Dermatophilosis (lumpy wool) in sheep: a review of pathogenesis, aetiology, resistance and vaccines
Ross L. Tellam A , Tony Vuocolo A , Stuart Denman A , Aaron Ingham A , Gene Wijffels A , Peter J. James B and Ian G. Colditz
B and Ian G. Colditz  C *
C *
A Agriculture and Food, CSIRO, 306 Carmody Road, St Lucia, Qld 4067, Australia.
B Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Brisbane, Qld 4072, Australia.
C Agriculture and Food, CSIRO, New England Highway, Armidale, NSW 2350, Australia.
Animal Production Science 62(2) 101-113 https://doi.org/10.1071/AN21119
Submitted: 2 March 2021 Accepted: 1 September 2021 Published: 16 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Lumpy wool (dermatophilosis) develops following prolonged wetting of sheep when bacterial proliferation in wool and on skin induce an exudative dermatitis, causing a superficial skin lesion and damage to wool follicles and fibres. The incidence of dermatophilosis is strongly dependent on wet and warm weather and, hence, infection is sporadic. While older animals are less at risk than are lambs, it is unclear whether this reflects naturally acquired immune resistance or the maturation of skin and wool fibres. Dermatophilosis directly causes wool production losses and it also is a risk factor for blowfly strike, which has a substantial economic impact and increasing challenges associated with current control procedures. This review assessed research on the bacterial causes of lumpy wool, the characteristics of the resulting immune defence reactions in sheep, current control strategies, and limitations of previous attempts to control lumpy wool by sheep vaccination.
Keywords: acquired immunity, antibiotics, dermatophilosis, dermatitis, Dermatophilus congolensis, genetic resistance, local immunity, lumpy wool, vaccines.
Introduction
Lumpy wool (dermatophilosis) is an exudative dermatitis of sheep skin characterised by inflammation of skin that results in crusting and matting of wool (Seddon 1927; Bull 1929; Roberts 1961, 1965, 1966; Gardiner 1971; Ellis et al. 1993; Norris et al. 2008; NSW Industry and Investment 2010; Moriello 2019). Skin lesions occur over the dorsal mid-line and spread laterally and ventrally (Rodostits et al. 2006; Berry and Watt 2017). Infections of sheep can be acute, chronic or sporadic. The major source of body infection is from chronically infected sheep with active lesions typically on the ears and face (Berry and Watt 2017). Despite intensive research efforts in the early 1990s, there are many features of the disease and its control that are unclear. This review assesses the causes of lumpy wool, including environmental, bacterial and host factors, and past research on vaccine development. This information will help identify new opportunities for the immunological control of lumpy wool, which indirectly may also aid in the control of blowfly strike in sheep (Denman et al. 2021). An electronic draft of this review was previously made available by Australian Wool Innovation as part of a larger commissioned industry report (Vuocolo et al. 2020). There are also accompanying reviews on fleece rot and the potential of vaccines to protect sheep from fleece rot and lumpy wool (Colditz et al. 2021; Denman et al. 2021).
Pathogenesis and aetiology
The causative microbial agent of lumpy wool is Dermatophilus congolensis, which is a facultative anaerobic actinomycete spread among sheep by contact transmission (Bull 1929; Roberts 1965; Zaria 1993; Leoni et al. 1993; Moriello 2019). The evidence for a causal involvement of the bacterium in generating lumpy wool includes (i) a strong association of the presence of this bacterial species with lumpy wool (Bull 1929; Roberts 1965; Ellis et al. 1993; Leoni et al. 1993; Awad et al. 2007; Moriello 2019), (ii) experimental infections of sheep skin with D. congolensis, resulting in lumpy wool (Sutherland et al. 1983, 1987; Ellis et al. 1987, 1991, 1993; Sutherland and Robertson 1988), (iii) antibodies specific to this bacterial species being induced in sheep with lumpy wool (Sutherland et al. 1987; Sutherland and Robertson 1988) and (iv) experimental vaccines containing components of D. congolensis as vaccine antigens showing some evidence of protection of sheep from lumpy wool. Results from these vaccination trials are summarised in Table 1. D. congolensis also causes a world-wide distribution of infectious dermatitis in a broad group of livestock animals including sheep, cattle, horses, goats, camels, as well as non-ruminant animals including dogs, cats and rabbits (references listed in Table 2). In the absence of adequate hygiene practices, humans can also be infected (Kaminski and Suter 1976; Zaria 1993; Faris and Hollis 2013).

|
|
After initial infection of the skin by D. congolensis, a hard scab is formed, which then lifts from the skin as the fleece grows to produce localised, crusted, hard pyramidal masses of often discoloured wool (Bull 1929; Roberts 1966; Gardiner 1971; Norris et al. 2008; Moriello 2019). These areas of affected wool impede shearing. Lumpy wool (dermatophilosis) is sometimes erroneously called mycotic dermatitis (D. congolensis is a bacterium and not a fungus). Wetting of fleece for an extended time encourages spread of the infection and thus sporadic outbreaks of lumpy wool are associated with seasonal rain (Gardiner 1971; Zaria 1993; NSW Industry and Investment 2010; Marsella 2016; Berry and Watt 2017). Lumpy wool is thought to be predominantly spread within a flock by contact transmission between infected and uninfected sheep, although there is a suggestion of potential transmission via sheep dips that contain no or inadequate antibacterial agents (Gardiner 1971). It is noteworthy that bovine dermatophilosis is spread by tick infestations (Ambrose 1996b). There is speculation that dermatophilosis in sheep could be spread by blowflies and lice, although there is no evidence for this means of disease transmission (Gardiner 1971; Awad et al. 2007).
D. congolensis has a complex life cycle involving two morphological forms, filamentous hyphae and motile zoospores (Ambrose 1996a, 1996b; Marsella 2016; Moriello 2019). Hyphae are composed of filaments coated by a mucoid capsule that develop into coccoid cells, which then mature into flagellated zoospores, the infective and motile biological agent. Persistent wetting of the sheep skin disperses the protective waxy layer on the skin and softens the skin making it vulnerable to zoospore infection from other animals. These conditions promote D. congolensis replication and repeated invasion of the epidermis and the wool follicular sheath by hyphae, resulting in a reactive infiltration of the tissue by immune cells, particularly neutrophils. The consequent inflammation of the infected tissue generates an exudate on the skin surface (Roberts 1961, 1966; Ellis et al. 1987). The combination of the exudate and epithelial tissue generates the fleece lesion. The exudate maintains hydration on the skin surface near wool follicles and may provide additional nutrition to the bacteria, thereby promoting a further cycle of D. congolensis proliferation (Gardiner 1971; Ellis et al. 1987; Ambrose 1996b). Roberts suggested that infection occurs in lambs before the skin wax layer is properly formed or as a result of wet weather that compromises skin barrier function (Roberts 1965, 1966). He also noted that various management practices could actively promote infection.
Ellis et al.(1987, p. 151) concluded from the research of Roberts (1965, 1966) that ‘resolution of lesions formed by D. congolensis was associated with delayed-type hypersensitivity whereas resistance (to infection) was associated with antibodies to somatic antigens of D. congolensis’. The latter conclusion is consistent with the development of resistance to infection via naturally acquired immunity although, as discussed below, the evidence is equivocal.
Sheep production losses
Sheep with lumpy wool are associated with lowered production of wool, decreased wool value, culling losses, treatment costs and difficulty in shearing (Edwards 1985; Edwards et al. 1985; Bateup and Edwards 1990; Berry and Watt 2017). Moreover, lumpy wool may be a predisposing factor for fleece rot and both conditions are predisposing factors for blowfly strike, particularly body strike (Wilkinson 1979; Gherardi et al. 1981, 1983; NSW Industry and Investment 2010). The latter results in lost wool productivity and direct preventative measures such as the use of insecticides and mulesing are increasingly unacceptable in the industry (Tellam and Bowles 1997; Norris et al. 2008; James et al. 2019). Thus, prevention of lumpy wool is a strategy that can potentially decrease the incidence of body strike in sheep. Lumpy wool may also reduce the efficacy of pour-on insecticides used for lice control (NSW Industry and Investment 2010). The incidence of sheep affected by lumpy wool in the Australian sheep industry has not been recently surveyed. Hence, the extent of current industry losses from dermatophilosis, changes in the geographical spread of the disease or changes resulting from the industry shifting toward fine and superfine wooled sheep are unclear.
