, liveweight gain and carcass composition in male and female progeny, the consequences of these decisions on carcass and meat quality traits must be known. Conversely, for producers of tropically adapted cattle who place selection pressure on carcass and meat quality traits, the consequences of such strategies on production traits in steers, and in heifers managed as a self-replacing herd, needs to be evaluated to determine reliable estimates of genetic parameters.
As demonstrated by the models developed for the MSA beef carcass grading system (Thompson 2002; Watson et al. 2008), selection is not the only means available to the beef producers and processors to influence meat quality traits. Tenderstretching (TS), the hanging of carcasses from the aitch-bone rather than hanging conventionally by the Achilles tendon (AT), has been demonstrated to be an effective means of improving tenderness, particularly in the high value cuts of the rump and loin (Hostetler et al. 1970; Harris and Shorthose 1988). However, genetic relationships between tenderness measurements in carcasses subjected to these hanging methods have yet to be examined.
The aims of this study were first to quantify the genetic parameters for carcass and meat quality traits in two tropically adapted genotypes of cattle (Brahman and Tropical Composite) and identify any genotype differences. The second aim was to identify the genetic and phenotypic relationships of carcass and meat quality traits with the feedlot and pre-feedlot entry performance of steers and their half sib sisters which were managed under the harsh and extensive production environments of northern Australia and to identify possible genetic indicator traits for meat quality. Finally, the experiment aimed to examine the improvement in meat quality achieved through the application of tenderstretching in the carcasses of tropically adapted steers, and the impact on genetic parameters for meat quality traits when assessed in tenderstretched carcasses.
Materials and methods
Animals and live measurements
Animals used for this study were part of the Cooperative Research Centre for Cattle and Beef Quality (Beef CRC) northern breeding project (Burrow and Bindon 2005), with genotypes selected to represent the beef cattle population present in subtropical and tropical environments of Northern Australia. Breeding and management of animals, and experimental treatments imposed on the steer and heifers have been described by Barwick et al. (2009a , 2009b). Briefly, animals of two tropically adapted genotypes, Brahman (BRAH) and Tropical Composite (TCOMP) were bred over a 4-year period (2000 to 2003) on eight co-operating properties (subsequently referred to as herds of origin), using a combination of artificial insemination (AI) and natural mating. The TCOMP genotype comprised ~50% tropically adapted Bos indicus or African Sanga, and 50% non-adapted Bos taurus genetics. Use of AI sires ensured genetic linkage across years and breeding locations.
As described by Barwick et al. (2009a), animals were weaned at an average age of 197.1 (BRAH) and 194.5 days (TCOMP). Shortly after weaning, steer progeny were transported to allocated grow-out properties. At a mean age of 284 days (BRAH) and 313 days (TCOMP), post-weaning (POSTW) measurements of liveweight (LWT), hip height (HH), ultrasound scanned eye muscle area (SEMA), flight time (FT) and blood concentration of insulin-like growth factor I (IGF-I) were collected. Animals were grown to feedlot entry at a mean liveweight of 393 kg and 406 kg for BRAH and TCOMP, respectively, which was achieved at an average age of 662 days for steers of both genotypes. Feedlot entry (ENTRY) measurements included all those collected at POSTW, plus condition score (CS), ultrasound scanned percent intramuscular fat (SIMF), P8 fat depth (SP8) and 12/13th rib fat depth (SRIB) and growth rate (ADG) calculated as the regression of multiple weights between weaning and feedlot entry, on time in days. Steers were finished on a high energy grain ration for an average of 119 days at the Beef CRC’s research feedlot (‘Tullimba’). Individual daily feed intake (DFI) was recorded for a proportion of the steers (700 BRAH and 787 TCOMP) in accordance with the guidelines specified by Exton (2001). Residual feed intake (RFI) was calculated as a function of DFI, growth rate and metabolic mid-weight during the test period (Barwick et al. 2009a ). Steers exited the feedlot at a mean liveweight of 539 kg for BRAH and 592 kg for TCOMP. Measurements collected at feedlot exit (EXIT) were a repeat of those described at ENTRY, with growth rate at exit (EXIT ADG) calculated as the regression of multiple weight measurements collected between ENTRY and EXIT, on time in days.
Heifers were weaned at the same time as steers and allocated, while maintaining genetic linkage, to one of four locations (Barwick et al. 2009b ). The harshest of the four environments was occupied only by BRAH, as it was considered unsuitable for TCOMP animals. At each location, heifers of the same year of birth (defined as a cohort) were managed as a single group. Barwick et al. (2009b) described the measurements collected at two times: (1) at the end of the animals’ first wet season following weaning (ENDWET) when BRAH and TCOMP heifers averaged 518 and 555 days of age and 288 and 314 kg liveweight, respectively; and (2) at the end of the second dry season following weaning (ENDDRY) when BRAH and TCOMP mean age and liveweight were 713 and 749 days and 320 and 354 kg, respectively. ENDWET and ENDDRY measurements examined for this paper were: LWT, HH, CS, SEMA, SIMF, SP8, SRIB, and IGF-I. ENDWET and ENDDRY growth rate (ADG) were calculated as the regression of multiple weight measurements collected between weaning and ENDWET (ENDWET ADG), and between ENDWET and ENDDRY measurement times (ENDDRY ADG), respectively.
Carcass and meat quality measurements
A description of all carcass and meat quality traits analysed for this experiment are in Table 1, and pre- and post-slaughter methods and measurement protocols are described in Perry et al. (2001). Briefly, following feedlot finishing, steers were transported to one of two commercial abattoirs, and slaughtered within 30 h of leaving the feedlot. Animals were stunned using a captive bolt and bled immediately. As steers had an average of 119 days on a high energy ration before slaughter, it was assumed that heavy carcass weight and fat deposition would prevent rapid chilling and cold-shortening. Carcasses were, therefore, not electrically stimulated, though a low voltage rigidity probe was applied during mechanical hide pulling. Carcasses were dressed in accordance with AUSMEAT standard specifications (AUSMEAT 1998), with the qualification that trimming of subcutaneous fat was limited to not influence fat depth measurement at the P8 or 12/13th rib measurement sites. Hot carcass weight (CWT) and hot P8 fat depth (P8h) was recorded by abattoir staff when the carcasses had been fabricated to sides, immediately before entry to the chiller, with the time from stunning to entry to the chillers being ~40 min. At this time, left sides were transferred to tenderstretch (TS) hooks, which secured the side by the aitch-bone (Hostetler et al. 1970; Thompson et al. 2006). The right sides remained conventionally hung by the Achilles tendon (AT). Sides were quartered 20–24 h post-slaughter. Quartering took place between the 12/13th ribs for a proportion of the carcasses (BRAH = 276 and TCOMP = 290) and between the 10/11th ribs for the remainder (BRAH = 710 and TCOMP = 903). Research staff measured cold P8 fat depth (P8c), while MSA certified graders recorded ossification score (OSS), hump height (HMP), cold rib fat depth (RIB), 12/13th rib eye muscle area (EMA), ultimate pH (pH_U), and MSA marbling score (MS). For a subset of the carcasses (274 BRAH and 289 TCOMP), the left sides were fabricated into 17 primal cuts, with fat trimmed to retail specifications. Retail beef yield (RBY) was calculated as the ratio of weight of total retail cuts, plus the weight of adjusted manufacturing trim, to side weight. Perry et al. (2001) provided a complete description of the RBY measurement procedures adopted for this experiment.
