Genetics of adaptive traits in heifers and their relationship to growth, pubertal and carcass traits in two tropical beef cattle genotypes
K. C. Prayaga A B E , N. J. Corbet A B , D. J. Johnston A C , M. L. Wolcott A C , G. Fordyce A D and H. M. Burrow AA Cooperative Research Centre for Beef Genetic Technologies, University of New England, Armidale, NSW 2351, Australia.
B CSIRO Livestock Industries, J. M. Rendel Laboratory, Rockhampton, Qld 4702, Australia.
C Animal Genetics and Breeding Unit 1 , University of New England, Armidale, NSW 2351, Australia.
D Queensland Department of Primary Industries and Fisheries, Charters Towers, Qld 4820, Australia.
E Corresponding author. Email: kishore.prayaga@csiro.au
Animal Production Science 49(6) 413-425 https://doi.org/10.1071/EA08247
Submitted: 30 September 2008 Accepted: 5 December 2008 Published: 13 May 2009
Abstract
Genetic analyses of tropical adaptive traits were conducted for two tropically adapted genotypes, Brahman (BRAH) and Tropical Composite (TCOMP). Traits included tick scores (TICK), faecal egg counts (EPG), buffalo fly-lesion scores (FLY), rectal temperatures under hot conditions (TEMP), coat scores (COAT), coat colour on a light to dark scale (COLOUR), navel scores (NAVEL) and temperament measured as flight time (FT). The data comprised adaptive measures recorded at specific times on 2071 heifers comprising 966 BRAH and 1105 TCOMP. The genetic correlations of these adaptive traits with heifer growth, scanned carcass, pubertal measures and steer growth and carcass traits were estimated. BRAH recorded significantly (P < 0.05) lower TICK, EPG, FLY and TEMP than did TCOMP. BRAH also had significantly sleeker coats, lighter coat colour, more pendulous navels and more docile temperament than did TCOMP. The heritability of TICK and FLY was low (<20%), that of EPG, TEMP, NAVEL and FT was moderate (20–50%) and that of COAT and COLOUR high (>50%). In general, phenotypic correlations between these adaptive traits were low and genetic correlations were non-significant, implying trait independence. Genetic correlations between EPG and weight traits (0.29 to 0.44) indicated a positive relationship, implying no deleterious effect of worms on the growth at a genetic level, especially in TCOMP. The negative genetic correlations between COAT and body-condition score across genotypes (–0.33 to –0.48) indicated genetic advantage of sleek coats in tropics. A positive genetic correlation between COAT and the age at the first-observed corpus luteum (0.73) in BRAH indicated that BRAH with sleeker coats were genetically early maturing. Further, sleeker coats were genetically indicative of lower weights and lower fat cover at puberty in BRAH. The scanned fat measures at rump and rib sites for feedlot steers showed strong genetic correlation (0.50–0.58) with heifer TEMP, indicating genetically fatter animals had genetically lower heat tolerance. In BRAH, a positive genetic association between heifer COLOUR and scanned fat measures in steers (0.50–0.54) implied increased fatness in genetically darker animals. Further, in BRAH, a strong negative genetic correlation (–0.97) was observed between steer retail beef yield and heifer TEMP, indicating a favourable genetic association. In general, genetic correlations between adaptive traits and other economic traits were genotype specific. Further, it can be concluded that selection for productive and pubertal traits in tropical beef cattle genotypes would not adversely affect their tropical adaptability.
Additional keywords: coat score, genetic correlation, heat resistance, heritability, parasite resistance, temperament.
Introduction
Adaptation in general, and tropical adaptation in particular, is gaining importance in beef cattle production because of the focus on sustainable agriculture with less reliance on chemicals and increased consumer concern about animal welfare practices. Through natural selection, cattle have evolved and adapted to their immediate environment over several centuries. However, because of the current trends in international cattle and meat trade and the consequent need for matching genotypes to their intended markets, cattle are now required to adapt to several different environments during their lives. Adaptation of cattle to their immediate environment is also perceived to be important for efficient beef production because of possible advantages of lowered physiological stress. Further, tropical adaptation is a topical issue now because of the concerns about climate change and global warming. In northern Australia, cattle are expected to survive, grow and reproduce while enduring various environmental stressors such as ecto- and endoparasites, heat, humidity and seasonal variation in pasture quality. To counter these environmental stressors, breeds with natural resistance to the stressors (e.g. Brahman) have been introduced. However, lower calf output (Prayaga 2004) and poorer meat-quality attributes (Gazzola et al. 1999) of these highly adapted breeds has led to the development of alternative breeding strategies such as the development of tropical composite breeds to improve overall herd productivity and profitability.
Several studies have reported genetic parameters of adaptive traits measured in northern Australia [see review by Davis (1993)]. Some recent studies have focussed on crossbred (Prayaga and Henshall 2005) and composite populations (Burrow 2001). Most studies were based on a specific region (Central Queensland) of northern Australia and thus did not represent the whole of tropical Australia. The Co-operative Research Centre for Cattle and Beef Quality (Beef CRC) undertook a project aimed at improving our understanding of the genetic links between beef quality and components of herd profitability in northern Australia. Components of herd profitability included a comprehensive list of productive, adaptive and reproductive traits of Brahman (BRAH) and Tropical Composite (TCOMP) cattle reared under four different environments of northern Australia (Barwick et al. 2009a , 2009b ). The present paper reports the means, variances, genetic and phenotypic correlations of tropical adaptive traits in both genotypes. Although reports on genetic parameters for these traits exist, studies exploring the genetic relationships between adaptive traits and other growth, carcass, meat-quality and pubertal traits are scarce. Hence, the further aim of the present paper is to examine the potential effects of selection for improvements in a range of economic traits on the tropical adaptability of beef cattle.
Materials and methods
Animals
A full description of the breeding program conducted to generate this resource population and the management and treatment of heifers and steers is outlined in the companion papers (Barwick et al. 2009a , 2009b ). In essence, 1032 BRAH and 1142 TCOMP heifers representing 54 and 51 sires, respectively, were bred between 1999 and 2002 by using AI and natural service at seven cooperating industry properties and CSIRO managed Belmont research station in Queensland and Northern Territory, Australia. AI sires ensured genetic linkage across years and properties of origin within genotype. TCOMP represented ~50% Bos indicus or African Sanga and 50% tropically non-adapted Bos taurus (British and European) breeds. Heifer progeny generated across 4 years for BRAH (1999–2002) and 3 years for TCOMP (2000–2002) were allocated at weaning according to property of origin and sire and transported to one of four research stations in Queensland. BRAH were allocated to Belmont (Rockhampton), Swans Lagoon (Ayr) and Toorak (Julia Creek), whereas TCOMP were allocated to Brian Pastures (Gayndah), Belmont and Toorak. The genotypes were allocated to each of these locations on the basis of their perceived ability to cope with the stressors experienced by cattle on each of the research stations. The environments at these four research stations were representative of the subtropical and tropical conditions prevailing in Australia’s northern production environments. These environments generally have a hot period of ~4–5 months (December to April) when 75% of the median rainfall occurs, referred to as wet season, followed by a usually dry cool period of ~3 months (May to July) and then a typically dry hot period of ~3–4 months (August to November). Average annual rainfall at the sites ranged from 439 mm at Toorak to 860 mm at Swans Lagoon. BRAH and TCOMP were raised as contemporaries from birth only at Belmont. At each location, all heifers weaned in the same year were managed as a single group (defined as a cohort) until mated as 2 year olds when they joined other cohorts in large multiple-sire mating groups. Following weaning at co-operating properties of origin, steer progeny (1007 BRAH, 1209 TCOMP) were allocated to grow-out properties on the basis of the property of origin, genotype and year of birth to maintain genetic linkages across contemporary groups (Barwick et al. 2009a ). Following grow-out, steers entered a feedlot in northern New South Wales when the average bodyweight of their cohort was ~400 kg.
Adaptive traits in heifers
Climatic conditions and environmental stressors on animals differed from location to location. However, in general most of the northern properties in Queensland are subjected to varying levels of environmental stressors such as high temperatures and humidity during summer, nutritional deficiency during dry season, ectoparasites (cattle tick, Boophilus microplus and buffalo fly, Haematobia irritans exigua), endoparasites (gastrointestinal helminths or worms, predominantly Haemonchus, Cooperia and Oesophagostomum species) and periodic exposure to diseases (e.g. bovine infectious kerato-conjunctivitis and ephemeral fever).
Measurement of adaptive traits was carried out by experienced project staff to enable consistency in recording. Although all measurements at each location were obtained on the same date, the date of measurement varied depending on natural challenge for the traits being recorded. Repeated-measures of all traits were recorded at regular postweaning intervals. From these postweaning measures, specific measures of the traits (Table 1) were defined, on the basis of the biological significance of age at which the measurement was taken and to maximise the number of records for analyses across cohorts.

