Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 3. Immunological parameters and gene expression
Hiep Thi Dao A B , Nishchal K. Sharma A , Sarbast K. Kheravii A , Emma J. Bradbury C , Shu-Biao Wu
A B , Nishchal K. Sharma A , Sarbast K. Kheravii A , Emma J. Bradbury C , Shu-Biao Wu  A and Robert A. Swick
A and Robert A. Swick  A *
A *
A School of Environmental and Rural Science, Faculty of Science, Agriculture, Business and Law, University of New England, Armidale, NSW 2351, Australia.
B Faculty of Animal Science, Vietnam National University of Agriculture, Trau Quy Town, Gia Lam District, Hanoi 100000, Vietnam.
C Ridley AgriProducts, Melbourne, Vic. 3000, Australia.
Animal Production Science 62(13) 1266-1279 https://doi.org/10.1071/AN21395
Submitted: 28 July 2021 Accepted: 23 May 2022 Published: 1 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The impact of necrotic enteritis (NE) on acute-phase proteins, interleukins, blood mineral profiles, and gene expression have not been well documented.
Aims: This study aimed to determine the effects of l-arginine (Arg) or l-citrulline (Cit) supplementation on serum immunological parameters, serum mineral composition and gene expression in broilers fed reduced-protein diets (RP) during subclinical NE challenge.
Methods: Ross 308 cockerels (n = 720) were randomly assigned to six experimental treatments, with eight replicates of 15 birds per pen. The treatments were standard protein without and with NE challenge (SP−, SP+); reduced protein (2% points lower crude protein) without and with NE challenge (RP−, RP+), RP plus added Arg (103% of Ross 308 requirement) with NE challenge (RPA+) and RPC+ where supplemental Arg in RPA+ was replaced with Cit. A 2 × 2 factorial arrangement was employed for the first four treatments. Additionally, treatments SP+, RP+, RPA+, and RPC+ were analysed by one-way ANOVA.
Key results: The NE × protein interactions indicated that serum calcium concentration decreased in birds fed the RP diets only when challenged with NE (P < 0.05). The NE × protein interactions showed that the NE challenge downregulated the mRNA expression of jejunal y+ L amino acid transporter-2, and mucin 2 only in birds fed the RP diets (P < 0.05). Feeding the RP decreased expression of catenin alpha 1, but increased expression of claudin 5 and tight junction protein genes compared with the SP (P < 0.05). Birds in the RPC+ treatment had increased gene expression of tight junction protein and claudin 5 compared with the SP+ treatment (P < 0.05).
Conclusions: Dietary protein level and infection with NE both have an impact on immune response and expression of genes involved in immunity and nutrient digestibility. In part replacement of Arg with Cit in the RPC diet may have beneficial effects on gene expression in NE-challenged birds.
Implications: Feeding RP diets may alleviate a decline in growth during subclinical NE by increasing gene expression of tight junction proteins compared with the SP diets.
Keywords: alpha-1 acid glycoprotein, arginine, calcium, citrulline, interleukins, low protein, minerals, ovotransferrin.
Introduction
Necrotic enteritis (NE), caused by Clostridium (C.) perfringens has been estimated to cause losses of USD6 billion annually to the global broiler industry (Wade and Keyburn 2015). The negative effects of NE on growth, feed conversion, intestinal morphology and permeability, production of short-chain fatty acids, and microbiota population have been extensively studied (Wu et al. 2016; Latorre et al. 2018; Gharib-Naseri et al. 2019; Hilliar et al. 2020). Besides, challenge with NE and infection with Newcastle disease have been shown to reduce serum calcium (Ca) and phosphorus (P); however, the effects on serum levels of other minerals such as sodium (Na), potassium (K), and zinc (Zn) have not been reported (Fernandez et al. 1994; Igwe et al. 2018; Zanu et al. 2020a). The impact of NE on blood immunological parameters such as acute-phase proteins and interleukins and blood mineral profiles have not been well documented. Acute-phase proteins including alpha-1 acid glycoprotein and ovotransferrin are primarily synthesised in the liver as part of the acute-phase response and serve as a key part of the innate immune response to external stresses such as tissue injury and infection (O’Reilly and Eckersall 2014; Zulkifli et al. 2014). Alpha-1 acid glycoprotein acts as an immune-regulator influencing T-cell function, while ovotransferrin modulates heterophil and macrophage function and possesses antimicrobial properties via sequestration of iron (Murata et al. 2004). Interleukins (IL) such as IL-1 and IL-6 are pro-inflammatory agents and influence the production of acute-phase proteins (Marinkovic et al. 1989; Zulkifli et al. 2014). A recent study by Xue et al. (2017) showed that NE challenge increased serum IL-1 and immunoglobulin G (IgG) concentrations.
There is evidence that feeding reduced-protein diets (17% and 15% crude protein in grower and finisher phases respectively) downregulates gene expression of tight junction proteins such as zonula occludens-2 and upregulate expression of Na+-dependent glucose transporter 1 in the ileum of broiler chickens (Barekatain et al. 2019a). Furthermore, supplementation of arginine (Arg) to reduced-protein diets (19.4% and 17.7% crude protein in grower and finisher phases respectively) has been reported to upregulate expression of the claudin-1 gene but did not affect the expression of the claudin-3 gene in broilers subjected to leaky-gut model, compared with those offered the reduced-protein diets alone or reduced-protein diets supplemented with either l-glutamine or glycine (Barekatain et al. 2019b). Claudins are important tight junction proteins associated with intestinal permeability and are considered as one of the most relevant immune-histochemical markers to evaluate the tight junction function (Guo et al. 2018). Dietary supplementation of Arg has been reported to increase the expression of antioxidant genes and reduce the expression of pro-inflammatory genes in the small intestine and adipose tissue in rats (Fu et al. 2005; Jobgen et al. 2009). In broiler chickens, Tan et al. (2014) found that Arg supplementation decreased mucosal secretory IgG concentrations and gene expression of jejunal pro-inflammatory interleukin (IL-1b) during a coccidial vaccine challenge. As a metabolite of Arg, citrulline (Cit) has been demonstrated to have Arg-sparing effects in chickens and even more effective than Arg in increasing blood Arg concentrations (Su and Austic 1999; Lassala et al. 2009; Dao et al. 2021a, 2021b). The objective of the current study was to determine the effects of Arg and Cit supplementation in reduced-protein diets on serum acute-phase protein, IL-6, immunoglobulins, mineral composition, and expression of nutrient-related, tight junction protein, mucin, and inflammatory-related genes in broilers during NE challenge.