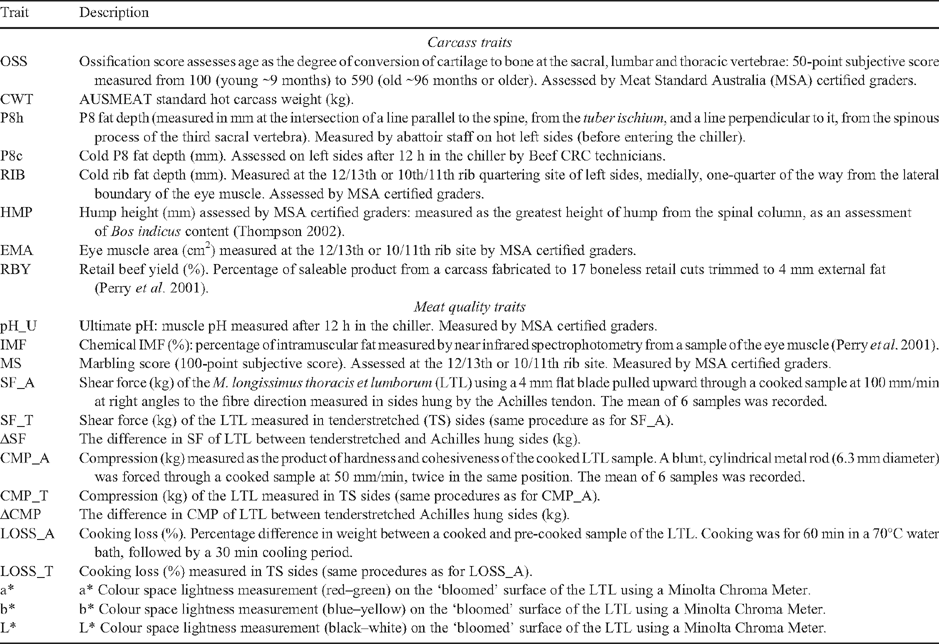
|
During carcass fabrication, a 15-cm sample was collected from the M. longissimus thoracis et lumborum (LTL), caudal from the 12/13th ribs, from both the TS and AT hung sides, and frozen for later objective meat quality measurement (Perry et al. 2001). Prior to cooking, Minolta a*, b* and L* (a*, b* and L*, respectively) values were recorded to provide an objective assessment of meat colour in samples of both the AT and TS hung sides (Wulf and Wise 1999). A standard portion (250 g, ~90 × 60 × 50 mm) was obtained from each sample, and cooked for 1 h in a 70°C water bath, and used to measure objective tenderness via shear force (SF) and compression (CMP) in both the AT (SF_A and CMP_A) and TS (SF_T and CMP_T) hung sides. To examine the impact of tenderstretching on the tenderness of BRAH and TCOMP LTL samples, two variables were calculated as the difference in SF (ΔSF) and CMP (ΔCMP) between conventionally hung and tenderstretched sides for each animal. Cooking loss (LOSS) was calculated as the ratio of post-cooked sample weight to sample weight before cooking (Perry et al. 2001). A second portion (~100 g) of each sample was freeze-dried for chemical fat (IMF) analysis using near infrared spectrophotometry (NIR) methods (Perry et al. 2001).
Statistical analysis
Data editing and fixed effect modelling
For each trait, initial data editing identified outliers, defined as records that were more than three standard deviations from the mean. Identification of outliers was conducted on a within-year and herd of origin basis. For most traits, numbers of outliers were low (generally ≤7 records) and these were not included in further analysis. The pH_U trait was an exception, with greater numbers of apparent outliers identified (n = 13 for BRAH and 14 for TCOMP). These occurred exclusively at the high end of the range (pH_U >5.61) and were considered potentially important records, so were retained in the analysis. Beef muscle pH is known to have a non-normal distribution (Page et al. 2001) and these high records may have identified animals displaying a documented biological condition known as ‘dark cutting’.
Significant fixed effects for each carcass and meat quality trait, first within genotype and then for a pooled dataset, were identified using mixed model procedures in SAS (SAS Institute 1989). The initial fixed effects tested included property of origin, month of birth (which accounted for age effects and the seasonal conditions at birth), age of dam in years, cohort (which defined the animal’s grow-out location, year of birth and feedlot allocation), and date of kill. As quartering site (10/11th or 12/13th rib) was confounded with slaughter location, and therefore date of kill, this effect was not included. For TCOMP, all models initially included the additional term of sire group (6 levels), dam group (7 levels), and their interactions, to account for possible TCOMP group effects, and differences in heterosis in these animals. Sire and dam groups defined the breed composition of TCOMP animals, as described by Barwick et al. (2009a). Initial models for each trait included main effects and all first order interactions. Sire was included as a random effect in all models. Final models were generated by sequentially dropping terms that were not significant (P > 0.05). Initial models for the pooled dataset included all significant terms from the individual genotype analyses, as well as genotype and its interactions. Sire was included as a random effect, and final models were determined by sequentially dropping terms that were not significant (P > 0.05).
Variance component estimation
Restricted maximum likelihood was used to estimate variance components for the BRAH, TCOMP and combined datasets via univariate analyses using ASReml (Gilmour et al. 1999). Variance components were estimated for models with animal fitted as a random genetic effect, and including the significant fixed effects identified above. A dam permanent environmental effect was tested for each trait by including it as a random effect and comparing log-likelihoods for models with and without the effect. Relationships between animals were accommodated using a three generation pedigree. A total of 53 BRAH and 50 TCOMP sires were represented with steer progeny.
Bivariate analyses were performed to calculate genetic and phenotypic correlations between carcass and meat quality traits. Genetic correlations were also estimated between the carcass and meat quality traits and the range of live steer and heifer measurements described by Barwick et al. (2009a , 2009b), respectively, for traits with a heritability greater than 0.10. Genetic correlations are presented for the combined dataset, but in instances where substantial differences existed for relationships between specific pairs of traits across genotypes (where the difference between genotype specific genetic correlations was greater than the sum of their standard errors) these results were also tabulated. To limit the genetic correlations presented to interpretable results, genotype specific correlations were only tabulated for estimates with standard errors less than or equal to 0.30 for both genotypes.
Model predicted means
Predicted genotype means were calculated for a subset of the steers, representing 32 BRAH and 27 TCOMP sires, which were born at one property of origin, and managed as contemporaries through to slaughter. Predicted means for genotype effects were estimated in ASReml as linear functions of the vector of fixed effects in the models developed for traits from the pooled dataset as described by Gilmour et al. (2004). Predicted means did not account for the influence of random effects. For CWT and RIB, fixed effect models were simplified by removing the first order interactions, to allow predicted means to be estimable. Predicted means were also calculated for two traits that quantified the difference between AT and TS tenderness, i.e. ΔSF and ΔCMP.
Results and discussion
Means, standard deviations, minimum and maximums for carcass and meat quality traits are in Table 2. The MSA grading system demands minimum standards for carcasses to be eligible for grading. Based on these requirements, and assessing each trait in isolation, 212 BRAH and 207 TCOMP carcasses would not have been graded on the basis of OSS (maximum OSS = 300), 39 BRAH and 16 TCOMP animals on pH_U (maximum pH_U = 5.7), and 7 BRAH and 6 TCOMP on the basis of RIB (minimum 12/13th rib fat depth = 3 mm).

