Early end of embryonic diapause and overall reproductive activity in roe deer populations from Bavaria
C. Ehrmantraut A * , T. Wild
A * , T. Wild  A , S.-A. Dahl
A , S.-A. Dahl  A , N. Wagner
A , N. Wagner  B and A. König
B and A. König  A
A
A
B
Animal Production Science 63(16) 1623-1632 https://doi.org/10.1071/AN23040
Submitted: 25 January 2023 Accepted: 4 September 2023 Published: 2 October 2023
Abstract
According to the established, older literature, the embryonic diapause (ED) in roe deer ends in late December. In many other species groups, phenological phases are shifting as a result of climatic changes. Whether this is the case in roe deer for the period of ED has not yet been explicitly investigated.
The aim of this study was to obtain an up-to-date overview of the reproduction of roe deer in Bavaria, particularly with regard to climate change.
For this purpose, uterus samples were taken from roe deer aged at least 1 year between September and January and examined macroscopically. The samples were collected during regular hunting activities in Bavaria in the years 2017–2020.
A large proportion of the female roe deer examined was found to have been already engaged in reproduction. In 98% of the animals, Corpora lutea (CL) were present in the ovaries. Adult does had significantly more CL than did subadult does just reaching sexual maturity. In 75 roe deer does, 128 embryos, in total, were detected. On average, 1.67 embryos were found per doe with visible embryos. Of these does, 30% carried a single embryo, 67% were bearing twins and 3% were bearing triplets. The animals with visible embryos ranged in age from 1 to 12 years. In a few cases, ED was already completed in November or early December. By the end of the hunting season, a correspondingly wide variety of developmental stages of embryos was visible. Our study suggests potential indications that older animals or those with a higher body mass end ED earlier.
Overall, the results indicated vigorous reproductive activity in the study area. The results showed that some individuals already end the ED in November or early December, so they may adapt to an earlier onset of the vegetation period. In contrast, we found a few individuals that ended the ED during January, which illustrates the temporal range of the ED.
Whether the early end of diapause in these roe deer is a reaction to climatic changes or whether there are other underlying causes is something that requires further investigation.
Keywords: Bavaria, Capreolus capreolus, Corpora lutea, embryo, embryonic diapause, Germany, reproduction, roe deer, uterus.
Introduction
The roe deer (Capreolus capreolus) occupies a special position among cloven-hoofed animals due to its reproductive biology, because it is the only representative of this group, apart from the Siberian roe deer, to certainly undergo the obligate embryonic diapause (Danilkin 1996; Smith-Jones 2022). The rutting season of roe deer reaches its peak at the end of July to the beginning of August. During this phase, the does ovulate and mating takes place (Ellenberg 1978; Kurt 2002). Successful fertilisation results in a blastocyst, which, after a few days of development, passes into the ED and remains free-floating in the uterus until late December (Short and Hay 1966; Aitken 1974; Lambert et al. 2001). During ED, the mitotic activity of the blastocyst is greatly reduced. Consequently, the end of ED is characterised by an increase in mitotic activity (Lengwinat and Meyer 1996; Lopes et al. 2004). Specifically, termination of ED can be assessed macroscopically to the extent that if an embryo is visible to the naked eye, ED has been terminated (Short and Hay 1966; Aitken 1977). In late December/early January, there is rapid elongation of the blastocyst, causing the chorionic sac to rapidly reach a length of about 30 cm (Aitken 1981). Finally, implantation follows in early January, during which a firm attachment is formed between the embryo and the uterus. From the end of the ED until parturition in May/June, embryonic development is comparable to the development of other mammals (Bischoff 1854; Short and Hay 1966).
This strategy allows the species to decouple the time between mating and embryonic development, so that the offspring is born during a period with favourable environmental conditions (Andersen et al. 1998). The roe deer thus has an optimum composition of energy-rich vegetation available to it during the energetically demanding lactation phase (König et al. 2020), which ultimately provides a good basis for the successful rearing of fawns (Andersen et al. 2000). As an income breeder, the roe deer is dependent on the availability of high-quality food during this phase (Williams et al. 2017).
The climatic changes of the past decades, reflected, for example, in an earlier onset of the vegetation period (Menzel and Fabian 1999; Cleland et al. 2007), interfere with this reproductive strategy (Kerby and Post 2013).
The negative consequences of changes in the phenological phases of plants for animal species adapted to them have been described numerous times already, for example, in great tits (Parus major), caribou (Rangifer tarandus), the northern wheatear (Oenanthe oenanthe L.), Ross’s geese (Chen rossii) and lesser snow geese (Chen caerulescens caerulescens) (Visser et al. 1998; Post and Forchhammer 2008; Arlt and Pärt 2017; Ross et al. 2017). In contrast, other species seem to be able to adapt to the changed environmental conditions (Marrot et al. 2018; Bonnet et al. 2019). These latter species include some ungulates, such as red deer (Cervus elaphus), Pyrenean chamois (Rupicapra pyrenaica pyrenaica), reindeer (R. tarandus) and moose (Alces alces), that have adapted their reproductive behaviour to the changed climatic conditions, for example, by bringing forward their mating season or shortening their gestation period (Moyes et al. 2011; Kourkgy et al. 2016; Peláez et al. 2017; Paoli et al. 2018; Neumann et al. 2020).
The extent to which such changes affect the reproductive behaviour of roe deer in the long term is still unclear, because previous studies have come to different conclusions. Plard et al. (2014a) observed no shift in timing of the birth date of roe deer over a 27-year study period in France, with negative consequences for reproduction. Two further studies also examined the relationship in time between plant phenology and fawning period over a period of several decades. The first study from Switzerland concluded that roe deer is very slow to respond in comparison with other ungulates, and that there is already a clear discrepancy between plant phenology and the fawning period (Rehnus et al. 2020). However, a second study from Germany saw a stronger adaptation of the species to climate change, because the fawning period had also shifted to an earlier date over the decades (Hagen et al. 2021).
The aim of the present study was to obtain an up-to-date overview of the reproduction of roe deer in different areas of Bavaria (Germany). Primarily, the end of ED was to be recorded. A comparison was to be made with statements made in the established literature indicating that the ED ends at the end of December (Short and Hay 1966; Aitken 1974; Wandeler 1975), and it was to be checked whether ED ends earlier nowadays. Because an early end of ED (from November onward) had been observed in some of the does, it was also investigated whether the age and body mass of these does had an influence on embryo size.