|
Natural tick challenge was low during the experimental period. The protocol for determining the accepted levels of challenge was to count the ticks on the first 20 animals at each muster and if the average of tick counts was less than 15, it was deemed to be not enough natural challenge and no further scoring was done. Hence, tick score (TICK) data suitable for genetic analysis were only available from cows with a mean age of 34 months from Belmont and Swans Lagoon stations during 2003–2004. In general, first postweaning records of faecal egg counts (EPG) at ~260 days of age were used as a measure of worm resistance. Buffalo fly-lesion scores (FLY) from a specific time period of March–April 2005 were selected because of the availability of maximum number of records during that period. Although, number of flies provides an objective measure of the level of infestation, it is the physical damage to the skin arising from lesions that is of economic importance and also an animal-welfare concern. Thus, fly-lesion scores were used to determine the resistance levels.
Rectal temperatures (TEMP) were recorded only during summer months when the ambient temperature was >30°C. Coat scores (COAT) were converted to a continuous 21-point scale to accommodate three subdivisions within each of the numeric 1–7 score. Coat colour (COLOUR) was recorded as the following six categories: white, cream, grey, red, tan and black. Under each of these categories, two shades of the basic colour, light and dark, and any other variations such as brindle, roan and markings were also recorded. However, for these quantitative analyses, all records were transformed to a light-to-dark scale of 1–6, with 1 being light and 6 being dark, irrespective of the actual colour of the animal. In the present study, COLOUR was investigated as an attribute of heat tolerance because of the reported greater absorption of solar radiation by darker-coloured animals than by lighter-coloured ones, leading to greater rate of environmental heat gain at the skin (Finch et al. 1984). Thus, the rationale for light-to-dark scale scoring was to examine the relationship of coat darkness with other adaptive attributes, especially resistance to heat. Navel score (NAVEL) represented on a continuum of 1 (very pendulous) to 9 (extremely tight skin) was used for analyses. Navel score, although not a conventional measure of adaptation, was scored as a possible indicator of skin looseness. For flight time (FT), higher-end outlier records were equated to the maximum measure within the distribution because those records represent highly docile animals that moved very slowly or even stopped in the race, after initially activating the recorder beam.
Heifer growth and composition traits
Barwick et al. (2009b) presented a full description of heifer growth and body-composition traits. In brief, heifer traits included growth and body-composition traits at the end of their first postweaning ‘wet season’ (ENDWET) when BRAH and TCOMP heifers averaged 518 and 555 days of age and 288 kg and 314 kg liveweight, respectively, and again at the end of the following ‘dry season’ (ENDDRY) when BRAH and TCOMP heifers averaged 713 and 749 days of age and 320 kg and 354 kg liveweight, respectively. At both time periods, liveweight (LWT), hip height (HH), average daily gain (ADG) estimated as individual animal regressions of liveweight on days for multiple weights recorded during the previous 6-month period, ultrasonically scanned eye muscle (M. longissimus thoracis et lumborum) area at 12/13th rib (SEMA), ultrasonically scanned fat depths at the rump P8 site (SP8) and the 12/13th rib site (SRIB), body-condition score (CS) and serum insulin-like growth factor-I concentration (IGF-I) were considered for analyses.
Pubertal traits in heifers
A full description of pubertal traits in heifers is given by Johnston et al. (2009). Briefly, pubertal traits in heifers included the age at the first-observed corpus luteum (AGECL), weight at the first-observed corpus luteum (WTCL), ultrasound-scanned P8 rump-fat depth at the first-observed corpus luteum (FATCL), condition score at the first-observed corpus luteum (CSCL), reproductive-tract score before joining with bulls (TSIZE), presence or absence of a corpus luteum before joining (CLPRIOR), presence or absence of a corpus luteum on the day of joining (CLJOIN).
Steer growth, carcass and meat-quality traits
To investigate the genetic relationships between heifer measures of adaptation and production traits in steers, the records of paternal half-sibs were used. Steer production traits recorded at weaning (WEAN), ~80 days postweaning (POSTW), feedlot entry (ENTRY) and after feedlot finishing (EXIT) were included in the analyses (Barwick et al. 2009a ). The steers were managed in several postweaning grow-out groups and entered the feedlot at a mean liveweight of 393 kg and 406 kg for BRAH and TCOMP, respectively. They were fed for an average of 119 days on a high-energy feedlot ration and slaughtered at an average liveweight of 568 kg.
The steer traits included steer LWT, SP8, SRIB, SEMA, scanned intramuscular fat% (SIMF), CS, HH, flight time (FT) and IGF-I. On a subset of steers, individual daily feed intake (DFI) was recorded over an average test period of 71.6 days. Residual feed intake (RFI) was calculated as the difference between the DFI of steers and their expected feed requirements based on their metabolic mid-test weight and test average daily liveweight gain (Barwick et al. 2009a ).
Carcass- and meat-quality measures are described by Wolcott et al. (2009). In brief, steers were slaughtered within 30 h of leaving the feedlot in one of two commercially operated abattoirs, where meat samples were removed from each carcass for subsequent meat-quality assessment. In the present paper, carcass- and meat-quality traits included hot carcass weight (CWT), carcass 12th/13th rib eye-muscle area (EMA), cold rib-fat depth (RIB), ossification score (OSS), Meat Standards Australia marbling score (MS), percentage of retail beef yield (RBY) and shear force (SF). OSS assesses maturity of the carcass as a degree of conversion of cartilage to bone at the sacral, lumbar and thoracic vertebrae on a 50-point subjective score measured from 100 (young ~9 months) to 590 (old ~96 months or older). MS is measured as a 100-point subjective score and RBY is computed as a percentage of saleable product from a carcass fabricated to 17 boneless retail cuts trimmed down to 4 mm of external fat. SF in kilograms is a measure of meat tenderness, by using a 4-mm flat blade pulled upward through a cooked sample of the M. longissimus thoracis et lumborum (LTL) at 100 mm/min at right angles to the fibre direction measured in sides hung by the Achilles tendon. For purposes of the analyses, the mean SF of six samples was used.
Statistical analyses
Fixed-effects modelling
For all adaptive traits, records beyond three standard deviations from the mean were scrutinised and checked for discrepancies. Unless obvious, these outliers were not deleted as they represent the natural biological variability of the adaptive traits. Fixed-effects models were developed for each genotype separately by using SAS Mixed Procedure (SAS Institute, Cary, NC, USA) to identify significant fixed effects and sire was included as a random effect. In general, the linear models included the effects of the property of origin, cohort (combined effects of weaning year and postweaning location), age of dam (in years) and birth month. Birth month included seasonal as well as age effects. In TCOMP, sire group (up to 7 levels) and dam group within herd of origin were fitted to account for the average additive differences between the composite groups and any heterotic effects among combinations of sire and dam groups. First- and second-order interactions were also fitted. Non-significant effects (P > 0.05) were sequentially omitted to yield the final models for each trait. For TEMP, time of recording within the date of recording was included to account for changing ambient air temperature during the measurement period. Wherever significant, the date of recording was also included in the model. Initial data edits deleted records with less than two sires in each cohort–origin combination. Data transformations were applied where required to normalise the data. FT data were log-transformed and EPG data were cube-root transformed. Finally, fixed-effects models were also generated for pooled data of both genotypes by including genotype as a fixed effect and its interactions with other significant fixed effects.
Predicted means
Predicted means of adaptive traits in heifers for each genotype were derived from the analyses of pooled data with Genstat (Payne et al. 2006) and by averaging over other fixed effects. EPG and FT data were back-transformed to the original scale, to enable sensible biological comparisons. Genotype means were derived only for heifers located at Belmont, where both genotypes were raised together from birth as contemporaries.
Variance-component estimation
Restricted maximum likelihood estimates of variance components were derived from univariate animal models using ASReml (Gilmour et al. 2005). The fixed effects identified as significant in the final model for each trait, along with animal and error as random effects were included in this univariate animal model. The relationship matrix was based on a 3-generation pedigree. Transformed data were used for the variance-components estimation. Insufficient data were available (Table 2) for meaningful genetic analyses of TICK and TEMP in TCOMP. Hence, those estimates were not presented in the results tables. However, this limited TCOMP data for these traits was included for deriving pooled estimates. A series of bivariate analyses was conducted for all possible pairs of traits to estimate genetic (r G) and phenotypic (r P) correlations among the adaptive traits. To estimate genetic and phenotypic correlations between the heifer adaptive traits and heifer growth, composition and pubertal traits and steer growth, and carcass- and meat-quality traits, a further series of bivariate analyses was performed. Bivariate analyses were performed separately for each genotype as well as for pooled data.

