Variability in practices for drinking water vaccination of meat chickens against infectious laryngotracheitis
Peter J. Groves A * , Awol M. Assen B , Ashley Etherington C , Mark Stillman D , Sheridan Alfirevich D , Priscilla F. Gerber B , Alex-Kate Langfield E and Stephen W. Walkden-Brown B
A * , Awol M. Assen B , Ashley Etherington C , Mark Stillman D , Sheridan Alfirevich D , Priscilla F. Gerber B , Alex-Kate Langfield E and Stephen W. Walkden-Brown B
A Sydney School of Veterinary Science, Faculty of Science, The University of Sydney, 425 Werombi Road, Camden, NSW 2570, Australia.
B School of Environmental and Rural Science, The University of New England, Armidale, NSW, Australia.
C Ingham’s Enterprises Pty Ltd, Burton, SA 5110, Australia.
D Baiada Farms Pty Limited, NSW, Pendle Hill, NSW 2145, Australia.
E Zootechny Pty Ltd, Austral, NSW 2179, Australia.
Animal Production Science 62(18) 1830-1838 https://doi.org/10.1071/AN21605
Submitted: 14 December 2021 Accepted: 10 August 2022 Published: 26 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Drinking water vaccination of young meat chickens with Infectious Laryngotracheitis (ILT) vaccine is problematic. Vaccine failure and adverse vaccine reactions are frequently reported. Variations in the technique of applying ILT vaccines by this mass vaccination method need to be understood to contribute to improving the success of vaccination.
Aims: This study aimed to examine variations in the techniques of application of Infectious Laryngotracheitis vaccines via drinking water for young meat chickens.
Methods: Drinking water vaccination techniques were observed and recorded across 52 broiler flocks during ILT outbreaks in three geographic areas of Australia. Descriptive statistics for all variables were computed and variations between integrator company procedures were statistically compared.
Key results: Despite rigorous standard operating procedures, wide variations were observed in time of water deprivation prior to vaccination (3–15 min), time drinking water was stabilised prior to addition of vaccine and the type of stabiliser product used, time to activate the flock following filling of the water lines with vaccine (10–127 min), time for the vaccine to be consumed (36–226 min) and the volume of drinking water per bird used to provide the vaccine (11–48 mL/bird).
Conclusions: Variation in vaccination technique can affect the success of drinking water vaccination against ILT in young meat chickens.
Implications: Understanding the importance of the variable factors in vaccine application method can improve the success of water vaccination against ILT.
Keywords: broiler, chicken, drinking water, immunisation, infectious laryngotracheitis, poultry, poultry diseases, vaccination.
Introduction
Infectious laryngotracheitis (ILT) is a serious respiratory disease of chickens worldwide, caused by infection with an alphaherpesvirus (Gallid alphaherpesvirus 1). Most live attenuated vaccines against ILT are registered for use by individual eye drop administration or via drinking water (Hilbink et al. 1987; Coppo et al. 2012). ILT vaccination of flocks of commercial meat chickens is generally only envisaged in the face of a local outbreak and the huge numbers of birds involved requires the use of mass vaccination techniques, usually via drinking water at between 1 and 2 weeks of age (Coppo et al. 2012; Groves et al. 2019). Although registered for application by this method, drinking water application can produce variable results in terms of the effective proportion of birds that take up the vaccine virus initially (Groves et al. 2019). This is likely due to challenges in ensuring that sufficient amounts of the vaccine virus come into contact with respiratory tissues to actually vaccinate the bird (Hilbink et al. 1981; Robertson and Egerton 1981; De Wit 2013). Laboratory studies often show successful protection against challenge with field strains of ILT virus with the available vaccines (Arzey and Arzey 2009; Korsa et al. 2015) but problems in achieving protection in the field are commonly described (De Wit 2013; Keck 2018). A previous study (Groves et al. 2019) conducted in commercial meat chicken flocks in Australia demonstrated marked variation in vaccine virus establishment in respiratory tissues associated with drinking water application factors. This previous study was limited in its ability to identify all the important administration factors as it included only eight flocks. During this and another subsequent study, an ability to estimate vaccine uptake success by quantitative polymerase chain reaction (qPCR) assay of dust samples was developed (Ahaduzzaman et al. 2020; Assen et al. 2020). Therefore, a larger field study involving 52 flocks across Australia was designed to look at the associations between variability in drinking water vaccine application and subsequent effectiveness of vaccination. The qPCR dust detection method (Ahaduzzaman et al. 2020) was used in this present field study but wild or vaccine strain ILT viral DNA was found to already be present in many flocks prior to vaccination (Assen et al. 2019). This compromised the ability to analyse the association of vaccination administration variables with vaccination success as virus may have been circulating in the flocks prior to vaccination. These associations will require further studies. Reported herein are the variations in drinking water vaccination techniques observed in this larger study of 52 flocks.