Current management of dermatophilosis
Management of lumpy wool on sheep is currently achieved by avoiding close-contact wetting events, removal and culling of chronically affected sheep and the use of more resistant sheep (Edwards 1991; Roberts and Graham 1966). Past practices for the application of insecticides on sheep that involved dipping and jetting could have increased the risk of infection; however, these practices have now largely been replaced by technologies that avoid the wetting of sheep. Management also includes the monitoring of spontaneous self-healing or, in the case of severe chronic infections, the use of intramuscular injection of sheep with long-lasting antibiotics (Berry and Watt 2017). The antibiotics used in the past were a combination of streptomycin and penicillin (Streptopen™) administered by a single intramuscular injection (Roberts and Graham 1966; Roberts 1967). Penicillin by itself was ineffective but provided a synergistic effect when combined with streptomycin. It is now recommended that antibiotics such as oxytetracycline be used for valuable animals (NSW Industry and Investment 2010). The use of long-lasting antibiotics ensures that animal handling occurs only once. The efficacy of antibiotic treatment is reported as variable (Zaria 1993; Scrivener and Vizard 1995; Norris et al. 2008; Berry and Watt 2017). Moreover, there is increasing public and human health care disquiet about the use of antibiotics in livestock animals, especially the promotion of antibiotic resistance in unrelated bacteria in the environment and the presence of antibiotic residues in sheep products. Therefore, the availability of antibiotics for the control of lumpy wool in the medium- to long-term future may be uncertain (Norris et al. 2008). Management of dermatophilosis also involves standard human hygiene practices after direct contact with infected animals and farm tools. Additional management practices in use include the isolation of infected sheep, the avoidance of penning groups of infected and uninfected sheep, clipping of wool, drying affected sheep, disinfection of lesions, disinfection of equipment, use of dips containing antibacterial additives, and zinc sulfate sprays (Gardiner 1971; Edwards 1991; NSW Industry and Investment 2010; Sheep CRC 2013). No commercial vaccines are currently available for protecting sheep from D. congolensis infection, despite some limited experimental efforts in the late 1980s and early 1990s (summarised in Table 1).
Susceptibility of sheep to D. congolensis
The susceptibility of sheep to D. congolensis is complex and likely strongly modified by interacting combinations of factors including environmental conditions (especially wet and warm weather), general immune responsiveness to infection, age, bacterial strain variation, bacterial pathogenicity and infectivity factors, specific protective immune responsiveness in sheep induced by infection, and genetic resistance of sheep to infection.
The genetic resistance factor could relate to both the genetics underpinning population variation in wool and skin structure and an effective immune system (Sheep CRC 2013). Sheep of all ages are susceptible to dermatophilosis; however, the infection rate is higher in younger animals (Norris et al. 2008; Berry and Watt 2017). The latter observation may reflect changes in wool fibre physical structure with age (particularly lower wax levels in young sheep and wool fibre diameter; Edwards 1985), maturation of the sheep immune system with age (Watson et al. 1994), and/or acquired immunity (Gardiner 1971; Norris et al. 2008). Teasing out the relative contributions of these factors to sheep resistance has been difficult.
Genetic susceptibility and resistance of sheep to dermatophilosis
Chronically infected sheep can act as a reservoir of infection in the flock and those sheep that are unresponsive to antibiotic treatment are sometimes culled to remove the risk of infection and potentially improve the genetics-based resistance of the flock to dermatophilosis (NSW Industry and Investment 2010). There is a lack of objective resistance testing in flocks and, hence, it is unclear whether culling of chronically infected sheep has progressively increased the genetic resistance to dermatophilosis in sheep flocks. Wool producers in some areas breed sheep for finer wool as a purported means of increasing resistance to dermatophilosis. However, as described below, there is no evidence to support this approach.
The identification of ovine phenotypic or genetic markers of resistance to dermatophilosis infection and, more specifically, the severity of infection may potentially be used in selective breeding programs to progressively enhance resistance and decrease the severity of the infection in a population of sheep over multiple generations. The advantages of this approach are that the increased dermatophilosis resistance or decreased severity are permanent in the selected population, incremental increases of resistance and decreases in severity can be obtained by selective breeding in each generation, and the use of genetic (DNA) markers has potential to markedly accelerate these phenotypic gains in each generation. In practice, however, the extent of the genetic contribution to the overall resistance of sheep to dermatophilosis is low and variable (Norris et al. 2008). In another study, the heritability of resistance using experimental infections of sheep was reported as being less than 0.14 (Lewer et al. 1987). The heritability of the severity of the D. congolensis infection in sheep ranged between 0.25 and 0.42, that is, it was moderately heritable (Raadsma et al. 1992). There have been no recent confirmatory measurements of these heritability values. Thus, sheep have a low level of heritable resistance to dermatophilosis infection but a greater genetic contribution to the potential minimisation of the severity of infection. It is notable that both investigations reporting the heritability estimates used artificial challenges of sheep skin (either extended wetting or skin clipped and wax removed before addition of spores). Consequently, it is likely that the measured heritability estimates corresponded with immune responsiveness or physical skin effects rather than any wool characteristics. Apart from the culling of chronically infected sheep unresponsive to antibiotic treatment from breeding programs, there has been no concerted effort to use this genetic information in widespread intensive selective breeding programs for the enhancement of sheep resistance to dermatophilosis. One prediction may be that selective breeding for resistance will not greatly reduce the infection rate in sheep but could affect the scale (severity) of the infection on sheep, especially the speed of dermatophilosis lesion healing. Greater emphasis on the dermatophilosis severity trait in selective breeding programs may be more beneficial than selecting for resistance due to the different heritabilities of these traits.
The wool industry is using different genetic lines of sheep to produce wool with a range of fibre diameters suitable for specialised markets. In the only study that investigated the relationship between dermatophilosis and fleece characteristics within flocks, Gherardi et al. (1984) found no association with mean fibre diameter, fibre diameter variation and yield. Other individual sheep factors have not been investigated. Moreover, Edwards (1991) concluded that the different incidences of dermatophilosis in lambs on different properties were not related to mean fibre diameter and fibre diameter variation. Comparisons of dermatophilosis incidence in sheep among different properties can be confounded by differences in climatic zones and other factors.
Genetic variants in ruminant species have been identified that are linked to resistance to various bacterial infections causing skin lesions. Genetic variants in the ovine MHC-DQA2 major histocompatibility locus have been associated with resistance to a bacterial dermatitis of the hoof (footrot) caused by the bacterium Dichelobacter nodosus and a commercial molecular marker is available for selective breeding for resistance to this disease (Escayg et al. 1997; Norris et al. 2008). A bovine genetic marker in the major histocompatibility region (boLA-DR/DQ) was linked with high susceptibility of cattle to bovine dermatophilosis (Maillard et al. 2003). A selective breeding program using this bovine genetic marker to eliminate affected animals resulted in ‘marked reduction in disease (dermatophilosis) prevalence’. However, the selective breeding of animals assisted by genetic variation in the major histocompatibility complex has potential to unintentionally adversely affect resistance of these animals to unrelated viral, bacterial or parasitic diseases (Norris et al. 2008). Hence, the general value of DNA markers located within the major histocompatibility genetic loci for use in livestock selective breeding programs for disease resistance enhancement is unclear.
