Shifting agriculture and a depleting aquifer: implications of row-crop farming on mule deer population performance
Levi J. Heffelfinger A * , David G. Hewitt A , Randy W. DeYoung A , Timothy E. Fulbright A , Louis A. Harveson B , Warren C. Conway C and Shawn S. Gray D
A * , David G. Hewitt A , Randy W. DeYoung A , Timothy E. Fulbright A , Louis A. Harveson B , Warren C. Conway C and Shawn S. Gray D
A Caesar Kleberg Wildlife Research Institute, Texas A&M University – Kingsville, Kingsville, TX, USA.
B Borderlands Research Institute, Sul Ross State University, Alpine, TX, USA.
C Department of Natural Resources Management, Texas Tech University, Lubbock, TX, USA.
D Texas Parks and Wildlife Department, Alpine, TX, USA.
Animal Production Science 63(16) 1633-1647 https://doi.org/10.1071/AN22408
Submitted: 2 November 2022 Accepted: 15 March 2023 Published: 11 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Conversion of native vegetation to cropland is one of the most widespread anthropogenic landscape alterations, particularly in the Great Plains region of the United States. Mule deer occur throughout the Great Plains; however, it is the south-eastern edge of their geographical distribution, and few populations coincide with dense cropland. The rapidly depleting Ogallala Aquifer supplies irrigation to row-crops throughout the region, which will likely shift towards dryland agricultural practices in the near future.
Aims: We sought to understand how cropland use influences morphology, body condition indices, reproductive output, and survival of free-ranging mule deer.
Methods: We accumulated a multi-year, longitudinal dataset of movement and morphology for 146 mule deer in the Texas Panhandle. We linked seasonal cropland use with observed morphology, body condition metrics, and reproductive output via linear mixed-effect modelling and assessed the influence of cropland on annual survival by using Cox proportional hazard models.
Key results: Mule deer that did not use cropland at any time during the year exhibited morphological and nutritional indices similar to those that did; except body-fat percentage being greater for mature (≥4-year-old) males that used cropland. Further, cropland use did not predict survival probability. Analyses of cropland use during seasons defined by life-stage showed context-dependent nutritional benefits. Use of cropland during winter following reproduction demonstrated an increase in young (≤3-year-old) male antler size and body mass and summer crop use increased body condition for all males. Female mule deer that utilised cropland before pregnancy had increased probability of successful reproduction, demonstrating a potential capital investment strategy in reproduction.
Conclusions: Cropland does not limit morphology or survival of mule deer; however, additive use of row-crops can provide a nutritional buffer and enhanced reproductive output for individuals that choose to utilise it.
Implications: Our study demonstrates important population-level interactions with the environment for a species near the extent of their geographical distribution. Conversion of row-crop farming from aquifer depletion or climate shifts may not diminish mule deer populations, but these changes may alter specific habitat-nutritional health relationships that can influence population performance and future conservation efforts.
Keywords: body condition, cropland, geographic distribution, Great Plains, morphology, Odocoileus hemionus, Ogallala Aquifer, survival.
Introduction
Anthropogenic landscape alterations impose population-level consequences on wildlife. As the human population continues to increase, the human footprint follows suit, and now nearly half of the global land cover is human altered in some capacity (Vitousek et al. 1986; Foley et al. 2005). Furthermore, increasing food and fibre demands induce stress on row-crop production, which is often limited by space and resources (de Fraiture and Wichelns 2010; Foley et al. 2011). The conversion of native ecological communities to row-crop farming is one of the most dramatic anthropogenic landscape alterations in the modern era (Federico 2005; Foley et al. 2005), and could be argued as a threat to terrestrial biodiversity (Pereira et al. 2010). Balancing the wellbeing of the human population with biodiversity conservation is difficult in the face of societal and political pressures (Cunningham et al. 2013). Understanding the interaction between row-crop farming and native wildlife populations will support better integration of conservation planning and land management.
Conversion of native rangeland to cropland is extensive in the Great Plains region of the United States. In the southern Great Plains, irrigation is necessary for many crops and irrigation water comes primarily from the Ogallala Aquifer. Greater than 90% of the water extracted from the Ogallala Aquifer is used for industrialised row-crop farming, and this region accounts for ~30% of the total crop and animal production for the United States (Maupin and Barber 2005; Cano et al. 2018). The Ogallala Aquifer does not easily recharge and is rapidly depleting at a rate of nearly 20 km3 per year, a rate that may accelerate under climate-change predictions (Rosenberg et al. 1999; Scanlon et al. 2012; Lauffenburger et al. 2018; Nozari et al. 2022). More recently, the diminishing water availability coupled with drought has negatively affected row-crop output and created dust storm events akin to the 1930s Dust Bowl (Stewart et al. 2010). Further, the southern region of the Ogallala Aquifer in northern Texas is depleting at rates that indicate a potential loss of available water within 20 years (Scanlon et al. 2012). Many landowners are forced to alter land use to dryland practices or switch to crops that require less irrigation (Cano et al. 2018; Deines et al. 2020). Greater human row-crop demand juxtaposed with water limitation in this region has implications for how the landscape may alter in the near future, directly influencing wildlife species that occupy it.
Cropland can create a patchy landscape where crops act as a potential source of higher nutrition in some seasons or as a reduction in available nutrition by replacing native forage with unpalatable crops (Newton 2004). Although row-crop farming can provide forage benefits, it can have negative consequences for herbivores if fields are fallow at crucial times or the nutrients provided do not meet the animals’ needs. For example, some bird species have high nutritional demands while raising altricial young and large-scale conversions of rangeland to cropland can have negative consequences on recruitment of young (Westmoreland et al. 1986; Pruitt et al. 2008). However, cropland can have positive influences on the population performance of other species. Cropland can increase habitat for species of small mammals and birds by introducing a heterogeneous element to the landscape (Hinsley and Bellamy 2000; Michel et al. 2006). Additionally, row-crop farming typically increases the overall nutritional value of the landscape, at least seasonally, and thus can have a positive influence on large ruminants such as elk (Cervus elaphus), white-tailed deer (Odocoileus virginianus), and pronghorn (Antilocapra americana), if nutritious crops are available during energetically demanding periods of the life cycle (O’Gara and Yoakum 2004; Brook 2009; Dechen Quinn et al. 2013).
Mule deer (Odocoileus hemionus) occur throughout most of western North America, including the Great Plains region of the United States. Mule deer are adaptable to widely disparate climatic conditions and vegetation communities (Wallmo 1981; Heffelfinger 2006), which can be valuable in evaluating population responses to many different landscape-level factors. Population performance is directly linked to demographic and individual animal parameters such as adult survival, reproduction, accumulated body fat, and recruitment of young into the population. Population performance of large mammals, particularly herbivores, is influenced by climate, forage quality and quantity, predation, human interactions, density dependence, and habitat fragmentation (Sinclair and Krebs 2002; Sinclair et al. 2003; Parker et al. 2009; Heffelfinger et al. 2018). Adult mule deer exhibit high and stable survival rates (Bishop et al. 2009; Hurley et al. 2011; Bender et al. 2012; Monteith et al. 2014). Thus, juvenile recruitment is typically the most important measure of population performance (Gaillard et al. 1998). If landscape alteration via cropland changes the ability of mule deer to acquire necessary resources, the inability to maintain daily nutrient intake or a proper level of endogenous reserves (body fat) could result in a drop in vital rates, with profound effects on population performance. Further, throughout the geographic range of mule deer, there are few instances where the species coincides with areas dominated by row-crop farming (Wallmo 1981; Heffelfinger 2006). Cropland abundance at the eastern edge of mule deer occurrence in the southern Great Plains may serve as a contributing factor limiting range expansion.