|
Effect of tenderstretching on objective tenderness
Table 3 presents the number of observations, model predicted means (P.Mean), additive and phenotypic variances (σ2 A and σP 2) and heritabilities (h 2) for meat quality traits estimated from the combined dataset, plus genetic correlations of meat quality traits with ΔSF. Heritabilities for SF and CMP measured in tenderstretched (SF_T and CMP_T) and conventionally hung sides (SF_A and CMP_A) were similar (Table 3) across hanging methods, with SF_A (h 2 = 0.31) and CMP_A (h 2 = 0.18) both moderately heritable. Tenderstretching reduced the phenotypic and additive variance of SF_A measurement, by 77%. These results were consistent with observations made by Thompson (2002) at the phenotypic level, where subjective taste panel scores of tenderness displayed greater variation in AT sides than those hung by TS.

|
ΔSF was moderately heritable (h 2 = 0.26), with no difference between genotypes, while ΔCMP (results not presented) was not heritable (h 2 = 0.04). The predicted mean ΔSF was 1.04 kg (Table 3) when estimated from the pooled dataset. Genetic correlations showed that ΔSF was highly, positively genetically related to SF_A (r g = 0.92), suggesting that genetically tougher (i.e. higher SF_A) animals benefited most from TS. Interestingly, ΔSF (results not presented) was not strongly or consistently genetically related to fat measurements or carcass weight, (P8c = 0.28, RIB = 0.16, CWT = 0.26), and phenotypic correlations between these traits were also low (r P < 0.10). This indicates that traits likely to impact the cooling rate of carcasses had little influence on the magnitude of the effect of tenderstretching. With the exception of a strong relationship between ΔSF and SF_A (r P = 0.88), phenotypic correlations of meat quality traits with ΔSF were low (r P < 0.10; results not presented).
Genetic correlations between tenderness measurements in AT and TS hung sides were 0.77 for SF and 0.72 for CMP (Table 6), and genetic relationships between the two measures of tenderness (SF and CMP) were moderate and positive, whether measured on AT or TS sides (r g = 0.69 and 0.67 respectively). This, combined with the comparable heritabilities for tenderness traits measured in AT and TS sides suggests that, if phenotypes were available, selection to improve tenderness based on objective measurements would result in improvement for the trait, though if measurements of SF were collected exclusively from TS sides, genetic progress would be slower than selection based on measurements from conventionally hung sides. Phenotypic correlations (Table 6) between TS and AT measurements of tenderness were positive, though of lower magnitude than the genetic relationships (r P = 0.26–0.28).
Genotype differences
Model predicted means for BRAH and TCOMP are in Table 4. Despite the similarity in slaughter ages (Table 2), BRAH carcasses had significantly higher OSS (a descriptor of physiological age) than TCOMP (BRAH = 242.6 and TCOMP = 223.3). BRAH steers had significantly lower carcass weights (BRAH = 298.2 kg, TCOMP = 327.0 kg), with less fat, at both the P8c (BRAH = 12.9 mm, TCOMP = 14.2 mm) and RIB sites (BRAH = 8.8 mm, TCOMP = 10.9 mm) and smaller EMA (BRAH = 71.5 cm2, TCOMP = 76.4 cm2). RBY differences between the genotypes were also significant despite the low numbers for the trait, with TCOMP (66.6%) yielding lower than BRAH (68.1%). These results reflect similar differences observed at feedlot exit between the genotypes (Barwick et al. 2009a ), with the exception of P8c fat depth, where EXIT SP8 was not significantly different between the genotypes (BRAH = 12.7 mm, TCOMP = 12.6 mm). Though the genotypes examined were not exactly the same, these results were consistent with those presented by Newman et al. (2002) where the progeny of Brahman cows joined to a British breed, European and non-Brahman tropically adapted sires produced heavier, fatter and lower yielding carcasses, than pure bred Brahman progeny.

|
TCOMP produced carcasses with significantly more marbling, measured as either IMF (BRAH = 2.17% and TCOMP = 3.00%) or MS (BRAH = 0.49 and TCOMP = 0.89). These were also consistent with the results of Newman et al. (2002) where pure-bred Brahmans produced carcasses with lower IMF than Brahman cross-breeds. BRAH were significantly less tender than TCOMP when measured as SF (BRAH SF_A = 5.29 kg, TCOMP SF_A = 4.55 kg) or CMP (BRAH CMP_A = 1.96 kg, TCOMP CMP_A = 1.76 kg). Genotype rankings and their significance were maintained for sides under TS, though the magnitude of the differences was reduced. These results were consistent with several studies contrasting the meat quality of Brahman cattle with less tropically adapted genotypes (Shackelford et al. 1994; Sherbeck et al. 1996; O’Connor et al. 1997; Ferguson et al. 2000). LOSS was also greater for BRAH than TCOMP, though this difference was only significant for sides under AT hanging. No significant difference was present in L* (BRAH = 38.9, TCOMP = 39.0) between the genotypes.
Genetic and phenotypic variances and heritabilities for carcass and meat quality traits
Table 5 presents the additive and phenotypic variances and heritabilities for carcass and meat quality traits in BRAH and TCOMP steers. Several studies (O’Connor et al. 1997; Elzo et al. 1998; Newman et al. 2002; Johnston et al. 2003b ; Reverter et al. 2003a ; Riley et al. 2003; Domingue 2005; Smith et al. 2007) have examined the genetic parameters of meat quality and carcass traits in tropically adapted beef cattle. Generally, levels of phenotypic variation and heritabilities in this study were consistent with those presented by Johnston et al. (2003b) and Reverter et al. (2003b) from previous studies conducted by the Beef CRC in Australia. Some traits were lowly heritable (h 2 < 0.10): pH_U, LOSS_A, LOSS_T, a* and b* in BRAH, and pH_U, a* and b* in TCOMP, and these were not included in further analyses. Results reported by Johnston et al. (2003a) also found pH_U (h 2 = 0.02) and a* (h 2 = 0.13) to be lowly heritable in tropically adapted cattle.

