Bitter compound quinine hydrochloride improved post-weaning pig performance in the absence of zinc oxide
Elisabet Garcia-Puig A , Fan Liu
A , Fan Liu  B , Rebecca Morrison B , Maximiliano Müller
B , Rebecca Morrison B , Maximiliano Müller  A , Allan Lisle A and Eugeni Roura
A , Allan Lisle A and Eugeni Roura  A *
A *
A
B
Abstract
Dietary zinc oxide (ZnO) (a bitter antimicrobial chemical) in pigs is being gradually phased out due to pollution and antibiotic resistance. Bitter compounds like quinine hydrochloride (HCl) have shown potential to enhance growth and feed efficiency by slowing gastric emptying and intestinal passage rates in pigs.
This study aimed to evaluate quinine’s ability to improve performance in weanling pigs without ZnO.
Two experiments were conducted. Experiment 1: 120 Landrace (LD) × Large White (LW) weaned piglets (initial BW 6.8 ± 0.1 kg) were randomly assigned to one of four diets in a 2 × 2 factorial design: with/without ZnO and copper sulfate (CuSO4) (3000 and 250 ppm, respectively) and two quinine levels (0 and 500 ppm). Parameters measured included average daily feed intake (ADFI), average daily gain (ADG), gain to feed ratio (G:F), and faecal score. Experiment 2: 1440 LD × LW piglets (initial BW 7.4 ± 0.2 kg) were housed in groups of 18 per pen and assigned the same four dietary treatments as in Experiment 1.
Growth performance parameters were recorded and analysed, showing that ZnO/CuSO4 supplement improved growth and feed efficiency (P < 0.05) compared to the ZnO/CuSO4-free diet group. In Experiment 1, pigs supplemented with quinine in non-ZnO/CuSO4 diets showed similar (P > 0.05) performance levels to the ZnO/CuSO4 fed group. In addition, an interaction (P < 0.05) was found, indicating that adding quinine improved or worsened ADG and G:F depending on the absence or presence of ZnO/CuSO4 in the diet, respectively. In Experiment 2, quinine inclusions in non-ZnO diets improved (P < 0.05) ADG but did not affect (P > 0.05) ADFI and G:F.
Our findings suggest that the anticipated deleterious effects of phasing out the use of dietary ZnO can be partially compensated by includingquinine in the diet of post-weaning pigs. The negative effect of quinine when provided together with ZnO is compatible with a competitive exclusion mechanism linked to both stimulating bitterness, a mechanism that warrants further investigation.
Quinine shows potential as a partial replacement for ZnO in post-weaning pig diets, providing a promising alternative to maintain piglet health and growth while transitioning away from ZnO.
Keywords: antimicrobials, bitter compounds, diarrhoea, feed efficiency, growth, piglets, quinine, ZnO.
Introduction
One of the main concerns raised by pork consumers is the use of in-feed antimicrobials, including pharmacological doses of zinc oxide (ZnO and copper sulfate (CuSO4 (Cromwell 2001; Bednorz et al. 2013)). High doses of zinc (Zn) and copper (Cu) can be used to prevent bacterial infections (i.e. diarrhoea), or as growth promoters in weanling pigs (Jacela et al. 2010). However, dietary ZnO is poorly absorbed, with only 14% uptake, leading to high levels of excretion. As a result, the use of pig manure as fertiliser pollutes farmlands and groundwater (Heo et al. 2013; Shen et al. 2014; Long et al. 2017; Feng et al. 2018). Furthermore, dietary ZnO has been linked to the proliferation of antibiotic-resistant genes and the development of multi-resistant pathogens such as Escherichia coli (Bednorz et al. 2013; Medardus et al. 2014; Vahjen et al. 2015). Consequently, several countries are phasing out or have banned the use of pharmacological doses of ZnO in pig production.
On a different note, ZnO has been reported to elicit bitterness in humans (Rodgers et al. 2006; Dagan-Wiener et al. 2019). Sensing of bitterness has been associated with toxic compounds or general synthetic chemicals such as pharmaceuticals thus eliciting repulsive resposes in mammalian species including pigs (Nelson and Sanregret 1997; Meyerhof et al. 2010). Some additional protective responses found in pigs include feed aversion, inhibition of gastric motility and decreased intestinal peristalsis (Roura and Fu 2017; Fu et al. 2018; Roura et al. 2019). The study of the bitter taste receptor (TAS2Rs) repertoire in pigs has resulted in the discovery of at least 14 active genes present in the oral cavity and along the intestinal mucosa (da Silva et al. 2014; Ribani et al. 2017). Some of these TAS2Rs responded to the bitterness of ZnO by increasing the expression in the small intestine in weaned pigs (Paniagua et al. 2023). In addition, dietary inclusion of bitter compounds, such as quinine HCl, improved growth and feed efficiency in finisher pigs, an effect that could potentially be related to the effect of ZnO (Fu et al. 2015, 2018; Roura and Fu 2017). The aim of this study was to evaluate the ability of dietary quinine as a potential replacement for ZnO and CuSO4 in post-weaning pigs. It was hypothesised that quinine supplementation would have a positive impact on growth, feed efficiency, and diarrhoea incidence in piglets, particularly in the absence of ZnO.