Materials and methods
Experimental design and diets
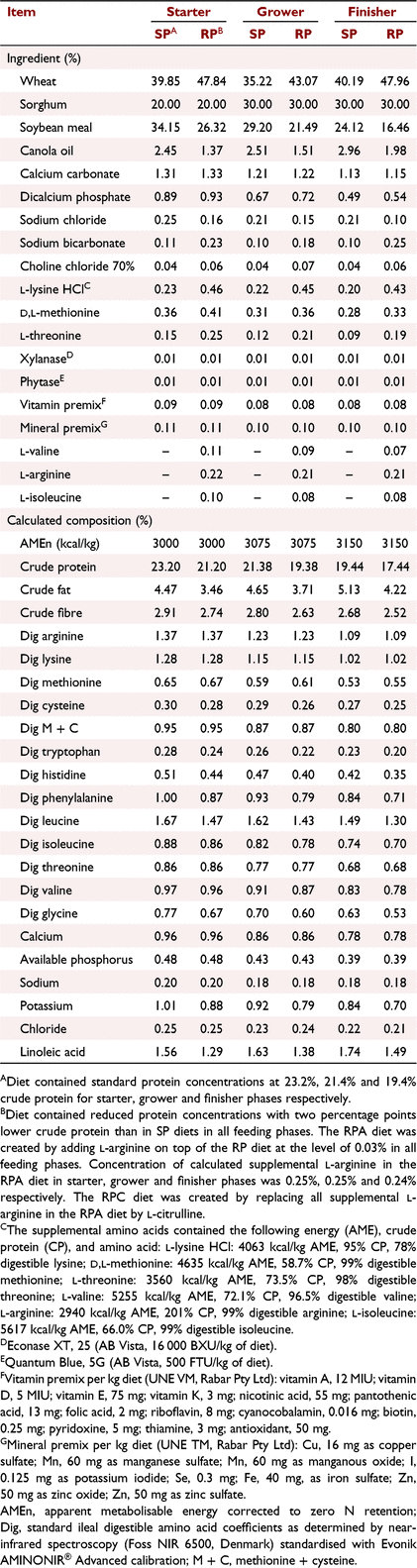
The study was implemented at the Centre of Animal Research and Teaching at the University of New England, Armidale, New South Wales, Australia, approved by its Animal Ethics Committee (Approval number AEC19-119), and met the requirements of the Australian code of practise to care and use of animals for scientific purposes (NHMRC 2013). Day-old Ross 308 cockerels (n = 720) were allocated to 48 equal-sized floor pens (120 × 80 cm) with 15 birds per pens. Starting pen weights were similar across treatments. Birds were grown to mimic commercial conditions with hardwood shavings as bedding material in environmentally controlled rooms. Feed and water were provided ad libitum throughout the 35-day feeding study. The temperature, lighting, and ventilation conditions followed Ross 308 recommendations (Aviagen 2014a). Six treatments were used in this study, with eight replicate pens per treatment. The treatments were standard protein diet without NE challenge (SP−), SP treatment with NE challenge (SP+), reduced-protein diet balanced with crystalline amino acids without NE challenge (RP−), RP− with NE challenge (RP+), RP+ with additional Arg to 103% of requirement (equal to 15% additional supplemental crystalline Arg, RPA+), RP+ with Cit replacing all supplemental Arg in RPA+ (RPC+). A 2 × 2 factorial arrangement was employed for the first four treatments. Factors were NE (− or +) and protein level (SP or RP). All six treatments were analysed by one-way ANOVA. Crude protein was 2% points lower in RP than in SP diets for all feeding phases. The concentrations of essential amino acids in the RP diet were equivalent to the SP diet and in accordance with Ross 308 broiler nutrition specifications (Aviagen 2014b). Feeds were provided as crumbles for starter (Days 0–10), and pellets for grower (Days 10–24), and finisher (Days 24–35). Supplementation levels of added crystalline Arg in the RP treatments in starter, grower, and finisher phases were 0.217%, 0.213%, and 0.212% respectively. Levels of added crystalline Arg in the RPA+ treatment in starter, grower, and finisher phases were 0.249%, 0.245%, and 0.244% respectively. Concentrations of Cit in the RPC+ treatment were equivalent to the supplemental Arg level in the RPA+ treatment. Details on diet composition and nutrient contents are presented in Tables 1 and 2. Arginine and Cit were supplemented to the RP diets at the expense of wheat. More detailed information on the feed analysis was provided in the first part of this series (Dao et al. 2022a).
|

|
Necrotic-enteritis challenge
Subclinical NE was introduced following procedures previously described by Rodgers et al. (2015). Birds in the RPA+ and RPC+ treatments and half of the birds in the SP and RP treatments (challenged) were orally inoculated with 1 mL of sterile phosphate-buffered solution (PBS) containing a vaccine strain of Eimeria, with 5000 sporulated oocysts of Eimeria acervulina, 5000 sporulated oocysts of Eimeria maxima, and 2500 sporulated oocysts of Eimeria brunetti (Eimeria Pty Ltd, Ringwood, Victoria, Australia) on Day 9 and 1 mL of C. perfringens with an approximate concentration of 108 CFU (EHE-NE18 strain, Commonwealth Scientific and Industrial Research Organization, Geelong, Victoria, Australia) in a starch thioglycollate broth on Day 14. The remaining birds in the SP and RP treatments were given 1 mL of sterile PBS on Day 9 and 1 mL of sterile thioglycollate broth media as a sham treatment on Day 14 (unchallenged control groups).
Measurements of serum immunological parameters and minerals
On Day 16, two birds per pen were randomly collected, weighed, electrically stunned (MEFE CAT 44N, Mitchell Engineering Food Equipment, Clontarf, Queensland, Australia), and euthanised by decapitation for sample collection. Blood samples (from a jugular vein) were collected in vacutainers (Becton, Dickinson UK Ltd, Plymouth, UK) that contained spray-coated silica and a polymer gel, and centrifuged at 3000g at 4°C for 10 min to separate the serum. Serum samples were stored at −20°C until further analysis. Concentrations of alpha-1 acid glycoprotein, ovotransferrin, IL-6, IgA, IgM, and IgG in serum were quantified using an indirect enzyme-linked immunosorbent assay (ELISA) commercial kits according to manufacturer’s instructions (catalogue numbers ab157690 (Abcam, Cambridge, MA, USA), ab157694 (Abcam), ELG-IL6 (RayBiotech, Norcross, GA, USA), ab157691 (Abcam), ab157692 (Abcam) and KT-619 (Kamiya Biomedical Company, Seattle, WA, USA) respectively). The concentrations of K, Na, Ca, and P in the serum were determined using the commercial kits in a Thermo Scientific™ Indiko™ Plus clinical chemistry analyser (Thermo Fisher Scientific Inc., Waltham, MA, USA) following the manufacturer’s instruction. The kits used were as follows: sodium (Na), Enzymatic Colorimetric Test, catalogue number NA 3851 (Randox Laboratories Ltd, County Antrim, UK); potassium (K), U.V. Test, catalogue number PT 3852 (Randox Laboratories Ltd); calcium, REF number 981772 (Thermo Fisher Scientific Inc., Waltham, MA, USA); and phosphorus, REF number 981890 (Thermo Fisher Scientific Inc.). The blood serum Zn concentration was determined using a Zinc (Zn) kit, catalogue number ZN 2341 (Randox Laboratories Ltd), following the manufacturer’s instruction, and the results were read on a SpectraMax M2e plate reader (Molecular Devices, California, USA).