The genetic resistance of sheep populations to infectious challenge by D. congolensis and the genetic contribution to dermatophilosis severity may be minor to moderate contributors to the total disease variation in the population due to strong environmental influences, such as weather and bacterial strain variations (see below). It is also possible that the genetic contribution to disease resistance in the sheep population could be modified in a complex interactive manner by the environmental effects or bacterial strain variation. In addition, in most animal models, the genetics of disease resistance is typically polygenic, that is, genetic resistance is due to many genetic variants, each of a small effect size (Hayes and Goddard 2001). In summary, there are no specific genetic markers currently available to assist in the efficient selective breeding of sheep populations for resistance to lumpy wool. Alternatively, a whole-genome selection strategy using genomic breeding values (GEBVs) will likely be required to achieve genetic progress in selective breeding programs for the enhancement of genetic resistance of sheep to dermatophilosis (Meuwissen et al. 2001; Goddard and Hayes 2007). The recent availability of high-density ovine single-nucleotide polymorhism chips, the ovine genome sequence and advancements in whole-genome sequencing technologies now enable the GEBV approach for selective breeding applications within sheep populations (Jiang et al. 2014; Al-Mamun et al. 2015; Naval Sanchez et al. 2018). A powerful addition to these ovine genetic resources will be the emerging genomic map of all gene regulatory regions (Andersson et al. 2015; Giuffra et al. 2019). The latter provides the potential to identify causal genetic variants underlying a trait in a population of sheep.
D. congolensis strain variation
Microbial strain variation is an evolutionary strategy that enhances the survival chances of the microbial population in a variable environment; hence, genetic variation is prevalent in many bacterial species. D. congolensis strain variation could potentially influence the prevalence and severity of lumpy wool in sheep populations. Moreover, vaccination of sheep against D. congolensis and acquired natural resistance in sheep after infection could potentially be confounded by D. congolensis strain variation. Thus, knowledge of the extent of strain variation is important for the management of dermatophilosis.
D. congolensis has considerable strain variation (Ellis et al. 1991, 1993; Gogolewski et al. 1992; Masters et al. 1997a, 1997b). The morphological and biochemical properties of 30 isolates of D. congolensis that infected sheep from throughout Australia showed substantial variation in haemolytic activity on blood agar, mucoid nature of colonies, motility, flagella density and polarity, restriction enzyme profiles of the bacterial DNA, proteins, carbohydrate content and a spectrum of enzyme activities (Ellis et al. 1993). The ranking of the infectivity of these isolates was associated with isolate haemolytic activity and three enzymatic activities; these activities may be highlighting the effects of infectivity factors. The differences in restriction enzyme profiles in bacterial isolates signify differences in the DNA present in each strain and, hence, a genetic basis to the different phenotypic characteristics of the isolates. In many cases in the past, the technologies used to detect strain variation at the DNA level often had low resolving power by current standards, that is, DNA restriction length polymorphisms, sodium dodecyl sulfate–polyacrylamide gel electrophoresis of polypeptides, immunoblots and PCR of ribosomal RNA. Thus, the past estimates of the extent of D. congolensis strain variation at the genetic level may have been substantially underestimated. Changes in strain frequencies with time, geographical distribution, climate and season have not been investigated. Moreover, the use of antibiotics to treat chronically infected sheep over the past three decades may have altered strain diversity. An alternative view is that antibiotics would not affect strain diversity as they are likely to affect only D. congolensis populations at the skin level, which is outweighed by the larger D. congolensis population in the fleece. Future experimental investigations of this potential issue are required.
D. congolensis strain variation is associated with differential pathogenicity in sheep (Ellis et al. 1991). However, the relative infectivity of each strain is unclear. Strain variation can also induce differential immune responses in sheep after natural infection, differential immune responses in experimentally infected sheep, and differential protective immune responses in sheep immunised with candidate vaccine antigens from different bacterial strains (Ellis et al. 1991). Similarly, dermatophilosis in cattle is associated with D. congolensis strain variation (Ambrose 1996a, 1996b; Ambrose et al. 1997, 1999; Larrasa et al. 2002, 2004; Hiraizumi and Tagawa 2014). Thus, a key gap in knowledge relates to the extent of D. congolensis strain variation and the strain-specific impacts on infectivity, pathology and immune responses in sheep. Currently available high-throughput DNA sequencing technologies have the capacity to rapidly and cheaply generate genome sequences of D. congolensis culture isolates or individual strains in complex communities of D. congolensis. Applications of this technology can rapidly survey changes in strain variation and determine whether field challenge strains are consistent with the structure of D. congolensis antigens used in potential vaccines. This capability and knowledge will be required to ensure optimum efficacy of future vaccines in the field.
Acquired immune resistance to D. congolensis in sheep
The presence of acquired natural immunity in an animal to a disease is the hallmark of induction of a protective immune response in the host to the infectious agent. This is highlighted by the ability of the immune system to retain the memory of a previous microbial infection and mount a rapid neutralising immune response to subsequent infections. This type of response provides a strong feasibility statement for the development of a vaccine that mimics this natural response but without the adverse effects of the disease agent.
Naturally acquired immunity is consistent with the decreasing prevalence of dermatophilosis with sheep age; however, there is contradictory information about whether dermatophilosis in sheep generates a natural protective immune response to subsequent infections. Moreover, the relevant scientific information is limited.
Most sheep develop resistance to dermatophilosis after 4–6 weeks of infection, which is consistent with the development of acquired immunity (Sheep CRC 2013). An alternative explanation is that older sheep have more protective waxes secreted around wool follicles and on the skin surface, making D. congolensis infection more difficult. Roberts (1966) also described acquired immunity of previously exposed sheep and guinea pigs when the animals were challenged with D. congolensis zoospores on scarified skin. The protective response was shown to be mediated by phagocytes and was ineffective against infection of unbroken skin, presumably because of the absence of phagocytes in this circumstance. Ellis and colleagues used three successive challenges of sheep with D. congolensis to ascertain changes in the infection rate and lesion severity at each challenge (Ellis et al. 1987). They demonstrated fewer lesions in the second challenge and faster-healing lesions in the third challenge. A subset of the sheep did not develop skin lesions after the third challenge. Dermatophilosis in cattle is also thought to generate a naturally acquired immunity (Ambrose et al. 1999). Thus, these multiple investigations provided evidence in support of acquired immunity to dermatophilosis in various animal species.
In another study, healthy unexposed sheep (sheep naive to D. congolensis) were compared with healthy sheep having a history of chronic D. congolensis infections (Ellis et al. 1992). After a controlled challenge of both sheep groups with D. congolensis zoospores, there were more lesions and weaker lymphocyte responses to the skin infection in the group with a history of chronic infection, despite this group having a stronger antibody response to D. congolensis. The sheep groups had no observed differences in fleece characteristics, skin wax and suint concentrations that could account for the differences in susceptibility of the two groups. Thus, there was no evidence of naturally acquired immunity in this experiment.
Comparison of a group of chronically infected merino sheep without active lesions with a group that had naturally recovered from dermatophilosis, after a reinfestation challenge with D. congolensis zoospores, showed similar reinfection rates, severities of lesions, rates of resolution of the disease and abilities of sheep sera to kill zoospores (Ellis et al. 1989). Despite these disease response similarities, there were several immunological and inflammatory response differences between the two sheep groups. This information suggests that the monitored immunological and inflammatory response differences were irrelevant to the ability of the sheep to control the D. congolensis challenge infections.
Collectively, these apparently contradictory studies are difficult to reconcile. Some studies have provided evidence of acquired immunity in sheep to D. congolensis infection (Roberts 1966; Ellis et al. 1987). However, other studies have demonstrated that chronically infected sheep have a weaker protective immune response than that of an unexposed sheep group (Ellis et al. 1992). One possibility is that chronically infected sheep may not be a good model for testing for acquired immunity to a specific disease because the prior infection may have resulted in a generalised immune response suppression. Although antibody titre to D. congolensis generated by the infected sheep was an indication of a functional humoral immune response, it seems to be irrelevant to protection from infection in these sheep. The presence of a strong and specific antibody response to D. congolensis is important as it demonstrates that the skin infection activates the immune system even though the infectious agent is present only in the surface layers of the skin. The lack of antibody-mediated protection could simply be a consequence of induction of a humoral immune response to the infectious zoospores rather than the invasive filamentous hyphae extending into the epidermis and wool follicles, which are likely to be responsible for generating skin inflammation and disease pathology (Roberts 1966; Ellis et al. 1987). In addition, there could be insufficient antibody response to specific and crucial D. congolensis antigens at the site of the infection. Thus, the protective role of antibodies to D. congolensis antigenic components is unclear. Another untested possibility is that sheep acquire immunity only after natural infection with a succession of different D. congolensis strains.