|
For most carcass and meat quality traits, there was more additive variation (σ2 A) for TCOMP than BRAH (Table 5). Ossification score, however, displayed higher additive and phenotypic variation (σP 2) in BRAH, with the heritability for the trait also greater in BRAH (h 2 = 0.48) than TCOMP (h 2 = 0.35). Hump height (HMP) was measured as a component of the MSA system, as an indicator of the B. indicus content of carcasses. The trait was more heritable in BRAH than TCOMP (h 2 = 0.21 and 0.06, respectively), though the estimate for BRAH was lower than those presented in the literature, where Smith et al. (2007) and Riley et al. (2003) reported heritabilities of 0.38 and 0.52, respectively.
There was substantially less variation observed for the marbling traits in BRAH (IMF and MS σP 2 = 0.50 and 0.13) than TCOMP (IMF and MS σP 2 = 0.97 and 0.25). Measurements of IMF were moderately heritable in BRAH (h 2 = 0.26), but for TCOMP, the trait was more highly heritable (h 2 = 0.64). Interestingly, the study of Reverter et al. (2003b), where estimates were calculated from data pooled across tropically adapted genotypes, reported a heritability of 0.39, which represents an approximate midpoint for the range described by the genotype specific results presented for this experiment.
Relatedness of carcass and meat quality traits
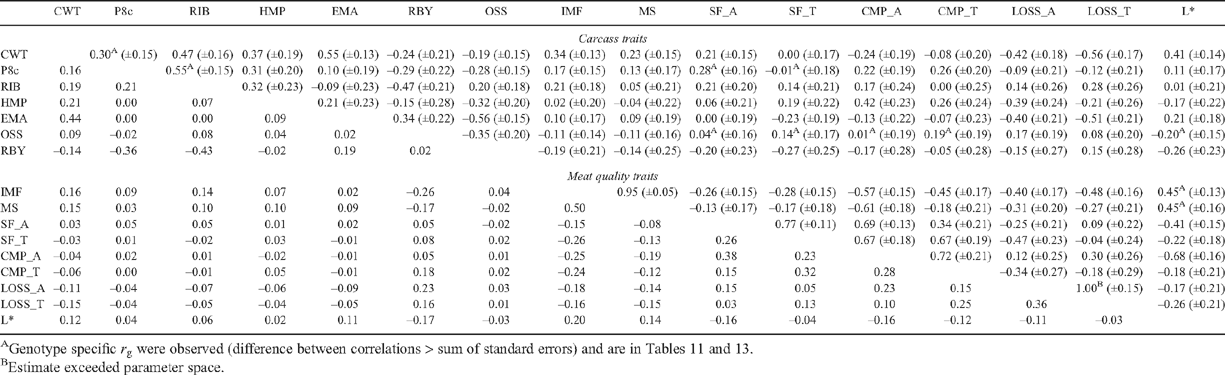
Table 6 presents the genetic and phenotypic correlations between carcass and meat quality traits calculated for the pooled dataset. Where significant differences existed in genetic correlations between specific pairs of traits for BRAH and TCOMP, these are in Tables 11–13.
|
Meat quality and marbling traits
Genetic correlations among tenderness measurements were generally high and positive (r g = 0.67–0.77), except for the relationship between SF_A and CMP_T, which was of a lower magnitude (r g = 0.34). Improved tenderness (decreased SF_A) was genetically related to increased LOSS_A (r g = –0.25). This was not consistent with the results presented by Johnston et al. (2003b) where this relationship in tropically adapted animals was reported to be low to moderate, but positive (r g = 0.15). In this study, cooking loss was negatively genetically correlated with most carcass and meat quality traits, most strongly with CWT, IMF EMA and HMP, suggesting that animals with genetically heavier carcasses, larger eye muscles higher percent intramuscular fat and lower hump height were genetically inclined to produce meat with lower LOSS. Steers with higher genetic merit for IMF were also those which tended to produce more favourable tenderness (Table 6), with this relationship stronger for CMP_A (r g = –0.57) than for SF_A (r g = –0.26). This may suggest the genetic predisposition to deposit intramuscular fat is genetically antagonistic to the development of connective tissue (Harper 1999), which has been demonstrated to have a negative impact on tenderness. The genetic correlations between shear force and other meat quality traits in this study were generally of greater magnitude than those reported by Reverter et al. (2003a), where they estimated a genetic correlation of only –0.09 between shear force and IMF in tropically adapted genotypes, though the direction of the relationship was consistent. Marble score (MS) was also negatively correlated with tenderness, though the magnitude of the correlations was lower than those between IMF and tenderness. Increased IMF was also genetically related to more favourable (lower) cooking loss (Table 6), whether measured in AT (r g = –0.40) or TS (r g = –0.48) hung sides. Phenotypically, the relationship between marbling and tenderness traits were lower (r P = –0.26 to –0.08), suggesting that limited opportunity exists to exploit marbling as an indirect means of assessing tenderness traits in individual carcasses of tropically adapted animals, particularly when the trait is measured subjectively. Meat colour L* measurements were moderately and positively genetically correlated with estimates of marbling (r g = 0.45 for both IMF and MS). However, genotype differences in these relationships show that for BRAH, IMF and MS (Table 13), had a stronger relationship with L* (r g = 0.80 and 1.00 for IMF and MS, respectively) than was the case for TCOMP (r g = 0.31 and 0.39 for IMF and MS, respectively). Therefore, L* tended to mirror the relationships with meat quality traits estimated for IMF, being favourably genetically correlated with measures of tenderness (SF_A = –0.41 and CMP_A = –0.68), which was consistent with the results of Reverter et al. (2003a).
Meat quality and carcass composition traits
In general, genetic relationships between carcass fat measurements and meat quality traits were positive and of low to moderate magnitude, consistent with the results of Reverter et al. (2003b), with the exception of the correlation between P8c and SF_A, which differed for the genotypes (Table 13: r g = 0.51 for TCOMP, and r g = –0.08 for BRAH). This suggested that genetically fatter TCOMP animals were also genetically tougher. Phenotypically, these relationships were low (r P = –0.06 to 0.05), which implies that processing conditions, and in particular chilling rate, did not influence meat quality traits in the animals involved in this experiment.
For post-slaughter measurements (Table 6 or Table 11 for genotype specific correlations), genetic and phenotypic correlations between measures related to carcass composition were generally in the direction expected from the literature (Reverter et al. 2003a ; Meyer 2007). Carcass fat depth (P8c and RIB) measurements were moderately genetically correlated (r g = 0.55), though this relationship was stronger for TCOMP than BRAH (Table 11: r g = 0.75 and 0.07, respectively). The result for BRAH contrasts with the genetic correlations between EXIT SP8 and EXIT SRIB reported by Barwick et al. (2009a) which were consistently high for both genotypes (r g = 0.90), and may point to lower accuracy of carcass rib fat depth measurement in BRAH cattle. Carcass fat depths were consistently positively correlated with carcass weight, though in the case of P8c (Table 11), this relationship was stronger for TCOMP (r g = 0.49) than BRAH (r g = 0.02) genotypes. EMA had a positive genetic relationship with CWT (r g = 0.55) for both genotypes. The genetic relationships between RBY and fat measurements were consistently negative (P8 = –0.29, RIB = –0.47), while EMA had a positive genetic relationship with RBY (r g = 0.34). Phenotypic correlations for these relationships (Table 6) were moderate, and in the same direction as the genetic correlations, and generally consistent with those reported by Reverter et al. (2003b) for tropically adapted animals. The genetic correlations between marbling and carcass traits were generally low, being positive with P8c, RIB and EMA (r g = 0.10 to 0.21).
Ossification score and meat quality traits
Ossification scores displayed different genetic relationships with meat tenderness measurements across genotypes (Table 13), with a consistent pattern for increased maturity (higher OSS) to be moderately genetically related to tenderness in TCOMP (r g = –0.26 and –0.40 for SF_A and CMP_A, respectively), but unfavourably correlated in BRAH (r g = 0.24 to 0.35 for SF_A and CMP_A, respectively). The difference in this relationship was not present in the phenotypic correlations, where OSS had virtually no impact on meat quality traits (Table 6: r P = –0.02 to 0.03) for either genotype.
Relatedness of carcass and live animal traits
Genetic correlations between carcass weight and liveweights measured in steers and heifers (Tables 7, 9–12) were consistently high, with weights measured in steers more highly genetically related to carcass weight as time of measurement approached slaughter (r g = 0.97, 0.83 0.63 and 0.56 for weights at EXIT, ENTRY POSTW and WEAN, respectively). Scanned measurements of fat depth (SP8 and SRIB) and SEMA collected at EXIT were highly genetically correlated with the corresponding carcass measurements (r g = 0.87 to 0.89), while EXIT SIMF had a genetic correlation of 0.61 with carcass IMF. These results were all within the range described by Crews et al. (2003), in reviewing the genetic relationships between live animal and carcass measurements, and confirm the efficacy of ultrasound scanning as a means of assessing carcass traits for genetic evaluation. Scanned measurements at ENTRY and POSTW (Table 9) were less reliable genetic indicators of carcass results, and genotype significantly influenced the correlations for ENTRY SP8 (Tables 9 and 11). Subjective condition scores (CS) at EXIT were highly genetically related to carcass P8 and RIB, which was consistent with the high genetic correlation between EXIT CS and EXIT P8 (r g = 0.91) and EXIT RIB (r g = 0.82) reported by Barwick et al. (2009a) in the same animals. Genetically, live animal measures of fat related traits at EXIT (SP8, SRIB, SIMF and CS), were negatively correlated with RBY (r g = –0.49, –0.30, –0.58 and –0.46, respectively). The genetic correlation between SEMA at EXIT and RBY was positive and low (r g = 0.10), but consistent with the results presented by Reverter et al. (2003a) in tropically adapted breeds.