|
Results and discussion
Adaptive-trait means
The number of records and the unadjusted means and standard deviations of these traits across all locations in each genotype and in the pooled data are presented in Table 2. High coefficients of variation (>50%) were observed in parasite-resistance traits and COAT and in general, lower coefficients of variation were observed for NAVEL and COLOUR traits.
Predicted means for all adaptive traits for BRAH and TCOMP at Belmont are presented in Table 3. The significantly (P < 0.05) lower predicted means for TICK, EPG and FLY in BRAH than in TCOMP indicate greater parasite resistance in Brahmans. The tick-count means in Zebu- and Sanga-derived breed groups in an earlier study (Prayaga 2003) at Belmont were 10 and 28, respectively, being comparable to the current tick-score estimates of 1.3 (BRAH) and 2.2 (TCOMP; see Table 1 for score definitions). The lower EPG means in BRAH and TCOMP were comparable to the earlier reported means of 319 and 781 in Zebu- and Sanga-derived breed groups, respectively (Prayaga 2003). Thus, the natural parasitic challenge during the course of the experiment could be regarded as low to moderate as per earlier studies at Belmont. Even though a significantly (P < 0.05) lower FLY was observed in BRAH than in TCOMP, the difference was minimal.

|
Despite the lower TEMP of BRAH (P < 0.05), the lower means indicated that TCOMP were also able to endure Belmont summer heat, reflecting their adaptability across several generations. However, significantly (P < 0.05) lower COAT and COLOUR recorded for BRAH indicated sleeker and lighter coats compared with TCOMP. BRAH also had looser navels. Significantly (P < 0.05) higher FT (favourable) in BRAH than in TCOMP in the present study contradicted earlier observations of Brahmans having excitable temperament under extensive management conditions (Burrow 1997). However, it was also reported that when handled early in life Brahmans become extremely docile because of a taming effect (Boissy et al. 2005). Thus, early life handling could be one of the reasons for relatively docile animals in the present study in both genotypes.
Genetic parameters of adaptive traits in heifers
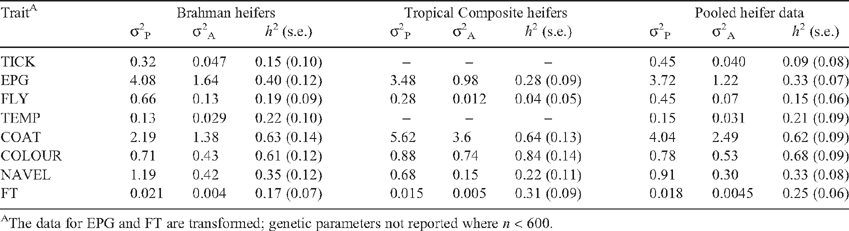
Genotype-specific and pooled estimates of variances and heritabilities (h 2) for adaptive traits are presented in Table 4. Variances for EPG and FT are derived from transformed data. In general, h 2 of TICK and FLY were low (<20%); EPG, TEMP and NAVEL were moderate (20–50%); and COAT and COLOUR were high (>50%). The h 2 of FT was moderate although lower in BRAH than in TCOMP. In general, trait and genotype-specific differences in variances and h 2 were observed. Phenotypic variances were higher for EPG, FLY, NAVEL and FT in BRAH and for COAT and COLOUR in TCOMP.
|
The low h 2 of TICK in the present study was comparable to those reported in a crossbred population (Prayaga and Henshall 2005), even though the later estimate was derived from repeated tick counts. However, the current h 2 estimates of TICK and FLY were lower than estimates reported by Burrow (2001) in a composite population. One of the reasons for these lower estimates of h 2 could be relatively low tick and fly challenge during the present study, compared with higher challenges in the earlier study (Burrow 2001). Moderate h 2 estimates for EPG were comparable to those in earlier studies (Burrow 2001; Prayaga and Henshall 2005). The immune response of ruminants changes with the age of the host, the length of the exposure of host to parasites, the number of parasites and plane of nutrition (Wakelin 1989; Gray and Gill 1993). In the present study, while EPG records were from relatively younger animals, TICK and FLY were from older animals and the differences in h 2 in these parasite-resistance traits might have been due to the differences in the age and maturity of animals.
The importance of heat tolerance in maintaining stable core body temperature under hot conditions has been well documented (Finch 1986). It was reported that cattle use a variety of strategies to cope with hot environment, such as transferring heat from body core to the skin through tissue conductance, raising the skin temperature by convection and radiation, increasing sweating rate to increase skin evaporative loss, and increasing respiratory rate to increase respiratory evaporative loss and a fall in metabolic rate. If these strategies fail, heat load exceeds body’s capacity to eliminate it and the body temperature rises. Hence, the traits TEMP, COAT and COLOUR recorded in the present study represent different aspects of heat tolerance. Earlier reports of low to moderate h 2 estimates of TEMP and high h 2 estimates of COAT in tropical beef cattle (Mackinnon et al. 1991; Burrow 2001; Prayaga and Henshall 2005) support the present estimates. Coat colour is recognised to be under the influence of a few major genes (Olson 1999), with no known h 2 estimates of coat colour recorded on a light-to-grey scale that are comparable to the present high estimates.
The h 2 estimates of postweaning navel scores were slightly higher than the previously reported estimate of 0.21 (Kriese et al. 1991) in Brangus cattle; however, they were lower than the 0.45 for a measurement of sheath/navel skin area in Brahman bulls and heifers reported by Franke and Burns (1985). The moderate h 2 estimate of NAVEL in BRAH was encouraging as selective breeding could be effectively used to breed animals with less pendulous navel skin. However, caution should be exercised because of the value attributed to breed characteristics by cattle breeders and its possible association with heat tolerance in tropical climates through increased surface area. Although temperament is not a tropical issue in particular, it relates to the adaptive ability of the animal to its immediate environment. Even though regular handling through routine management practices may reduce the excitable or aggressive behaviour of cattle, temperament is still an issue because of limited human interaction of cattle under extensive farming practices in tropical northern Australia. Although it is desirable to select animals with better temperament, information about behavioural genetics in ruminant livestock is lacking (Boissy et al. 2005). Temperament has been defined in various methods of subjective scoring and objective measures (Fordyce et al. 1982; Burrow 1997). The current h 2 of objectively measured temperament in BRAH was comparable to the estimates from earlier similar measures in a crossbred population (Prayaga and Henshall 2005) and the TCOMP estimates were comparable to the estimates from similar genotypes (Burrow 2001; Kadel et al. 2006). Similar estimates (0.22) were also reported in Bos taurus beef cattle, even when temperament was measured subjectively as reactions to handling (Morris et al. 1994; Le Neindre et al. 1995). Although it might be difficult to assess whether the trait measured as flight time (Table 1) was similar to temperament traits measured in several other studies by different methods, it could be argued that the psychological process driving this reaction to human handling and proximity would be the same.
Phenotypic and genetic correlations (r P and r G, respectively) among the adaptive traits are presented in Table 5. In general, r P between all pairs of traits was low except that between TEMP and FT (–0.23 ± 0.04) in BRAH. The negative r P between TEMP and FT could be related to excitable animals having high rectal temperatures because of increased physical activity owing to agitation during mustering and handling. Even though such an association at a genetic level was observed in an earlier study (Burrow 2001; Prayaga and Henshall 2005), no such genetic correlation was observed in the present study. Standard errors associated with the estimates of all genetic correlations were generally as high as the estimates themselves. Moderate r G was observed between EPG and FLY (0.39 ± 0.31) in BRAH and EPG and TEMP (0.48 ± 0.25) in the pooled data. A moderate r G (0.49 ± 0.32) was observed between TICK and COAT in pooled data, indicating a relationship between the genes responsible for sleek coats and fewer ticks. Similarly, a moderate r G was observed, albeit with high standard errors, between FLY and COAT (0.47 ± 0.25) in BRAH, suggesting sleek coat as a genetic indicator trait for buffalo fly resistance. These genetic relationships between ectoparasite resistance and coat score warrant further investigation.