To give a general overview of recent reproductive activity, the effects of doe age and body mass on the number of embryos were also considered. Furthermore, the data set was divided into subadult and adult does, to test whether the number of CL and embryos differed between these two groups.
Materials and methods
Our study was conducted in the federal state of Bavaria in south-eastern Germany. Bavaria is located in the warm-temperate climate zone, with an average temperature of 7.9°C and average annual precipitation of 941 mm (in the period 1971–2000) (Bayerisches Landesamt für Umwelt 2022). However, the study area is characterised by sometimes very different climatic conditions and also has a diverse vegetation structure, including deciduous forests, coniferous forests, mixed mountain forests, grassland and agricultural areas (Bayerische Staatsforsten AöR 2019).
The study was based on uterus samples taken from roe deer. The sample material originated from different areas of Bavaria, which are distributed over the entire state and thus represent all typical habitats.
Sampling took place from September 2017 to January 2020. The material was collected during the regular hunting activities of the forestry enterprises and private hunters. The uterus and ovaries of the harvested deer were removed after field dressing and stored at −18°C until examination. For each doe, the date and place on which the animal was shot were recorded, as well as its live body mass (body mass total) and the mass after field dressing (body mass eviscerated). The animals’ lower jaws were also removed to determine the age by cementum annuli technique (Mitchell 1967).
The focus of this study was on the macroscopic examination of the uterus to determine the number of embryos and embryo sizes. For this purpose, the uterus was opened along the uterine horns with surgical scissors and tweezers to examine the uterine lumen for the presence of macroscopically visible embryonic tissue. The crown–rump length (CRL) or the greatest length (GL) of any embryos was measured using callipers (Mall 1907).
Ovulation results in the formation of one or several CL, which can indicate a potential pregnancy (Andersen 1953; Short and Hay 1966). A CL regresses until the beginning of the next oestrus cycle (Bischoff 1854; Horak 1989). To determine the number of CL, representing the ovulation rate, horizontal sections were made of the ovaries with a scalpel. These were examined under a binocular microscope for the presence of CL (Wandeler 1975).
In addition, it should be determined whether the ED had ended or not. The ED was terminated when the first macroscopically visible embryonic tissue was detectable (Short and Hay 1966; Aitken 1977). According to the literature (Short and Hay 1966; Aitken 1974; Wandeler 1975), does in temperate latitudes exit the ED at the end of December. This period aligns with the winter solstice (21 December), which serves as a significant photoperiod signal for the reproduction of various species (Bronson 2009).
Overall reproductive activity
The animals were divided into subadult (age 1 year) and adult (from the age of 2 years) does, because the animals reach sexual maturity between the first and second year of life (Andersen et al. 1998). Mann–Whitney U tests were used to investigate whether the number of CL and embryos differed between subadults and adults.
Animals with and without visible embryos were compared regarding their age (years), total body mass and eviscerated body mass using a Mann–Whitney U test.
Using a linear regression model, we investigated which predictors are associated with the number of embryos. The independent variables utilised for this investigation were body mass eviscerated, age (years), and number of CL.
End of ED
For the group of animals with visible embryos, a linear regression model was developed using the embryo size in centimetres as the dependent variable and time as the independent variable. We considered 4 November as the reference day (labelled as Day 1), because it marks the date when the first visible embryos were detected in a doe. Because of the non-normal distribution of embryo sizes, a logarithmic transformation was applied prior to conducting the analysis.
In a subsequent analysis, additional predictors (eviscerated body mass, age (years), the count of CL and embryos) were included in the linear regression model. For instances where the eviscerated body mass values were missing, they were imputed using the total body mass, which had a strong linear correlation with it (r = 0.86).
All statistical analyses were conducted using the software ‘R’ (ver. 4.1.3) provided by the R Foundation for Statistical Computing. The software can be accessed at https://www.r-project.org/. A consistent significance level of α = 0.05 was set for all statistical tests.
Results
In the course of this study, sample material from a total of 448 individual animals was examined. Of the samples, 28 were taken in September, 17 in October, 190 in November, 165 in December and 48 in January.
Overall reproductive activity
In 80 animals, macroscopically visible embryonic stages were detected during the dissection (5 of 80 uterus samples were damaged, but amniotic sheaths could be detected as a sign of pregnancy and, for two animals with visible embryos, the age is unknown). In total, 128 embryos were recorded.
The subadult does with visible embryos had an average total body mass of 21.1 kg (s.d. = 3.89), whereas the adult animals had an average total body mass of 21.8 kg (s.d. = 4.04). The age of these animals ranged from 1 to 12 years. In all, 30% had one embryo, 67% were bearing twins, and 3% were bearing triplets. In the case of the triplets, we found the ratio of two CL to three embryos once. In the other case, the ovaries were incomplete, which does not allow any conclusion. CL were detected in the ovaries of 98% of the animals examined.