RNA extraction and cDNA synthesis
Jejunal tissues from two birds per pen were collected for gene expression on Day 16. Approximately 2 cm of the proximal jejunal tissues were excised, carefully flushed with chilled sterile phosphate-buffered saline (PBS), then placed in 2-mL Eppendorf tubes containing RNAlater™ Solution (Invitrogen by Thermo Fisher Scientific). The jejunal tissues were stored at 4°C for 4 h, then at −20°C until further analysis. For RNA extraction, approximately 25 mg of the tissue sample was weighed in a 2-mL Eppendorf tube containing a 3-mm bead. Then, 350 μL of lysis buffer RLY-β-ME was added into the tubes, and samples were homogenised for 5 min by using a Tissuelyser II. Total RNA of the tissue sample was extracted using ISOLATE II RNA Mini Kit (Bioline, Sydney, NSW, Australia), as per the manufacturer’s instructions, with the inclusion of a DNase I digestion step to eliminate the genomic DNA. After extraction, the RNA concentration and purity were checked using a NanoDrop ND-8000 spectrophotometer (Thermo Fisher Scientific). The integrity of RNA samples was checked on the Agilent 2100 Bioanalyser (Agilent Technologies Inc., Waldbronn, Germany) by using an RNA 6000 Nano kit (Agilent Technologies Inc., Palo Alto, CA, USA), following the manufacturer’s instructions. The RIN values of the samples were ranged between 8 and 9.8 in the present study. The total RNA samples were reverse-transcribed to cDNA by using the SensiFAST cDNA Synthesis Kit (Bioline, Sydney, NSW, Australia) in a Rotorgene 6000 real-time (RT) polymerase chain reaction instrument (Corbett, Sydney, NSW, Australia), as per the manufacturer’s instructions. Synthesised cDNA samples were diluted 10 times with nuclease-free water and kept at −20°C until further analysis.
Quantitative polymerase chain reaction (RT-qPCR)
Quantitative polymerase chain reaction (qPCR) was performed using an SYBR Green kit SensiFAST SYBR No-ROX (Bioline, Sydney, NSW, Australia) on a Rotorgene 6000 real-time PCR machine (Corbett Research, Sydney, NSW, Australia). The PCR reaction was performed in a volume of 10 μL containing 5 μL of 2 × SensiFAST, 400 mmol/L of each primer, 2.2 μL of nuclease-free water, and 2 μL of 10 × diluted cDNA sample. To determine two suitable reference genes for the analysis, the geNorm module in qbase+ software ver. 3.0 (Biogazelle, Zwijnbeke, Belgium) was used to calculate the gene-expression stability (geNorm M) from 10 widely used house-keeping genes (hydroxymethylbilane synthase (HMBS), succinate dehydrogenase complex flavoprotein subunit A (SDHA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), hypoxanthine-guanine phosphoribosyltransferase (HPRT1), thyroxine-binding protein (TBP), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ), β-actin (ACTB), ribosomal protein L4 (RPL4), nuclear ribosomal RNA small subunit (18S), and albumin (ALB)). On the basis of the expression stability of the reference genes, HPRT1 and TBP were chosen to normalise the target genes in the jejunum because they had the lowest M values compared with the other reference genes (M = 0.311 for both genes). The candidate genes analysed were aminopeptidase N (APN), solute carrier family 7, member 9 (bo,+AT), cationic amino acid transporter-1 (CAT1), cationic amino acid transporter-2 (CAT2), claudin 1 (CLDN1), claudin 5 (CLDN5), excitatory amino acid transporter 3 (EEAT3), junctional adhesion 2 (JAM2), mucin 2 (MUC2), peptide transporter-1 (PepT1), peptide transporter-2 (PepT2), tight junction protein (TJP1), y+ L amino acid transporter-2 (y+LAT2), E-cadherin (CDH1), interferon-gamma (IFN-γ), nitric oxide synthase 2 (NOS2), and protein kinase AMP-activated non-catalytic subunit gamma 2 (PRKAG2). The relative quantification of genes using the arithmetic mean method in the qBase+ software was exported to SAS 9.3 package (SAS Institute, Inc., Cary, NC, USA) for further analysis.
Primers for qPCR were either sourced from published papers or designed using the NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), as shown in Table 3. All primers used in this study were checked for specificity by using an Agilent DNA 1000 Kit (Agilent Technologies, Inc., Waldron, Germany) on an Agilent 2100 Bioanalyser (Agilent Technologies, Inc.).

|
Data analyses
R Commander (ver. 3.3.1, R Foundation for Statistical Computing, Vienna, Austria) was used to analyse data. All data were tested for normality and variance homogeneity before analysis. First, a two-way ANOVA was used to test the interaction between NE challenge (no or yes) and protein level (SP or RP), excluding RPA+ and RPC+ treatments (2 × 2 factorial arrangement of treatments). Then, one-way ANOVA was used to test statistical differences among the four NE-challenged treatments (SP+, RP+, RPA+, and RPC+). Tukey’s post hoc test was used to identify pairwise differences between the treatments from significant ANOVA results. The P-value of <0.05 was considered significant.
Results
Serum immunological parameters
Results on concentrations of immunological parameters, including IL-6, IgA, IgM, IgG, alpha-1 acid glycoprotein, and ovotransferrin in the blood serum on Day 16 are presented in Table 4. No NE × protein interactions were detected for any of the above immunological parameters. Necrotic-enteritis challenge as the main effect increased the concentrations of serum alpha-1 acid glycoprotein (P < 0.05) and IgA (P < 0.05) on Day 16. Feeding the RP diets decreased both alpha-1 acid glycoprotein (P < 0.001) and ovotransferrin (P < 0.001) concentrations in the blood serum on Day 16, as shown by the main effect of protein supplementation level in Table 4. Concentrations of serum IL-6, IgM, and IgG were not affected by either NE challenge or protein supplementation level (Table 4). Supplementation of either Arg or Cit to the RP+ treatment did not affect concentrations of serum IL-6, IgA, IgM, IgG, alpha-1 acid glycoprotein, and ovotransferrin in the respective groups (P > 0.05; Table 4).