|
Whereas positive r G between COAT and TEMP, and COLOUR and TEMP was expected because of their presumed association with heat tolerance, no significant relationships were observed in BRAH. This supported the theory that lighter coat colour in itself might not influence heat tolerance (Finch et al. 1984) and the better thermal balance could be aided by other thermoregulatory attributes, such as increased sweating rate and respiratory rate. Negative genetic correlations between EPG and COLOUR in TCOMP (–0.52 ± 0.17) and in pooled data (–0.36 ± 0.14) indicated that as coat colour darkened, resistance to worms increased, especially in TCOMP. In general, the lack of significant phenotypic and genetic correlations among adaptive traits indicates their relative independence. Thus, selection to improve any one of these traits would not have an impact on other adaptive traits to a greater extent.
Genetic relationships between adaptation and growth in heifers
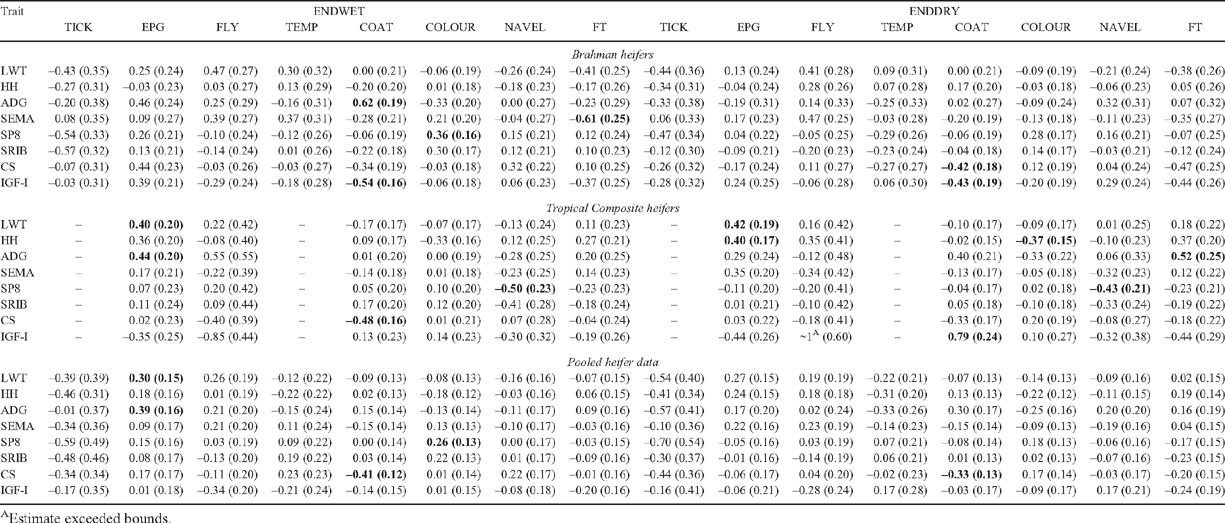
Genetic correlations between adaptive and growth traits at ENDWET and ENDDRY (Barwick et al. 2009b ) in heifers are presented in Table 6. The r P values among most traits were low (not presented). TICK showed moderate negative r G with fatness traits in BRAH during both ENDWET (–0.54 ± 0.33 with SP8; –0.57 ± 0.32 with SRIB) and ENDDRY (–0.47 ± 0.34 with SP8) seasons, indicating that tick score increased with decreased fat cover at a genetic level. However, no such association between fat cover and other parasite resistance traits (EPG and FLY) was observed.
|
Moderate positive r G between EPG and LWT (0.40 ± 0.20 in ENDWET; 0.42 ± 0.19 in ENDDRY) and similar r G between EPG and ADG (0.44 ± 0.20 in ENDWET) in TCOMP indicated that genetically heavier animals had higher worm burdens. Similar relationships were found in BRAH, although only at ENDWET. One interpretation of this correlation could be that the worm burden on its own had no negative relationship with growth. Mackinnon et al. (1991) and Mackinnon and Meyer (1992) also reported low to moderate positive correlations between faecal egg counts and growth traits in a composite line of tropical cattle. Even though Mackinnon and Meyer (1992) reported a significantly negative r G between EPG and wet-season gain in their study, reflecting negative effects on the growth during greater worm-challenge periods of wet season, no such association was observed in the present study. Horn fly (Haematobia irritans irritans L.) was reported to be the most economically damaging external parasite of pastured cattle in US and Canada because of reduced weight gains, seasonal weight loss and reductions in calf weaning weights (Steelman et al. 1996). However, under Australian tropical conditions, buffalo-fly infestation did not have a negative impact on bodyweights with non-significant genetic correlations between FLY and LWT in the present study and positive correlations reported by Burrow (2001). These results support the earlier conclusion (Davis 1993) that selection for growth in tropically adapted breeds will not genetically reduce resistance to parasites.
Even though earlier studies (Burrow 2001; Prayaga and Henshall 2005) reported favourable (negative) r G between growth traits and TEMP, this relationship was not evident in the present study. However, a low to moderate r P was observed between TEMP and LWT, ranging between –0.15 ± 0.04 and –0.28 ± 0.06 across both genotypes and seasons, indicating a favourable association between heat resistance and bodyweight at a phenotypic level. In TCOMP, moderate r G (not shown in the tables because of fewer records for TEMP) between TEMP and SP8 (0.53 ± 0.36 in ENDWET; 0.45 ± 0.33 in ENDDRY) and TEMP and SRIB (0.50 ± 0.37 in ENDWET; 0.48 ± 0.36 in ENDDRY) indicated that fatter animals were genetically more susceptible to heat. The fact that such a relation was not seen in BRAH animals supported the theory of differential fat deposition between BRAH and TCOMP (Barwick et al. 2009a ; Wolcott et al. 2009).