Companies provided specific ILT vaccination standard operating procedures (SOP) to farms involved in the current study, but nevertheless, substantial variations are thought to occur in application for a variety of reasons.
Materials and methods
Collaborators
Three regions of Australia were experiencing ILT outbreaks in meat chickens in 2018–2019. The companies farming in these areas were integrated operations, all operating hatcheries and abattoirs and using contracted farms to grow meat chickens. Two of these companies also operate their own breeding operations and feed mills. The integrator companies employ service personnel and veterinarians to provide supervision and advice to the contracted meat chicken growers. The companies supply chickens, feed and service; the contracted farmer provides facilities and labour. Either Cobb 500 or Ross 308 strain meat chickens were used. The service personnel from the companies supervised or performed the administration of ILT vaccines on the contracted farms, following a prescribed SOP. The companies also choose and supply the vaccine type to be used.
Vaccines
There are currently three attenuated, live chicken-embryo origin (CEO) vaccines available in Australia (García 2017; Fraser 2019). Two were developed in Australia (SA2 and A20 strains, Zoetis Poulvac Laryngo) and the third is imported (Serva strain, NOBILIS®ILT, MSD). Of these, only A20 and Serva strains are used in meat chickens as SA2, although genetically very similar to A20 (which was derived from SA2), is considered too pathogenic in this type of bird (Ou and Giambrone 2012). A20 and Serva strains are registered in Australia for use via drinking water (MSD undated; Zoetis undated). Strains SA2 and A20 are classified as Class 1 while Serva strain is designated as Class 7 using a restriction fragment frame length polymorphism (RFLP) technique (Kirkpatrick et al. 2006) which was subsequently modified to a multiplex polymerase chain reaction (PCR)-RFLP typing method (Williamson et al. 2019).
Procedures
Three different sites in Australia that were vaccinating commercial meat chickens against ILT were involved in the study. Two sites were in New South Wales: these were the greater Sydney basin, and a regional area in the Riverina district. The third site was in South Australia. Chickens were vaccinated using Serva strain in the greater Sydney region or A20 vaccine in the Riverina and South Australia.
Vaccination procedures followed SOPs according to each integrator company’s requirements which were all closely based on guidelines specified by the vaccine manufacturers (MSD undated; Zoetis undated). Briefly these feature the following specifications:
-
Vaccinate early in the day.
-
Clean and rinse drinkers and avoid the presence of disinfectants in the drinking system.
-
Adjust the water volume in the tank to the designated level using a formula to calculate required volume for vaccination based on the age and number of birds to provide water to be consumed within 1.5–2 h (volume (L) = the number of birds multiplied by their age in days multiplied by two). Where a medication tank is used, the volume is estimated in the tank. Some houses use automatic proportioners for provision of prepared vaccine directly into the water supply line. Typically, this method requires a water volume estimate (calculated as above or determined by measuring 2 h consumption the day before) and setting the proportioner to deliver the required volume of the prepared mixture of vaccine and water over that time.
-
Withdrawal of drinking water from the birds for a specified time, either by shutting off the drinker lines or, more frequently, by raising the drinker lines out of reach of the birds.
-
Adding a product to stabilise the water (i.e. to neutralise chlorine or salts that may inactivate the vaccine virus) such as skim milk powder (2.5 g/L) or a proprietary product containing a dye to protect the vaccine. A waiting time for stabilisation to occur is specified (commonly 20 min for skim milk but the proprietary dye products claim instant stabilisation).
-
Preparing the vaccine in a small volume of stabilised water and then adding this to the medication tank
-
Flushing the drinker lines with the water so that the skim milk or dye colour is seen at the end of the line to ensure vaccinated water is immediately available to all birds.
-
Drive the birds towards the drinkers by walking through the flock.
-
The vaccine mixture should be consumed within 2 h.
The target age for vaccination was between 7 and 14 days. Farms varied in their choice of stabiliser product, using skim milk powder (2.5 g/L water), liquid skim milk (approximately 17 mL/L water), or a proprietary stabiliser containing a blue dye: Vac-Pac Plus® (Animal Science Products Inc. undated) at 10 g/100 L drinking water; or DeCHLOR® (Feedwater undated) at 10 mL/100 L drinking water.