|
Relatedness of meat quality and live animal traits
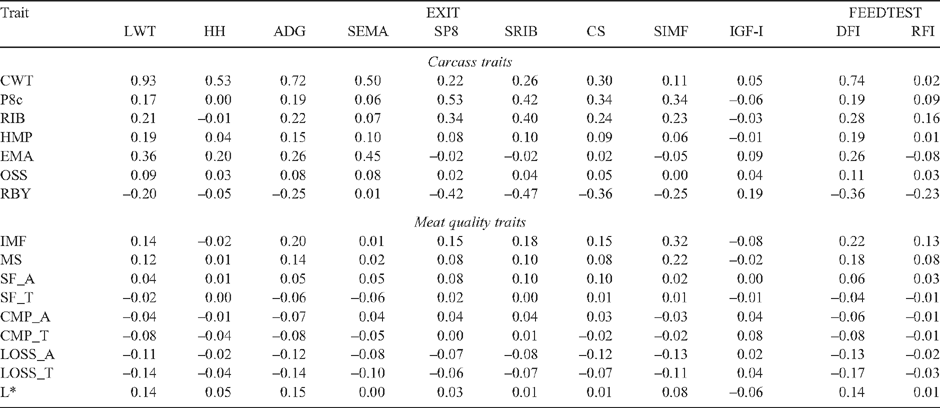
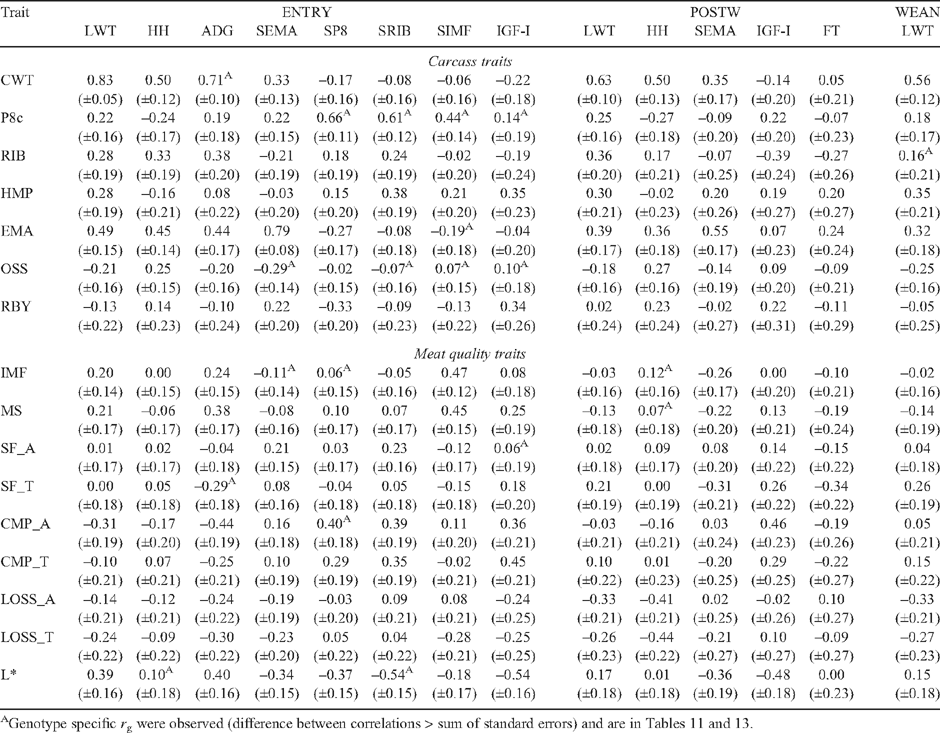
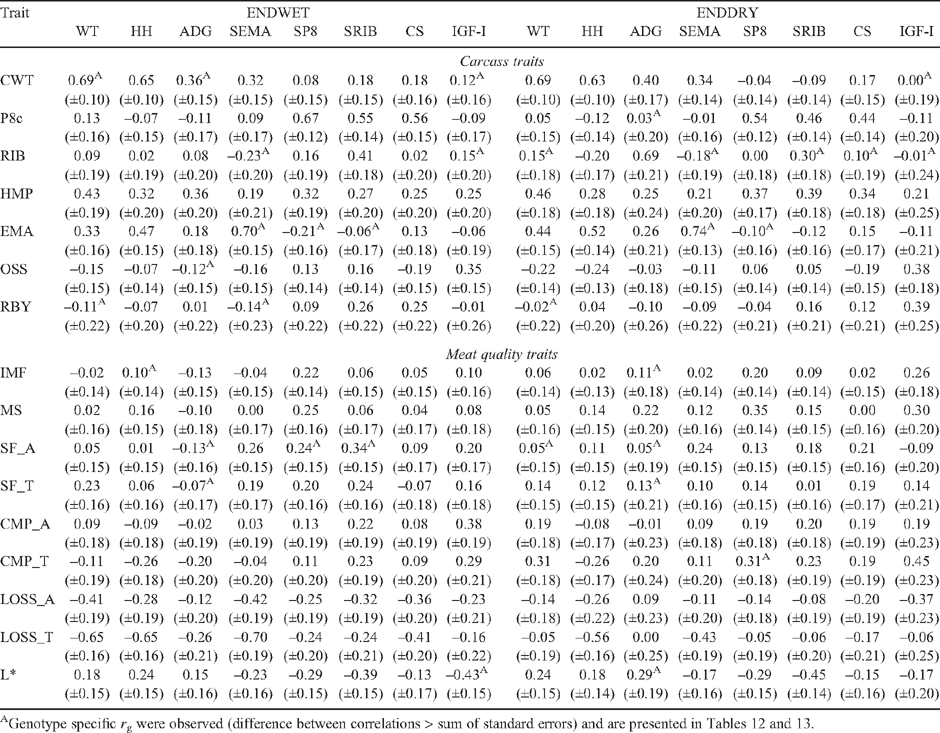
Tables 7–12 present genetic and phenotypic correlations of carcass and meat quality traits with live measures of steers, and their heifer paternal half-sibs.
|
|
|