Large herbivores, such as mule deer, generally exhibit slow-paced life-history strategies, and timing of nutritional limitations vary by sex. Late-gestation and lactation are the most energetically and nutritionally demanding periods throughout the life cycle of a female mule deer (Barboza et al. 2009; Parker et al. 2009). Females may forego investment in current reproduction when they are in poor nutritional condition and instead use ingested energy to meet maintenance needs and build reserves for future reproductive output (Clutton-Brock et al. 1983). Therefore, female mule deer must nutritionally balance current reproductive output versus future investment where tradeoffs may occur within the annual reproductive cycle (Stearns 1989; Heffelfinger et al. 2020), all of which fluctuate with resource availability (Monteith et al. 2014). The outcome of these tradeoffs may vary depending on whether females occupy rangeland or rangeland–cropland interfaces. Mule deer males do not experience the nutritional drain of offspring development and provisioning. However, male mule deer face nutritional constraints during antler growth and when acquiring energy stores to support mating activity (Heffelfinger 2006; Foley et al. 2018). Nutritional limitations and acquisition could vary drastically depending on mule deer interaction with cropland landscapes. The ability of males to enhance their morphology (i.e. grow larger antlers) or elevate their nutritional state can increase lifetime fitness and potentially influence overall population performance (Morina et al. 2018; Bowyer et al. 2020).
We tested the link between row-crop farming land cover and population health metrics of mule deer at their south-eastern range limit in the southern Great Plains, an area threatened by the depletion of the Ogallala Aquifer. By combining a large dataset of longitudinal movement and morphology metrics, we explored the relationships between space use of cropland and subsequent nutritional and reproductive measures. We hypothesise that individuals that use cropland versus those that do not will have greater body condition and size indices. Further, of those individuals that use cropland, we hypothesise that an additive effect will be present that may provide a nutritional buffer or enhancement. We posit that the large energy demands of reproduction in ungulates will cause an increase in reproductive output as individuals increase use of cropland. Last, evidence suggests that adult survival is not limiting population performance in our area. Thus, we hypothesise that cropland does not influence natural mortality, despite potentially enhancing morphology or providing a nutritional buffer.
Methods
Study site
We selected four representative sites of the southern Great Plains in the Texas Panhandle, the north-western-most region of Texas. Our sites offer a mosaic of differing cropland densities relative to native rangeland exhibited throughout the Texas Panhandle. Generally, the Texas Panhandle is dominated by cropland; however, areas of sandy shortgrass prairie, deciduous shrubland, caprock escarpments, and river drainage systems are scattered throughout the region (Fig. 1). Historic average temperature ranges from a low of −14°C during winter to a high of 33°C during the summer (National Oceanic and Atmospheric Association (NOAA) 2021). Annual precipitation is 54 cm regionally, of which up to 25 cm can be snowfall (National Oceanic and Atmospheric Association (NOAA) 2021). Common grass and forb species found throughout the southern Great Plains are present in our study sites, including, but not limited to, sideoats gramma (Bouteloua curtipendula), blue gramma (Bouteloua gracilis), buffalo grass (Bouteloua dactyloides), big bluestem (Andropogon gerardii), sand verbena (Abronia villosa), bush sunflower (Encelia californica), prairie clovers (Dalea spp.), and scarlet globemallow (Sphaeralcea coccinea; Griffith et al. 2007). The Texas Panhandle does not support many tree species; however, primary woody plant species in our sites were sand plum (Prunus texana), honey mesquite (Prosopis glandulosa), netleaf hackberry (Celtis reticulata), shinnery oak (Quercus havardii), sand sage (Artemesia filifolia), Mohr oak (Quercus mohriana), western soapberry (Sapindus saponaria), western hackberry (Celtis occidentalis), one-seed juniper (Juniperus monosperma), and four-winged saltbush (Atriplex canescens; Griffith et al. 2007). In descending order, primary crop species in the region consist of cotton (Gossypium herbaceum), corn (Zea mays), winter wheat (Triticum aestivum), alfalfa (Medicago sativa), milo or sorghum (Sorghum bicolor), peanuts (Arachis hypogaea), potatoes (Solanum tuberosum), and soybeans (Glycine max). Winter wheat and alfalfa were the only crops available during the winter months, whereas the rest are available during the summer months.
Our first study site was in the south-eastern portion of the Texas Panhandle in the Western Rolling Plains ecoregion off the eastern edge of the Llano Estacado escarpment (Gould 1975). This site is characterised by interspersed cropland among patches of native rangeland with the north-eastern portion of the site exhibiting primarily rolling plains and shrubland. Primary crop species are cotton and winter wheat. Cropland was 14.5% of land cover, with a mean distance to cropland of 2062 m within the area we focused sampling efforts (Fig. 1). The second study site was in the north-eastern portion of the Texas Panhandle along the Canadian River Breaks, a region characterised by riparian corridors along the river. Moving up in elevation to the north, the site has rolling hills of short grass prairie, which eventually lead to steep canyon escarpments. Sitting atop the Canadian River system are areas of dense cropland. Crop species in the region consist of cotton, corn, winter wheat, and milo or sorghum. We focused sampling efforts on the native rangeland within the Canadian River Breaks, resulting in a cropland density of 11.3% land cover, with a mean distance to cropland of 5166 m in the areas where collared deer occupied (Fig. 1). Our third and fourth study sites were in the south-western Texas Panhandle. The third site is characterised by highly dense cropland with interspersed sandy areas where remnant native vegetation communities persist. This area has few trees and is dominated by short-grass prairie species and shrubs. Crop species in the region consist of cotton, corn, winter wheat, alfalfa, and milo or sorghum. Cropland density within our region of interest was 37.0% land cover, with a mean distance to cropland of 603 m (Fig. 1). Last, our fourth study site is dominated by rolling dunes, many of which were vegetated. Primary crop species are cotton, winter wheat, peanuts, and corn. The relative cropland density within this site was 10.4% land cover, with a mean distance to cropland of 2427 m (Fig. 1). Study-site boundaries were defined by buffering a minimum convex polygon surrounding all mule deer locations by the observed upper-third quartile distance between successive GPS locations (Heffelfinger 2021).
Data collection
During October 2015–2019, we captured adult (>1 year old) male and female mule deer via the helicopter net-gun technique and flew individuals to a central processing station (Krausman et al. 1985; Heffelfinger 2021). We recorded a suite of morphometric attributes for each individual, including body mass, age, percentage body fat, antler size, and lactation status. Body mass was measured with a digital platform scale to the nearest 0.1 kg. We estimated the age of each individual via tooth wear and replacement (Severinghaus 1949). We also used standard protocols validated and developed for mule deer for determining nutritional condition of each animal (Monteith et al. 2013). We used palpation of the rump, ribs, and withers to assign an overall body condition score ranging from 1 (emaciated) to 5 (obese) for each animal (Gerhart et al. 1996; Stephenson et al. 2002). Further, we measured maximum rump fat thickness via ultrasonography. Rump fat thickness was used in combination with body mass and body condition score to calculate ingesta-free body fat (IFBF; Cook et al. 2007, 2010; Monteith et al. 2013). Because capture efforts occurred in October, IFBF calculations were not influenced by inclusion of fetus and other products of conceptus (Cook et al. 2010). Antler measurements followed the measurement criteria from the Boone and Crockett Club records book system (Nesbitt et al. 2009). Lactation status was used as an indicator of a female successfully reproducing during the summer, immediately prior to autumn handling events. A female was classified as lactating if milk could be expressed from a teat or if her udder was noticeably swollen.
On initial capture, adults were fitted with GPS radio collars programmed to collect one location every 2 h, emit a very high-frequency (VHF) signal, and increase the VHF signal pulse-rate if the deer died (GPS3300, and GPS6000, Lotek Wireless, Ontario, Canada). Collaring efforts were staggered, where each study site had active collars on individuals for a maximum of 2 years. Following the initial capture and after 1 year of monitoring in each site, we performed an additional capture to download GPS location data, collect all aforementioned measurements, and add individuals to our sample to replace mortalities or failed collars. A third and final capture event took place after 2 years in each site to collect a final suite of measurements and remove GPS collars. All individuals were monitored bi-monthly for survival status via radio telemetry. Assuming a given individual survived the entirety of the study, our data collection resulted in three measurement events and 2 years of location data in between respective handling occasions. All movement data were screened for locations associated with mortality by using either known mortality events or when step lengths never exceeded 25 m for a 48-h timeline. Further, we also screened locations that lacked precision confidence (HDOP > 10) or speeds exceeding 5 km per hour (Dussault et al. 2001).