Measurements and records
Service personnel from the company supervised or conducted all vaccinations and then completed a detailed and standardised record sheet on the practices used. Descriptions of the house and procedures used were recorded, including flock size, proportion of the house available to the birds at the time of vaccination and number of drinker lines used, ventilation system, number of birds present, bird age at time of vaccination, vaccine strain used, and number of label doses delivered, and water volume used for vaccination. The duration of each procedure was recorded for time of water withdrawal, time at which stabiliser was added to the water supply, time at which vaccine was prepared, and time this was added to the drinker system, time that flushing of the lines to fill them with vaccinated water was completed, time that the staff walked through the house and time that vaccine was completely consumed.
Statistical analyses
All recorded data were entered into a computerised statistics package (Statistica v6.1, StatSoft Inc. 2003). Descriptive statistics were generated for each variable which consisted of the number of valid entries, means, standard deviation and coefficients of variation, the 95% confidence intervals of the mean, minimum, median, lower and upper quartiles, maximum values, skewness and kurtosis. Pearson correlation coefficients were calculated between quantitative variables. Comparison between practices in each company were compared using one-way Analysis of Variance with means separated using Tukey’s HSD test. Where variables did not show homogeneity of variance (significant Brown–Forsythe test) then the non-parametric Kruskal–Wallis ANOVA was used. Results were considered significant at P < 0.05.
Animal ethics
The study was conducted under the supervision of the Animal Ethics Committee of the University of New England (authority number AEC19-011). All birds were held under normal commercial conditions within the operations of large integrated meat chicken companies and were subject to their animal welfare requirements and controls. Many of the farms used were Royal Society for Prevention of Cruelty to Animals (RSPCA)-accredited establishments. This was an observational study only; no experimental interventions were performed.
Results
Table 1 shows qualitative factors that were fixed for the farm at the time of vaccination (i.e. location, integrator company, house design, strain of chicken supplied, hatchery supplying chicks and hatchery vaccinations applied). The strain of ILT virus (ILTV) vaccine used is also chosen by the integrator company for the location of the farms.

|
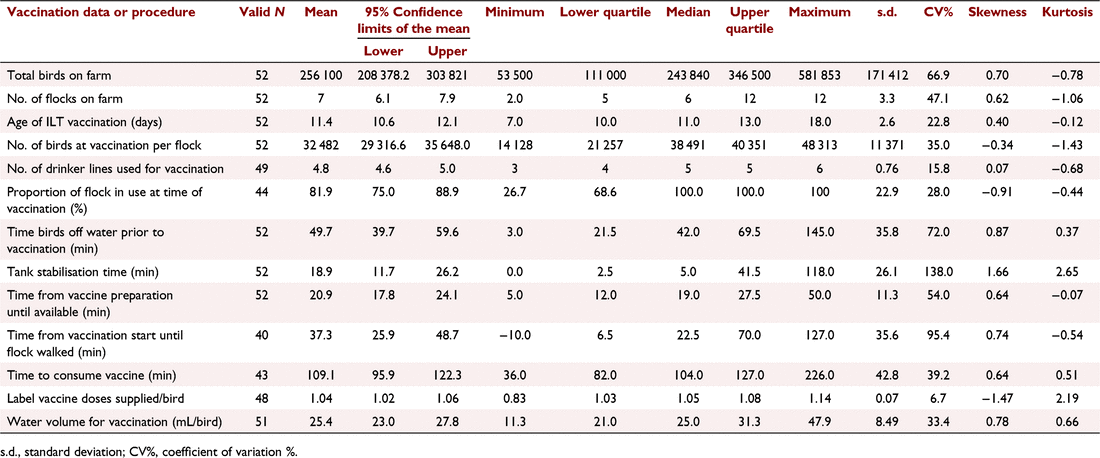
A variety of descriptive statistics for quantitative variables are displayed in Table 2 (number of flocks supplying data, mean value and 95% confidence intervals for the mean, minimum value, upper and lower quartile, median and maximum values, standard deviation, coefficient of variation, skewness and kurtosis). The majority of the data distributions were moderately positively skewed (skewness greater than +0.5). Thus, most of the values in the distributions are less than the mean, the mean being elevated by a few very high values. All of the distributions are platykurtic (Kurtosis <3.0), as the values towards the extremities are less than would be expected in a normal distribution (Dugar 2018). Table 2 also shows the ranges and variation in the recorded variables across the 52 flocks in the study. The factors involved in the practice of vaccination for ILT showed marked variation with coefficients of variation for the time observations ranging from 39.2 to 95.4% (Table 2). The key variables of concern are noted below.