|
Serum mineral composition
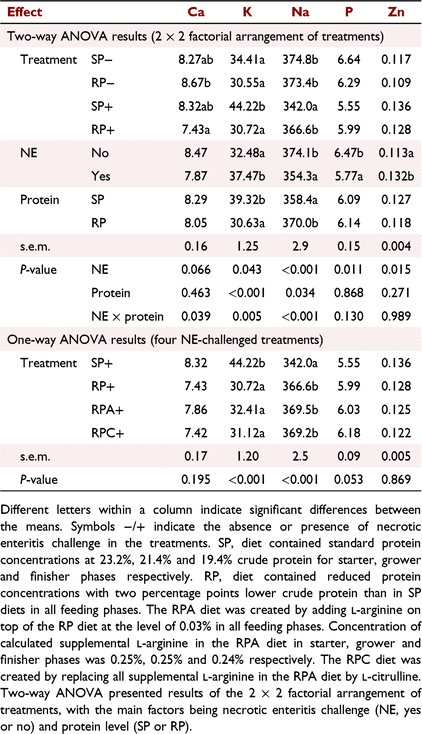
Results for serum mineral concentrations of Ca, K, Na, P, and Zn on Day 16 are shown in Table 5. NE × protein interactions were obtained for Ca (P < 0.05), K (P < 0.01) and Na (P < 0.001) concentration. The results indicated that the NE challenge decreased serum Ca concentration only in birds fed the RP diets. Whereas the NE challenge increased serum K concentration and decreased serum Na concentration only in birds fed the SP diets (Table 5). Necrotic-enteritis challenge as the main effect increased Zn concentration (P < 0.05) but decreased P concentration (P < 0.05) in the blood serum. Supplementation of either Arg or Cit to the RP+ treatment did not affect serum Ca, K, Na, P, and Zn concentrations in the respective groups (P > 0.05; Table 5). A negative correlation was found between serum Na and K (P < 0.001) concentration, while serum Na concentration was positively correlated with serum P concentration (P < 0.001; Fig. 1).
|
|
|
Expression of jejunal nutrient-related genes
Relative mRNA expression of nutrient-related genes in the jejunum on Day 16, including APN, bo,+AT, PepT1, PepT2, CAT1, CAT2, EEAT3, y+ LAT2, and PRKAG2 are shown in Table 6. NE × protein interactions were observed for bo,+AT (P < 0.05) and y+ LAT2 (P < 0.05). The NE challenge downregulated the mRNA expression of bo,+AT in both SP- and RP-fed birds, but greater downregulation was observed in RP-fed birds. The NE challenge downregulated the mRNA expression of y+ LAT2 only in birds fed the RP diets (Table 6). Necrotic-enteritis challenge as the main effect decreased the expression of APN (P < 0.001), PepT1 (P < 0.001), EEAT3 (P < 0.001), and PRKAG2 (P < 0.01) and increased the expression of PepT2 (P < 0.05) and CAT1 (P < 0.01; Table 6). Additional supplementation of Arg to the RP+ treatment decreased PepT2 expression compared with the RP+ treatment (P < 0.01), and increased CAT1 expression compared with the SP+ treatment (P < 0.001; Table 6). Supplementation of Cit to the RP+ treatment increased PRKAG2 expression compared with the SP+ treatment (P < 0.05; Table 6).

|
Expression of jejunal tight junction protein, mucin, and inflammatory-related genes
Results on relative mRNA expressions of tight junction protein and inflammatory-related genes in the jejunum on Day 16, including CLDN1, CLDN5, JAM2, TJP1, CDH1, NOS2, IFN-γ, and MUC2 are presented in Table 7. A NE × protein interaction was detected for MUC2 (P < 0.05), where NE challenge downregulated MUC2 expression only in birds fed the RP diets (Table 7). Necrotic-enteritis challenge as the main effect decreased the expression of CLDN5 (P < 0.05) and TJP1 (P < 0.001) and increased the expression of NOS2 (P < 0.001) and IFN-γ on Day 16 (P < 0.001; Table 7). Feeding the RP diet increased the expression of CLDN5 (P < 0.01) and TJP1 (P < 0.01), and decreased the expression of CDH1 (P = 0.05), regardless of the NE challenge on Day 16 (Table 7). Supplementation of both Arg and Cit to the RP+ treatment increased TJP1 expression and Cit supplementation increased CLDN5 expression compared with the SP+ treatment (P < 0.05; Table 7).

|
Discussion
Interleukin-6 is known as a primary initiator for the production of acute-phase proteins (Marinkovic et al. 1989; Le Floc’h et al. 2004). Increasing concentrations of pro-inflammatory cytokines, including IL-1 and IL-6 following an infection, act as a signal for the liver to increase the production of acute-phase proteins such as alpha-1 acid glycoprotein and ovotransferrin to prepare the host to fight against the infection, resulting in the increased concentrations of these proteins in the blood stream (Tosi 2005). In the current study, although serum IL-6 concentration was not altered following the NE challenge, the increased serum alpha-1 acid glycoprotein and IgA concentrations in NE-challenged birds indicated that there was an inflammatory response. The absence of treatment effects on IL-6 concentration in the current study may be attributed to the following factors: (1) collection time of the serum sample because it can be rapidly cleared from circulation after the stimulus (O’Reilly and Eckersall 2014); (2) the upregulation of jejunal IFN-γ gene in NE-challenged birds might decrease the synthesis of IL-6 (Schroder et al. 2004).
Acute-phase proteins have been used as disease biomarkers in humans and veterinary medicine (O’Reilly and Eckersall 2014). The results of the current study and Saleem (2013) suggest that serum alpha-1 acid glycoprotein concentration may be more sensitive than is ovotransferrin to evaluate disease status in birds. Likewise, determination of serum IgA concentration may be more effective than is determination of IgM or IgG to assess immune response in NE-challenged birds, and that might be the reason for it being widely used in diagnosing Eimeria infection (Yun et al. 2000). Noticebly, reduced serum Ca and P concentrations have been reported in birds infected with NE and Newcastle disease, and have been considered as an indicator for the disease infection (Fernandez et al. 1994; Igwe et al. 2018; Zanu et al. 2020a). In the current study, the decreased serum Ca and P concentrations in NE-challenged birds might be attributed to reductions in feed intake, villus height, and apparent villus area, and increases in gut permeability compared with the unchallenged group, as reported in previous parts of this series (Dao et al. 2022a, 2022b). Reduced Ca and P digestibility was also reported in NE-infected birds compared with the uninfected birds by Paiva et al. (2014).