Antibody to D. congolensis on sheep skin
Healthy skin has a strong barrier function that protects an animal from microbial infection and prevents leakage of tissue fluids and dehydration. D. congolensis infection of sheep is likely to be initiated by zoospores that exploit partially broken skin or an absence of protective waxes on skin. The filamentous hyphae then superficially penetrate the skin and wool follicles, causing an exudative dermatitis and inflammation of the skin (Roberts 1966; Ellis et al. 1989; Marsella 2016). Although the skin is known to contain immune surveillance cells (Salmon et al. 1994), it was previously unclear whether specific antibodies to D. congolensis could be located on the skin surface, the primary infection site. The research of Sutherland and colleagues addressed this issue (Sutherland et al. 1987).
Specific antibody responses to three D. congolensis antigens (flagella, filament and soluble antigen) from different life stages of D. congolensis were investigated in sera and skin surface washings from sheep experimentally infected with three temporally separated inoculations of D. congolensis (Sutherland et al. 1987). The serology demonstrated that there were strong and rapid (7–21 days) antibody responses in sheep sera to each of the tested antigens. Antibody was also present in skin washings but it was detected later (28–42 days) than in sera and was more variable in titre. This investigation demonstrated that D. congolensis life stage-specific antigens can induce a strong and specific antibody response in the sera of sheep and on the inflamed surface of sheep skin. The latter may be mediated by transudative movement of the antibody isotypes IgG1 and IgG2 from serum to the skin surface (Lloyd et al. 1987; Colditz et al. 1992). In cattle, there was also active transport of IgA and IgM antibodies onto the skin through a local secretory process (Lloyd et al. 1987). Notably, new technologies have been developed for inducing strong local immune responses in the skin by intradermal injection of antigens; however, it is unclear whether these approaches are practical or efficacious for the protection of sheep from lumpy wool (Colditz et al. 1992; Colditz and Watson 1993; Wallis et al. 2019).
In general terms, there is potential for an induced antibody to a D. congolensis antigen(s) to neutralise the infectivity of D. congolensis at an infection site and thereby generate a protective immunity resulting in control of the incidence of lumpy wool in sheep. There are several factors crucial for success that are not yet clear, including identification of specific and effective D. congolensis life stage-specific antigen(s), induction of a relevant antibody isotype in and onto skin, production of sufficient quantity of neutralising antibody at the skin infection site, and the period of protection of sheep. In practical terms for the sheep industry, the latter should be at least one season. In addition, there needs to be an easy, safe, reproducible and cost-effective means of scaling up antigen production and, ultimately, a commercially acceptable value proposition for vaccine development.
Acquired immunity to a disease agent can also be mediated by specific immune cells. Lymphocytes and macrophages are enriched in underlying skin tissue at sites of D. congolensis infection (Ellis et al. 1987). However, there is little direct evidence that these immune cells in skin kill or inhibit the reproduction of D. congolensis. These immune cells are likely to infiltrate the infected tissue region in response to disease pathology signals. It is also unlikely that live biologically functional lymphocytes or other immune-related cells are present in dermatophilosis exudate. Hence, cell-mediated immunity is an unlikely mechanism for control of dermatophilosis.
Experimental vaccines
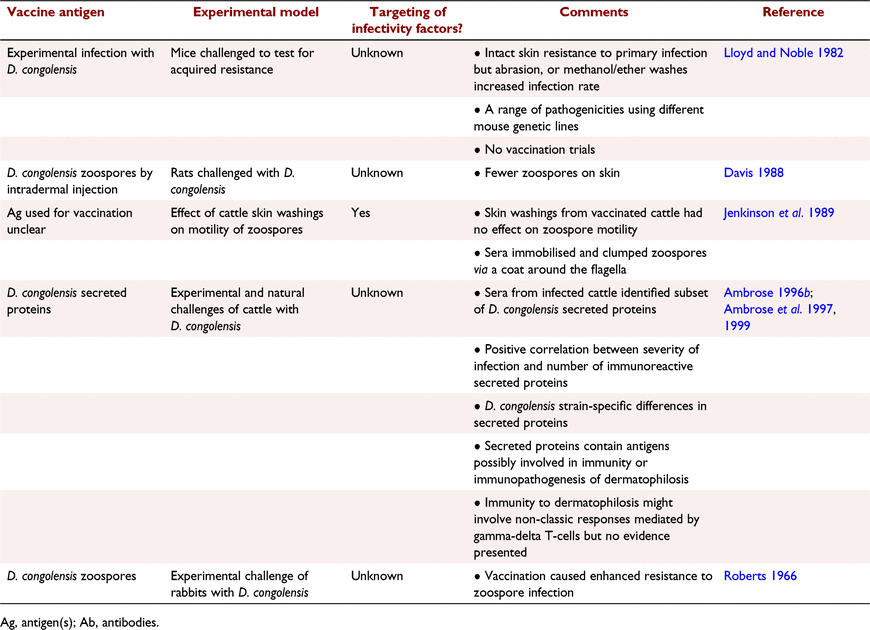
Vaccination of sheep against dermatophilosis could be an important control strategy. During the late 1980s and early 1990s, there were attempts to produce experimental vaccines that protected sheep from D. congolensis infection (Norris et al. 2008). Table 1 summarises these investigations. Table 2 summarises, more briefly, parallel investigations in other species.
The sheep dermatophilosis vaccine research was largely undertaken by Sutherland, Ellis and colleagues (Ellis et al. 1987, 1991; Sutherland et al. 1987, 1991; Sutherland and Robertson 1988). There is an unfiled provisional Australian Patent application (Australian Patent Office Number 1986908866; provisional patent, PH8866; Applicant, The State of Western Australia; 1986) entitled Dermatophilosis Vaccine, although its details are unavailable. The absence of full patent filing and, particularly, the absence of a past attempt at full commercialisation of the vaccine suggests that there were significant technical issues and/or market constraints at the time.
The antigens used in the experimental sheep vaccine trials included live and dead zoospores, filaments of live and dead hyphae, and crude soluble antigens secreted from hyphae or zoospores (Table 1). There was no evidence for a protective immune response induced by vaccination of sheep with zoospores (Sutherland et al. 1987, 1991; Sutherland and Robertson 1988). Zoospores are the infective and motile life stage but they may not have a sufficiently intimate interaction with antibody present on or in inflamed sheep skin to be affected by vaccination. In contrast, filamentous hyphae penetrate the outer epidermis and wool follicles, generate local inflammation, and induce a skin lesion containing serous exudate. The latter life stage has more intimate contact with the immune system and therefore antibody. Crude filamentous-hyphae antigens tended to induce immune responses in sheep that provided partial protection for disease incidence and/or reduced lesion severity (Sutherland et al. 1987, 1991; Sutherland and Robertson 1988; Sanders et al. 1991). However, the effects of the experimental vaccines were generally weak and there was no demonstration of vaccine efficacy in field trials (Sutherland et al. 1991). Vaccine-induced resistance to infection was not associated with serum antibody concentrations or skin test reactivity to D. congolensis antigens (Sanders et al. 1991). Field trials are presumably difficult to undertake due to the sporadic incidence of natural infections due to the vagaries of conducive weather and the low infection rate within a flock. It was also suggested that vaccine efficacy was dependent on the challenge strain of D. congolensis and nutrition (Ellis et al. 1991; Sanders et al. 1991). D. congolensis may secrete proteins, especially proteases to aid removal of the protective outer keratin layer of skin, lipases to remove skin wax and haemolysins to allow bacterial invasion of cells, that collectively facilitate the invasion of skin (How et al. 1990). These enzymes may be essential bacterial infectivity or pathogenicity factors and, hence, could be specifically targeted using a vaccine to generate antibody-mediated neutralisation of the functions of these factors, potentially leading to decreased infectivity and lesion severity (How et al. 1990). However, the identification and testing of these factors in vaccines have not been systematically undertaken in the past. Roberts concluded that D. congolensis did not produce any factors that resulted in the killing of host phagocytes or leukocytes (Roberts 1965, 1966). He concluded that D. congolensis does not secrete factors that contribute to the pathogenesis of infection as measured by a specific immune cell killing assay. The inference from this research is that the strong skin inflammatory response to infection may be attributable to products arising from mild cellular damage in the skin caused by the penetrating D. congolensis filamentous hyphae. However, there are many additional ways in which bacteria could generate pathology via the secretion of bacterial proinflammatory products and, hence, the absence of secreted pathogenicity factors is unlikely. Purified pathogenicity or infectivity factors may be high-priority candidates for testing as vaccine antigens.