-
The length of water deprivation prior to vaccination ranged between 3 and 145 min with a median time of 42 min.
-
Time of stabilisation of the drinking water ranged from 0 to 118 min with a median time of 5 min. This would reflect the choice of stabiliser, with skim milk requiring 20 min but the proprietary dye products claiming instant stabilisation. Thirty flocks (58%) had a stabilisation time between 0 and 20 min.
-
Time from the start of vaccine availability until the birds were activated by staff walking the house ranged from 10 to 127 min with a median of 22.5 min. One operator walked the flock prior to vaccination beginning and the task was completed in 19 flocks (37%) within 20 min of vaccine availability to the birds.
-
Time to consume the vaccine varied from 36 to 226 min with a median time of 104 min. This is within the target time of <120 min according to the SOPs. This may have been affected by when the farmer deemed the process ‘finished’. Some tanks were empty as soon as the drinker lines were flushed, while others took some time to empty.
-
Nearly all birds were vaccinated between 7 and 13 days of age but one flock was not vaccinated until 18 days. The proportion of the house in use at the time of vaccination varied from 26.7% to the full house.
-
The volume of water used to vaccinate varied between 11.3 and 47.9 mL per bird. This was confounded by company and by the variation in age of bird vaccinated across the sampled population.
-
Delivery of a full label dose is a recommendation of the manufacturer with vaccines registered for drinking water delivery (APVMA undated; Zoetis undated). The actual number of doses applied (as specified on the label) depended on the vial size (either 2000 or 5000 doses per vial) and the actual number of birds present. The distribution of values of the number of label doses of vaccine supplied per bird was strongly negatively skewed (skewness = −1.47) illustrating the understandable tendency of the administrators to slightly overdose rather than underdose.
|
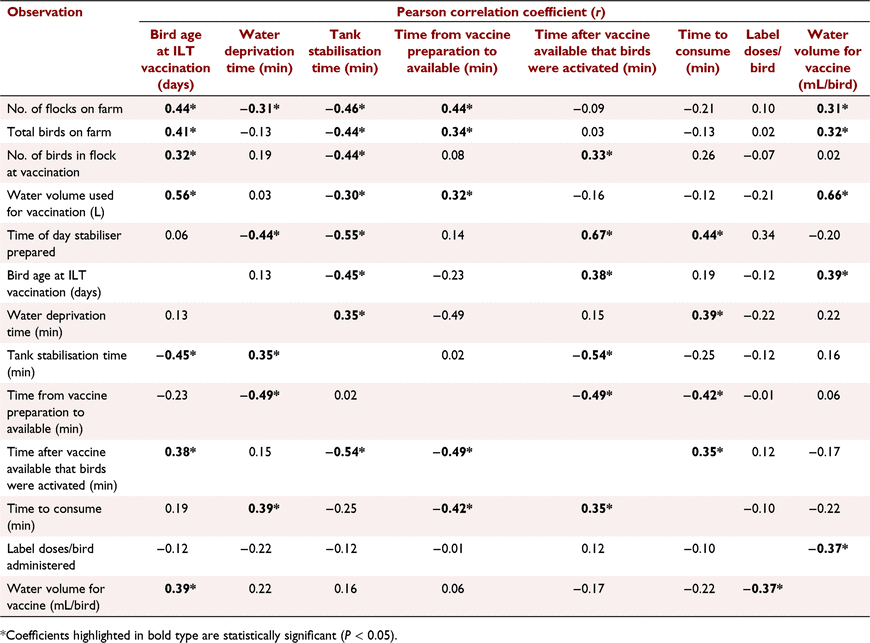
Table 3 is a rectangular matrix displaying Pearson coefficients of correlation between quantitative variables. Table 3 displays 68 individual correlation coefficients and hence, by definition, at least three to four of these could have shown significance by chance alone. Many of the coefficients were statistically significant but most were weak correlations (−0.5 < r < +0.5). Only the variables of age of ILT vaccination, time of day that vaccine preparation began, the time from vaccine being available to the birds until they were activated by staff walking through the flock, and the time to consume vaccine were normally distributed (Kolmogorov–Smirnov and Lilliefors tests of normality P > 0.05 – data not shown), hence some correlations may be unreliable with other variables.
|
Age of the birds at vaccination was positively correlated to flock size variables (farm size and number of birds per flock) which would indicate that larger farms tended to be vaccinated at slightly older ages.