|

|
Live measures of body composition and meat quality traits
Live animal ultrasound scanned measurements of fat depth (SP8 and SRIB), eye muscle area (SEMA) and intramuscular fat (SIMF) in steers (at EXIT, ENTRY and POSTW) and heifers at ENDWET (the end of their first wet season following weaning) and ENDDRY (the end of their second dry season following weaning), tended to follow the genetic trends observed for the corresponding carcass measurements, though relationships were generally of a lower magnitude. The trend was that the implementation of a breeding objective to decrease fatness in both steers and heifers would tend to genetically improve tenderness, meat colour and IMF. This relationship was strongest for EXIT SRIB in steers (Table 7: SF_A = 0.42, CMP_A = 0.38), but extended across fat measurement sites and measurement times in both sexes (r g CMP_A with ENTRY SP8 and SRIB = 0.40, and 0.39, respectively; and r g SF_A with ENDWET SRIB = 0.34, Tables 9 and 10, respectively). As was seen for carcass measurements, however, there were differences across genotypes in the genetic relationship between fat depths and tenderness (Table 13), with the correlations tending to be more positive for TCOMP steers following feedlot finishing (r g of SF_A with SP8 at EXIT = 0.39 for TCOMP, 0.03 for BRAH). Correlations between fat depths in heifers, and tenderness measured in their steer half-sibs were more positive for BRAH at ENDWET (r g SF_A with ENDWET SRIB = 0.53, and with ENDWET SP8 = 0.36) than TCOMP, where these relationships were virtually zero (r g = –0.02 and –0.07, respectively). Similar trends were observed for CMP_A and SP8 at ENTRY (r g = 0.59 for BRAH and 0.10 for TCOMP) and SP8 at ENDDRY and CMP_T (r g = 0.41 for BRAH and –0.15 for TCOMP). Literature examining genetic correlations between live body composition measurements and meat quality traits are sparse, though Crews and Kemp (2001) reported a positive genetic relationship between yearling heifer scans of 12/13th rib fat depth and Warner-Bratzler shear force (r g = 0.27 ± 0.23), though this correlation reversed when heifers were scanned ~50 days later, after entering the breeding phase of their lives (r g = –0.28 ± 0.22). While differences in magnitude and across genotypes were present, the generally positive genetic correlations between live fat measurements and SF_A found in this study suggest that selection to reduce fatness, will not negatively impact genetic tenderness in tropically adapted genotypes.