We monitored row-crop fields monthly to create spatio-temporal availability of active cropland (Heffelfinger 2021). Briefly, monitored fields were spatially referenced and assigned the monthly crop species in ArcGIS (ArcGIS 10.8.1, ESRI, Redlands, CA, USA). Row-crop fields that were outside of our field sampling regime, but were within the study areas, were also georeferenced. Each monthly crop layer was then overlaid onto native land-cover data updated annually from the Texas Ecological Mapping Systems program (Elliott et al. 2014). We then summarised all active crop availability into a general ‘cropland’ category. Location data were overlaid onto appropriate monthly spatial layers and values were extracted using the ‘sf’ and ‘amt’ packages in R ver. 4.1.1 (R Core Team 2022). We divided the number of locations in cropland by the total number of locations collected for each individual to create a ‘proportional cropland use’ metric. Proportional cropland use is analogous to the proportion of time an individual used cropland (Heffelfinger 2021).
Cropland use versus no cropland use
To assess how time spent accessing cropland forage sources influenced morphology and health metrics, we developed linear mixed-effects models. First, we assigned a categorical variable to individuals who had used cropland in a given year versus those that did not (Fig. 2a). We assigned an age class to each adult where all individuals ≤3 years old were assigned a ‘young’ classification and those ≥4 years old were assigned a ‘mature’ classification at the time of capture in autumn. We then combined morphological data with each year’s preceding crop use for each individual. For example, a male classified as 2 years old would be evaluated using the preceding year’s crop use when the animal was ≥1 year old. We then developed separate linear mixed-effect models for each morphological metric of interest, including body mass, IFBF, and antler size. For males, each morphological metric was predicted by fixed effects, including our dichotomous crop-use category, age class, and an interaction between age class and crop group. However, females are influenced nutritionally by reproductive status more so than age (Barboza et al. 2009). Specifically, females cease structural growth sooner in life than do males, to facilitate energy allocation towards reproductive output. Therefore, our modelling for females included both age class and lactation status as a fixed effect and inclusion as an interaction variable with our dichotomous crop-use category. Further, to account for multiple observations from individuals and our several study sites, we included a nested random effect in each model where the individual identification number was nested within the study site. We then assessed pairwise comparisons between crop use and age class or lactation status groups and calculated marginal means using the ‘emmeans’ package (Lenth 2021) in R ver. 4.1.1 (R Core Team 2022) using Satterthwaite’s approximation of degrees of freedom (Gałecki and Burzykowski 2013).
Additive use of cropland and morphology
We separated the annual life cycle of each sex into relevant biological periods to evaluate how relevant seasonal use of cropland may influence deer morphology and survival. Peak conception or reproductive attempts in the region are at the end of December (i.e. rut; Pittman and Bone 1987). To verify this estimate, we calculated daily step length for males and visually inspected population-level means to identify movement peaks when males are actively searching for receptive females. Further, we visually inspected daily step length for females during the likely summer parturition period. Reductions in female movement during this time are indicative of parturition (DeMars et al. 2013; Nicholson et al. 2019). Peak male movement was during late December, which corresponded to a reduction in female movement roughly 203 days later (average gestation length; Robinette et al. 1973), indicating that late December was likely to be the peak conception period. We censored movement data during October to avoid bias of habitat use that may have been influenced by capture (Dechen Quinn et al. 2012). Therefore, following October captures, annual life-cycle time-frames for males were as follows; November‒January = rut, February‒April = post-rut, May‒July = antlerogenesis, and August‒September = rut preparation. For females, annual life-cycle periods were November‒December = pre-reproduction, January‒March = early gestation, April‒June = late gestation, and July‒September = provisioning young. We then re-calculated proportional cropland use for each individual within each respective annual life-cycle period (Fig. 2b, c).
To assess how proportional cropland use during each annual life cycle stage may influence morphology and health metrics, we developed similar linear mixed-effect models as mentioned above. Further, we subsampled our dataset to include only individuals that used cropland at some point during the preceding year before each capture event. By doing this, we sought to address our hypothesis that additive cropland use may describe the relationship between cropland and morphology more so than a dichotomous assignment of cropland use versus no cropland use. Our life-stage-specific measures of proportional cropland use (early gestation, late gestation, etc.) generally violated multicollinearity assumptions to be included in the same model. Therefore, we ran separate linear mixed-effects models for each life-stage and sex. Our response variables of interest included body mass, IFBF, and antler size (males only). Similar to above, morphology was predicted by proportional cropland use during each life-stage, age class, or lactation status (for males or females respectively) and an interaction between the two. We also included a nested random effect in each model where the individual identification number was nested within the study site.
Additive use of cropland and reproductive output
To assess how cropland influences the probability of successful reproduction, we ran generalised linear mixed-effects models. Successful reproduction (lactating females) was coded as 1 and non-reproductive females were coded as 0. Similar to above, we assessed proportional crop use during each annual life stage separately and included nested random effects of individual identifiers within study site.
Cropland use and survival
We estimated annual survival rates using a modified Kaplan–Meier curve (Kaplan and Meier 1958; Pollock et al. 1989) and the ‘survival’ package in R ver. 4.1.1 (Therneau 2021; R Core Team 2022). We calculated annual survival rates using estimates of individuals that did not use cropland, mean cropland use, and the maximum observed cropland use. We also developed Cox proportional hazards regression models to assess the relationship between cropland use and survival (Cox 1972; Fan and Li 2002). We additively modelled effects on mortality risk by proportional cropland use, sex, age class, lactation status, and study site. We also tested all combinations of predictors and included all possible interactions. Many individuals were tracked for 2 years, which resulted in >1 entry in the encounter occasion dataset for these individuals. We therefore clustered by individual to obtain robust standard errors for parameter estimates, which accounts for the lack of independent observations (Therneau and Grambsch 2000; Blackburn et al. 2021). To assess how cropland influences natural mortality rates, we censored all adults that succumbed to legal harvest.
Results
From 2015 to 2019, we captured, collared, and monitored 77 adult female and 69 adult male mule deer in the Texas Panhandle. Our data set totalled 480 212 GPS locations for adult females and 353 510 locations for adult males. Our data were reliant on an individual surviving ≥1 year or collars properly functioning to obtain morphological and health metrics following each respective year’s location-data collection. Thus, our dataset resulted in 188 unique deer–year combinations of associated space use and morphological measurements. Of these 188 deer–year combinations, 67 demonstrated no use of cropland throughout the annual cycle (Fig. 2a). For individuals that accessed cropland in a given life-history stage, overall proportional cropland use varied and population means generally ranged between 10% and 21% use in males and 9% to 41% in females (Fig. 2b, c). Greater use during winter coincides with winter wheat and alfalfa prevalence and summer use coincides with primarily cotton and corn (Heffelfinger 2021).
Cropland use versus no cropland use
Our dichotomous testing of individuals that used cropland in a given year versus those that did not use cropland showed few biologically significant benefits for males. Mature males that used cropland had an average of 2.5% greater IFBF (P = 0.03) than did those that did not, but this relationship was not apparent for young males (P = 0.19; Fig. 3). Body mass and antler size were similar between males that used cropland and those that did not for both age classes (Fig. 3). Finally, health metrics (IFBF and body mass) were similar between females that used cropland versus those that did not (Fig. 3).
Additive use of cropland and morphology
Our linear mixed-effects models of additive cropland use showed several annual life-cycle stage-dependent relationships. Regardless of age class, IFBF increased by 1.8% for every 10% increase in proportional crop use by males during the May‒July summer antler-growing period (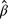 = 18.45 95% CI = 0.45–36.27; Fig. 4a). There was no additive relationship between cropland use and IFBF for males during the pre-reproduction, rut, and post-rut time-frames. Young males demonstrated a 7.3 kg greater body mass (
= 18.45 95% CI = 0.45–36.27; Fig. 4a). There was no additive relationship between cropland use and IFBF for males during the pre-reproduction, rut, and post-rut time-frames. Young males demonstrated a 7.3 kg greater body mass (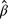 = 72.91 95% CI = 0.75–146.6) and 32 cm greater antler size (
= 72.91 95% CI = 0.75–146.6) and 32 cm greater antler size (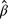 = 320 95% CI = 23–663) for every 10% increase in cropland use during the February‒April post-rut maintenance period, whereas there was no similar relationship for mature males (Fig. 4b, c). There was no additive relationship between cropland use and body mass for males during the pre-reproduction, rut, and antlerogenesis time-frames.