COAT was genetically correlated with IGF-I in the present study; however, this relationship was in the opposite direction in the two genotypes. In BRAH, the r G between COAT and IGF-I was negative (–0.54 ± 0.16 in ENDWET; –0.43 ± 0.19 in ENDDRY), whereas in TCOMP, the r G was positive (0.79 ± 0.24 in ENDDRY). These relationships indicated that in BRAH sleeker-coat animals had genetically higher IGF-I levels, whereas in TCOMP, genes causing woollier coats had a relationship with those causing higher IGF-I levels. In addition, COAT was highly genetically related to ADG (0.62 ± 0.19) at ENDWET in BRAH, but not in TCOMP, indicating that genetically sleeker Brahman animals grew slower during the wet season. Despite this, there was a negative r G between COAT and CS across seasons and genotypes (–0.41 ± 0.12 in ENDWET; –0.33 ± 0.13 in ENDDRY in pooled), indicating that animals with sleeker coats had better condition scores at a genetic level throughout the year. This highlighted the genetic potential of sleek-coated animals to excel and retain better condition under heat-stress conditions, probably owing to better heat-loss mechanisms associated with sleek coats. Further, the r P between COAT and CS was also negative, albeit of lower magnitude, ranging between –0.13 ± 0.03 and –0.20 ± 0.04 across seasons and genotypes. Similarly, r P between COAT and EMA (ranging between –0.13 ± 0.04 and –0.18 ± 0.04) and COAT and LWT (ranging between –0.13 ± 0.03 and –0.16 ± 0.03 in Pooled) across seasons were low, although significant, indicating an advantage for animals with a sleeker coat even at a phenotypic level.
In BRAH, moderate r G between COLOUR and SP8 (0.36 ± 0.16) and COLOUR and SRIB (0.30 ± 0.17) was estimated during ENDWET, indicating that darker-coloured Brahmans had genetically more P8 and RIB fat. A similar relationship was observed during ENDDRY, albeit of lower magnitude. Similarly, a biological explanation for a negative r G (–0.50 ± 0.23 in ENDWET; –0.43 ± 0.21 in ENDDRY) between SP8 and NAVEL in TCOMP, indicating pendulous navels in genetically fatter animals, was not obvious. In BRAH, a negative r G between FT and SEMA (–0.61 ± 0.25 in ENDWET; –0.35 ± 0.27 in ENDDRY) was observed, indicating that genes contributing to aggressive temperament, i.e. low FT, tend to be associated with genes contributing to increased SEMA. Reverter et al. (2003) reported no such relationship in their study with tropically adapted genotypes. Genotype variations in correlations were also evidenced by a significant r G between FT and ADG (0.52 ± 0.25 in ENDDRY), indicating a growth rate advantage for docile animals, especially during dry season in TCOMP; however, this was not the case for BRAH. Hence, as emphasised in companion publications of this work, specific parameters are required for specific populations, genotypes and measurement times when selecting traits as genetic predictors of correlated performance.
Genetic relationship between adaptation and pubertal traits in heifers
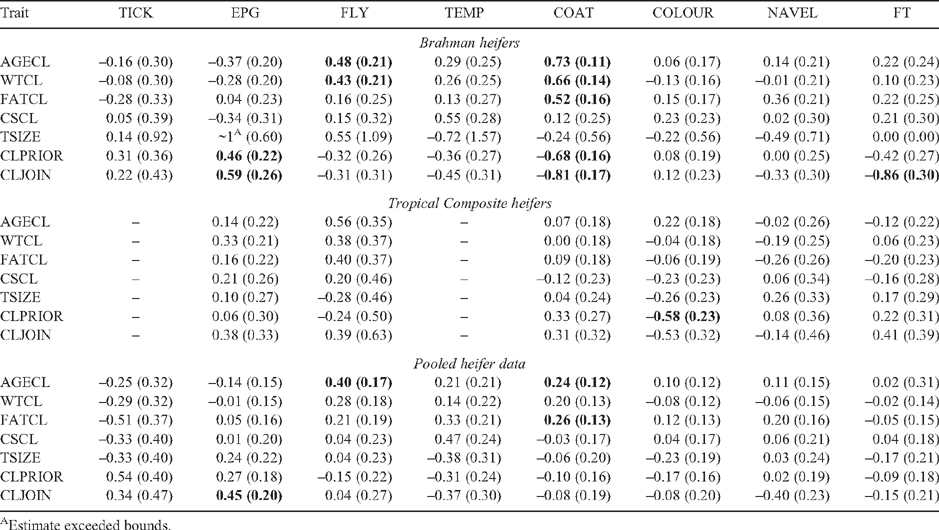
The genetic correlations between adaptive and pubertal traits in heifers are presented in Table 7. Most of the phenotypic correlations are low and insignificant (not presented). As pubertal traits were determined on the basis of regular ovarian scanning of heifers (Johnston et al. 2009) in the present study, most of these r G values between adaptive and pubertal traits are the first reports in beef cattle. Even though standard errors were high for r G between TICK and FATCL (–0.51 ± 0.37), the general trend of negative correlations between TICK and fat traits as observed during heifer growth phase was repeated. The positive r G between EPG and CLPRIOR (0.46 ± 0.22) and EPG and CLJOIN (0.59 ± 0.26) in BRAH supported the argument that higher worm burdens had no negative genetic impact especially in a Bos indicus breed. The moderate r G between FLY and WTCL (0.43 ± 0.21) in general followed the positive relation between LWT and FLY in BRAH (Table 6) and was also reported in earlier studies (Davis 1993; Burrow 2001). These earlier reports suggested that metabolic products in heavier animals could be attracting buffalo flies. Thus, fly infestation could be a concern only for animal welfare, rather than a production issue under tropical Australian conditions using tropically adapted