Harnessing plant bioactivity for enteric methane mitigation in Australia
Z. Durmic A C , J. L. Black B , G. B. Martin A and P. E. Vercoe A
A C , J. L. Black B , G. B. Martin A and P. E. Vercoe A
A UWA School of Agriculture and Environment and UWA Institute of Agriculture, The University of Western Australia, M085, 35 Stirling Highway, Crawley, WA 6009, Australia.
B John L Black Consulting, Warrimoo, NSW 2774, Australia.
C Corresponding author. Email: zoey.durmic@uwa.edu.au
Animal Production Science 62(12) 1160-1172 https://doi.org/10.1071/AN21004
Submitted: 5 January 2021 Accepted: 21 October 2021 Published: 16 December 2021
Journal compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
This review provides examples of the utilisation of plant bioactivity to mitigate enteric methane (CH4) emissions from the Australian ruminant production systems. Potential plant-based mitigation strategies that reduce CH4 without major impacts on forage digestibility include the following: (i) low methanogenic tropical and temperate grass, legume and shrub forage species, which offer renewable and sustainable solutions and are easy to adopt, but may have restricted geographical distribution or relatively high costs of establishment and maintenance; (ii) plant-based agricultural by-products including grape marc, olive leaves and fruit, and distiller’s grains that can mitigate CH4 and provide relatively cheap high-nutrient supplements, while offsetting the impact of agricultural waste, but their use may be limited due to unfavourable characteristics such as high protein and water content or cost of transport; (iii) plant extracts, essential oils and pure compounds that are abundant in Australian flora and offer exciting opportunities on the basis of in vitro findings, but require verification in ruminant production systems. The greatest CH4 mitigation potential based on in vitro assays come from the Australian shrubs Eremophila species, Jasminum didymium and Lotus australis (>80% CH4 reduction), tropical forages Desmanthus leptophyllus, Hetropogon contortus and Leucaena leucocephala (~40% CH4 reduction), temperate forages Biserrula pelecinus (70–90% CH4 reduction), perennial ryegrass and white clover (~20% CH4 reduction), and plant extracts or essential oils from Melaleuca ericifolia, B. pelecinus and Leptospermum petersonii (up to 80% CH4 reduction). Further research is required to confirm effectiveness of these plant-based strategies in vivo, determine optimal doses, practical modes of delivery to livestock, analyse benefit–cost ratios and develop pathways to adoption.
Keywords: methane, mitigation, rumen, forages, plant-based feed additives, plant bioactive compounds.
Introduction
Ruminants can consume and digest large amounts of plant material and convert it into high-quality products such as meat, milk and wool, by virtue of microbial fermentation in their gut, where volatile fatty acids (VFA) and methane (CH4) are produced as end products of the fermentation. While VFA present a major energy source for the animal, the CH4 serves as the main pathway of eliminating hydrogen in the rumen (Beijer 1952). In the past century, enteric CH4 has been regarded simply as a loss of energy from ingested feed, but in more recent times, it has become of concern as a major greenhouse gas (GHG; Johnson and Johnson 1995). About 70% of the CH4 produced on Earth is generated from anthropogenic sources, and ruminant livestock is the single most significant contributor (Moss et al. 2000). Each ruminant animal produces and eructates 5–130 kg of CH4 year, depending on its size and the amount of fibrous material consumed in the diet (Johnson and Johnson 1995). Enteric CH4 has been estimated to contribute half of the total GHG emissions from the agricultural sector (Swainson et al. 2018; Dong et al. 2019).
Methane is a highly potent GHG, being more effective than carbon dioxide (80 times over 10–20 years, or 28 times over 100 years from release), but has a shorter atmospheric lifetime of 12 years from release, compared with either carbon dioxide at 50–200 years from release, or nitrous oxide at 120 years from release (EPA 2003). Thus, enteric CH4 is an attractive target for reducing overall GHG production. A reduction of 10% in enteric CH4 production could be sufficient to prevent further accumulation of CH4 in the atmosphere (Moss et al. 2000). In Australia, CH4 emissions from livestock account for 60–70% of total GHG emissions from the agricultural sector (Cottle et al. 2011; Taylor et al. 2016). The size of this contribution is partly due to the large number of ruminants; Australia is a significant player in global food supply, accounting for ~4% of global beef production (Suybeng et al. 2019). Australian CH4 emissions from livestock are also significant because most ruminants are managed under extensive conditions where the animals usually graze low-quality, fibrous diets that promote relatively high CH4 production in the rumen compared with feedlot or dairy cattle offered high-grain diets (Charmley et al. 2008).
Early CH4 mitigation strategies focussed on removal of protozoa from the rumen, because a portion of the methanogenic archaea in the rumen live in close association with them (Blaxter and Czerkawski 1966; Whitelaw et al. 1984). Subsequently, concepts and approaches broadened in the recognition that enteric CH4 is a significant contributor to atmospheric pollution and global warming. The outcome was global development of solutions, including manipulation of animal physiology, genetics and management of the rumen microbiome by manipulation of diet composition and the use of feed additives (reviews: Moss et al. 2000; Busquet et al. 2006; Calsamiglia et al. 2007; McAllister and Newbold 2008; Hristov et al. 2013; Patra et al. 2017; Beauchemin et al. 2020; Min et al. 2020).
Early attempts to reduce enteric CH4 emissions frequently relied on synthetic chemicals and antibiotics, but often with only modest benefits (reduction by only 10% in vivo), that were also variable (Johnson and Johnson 1995) and mostly ineffective in grazing animals (Grainger et al. 2010). Emerging concerns over the impact of antibiotics in feed on human, animal and environmental health led to legislation of restrictions on their use. The result was a worldwide search for natural, safe and sustainable alternatives, with a particular focus on plants and plant products.