Comparison between subadults and adults in number of CL and embryos
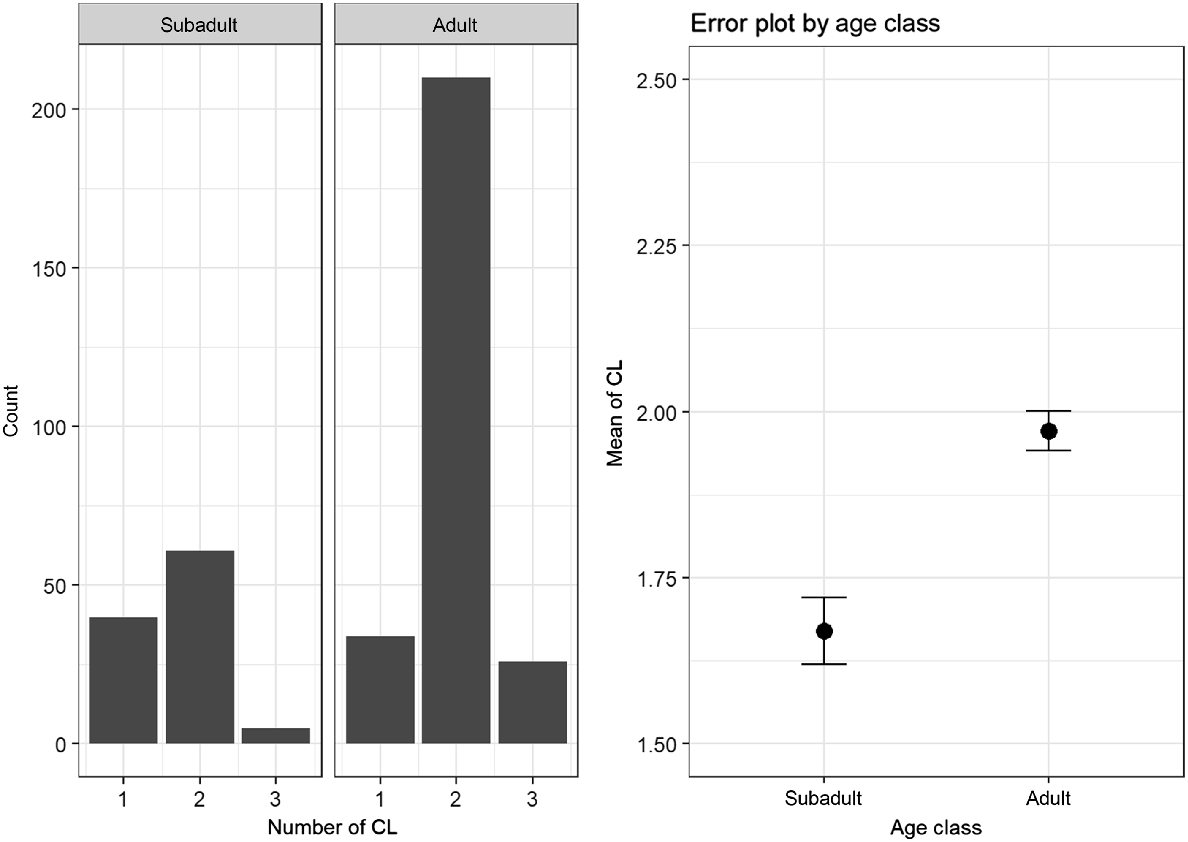
A total of 379 animals could be used to compare the number of CL in adult (n = 270) and subadult animals (n = 109). Adults showed a higher mean number of CL (M = 1.97, s.d. = 0.47) than did subadults (M = 1.67, s.d. = 0.56). This difference turned out to be statistically significant (Mann–Whitney U test: P < 0.001). The differences and distribution of the number of CL between the subadults and adults is shown in Fig. 1.
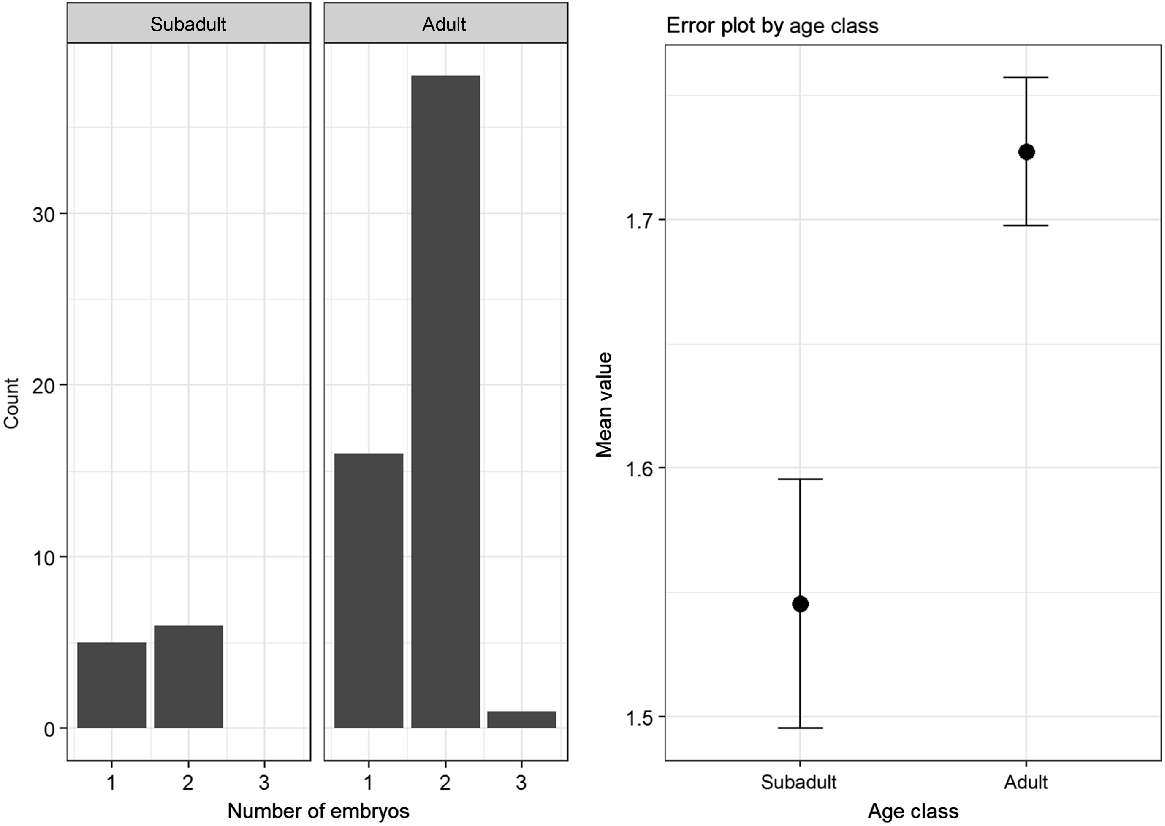
For the comparison of the embryo numbers in adult does (n = 59) and subadult does (n = 14), 73 samples could be used. The average number of embryos was slightly higher for adults (M = 1.75, s.d. = 0.50), than for subadults (M = 1.57, s.d. = 0.56). However, this difference was not statistically significant (Mann–Whitney U test: P = 0.27). The differences and distribution of embryos within the subadult and adult animals is shown in Fig. 2.
Comparison between groups with and without visible embryos
The group of animals with visible embryos was slightly older, on average (M = 3.37), than was the group without visible embryos (M = 3.09), but no statistically significant differences could be found for either of the characteristics.