genotypes. However, the r G between FLY and AGECL (0.48 ± 0.21 in BRAH and 0.56 ± 0.35 in TCOMP) indicated that animals with genetic susceptibility to fly lesions tend to show the first CL at a later age. This relation is not seen at a phenotypic level, with low and insignificant phenotypic correlation estimates. There were no comparable reports in the literature. A significant r G between TEMP and FATCL in TCOMP (0.90 ± 0.35, not presented in the table), although based on a smaller number of records, confirmed the earlier observation of relatively high r G between TEMP and postweaning scanned fat measures in TCOMP. Given the correlated responses of high marbling scores in carcasses of tropically adapted composite lines (similar to TCOMP) selected for lower rectal temperatures (Burrow and Prayaga 2004), the genetic link between fat deposition and heat resistance, especially in tropically adapted Bos taurus, needs to be explored further.
|
Significant genotype differences between BRAH and TCOMP were observed in their r G between pubertal traits in heifers and COAT. It was evident from predicted means that BRAH had significantly sleeker coats than TCOMP (Table 3). Given this, the high r G estimates between COAT and a majority of pubertal measures in heifers only in BRAH emphasised the importance of sleek coats. These genotype differences in r G between COAT and pubertal measures could also be driven by the breed-specific genetic differences between BRAH and TCOMP in their reproductive traits. The r G in BRAH suggested that animals with sleeker coats were genetically early maturing by reaching puberty at a younger age (0.73 ± 0.11) and showed a CL before (–0.68 ± 0.16) or at (–0.81 ± 0.17) mating. The positive r G between WTCL and COAT (0.66 ± 0.14) and between FATCL and COAT (0.52 ± 0.16) suggested that animals with genetically sleeker coats had genetically lower weight and P8 fat cover at the time of the first CL. These breed-specific differences in correlations were also evident at the phenotypic level, with r P between COAT and AGECL of 0.25 ± 0.04 in BRAH and 0.07 ± 0.18 in TCOMP.
COLOUR and NAVEL did not show any significant relation with the studied pubertal measures in heifers, except for a significant negative r G between COLOUR and CLPRIOR (–0.58 ± 0.23) in TCOMP, indicating that light-coloured composites had a greater genetic predisposition for showing a CL before mating than did darker-coloured animals. In general, FT was not genetically correlated with the pubertal measures in heifers, except for a significant r G with CLJOIN (–0.86 ± 0.30) in BRAH, signifying the genetic predisposition for showing a CL at mating by the animals with poor temperament.
Genetic relationships between adaptive traits in heifers and steer-growth, carcass- and meat-quality traits
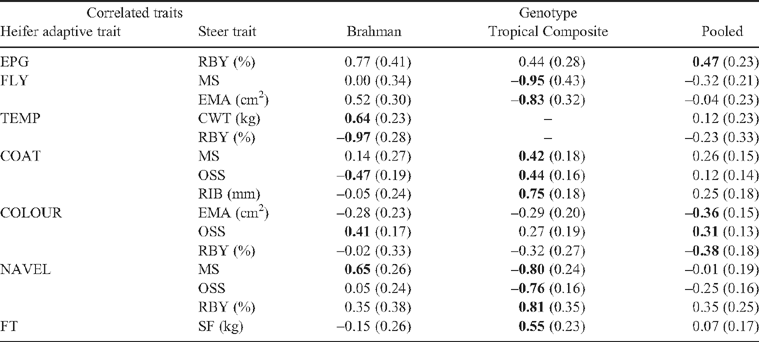
A subset of selected r G between adaptive traits in heifers and growth and scanned measures of their paternal half-sib steers (Table 8), and carcass- and meat-quality traits (Table 9) are presented. These correlations were selected on the basis of the magnitude and significance of the relationship, with the estimates with high standard errors not being presented. Most of these estimates are also the first reports in beef cattle. Positive r G estimates between heifer TEMP and scanned fat measures in steers at EXIT supported the earlier observation of genetic susceptibility to heat of fatter animals. This relationship was stronger in TCOMP (0.74 ± 0.35, not presented in the table) than in BRAH (0.52 ± 0.25). In BRAH, a high r G (0.84 ± 0.29) between heifer TEMP and steers EXIT SIMF indicated that as scanned intramuscular fat increased at a genetic level, the heat susceptibility increased. In BRAH, a strong negative r G (–0.97 ± 0.28) between steer RBY and heifer TEMP indicated a favourable genetic relationship between increased beef yield and increased heat resistance. However, it should be noted that r G between steer CWT and heifer TEMP was significantly positive (0.64 ± 0.23). This could be due to lack of genetic relationship between CWT and RBY (–0.24 ± 0.21), as reported in a companion paper (Wolcott et al. 2009).