The time allowed for the drinking water to be stabilised was weakly negatively correlated to larger farm and flock size variables, and to bird age and the time after vaccine availability that the birds were activated. This may indicate that staff were more hurried on larger farms. This is supported by the significant but weak positive correlation of stabilisation time with water deprivation time (i.e. shorter stabilisation time was associated with shorter water deprivation times). Stabilisation time was also negatively associated with time of day that it was conducted, indicating shorter stabilisation times as the day proceeded, again possibly a factor of flock size (taking longer to vaccinate a larger farm). The time of day that vaccination began (as evidenced by the time when vaccine stabiliser was added to the water) was moderately positively correlated (r = 0.67) with the time after vaccine was made available that the farmer walked through the flock, activating the birds. This may also be associated with larger farms, as busier staff may take longer to access the flocks.
The time between preparation of the vaccine (in a small volume of water to be added to the total volume) was weakly negatively correlated with the time that staff walked through the flock to activate the birds to drink after vaccine was available to the birds (r = −0.49) and the time for the birds to consume the vaccine (r = −0.42).
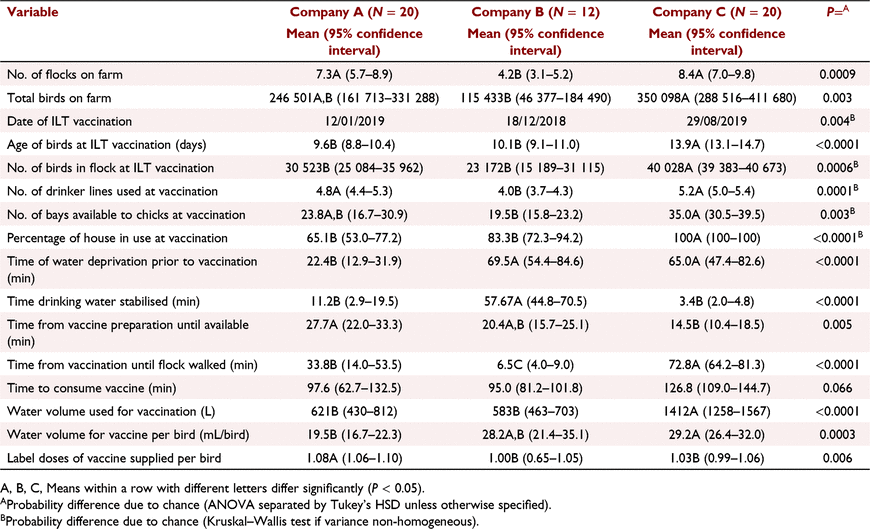
Table 4 displays comparative practices between the three meat chicken companies that participated in the studies. Despite very similar SOPs for ILT drinking water vaccination, the details of their practices differed significantly in many aspects. A major contributing factor here was comparative farm and flock size, with company C having very large houses and farms with more flocks, company B being much smaller and company A being between these extremes. The studies were also conducted at different times, with companies A and B studied in late 2018 to early 2019 and company C being involved later in 2019. Average age of application of the vaccine was around 10 days for companies A and B but tended to be older for company C (about 14 days). The size of the flocks dictated the number of drinkers in use at time of vaccination and the total volume of drinking water used for each flock. Age of vaccination would also have been a factor in water volume used. The proportion of the house available for the chicks also varied with company management style with company C using the full house while the other companies had restriction of amount of space utilised (65–83% in companies A and B). Company A used a much shorter period of water deprivation prior to vaccination than did companies B and C (22 min compared to 69–65 min respectively). Time allowed for the water to be stabilised prior to the addition of vaccine varied markedly with company C averaging only 3.4 min, as did the time from vaccine preparation until its presentation to the birds, but this was probably due to this operation using proportioners to dose water rather than a medication header tank. Company A also had shorter stabilisation time (11 min) than company B (58 min) but this reflects the choice of stabiliser where the proprietary dye does not require a lengthy time compared with skim milk products. Company B personnel walked through the flock earlier following vaccination application than either companies A or C. Time to consume the vaccine was not significantly different between companies, generally taking between 1.5 and 2 hours. The amount of water used per bird to supply the vaccine varied but may have been confounded by the bird age at the time for company C compared to company A. Company B used a higher water allocation than company A despite similar bird age. The actual vaccine supplied per bird was close to one label dose although company A seemed likely to oversupply slightly, but significantly, compared to the other two companies.