Intestinal tight junctions are a major defence against pathogenic bacteria but also regulate nutrient absorption and homeostasis in the gut (Gasbarrini and Montalto 1999). In the current study, the lower expression levels of tight junction genes, including CLDN5 and TJP1, indicate the impairment of tight junction function and gut permeability in NE-challenged birds. This is in agreement with the observations reported in the previous studies (Park et al. 2008; Gharib-Naseri et al. 2020). Similarly, necrotic enteritis downregulated various nutrient-related genes examined in the current study including PepT1, EEAT3, and PRKAG2. This was consistent with the reduced feed efficiency and impaired jejunal morphology (reduced villus height, villus height to crypt depth ratio, and apparent villus area) observed in NE-challenged birds compared with unchallenged birds, as has been reported in previous parts of this series (Dao et al. 2022a, 2022b). Various nutrient transporters are located on the tip of the villi (Obst and Diamond 1992). The alteration in jejunal morphology, as shown by villi atrophy and sloughing of the brush border membrane of the enterocytes due to NE challenge (as reported in the second part of this series, Dao et al. 2022b), was likely to be the main reason for the lower expression levels of nutrient-related genes in NE-challenged birds in the current study. The digestive enzyme APN cleaves neutral and basic amino acids from the N-terminal end of peptides (Sanderink et al. 1988). The lower APN expression in NE-challenged birds indicates decreased amino acid digestion at the brush border membrane that may reduce substrates for the peptide and amino acid transport system and expression of related genes. In addition, the expression levels of amino acid transporter genes might be associated with feed intake. For instance, higher PepT1 gene expression has been reported in feed-restricted chicks (3–14 days old) raised under thermoneutral conditions, than in those offered the ad libitum feed access (Gilbert et al. 2008). In the current study, PepT1 was downregulated while PepT2 and CAT1 were upregulated in NE-challenged birds compared with the unchallenged group. The differences in the bird age and experimental design best explain the discrepancies between the studies.
The expression of tight junction proteins can be influenced by the secretion of inflammatory cytokines such as IFN-γ (Sakaguchi et al. 2002). Also, there is evidence that expression of NOS2 in macrophages and other cell types was induced by IFN-γ concentration and/or bacterial lipoproteins/exotoxins (Braun et al. 1999; Flak and Goldman 1999). In the current study, increased jejunal IFN-γ and NOS2 expression levels were observed in NE-challenged birds compared with the unchallenged group, suggesting increased inflammatory responses in the NE group, similar to what has been reported by others (Collier et al. 2008; Lee et al. 2018; Emami et al. 2019). The increased production of IFN-γ is needed for the re-arrangement and redistribution of the intestinal actin cytoskeleton that may help increase paracellular permeability (Bruewer et al. 2005). Nitric oxide is involved in innate immunity as a toxic agent against pathogenic organisms. Equally, nitric oxide has been implicated as an anti-inflammatory or immunosuppressive agent through its inhibitory or apoptotic effects on cells (Coleman 2001). The beneficial effect of Arg on gut permeability is associated with the activity of NOS2 (Meng et al. 2017; He et al. 2018). However, no difference in NOS2 gene expression was observed in the current study when Arg or Cit was added to the RP diet for challenged birds. Higher Arg supplemental concentrations as well as effects of intestinal microorganisms on the Arg metabolism and interactions among Arg, cytokines, pro-inflammatory agents, and other amino acids during NE challenge should be investigated to clarify possible mechanisms.
There are few if any reports that have examined the effects of protein concentration, Arg/Cit supplementation, and/or NE challenge on the concentrations of serum alpha-1 acid glycoprotein, ovotransferrin and minerals. Feeding the RP diets decreased both serum alpha-1 acid glycoprotein and ovotransferrin concentrations compared with the SP diet in the current study. The lower concentrations of phenylalanine, tyrosine, and tryptophan in the RP diets than in the SP diets might partly explain the observations, as they are essential components of acute-phase proteins (Reeds et al. 1994). Also, the results of the current study illustrated that dietary protein concentrations could interact with the NE challenge and alter serum mineral concentrations in birds. This was shown by the decreased serum Ca concentration in RP-fed birds, and decreased serum Na and increased K concentrations in SP-fed birds during challenge with NE. This might be due to altered mineral metabolism during the NE challenge, and/or differences in mineral composition between the SP and RP diet.
In the current study, the NE challenge downregulated gene expression of bo,+AT in both SP- and RP-fed birds, but greater downregulation was observed in RP-fed birds. Also, feeding RP diets downregulated gene expression of y+ LAT2 only during the NE challenge. It has been known that bo,+AT is responsible for transporting Na+-independent cationic and zwitterionic amino acids at the brush border membrane, and y+ LAT2 is a Na+-independent cationic and Na+-dependent neutral amino acid transporter at the basolateral membrane (Verrey et al. 2004; Gilbert et al. 2008). The lower feed intake in NE-challenged birds than in the unchallenged group and lower concentrations of amino acids such as histidine, alanine, serine, glycine, leucine, and phenylalanine in the RP diets than in the SP diets in the current study might reduce substrates for amino acid transport systems and, consequently, lead to the downregulation of these genes. Lower bo,+AT gene expression has been reported in birds fed a corn gluten meal-based diet than in those offered a soybean meal-based diet (Gilbert et al. 2008). In a similar manner, the lower inclusion of soybean meal and a higher inclusion of wheat in the RP diets than in the SP diets might influence the expression of jejunal bo,+AT gene in respective groups in the current study. Additionally, differential cell composition in the gut mucosa during NE challenge may also alter relative gene expression of nutrient transporters in this study.
Mucin-2 as a major component of intestinal mucus plays a crucial role in maintaining the thickness of the mucous layer and serves as a physical barrier preventing epithelial cells from direct contact with the intestinal microorganisms (Sovran et al. 2016). E-cadherin encoding by the CDH1 gene is responsible for cell to cell adhesion, the organisation, and maintenance of epithelial cells, and plays essential roles in mediating intercellular cohesion and tissue development (Jiang 1996; Bhatt et al. 2013). In the current study, the jejunal MUC2 gene was downregulated in birds fed the RP diets only when they were challenged with NE. Also, feeding the RP diets downregulated the expression of CDH1 compared with the SP diets, regardless of the NE challenge. These results reflect unfavourable effects of the RP diets on the mucosal layer and intercellular strength during the NE challenge. However, feeding the RP diets resulted in a higher expression of CLDN5 and TJP1 than feeding the SP diets. This result was consistent with the increased growth performance in RP-fed birds compared with those offered the SP diet, as reported in the first part of this series (Dao et al. 2022a).
Supplementation of Arg to the RP diets for challenged birds decreased PepT2 gene expression, and increased CAT1 gene expression, whereas Cit supplementation to the RP diet for challenged birds did not affect CAT1 gene expression in the current study. This differential effect of Arg and Cit might be attributed to the difference in transportation/absorption routes of Arg and Cit in the small intestine, and the lower concentration of Arg in the RPC diet than the RPA diet in the current study. It is well known that CAT1 mediates the transport of cationic amino acids such as lysine, Arg, and histidine with high affinity (Gilbert et al. 2007), whereas Cit is mainly transported and absorbed in the intestine by transporters belonging to the B0,+, L, and b0,+ systems, such as bo,+AT, and LAT1 (Bahri et al. 2013). The gene expression of the CAT family has been reported to depend on substrate availability (Zhang et al. 2019). However, Cit supplementation to the RP diet for challenged birds increased PRKAG2 expression compared with the SP+ treatment in the current study. Expression of the PRKAG2 gene has been reported to be closely associated with the feed intake and/or feed efficiency in chickens and cattle (Lindholm-Perry et al. 2014; Jin et al. 2016). Citrulline supplementation to the RP+ treatment also increased expression of CLDN5 and TJP1, which are important tight junction proteins regulating nutrient absorption and homeostasis. Thus, the upregulation of PRKAG2, CLDN5, and TJP1 genes might partly explain the higher feed efficiency in challenged birds fed the RPC diets than in those fed the SP diets, as reported in the first part of this series (Dao et al. 2022a). Additional supplementation of Arg and Cit to the RP diet for challenged birds did not affect jejunal MUC2 expression in the present study. However, a higher dietary Arg supplemental level may increase MUC2 expression and is a worthwhile subject for further studies.