|
|
A moderate negative r G in BRAH between heifer COAT and the postweaning IGF-I in steers (–0.55 ± 0.25) was consistent with a similar relationship between COAT and IGF-I in BRAH heifers. Thus, sleeker coat could be considered a genetic indicator of higher IGF-I levels in Brahmans. Genotype differences were evident in genetic relationships between heifer COAT and steer EXIT RFI (–0.55 in BRAH, –0.20 in TCOMP), steer POSTW CS (–0.93 in BRAH, –0.02 in TCOMP), steer ENTRY SEMA (–0.66 in BRAH, 0.18 in TCOMP) and steer EXIT SEMA (–0.61 in BRAH, 0.05 in TCOMP), implying that inherent genotype differences were driving these genetic relationships. Although BRAH were reported to be more efficient than TCOMP in feed use, as measured by their lower RFI (Barwick et al. 2009a ), within BRAH, animals with sleeker coats were genetically less efficient utilisers of feed, as reflected by r G estimates with RFI (–0.55 ± 0.26). A positive r G (0.54 ± 0.27) between heifer TEMP and steer EXIT DFI indicated an unfavourable association in BRAH. This could be viewed as an adaptive measure of reduced feed-intake requirement in Brahmans to maintain lower rectal temperatures under hot, humid conditions. This observation was further strengthened by a positive r G between heifer TEMP and steer EXIT fatness attributes (SP8, SIMF), especially in BRAH, indicating a genetic predisposition for reduced fat in half-sib animals with greater heat-resistance abilities.
The high negative r G (–0.93 ± 0.45) in BRAH between heifer COAT and steer postweaning CS further emphasised that Brahmans with sleeker coats had the genetic predisposition to achieve better condition score in tropics, as observed earlier in heifers. The positive r G between heifer COLOUR and scanned fat measures in BRAH steers (Table 8), similar to the r G observed between COLOUR and SP8 in heifers (Table 6), indicated that darker-coloured Brahmans were genetically fatter. In TCOMP, strong negative r G values were observed between heifer NAVEL and steer POSTW HH, POSTW SEMA, ENTRY SEMA, ENTRY LWT and EXIT SIMF. Moderate to high negative r G between heifer navel and steer weights at weaning and postweaning in both genotypes (–0.50 to –0.71) also indicated a growth advantage for animals with genetically loose navel skin. This could also be attributed to the growth advantage because of better heat-tolerance capability owing to increased surface area through pendulous navels. This genetic relationship needs to be considered when making selection decisions for growth in the tropics because bulls with an extremely pendulous sheath/navel have a greater risk of preputial injury or prolapse (McGowan et al. 2002). As expected, a strong positive r G (0.92 ± 0.16) was observed between temperament measures (flight time) of heifers and their half-sib steers.
In TCOMP, a moderately significant r G was observed between COAT and steer MS (0.42 ± 0.18) and RIB (0.75 ± 0.18), implying sleeker coats to be genetically associated with lower marbling and rib fat in tropically adapted predominantly Bos taurus genotypes. Such an association was not evident in BRAH. Such a difference between BRAH and TCOMP in r G was also evident for steer MS and heifer FLY (0.00 in BRAH, –0.95 in TCOMP) and for steer MS and heifer NAVEL (0.65 in BRAH, –0.80 in TCOMP). This could be attributed to inherent differences in tropical adaptation between BRAH and TCOMP, as identified in the present paper, and the significant genotype differences in marbling scores between BRAH (0.51) and TCOMP (0.89) reported by Wolcott et al. (2009). Although, a favourable association between FT and meat tenderness was reported earlier in tropically adapted genotypes (Reverter et al. 2003), an unfavourable r G (0.55 ± 0.23) between heifer FT and steer SF was estimated in TCOMP in the present study, indicating a genetic association between increased docility in heifers and increased toughness of meat in their half-sib steers. A low, negative, non-significant r G was reported between steer POSTW FT and steer SF in our companion paper (Wolcott et al. 2009). In general, these r G values between adaptive traits in heifers and those in steers also strengthen the need for genotype-specific parameter estimates owing to the differences in genetic relationships.
Conclusions
A distinct advantage for BRAH in tropical adaptation over TCOMP, as demonstrated by lower TICK, EPG, FLY, TEMP and COAT, was evident in the present study. Genetic variances and h 2 of adaptive traits and r G between adaptive traits, growth and reproductive traits in heifers and production traits in steers generally differed among genotypes, and may reflect genotype differences in underlying physiological processes affecting the ability of animals to cope with prevailing environmental stresses. The genetic relationship between fat deposition and heat tolerance needs further investigation, especially in heat-susceptible tropical genotypes such as tropically adapted Bos taurus. Further, sleeker coat was identified as a genetic indicator of better condition scores in both genotypes and as a genetic indicator of early puberty in Brahmans. In general, selection for improved productive and reproductive performance in tropical beef genotypes will not jeopardise tropical adaptability. This is of significance for the northern beef industry, given the emphasis on improving female reproductive performance by identifying molecular genetic markers associated with reproductive measures. Hence, given the overall favourable associations of tropical adaptive measures, and in some cases, lack of significant associations with other productive and reproductive measures, selection decisions on female pubertal measures can be made without serious concerns about compromising tropical adaptation. Further, the phenotypic expression of tropical adaptation as measured in the present study is dependent on various factors such as environmental conditions, innate and acquired resistance levels of the animals and the selection history of the population concerned. Hence, breed- and population-specific genetic parameters of adaptive traits need to be developed and their relationships with other important economic traits revisited periodically.
Acknowledgements
The authors acknowledge the Cooperative Research Centre of Cattle and Beef Quality (and its core partners: The University of New England, NSW Department of Primary Industries, CSIRO, and Queensland Department of Primary Industries and Fisheries), the Commonwealth Government funding through CRC program, and the financial support of Meat and Livestock Australia and the Australian Centre for International Agricultural Research. The cattle used for this experiment were contributed by producers from the Northern Pastoral Group, and their financial support of this project is gratefully acknowledged. We also acknowledge contributions of all those staff involved in the CRC network, particularly Paul Williams, Warren Sim, Dick Holroyd, Tracy Longhurst, Mick Sullivan, Andrew McCann, research station managers and technical staff.
Barwick SA,
Wolcott ML,
Johnston DJ,
Burrow HM, Sullivan MT
(2009a) Genetics of steer daily and residual feed intake in two tropical beef genotypes, and relationships among intake, body composition, growth and other post-weaning measures. Animal Production Science 49, 351–366.
| Crossref | GoogleScholarGoogle Scholar |