Materials and methods
Experiment 1
Post-weaning piglets (n = 120, breed Landrace (LD) × Large White (LW), average 26 days of age, 6.8 ± 0.1 kg) were housed in the individual weaner facility at a research farm in New South Wales, Australia. Pens were grouped into complete blocks based on their position in the shed. The experimental diets (Table 1) were designed and prepared at Rivalea feed mill as a 2 × 2 factorial arrangement, consisting of two levels of antimicrobials without or with 3000 ppm ZnO and 250 ppm CuSO4 (Riverina Pty Ltd, Brisbane, Queensland, Australia), by two levels (0 and 500 ppm) of quinine HCl (Lucta S.A, Montornés del Vallès, Catalonia, Spain). The trial was conducted in two runs, with 15 pigs per treatment in each run. The piglets were given ad-libitum access to one of the four dietary treatments in a randomised complete design blocked by run. Every day, a pre-weighed amount of feed was added to each feeder, on top of any remaining feed from the previous day. Once a week, the total feed disappearance was calculated by measuring the remaining feed in each feeder and determining the difference between this value and the total feed offered over the week. Body weights of the pigs were recorded individually at the beginning of the experiment (Day 0) and at the end of each weekly interval (Days 7 and 14). These measurements were used to calculate ADFI, ADG and gain:feed ratio (G:F) for each pig. In addition, all animals were individually monitored for signs of clinical diarrhoea at 0, 8 and 15 days. Monitoring was performed by a single trained individual to ensure consistency in scoring. Observations were conducted early in the morning, using faeces present on the floor of each pen. Faecal consistency was recorded to identify the presence/absence of diarrhoea using the score (FCS) published by O’Shea et al. (2016). The scoring system consists of a five-grade scale where the lower the value the higher the consistency of the faeces: 1 = hard firm faeces; 2 = slightly soft faeces; 3 = soft, partially formed faeces; 4 = loose, semi-liquid faeces (diarrhoea); and 5 = watery, mucous-like faeces (severe diarrhoea).
| Ingredients | Content (%) | Nutrient | Content | |
|---|---|---|---|---|
| Wheat | 28.95 | DE, MJ/kg | 14.99 | |
| Steam flaked wheat | 30.0 | NE, MJ/kg | 11.27 | |
| Barley | 5.0 | Crude protein, % | 21.93 | |
| Canola meal (38%) | 3.0 | Calcium, % | 0.67 | |
| Soybean meal (46%) | 8.0 | Available P, % | 0.53 | |
| Meat meal (58%) | 5.0 | SID lysine/DE, g/MJ | 0.90 | |
| Fish meal (60%) | 2.5 | Methionine, % | 0.19 | |
| Blood meal | 1.5 | Methionine + cysteine, % | 0.13 | |
| Soy protein concentrate | 1.25 | Threonine, % | 0.39 | |
| Lactose | 8.0 | Isoleucine, % | 0.38 | |
| Water | 1.0 | Tryptophan, % | 0.12 | |
| Tallow | 1.0 | Valine, % | 0.41 | |
| DL-Methionine | 0.16 | |||
| Valine | 0.06 | |||
| Tiamulin hydrogen fumarate (20%) | 0.1 | |||
| Benzoic acid | 0.4 | |||
| Enzyme premix A | 0.02 | |||
| Lysine | 0.6 | |||
| Threonine | 0.235 | |||
| Isoleucine | 0.16 | |||
| Tryptophan | 0.05 | |||
| Vitamin and mineral premix B | 0.175 |
SID, standard Ileal digestibility; DE, digestible energy; NE, net energy.
Experiment 2
This experiment was a repetition of Experiment 1 but with a larger number of animals raised in groups, aiming for a higher commercial relevance. Weaned piglets (n = 1440, breed LD × LW, 23 to 37 day-old; 7.4 ± 0.2 kg mean ± s.e.) were housed in the group weaner shed at the research farm in New South Wales, Australia. Each of the 80 pens (5.4 m2 each) housed 18 piglets of the same sex (40 pens for each sex). Pens were grouped into complete blocks based on their position in the shed. The experimental diets (Table 2) were designed and prepared at Rivalea feed mill as a 2 × 2 factorial arrangement, consisting of two levels of ZnO (0 or 3000 ppm, Riverina Pty Ltd, Queensland, Australia), by two levels of quinine (0 or 500 ppm, Lucta S.A., Montornés del Vallès, Catalonia, Spain). The piglets were given ad-libitum access to one of the four dietary treatments in a randomised complete block design. Feed disappearance was calculated weekly by determining the difference between the total feed provided to each pen over the week and the feed remaining at the end of the week. To estimate feed disappearance per pig, the weekly feed disappearance was divided by the number of piglets present in the pen, adjusting for mortalities during the week. Body weights were recorded on Days 0, 7, and 21 of the experiment, and ADG, ADFI, and G:F ratio were calculated per pig for each period. Mortalities were recorded, and the data for affected pens were adjusted accordingly to maintain accurate performance calculations.