= 320 95% CI = 23–663) for every 10% increase in cropland use during the February‒April post-rut maintenance period, whereas there was no similar relationship for mature males (Fig. 4b, c). There was no additive relationship between cropland use and body mass for males during the pre-reproduction, rut, and antlerogenesis time-frames.
Both IFBF (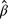 = −3.40 95% CI = −10.54 to 3.75) and body mass (
= −3.40 95% CI = −10.54 to 3.75) and body mass (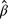 = −16.3 95% CI = −27.82 to 4.74) had a negative relationship with pre-reproduction cropland use for reproductive females, whereas non-reproductive females demonstrated no relationship (Fig. 5a, b). There was no additive relationship between cropland use and body mass or IFBF for females during early gestation, late gestation, or provisioning young time-frames.
= −16.3 95% CI = −27.82 to 4.74) had a negative relationship with pre-reproduction cropland use for reproductive females, whereas non-reproductive females demonstrated no relationship (Fig. 5a, b). There was no additive relationship between cropland use and body mass or IFBF for females during early gestation, late gestation, or provisioning young time-frames.
Additive use of cropland and reproductive output
Our generalised linear mixed-effects models predicting reproductive status as a function of cropland use showed that pre-reproduction use had a positive influence on the probability of producing young later in the year (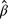 = 4.06 95% CI = 0.09–8.22) and early gestational crop use demonstrated a moderately positive relationship (
= 4.06 95% CI = 0.09–8.22) and early gestational crop use demonstrated a moderately positive relationship (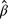 = 3.27 95% CI = −1.33 to 7.88; Fig. 6a, b). Thereafter, cropland use diminished, and we found no relationship between cropland use and reproductive success during late gestation (
= 3.27 95% CI = −1.33 to 7.88; Fig. 6a, b). Thereafter, cropland use diminished, and we found no relationship between cropland use and reproductive success during late gestation (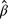 = 4.37 95% CI = −6.23 to 14.97) and while provisioning young life stages (
= 4.37 95% CI = −6.23 to 14.97) and while provisioning young life stages (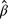 = −5.31 95% CI = −4.62 to 13.36; Fig. 6c, d).
= −5.31 95% CI = −4.62 to 13.36; Fig. 6c, d).
Cropland use and survival
We monitored 146 unique adult mule deer for up to 2 years, resulting in 223 unique deer–year combinations to assess annual survival rates. Overall, we observed 14 natural mortality events. Adult survival modelling identified no relationship between cropland use and survival estimates among all predictor variables (proportional crop use, sex, age class, lactation status, and study site). The high and stable annual adult survival estimate was 93.3% (95% CI = 90.1–96.8; Fig. 7).
Discussion
Anthropogenically altered landscapes can shift species’ behaviour and resources (Pereira et al. 2010). Row-crop farming may replace native vegetation communities, which could have implications for the nutritional status of large herbivores. Here, we have demonstrated that a cropland-dominated landscape can influence population parameters of mule deer. Not all mule deer used row-crops as a source of forage and there was little difference in morphological or body condition parameters for those that do. Even for those deer that do access cropland forage sources, the proportional time spent in crop fields and therefore the time available for foraging in these fields is small on the basis of the population mean. Albeit, a few individuals exhibited a substantial amount of time in crop fields during certain times of the year; however, without direct behavioural observations, we cannot deduce the time was spent foraging. Nonetheless, at the population scale, short-term access during important biological periods to a potentially greater nutritional source benefits mule deer by providing a nutritive buffer and enhancing reproductive output. If cropland densities or crop species availability shifts as a result of a rapidly declining aquifer (or other market driven landscape changes), these relationships shed light on factors that may become limiting to population performance of this endemic species at the fringe of their distribution in the southern Great Plains.
Our hypothesis that individuals that used cropland versus those that did not would demonstrate greater morphology and body condition metrics, was not fully supported, apart from mature male IFBF. The similarity between health metrics (body-fat percentage, body mass, and antler size) between both crop use groups suggested that, from a broad perspective, crops did not measurably improve the nutritional status of deer in this population. However, the assumption is that time spent in cropland is directly correlative to forage intake. Perhaps cropland use facilitates other necessities, such as open areas for resting or predator avoidance. Furthermore, most individuals may be able to meet their energetic requirements with native forage sources and no cropland use. For example, values of body-fat percentage reported here are greater than are those of other mule deer populations that have little to no access to cropland (Bender et al. 2007; Bergman et al. 2014, 2018; Monteith et al. 2014; Merems et al. 2020). Row-crop farming and mule deer occurrence rarely overlap throughout the species’ geographic range. Perhaps mule deer rely more heavily and have a greater adaptation to native vegetative communities as opposed to relatively new sources of forage on the landscape. Cropland availability may not be the limiting variable at the landscape level or may pose areas of greater risk. Nonetheless, cropland may be a source of additive nutrition for those deer that accessed crops during certain biological stages more than others.
In northern latitudes, large herbivores often experience dramatic seasonal changes in food availability that may cause a nutritional bottleneck during winter (Hobbs 1989; Parker et al. 2005; Bishop et al. 2009; Monteith et al. 2013). Individuals allocate a large percentage of their energy budget towards maintenance during these nutritionally limiting periods and can no longer allocate energetic stores towards growth and reproduction (Bårdsen et al. 2008, 2010; Monteith et al. 2014). However, in the southern Great Plains, winters are generally mild and cropland areas often contain only winter wheat (T. aestivum) as a form of cover crop and alfalfa (M. sativa). Additive use of cropland may provide a means to enhance an individual’s energetic stores, thereby providing a buffer against future nutritional limitations that deer may experience in the southern Great Plains (e.g. drought, crop rotation, landownership shifts, dryland farming practices). Winter wheat is in its early phenological growth stage in January–March and provides a protein and energy source that exceeds native rangeland forage (Lampman 2019). Furthermore, mule deer in this system are attracted to winter wheat (Heffelfinger 2021). The early phenological growth stages of winter wheat coincide with early gestation for females and post-rut for males which has an additive improvement on reproduction and morphology, respectively. Therefore, winter wheat availability may act as the additive enhancement unavailable to mule deer in other populations.
We show that males in this system can enhance both antler size and nutritional condition by accessing these crops, particularly during post-rut after the nutritionally demanding period of reproduction. For instance, young males exhibited greater body mass and antler size when accessing cropland following rut. Young males are particularly responsive to greater nutrient availability (Gao et al. 2002), and enhancement of these nutritional signals can benefit fitness measures and ontogeny in early life. For example, ungulates have a complex social structure, and the reproductive period is energetically expensive where individuals’ success and performance is influenced by age, dominance, antler size, and nutritional status or expenditure (Yoccoz et al. 2002; DeYoung et al. 2006; Foley et al. 2018; Morina et al. 2018; Bowyer et al. 2020). Young males are often less competitive for breeding opportunities because they invest more energy in body growth and less in antler growth and fat stores (Yoccoz et al. 2002; Airst and Lingle 2020). However, young males still seek potential mates and sire up to 30% of the offspring (Mysterud et al. 2004, 2008; DeYoung et al. 2009; Foley et al. 2018). Conversely, young males must balance future and current reproductive investment where energy is partitioned between maximising growth, which benefits future efforts, and current reproductive efforts to maximise lifetime fitness (Stearns 1992). Therefore, an enhancement of body mass and antler size resulting from post-rut cropland use may serve both as energetic recovery from early life reproductive attempts, thereby enabling young males to invest in current reproduction while allowing nutritional acquisition for growth, which benefits future reproduction.