A serine protease secreted by D. congolensis has been identified and a corresponding recombinant protein produced, which induced specific antibodies in vaccinated sheep (Mine 1996). The study provided no evidence that the protease was a pathogenicity factor. There was also no testing of the vaccinated sheep to determine whether they were protected from dermatophilosis. A related investigation identified peptides from phage libraries that bound antibody (Ig) to a recombinant serine protease. The native protease was secreted by D. congolensis. Sheep vaccinated with these peptides or the recombinant serine protease showed accelerated resolution of skin lesions but no effect on infectivity after challenge with D. congolensis (Tabar 1998; Tabar and Carnegie 2002). It is unclear whether the serine proteases used in the three investigations were identical.
A potential infectivity factor and therefore candidate vaccine antigen could be the flagellar protein of zoospores (Hiraizumi and Tagawa 2014). It is speculated that binding of specific antibodies to this protein could hinder zoospore motility and therefore infectivity if sufficient antibody to zoospores was available on the skin surface. Another potential infectivity factor is the non-proteinaceous mucoid material coating hyphae filaments. D. congolensis is an opportunist pathogen strongly reliant on moisture for its survival, infectivity and proliferation. Hence, mucoid material coating hyphae filaments, which primarily prevents dehydration, may be an important target for disruption by antibodies induced by vaccination. The mucoid material has been indirectly tested in vaccination trials using crude D. congolensis filaments as antigens and was associated with some protection against infection (Sutherland et al. 1991). However, a more robust and targeted immune response could be generated using purified mucoid material as the vaccine antigen. In summary, there has been no systematic identification of D. congolensis pathogenicity or infectivity factors and only one secreted protein was tested in a vaccination trial. Indirect testing of potential pathogenicity or infectivity factors has potentially occurred in some experimental vaccine trials that used crude antigen fractions, particularly crude soluble antigens. At best, these trials demonstrated only weak protective responses (Sutherland et al. 1987, 1991; Sutherland and Robertson 1988).
Experimental vaccination trials have also been undertaken in other mammalian species beside sheep (Table 2). One investigation using cattle vaccinated with D. congolensis secreted antigens demonstrated a positive correlation between lesion severity and the number of immunoreactive secreted proteins present in the crude antigen preparation (Ambrose 1996a; Ambrose et al. 1997, 1999). This result suggests the induced immune response reduced the pathology associated with D. congolensis infection, although the vaccine antigen was not clearly defined. It is suggested that small differences in the growth conditions for D. congolensis could alter the composition of secreted components, which may be a reason for variable results.
Commercial viability of a dermatophilosis vaccine
A successful vaccine against dermatophilosis would potentially enhance wool yield and quality, decrease reliance on antibiotics for treatment of chronically infected sheep and decrease the risk of blowfly strike. Dermatophilosis is often a sporadic disease of sheep as incidence is strongly dependent on climate (Gardiner 1971; Zaria 1993; NSW Industry and Investment 2010; Marsella 2016; Berry and Watt 2017). Moreover, the disease typically affects only a small number of individuals within a flock and is usually managed by sheep isolation and the monitoring of self-healing. Thus, the cost of routine seasonal vaccination of a flock would likely exceed the production losses from dermatophilosis. These considerations suggest that the major justification for the development of a vaccine to protect sheep from dermatophilosis relates to the potential ability of the vaccine to indirectly reduce the risk of blowfly strike, which has a substantial economic impact in the sheep industry (Tellam and Bowles 1997). Some current practices used for control of blowfly strike, including insecticides and mulesing, are increasingly problematic due to the continuing development of insecticide resistance, and market and industry sensitivities relating to mulesing (Tellam and Bowles 1997). Selective breeding of sheep for breech cover and breech wrinkle are also important flystrike control strategies but genetic progress has been slow. In particular, what is unknown is the quantitative relationship between the incidence and severity of dermatophilosis with the risk of flystrike, particularly body strike. This information should be a priority for future research as it will underpin the commercial value proposition for development of a dermatophilosis vaccine. The commercial viability of a vaccine would also depend on its efficacy, protective period, number of injections, cost, ease of application, and market uptake and size. One future ambitious possibly is a single combined antigen vaccine that protects sheep from flystrike, fleece rot and lumpy wool.
There have been massive technological advances since the investigations of the feasibility of the development of dermatophilosis vaccines undertaken nearly 30 years ago. First, it is now possible to identify D. congolensis strain variation at high resolution by using genome sequencing. It is likely that previous vaccine formulations and challenges were confounded by strain variation (Ellis et al. 1991, 1993). The use of antibiotics to treat chronically infected sheep may also have been a driver of strain variation. Second, molecular technologies now provide an ability to rapidly isolate and identify D. congolensis antigens, particularly secreted factors that promote infectivity, lesion pathology and immune responses. The complete genome sequence (2.63 Mb) of D. congolensis is available, which rapidly provides access to all 2221 D. congolensis genes encoding proteins, some of which may be candidate vaccine antigens (NCBI 2017). Third, there is a greatly enhanced ability to artificially produce vaccine antigens. For example, recombinant protein antigens can be produced in a variety of ways that optimise the strength, specificity and type of an immune response to the antigen as described in an accompanying review (Denman et al. 2021). Fourth, there have been significant advances in vaccine formulation technologies that enhance humoral immune responses particularly the strength, type and duration of humoral immune responses (Denman et al. 2021).
Conclusions
Lumpy wool is an infectious exudative dermatitis of sheep skin characterised by crusting and matting of wool that results in lower wool value and yield per animal and an increased risk of blowfly strike. The incidence is highly variable and primarily dependent on a wet and warm period of weather. The causative bacterial agent is D. congolensis, which is spread among sheep by contact transmission. The bacterium can infect many animal species, particularly livestock, and in rare instances, humans. D. congolensis has a complex life cycle, with one stage, the hyphae, able to penetrate the outer layers of skin and wool follicles. Repeated infection and natural clearance of dermatophilosis occur in sheep as D. congolensis often remains in healthy fleece. Scabs on the ears and face are also likely to be a significant reservoir underpinning disease transmission to other sheep. Currently, lumpy wool is managed by a combination of hygiene practices, isolation of affected sheep, monitored self-healing of sheep, and treatment of severely infected animals with antibiotics. There is little information available about D. congolensis strain variation or antibiotic resistance, the current extent of dermatophilosis in the Australian sheep industry, and the relationship between dermatophilosis incidence and increased risk of flystrike. These are important prerequisites before future vaccine research is undertaken.
There is some evidence for naturally acquired immunity as older sheep are less susceptible. However, this effect could also be due to wool follicle and immune system maturation. Experimental vaccinations of sheep with antigens derived from specific D. congolensis life stages produced antibodies to these antigens in sheep sera and in washings of the skin from areas of the fleece affected by lumpy wool. However, it is unclear whether these antibodies protected sheep from infection. Vaccination of sheep with crude antigens from the filamentous life stage of D. congolensis resulted in fewer and less severe incidences of lumpy wool in some experimental trials. D. congolensis strain variation may be a significant factor in the inconsistent results of past vaccination trials.