|
Discussion
The wild strain of ILT virus causing the outbreak in the greater Sydney region was identified as Class 9 (Fraser 2019), which had been the predominant strain in Australia since 2009 (Agnew-Crumpton et al. 2016). However, the outbreak strain in the Riverina and in South Australia was identified as Class 7 (Fraser 2019; Williamson et al. 2019) which may be a recombinant strain derived from the Serva vaccine which was subsequently identified as Class 7b by whole genome analysis (Sabir et al. 2020). The A20 vaccine strain was in use in the Riverina region of NSW and in South Australia while Serva vaccine strain was used in the greater Sydney region of NSW. In many of the flocks in the study, ILTV DNA of Classes 7 and 9 were detected in dust samples from the houses prior to vaccination being administered in the region being vaccinated with Serva strain, and from Class 7 in the regions vaccinated with A20 strain (Assen et al. 2019). It is not known whether the Class 7 detections were actually Class 7b (Sabir et al. 2020) as this nomenclature was not recognised at the time of testing.
The ILT vaccine manufacturers specify that a full label dose must be delivered per bird for effectiveness (MSD undated; Zoetis undated). However mass administration techniques do not guarantee that the complete designed dose will actually reach the respiratory target tissues. It has also been shown that it may require at least a ten-fold higher virus dose for drinking water application to achieve a similar effect to a single dose via individual eye drop (De Wit 2013). The ability of ILT vaccine virus to contact respiratory tissue (conjunctiva, nasal mucosa, inner choanae, larynx or trachea) is imperative for effective vaccination to occur (Robertson and Egerton 1981) but this is highly variable between birds using mass administration (Groves et al. 2019). Mass vaccination via drinking water application provides variable outcomes in this respect (Coppo et al. 2012) and relies extensively on bird to bird spread following successful initial vaccine uptake by only a proportion of the flock (Groves et al. 2019). Some of this wide variation in initial vaccine uptake may perhaps be due to subtle variations in the drinking water administration technique. The present study has shown that many variations in details of the vaccine administration method may occur in spite of rigorous SOP instructions. Many significant variations in process were observed between companies, as evidenced by the large coefficients of variation in all procedures, much of which was due to differences in farm and flock size, the method of water dosing (medication tanks compared to proportioners), differences in age that birds were vaccinated and choice of water stabilisation product. It has previously been shown that the proportion of birds taking up the vaccine quickly following vaccination can be affected by the application method and also by the stabiliser used (Groves et al. 2019; Assen et al. 2020) and this can affect the adequacy of vaccine protection and the occurrence of vaccine reactions. Hence the extent of variations in these techniques can have major effects on vaccination success. Further studies need to focus on the actual contributions of the various application factors on the uptake of the vaccine by birds at the time of administration.
It was unfortunate that the detection of the presence of ILTV DNA in dust prior to vaccination on many farms eliminated the ability of the study to make associations between variation in administration technique and subsequent vaccine uptake by the birds. Further studies to understand the association of drinking water vaccination practices with ILT vaccination success are needed where vaccine uptake can be assessed without complication from unintended presence of virus (either wild or vaccine strains) prior to vaccine administration. The present study detected the presence of extraneous virus on the day of vaccination using environmental dust samples (Ahaduzzaman et al. 2020; Assen et al. 2020). Collection of individual bird samples such as tracheal swabs or feather Davidson et al. (2018) may have provided additional insight but would have required a significantly greater number of samples and, if the chickens were also positive for ILTV prior to vaccination, would not have overcome the problem of determining vaccination success in chickens already infected with ILTV. Indeed, we have subsequently shown that many flocks with positive dust samples prior to vaccination harbour active infection with ILTV as determined by qPCR of tracheal swabs (Assen et al. 2022). Studies on ILTV detection in feather shaft have occurred in older layer chickens, and the delay in time of detection using this method following vaccination may limit the value of this in young broilers where an assessment of vaccine uptake within 4–7 days is essential. Further studies to understand the most important factors involved in achieving a better initial flock uptake of the vaccine virus will lead to more efficacious field vaccination.
Conclusions
Even when an SOP is followed, variation in vaccination practices with ILT vaccines via drinking water shows marked flock to flock variation. The variations, for both fixed and variable factors, need to be assessed for associations with an accurate estimate of effective vaccine ‘take’ in each flock if the complication of an existing circulating ILT virus before vaccination can be understood and controlled.
This will assist in optimising ILT vaccination in future.
Data availability
The dataset used for this analysis is not available due to privacy requirements.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This project was funded by AgriFutures Australia (project number 010639).
Acknowledgements
The assistance and generous contributions from the collaborating companies, Cordina Farms, Baiada Poultry, Ingham’s Enterprises, Birling Avian Laboratories and Zootechny, in collecting and providing observations and data is gratefully acknowledged. In particular assistance provided by Sue Ball and Danielle Stubbs was highly appreciated.