Conclusions
The NE challenge reduced nutrient digestion and absorption and induced an immune response in infected birds by downregulating digestive enzyme, nutrient-related and tight junction protein genes, increasing serum alpha-1 acid glycoprotein concentration and upregulating gene expression of pro-inflammatory agents including NOS2 and IFN-γ. Increased gene expressions of CLDN5 and TJP1 were observed in birds fed the RP diets compared with those fed the SP diets. The NE challenge downregulated the expression of bo,+AT in both SP- and RP-fed birds, but greater downregulation was observed in the RP-fed birds. These results were most likely due to the differences in the composition and amino acid concentrations of the RP diets compared with the SP diets. Supplementation of Arg to the RP diet for challenged birds decreased PepT2 gene expression and increased CAT1 and TJP1 gene expression. Whereas Cit supplementation to the RP diet for challenged birds increased the expression of PRKAG2, CLDN5, and TJP1. Thus, in part replacement of Arg with Cit in the RPC diet may have beneficial effects on gene expression of broiler chickens during the NE challenge.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Declaration of funding
The authors thank Poultry Hub Australia for their financial support for this study (grant number: 18-414).
Acknowledgements
We thank Australian Proteome Analysis Facility, University of Macquarie, Australia, for feed analysis. We thank Evonik (South East Asia) Pte. Ltd. (Singapore) for NIRS feed analysis. Also, the authors thank Mr Jonathon Clay studying at School of Environmental and Rural Science and the Poultry Research and Teaching Unit, the University of New England, Australia, for their help during the experiment and laboratory analysis.
References
Aviagen (2014a) ‘Ross 308 broiler management handbook.’ (Ross Breeders Ltd: Newbridge, Midlothian, Scotland)Aviagen (2014b) ‘Ross 308 broiler nutrition specification.’ (Ross Breeders Ltd: Newbridge, Midlothian, Scotland)
Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E, Chaumeil J-C, Cynober L, Sfar S (2013) Citrulline: from metabolism to therapeutic use. Nutrition 29, 479–484.
| Citrulline: from metabolism to therapeutic use.Crossref | GoogleScholarGoogle Scholar | 23022123PubMed |
Barekatain R, Nattrass G, Tilbrook AJ, Chousalkar K, Gilani S (2019a) Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poultry Science 98, 3662–3675.
| Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid.Crossref | GoogleScholarGoogle Scholar | 30597073PubMed |
Barekatain R, Chrystal PV, Howarth GS, McLaughlan CJ, Gilani S, Nattrass GS (2019b) Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poultry Science 98, 6761–6771.
| Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model.Crossref | GoogleScholarGoogle Scholar | 31328774PubMed |
Bhatt T, Rizvi A, Batta SPR, Kataria S, Jamora C (2013) Signaling and mechanical roles of E-cadherin. Cell Communication & Adhesion 20, 189–199.
| Signaling and mechanical roles of E-cadherin.Crossref | GoogleScholarGoogle Scholar |
Braun JS, Novak R, Gao G, Murray PJ, Shenep JL (1999) Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infection and Immunity 67, 3750–3756.
| Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages.Crossref | GoogleScholarGoogle Scholar | 10417133PubMed |
Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A (2005) Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. The FASEB Journal 19, 923–933.
| Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process.Crossref | GoogleScholarGoogle Scholar | 15923402PubMed |
Coleman JW (2001) Nitric oxide in immunity and inflammation. International Immunopharmacology 1, 1397–1406.
| Nitric oxide in immunity and inflammation.Crossref | GoogleScholarGoogle Scholar | 11515807PubMed |
Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR (2008) Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Veterinary Immunology and Immunopathology 122, 104–115.
| Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth.Crossref | GoogleScholarGoogle Scholar | 18068809PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021a) Response of meat chickens to different sources of arginine in low-protein diets. Journal of Animal Physiology and Animal Nutrition 105, 731–746.
| Response of meat chickens to different sources of arginine in low-protein diets.Crossref | GoogleScholarGoogle Scholar | 33410556PubMed |
Dao HT, Sharma NK, Bradbury EJ, Swick RA (2021b) Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature. Animal Nutrition 7, 927–938.
| Effects of l-arginine and l-citrulline supplementation in reduced protein diets for broilers under normal and cyclic warm temperature.Crossref | GoogleScholarGoogle Scholar | 34703910PubMed |
Dao HT, Sharma NK, Daneshmand A, Kumar A, Bradbury EJ, Wu S-B, Swick RA (2022a) Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 1. Growth, carcass yield, and intestinal lesion scores. Animal Production Science
| Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 1. Growth, carcass yield, and intestinal lesion scores.Crossref | GoogleScholarGoogle Scholar |
Dao HT, Sharma NK, Barekatain R, Kheravii SK, Bradbury EJ, Wu S-B, Swick RA (2022b) Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 2. Intestinal permeability, microbiota, and short-chain fatty acid production. Animal Production Science
| Supplementation of reduced protein diets with l-arginine and l-citrulline for broilers challenged with subclinical necrotic enteritis. 2. Intestinal permeability, microbiota, and short-chain fatty acid production.Crossref | GoogleScholarGoogle Scholar |
Emami NK, Calik A, White MB, Young M, Dalloul RA (2019) Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms 7, 231
| Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease.Crossref | GoogleScholarGoogle Scholar |
Fan SP, Liberman Z, Keysar B, Kinzler KD (2015) The exposure advantage: early exposure to a multilingual environment promotes effective communication. Psychological Science 26, 1090–1097.
| The exposure advantage: early exposure to a multilingual environment promotes effective communication.Crossref | GoogleScholarGoogle Scholar | 25956911PubMed |
Fernandez A, Verde MT, Gascon M, Ramos J, Gomez J, Luco DF, Chavez G (1994) Variations of clinical biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed. Avian Pathology 23, 37–47.
| Variations of clinical biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed.Crossref | GoogleScholarGoogle Scholar | 18671070PubMed |
Flak TA, Goldman WE (1999) Signalling and cellular specificity of airway nitric oxide production in pertussis. Cellular Microbiology 1, 51–60.
| Signalling and cellular specificity of airway nitric oxide production in pertussis.Crossref | GoogleScholarGoogle Scholar | 11207540PubMed |
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. The Journal of Nutrition 135, 714–721.
| Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats.Crossref | GoogleScholarGoogle Scholar | 15795423PubMed |
Gasbarrini G, Montalto M (1999) Structure and function of tight junctions. Role in intestinal barrier. Italian Journal of Gastroenterology and Hepatology 31, 481–488.