Predictive factors for embryo count: influence of age, body mass, and CL
Age (years) showed a significantly positive effect (β = 0.34, P < 0.001), indicating that older animals, on average, had a higher number of embryos. However, neither the eviscerated body mass (β = 0.01, P = 0.96) nor the number of CL (β = 0.06, P = 0.51) was found to be a significant predictor. It is important to note that this does not contradict previous results, where no significant difference was found between subadults and adults in terms of their embryo count. The significant effect in the regression model mainly comes from the older animals (10 years and older), which are less represented in the data. For instance, there are minor differences in embryo count between subadults and young adults. However, all animals aged 10 or older had at least two embryos. Likewise, no animal younger than 10 years was observed to have more than two embryos.
End of ED
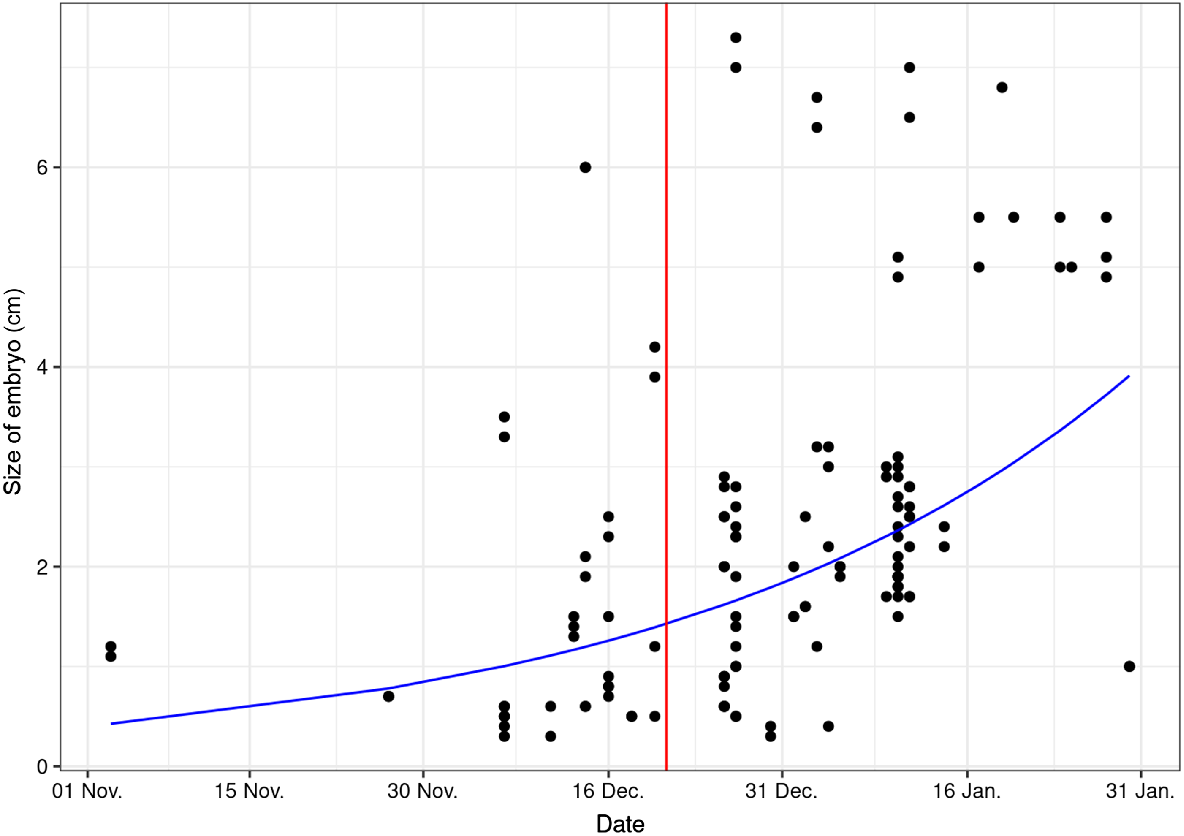
The earliest embryos were found in a doe shot on 4 November 2017 in northern Bavaria (Haßberge). The two embryos measured 11 and 12 mm respectively. Over the entire study period, the size of the embryos varied between 3 and 73 mm (Fig. 3).
The plot depicts the embryo sizes (measured as CRL in centimetres) for samples taken from November to January. The red line represents the winter solstice, and the blue line illustrates the fitted trend from the exponential regression model.
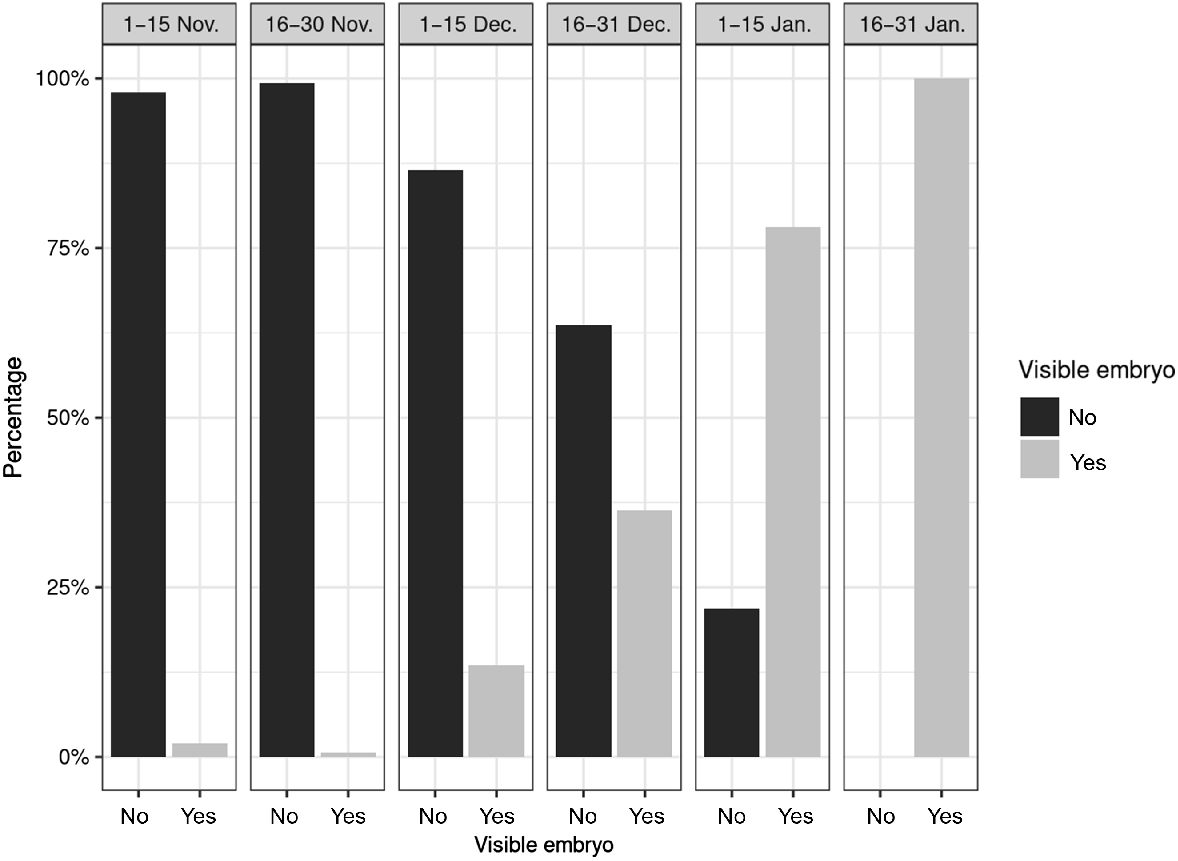
The proportion of animals in which visible embryos could be detected increased during the study period and, from mid-January onward, visible embryos were detected in all animals examined (Fig. 4).