Justification for the development of a vaccine that protects sheep from dermatophilosis is likely to be strongly dependent on the indirect ability of the vaccine to decrease the risk of blowfly strike. Technological advances since previous vaccination trials that were undertaken about 30 years ago increase the likelihood of the production of an efficacious vaccine against dermatophilosis that may also decrease the incidence and severity of flystrike in sheep.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
All authors were involved in writing a related commissioned industry report that was submitted to Australian Wool Innovation, but have no other conflicts of interest.
Declaration of funding
This research was funded by Australian Wool Innovation (AWI) Project ON-00723. AWI is funded primarily by Australian woolgrowers through a wool levy and by the Australian Government, which provides a matching contribution for eligible R&D activities.
Acknowledgements
We are very grateful to two reviewers who provided expert comments.
References
Al-Mamun HA, Kwan P, Clark SA, Ferdosi MH, Tellam R, Gondro C (2015) Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight. Genetics Selection Evolution 47, 66| Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight.Crossref | GoogleScholarGoogle Scholar |
Ambrose N (1996a) Antigenic, pathogenic and virulence factors of Dermatophilus congolensis. Available at http://www.vet.ed.ac.uk/ctvm/AHP/ahp/finalAHPelectronic/025.pdf
Ambrose NC (1996) The pathogenesis of dermatophilosis. Tropical Animal Health and Production 28, 29S–37S.
| The pathogenesis of dermatophilosis.Crossref | GoogleScholarGoogle Scholar | 8809989PubMed |
Ambrose NC, El Jack MA, McOrist S, Boid R (1997) Electrophoretic and antigenic characterisation of Dermatophilus congolensis extracellular products. Veterinary Microbiology 59, 37–51.
| Electrophoretic and antigenic characterisation of Dermatophilus congolensis extracellular products.Crossref | GoogleScholarGoogle Scholar | 9460195PubMed |
Ambrose N, Lloyd D, Maillard J-C (1999) Immune responses to Dermatophilus congolensis infections. Parasitology Today 15, 295–300.
| Immune responses to Dermatophilus congolensis infections.Crossref | GoogleScholarGoogle Scholar | 10377534PubMed |
Andersson L, Archibald AL, Bottema CD, Brauning R, Burgess SC, Burt DW, et al. (2015) Coordinated international action to accelerate genome-to-phenome with FAANG, the Functional Annotation of Animal Genomes project. Genome Biology 16, 57
| Coordinated international action to accelerate genome-to-phenome with FAANG, the Functional Annotation of Animal Genomes project.Crossref | GoogleScholarGoogle Scholar | 25854118PubMed |
Awad W, Abdou NE, El-Sayed A (2007) Diagnosis and treatment of bovine, ovine and equine dermatophilosis. Journal of Applied Sciences Research 4, 367–374.
Bateup B, Edwards J (1990) Processing of wool contaminated with dermatophilosis scab. Australian Veterinary Journal 67, 154–155.
Berry W, Watt B (2017) High prevalence mild dermatophilosis (lumpy wool) in unweaned merino lambs following a wet spring. Available at http://www.flockandherd.net.au/sheep/ireader/dermatophilosis.html
Bull LB (1929) Dermatomycosis of the sheep (lumpy or matted wool) due to Actinomyces dermatonomus. Australian Journal of Experimental Biology and Medical Science 6, 301–314.
Colditz IG, Watson DL (1993) Immunisation to induce antibody production in skin of sheep. In ‘Proceedings of Vaccines 1993, Lorne House, Victoria’. (Abstract J94).
Colditz IG, Altmann KG, Watson DL (1992) Intradermal and percutaneous transudation of IgG1 and transferrin in sheep. Immunology and Cell Biology 70, 323–327.
| Intradermal and percutaneous transudation of IgG1 and transferrin in sheep.Crossref | GoogleScholarGoogle Scholar | 1478698PubMed |
Colditz IG, Vuocolo T, Ingham AB, Wijffels G, James PJ, Tellam RL (2021) Fleece rot in sheep: a review of pathogenesis, aetiology, resistance and vaccines. Animal Production Science (submitted).
Davis D (1988) Experimental vaccination of rats with Dermatophilus congolensis zoospores. Research in Veterinary Science 44, 400–401.
| Experimental vaccination of rats with Dermatophilus congolensis zoospores.Crossref | GoogleScholarGoogle Scholar | 3406539PubMed |
Denman SE, Tellam RL, Vuocolo T, Ingham AB, Wijffels G, James PJ, Colditz IG (2021) Fleece rot and lumpy wool in sheep: opportunities and challenges for new vaccines. Animal Production Science (submitted).
Edwards JR (1985) Sale and processing of wool affected with dermatophilosis. Australian Veterinary Journal 62, 173–174.
| Sale and processing of wool affected with dermatophilosis.Crossref | GoogleScholarGoogle Scholar | 4038229PubMed |
Edwards JR (1991) Studies on the epidemiology of ovine dermatophilosis. PhD Thesis, Murdoch University, Murdoch, WA, Australia. Available at https:nla.gov.au/nla.cat-vn1491700
Edwards JR, Gardner JJ, Norris RT, Love RA, Spicer P, Bryant R, Gwynn RV, Hawkins CD, Swan RA (1985) A survey of ovine dermatophilosis in Western Australia. Australian Veterinary Journal 62, 361–365.
| A survey of ovine dermatophilosis in Western Australia.Crossref | GoogleScholarGoogle Scholar | 3834898PubMed |
Ellis TM, Robertson GM, Sutherland SS, Gregory AR (1987) Cellular responses in the skin of merino sheep to repeated inoculation with Dermatophilus congolensis. Veterinary Microbiology 15, 151–162.
| Cellular responses in the skin of merino sheep to repeated inoculation with Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar | 3439011PubMed |
Ellis TM, Sutherland SS, Gregory AR (1989) Inflammatory cell and immune function in merino sheep with chronic dermatophilosis. Veterinary Microbiology 21, 79–93.
| Inflammatory cell and immune function in merino sheep with chronic dermatophilosis.Crossref | GoogleScholarGoogle Scholar | 2623798PubMed |
Ellis TM, Sutherland SS, Davies G (1991) Strain variation in Dermatophilus congolensis demonstrated by cross-protection studies. Veterinary Microbiology 28, 377–383.
| Strain variation in Dermatophilus congolensis demonstrated by cross-protection studies.Crossref | GoogleScholarGoogle Scholar | 1949551PubMed |
Ellis TM, Sutherland SS, Turnor R, Krueger R (1992) Relationship between chronic ovine dermatophilosis and levels of T6 lymphocyte antigen staining in peripheral blood mononuclear cells. Veterinary Microbiology 30, 281–287.
| Relationship between chronic ovine dermatophilosis and levels of T6 lymphocyte antigen staining in peripheral blood mononuclear cells.Crossref | GoogleScholarGoogle Scholar | 1557900PubMed |
Ellis TM, Masters AM, Sutherland SS, Carson JM, Gregory AR (1993) Variation in cultural, morphological, biochemical properties and infectivity of Australian isolates of Dermatophilus congolensis. Veterinary Microbiology 38, 81–102.
| Variation in cultural, morphological, biochemical properties and infectivity of Australian isolates of Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar | 8128605PubMed |
Escayg AP, Hickford JG, Bullock DW (1997) Association between alleles of the ovine major histocompatibility complex and resistance to footrot. Research in Veterinary Science 63, 283–287.
| Association between alleles of the ovine major histocompatibility complex and resistance to footrot.Crossref | GoogleScholarGoogle Scholar | 9491458PubMed |
Faris B, Hollis L (2013) Dermatophilosis in sheep and goats. Kansas State University Agricultural Experiment Station and Cooperative Extension Service, Manhattan, KS, USA. Available at https://bookstore.ksre.ksu.edu/pubs/MF3118.pdf
Gardiner MR (1971) Mycotic dermatitis (lumpy wool) of sheep. Journal of the Department of Agriculture, Western Australia, Series 4 12, 16
Gherardi SG, Monzu N, Sutherland SS, Johnson KG, Robertson GM (1981) The association between body strike and dermatophilosis of sheep under controlled conditions. Australian Veterinary Journal 57, 268–271.
| The association between body strike and dermatophilosis of sheep under controlled conditions.Crossref | GoogleScholarGoogle Scholar | 6895592PubMed |
Gherardi SG, Sutherland SS, Monzu N, Johnson KG (1983) Field observations on body strike in sheep affected with dermatophilosis and fleece-rot. Australian Veterinary Journal 60, 27–28.
| Field observations on body strike in sheep affected with dermatophilosis and fleece-rot.Crossref | GoogleScholarGoogle Scholar | 6830546PubMed |
Gherardi SG, Harris DJ, Rolls SW (1984) The association of fleece characters and susceptibility to dermatophilosis. Animal Production in Australia 15, 357–360.