|
The genetic relationship between fatness traits and L* was stronger for measurements collected while animals were on pasture than was the case when fatness was measured in the carcass or steers at the end of feedlot finishing. Genetic correlations of L* with SRIB at ENTRY (Table 9), and in heifers at ENDWET and ENDDRY (Table 10) were consistently negative and of moderate magnitude (r g = –0.54 to –0.39), though again, genotype specific relationships did exist (Table 13). These results demonstrate that selection to reduce fatness will not negatively impact on meat quality traits, and may genetically improve tenderness and L* in tropically adapted animals, but that fat measurement time will impact the magnitude of this effect, and will influence the degree to which different genotypes demonstrate this response.
Steer and heifer growth rates and meat quality traits
Steer growth rate over the finishing phase (EXIT ADG; Tables 7 and 8) had little relationship with tenderness and other meat quality traits either genetically or phenotypically. For the pooled dataset, ENTRY ADG (Table 9), ENDWET ADG and ENDDRY ADG (Table 10), tended to be negatively genetically correlated with tenderness measurements, though for several these relationships there were differences between genotypes (Table 13). In general, increased ADG was genetically associated with lower SF in BRAH (for ADG at ENTRY and SF_T r g = –0.75, for ENDWET ADG and SF_A r g = –0.41, and for ENDDRY ADG and SF_A r g = –0.35). However, the effect was substantially smaller, or even reversed in TCOMP (for ENTRY ADG and SF_T r g = –0.16, for ENDWET ADG and SF_A r g = 0.21, and for ENDDRY ADG and SF_A r g = 0.56). Perry and Thompson (2005) reported that ADG was phenotypically related to tenderness, with increased ADG associated with improved meat quality (decreased shear force, compression and increased palatability scores) in both temperate and tropically adapted cattle. At the genetic level, Shackelford et al. (1994) showed that ADG had a negative relationship with SF (r g = –0.40). These results are consistent with those reported for BRAH in the current study. The significant and positive genetic relationships between SF_A and ADG at ENDDRY for TCOMP, was in contrast to the literature however, and suggested that TCOMP heifers which maintain ADG through the harsh conditions prevailing during their second dry season, were likely to be related to steers which perform less well for SF_A and SF_T. When measured in heifers at ENDDRY, genetic increases in ADG were strongly correlated with increased L* and IMF in BRAH, (r g = 0.60 and 0.77, respectively), but there was no such relationship (r g = 0.04 and –0.14, respectively) in TCOMP.
There were consistent trends across measurement times, in both steers and heifers, for LWT, ADG, HH and SEMA traits measured in the live animals to be negatively genetically related to cooking (LOSS). This, combined with the moderate favourable genetic correlation of LOSS with SF (r g = –0.47 to 0.09) and IMF (r g = –0.40 and –0.48 for LOSS_A and LOSS_T, respectively) suggests that selection to improve growth would not adversely affect the genetics of cooking loss or other meat quality traits in tropically adapted animals.
Insulin-like growth factor I and meat quality traits
Measures of plasma concentration of insulin-like growth factor I (IGF-I) was not genetically related to tenderness traits when blood samples were collected at feedlot exit (Table 7; EXIT IGF-I), but had consistently positive and moderate genetic relationships with CMP for steers at feedlot entry and post weaning (Table 9) (r g = 0.36 and 0.46, respectively), and heifers at ENDWET (Table 10; r g = 0.38). These correlations were complimented by genetic relationships between L* and IGF-I at ENTRY, POSTW and ENDWET (r g = –0.54, –0.48 and –0.43, respectively) indicating that lower concentrations of IGF-I were genetically consistent with more favourable meat colour in steer carcasses. However, for ENDWET IGF-I (Table 13), the correlation was stronger for BRAH (r g = –0.72) than for TCOMP (r g = –0.21). Genetic correlations between IGF-I and carcass fatness varied with sampling times and tended to be different across genotypes. For TCOMP, IGF-I measured in heifers displayed a negative relationship with carcass fat measurements (Table 12: ENDWET and ENDDRY IGF-I with RIB = 0.57 and 0.50, respectively), while measurements taken in steers (Table 11) at both ENTRY (IGF-I with P8c = –0.43) and EXIT (IGF-I with P8c = –0.52) were negative. For BRAH, the genetic correlations between IGF-I and carcass fatness were of a lower magnitude and tended to be more positive, again, except for ENDDRY IGF-I (Table 12), where BRAH displayed a more negative relationship between the traits (r g ENDDRY IGF-I with RIB = –0.25). Johnston et al. (2001) reported a positive genetic correlation (r g = 0.38) between P8c and IGF-I samples collected post weaning in B. taurus animals which was reasonably consistent with results of this study for POSTW IGF-I in the pooled dataset (r g = 0.22). These results suggest that the exploitation of IGF-I as a genetic indicator of meat quality traits may be possible, but that these results would need to be interpreted on a genotype specific basis.
Residual feed intake and meat quality and carcass traits
Genetic relationships between residual feed intake (RFI) and meat quality traits were consistent across genotypes and of low to moderate magnitude (Table 7), suggesting that aiming to genetically improve RFI, measured in finishing steers on a feedlot ration, would not negatively impact genetic tenderness, cooking loss, L* or IMF. This result is consistent with the observation of Baker et al. (2006) that, at a phenotypic level, reported RFI measured in Angus steers had no significant effect on objective tenderness measurements or taste panel scores. RFI had moderate genetic relationships with carcass composition traits (Table 7: r g = 0.49 with RIB, –0.42 with EMA and –0.61 with RBY), which were consistent across genotypes, suggesting that selection to improve RFI would produce carcasses which were genetically leaner, with larger EMA and higher RBY. This agrees with the results of Robinson and Oddy (2004), who reported a similar genetic relationship between RFI and scanned 12/13th rib fat depth (r g = 0.48) and EMA (r g = –0.24) measured immediately before slaughter. DFI had low genetic correlations with SF and CMP traits (r g = –0.20 to 0.16), though there was an indication that animals which had genetically higher DFI would generate lower LOSS (r g = –0.33 for AT sides and –0.48 for TS), and produce carcasses with more favourable L* (r g = 0.54) and IMF (r g = 0.32). The genetic relationship between DFI and fatness and RBY mirrored those reported for RFI, though the relationships with P8c and RIB differed across genotypes (Table 11) and showed that the positive correlation between DFI and fat traits was stronger in TCOMP than BRAH. DFI also had a strong genetic relationship with CWT (r g = 0.87) and was one of the few pairs of traits (Table 8) which displayed a strong phenotypic relationship (r P = 0.74).
Flight time and tenderness traits
Flight time measured in steers post weaning (POSTW FT) was lowly to moderately heritable (Barwick et al. 2009a ), and had low but consistently negative genetic correlations with tenderness (Table 9: r g = –0.15 and –0.19 for SF_A and CMP_A, respectively). These results were of a lower magnitude than those presented by Kadel et al. (2006) and Reverter et al. (2003a), where the genetic correlation between PWFT and SF_A was –0.42 for tropically adapted animals, but supported the conclusion that animals identified as having more desirable temperament (higher POSTW FT), tended to produce progeny with meat which was more tender.
Conclusions
This study has provided significant new insight into the genetic influences on meat quality and carcass traits in tropically adapted cattle under Australian production conditions. BRAH and TCOMP genotypes were significantly different for some key meat quality and carcass traits, supporting the MSA position that Brahman content be included as a factor in meat quality grading. Similarly, tenderstretching was confirmed as a processing option to improve tenderness, and reduce the degree of variation for the trait in both genotypes, particularly in animals which were genetically tougher.
Moderate heritabilities and the presence of adequate phenotypic variation for tenderness, meat colour, marbling and cooking loss showed that meat quality traits could be successfully improved by selection in tropically adapted animals. However, the difficulty and cost associated with collecting direct measurements remains a constraint to the genetic improvement of these traits. Genetic correlations indicated that selection to improve percent intramuscular fat, meat colour and in the case of TCOMP animals, to decrease P8 fat depth, would have a positive impact on genetics tenderness.
Differences in the genetic relationships between meat quality, carcass, and live animal traits, demonstrated the importance of genotype specific genetic evaluations for tropically adapted cattle. Additionally, the developmental stage when live measurements were collected, and the production environment under which animals were managed, influenced the magnitude and in some cases, the direction, of genetic relationships between carcass and meat quality traits and live animal measurements. This emphasises the importance of thoroughly defining measurement protocols, including animal age and condition at time of assessment, for live animal traits which are to be included in a genetic evaluation analysis.
Acknowledgements
The authors acknowledge the Cooperative Research Centre for Cattle and Beef Quality (and its core partners: The University of New England, NSW DPI, CSIRO, and Queensland DPI), the Commonwealth funding through the CRC Program, and the financial support of Meat and Livestock Australia and the Australian Centre for International Agricultural Research. The cattle used for this experiment were contributed by producers from the Northern Pastoral Group, and their financial support of this project is gratefully acknowledged. We also acknowledge all Beef CRC scientific and technical staff that contributed to or supported the work, including those involved in the cattle management, data collection, laboratory analyses, and data handling and management. We especially acknowledge the contributions of Diana Perry, Paul Reynolds, Andrew Blakely, Andrew Slack-Smith, Jason Siddell, Sheridan Moll and Felix Graser for their work in coordinating and conducting the carcass measurements, meat sample collection and meat quality laboratory analyses. Also, the contributions of Vince Edmonston, Rebecca Farrell, Roger Sneath and John Bertrand, of the Queensland Department of Primary Industries, in the co-ordination of kills, and collection of carcass measurements are gratefully acknowledged.
Baker SD,
Szasz JI,
Klein TA,
Kuber PS,
Hunt CW,
Glaze JB,
Falk D,
Richard R,
Miller JC,
Battaglia RA, Hill RA
(2006) Residual feed intake of purebred Angus steers: Effects on meat quality and palatability. Journal of Animal Science 84, 938–945.
[Verified 17 March 2009]
Ferguson DM,
Jiang S,
Hearnshaw H,
Rymill SR, Thompson JM
(2000) Effect of electrical stimulation on protease activity and tenderness of M. longissimus from cattle with different proportions of Bos indicus content. Meat Science 55, 265–272.
| Crossref | GoogleScholarGoogle Scholar |

Gilmour AR,
Cullis BR,
Welham SJ,
Gogel B, Thompson R
(2004) An efficient computing strategy for prediction in mixed linear models. Computational Statistics & Data Analysis 44, 571–586.
| Crossref | GoogleScholarGoogle Scholar |

Harper GS
(1999) Trends in skeletal muscle biology and the understanding of toughness in beef. Australian Journal of Agricultural Research 50, 1105–1129.
| Crossref | GoogleScholarGoogle Scholar |

Hostetler RL,
Landmann WA,
Link BA, Fitzhugh HA
(1970) Influence of carcass position during rigour on tenderness of beef muscles: comparison of two treatments. Journal of Animal Science 31, 47–50.