Gharib-Naseri K, Kheravii SK, Keerqin C, Morgan N, Swick RA, Choct M, Wu S-B (2019) Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poultry Science 98, 6422–6432.
| Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 31424518PubMed |
Gharib-Naseri K, de Paula Dorigam JC, Doranalli K, Kheravii S, Swick RA, Choct M, Wu S-B (2020) Modulations of genes related to gut integrity, apoptosis, and immunity underlie the beneficial effects of Bacillus amyloliquefaciens CECT 5940 in broilers fed diets with different protein levels in a necrotic enteritis challenge model. Journal of Animal Science and Biotechnology 11, 104
| Modulations of genes related to gut integrity, apoptosis, and immunity underlie the beneficial effects of Bacillus amyloliquefaciens CECT 5940 in broilers fed diets with different protein levels in a necrotic enteritis challenge model.Crossref | GoogleScholarGoogle Scholar | 33088501PubMed |
Gilbert ER, Li H, Emmerson DA, Webb KE, Wong EA (2007) Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poultry Science 86, 1739–1753.
| Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers.Crossref | GoogleScholarGoogle Scholar | 17626820PubMed |
Gilbert ER, Li H, Emmerson DA, Webb KE, Wong EA (2008) Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. The Journal of Nutrition 138, 262–271.
| Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks.Crossref | GoogleScholarGoogle Scholar | 18203889PubMed |
Guo S, Liu D, Zhao X, Li C, Guo Y (2014) Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poultry Science 93, 94–103.
| Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens.Crossref | GoogleScholarGoogle Scholar | 24570428PubMed |
Guo W, Wang P, Liu Z-H, Ye P (2018) Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. International Journal of Oral Science 10, e8
| Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate.Crossref | GoogleScholarGoogle Scholar | 29319048PubMed |
Hangalapura BN, Kaiser MG, van der Poel JJ, Parmentier HK, Lamont SJ (2006) Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Developmental & Comparative Immunology 30, 503–511.
| Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses.Crossref | GoogleScholarGoogle Scholar |
He F, Wu C, Li P, Li N, Zhang D, Zhu Q, Ren W, Peng Y (2018) Functions and signaling pathways of amino acids in intestinal inflammation. BioMed Research International 2018, 1–13.
| Functions and signaling pathways of amino acids in intestinal inflammation.Crossref | GoogleScholarGoogle Scholar |
Hilliar M, Keerqin C, Girish CK, Barekatain R, Wu S-B, Swick RA (2020) Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poultry Science 99, 2048–2060.
| Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 32241490PubMed |
Igwe AO, Ihedioha JI, Okoye JOA (2018) Changes in serum calcium and phosphorus levels and their relationship to egg production in laying hens infected with velogenic Newcastle disease virus. Journal of Applied Animal Research 46, 523–528.
| Changes in serum calcium and phosphorus levels and their relationship to egg production in laying hens infected with velogenic Newcastle disease virus.Crossref | GoogleScholarGoogle Scholar |
Jiang WG (1996) E-cadherin and its associated protein catenins, cancer invasion and metastasis. British Journal of Surgery 83, 437–446.
| E-cadherin and its associated protein catenins, cancer invasion and metastasis.Crossref | GoogleScholarGoogle Scholar | 8665230PubMed |
Jin S, Moujahid EME, Duan Z, Zheng J, Qu L, Xu G, Yang N, Chen S (2016) Association of AMPK subunit gene polymorphisms with growth, feed intake, and feed efficiency in meat-type chickens. Poultry Science 95, 1492–1497.
| Association of AMPK subunit gene polymorphisms with growth, feed intake, and feed efficiency in meat-type chickens.Crossref | GoogleScholarGoogle Scholar | 27143764PubMed |
Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary l-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. The Journal of Nutrition 139, 230–237.
| Dietary l-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats.Crossref | GoogleScholarGoogle Scholar | 19106310PubMed |
Lassala A, Bazer FW, Cudd TA, Li P, Li X, Satterfield MC, Spencer TE, Wu G (2009) Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus. The Journal of Nutrition 139, 660–665.
| Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus.Crossref | GoogleScholarGoogle Scholar | 19225132PubMed |
Latorre JD, Adhikari B, Park SH, Teague KD, Graham LE, Mahaffey BD, Baxter MFA, Hernandez-Velasco X, Kwon YM, Ricke SC, Bielke LR, Hargis BM, Tellez G (2018) Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Frontiers in Veterinary Science 5, 199
| Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 30186844PubMed |
Le Floc’h N, Melchior D, Obled C (2004) Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livestock Production Science 87, 37–45.
| Modifications of protein and amino acid metabolism during inflammation and immune system activation.Crossref | GoogleScholarGoogle Scholar |
Lee YS, Lee SH, Gadde UD, Oh ST, Lee SJ, Lillehoj HS (2018) Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poultry Science 97, 1899–1908.
| Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens.Crossref | GoogleScholarGoogle Scholar | 29538713PubMed |
Li YP, Bang DD, Handberg KJ, Jorgensen PH, Zhang MF (2005) Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Veterinary Microbiology 110, 155–165.
| Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus.Crossref | GoogleScholarGoogle Scholar | 16159698PubMed |
Lindholm-Perry AK, Kuehn LA, Oliver WT, Kern RJ, Cushman RA, Miles JR, McNeel AK, Freetly HC (2014) DNA polymorphisms and transcript abundance of PRKAG2 and phosphorylated AMP-activated protein kinase in the rumen are associated with gain and feed intake in beef steers. Animal Genetics 45, 461–472.
| DNA polymorphisms and transcript abundance of PRKAG2 and phosphorylated AMP-activated protein kinase in the rumen are associated with gain and feed intake in beef steers.Crossref | GoogleScholarGoogle Scholar | 24730749PubMed |
Marinkovic S, Jahreis GP, Wong GG, Baumann H (1989) IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. The Journal of Immunology 142, 808–812.
Meng Q, Cooney M, Yepuri N, Cooney RN (2017) l-arginine attenuates Interleukin-1β (IL-1β) induced Nuclear Factor Kappa-Beta (NF-κB) activation in Caco-2 cells. PLoS ONE 12, e0174441
| l-arginine attenuates Interleukin-1β (IL-1β) induced Nuclear Factor Kappa-Beta (NF-κB) activation in Caco-2 cells.Crossref | GoogleScholarGoogle Scholar | 28334039PubMed |
Murata H, Shimada N, Yoshioka M (2004) Current research on acute phase proteins in veterinary diagnosis: an overview. The Veterinary Journal 168, 28–40.
| Current research on acute phase proteins in veterinary diagnosis: an overview.Crossref | GoogleScholarGoogle Scholar | 15158206PubMed |
Nafari SB (2019) Metabolism of energy and implementation of net energy system in laying hens. PhD thesis, University of New England, NSW, Australia.