Proportion of animals with and without visible embryos from November till January in Bavaria, Germany.
Regression analysis of embryo size over time
This analysis showed a statistically significant positive relationship (P < 0.001, β = 0.49), indicating that, on average, embryos tend to be larger as time progresses.
Extended analysis with multiple predictors and data imputation
In this enhanced model, time remained a significant predictor (β = 0.53, P < 0.001). Additionally, there was a small, yet non-significant, positive relationship with the eviscerated body mass (β = 0.15, p = 0.07). Other predictors such as age (β = 0.11), number of CL (β = 0.05), and number of embryos (β = 0.08) exhibited smaller effects.
To summarise, the only noteworthy effect identified was for time, and it had a moderate strength.
Discussion
The data collected in this study provide an up-to-date insight into the reproduction of roe deer for the period from September to January.
In our study, CL was detected in 98% of the does examined. These animals were actively involved in reproduction (Andersen 1953; Bischoff 1854; Short and Hay 1966). Similarly high rates of CL have been observed in other studies (Bischoff 1854; Strandgaard 1972; Horak 1989; Hewison and Gaillard 2001; Flajšman et al. 2017). In keeping with the established literature, it was found that the subadult animals have less CL than do the adult roe deer (Borg 1971; Ellenberg 1978; Horak 1989; Flajšman et al. 2017).
An average of 1.57 embryos was found in subadult, and 1.75 embryos in adult animals with visible embryos. This matches the data in the established literature (Ellenberg 1978). However, because the number of embryos did not differ significantly between the two age groups (in contrast to the number of CL), it could be assumed that losses during embryo development were higher in the adult animals. Chirichella et al. (2019) demonstrated that implantation failure increased with age, supporting our assumption. All in all, the collected parameters indicated vigorous reproductive activity and corresponded to the results of previous studies (Ellenberg 1978; Horak 1989; Flajšman et al. 2017).
Our study may provide indications of the potential for an earlier termination of embryonic development in older animals and in those with a higher body mass. Initially, a small but statistically non-significant positive correlation was observed between embryo size and body mass. Similarly, the group of animals with visible embryos exhibited slightly older ages than did the group without visible embryos. Nevertheless, these differences did not reach statistical significance. Several studies have already observed that female characteristics, such as age and body mass, influence the timing of rut, embryonic development, and the date of parturition. It has been observed that older animals and those with a higher body mass within a population tend to enter the mating season earlier (Bischoff 1854; Ellenberg 1978; Horak 1989) and give birth earlier in the subsequent spring (Plard et al. 2014b). Given these observations, it is not unreasonable to speculate that our results hint at the influence of age and body mass on the end of ED.
Moreover, the end of the ED is not a synchronous process but takes place within a certain time span (Bischoff 1854; Stubbe et al. 1981). In contrast to the relatively constant rutting season, the length of gestation and the time of parturition can also vary from year to year among individuals (Prell 1938; Ellenberg 1978, Danilkin 1996). A correspondingly wide range of stage of development is thus also found in the embryos (Fig. 3). In samples taken in the same population on the same date at the end of December, for example, two embryos with a length of 5 mm were detected in one doe, whereas in another doe, two embryos already measuring over 70 mm each were found. Similar observations were described by Borg (1971), which can also occur between individuals of the same age (Bischoff 1854).
The main objective of the study was to record the end of ED in roe deer in Bavaria. For this purpose, the time from which the first macroscopically visible embryos were detectable was investigated.
Our results showed that some does had already finished the ED in November or early December. It can also be assumed on the basis of the size of some embryos from the month of January that they had started to develop early in December (Bischoff 1854; Bubenik 1984). In studies from Germany from the past, the first macroscopically visible embryos were usually found at the end of December or beginning of January (Pockels 1836; Ziegler 1843; Bischoff 1854; Keibel 1899; Sakurai 1906; Stieve 1950). A study from Switzerland (Canton Bern) concluded that 50% of does have finished ED by mid-January (Wandeler 1975). Up to the same point in time, we found a significantly higher rates in our study (Fig. 4). The results of the above studies differ from our findings. Unfortunately, there is only a limited amount of data from the past, so it is difficult to make a systematic comparison.
Stieve (1950) and Raesfeld (1956) described that in a few cases a second fertile oestrus occurs in November, with subsequent embryonic development without ED. This could be a possible explanation for the early presence of embryos. However, numerous other studies have not been able to confirm the existence of a second rut or have also classified it as a rare event of minor importance (Ziegler 1843; Short and Mann 1966; Wandeler 1975; Ellenberg 1978; Hoffmann et al. 1978; Horak 1989). Therefore, it is unlikely that this phenomenon is the cause for the presence of embryos in November or early December in our study.
Climate change could be considered as a possible cause for the early end of ED in roe deer. The gestation period and the onset of lactation are associated with high energy costs (Mauget et al. 1997). Therefore, the beginning of the vegetation period is the optimal time for most large herbivores to give birth (Merkle et al. 2016). Shifting phenological phases is therefore a challenge for these species, especially for income breeders (Kerby and Post 2013). As some examples show, there is currently no clear pattern of how large herbivores react to an earlier onset of spring. Pettorelli et al. (2007) observed negative effects on recruitment in bighorn sheep, mountain goat and ibex. Improved individual performance have been reported for red deer (Pettorelli et al. 2005) and chamois (Garel et al. 2011). Even within species, effects of climate change are not consistent. Helle and Kojola (2008) reported that an earlier spring has a positive effect on reindeer in Finland. However, in Greenland negative effects on reindeer were observed (Post and Forchhammer 2008). Moose respond in some regions with range expansion and population growth, whereas in other regions the range is decreasing, and the population is declining (Grøtan et al. 2009; Murray et al. 2012).