| Ingredients | Content (%) | Nutrient | Content | |
|---|---|---|---|---|
| Wheat | 29.18 | DE, MJ/kg | 15.43 | |
| Steam flaked wheat | 30.0 | NE, MJ/kg | 11.27 | |
| Barley | 5.0 | Crude protein, % | 22.01 | |
| Canola meal (37%) | 3.0 | Calcium, % | 0.94 | |
| Soybean meal (46%) | 8.0 | Available P, % | 0.78 | |
| Meat meal (60%) | 5.0 | SID lysine/DE, g/MJ | 0.80 | |
| Fish meal (60%) | 2.5 | Methionine, % | 0.19 | |
| Blood meal | 1.5 | Methionine + cysteine, % | 0.13 | |
| Soy protein concentrate | 1.25 | Threonine, % | 0.41 | |
| Lactose | 8.0 | Isoleucine, % | 0.38 | |
| Water | 1.0 | Tryptophan, % | 0.12 | |
| Tallow | 1.0 | Valine, % | 0.41 | |
| DL-Methionine | 0.16 | |||
| Valine | 0.06 | |||
| Tiamulin hydrogen fumarate (20%) | 0.1 | |||
| Benzoic acid | 0.4 | |||
| Enzyme premix A | 0.03 | |||
| Lysine | 0.6 | |||
| Threonine | 0.235 | |||
| Isoleucine | 0.16 | |||
| Tryptophan | 0.05 | |||
| Vitamin and mineral premix B | 0.175 |
SID, standard ileal digestibility; DE, digestible energy; NE, net energy.
Statistical analyses
The performance (ADFI, ADG and G:F) data were analysed by using an analysis of covariance (ANCOVA) model implemented in R software (version 3.6.3., R Core Team 2023), developed by R Foundation for Statistical Computing (Vienna, Austria). Initial body weight was included as a covariate. The model considered main effects of ZnO/CuSO4, and quinine, and their interaction, while also considering Ssex as a fixed effect. Additionally, Run and Block were incorporated as random effects, with Block nested within Run. Comparisons between treatments were assessed using the least significant differences (l.s.d.) multiple comparisons test. Faecal scores were analysed using a linear mixed model (lme4 package in R) to incorporate observation days as a repeated measurement. The number of samples refers to the number of pigs (Experiment 1, n = 30) or pens (Experiment 2, n = 20) used. Statistical significance was set at P ≤ 0.05.
Animal welfare statement
This study was performed in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes, 8th Edition’ (National Health and Medical Research Council; Canberra, 2013. All protocols were approved by the Animal Ethics Committee of the University of Queensland-RIVALEA/156/20 and RIVALEA/345/20- (Animal Ethics Unit, St Lucia, Queensland, Australia) and the Animal Care and Ethics Committee of Rivalea-19V059C and 20N046- (Corowa, New South Wales, Australia).
Results
Experiment 1
The effects of the four dietary treatments in Experiment 1 on performance parameters are summarised in Table 3. Piglets fed ZnO/CuSO4 had significantly (P < 0.05) improved ADG and feed efficiency measured as G:F ratio during the second week of the experiment (between 8 and 14 days of age). In addition, also during Week 2, there was a trend (P = 0.066) showing quinine-treated pigs having higher feed consumption compared to non-quinine pigs, independent of ZnO/CuSO4. Furthermore, a significant (P < 0.05) interaction was observed between ZnO/CuSO4 and quinine supplements, indicating that the impact of quinine on performance (i.e. ADG and G:F ratio) was negative in ZnO/CuSO4 supplemented pigs, but positive in the absence of the antimicrobials.
| ZnO/CuSO4 3000/250 ppm | ZnO/CuSO4 0 ppm | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm quinine | 500 ppm quinine | 0 ppm quinine | 500 ppm quinine | ZnO/CuSO4 | Quinine | ZnO/CuSO4 × quinine | |||
| Week 1 | ADG, g | 54.6 ± 12.7 | 40.2 ± 12.9 | 27.5 ± 13.4 | 41.3 ± 13.2 | 0.385 | 0.893 | 0.283 | |
| ADFI, g | 123 ± 9.59 | 114 ± 9.60 | 111 ± 9.97 | 133 ± 9.78 | 0.599 | 0.504 | 0.435 | ||
| G:F | 0.31 ± 0.20 | 0.26 ± 0.20 | 0.04 ± 0.20 | 0.14 ± 0.20 | 0.426 | 0.892 | 0.69 | ||
| Week 2 | ADG, g | 263 ± 13.7b | 241 ± 14.3ab | 203 ± 14.5a | 238 ± 14.3ab | 0.031 | 0.663 | 0.047 | |
| ADFI, g | 340 ± 15.6 | 366 ± 16.3 | 310 ± 16.9 | 346 ± 16.6 | 0.147 | 0.066 | 0.767 | ||
| G:F | 0.81 ± 0.03b | 0.66 ± 0.03a | 0.62 ± 0.03a | 0.67 ± 0.03a | 0.008 | 0.104 | 0.004 | ||
| Overall | ADG, g | 166 ± 11.4 | 149 ± 11.8 | 121 ± 12.1 | 146 ± 11.8 | 0.054 | 0.769 | 0.079 | |
| ADFI, g | 239 ± 11.6 | 249 ± 11.8 | 217 ± 12.3 | 244 ± 12.0 | 0.309 | 0.119 | 0.475 | ||
| G:F | 0.69 ± 0.03b | 0.60 ± 0.03a | 0.51 ± 0.04a | 0.55 ± 0.04a | 0.001 | 0.346 | 0.046 | ||
A total of 120 weaned pigs (LD × LW) were used in a 14-day trial. Each mean represents the average of 30 pigs per treatment. Values are expressed as mean ± s. e. average daily gain (ADG), average daily feed intake (ADFI), and efficiency expressed as gain to feed ratio (G:F), following a 2 × 2 factorial design with ‘ZnO and CuSO4’ or ‘quinine’ as main effects and ‘ZnO and CuSO4 by quinine’ as interaction effect in post-weaned piglets.