Summer often serves as a period of native forage abundance, which can aid in building endogenous reserves before the next reproductive cycle or in replenishing stores from previous reproductive attempts (Mautz 1978; Bårdsen and Tveraa 2012). Further, in northern latitudes, spring and summer green-up provides access to nutrition to replenish body mass and fat loss over winter (Parker et al. 1993) During winter, we demonstrated that crop availability can elevate individual nutritional plane, unlike in northern environments where winter is a time of poor nutrient availability (Bishop et al. 2009). Summer crop availability may also play an important role in nutritional maintenance in the southern Great Plains. Both young and mature males that additively used crops during late spring and summer (the period of antlerogenesis) had increased body fat during autumn, perhaps from reduced reliance on fat reserves or increased fat deposition during those seasons. Following summer, during the reproductive period, males can lose a substantial amount of weight and energetic reserves (up to 40% body mass) because they alter their time-energy budget from foraging toward mate-search (Warren et al. 1981; Bartoskewitz et al. 2007; Foley et al. 2018). Yearling males tend to not energetically invest in reproduction; however, 2- and 3-year-olds dramatically increase effort, despite the greatest effort occurring at older age classes in white-tailed deer, for example (Foley et al. 2018). Our young age class included individuals in their second and third reproductive seasons, meaning that those individuals were likely to demonstrate a relatively high degree of effort, despite being at a younger age. Beyond native forage sources, summer crop availability can act as an additional source of energetic income or maintenance before a period of fat accumulation immediately before breeding.
Although cropland is not likely to be a limiting factor for overall nutritional maintenance in our system, it may provide excess nutrition to boost overall population performance via reproductive output. Gestation and lactation are the most nutritionally draining periods throughout a female ungulate’s annual life cycle (Barboza et al. 2009; Parker et al. 2009); therefore, state-dependent foraging is necessary to provide nutritional means for current or future development and provisioning of young. Female mule deer rely on immediate nutritional income to facilitate energetic needs (income breeder; Johnstone-Yellin et al. 2009; Tollefson et al. 2010). Like other studies assessing mule deer along the capital–income breeder continuum (Stephens et al. 2009; Monteith et al. 2014), we show that female mule deer that use cropland before conception were more likely to be reproductively successful, demonstrating more of a capital investment strategy. Additionally, our data suggested that greater cropland use during early gestation also positively influences reproduction. Surprisingly, we found no relationship between reproductive success and crop use during late gestation or while provisioning young, which, if it did occur, would support an income breeding strategy. Crop availability during late gestation is generally low and nutritional value of available crop species is generally less than that of native rangeland (Lampman 2019). Further, row-crop fields may not be worth foraging in while provisioning young, as this may increase susceptibility to predation for dependent young. Female mule deer in our system are likely to employ income-breeding mechanisms, but we were unlikely to observe them through our measures of cropland use and the general rotational cropland scheme in our study system. Nonetheless, our hypothesis that cropland would benefit reproductive output was generally supported.
Interestingly, cropland use during the pre-reproductive period by females that successfully reproduced exhibited a slight decrease in body-fat percentage and body mass the following autumn, whereas cropland use pre-reproduction enhanced the probability of successfully producing young. Perhaps females that exhibited greater cropland use pre-reproduction had a greater propensity to produce and raise multiple offspring litters, which would increase nutritional demands during lactation (Johnstone-Yellin et al. 2009). These increased nutritional demands would then negatively influence body-fat percentage and body mass prior to our handling event the following autumn (Barboza et al. 2009; Parker et al. 2009). Using lactation status as a measure of reproductive success provided no information on litter size for each reproductive female. Indeed, additional research is needed to determine the effects of cropland use on litter size.
Mule deer that chose to use cropland as part of their forage acquisition experienced additive nutritional benefits. Males had greater body-fat reserves, body mass, and antler size (dependent on age class) and females had higher reproductive success, but cropland is not likely to be the limited resource in this landscape, as mule deer that did not access crops still exhibited viable health metrics. If excessive land cover by cropland drastically limited population performance, we would have observed reduced survival probability based on cropland use. Furthermore, mule deer in our study area did not use areas of >40% cropland density (Heffelfinger 2021). Perhaps the narrow breadth of cropland use we observed was pre-determined at a grander landscape scale of mule deer occurrence. Nevertheless, row-crop farming may be acting as a nutritional buffer or enhancement source in these populations, where fitness measures can be greater for individuals that use the resource dependent on the annual life stage. We highlight the importance of accounting for spatio-temporal fluctuations in cropland forage availability towards future management and conservation of mule deer.
Depletion of the Ogallala Aquifer will likely shift the land cover makeup and availability of row-crop farming in the near future. Although conversions of row-crop farming to dryland practices may not diminish mule deer populations, these shifts may alter specific habitat–nutritional health relationships that can influence population performance. Our study demonstrated important population-level interactions with the environment for a species at the extent of its geographical distribution. Understanding the complex relationships between anthropogenic land use and wildlife populations will prove crucial for conserving wildlife as cropland policy and land-use regimes change the landscape.
Data availability
Data for this publication will be made available online at the Dryad Digital Repository upon acceptance for publication.
Conflicts of interest
The authors declare that they have no competing interests.
Declaration of funding
This research was funded by the Texas Parks and Wildlife Department as part of Federal Aid in Wildlife Restoration Grant #TX W-157-R-1. Additional direct and in-kind support was also contributed by the Boone and Crockett Club, Mule Deer Foundation, Houston Safari Club, and Mr. Rene Barrientos.
Acknowledgements
We acknowledge Texas Parks and Wildlife Department employees J. Bonner, S. Harryman, J. Hoskins, M. Lockwood, C. Malone, T. Montandon, C. Richardson, C. Ruthven, B. Simpson, D. Wright, and others that supported this research. Additional field support was provided by K. Catter, J. Dykes, J. Fuess, O. Gray, A. Killam, M. Mahurin, A. Menefee, T. Opatz, A. Ryan, A. Veals, J. VonBank, C. Wilson, and others. Previous versions of this paper benefited from comments by A. Foley, J Lombardi, and two anonymous reviewers. This is publication #22-130 of the Caesar Kleberg Wildlife Research Institute.
References
Airst JI, Lingle S (2020) Male size and alternative mating tactics in white-tailed deer and mule deer. Journal of Mammalogy 101, 1231–1243.| Male size and alternative mating tactics in white-tailed deer and mule deer.Crossref | GoogleScholarGoogle Scholar |
Barboza PS, Parker KL, Hume ID (2009) ‘Integrative wildlife nutrition.’ (Springer Science and Business Media: Berlin, Germany)
Bartoskewitz ML, Hewitt DG, Laurenz JC, Pitts JS, Bryant FC (2007) Effect of dietary copper and zinc concentrations on white-tailed deer antler growth, body size, and immune system function. Small Ruminant Research 73, 87–94.
| Effect of dietary copper and zinc concentrations on white-tailed deer antler growth, body size, and immune system function.Crossref | GoogleScholarGoogle Scholar |
Bender LC, Lomas LA, Browning J (2007) Condition, survival, and cause-specific mortality of adult female mule deer in north-central New Mexico. Journal of Wildlife Management 71, 1118–1124.
| Condition, survival, and cause-specific mortality of adult female mule deer in north-central New Mexico.Crossref | GoogleScholarGoogle Scholar |
Bender LC, Hoenes BD, Rodden CL (2012) Factors influencing survival of desert mule deer in the greater San Andres Mountains, New Mexico. Human-Wildlife Interactions 6, 245–260.