NHMRC (2013) ‘Australian code of practice for the care and use of animals for scientific purposes.’ 8th edn. (The National Health and Medical Research Council: Australia)
Obst BS, Diamond J (1992) Ontogenesis of intestinal nutrient transport in domestic chickens (Gallus gallus) and its relation to growth. The Auk 109, 451–464.
| Ontogenesis of intestinal nutrient transport in domestic chickens (Gallus gallus) and its relation to growth.Crossref | GoogleScholarGoogle Scholar |
O’Reilly EL, Eckersall PD (2014) Acute phase proteins: a review of their function, behaviour and measurement in chickens. World’s Poultry Science Journal 70, 27–44.
| Acute phase proteins: a review of their function, behaviour and measurement in chickens.Crossref | GoogleScholarGoogle Scholar |
Paiva D, Walk C, McElroy A (2014) Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poultry Science 93, 2752–2762.
| Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode.Crossref | GoogleScholarGoogle Scholar | 25143591PubMed |
Paris NE, Wong EA (2013) Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poultry Science 92, 1331–1335.
| Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens.Crossref | GoogleScholarGoogle Scholar | 23571343PubMed |
Park SS, Lillehoj HS, Allen PC, Park DW, FitzCoy S, Bautista DA, Lillehoj EP (2008) Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Diseases 52, 14–22.
| Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 18459290PubMed |
Reeds PJ, Fjeld CR, Jahoor F (1994) Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? The Journal of Nutrition 124, 906–910.
| Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states?Crossref | GoogleScholarGoogle Scholar | 7515956PubMed |
Rodgers NJ, Swick RA, Geier MS, Moore RJ, Choct M, Wu S-B (2015) A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Diseases 59, 38–45.
| A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 26292532PubMed |
Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker H-C (2002) Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1α. Journal of Biological Chemistry 277, 21361–21370.
| Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1α.Crossref | GoogleScholarGoogle Scholar | 11934881PubMed |
Saleem G (2013) Necrotic enteritis, disease induction, predisposing factors and novel biochemical markers in broilers chickens. PhD thesis, University of Glasgow, UK.
Sanderink G-J, Artur Y, Siest G (1988) Human aminopeptidases: a review of the literature. Journal of Clinical Chemistry and Clinical Biochemistry 26, 795–807.
| Human aminopeptidases: a review of the literature.Crossref | GoogleScholarGoogle Scholar |
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology 75, 163–189.
| Interferon-γ: an overview of signals, mechanisms and functions.Crossref | GoogleScholarGoogle Scholar | 14525967PubMed |
Sovran B, Lu P, Loonen LMP, Hugenholtz F, Belzer C, Stolte EH, Boekschoten MV, van Baarlen P, Smidt H, Kleerebezem M, de Vos P, Renes IB, Wells JM, Dekker J (2016) Identification of commensal species positively correlated with early stress responses to a compromised mucus barrier. Inflammatory Bowel Diseases 22, 826–840.
| Identification of commensal species positively correlated with early stress responses to a compromised mucus barrier.Crossref | GoogleScholarGoogle Scholar | 26926038PubMed |
Su CL, Austic RE (1999) The recycling of l-citrulline to l-arginine in a chicken macrophage cell line. Poultry Science 78, 353–355.
| The recycling of l-citrulline to l-arginine in a chicken macrophage cell line.Crossref | GoogleScholarGoogle Scholar | 10090261PubMed |
Su S, Miska KB, Fetterer RH, Jenkins MC, Wong EA (2014) Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers. Poultry Science 93, 1217–1226.
| Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers.Crossref | GoogleScholarGoogle Scholar | 24795315PubMed |
Tan J, Applegate TJ, Liu S, Guo Y, Eicher SD (2014) Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. British Journal of Nutrition 112, 1098–1109.
| Supplemental dietary l-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 25181320PubMed |
Tosi MF (2005) Innate immune responses to infection. Journal of Allergy and Clinical Immunology 116, 241–249.
| Innate immune responses to infection.Crossref | GoogleScholarGoogle Scholar | 16083775PubMed |
Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Archiv 447, 532–542.
| CATs and HATs: the SLC7 family of amino acid transporters.Crossref | GoogleScholarGoogle Scholar | 14770310PubMed |
Wade B, Keyburn A (2015) The true cost of necrotic enteritis. World Poultry 31, 16–17.
Wu S-B, Rodgers NJ, Cui G, Sun Y, Choct M (2016) Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens. Avian Pathology 45, 346–356.
| Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 27245303PubMed |
Xue G-D, Wu S-B, Choct M, Swick RA (2017) Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Animal Nutrition 3, 399–405.
| Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model.Crossref | GoogleScholarGoogle Scholar | 29767160PubMed |
Yang F, Lei X, Rodriguez-Palacios A, Tang C, Yue H (2013) Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup J. BMC Research Notes 6, 402
| Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup J.Crossref | GoogleScholarGoogle Scholar | 24099561PubMed |
Yun CH, Lillehoj HS, Lillehoj EP (2000) Intestinal immune responses to coccidiosis. Developmental & Comparative Immunology 24, 303–324.
| Intestinal immune responses to coccidiosis.Crossref | GoogleScholarGoogle Scholar |
Zanu HK, Kheravii SK, Morgan NK, Bedford MR, Swick RA (2020a) Interactive effect of dietary calcium and phytase on broilers challenged with subclinical necrotic enteritis: 3. Serum calcium and phosphorus, and bone mineralization. Poultry Science 99, 3617–3627.
| Interactive effect of dietary calcium and phytase on broilers challenged with subclinical necrotic enteritis: 3. Serum calcium and phosphorus, and bone mineralization.Crossref | GoogleScholarGoogle Scholar | 32616258PubMed |
Zanu HK, Keerqin C, Kheravii SK, Morgan N, Wu S-B, Bedford MR, Swick RA (2020b) Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. Intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression. Poultry Science 99, 2581–2594.
| Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. Intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression.Crossref | GoogleScholarGoogle Scholar | 32359594PubMed |
Zhang B, Gan L, Shahid MS, Lv Z, Fan H, Liu D, Guo Y (2019) In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. Journal of Animal Science and Biotechnology 10, 73
| In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 31428367PubMed |
Zulkifli I, Najafi P, Nurfarahin AJ, Soleimani AF, Kumari S, Aryani AA, O’Reilly EL, Eckersall PD (2014) Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poultry Science 93, 3112–3118.
| Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone.Crossref | GoogleScholarGoogle Scholar | 25306460PubMed |