Mean values in the same row with different letters (a, b) differ significantly according to the l.s.d. multiple comparison (P ≤ 0.05).
The accumulated results of the 2-week experiment (0 to 14 days) reflected mainly the results of the 2nd week. Pigs supplemented with ZnO/CuSO4 had higher ADG (P = 0.05) and better G:F (P < 0.01) ratio compared to the non-ZnO/CuSO4 treated group. Similar to Week 2, a significant (P < 0.05) interaction was observed affecting the G:F ratio, where quinine had a negative impact in the ZnO/CuSO4 supplemented group but a positive effect in the absence of the antimicrobial.
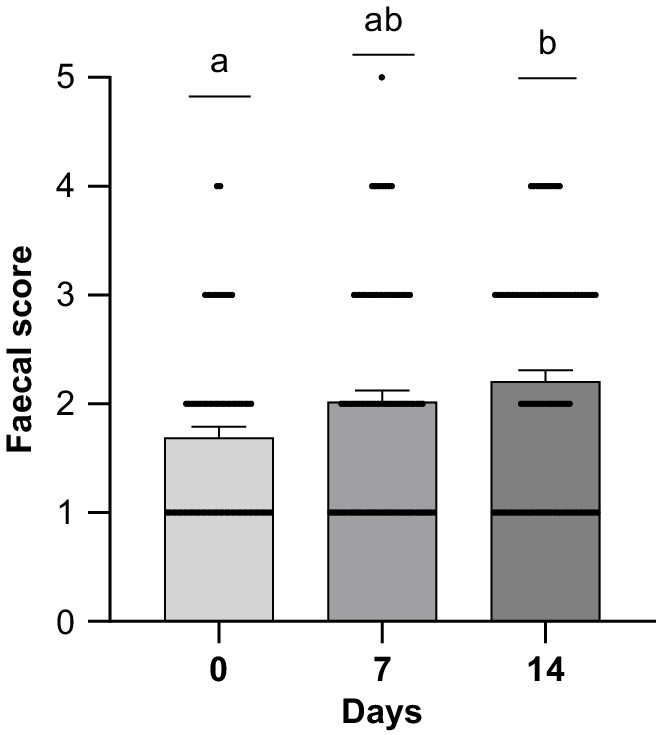
Independent of dietary treatments, FCS increased (P ≤ 0.01) over the three time points shown in Fig. 1. In addition, the FCS on Day 14 showed a trend (P = 0.071) indicating lower FCS (i.e. more consistent faeces) 2.10 ± 0.29 vs 2.32 ± 0.32 in the antimicrobial compared to the non-antimicrobial fed groups, respectively (Table 4).
Experiment 1: effect of post-weaning day 0, 7 or 14 on faecal scores of weaned pigs across treatments. A total of 120 weaned pigs (LD × LW) were used in a 14-day trial with one pig per pen. A scoring system was used: 1 = hard firm faeces; 2 = slightly soft faeces; 3 = soft faeces; 4 = loose faeces; and 5 = watery faeces. The bars represent the mean faecal score for each day. The length of the lines reflects the number of replicates at each score value, with longer lines indicating more replicates at that value. a,bMean values between days with different letters differ significantly according to the l.s.d. multiple comparison (P ≤ 0.01).
| ZnO/CuSO4 3000/250 ppm | ZnO/CuSO4 0 ppm | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 ppm quinine | 500 ppm quinine | 0 ppm quinine | 500 ppm quinine | ZnO/CuSO4 | Quinine | ZnO/CuSO4 × quinine | ||
| Day 0 | 1.88 ± 0.18 | 1.77 ± 0.18 | 1.64 ± 0.25 | 1.70 ± 0.22 | 0.349 | 0.727 | 0.678 | |
| Day 7 | 1.94 ± 0.14 | 2.01 ± 0.14 | 1.76 ± 0.15 | 2.07 ± 0.15 | 0.828 | 0.187 | 0.391 | |
| Day 14 | 2.00 ± 0.13 | 2.10 ± 0.13 | 2.25 ± 0.13 | 2.39 ± 0.13 | 0.071 | 0.451 | 0.868 | |
A total of 120 weaned pigs (LD × LW) were used in a 14-day trial with one pig per pen. Each mean represents the average of 30 pigs per treatment. Values are expressed as mean ± s.e. A scoring system was used: 1 = hard firm faeces; 2 = slightly soft faeces; 3 = soft faeces; 4 = loose faeces; and 5 = watery faeces.