Johnston DJ,
Reverter A,
Robinson DL, Ferguson DM
(2001) Sources of variation in mechanical shear force measurements of tenderness in beef from tropically adapted genotypes, effects of data editing and their estimation for genetic parameter estimation. Australian Journal of Experimental Agriculture 41, 991–996.
| Crossref | GoogleScholarGoogle Scholar |

Johnston DJ,
Reverter A,
Burrow HM,
Oddy VH, Robinson DL
(2003a) Genetic and phenotypic characterisation of animal, carcass and meat quality traits for temperate and tropically adapted beef breeds. 1. Animal measures. Australian Journal of Agricultural Research 54, 107–118.
| Crossref | GoogleScholarGoogle Scholar |

Johnston DJ,
Reverter A,
Ferguson DM,
Thompson JM, Burrow HM
(2003b) Genetic and phenotypic characterisation of animal, carcass and meat quality traits for temperate and tropically adapted beef breeds. 3. Meat quality traits. Australian Journal of Agricultural Research 54, 135–147.
| Crossref | GoogleScholarGoogle Scholar |

Johnston DJ,
Barwick SA,
Corbet NJ,
Fordyce G,
Holroyd RG,
Williams PJ, Burrow HM
(2009) Genetics of heifer puberty in two tropical beef genotypes in northern Australia and associations with heifer‐ and steer‐production traits. Animal Production Science 49, 399–412.
| Crossref | GoogleScholarGoogle Scholar |

Kadel MJ,
Johnston DJ,
Burrow HM,
Graser H-U, Ferguson DM
(2006) Genetics of flight time and other measures of temperament and thief value as selection criteria for improving meat quality traits in tropically adapted breeds of beef cattle. Australian Journal of Agricultural Research 57, 1029–1035.
| Crossref | GoogleScholarGoogle Scholar |

Mackinnon MJ,
Meyer K, Hetzel DJS
(1991) Genetic variation and covariation for growth, parasite resistance and heat tolerance in tropical cattle. Livestock Production Science 27, 105–122.
| Crossref | GoogleScholarGoogle Scholar |

Meyer K
(2007) Multivariate analyses of carcass traits for Angus cattle fitting reduced rank and factor analytic models. Journal of Animal Breeding and Genetics 124, 50–64.
| Crossref | GoogleScholarGoogle Scholar |

Newman S,
Reverter A, Johnston DJ
(2002) Purebred–crossbred performance and genetic evaluation of postweaning growth and carcass traits in Bos indicus × Bos taurus crosses in Australia. Journal of Animal Science 80, 1801–1808.

O’Connor SF,
Tatum JD,
Wulf DM,
Greed RD, Smith GC
(1997) Genetic effects on beef tenderness in Bos indicus composite and Bos taurus cattle. Journal of Animal Science 57, 1822–1830.

Page JK,
Wulf DM, Schwotzer TR
(2001) A survey of beef muscle colour and pH. Journal of Animal Science 797, 678–687.

Perry D, Thompson JM
(2005) The effect of growth during backgrounding and finishing on meat quality traits in beef cattle. Meat Science 69, 691–702.
| Crossref | GoogleScholarGoogle Scholar |

Perry D,
Thompson JM,
Hwang IH,
Butchers A, Egan AF
(2001) Relationship between objective measurements and taste panel assessment of beef quality. Australian Journal of Experimental Agriculture 41, 981–989.
| Crossref | GoogleScholarGoogle Scholar |

Pringle TD,
Williams SE,
Lamb BS,
Johnson DD, West RL
(1997) Carcass characteristics, the calpain proteinase system and aged tenderness in Angus and Brahman crossbred steers. Journal of Animal Science 75, 2955–2961.

Reverter A,
Johnston DJ,
Ferguson DM,
Perry D,
Goddard ME,
Burrow HM,
Oddy VH,
Thompson JM, Bindon BM
(2003a) Genetic and phenotypic characterisation of animal, carcass and meat quality traits for temperate and tropically adapted beef breeds. 4. Correlations among animal, carcass and meat quality traits. Australian Journal of Agricultural Research 54, 149–158.
| Crossref | GoogleScholarGoogle Scholar |

Reverter A,
Johnston DJ,
Perry D,
Goddard ME, Burrow HM
(2003b) Genetic and phenotypic characterisation of animal, carcass and meat quality traits for temperate and tropically adapted beef breeds. 2. Abattoir carcass traits. Australian Journal of Agricultural Research 54, 119–134.
| Crossref | GoogleScholarGoogle Scholar |

Riley DG,
Chaser CC,
Hammond AC,
West RL,
Johnson DD,
Olson TA, Coleman SW
(2003) Estimated genetic parameters for palatability traits of steaks for Brahman cattle. Journal of Animal Science 81, 54–60.

Robinson DL, Oddy VH
(2004) Genetic parameters for feed efficiency, fatness, muscle area and feeding behavior of feedlot finished beef cattle. Livestock Production Science 90, 255–270.
| Crossref | GoogleScholarGoogle Scholar |

Shackelford SD,
Koohmaraie M,
Cundiff LV,
Gregory KE,
Rohrer GA, Savell JW
(1994) Heritabilities and phenotypic and genetic correlations for bovine postrigor calpastatin activity, intramuscular fat content, Warner-Bratzler shear force, retail product yield, and growth rate. Journal of Animal Science 72, 857–863.

Shackelford SD,
Wheeler TL, Koohmaraie M
(1995) Relationship between shear force and trained sensory panel tenderness ratings of 10 major muscles from Bos indicus and Bos taurus cattle. Journal of Animal Science 73, 3333–3340.

Sherbeck JA,
Tatum JD,
Field TG,
Morgan JB, Smith CG
(1996) Effect of phenotypic expression of Brahman breeding on marbling and tenderness traits. Journal of Animal Science 74, 304–309.

Smith T,
Domingue JD,
Paschal JC,
Franke DE,
Bidner TD, Whipple G
(2007) Genetic parameters for growth and carcass traits of Brahman steers. Journal of Animal Science 85, 1377–1384.
| Crossref | GoogleScholarGoogle Scholar |

Thompson JM
(2002) Managing meat tenderness. Meat Science 62, 295–308.
| Crossref | GoogleScholarGoogle Scholar |

Thompson JM,
Perry D,
Daly B,
Gardner GE,
Johnston DJ, Pethick DW
(2006) Genetic and environmental effects on the muscle structure response post mortem. Meat Science 74, 59–65.
| Crossref | GoogleScholarGoogle Scholar |

Watson R,
Polkinghorne R, Thompson JM
(2008) Development of the Meat Standards Australia (MSA) model for beef palatability. Australian Journal of Experimental Agriculture 48, 1368–1379.
| Crossref | GoogleScholarGoogle Scholar |

Wulf DM, Wise JW
(1999) Measuring muscle colour on beef carcasses using the L* a* b* colour space. Journal of Animal Science 77, 2418–2427.

1 Animal Genetics and Breeding Unit is a joint venture of the New South Wales Department of Primary Industries and the University of New England.