Bergman EJ, Doherty PF, Bishop CJ, Wolfe LL, Banulis BA (2014) Herbivore body condition response in altered environments: mule deer and habitat management. PLoS ONE 9, e106374
| Herbivore body condition response in altered environments: mule deer and habitat management.Crossref | GoogleScholarGoogle Scholar |
Bergman EJ, Anderson CR, Bishop CJ, Holland AA, Northrup JM (2018) Variation in ungulate body fat: individual versus temporal effects. The Journal of Wildlife Management 82, 130–137.
| Variation in ungulate body fat: individual versus temporal effects.Crossref | GoogleScholarGoogle Scholar |
Bishop CJ, White GC, Freddy DJ, Watkins BE, Stephenson TR (2009) Effect of enhanced nutrition on mule deer population rate of change. Wildlife Monographs 172, 1–28.
| Effect of enhanced nutrition on mule deer population rate of change.Crossref | GoogleScholarGoogle Scholar |
Blackburn A, Heffelfinger LJ, Veals AM, Tewes ME, Young JH (2021) Cats, cars, and crossings: the consequences of road networks for the conservation of an endangered felid. Global Ecology and Conservation 27, e01582
| Cats, cars, and crossings: the consequences of road networks for the conservation of an endangered felid.Crossref | GoogleScholarGoogle Scholar |
Bowyer RT, McCullough DR, Rachlow JL, Ciuti S, Whiting JC (2020) Evolution of ungulate mating systems: integrating social and environmental factors. Ecology and Evolution 10, 5160–5178.
| Evolution of ungulate mating systems: integrating social and environmental factors.Crossref | GoogleScholarGoogle Scholar |
Brook RK (2009) Historical review of elk–agriculture conflicts in and around Riding Mountain National Park, Manitoba, Canada. Human–Wildlife Conflicts 3, 72–87.
Bårdsen B-J, Tveraa T (2012) Density dependence vs. density independence – linking reproductive allocation to population abundance and vegetation greenness. Journal of Animal Ecology 81, 364–376.
| Density dependence vs. density independence – linking reproductive allocation to population abundance and vegetation greenness.Crossref | GoogleScholarGoogle Scholar |
Bårdsen B-J, Fauchald P, Tveraa T, Langeland K, Yoccoz NG, Ims RA (2008) Experimental evidence of a risk-sensitive reproductive allocation in a long-lived mammal. Ecology 89, 829–837.
| Experimental evidence of a risk-sensitive reproductive allocation in a long-lived mammal.Crossref | GoogleScholarGoogle Scholar |
Bårdsen B-J, Tveraa T, Fauchald P, Langeland K (2010) Observational evidence of risk-sensitive reproductive allocation in a long-lived mammal. Oecologia 162, 627–639.
| Observational evidence of risk-sensitive reproductive allocation in a long-lived mammal.Crossref | GoogleScholarGoogle Scholar |
Cano A, Núñez A, Acosta-Martinez V, Schipanski M, Ghimire R, Rice C, West C (2018) Current knowledge and future research directions to link soil health and water conservation in the Ogallala Aquifer region. Geoderma 328, 109–118.
| Current knowledge and future research directions to link soil health and water conservation in the Ogallala Aquifer region.Crossref | GoogleScholarGoogle Scholar |
Clutton-Brock TH, Guinness FE, Albon SD (1983) The costs of reproduction to red deer hinds. Journal of Animal Ecology 52, 367–383.
| The costs of reproduction to red deer hinds.Crossref | GoogleScholarGoogle Scholar |
Cook RC, Stephenson TR, Myers WL, Cook JG, Shipley LA (2007) Validating predictive models of nutritional condition for mule deer. Journal of Wildlife Management 71, 1934–1943.
| Validating predictive models of nutritional condition for mule deer.Crossref | GoogleScholarGoogle Scholar |
Cook RC, Cook JG, Stephenson TR, Myers WL, Mccorquodale SM, Vales DJ, Irwin LL, Hall PB, Spencer RD, Murphie SL, Schoenecker KA, Miller PJ (2010) Revisions of rump fat and body scoring indices for deer, elk, and moose. Journal of Wildlife Management 74, 880–896.
| Revisions of rump fat and body scoring indices for deer, elk, and moose.Crossref | GoogleScholarGoogle Scholar |
Cox DR (1972) Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological) 34, 187–202.
| Regression models and life-tables.Crossref | GoogleScholarGoogle Scholar |
Cunningham SA, Attwood SJ, Bawa KS, Benton TG, Broadhurst LM, Didham RK, McIntyre S, Perfecto I, Samways MJ, Tscharntke T, Vandermeer J, Villard M-A, Young AG, Lindenmayer DB (2013) To close the yield-gap while saving biodiversity will require multiple locally relevant strategies. Agriculture, Ecosystems & Environment 173, 20–27.
| To close the yield-gap while saving biodiversity will require multiple locally relevant strategies.Crossref | GoogleScholarGoogle Scholar |
de Fraiture C, Wichelns D (2010) Satisfying future water demands for agriculture. Agricultural Water Management 97, 502–511.
| Satisfying future water demands for agriculture.Crossref | GoogleScholarGoogle Scholar |
Dechen Quinn AC, Williams DM, Porter WF (2012) Postcapture movement rates can inform data-censoring protocols for GPS-collared animals. Journal of Mammalogy 93, 456–463.
| Postcapture movement rates can inform data-censoring protocols for GPS-collared animals.Crossref | GoogleScholarGoogle Scholar |
Dechen Quinn AC, Williams DM, Porter WF (2013) Landscape structure influences space use by white-tailed deer. Journal of Mammalogy 94, 398–407.
| Landscape structure influences space use by white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Deines JM, Schipanski ME, Golden B, Zipper SC, Nozari S, Rottler C, Guerrero B, Sharda V (2020) Transitions from irrigated to dryland agriculture in the Ogallala Aquifer: land use suitability and regional economic impacts. Agricultural Water Management 233, 106061
| Transitions from irrigated to dryland agriculture in the Ogallala Aquifer: land use suitability and regional economic impacts.Crossref | GoogleScholarGoogle Scholar |
DeMars CA, Auger-Méthé M, Schlägel UE, Boutin S (2013) Inferring parturition and neonate survival from movement patterns of female ungulates: a case study using woodland caribou. Ecology and Evolution 3, 4149–4160.
| Inferring parturition and neonate survival from movement patterns of female ungulates: a case study using woodland caribou.Crossref | GoogleScholarGoogle Scholar |
DeYoung RW, Demarais S, Honeycutt RL, Gee KL, Gonzales RA (2006) Social dominance and male breeding success in captive white-tailed deer. Wildlife Society Bulletin 34, 131–136.
| Social dominance and male breeding success in captive white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
DeYoung RW, Demarais S, Gee KL, Honeycutt RL, Hellickson MW, Gonzales RA (2009) Molecular evaluation of the white-tailed deer (Odocoileus virginianus) mating system. Journal of Mammalogy 90, 946–953.
| Molecular evaluation of the white-tailed deer (Odocoileus virginianus) mating system.Crossref | GoogleScholarGoogle Scholar |
Dussault C, Courtois R, Ouellet J-P, Huot J (2001) Influence of satellite geometry and differential correction on GPS location accuracy. Wildlife Society Bulletin 29, 171–179.
Elliott LF, Treuer-Kuehn A, Blodgett CF, True CD, German D, Diamond DD (2014) Ecological systems of Texas: 391 mapped types. Phase 1–6, 10-meter resolution geodatabase, interpretive guides, and technical type descriptions. Documents and Data. Texas Parks and Wildlife Department and Texas Water Development Board, Austin, Texas, USA. Available at http://www.tpwd.state.tx.us/gis/data/downloads#EMS-T
Fan J, Li R (2002) Variable selection for Cox’s proportional hazards model and frailty model. The Annals of Statistics 30, 74–99.
| Variable selection for Cox’s proportional hazards model and frailty model.Crossref | GoogleScholarGoogle Scholar |
Federico G (2005) Not guilty? Agriculture in the 1920s and the Great Depression. The Journal of Economic History 65, 949–976.
| Not guilty? Agriculture in the 1920s and the Great Depression.Crossref | GoogleScholarGoogle Scholar |
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309, 570–574.
| Global consequences of land use.Crossref | GoogleScholarGoogle Scholar |
Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC, Balzer C, Bennett EM, Carpenter SR, Hill J, Monfreda C, Polasky S, Rockström J, Sheehan J, Siebert S, Tilman D, Zaks DPM (2011) Solutions for a cultivated planet. Nature 478, 337–342.
| Solutions for a cultivated planet.Crossref | GoogleScholarGoogle Scholar |
Foley AM, Hewitt DG, DeYoung RW, Schnupp MJ, Hellickson MW, Lockwood MA (2018) Reproductive effort and success of males in scramble-competition polygyny: evidence for trade-offs between foraging and mate search. Journal of Animal Ecology 87, 1600–1614.
| Reproductive effort and success of males in scramble-competition polygyny: evidence for trade-offs between foraging and mate search.Crossref | GoogleScholarGoogle Scholar |
Gaillard J-M, Festa-Bianchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends in Ecology & Evolution 13, 58–63.
| Population dynamics of large herbivores: variable recruitment with constant adult survival.Crossref | GoogleScholarGoogle Scholar |
Gao X, Yang F, Li C, Stevens DR (2002) Progress on nutritional requirements of deer farmed for velvet production in China. NZGA: Research and Practice Series 9, 69–72.