Experiment 2
The effects of the four dietary treatments in Experiment 2 on ADG, ADFI, and G:F ratio are summarised in Table 5. Positive effects (P < 0.001) of ZnO on ADG and ADFI were observed in Weeks 2 and 3, as well as over the entire experimental period (0 to 21 days). The ZnO supplement increased ADG and ADFI by 15% and 16%, respectively, compared to the negative control group.
| ZnO 3000 ppm | ZnO 0 ppm | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm quinine | 500 ppm quinine | 0 ppm quinine | 500 ppm quinine | ZnO | Quinine | ZnO × quinine | |||
| Day 0 to 7 | ADG, g | 79.6 ± 7.65b | 51.3 ± 7.65a | 65.0 ± 7.65ab | 68.1 ± 7.65ab | 0.883 | 0.093 | 0.038 | |
| ADFI, g | 150 ± 6.48 | 145 ± 6.48 | 143 ± 6.48 | 147 ± 6.48 | 0.769 | 0.956 | 0.482 | ||
| G:F | 0.55 ± 0.05b | 0.33 ± 0.05a | 0.45 ± 0.05ab | 0.46 ± 0.05b | 0.720 | 0.034 | 0.015 | ||
| Day 7 to 21 | ADG, g | 346 ± 12.7c | 309 ± 12.7b | 261 ± 12.7a | 299 ± 12.7b | <0.001 | 0.955 | <0.001 | |
| ADFI, g | 426 ± 9.95b | 410 ± 9.95b | 360 ± 9.95a | 381 ± 9.95a | <0.001 | 0.763 | <0.001 | ||
| G :F | 0.82 ± 0.03 | 0.77 ± 0.03 | 0.74 ± 0.03 | 0.78 ± 0.03 | 0.314 | 0.981 | 0.143 | ||
| Day 0 to 21 | ADG, g | 252 ± 8.82c | 222 ± 8.82b | 194 ± 8.82a | 219 ± 8.82b | <0.001 | 0.791 | <0.001 | |
| ADFI, g | 329 ± 7.44c | 317 ± 7.44bc | 284 ± 7.44a | 299 ± 7.44ab | <0.001 | 0.839 | 0.057 | ||
| G:F | 0.77 ± 0.03 | 0.71 ± 0.03 | 0.69 ± 0.03 | 0.73 ± 0.03 | 0.252 | 0.829 | 0.053 | ||
A total of 1440 weaned pigs (LD × LW) were used in a 21-day trial. Each mean represents the average of 20 replicates (18 pigs/pen) per treatment. Values are expressed as mean ± s.e. average daily gain (ADG), average daily feed intake (ADFI), and efficiency expressed as gain to feed ratio (G:F), in post-weaned piglets.
Mean values in the same row with different letters (a, b, c) differ significantly according to the l.s.d. multiple comparison (P ≤ 0.05).
Quinine supplementation as a main effect worsened the G:F ratio (P < 0.05) during the first week (0 to 7 days post-weaning). In addition, significant interactions between the two main effects (ZnO and quinine supplements) were observed throughout the experiment, affecting ADG, ADFI or G:F. These interactions indicate that quinine supplementation negatively impacted performance in the presence of ZnO, whereas a positive effect was recorded in the absence of ZnO (Table 5). Quinine supplementation significantly (P < 0.001) improved the cumulative ADG (0 to 21 days) of pigs fed ZnO-free feed.
Discussion
This research project aimed at studying bitter compounds (quinine) as additives to mitigate the potential absence/banning of high doses of ZnO in piglet feeds. The study consisted of two experiments, where in Experiment 1 pigs were housed individually to test the dietary treatments under controlled research conditions; and in Experiment 2 piglets were housed in groups more reflective of commercial conditions. Dietary supplementation with ZnO at 3000 ppm significantly improved performance in post-weaning piglets. ZnO has been widely used as an antimicrobial against gut pathogens including E. coli (Pieper et al. 2012; Swain et al. 2016; Wang et al. 2019; Bonetti et al. 2021). These results confirmed previous studies showing that dietary pharmaceutical doses of ZnO (2000 to 4000 ppm) improve growth performance and feed efficiency in weaned pigs (Smith et al. 1997; Poulsen 1998; Hill et al. 2001; Heo et al. 2010; Hu et al. 2012; Walk et al. 2015). In addition, the results show a statistical trend indicating a decreased risk of diarrhoea in ZnO/CuSO4-fed piglets, which is consistent with previous reports (Cho et al. 2015; Rattigan et al. 2020; Wang et al. 2021). The relatively low FCS across treatments was likely the result of the use of an in-feed antibiotic (tiamulin) (Burch 1982). Interestingly, the use of an in-feed antibiotic did not prevent the positive effect of ZnO (and quinine), indicating that the antimicrobial activity of these additives alone does not fully explain the positive results observed.