Some examples have shown that large herbivores can respond to climate change by adapting their reproductive cycle. Red deer (Clements et al. 2011; Moyes et al. 2011) and reindeer (Mysterud et al. 2009; Paoli et al. 2018) react by shortening the duration of gestation. Other studies have shown that the rutting season of Pyrenean chamois (Kourkgy et al. 2016) and red deer (Peláez et al. 2017) take place earlier as a result of climatic changes.
Owing to the special reproductive biology of roe deer, a direct comparison with the above-mentioned species is probably only possible to a very limited extent. Previous studies on roe deer have come to different conclusions. Gaillard et al. (2013) concluded that roe deer in forest habitats is currently unable to adapt to an earlier onset of spring and may therefore migrate to more favourable habitats. Another study from north-eastern France suggests the lack of phenotypic plasticity in birth timing. The discrepancy between the onset of the vegetation period and the parturition date has negative consequences for fawn survival (Plard et al. 2014a). Rehnus et al. (2020) found a consistent but slow advance towards earlier parturition dates across all scales and most elevational ranges in Switzerland. The authors also noted that mean birth dates at low elevations are already outside the window of optimal forage quality. The results of Hagen et al. (2021) speak for an adaptation to changing climatic conditions because they found a shift in parturition dates by 1–2 weeks within 47 years in southern Germany.
Despite these different results, the potential of roe deer to adapt to different environmental conditions is reflected by their large distribution. This extends in Europe from the south of Spain (36°N) to Norway (68°N). In this range, the species finds all sorts of different habitats and environmental conditions (Andersen et al. 1998). It has also been shown that roe deer is able to adapt to new habitats, such as, for example, agricultural landscapes (Kałuziński 1982), or to colonise higher altitudes (Acevedo et al. 2005).
There are temporal shifts of several weeks from north to south in terms of fawning period (Rieck 1955). The mean parturition dates of the populations in the south and on the Atlantic coast are earlier than those of the populations from the continental areas. In Spain, 80% of fawns are born in April. In Denmark, Norway and Sweden, the focus is between the second half of May and the beginning of June (Linnell et al. 1998). Differences can also occur on a regional scale. In the Danube valley, birth dates are about 10–12 days earlier than in the Jura, which is only 20 km away but at a higher altitude (Ellenberg 1978). A study from Denmark showed that embryonic development was delayed about 2 weeks in West Jutland compared with East Jutland (Strandgaard 1972). Plard et al. (2013) concluded that roe deer can adapt their parturition dates to different geographical locations and the associated climate.
Our results showed that some does had already finished the ED in November or early December. It is known that the occurrence of ED is quite variable and temporally flexible in species that perform obligate diapause (Enders 1952; Wade-Smith et al. 1980). In some cases, the absence of the ED has also been observed in roe deer, resulting in premature birth (Aitken 1981). For this reason, the definition of ‘obligate’ diapause is not without controversy (Ptak et al. 2012). In recent years, several molecular processes have been identified in roe deer that occur during ED and during the resumption of embryonic development (van der Weijden et al. 2021). Despite this, in comparison to other species with ED, less is known about the control of ED in roe deer. Various factors (e.g. photoperiod, temperature, onset of the growing season) have been discussed in the past (Sempéré et al. 1993; Danilkin 1996). However, it has not been conclusively investigated which external and endogenous factors significantly influence ED in roe deer.
Whether these early cases we found are based on a selection process owing to climatic changes and whether climate change influences the course of ED in roe deer is a topic that requires especially further long-term investigation (Lindenmayer et al. 2022).
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
Declaration of funding
This research was funded by the Bavarian Ministry of Agriculture, Forestry and Nutrition.
Acknowledgements
We gratefully acknowledge the financial support given by the Bavarian Ministry of Agriculture, Forestry and Nutrition. We also thank the colleagues of the Bavarian State Forest and all students involved for their practical support. We thank M. Clauss and an anonymous reviewer for valuable comments on a previous version of the manuscript. We also thank T. Zwanowetz for his valuable comments on the manuscript.
References
Acevedo P, Delibes-Mateos M, Escudero MA, Vicente J, Marco J, Gortazar C (2005) Environmental constraints in the colonization sequence of roe deer (Capreolus capreolus Linnaeus, 1758) across the Iberian Mountains, Spain. Journal of Biogeography 32, 1671-1680.
| Crossref | Google Scholar |
Aitken RJ (1974) Delayed implantation in roe deer (Capreolus capreolus). Journal of Reproduction and Fertility 39, 225-233.
| Crossref | Google Scholar | PubMed |
Aitken RJ (1981) Aspects of delayed implantation in the roe deer (Capreolus capreolus). Journal of Reproduction and Fertility 29, 83-95.
| Google Scholar | PubMed |
Andersen J (1953) Analysis of a Danish roe-deer population. Danish Review of Game Biology 2, 131-155.
| Google Scholar |
Andersen R, Gaillard J-M, Linnell JDC, Duncan P (2000) Factors affecting maternal care in an income breeder, the European roe deer. Journal of Animal Ecology 69, 672-682.
| Crossref | Google Scholar |
Arlt D, Pärt T (2017) Marked reduction in demographic rates and reduced fitness advantage for early breeding is not linked to reduced thermal matching of breeding time. Ecology and Evolution 7, 10782-10796.
| Crossref | Google Scholar | PubMed |
Bayerisches Landesamt für Umwelt (2022) Klima in Bayern. Available at https://www.lfu.bayern.de/klima/klimawandel/klimafaktenblaetter/index.html. [Verified 9 January 2023]
Bayerische Staatsforsten AöR (2019) Standorte. Available at https://www.baysf.de/de/ueber-uns/standorte.html [Verified 9 January 2023]
Bonnet T, Morrissey MB, Morris A, Morris S, Clutton-Brock TH, Pemberton JM, Kruuk LEB (2019) The role of selection and evolution in changing parturition date in a red deer population. PLoS Biology 17, e3000493.