Barwick SA,
Johnston DJ,
Burrow HM,
Holroyd RG,
Fordyce G,
Wolcott ML,
Sim WD, Sullivan MT
(2009b) Genetics of heifer performance in ‘wet’ and ‘dry’ seasons and their relationships with steer performance in two tropical beef genotypes. Animal Production Science 49, 367–382.
| Crossref | GoogleScholarGoogle Scholar |

Boissy A,
Fisher AD,
Bouix J,
Hinch GN, Le Neindre P
(2005) Genetics of fear in ruminant livestock. Livestock Production Science 93, 23–32.
| Crossref | GoogleScholarGoogle Scholar |

Burrow HM
(1997) Measurements of temperament and their relationships with performance traits of beef cattle. Animal Breeding Abstracts 65, 477–495.

Burrow HM
(2001) Variances and covariances between productive and adaptive traits and temperament in a composite breed of tropical beef cattle. Livestock Production Science 70, 213–233.
| Crossref | GoogleScholarGoogle Scholar |

Burrow HM, Prayaga KC
(2004) Correlated responses in productive and adaptive traits and temperament following selection for growth and heat resistance in tropical beef cattle. Livestock Production Science 86, 143–161.
| Crossref | GoogleScholarGoogle Scholar |

Burrow HM,
Seifert GW, Corbet NJ
(1988) A new technique for measuring temperament in cattle. Proceedings of the Australian Society of Animal Production 17, 154–157.

Davis GP
(1993) Genetic parameters for tropical beef cattle in Northern Australia: a review. Australian Journal of Agricultural Research 44, 179–198.
| Crossref | GoogleScholarGoogle Scholar |

Finch VA
(1986) Body temperature in beef cattle: Its control and relevance to production in the tropics. Journal of Animal Science 62, 531–542.

Finch VA,
Bennett IL, Holmes CR
(1984) Coat colour in cattle: effect on thermal balance, behaviour and growth, and relationship with coat type. Journal of Agricultural Science 102, 141–147.
| Crossref |

Fordyce G,
Goddard ME, Seifert GW
(1982) The measurement of temperament in cattle and the effect of experience and genotype. Proceedings of the Australian Society of Animal Production 14, 329–332.

Franke DE, Burns WC
(1985) Sheath area in Brahman and grade Brahman calves and its association with preweaning traits. Journal of Animal Science 61, 398–401.
|
CAS |
PubMed |

Gazzola C,
O’Neill CJ, Frisch JE
(1999) Comparative evaluation of the meat quality of beef cattle breeds of Indian, African and European origins. Animal Science 69, 135–142.

Gray GD, Gill HS
(1993) Host genes, parasites and parasitic infections. International Journal for Parasitology 23, 485–494.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Johnston DJ,
Barwick SA,
Corbet NJ,
Fordyce G,
Holroyd RG,
Williams PJ, Burrow HM
(2009) Genetics of heifer puberty in two tropical beef genotypes in northern Australia and associations with heifer- and steer-production traits. Animal Production Science 49, 399–412.
| Crossref | GoogleScholarGoogle Scholar |

Kadel MJ,
Johnston DJ,
Burrow HM,
Graser H-U, Ferguson DM
(2006) Genetics of flight time and other measures of temperament and their value as selection criteria for improving meat quality traits in tropically adapted breeds of beef cattle. Australian Journal of Agricultural Research 57, 1029–1035.
| Crossref | GoogleScholarGoogle Scholar |

Kriese LA,
Bertrand JK, Benyshek LL
(1991) Genetic and environmental growth trait parameter estimates for Brahman and Brahman-derivative cattle. Journal of Animal Science 69, 2362–2370.
|
CAS |
PubMed |

Le Neindre P,
Trillat G,
Sapa J,
Menissier F,
Bonnet JN, Chupin JM
(1995) Individual differences in docility in Limousin cattle. Journal of Animal Science 73, 1–5.

Mackinnon MJ, Meyer K
(1992) Direct and maternal components of the relationship between growth and worm resistance in cattle. Proceedings of the Association for the Advancement of Animal Breeding and Genetics 10, 408–411.

Mackinnon MJ,
Meyer K, Hetzel DJS
(1991) Genetic variation and covariation for growth, parasite resistance and heat tolerance in tropical cattle. Livestock Production Science 27, 105–122.
| Crossref | GoogleScholarGoogle Scholar |

McGowan MR,
Bertram JD,
Fordyce G,
Fitzpatrick LA,
Miller RG,
Jayawardhana GA,
Doogan VJ,
De Faveri J, Holroyd RG
(2002) Bull selection and use in northern Australia. 1. Physical traits. Animal Reproduction Science 71, 25–37.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morris CA,
Cullen NG,
Kilgour R, Bremner KJ
(1994) Some genetic factors affecting temperament in Bos taurus cattle. New Zealand Journal of Agricultural Research 37, 167–175.

Prayaga KC
(2003) Evaluation of beef cattle genotypes and estimation of direct and maternal genetic effects in a tropical environment. 2. Adaptive and temperament traits. Australian Journal of Agricultural Research 54, 1027–1038.
| Crossref |

Prayaga KC
(2004) Evaluation of beef cattle genotypes and estimation of direct and maternal genetic effects in a tropical environment. 3. Fertility and calf survival traits. Australian Journal of Agricultural Research 55, 811–824.
| Crossref | GoogleScholarGoogle Scholar |

Prayaga KC, Henshall JM
(2005) Adaptability in tropical beef cattle: genetic parameters of growth, adaptive and temperament traits in a crossbred population. Australian Journal of Experimental Agriculture 45, 971–983.
| Crossref | GoogleScholarGoogle Scholar |

Reverter A,
Johnston DJ,
Ferguson DM,
Perry D,
Goddard ME,
Burrow HM,
Oddy VH,
Thompson JM, Bindon BM
(2003) Genetic and phenotypic characterisation of animal, carcass, and meat quality traits from temperate and tropically adapted beef breeds. 4. Correlations among animal, carcass, and meat quality traits. Australian Journal of Agricultural Research 54, 149–158.
| Crossref | GoogleScholarGoogle Scholar |

Roberts FHS, O’Sullivan PJ
(1950) Methods for egg counts and larval cultures for strongyles infesting the gastrointestinal tract of cattle. Australian Journal of Agricultural Research 1, 99–103.
| Crossref | GoogleScholarGoogle Scholar |

Steelman CD,
Brown CJ,
McNew RW,
Gbur EE,
Brown MA, Tolley G
(1996) The effects of selection for size in cattle on horn fly population density. Medical and Veterinary Entomology 10, 129–136.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Turner HG, Schleger AV
(1960) The significance of coat type in cattle. Australian Journal of Agricultural Research 11, 645–663.
| Crossref | GoogleScholarGoogle Scholar |

Wakelin D
(1989) Nature and nurture: overcoming constraints on immunity. Parasitology 99, S21–S35.
| PubMed |

Wharton RH,
Utech KBW, Turner HG
(1970) Resistance to the cattle tick, Boophilus microplus, in a herd of Australian Illawarra Shorthorn cattle: its assessment and heritability. Australian Journal of Agricultural Research 21, 163–181.
| Crossref | GoogleScholarGoogle Scholar |

Wolcott ML,
Johnston DJ,
Barwick SA,
Iker CL,
Thompson JM, Burrow HM
(2009) Genetics of meat quality and carcass traits and the impact of tenderstretching in two tropical beef genotypes. Animal Production Science 49, 383–398.
| Crossref | GoogleScholarGoogle Scholar |

1 Animal Genetics and Breeding Unit is a joint venture of New South Wales Department of Primary Industries and the University of New England.