The experiments aimed to test whether the addition of quinine could partially substitute pharmacological doses of ZnO (with or without CuSO4 in Experiments 1 or 2, respectively). The results from the two experiments were consistent in that the absence of ZnO significantly reduced ADG and ADFI, whereas quinine was able to compensate the growth depression associated with the ZnO-free group. The only exception was the G:F ratio which was significantly improved in favour of the ZnO group in Experiment 1 only. The results highlighted that the effect of quinine was highly dependent on the presence/absence of ZnO in the diet. Across the two experiments, consistent interactions between ZnO and quinine were identified, indicating that when quinine was provided alone, piglets presented improved performance compared to the negative control. In contrast, quinine negatively affected piglet performance in the presence of ZnO.
Quinine was selected for this study based on previous results associating dietary bitterants and improved performance in pigs (Fu et al. 2015; Roura and Fu 2017). Although quinine is one of the referential bitter compounds known in mammals, ZnO has also been reported to elicit bitter taste (Rodgers et al. 2006; Dagan-Wiener et al. 2019). These could be interpreted as a potential overlapping/blocking effect associated with the bitterness of both compounds when delivered together (Rodgers et al. 2006). Several studies have reported a low preference and decreased feed intake in diets rich in bitter compounds (Mawson et al. 1994; Bugnacka and Falkowski 2001; Roura et al. 2012). In previous studies, pigs have been reported to show aversion to quinine due to its bitter taste (Nelson and Sanregret 1997; Danilova et al. 1999). However, bitter extracts in post-weaned diets improved ADG, ADFI and feed conversion ratio (FCR) (Straub et al. 2005; Lien et al. 2007).
To the best of our knowledge, this is the first time that a bitterant has been able to compensate the withdrawal of ZnO in piglet diets. Several studies in humans and laboratory animals have reported that bitter compounds, such as quinine and denatonium benzoate, stimulate cholecystokinin (CCK) secretion through the activation of a gastrointestinal network of bitter taste receptors (TAS2Rs). The TAS2R activation (and CCK release) has been associated with delayed gastric emptying, decreased appetite and early satiety (Wicks et al. 2005; Glendinning et al. 2008; Andreozzi et al. 2015). A network of receptors controlling sensory perception in the gut, including bitter taste receptors (pTAS2R), has been described in pigs and other mammals. Bitterants, such as quinine, were shown to release gut peptides including CCK, and slowing down gastric emptying and passage rate (da Silva et al. 2014; Roura and Fu 2017; Fu et al. 2018; Roura and Navarro 2018; Roura et al. 2019). Thus, consistent with the existing literature, it could be speculated that the impact of quinine on performance presented in this article might be a consequence of the slowed-down emptying of the stomach and a decrease in the peristaltic movements of the small intestine in piglets. In addition, the bitterness of ZnO and not only the antimicrobial activity may also be involved in the growth promoting effect of this compound. However, the potential effect of ZnO as a bitter agonist has not been investigated to date and may warrant further investigation.
Conclusions
Pharmacological doses of ZnO/CuSO4 improved growth performance in post-weaning pigs regardless of the presence of a wide-spectrum antimicrobial in the feed. In the absence of ZnO, dietary quinine (500 ppm) improved piglet ADG to a similar level as ZnO and CuSO4, however the combination of dietary quinine and ZnO negatively impacted performance in weaned pigs indicating a potential competitive interaction between the two compounds possibly associated with sensing of bitterness. This interaction warrants further investigation. Overall, quinine showed a promising potential as a partial substitute for ZnO in post-weaning pig diets, providing an alternative strategy for maintaining growth performance without relying on high doses of ZnO.
Data availability
The data that support the current study are available upon reasonable request to the corresponding author.
Declaration of funding
This project was supported by Australian Pork Limited through project number 2018/0024.
Acknowledgements
The authors would like to thank Australian Pork Limited for their funding support, as well as Lucta S.A. for supplying quinine HCl and Riverina Pty Ltd for supplying ZnO for this research. Additionally, they acknowledge Mr Chris Brewster for assistance with feed formulation of Experiments 1 and 2. Some of the data in this manuscript was presented at the Australasian Pig Association Conference in 2021 and 2023 and published as peer-reviewed conference papers (Garcia-Puig et al. 2021, 2023).
References
Andreozzi P, Sarnelli G, Pesce M, Zito FP, Alessandro AD, Verlezza V, Palumbo I, Turco F, Esposito K, Cuomo R (2015) The bitter taste receptor agonist quinine reduces calorie intake and increases the postprandial release of cholecystokinin in healthy subjects. Journal of Neurogastroenterology and Motility 21, 511-519.
| Crossref | Google Scholar | PubMed |
Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, Bethe A, Semmler T, Tedin K, Schierack P, Wieler LH, Guenther S (2013) The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. International Journal of Medical Microbiology 303, 396-403.
| Crossref | Google Scholar | PubMed |
Bonetti A, Tugnoli B, Piva A, Grilli E (2021) Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals 11, 642.
| Crossref | Google Scholar | PubMed |
Bugnacka D, Falkowski J (2001) The effect of dietary levels of yellow lupin seeds (Lupinus luteus L.) on feed preferences and growth performance of young pigs. Journal of Animal and Feed Sciences 10, 133-142.