Gałecki A, Burzykowski T (2013) ‘Linear mixed-effects models using R.’ (Springer: New York, NY, USA)
Gerhart KL, White RG, Cameron RD, Russell DE (1996) Estimating fat content of caribou from body condition scores. The Journal of Wildlife Management 60, 713–718.
| Estimating fat content of caribou from body condition scores.Crossref | GoogleScholarGoogle Scholar |
Gould FW (1975) ‘Texas plants: a check list and ecological summary.’ (Texas Agricultural Experiment Station: College Station, TX, USA)
Griffith GE, Bryce SA, Omernik JM, Comstock JA, Rogers AC, Harrison B, Hatch SL, Bezanson D (2007) Ecoregions of Texas. Texas Commission on Environmental Quality, Austin, TX, USA.
Heffelfinger J (2006) ‘Deer of the Southwest: a complete guide to the natural history, biology, and management of southwestern mule deer and white-tailed deer.’ (Texas A&M University Press: College Station, TX, USA)
Heffelfinger LJ (2021) Resource selection, habitat influences on population performance, and body size trade-offs of cervids in a nutritionally variable environment. PhD dissertation, Texas A&M University-Kingsville, Kingsville, TX, USA.
Heffelfinger LJ, Stewart KM, Bush AP, Sedinger JS, Darby NW, Bleich VC (2018) Timing of precipitation in an arid environment: effects on population performance of a large herbivore. Ecology and Evolution 8, 3354–3366.
| Timing of precipitation in an arid environment: effects on population performance of a large herbivore.Crossref | GoogleScholarGoogle Scholar |
Heffelfinger LJ, Stewart KM, Shoemaker KT, Darby NW, Bleich VC (2020) Balancing current and future reproductive investment: variation in resource selection during stages of reproduction in a long-lived herbivore. Frontiers in Ecology and Evolution 8, 163
| Balancing current and future reproductive investment: variation in resource selection during stages of reproduction in a long-lived herbivore.Crossref | GoogleScholarGoogle Scholar |
Hinsley SA, Bellamy PE (2000) The influence of hedge structure, management and landscape context on the value of hedgerows to birds: a review. Journal of Environmental Management 60, 33–49.
| The influence of hedge structure, management and landscape context on the value of hedgerows to birds: a review.Crossref | GoogleScholarGoogle Scholar |
Hobbs NT (1989) Linking energy balance to survival in mule deer: development and test of a simulation model. Wildlife Monographs 101, 3–39.
Hurley MA, Unsworth JW, Zager P, Hebblewhite M, Garton EO, Montgomery DM, Skalski JR, Maycock CL (2011) Demographic response of mule deer to experimental reduction of coyotes and mountain lions in southeastern Idaho. Wildlife Monographs 178, 1–33.
| Demographic response of mule deer to experimental reduction of coyotes and mountain lions in southeastern Idaho.Crossref | GoogleScholarGoogle Scholar |
Johnstone-Yellin TL, Shipley LA, Myers WL, Robinson HS (2009) To twin or not to twin? Trade-offs in litter size and fawn survival in mule deer. Journal of Mammalogy 90, 453–460.
| To twin or not to twin? Trade-offs in litter size and fawn survival in mule deer.Crossref | GoogleScholarGoogle Scholar |
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53, 457–481.
| Nonparametric estimation from incomplete observations.Crossref | GoogleScholarGoogle Scholar |
Krausman PR, Hervert JJ, Ordway LL (1985) Capturing deer and mountain sheep with a net-gun. Wildlife Society Bulletin 13, 71–73.
Lampman JR (2019) Mule deer diet and nutrition in the western rolling plains ecoregion of Texas. MS thesis, Sul Ross State University, Alpine, TX, USA.
Lauffenburger ZH, Gurdak JJ, Hobza C, Woodward D, Wolf C (2018) Irrigated agriculture and future climate change effects on groundwater recharge, northern High Plains aquifer, USA. Agricultural Water Management 204, 69–80.
| Irrigated agriculture and future climate change effects on groundwater recharge, northern High Plains aquifer, USA.Crossref | GoogleScholarGoogle Scholar |
Lenth RV (2021) emmeans: estimated marginal means, aka least-squares means. R package version 1.6.3. Available at https://CRAN.R-project.org/package=emmeans
Maupin MA, Barber NL (2005) Estimated withdrawals from principal aquifers in the United States, 2000 (Vol. 1279). US Department of the Interior, US Geological Survey.
Mautz WW (1978) Sledding on a bushy hillside: the fat cycle in deer. Wildlife Society Bulletin 6, 88–90.
Merems JL, Shipley LA, Levi T, Ruprecht J, Clark DA, Wisdom MJ, Jackson NJ, Stewart KM, Long RA (2020) Nutritional-landscape models link habitat use to condition of mule deer (Odocoileus hemionus). Frontiers in Ecology and Evolution 8, 98
| Nutritional-landscape models link habitat use to condition of mule deer (Odocoileus hemionus).Crossref | GoogleScholarGoogle Scholar |
Michel N, Burel F, Butet A (2006) How does landscape use influence small mammal diversity, abundance and biomass in hedgerow networks of farming landscapes? Acta Oecologica 30, 11–20.
| How does landscape use influence small mammal diversity, abundance and biomass in hedgerow networks of farming landscapes?Crossref | GoogleScholarGoogle Scholar |
Monteith KL, Stephenson TR, Bleich VC, Conner MM, Pierce BM, Bowyer RT (2013) Risk-sensitive allocation in seasonal dynamics of fat and protein reserves in a long-lived mammal. Journal of Animal Ecology 82, 377–388.
| Risk-sensitive allocation in seasonal dynamics of fat and protein reserves in a long-lived mammal.Crossref | GoogleScholarGoogle Scholar |
Monteith KL, Bleich VC, Stephenson TR, Pierce BM, Conner MM, Kie JG, Bowyer RT (2014) Life-history characteristics of mule deer: effects of nutrition in a variable environment. Wildlife Monographs 186, 1–62.
| Life-history characteristics of mule deer: effects of nutrition in a variable environment.Crossref | GoogleScholarGoogle Scholar |
Morina DL, Demarais S, Strickland BK, Larson JE (2018) While males fight, females choose: male phenotypic quality informs female mate choice in mammals. Animal Behaviour 138, 69–74.
| While males fight, females choose: male phenotypic quality informs female mate choice in mammals.Crossref | GoogleScholarGoogle Scholar |
Mysterud A, Langvatn R, Stenseth NC (2004) Patterns of reproductive effort in male ungulates. Journal of Zoology 264, 209–215.
| Patterns of reproductive effort in male ungulates.Crossref | GoogleScholarGoogle Scholar |
Mysterud A, Bonenfant C, Loe LE, Langvatn R, Yoccoz NG, Stenseth NC (2008) The timing of male reproductive effort relative to female ovulation in a capital breeder. Journal of Animal Ecology 77, 469–477.
| The timing of male reproductive effort relative to female ovulation in a capital breeder.Crossref | GoogleScholarGoogle Scholar |
National Oceanic and Atmospheric Association (NOAA) (2021) National Climatic Data Center. Annual precipitation and temperature for Turkey, Canadian, Littlefield, and Levelland, TX, USA. Available at http://www.ncdc.noaa.gov/cdo-web/
Nesbitt WH, Wright PL, Buckner EL, Byers CR, Reneau J (2009) ‘Measuring and scoring North American big game trophies.’ (Boone and Crockett Club: Missoula, MT, USA)
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146, 579–600.