Giuffra E, Tuggle CK Giuffra E, Tuggle CK (2019) Functional annotation of animal genomes (FAANG): current achievements and roadmap. Annual Review of Animal Bioscience 15, 65–88.
| Functional annotation of animal genomes (FAANG): current achievements and roadmap.Crossref | GoogleScholarGoogle Scholar |
Goddard ME, Hayes BJ (2007) Genomic selection. Journal of Animal Breeding and Genetics 124, 323–330.
| Genomic selection.Crossref | GoogleScholarGoogle Scholar |
Gogolewski RP, Mackintosh JA, Wilson SC, Chin JC (1992) Immunodominant antigens of zoospores from ovine isolates of Dermatophilus congolensis. Veterinary Microbiology 32, 305–318.
| Immunodominant antigens of zoospores from ovine isolates of Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Hayes B, Goddard ME (2001) The distribution of the effects of genes affecting quantitative traits in livestock. Genetics Selection Evolution 33, 209–229.
| The distribution of the effects of genes affecting quantitative traits in livestock.Crossref | GoogleScholarGoogle Scholar |
Hiraizumi M, Tagawa Y (2014) Isolation and characterization of flagellar filament from zoospores of Dermatophilus congolensis. Veterinary Microbiology 173, 141–146.
| Isolation and characterization of flagellar filament from zoospores of Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
How SJ, Lloyd DH, Sanders AB (1990) ‘Advances in veterinary dermatology. Vol. 1.’ (Bailliere Tindall: London, UK)
James P, Forbes B, Anderson A (2019) Review of predisposing factors for breech strike. Australian Wool Innovation Limited, The Rocks, Australia. Available at https://www.wool.com/globalassets/wool/sheep/research-publications/welfare/flystrike-research-update/review-predisposing-factors-breech-flystrike-final-report.pdf
Jenkinson DM, Menzies JD, Pow IA, Inglis L, Lloyd DH, Mackie A (1989) Actions of bovine skin washings and sera on the motile zoospores of Dermatophilus congolensis. Research in Veterinary Science 47, 241–246.
Jiang Y, Xie M, Chen W, Talbot R, Maddox JF, Faraut T, Wu C, Muzny DM, Li Y, Zhang W, Stanton J-A, Brauning R, Barris WC, Hourlier T, Aken BL, Searle SMJ, Adelson DL, Bian C, Cam GR, Chen Y, Cheng S, DeSilva U, Dixen K, Dong Y, Fan G, Franklin IR, Fu S, Guan R, Highland MA, Holder ME, Huang G, Ingham AB, Jhangiani SN, Kalra D, Kovar CL, Lee SL, Liu W, Liu X, Lu C, Lv T, Mathew T, McWilliam S, Menzies M, Pan S, Robelin D, Servin B, Townley D, Wang W, Wei B, White SN, Yang X, Ye C, Yue Y, Zeng P, Zhou Q, Hansen JB, Kristensen K, Gibbs RA, Flicek P, Warkup CC, Jones HE, Oddy VH, Nicholas FW, McEwan JC, Kijas J, Wang J, Worley KC, Archibald AL, Cockett N, Xu X, Wang W, Dalrymple BP (2014) The sheep genome illuminates biology of the rumen and lipid metabolism. Science 344, 1168–1173.
| The sheep genome illuminates biology of the rumen and lipid metabolism.Crossref | GoogleScholarGoogle Scholar |
Kaminski GW, Suter II (1976) Human infection with Dermatophilus congolensis. Medical Journal of Australia 1, 443–447.
Larrasa J, Garcia A, Ambrose NC, Alonso JM, Parra A, de Mendoza MH, Salazar J, Rey J, de Mendoza JH (2002) A simple random amplified polymorphic DNA genotyping method for field isolates of Dermatophilus congolensis. Journal of Veterinary Medicine Series B 49, 135–141.
| A simple random amplified polymorphic DNA genotyping method for field isolates of Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Larrasa J, García-Sánchez A, Ambrose NC, Parra A, Alonso JM, Rey JM, Hermoso-de-Mendoza M, Hermoso-de-Mendoza J (2004) Evaluation of randomly amplified polymorphic DNA and pulsed field gel electrophoresis techniques for molecular typing of Dermatophilus congolensis. FEMS Microbiology Letters 240, 87–97.
| Evaluation of randomly amplified polymorphic DNA and pulsed field gel electrophoresis techniques for molecular typing of Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Leoni A, Fadda M, Nieddu AM, Pittau M, Sanna E, Pirino S, Contini A (1993) Dermatophilosis in sheep: first report in Italy, experimental reproduction and evaluation of immune response. Bollettino Della Societa Italiana Di Biologia Sperimentale 69, 775–782.
Lewer RP, Gherardi SG, Sutherland SS (1987) Realised heritability estimates for resistance to dermatophilosis in Merino sheep. In ‘Merino improvement programs in Australia: Proceedings of a National Symposium’, Leura, NSW, Australia. pp. 347–350. (Ed. BJ McGuirk). (Australian Wool Corporation: Melbourne Australia)
Lloyd DH, Noble WC (1982) Dermatophilus congolensis as a model pathogen in mice for the investigation of factors Influencing skin infection. British Veterinary Journal 138, 51–60.
| Dermatophilus congolensis as a model pathogen in mice for the investigation of factors Influencing skin infection.Crossref | GoogleScholarGoogle Scholar |
Lloyd DH, Jenkinson DM, Miller PN, Sykes TJ (1987) Sequential production of specific antibodies in serum and at the skin surface of cattle following intradermal vaccination with Dermatophilus congolensis. Veterinary Immunology and Immunopathology 16, 251–257.
| Sequential production of specific antibodies in serum and at the skin surface of cattle following intradermal vaccination with Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Maillard J-C, Berthier D, Chantal I, Thevenon S, Sidibé I, Stachurski F, Belemsaga D, Razafindraïbé H, Elsen J-M (2003) Selection assisted by a BoLA-DR/DQ haplotype against susceptibility to bovine dermatophilosis. Genetics Selection Evolution 35, S193–S200.
| Selection assisted by a BoLA-DR/DQ haplotype against susceptibility to bovine dermatophilosis.Crossref | GoogleScholarGoogle Scholar |
Marsella R (2016) Dermatophilosis: Veterian Key. Available at https://veteriankey.com/dermatophilosis/
Masters AM, Ellis TM, Grein SB (1997) Dermatophilus congolensis: strain differences in expression of phospholipase activities. Veterinary Microbiology 57, 199–213.
| Dermatophilus congolensis: strain differences in expression of phospholipase activities.Crossref | GoogleScholarGoogle Scholar |
Masters AM, Ellis TM, Groth D (1997) Difference between lambs with chronic and mild dermatophilosis in frequency of alleles of CD3γ. Veterinary Microbiology 57, 337–345.
| Difference between lambs with chronic and mild dermatophilosis in frequency of alleles of CD3γ.Crossref | GoogleScholarGoogle Scholar |
Meuwissen TH, Hayes BJ, Goddard ME (2001) Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829.
| Prediction of total genetic value using genome-wide dense marker maps.Crossref | GoogleScholarGoogle Scholar |
Mine OM (1996) Molecular cloning and characterisation of Dermatophilus congolensis serine protease genes. PhD Thesis, Murdoch University, Murdoch, WA, Australia.