| Crossref | Google Scholar |
Burch DG (1982) Tiamulin feed premix in the prevention and control of swine dysentery under farm conditions in the UK. Veterinary Record 110, 244-246.
| Crossref | Google Scholar | PubMed |
Cho JH, Upadhaya SD, Kim IH (2015) Effects of dietary supplementation of modified zinc oxide on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding and fecal score in weanling pigs. Animal Science Journal 86, 617-623.
| Crossref | Google Scholar | PubMed |
Cromwell G (2001) Chapter 18. Antimicrobial and promicrobial agents. In ‘Swine nutrition’. (Ed. AJ Lewis, LL Southern) pp. 401–406. (CRC Press: Boca Raton, FL, USA) 10.1201/9781420041842
da Silva EC, de Jager N, Burgos-Paz W, Reverter A, Perez-Enciso M, Roura E (2014) Characterization of the porcine nutrient and taste receptor gene repertoire in domestic and wild populations across the globe. BMC Genomics 15, 1057.
| Crossref | Google Scholar | PubMed |
Dagan-Wiener A, Di Pizio A, Nissim I, Bahia MS, Dubovski N, Margulis E, Niv MY (2019) BitterDB: taste ligands and receptors database in 2019. Nucleic Acids Research 47, D1179-D1185.
| Crossref | Google Scholar | PubMed |
Danilova V, Roberts T, Hellekant G (1999) Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chemical Senses 24, 301-316.
| Crossref | Google Scholar | PubMed |
Feng Z, Zhu H, Deng Q, He Y, Li J, Yin J, Gao F, Huang R, Li T (2018) Environmental pollution induced by heavy metal(loid)s from pig farming. Environmental Earth Sciences 77, 103.
| Crossref | Google Scholar |
Fu M, Collins CL, Henman DJ, Roura E (2015) Some bitter compounds show potential for decreasing feed intake and fat deposition while others improve growth and feed conversion ratio in finishing pigs. Animal Production Science 55, 1543.
| Crossref | Google Scholar |
Fu M, Val-Laillet D, Guerin S, Roura E (2018) Dietary bitter compounds delayed gastric emptying and glucose uptake while increased plasma insulinotropic hormone GLP-1 in pigs. 14th International Symposium of Digestive Physiology of Pigs. Advances in Animal Biosciences 9(S2), S84.
| Google Scholar |
Garcia-Puig E, Liu F, Morrison RS, Lisle A, Roura E (2021) In-feed quinine improved performance to a similar level as ZnO and CuSO4 in post-weaning pigs. Animal - Science Proceedings 12, 195.
| Crossref | Google Scholar |
Garcia-Puig E, Liu F, Morrison RS, Lisle A, Roura E (2023) In-feed quinine hydrochloride supplementation improved piglet growth in the absence of zinc oxide under commercial conditions. Animal - Science Proceedings 14, 882-883.
| Crossref | Google Scholar |
Glendinning JI, Yiin YM, Ackroff K, Sclafani A (2008) Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiology & Behavior 93, 757-765.
| Crossref | Google Scholar | PubMed |
Heo JM, Kim JC, Hansen CF, Mullan BP, Hampson DJ, Pluske JR (2010) Effects of dietary protein level and zinc oxide supplementation on performance responses and gastrointestinal tract characteristics in weaner pigs challenged with an enterotoxigenic strain of Escherichia coli. Animal Production Science 50, 827-836.
| Crossref | Google Scholar |
Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM (2013) Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. Journal of Animal Physiology and Animal Nutrition 97, 207-237.
| Crossref | Google Scholar | PubMed |
Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL (2001) Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. Journal of Animal Science 79, 934-941.
| Crossref | Google Scholar | PubMed |
Hu CH, Gu LY, Luan ZS, Song J, Zhu K (2012) Effects of montmorillonite–zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs. Animal Feed Science and Technology 177, 108-115.
| Crossref | Google Scholar |
Jacela JY, DeRouchey JM, Tokach MD, Goodband RD, Nelssen JL, Renter DG, Dritz SS (2010) Feed additives for swine: fact sheets – high dietary levels of copper and zinc for young pigs, and phytase. Journal of Swine Health and Production 18, 87-91.
| Crossref | Google Scholar |
Lien TF, Horng YM, Wu CP (2007) Feasibility of replacing antibiotic feed promoters with the Chinese traditional herbal medicine Bazhen in weaned piglets. Livestock Science 107, 97-102.
| Crossref | Google Scholar |
Long L, Chen J, Zhang Y, Liang X, Ni H, Zhang B, Yin Y (2017) Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 12, e0182550.
| Crossref | Google Scholar | PubMed |
Mawson R, Heaney RK, Zduńczyk Z, Kozłowska H (1994) Rapeseed meal-glucosinolates and their antinutritional effects. Part 5. Animal reproduction. Nahrung 38, 588-598.
| Crossref | Google Scholar | PubMed |
Medardus JJ, Molla BZ, Nicol M, Morrow WM, Rajala-Schultz PJ, Kazwala R, Gebreyes WA (2014) In-feed use of heavy metal micronutrients in U.S. swine production systems and its role in persistence of multidrug-resistant salmonellae. Applied and Environmental Microbiology 80, 2317-2325.