| The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions.Crossref | GoogleScholarGoogle Scholar |
Nicholson KL, Warren MJ, Rostan C, Månsson J, Paragi TF, Sand H (2019) Using fine-scale movement patterns to infer ungulate parturition. Ecological Indicators 101, 22–30.
| Using fine-scale movement patterns to infer ungulate parturition.Crossref | GoogleScholarGoogle Scholar |
Nozari S, Bailey RT, Haacker EMK, Zambreski ZT, Xiang Z, Lin X (2022) Employing machine learning to quantify long-term climatological and regulatory impacts on groundwater availability in intensively irrigated regions. Journal of Hydrology 614, 128511
| Employing machine learning to quantify long-term climatological and regulatory impacts on groundwater availability in intensively irrigated regions.Crossref | GoogleScholarGoogle Scholar |
O’Gara BW, Yoakum JD (2004) ‘Pronghorn ecology and management.’ (Ed. RE McCabe) (University Press of Colorado: Boulder, CO, USA)
Parker KL, Gillingham MP, Hanley TA, Robbins CT (1993) Seasonal patterns in body mass, body composition, and water transfer rates of free-ranging and captive black-tailed deer (Odocoileus hemionus sitkensis) in Alaska. Canadian Journal of Zoology 71, 1397–1404.
| Seasonal patterns in body mass, body composition, and water transfer rates of free-ranging and captive black-tailed deer (Odocoileus hemionus sitkensis) in Alaska.Crossref | GoogleScholarGoogle Scholar |
Parker KL, Barboza PS, Stephenson TR (2005) Protein conservation in female caribou (Rangifer tarandus): effects of decreasing diet quality during winter. Journal of Mammalogy 86, 610–622.
| Protein conservation in female caribou (Rangifer tarandus): effects of decreasing diet quality during winter.Crossref | GoogleScholarGoogle Scholar |
Parker KL, Barboza PS, Gillingham MP (2009) Nutrition integrates environmental responses of ungulates. Functional Ecology 23, 57–69.
| Nutrition integrates environmental responses of ungulates.Crossref | GoogleScholarGoogle Scholar |
Pereira HM, Leadley PW, Proença V, Alkemade R, Scharlemann JPW, Fernandez-Manjarrés JF, Araújo MB, Balvanera P, Biggs R, Cheung WWL, Chini L, Cooper HD, Gilman EL, Guénette S, Hurtt GC, Huntington HP, Mace GM, Oberdorff T, Revenga C, Rodrigues P, Scholes RJ, Sumaila UR, Walpole M (2010) Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501.
| Scenarios for global biodiversity in the 21st century.Crossref | GoogleScholarGoogle Scholar |
Pittman MT, Bone TL (1987) Mule deer reproduction. Texas Parks and Wildlife Department, Fed. Aid Proj. Texas W-109-R-10/Job 51 Final Report, Austin, TX, USA.
Pollock KH, Winterstein SR, Bunck CM, Curtis PD (1989) Survival analysis in telemetry studies: the staggered entry design. The Journal of Wildlife Management 53, 7–15.
| Survival analysis in telemetry studies: the staggered entry design.Crossref | GoogleScholarGoogle Scholar |
Pruitt KD, Hewitt DG, Silvy NJ, Benn S (2008) Importance of native seeds in white-winged dove diets dominated by agricultural grains. Journal of Wildlife Management 72, 433–439.
| Importance of native seeds in white-winged dove diets dominated by agricultural grains.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Robinette WL, Baer CH, Pillmore RE, Knittle CE (1973) Effects of nutritional change on captive mule deer. The Journal of Wildlife Management 37, 312–326.
| Effects of nutritional change on captive mule deer.Crossref | GoogleScholarGoogle Scholar |
Rosenberg NJ, Epstein DJ, Wang D, Vail L, Srinivasan R, Arnold JG (1999) Possible impacts of global warming on the hydrology of the Ogallala Aquifer region. Climatic Change 42, 677–692.
| Possible impacts of global warming on the hydrology of the Ogallala Aquifer region.Crossref | GoogleScholarGoogle Scholar |
Scanlon BR, Faunt CC, Longuevergne L, Reedy RC, Alley WM, McGuire VL, McMahon PB (2012) Groundwater depletion and sustainability of irrigation in the US High Plains and Central Valley. Proceedings of the National Academy of Sciences 109, 9320–9325.
| Groundwater depletion and sustainability of irrigation in the US High Plains and Central Valley.Crossref | GoogleScholarGoogle Scholar |
Severinghaus CW (1949) Tooth development and wear as criteria of age in white-tailed deer. The Journal of Wildlife Management 13, 195–216.
| Tooth development and wear as criteria of age in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Sinclair ARE, Krebs CJ (2002) Complex numerical responses to top-down and bottom-up processes in vertebrate populations. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 357, 1221–1231.
| Complex numerical responses to top-down and bottom-up processes in vertebrate populations.Crossref | GoogleScholarGoogle Scholar |
Sinclair ARE, Mduma S, Brashares JS (2003) Patterns of predation in a diverse predator–prey system. Nature 425, 288–290.
| Patterns of predation in a diverse predator–prey system.Crossref | GoogleScholarGoogle Scholar |
Stearns SC (1989) Trade-offs in life-history evolution. Functional Ecology 3, 259–268.
| Trade-offs in life-history evolution.Crossref | GoogleScholarGoogle Scholar |
Stearns SC (1992) ‘The evolution of life histories.’ (Oxford University Press: Oxford, UK)
Stephens PA, Boyd IL, McNamara JM, Houston AI (2009) Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90, 2057–2067.
| Capital breeding and income breeding: their meaning, measurement, and worth.Crossref | GoogleScholarGoogle Scholar |
Stephenson TR, Bleich VC, Pierce BM, Mulcahy GP (2002) Validation of mule deer body composition using in vivo and post-mortem indices of nutritional condition. Wildlife Society Bulletin 30, 557–564.
Stewart BA, Baumhardt RL, Evett SR (2010) Major advances of soil and water conservation in the US Southern Great Plains. In ‘Soil and water conservation advances in the United States, Vol. 60’. (Eds TM Zoebeck, WF Schillinger) pp. 103–129. (Soil Science Society of America)
Therneau TM (2021) A package for survival analysis in R. R package version 3.2-11. Available at https://CRAN.R-project.org/package=survival
Therneau TM, Grambsch PM (2000) The Cox model. In ‘Modeling survival data: extending the Cox model’. (Eds TM Therneau, PM Grambsch) pp. 39–77. (Springer: New York, NY, USA)
Tollefson TN, Shipley LA, Myers WL, Keisler DH, Dasgupta N (2010) Influence of summer and autumn nutrition on body condition and reproduction in lactating mule deer. Journal of Wildlife Management 74, 974–986.
| Influence of summer and autumn nutrition on body condition and reproduction in lactating mule deer.Crossref | GoogleScholarGoogle Scholar |
Vitousek PM, Ehrlich PR, Ehrlich AH, Matson PA (1986) Human appropriation of the products of photosynthesis. BioScience 36, 368–373.
| Human appropriation of the products of photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Wallmo OC (1981) ‘Mule and black-tailed deer in North America. A Wildlife Management Institute book.’ (University of Nebraska Press: Lincoln, NE, USA)
Warren RJ, Kirkpatrick RL, Oelschlaeger A, Scanlon PF, Gwazdauskas FC (1981) Dietary and seasonal influences on nutritional indices of adult male white-tailed deer. The Journal of Wildlife Management 45, 926–936.
| Dietary and seasonal influences on nutritional indices of adult male white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Westmoreland D, Best LB, Blockstein DE (1986) Multiple brooding as a reproductive strategy: time-conserving adaptations in mourning doves. The Auk 103, 196–203.
Yoccoz NG, Mysterud A, Langvatn R, Stenseth NC (2002) Age- and density-dependent reproductive effort in male red deer. Proceedings of the Royal Society of London B: Biological Sciences 269, 1523–1528.
| Age- and density-dependent reproductive effort in male red deer.Crossref | GoogleScholarGoogle Scholar |