| Crossref | Google Scholar | PubMed |
Borg K (1971) On mortality and reproduction of roe deer in Sweden during the period 1948-1969. Viltrevy 7, 121-149.
| Google Scholar |
Bronson FH (2009) Climate change and seasonal reproduction in mammals. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 3331-3340.
| Crossref | Google Scholar |
Chirichella R, Pokorny B, Bottero E, Flajšman K, Mattioli L, Apollonio M (2019) Factors affecting implantation failure in roe deer. The Journal of Wildlife Management 83, 599-609.
| Crossref | Google Scholar |
Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant phenology in response to global change. Trends in Ecology & Evolution 22, 357-365.
| Crossref | Google Scholar | PubMed |
Clements MN, Clutton-Brock TH, Albon SD, Pemberton JM, Kruuk LEB (2011) Gestation length variation in a wild ungulate. Functional Ecology 25, 691-703.
| Crossref | Google Scholar |
Ellenberg H (1978) Zur Populationsökologie des Rehes (Capreolus capreolus L., Cervidae) in Mitteleuropa. Spixiana, Zeitschrift für Zoologie Supplement 2, 1-211.
| Google Scholar |
Enders RK (1952) Reproduction in the mink (Mustela visori). Proceedings of the American Philosophical Society 96, 691-755.
| Google Scholar |
Flajšman K, Jerina K, Pokorny B (2017) Age-related effects of body mass on fertility and litter size in roe deer. PLoS ONE 12(4), e0175579.
| Crossref | Google Scholar | PubMed |
Gaillard J-M, Mark Hewison AJ, Klein F, Plard F, Douhard M, Davison R, Bonenfant C (2013) How does climate change influence demographic processes of widespread species? Lessons from the comparative analysis of contrasted populations of roe deer. Ecology Letters 16, 48-57.
| Crossref | Google Scholar | PubMed |
Garel M, Gaillard J-M, Jullien J-M, Dubray D, Maillard D, Loison A (2011) Population abundance and early spring conditions determine variation in body mass of juvenile chamois. Journal of Mammalogy 92, 1112-1117.
| Crossref | Google Scholar |
Grøtan V, Sæther B-E, Lillegård M, Solberg EJ, Engen S (2009) Geographical variation in the influence of density dependence and climate on the recruitment of Norwegian moose. Oecologia 161, 685-695.
| Crossref | Google Scholar | PubMed |
Hagen R, Ortmann S, Elliger A, Arnold J (2021) Advanced roe deer (Capreolus capreolus) parturition date in response to climate change. Ecosphere 12, e03819.
| Crossref | Google Scholar |
Helle T, Kojola I (2008) Demographics in an alpine reindeer herd: effects of density and winter weather. Ecography 31, 221-230.
| Crossref | Google Scholar |
Hewison AJM, Gaillard JM (2001) Phenotypic quality and senescence affect different components of reproductive output in roe deer. Journal of Animal Ecology 70(4), 600-608.
| Crossref | Google Scholar |
Hoffmann B, Barth D, Karg H (1978) Progesterone and estrogen levels in peripheral plasma of the pregnant and nonpregnant roe deer (Capreolus capreolus). Biology of Reproduction 19, 931-935.
| Crossref | Google Scholar | PubMed |
Kałuziński J (1982) Dynamics and structure of a field roe deer population. Acta Theriologica 27, 385-408.
| Crossref | Google Scholar |
Keibel F (1899) Zur Entwicklungsgeschichte des Rehes. Anatomischer Anzeiger 16, 64-65.
| Google Scholar |
Kerby J, Post E (2013) Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120484.
| Crossref | Google Scholar |
König A, Hudler M, Dahl S-A, Bolduan C, Brugger D, Windisch W (2020) Response of roe deer (Capreolus capreolus) to seasonal and local changes in dietary energy content and quality. Animal Production Science 60(10), 1315-1325.
| Crossref | Google Scholar |
Kourkgy C, Garel M, Appolinaire J, Loison A, Toïgo C (2016) Onset of autumn shapes the timing of birth in Pyrenean chamois more than onset of spring. Journal of Animal Ecology 85(2), 581-590.
| Crossref | Google Scholar | PubMed |
Lambert RT, Ashworth CJ, Beattie L, Gebbie FE, Hutchinson JS, Kyle DJ, Racey PA (2001) Temporal changes in reproductive hormones and conceptus–endometrial interactions during embryonic diapause and reactivation of the blastocyst in European roe deer (Capreolus capreolus). Reproduction 121(6), 863-871.
| Crossref | Google Scholar |
Lengwinat T, Meyer HHD (1996) Investigations of BrdU incorporation in roe deer blastocysts in vitro. Animal Reproduction Science 45, 103-107.
| Crossref | Google Scholar |
Lindenmayer DB, Lavery T, Scheele BC (2022) Why we need to invest in large-scale, long-term monitoring programs in landscape ecology and conservation biology. Current Landscape Ecology Reports 7, 137-146.
| Crossref | Google Scholar |
Lopes FL, Desmarais JA, Murphy BD (2004) Embryonic diapause and its regulation. Reproduction 128, 669-678.
| Crossref | Google Scholar | PubMed |
Mall FP (1907) On measuring human embryos. The Anatomical Record 1, 129-140.
| Crossref | Google Scholar |
Marrot P, Charmantier A, Blondel J, Garant D (2018) Current spring warming as a driver of selection on reproductive timing in a wild passerine. Journal of Animal Ecology 87, 754-764.
| Crossref | Google Scholar | PubMed |
Mauget C, Mauget R, Sempéré A (1997) Metabolic rate in female European roe deer (Capreolus capreolus): incidence of reproduction. Canadian Journal of Zoology 75, 731-739.
| Crossref | Google Scholar |
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397, 659.
| Crossref | Google Scholar |
Merkle JA, Monteith KL, Aikens EO, Hayes MM, Hersey KR, Middleton AD, Oates BA, Sawyer H, Scurlock BM, Kauffman MJ (2016) Large herbivores surf waves of green-up during spring. Proceedings of the Royal Society B: Biological Sciences 283, 20160456.
| Crossref | Google Scholar |
Mitchell B (1967) Growth layers in dental cement for determining the age of red deer (Cervus elaphus L.). Journal of Animal Ecology 36(2), 279-293.