| Crossref | Google Scholar | PubMed |
Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chemical Senses 35, 157-170.
| Crossref | Google Scholar | PubMed |
Nelson SL, Sanregret JD (1997) Response of pigs to bitter-tasting compounds. Chemical Senses 22, 129-132.
| Crossref | Google Scholar | PubMed |
O’Shea CJ, O’Doherty JV, Callanan JJ, Doyle D, Thornton K, Sweeney T (2016) The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. Journal of Nutritional Science 5, e15.
| Crossref | Google Scholar |
Paniagua M, Villagómez-Estrada S, Crespo FJ, Pérez JF, Arís A, Devant M, Solà-Oriol D (2023) Citrus flavonoids supplementation as an alternative to replace zinc oxide in weanling pigs’ diets minimizing the use of antibiotics. Animals 13, 967.
| Crossref | Google Scholar | PubMed |
Pieper R, Vahjen W, Neumann K, Van Kessel AG, Zentek J (2012) Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. Journal of Animal Physiology and Animal Nutrition 96, 825-833.
| Crossref | Google Scholar | PubMed |
Poulsen HD (1998) Zinc and copper as feed additives, growth factors or unwanted environmental factors. Journal of Animal and Feed Sciences 7, 135-142.
| Crossref | Google Scholar |
Rattigan R, Sweeney T, Vigors S, Rajauria G, O’Doherty JV (2020) Effects of reducing dietary crude protein concentration and supplementation with laminarin or zinc oxide on the faecal scores and colonic microbiota in newly weaned pigs. Journal of Animal Physiology and Animal Nutrition 104, 1471-1483.
| Crossref | Google Scholar | PubMed |
Ribani A, Bertolini F, Schiavo G, Scotti E, Utzeri VJ, Dall’Olio S, Trevisi P, Bosi P, Fontanesi L (2017) Next generation semiconductor based sequencing of bitter taste receptor genes in different pig populations and association analysis using a selective DNA pool-seq approach. Animal Genetics 48, 97-102.
| Crossref | Google Scholar | PubMed |
Rodgers S, Glen RC, Bender A (2006) Characterizing bitterness: identification of key structural features and development of a classification model. Journal of Chemical Information and Modeling 46, 569-576.
| Crossref | Google Scholar | PubMed |
Roura E, Fu M (2017) Taste, nutrient sensing and feed intake in pigs (130 years of research: then, now and future). Animal Feed Science and Technology 233, 3-12.
| Crossref | Google Scholar |
Roura E, Navarro M (2018) Physiological and metabolic control of diet selection. Animal Production Science 58, 613-626.
| Crossref | Google Scholar |
Roura E, Depoortere I, Navarro M (2019) Review: Chemosensing of nutrients and non-nutrients in the human and porcine gastrointestinal tract. Animal 13, 2714-2726.
| Crossref | Google Scholar | PubMed |
Shen J, Chen Y, Wang Z, Zhou A, He M, Mao L, Zou H, Peng Q, Xue B, Wang L, Zhang X, Wu S, Lv Y (2014) Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. British Journal of Nutrition 111, 2123-2134.
| Crossref | Google Scholar | PubMed |
Smith JW, Tokach MD, Goodband RD, Nelssen JL, Richert BT (1997) Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. Journal of Animal Science 75, 1861-1866.
| Crossref | Google Scholar | PubMed |
Straub R, Gebert S, Wenk C, Wanner M (2005) Growth performance, energy, and nitrogen balance of weanling pigs fed a cereal-based diet supplemented with Chinese rhubarb. Livestock Production Science 92, 261-269.
| Crossref | Google Scholar |
Swain PS, Rao SBN, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Animal Nutrition 2, 134-141.
| Crossref | Google Scholar | PubMed |
Vahjen W, Pietruszyńska D, Starke IC, Zentek J (2015) High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathogens 7, 23.
| Crossref | Google Scholar | PubMed |
Walk CL, Wilcock P, Magowan E (2015) Evaluation of the effects of pharmacological zinc oxide and phosphorus source on weaned piglet growth performance, plasma minerals and mineral digestibility. Animal 9, 1145-1152.
| Crossref | Google Scholar | PubMed |
Wang W, Van Noten N, Degroote J, Romeo A, Vermeir P, Michiels J (2019) Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. Journal of Animal Physiology and Animal Nutrition 103, 231-241.
| Crossref | Google Scholar |
Wang H, Kim KP, Kim IH (2021) Evaluation of the combined effects of different dose levels of zinc oxide with probiotics complex supplementation on the growth performance, nutrient digestibility, faecal microbiota, noxious gas emissions and faecal score of weaning pigs. Journal of Animal Physiology and Animal Nutrition 105, 286-293.
| Crossref | Google Scholar | PubMed |
Wicks D, Wright J, Rayment P, Spiller R (2005) Impact of bitter taste on gastric motility. European Journal of Gastroenterology & Hepatology 17, 961-965.
| Crossref | Google Scholar | PubMed |