Moriello K (2019) Dermatophilosis in animals – integumentary system. Merck Veterinary Manual. Available at https://www.merckvetmanual.com/integumentary-system/dermatophilosis/dermatophilosis-in-animals
Naval-Sanchez M, Nguyen Q, McWilliam S, Porto-Neto LR, Tellam R, Vuocolo T, Reverter A, Perez-Enciso M, Brauning R, Clarke S, McCulloch A, Zamani W, Naderi S, Rezaei HR, Pompanon F, Taberlet P, Worley KC, Gibbs RA, Muzny DM, Jhangiani SN, Cockett N, Daetwyler H, Kijas J (2018) Sheep genome functional annotation reveals proximal regulatory elements contributed to the evolution of modern breeds. Nature Communications 9, 859
| Sheep genome functional annotation reveals proximal regulatory elements contributed to the evolution of modern breeds.Crossref | GoogleScholarGoogle Scholar |
NCBI (2017) Genome List - Genome - NCBI. Available at https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/12627/
Norris BJ, Colditz IG, Dixon TJ (2008) Fleece rot and dermatophilosis in sheep. Veterinary Microbiology 128, 217–230.
| Fleece rot and dermatophilosis in sheep.Crossref | GoogleScholarGoogle Scholar |
NSW Industry and Investment (2010) Lumpy wool – a skin disease of sheep. Primefacts. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0013/314320/9819-Lumpy-wool---Primefact-986.pdf
Raadsma HW, Sandeman RM, Sasiak AB, Engwerda CR, Omeara TJ (1992) Genetic improvement in resistance to body strike in Merino sheep: where are we at with indirect selection. Proceedings of the Association of Advancement of Animal Breeding and Genetics 10, 143–146.
Roberts DS (1961) The life cycle of Dermatophilus dermatonomus, the causal agent of ovine mycotic dermatitis. Australian Journal of Experimental Biology and Medical Science 39, 463–476.
| The life cycle of Dermatophilus dermatonomus, the causal agent of ovine mycotic dermatitis.Crossref | GoogleScholarGoogle Scholar |
Roberts DS (1965) Penetration and irritation of the skin by Dermatophilus congolensis. British Journal of Experimental Pathology 46, 635–642.
Roberts DS (1966) The phagocytic basis of acquired resistance to infection with Dermatophilus congolensis. British Journal of Experimental Pathology 47, 372–382.
Roberts DS (1967) Chemotherapy of epidermal infection with Dermatophilus congolensis. Journal of Comparative Pathology 77, 129–136.
| Chemotherapy of epidermal infection with Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Roberts DS, Graham NPH (1966) Control of ovine cutaneous actinomycosis. Australian Veterinary Journal 42, 74–78.
| Control of ovine cutaneous actinomycosis.Crossref | GoogleScholarGoogle Scholar |
Rodostits OM, Gay CC, Hinchcliff KW, Constable PD (2006) ‘Veterinary Medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats.’ (Elsevier Health Sciences: Oxford, UK)
Salmon JK, Armstrong CA, Ansel JC (1994) The skin as an immune organ. Western Journal of Medicine 160, 146–152.
Sanders AB, How SJ, Lloyd DH, Hill R (1991) The effect of malnutrition on vaccination against Dermatophilus congolensis infection in ruminants. Journal of Comparative Pathology 105, 37–48.
| The effect of malnutrition on vaccination against Dermatophilus congolensis infection in ruminants.Crossref | GoogleScholarGoogle Scholar |
Scrivener CJ, Vizard AL (1995) Efficacy of a single dose of erythromycin or penicillin/streptomycin for the treatment of ovine dermatophilosis. Australian Veterinary Journal 72, 475–476.
| Efficacy of a single dose of erythromycin or penicillin/streptomycin for the treatment of ovine dermatophilosis.Crossref | GoogleScholarGoogle Scholar |
Seddon HR (1927) Dermatitis of sheep. NSW Department of Agriculture Veterinary Research Reports 5, 10–17.
Sheep CRC (2013) Dermatitis (lumpy wool). Flyboss. Available at http://www.flyboss.com.au/sheep-goats/files/pages/susceptibility/body-strike/Dermatitis_Pdf_download_130410.pdf
Sutherland SS, Robertson GM (1988) Vaccination against ovine dermatophilosis. Veterinary Microbiology 18, 285–295.
| Vaccination against ovine dermatophilosis.Crossref | GoogleScholarGoogle Scholar |
Sutherland SS, Gherardi SG, Monzu N (1983) Body strike in sheep affected with dermatophilosis with or without fleece rot. Australian Veterinary Journal 60, 88–89.
| Body strike in sheep affected with dermatophilosis with or without fleece rot.Crossref | GoogleScholarGoogle Scholar |
Sutherland SS, Ellis TM, Robertson GM, Gregory AR (1987) Serum and skin surface antibody responses in Merino sheep given three successive inoculations with Dermatophilus congolensis. Veterinary Microbiology 15, 209–218.
| Serum and skin surface antibody responses in Merino sheep given three successive inoculations with Dermatophilus congolensis.Crossref | GoogleScholarGoogle Scholar |
Sutherland SS, Ellis TM, Edwards JR (1991) Evaluation of vaccines against ovine dermatophilosis. Veterinary Microbiology 27, 91–99.
| Evaluation of vaccines against ovine dermatophilosis.Crossref | GoogleScholarGoogle Scholar |
Tabar GRH (1998) Selection of peptides from random peptide libraries for a recombinant vaccine against dermatophilosis. PhD Thesis, Murdoch University, Murdoch, WA, Australia.
Tabar GRH, Carnegie P (2002) Immunization of sheep with phage mimotopes against dermatophilosis. Iranian Biomedical Journal 6, 129–134.
Tellam RL, Bowles VM (1997) Control of blowfly strike in sheep: current strategies and future prospects. International Journal for Parasitology 27, 261–273.
| Control of blowfly strike in sheep: current strategies and future prospects.Crossref | GoogleScholarGoogle Scholar |
Vuocolo T, Denman SE, Ingham AB, Wijffels G, Colditz I, Tellam RL, James PJ (2020) Control of fleece-rot and lumpy wool by vaccination: feasibility review and options. Australian Wool Innovation Limited, The Rocks, NSW, Australia. Available at https://www.wool.com/globalassets/wool/sheep/research-publications/welfare/flystrike-control/200702-on-00723-fleece-rot-final-report.pdf
Wallis J, Shenton DP, Carlisle RC (2019) Novel approaches for the design, delivery and administration of vaccine technologies. Clinical and Experimental Immunology 196, 189–204.
| Novel approaches for the design, delivery and administration of vaccine technologies.Crossref | GoogleScholarGoogle Scholar |
Watson DL, Colditz IG, Andrew M, Gill HS, Altmann KG (1994) Age-dependent immune response in Merino sheep. Research in Veterinary Science 57, 152–158.
| Age-dependent immune response in Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Wilkinson FC (1979) Dermatophilosis of sheep association with dipping and effects on production. Australian Veterinary Journal 55, 74–76.
| Dermatophilosis of sheep association with dipping and effects on production.Crossref | GoogleScholarGoogle Scholar |
Zaria LT (1993) Dermatophilus congolensis infection (dermatophilosis) in animals and man! An update. Comparative Immunology, Microbiology and Infectious Diseases 16, 179–222.
| Dermatophilus congolensis infection (dermatophilosis) in animals and man! An update.Crossref | GoogleScholarGoogle Scholar |