| Crossref | Google Scholar |
Moyes K, Nussey DH, Clements MN, Guiness FE, Morris A, Morris S, Pemberton JM, Kruuk LEB, Clutton-Brock TH (2011) Advancing breeding phenology in response to environmental change in a wild red deer population. Global Change Biology 17, 2455-2469.
| Crossref | Google Scholar |
Murray DL, Hussey KF, Finnegan LA, et al. (2012) Assessment of the status and viability of a population of moose (Alces alces) at its southern range limit in Ontario. Canadian Journal of Zoology 90, 422-434.
| Crossref | Google Scholar |
Mysterud A, Røed KH, Holand Ø, Yoccoz NG, Nieminen M (2009) Age-related gestation length adjustment in a large iteroparous mammal at northern latitude. Journal of Animal Ecology 78, 1002-1006.
| Crossref | Google Scholar | PubMed |
Neumann W, Singh NJ, Stenbacka F, Malmsten J, Wallin K, Ball JP, Ericsson G (2020) Divergence in parturition timing and vegetation onset in a large herbivore-differences along a latitudinal gradient. Biology Letters 16, 20200044.
| Crossref | Google Scholar | PubMed |
Paoli A, Weladji RB, Holand Ø, Kumpula J (2018) Winter and spring climatic conditions influence timing and synchrony of calving in reindeer. PLoS ONE 13(4), e0195603.
| Crossref | Google Scholar |
Peláez M, San Miguel A, Rodríguez-Vigal C, Perea R (2017) Climate, female traits and population features as drivers of breeding timing in Mediterranean red deer populations. Integrative Zoology 12(5), 396-408.
| Crossref | Google Scholar | PubMed |
Pettorelli N, Mysterud A, Yoccoz NG, Langvatn R, Stenseth NC (2005) Importance of climatological downscaling and plant phenology for red deer in heterogeneous landscapes. Proceedings of the Royal Society B: Biological Sciences 272, 2357-2364.
| Crossref | Google Scholar |
Pettorelli N, Pelletier F, Hardenberg Av, Festa-Bianchet M, Côté SD (2007) Early onset of vegetation growth vs. rapid green-up: impacts on juvenile mountain ungulates. Ecology 88, 381-390.
| Crossref | Google Scholar | PubMed |
Plard F, Gaillard J-M, Bonenfant C, Hewison AJM, Delorme D, Cargnelutti B, Kjellander P, Nilsen EB, Coulson T (2013) Parturition date for a given female is highly repeatable within five roe deer populations. Biology Letters 9, 20120841.
| Crossref | Google Scholar | PubMed |
Plard F, Gaillard J-M, Coulson T, Hewison AJM, Delorme D, Warnant C, Bonenfant C (2014a) Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biology 12, e1001828.
| Crossref | Google Scholar | PubMed |
Plard F, Gaillard J-M, Coulson T, Hewison AJM, Delorme D, Warnant C, Nilsen EB, Bonenfant C (2014b) Long-lived and heavier females give birth earlier in roe deer. Ecography 37, 241-249.
| Crossref | Google Scholar |
Post E, Forchhammer MC (2008) Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 2367-2373.
| Crossref | Google Scholar |
Prell H (1938) Die Tragzeit des Rehes. Züchtungskunde 13, 325-345.
| Google Scholar |
Ptak GE, Tacconi E, Czernik M, Toschi P, Modlinski JA, Loi P (2012) Embryonic diapause is conserved across mammals. PLoS ONE 7(3), e33027.
| Crossref | Google Scholar |
Rehnus M, Peláez M, Bollmann K (2020) Advancing plant phenology causes an increasing trophic mismatch in an income breeder across a wide elevational range. Ecosphere 11, e03144.
| Crossref | Google Scholar |
Rieck W (1955) Die Setzzeit bei Reh-, Rot-, und Damwild in Mitteleuropa. Zeitschrift für Jagdwissenschaft 1(2), 69-75.
| Crossref | Google Scholar |
Ross MV, Alisauskas RT, Douglas DC, Kellett DK (2017) Decadal declines in avian herbivore reproduction: density-dependent nutrition and phenological mismatch in the Arctic. Ecology 98, 1869-1883.
| Crossref | Google Scholar | PubMed |
Short RV, Mann T (1966) The sexual cycle of a seasonally breeding mammal, the roe buck (Capreolus capreolus). Journal of Reproduction and Fertility 12, 337-351.
| Crossref | Google Scholar |
Stieve H (1950) Anatomisch-biologische Untersuchungen über die Fortpflanzungstätigkeit des europäischen Rehes (Capreolus capreolus). Zeitschrift für Mikroskopisch-Anatomische Forschung 55, 427-530.
| Google Scholar |
Strandgaard H (1972) An investigation of Corpora lutea, embryonic development, and time of birth of roe deer (Capreolus capreolus) in Denmark. Danish Review of Game Biology 6(7), 1-22.
| Google Scholar |
Stubbe C, Stubbe M, Stubbe I (1981) Zur Reproduktion der Rehwildpopulation – Capreolus c. capreolus (L., 1758) – des Wildforschungsgebietes Hakel. Hercynia N. F 19(1), 97-109.
| Crossref | Google Scholar |
van Der Weijden VA, Bick JT, Bauersachs S, Rüegg AB, Hildebrandt TB, Goeritz F, Jewgenow K, Giesbertz P, Daniel H, Derisoud E, Chavatte-Palmer P, Bruckmaier RM, Drews B, Ulbrich SE (2021) Amino acids activate mTORC1 to release roe deer embryos from decelerated proliferation during diapause. Proceedings of the National Academy of Sciences 118, e2100500118.
| Crossref | Google Scholar |
Visser ME, Noordwijk AJv, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society of London. Series B: Biological Sciences 265, 1867-1870.
| Crossref | Google Scholar |
Wade-Smith J, Richmond ME, Mead RA, Taylor H (1980) Hormonal and gestational evidence for delayed implantation in the striped skunk, Mephitis mephitis. General and Comparative Endocrinology 42, 509-515.
| Crossref | Google Scholar | PubMed |
Wandeler AI (1975) Die Fortpflanzungsleistung des Rehes (Capreolus capreolus L.) im Berner Mittelland. Jahrbuch des Naturhistorischen Museums Bern 5, 245-296.
| Google Scholar |
Williams CT, Klaassen M, Barnes BM, Buck CL, Arnold W, Giroud S, Vetter SG, Ruf T (2017) Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160250.
| Crossref | Google Scholar |