References
Agnew-Crumpton R, Vaz PK, Devlin JM, O’Rourke D, Blacker-Smith HP, Konsak-Ilievski B, Hartley CA, Noormohammadi AH (2016) Spread of the newly emerging infectious laryngotracheitis viruses in Australia. Infection, Genetics and Evolution 43, 67–73.| Spread of the newly emerging infectious laryngotracheitis viruses in Australia.Crossref | GoogleScholarGoogle Scholar |
Ahaduzzaman M, Groves PJ, Sharpe SM, Williamson SL, Gao YK, Nguyen TV, Gerber PF, Walkden-Brown SW (2020) A practical method for assessing infectious laryngotracheitis vaccine take in broilers following mass administration in water: spatial and temporal variation in viral genome content of poultry dust after vaccination. Veterinary Microbiology 241, 108545
| A practical method for assessing infectious laryngotracheitis vaccine take in broilers following mass administration in water: spatial and temporal variation in viral genome content of poultry dust after vaccination.Crossref | GoogleScholarGoogle Scholar |
Animal Science Products Inc. (undated) Vac-Pac-Plus®. Available at https://www.asp-inc.com/vac-pac-plus-2/ [Accessed 16 July 2021]
APVMA (undated) Australian Pesticides and Veterinary Medicines Authority. Available at https://portal.apvma.gov.au [Accessed 12 September 2022]
Arzey GG, Arzey KE (2009) ILT-protection against Class 8 NSW ILTV – challenge trial 2008. In ‘Proceedings scientific meeting of Australasian Veterinary Poultry Association.’ 11–12 February. (AVPA: Sydney, Australia)
Assen AW, Etherington A, Stillman M, Alfirevich S, Gerber FP, Groves PJ, Langfield A-K, Walkden-Brown SW (2019) Use of dust samples for assessing infectious largyngotracheitis virus status in meat chickens. In ‘Proceedings scientific meeting of Australasian Veterinary Poultry Association’. (AVPA: Adelaide, Australia)
Assen AM, Stillman M, Alfirevich S, Gerber PF, Groves PJ, Walkden-Brown SW (2020) Assessment of A20 infectious laryngotracheitis vaccine take in meat chickens using swab and dust samples following mass vaccination in drinking water. Veterinary Microbiology 251, 108903
| Assessment of A20 infectious laryngotracheitis vaccine take in meat chickens using swab and dust samples following mass vaccination in drinking water.Crossref | GoogleScholarGoogle Scholar |
Assen A, Groves P, Etherington A, Gerber P, Sexton M, Williamson S, Walkden-Brown S (2022) Field application of qPCR monitoring of infectious laryngotracheitis virus in chicken house dust and its role in control of a major outbreak. Avian Diseases 66, 1–9.
| Field application of qPCR monitoring of infectious laryngotracheitis virus in chicken house dust and its role in control of a major outbreak.Crossref | GoogleScholarGoogle Scholar |
Coppo MJC, Devlin JM, Noormohammadi AH (2012) Comparison of the replication and transmissibility of an infectious laryngotracheitis virus vaccine delivered via eye-drop or drinking-water. Avian Pathology 41, 99–106.
| Comparison of the replication and transmissibility of an infectious laryngotracheitis virus vaccine delivered via eye-drop or drinking-water.Crossref | GoogleScholarGoogle Scholar |
Davidson I, Natour-Altory A, Raibstein I, Kin E, Dahan Y, Krispin H, Elkin N (2018) Monitoring the uptake of live avian vaccines by their detection in feathers. Vaccine 36, 637–643.
| Monitoring the uptake of live avian vaccines by their detection in feathers.Crossref | GoogleScholarGoogle Scholar |
De Wit S (2013) Underestimation of the difficulties of vaccination against viral respiratory diseases by mass application methods. In ‘Proceedings XVIIIth congress of the World Veterinary Poultry Association, Session J: Viral respiratory diseases 2: IBV, metapneumovirus, others’. Vol. 18, pp. 63–67. 19–23 August. (WVPA: Nantes, France). Available at https://en.engormix.com/MA-poultry-industry/eventos/xviii-congress-2013-wvpa-t1658-conferences.htm [Accessed 16 July 2021]
Dugar D (2018) Skewness and Kurtosis. Available at https://codeburst.io/2-important-statistics-terms-that-you-need-to-know-in-data-science-skewness-and-kurtosis-388fe94eeaa [Accessed 10 July 2021]
Feedwater (undated) DeCHLOR®. Available at https://feedwater.co.uk/product/dechlor-dechlorination/ [Accessed 16 July 2021]
Fraser J (2019) Infectious laryngotracheitis in NSW chickens. NSW Animal Health Surveillance. NSW Government quarterly, July to September. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0009/1190718/animal-health-surveillance-2019-3.pdf [Accessed 13 July 2021]
García M (2017) Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Veterinary Microbiology 206, 157–162.
| Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry.Crossref | GoogleScholarGoogle Scholar |
Groves PJ, Williamson SL, Sharpe SM, Gerber PF, Gao YK, Hirn TJ, Walkden-Brown SW (2019) Uptake and spread of infectious laryngotracheitis vaccine virus within meat chicken flocks following drinking water vaccination. Vaccine 37, 5035–5043.
| Uptake and spread of infectious laryngotracheitis vaccine virus within meat chicken flocks following drinking water vaccination.Crossref | GoogleScholarGoogle Scholar |
Hilbink F, Smit T, Yadin Y (1981) Drinking water vaccination against infectious laryngotracheitis. Canadian Journal of Comparative Medicine 45, 120–123.
Hilbink FW, Oei HL, van Roozelaar DJ (1987) Virulence of five live vaccines against avian infectious laryngotracheitis and their immunogenicity and spread after eyedrop or spray application. Veterinary Quarterly 9, 215–225.
| Virulence of five live vaccines against avian infectious laryngotracheitis and their immunogenicity and spread after eyedrop or spray application.Crossref | GoogleScholarGoogle Scholar |
Keck L (2018) ILT vaccination decisions can be difficult balancing act in broilers. Poultry Health Today. Available at https://poultryhealthtoday.com/ilt-vaccination-decisions-can-be-difficult-balancing-act-in-broilers/ [Accessed 13 May 2018]
Kirkpatrick NC, Mahmoudian A, O’Rourke D, Noormohammadi AH (2006) Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes. Avian Diseases 50, 28–33.
| Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes.Crossref | GoogleScholarGoogle Scholar |
Korsa MG, Browning GF, Coppo MJC, Legione AR, Gilkerson JR, Noormohammadi AH, Vaz PK, Lee S-W, Devlin JM, Hartley CA (2015) Protection induced in broiler chickens following drinking-water delivery of live infectious laryngotracheitis vaccines against subsequent challenge with recombinant field virus. PLoS ONE 10, e0137719
| Protection induced in broiler chickens following drinking-water delivery of live infectious laryngotracheitis vaccines against subsequent challenge with recombinant field virus.Crossref | GoogleScholarGoogle Scholar |
MSD (undated) Nobilis ILT vaccine approved label. Available at Australian Pesticides and Veterinary Medicines Authority. Available at https://websvr.infopest.com.au/LabelRouter?LabelType=L&Mode=1&ProductCode=59802 [Accessed 13 July 2021]
Ou S-C, Giambrone JJ (2012) Infectious laryngotracheitis virus in chickens. World Journal of Virology 1, 142–149.
| Infectious laryngotracheitis virus in chickens.Crossref | GoogleScholarGoogle Scholar |
Robertson GM, Egerton JR (1981) Replication of infectious laryngotracheitis virus in chickens following vaccination. Australian Veterinary Journal 57, 119–123.
| Replication of infectious laryngotracheitis virus in chickens following vaccination.Crossref | GoogleScholarGoogle Scholar |
Sabir AJ, Olaogun OM, O’Rourke D, Fakhri O, Coppo MJC, Devlin JM, Konsak-Ilievski B, Noormohammadi AH (2020) Full genomic characterisation of an emerging infectious laryngotracheitis virus class 7b from Australia linked to a vaccine strain revealed its identity. Infection, Genetics and Evolution 78, 104067
| Full genomic characterisation of an emerging infectious laryngotracheitis virus class 7b from Australia linked to a vaccine strain revealed its identity.Crossref | GoogleScholarGoogle Scholar |
StatSoft, Inc. (2003) STATISTICA (data analysis software system), version 6. Available at www.statsoft.com
Williamson SL, Jones M, Sharpe SM, Pavic A (2019) Typing NSDW ILT: 2013–present. In ‘Proceedings scientific meeting of Australasian Veterinary Poultry Association’. 20–21 February. (Sydney, Australia)
Zoetis (undated) Zoetis Poulvac Laryngo product brochure: drinking water vaccination technique. Available at https://www.zoetis.com.au/product-class-new/vaccines/poulvac-laryngo-a20.aspx [Accessed 12 July 2021]