The primary constituents of forage plants, including soluble and insoluble carbohydrates and oils, can drive the ‘methanogenic potential’, namely, amount of CH4 produced when consumed and fermented by rumen microbes, but many plant secondary compounds (PSC) can also affect methanogenesis by acting directly and specifically on methanogens, or by acting indirectly on the overall processes of fermentation in the rumen (Bodas et al. 2012). The aim of a CH4 mitigation strategy is to reduce CH4 emissions, while not reducing overall digestibility/fermentability of the feed consumed. In that respect, a reduction in the concentration of VFA in the rumen can be used as an approximation for assessing negative effects of a strategy on overall feed digestibility/fermentability.
The present paper reviews current developments and strategies for the use of plant bioactivity to reduce enteric CH4 emissions in Australia. We focussed on mitigation approaches based on the grazing of low methanogenic forages, the feeding of plant by-products as supplements, the use of whole plant extracts, essential oils and pure PSC, and also considered some commercially available plant-based products. We first illustrated each of these mitigation strategies with some of the work done globally, and then focussed on work in Australia, critically evaluating the prospects for local success. We compared the strategies in terms of national potential for CH4 mitigation, agronomic and animal production benefits, and barriers and limitations to their use. This structured approach allowed us to finally analyse the options and prospects for practical applications in Australia.
Grazing low-methanogenic forages
Enteric CH4 emissions from grazing animals can be targeted by manipulating the forage they consume, because there is significant variation among plant species in their methanogenic potential. There is worldwide interest in commonly used forages, but also in region-specific and novel grazing plants. Among the mainstream forages, the most prominent candidates with low-to-moderate methanogenic potential are lotus (Lotus corniculatus, L. pedunculatus), sulla (Hedysarum coronarium), lucerne (Medicago sativa), chicory (Cichorium intybus), white clover (Trifolium repens) and red clover (Trifolium pratense; Tavendale et al. 2005; Navarro-Villa et al. 2011; Hammond et al. 2013). Tropical forages have been found to be particularly effective at reducing rumen CH4 production, in vitro and in vivo, with a wide variety of effective fodders from diverse plant families being reported (Hariadi and Santoso 2010; Silivong et al. 2013; Pal et al. 2015). The reduction in CH4 production in these forages was generally attributed to the presence of PSC, particularly tannins, with reductions by up to 25% (kJ/MJ gross energy intake) seen with tannin-rich shrubs and trees (Soliva et al. 2008; Tiemann et al. 2008).
Work on low-methanogenic forages has been particularly strong in Australia, because feeding systems rely so heavily on pasture-based grazing, with minimal grain supplementation. In that respect, native woody perennial plants (trees and shrubs) seem able to play an important role, especially in low-rainfall areas, while tropical forages are utilised in the tropical and subtropical regions. There is also emerging evidence that grazing ruminants in Australia have a high methanogen diversity and harbour some unique methanogen populations (Wright et al. 2004; Rea et al. 2007; McSweeney and Tomkins 2015).
The search for variability in methanogenic properties in grazing plants in Australia began with an investigation of forage shrubs when 128 Australian native forage shrubs were assessed using in vitro 24-h batch culture (Durmic et al. 2010). Several highly potent candidates were revealed, with almost half of the species tested producing less CH4 than with oaten chaff, a common supplementary feed. One plant in particular, commonly known as tar bush (Eremophila glabra), reduced CH4 production by 81%. This CH4 mitigation effect was subsequently confirmed using a continuous in vitro system (Li et al. 2014) and in vivo using sheep (Li 2013, K. Lund, pers. comm.). Eremophila species produce abundant terpenes and flavones that are potent CH4 inhibitors (Oskoueian et al. 2013), and the effect in E. glabra was linked to direct inhibition of methanogenic populations (Li et al. 2014). However, E. glabra had a general inhibitory effect on fermentation, with a 15% reduction in VFA concentrations when it is used as the sole substrate in vitro (Durmic et al. 2010). However, the anti-methanogenic effect of E. glabra is sufficiently potent so that it can be used in a mix with other forages, thus moderating negative effects while still significantly reducing CH4 (Li et al. 2014). E. glabra is well adapted to drought and infertile soils, two critical issues in our grazing systems, and it has an advantageous mineral profile, but it may be constrained by relatively low biomass production and poor palatability compared with some mainstream pastures (Revell et al. 2013). Such problems are likely to respond to plant improvement, or by just ensuring it is integrated as part of a mixed forage base in grazing systems; however, further research is needed for E. glabra to be widely adopted in grazing systems.
Research has been conducted on the plants from the Australian tropics and subtropics. These species are particularly important because that region is home to half of Australia’s beef cattle, so it is responsible for the majority of the nation’s enteric CH4 emissions (AGEIS 2017). Among those with low-to-moderate methanogenic potential are both grasses (Andropogon gayanus, Brachiaria ruziziensis, Bothriochloa decipiens, Sorghum plumosum, Urochloa mosambicensis) and leguminous forages (Calliandra calothyrsus, Desmanthus leptophyllus, Gliricidia sepium, Stylosanthes scabra, Leucaena leucocephala; Meale et al. 2012; Durmic et al. 2017; Suybeng et al. 2019). These species often contain tannins that can directly reduce the amount of CH4 produced (Piñeiro-Vázquez et al. 2018), but these forages can also reduce CH4 emission intensity because they improve growth rates and thus animal productivity (Taylor et al. 2016). There are some potential limitations, including eco-geographical constraints and some anti-nutritive or toxic PSC that impede feed intake or affect animal health (Dalzell et al. 2012), but as with all novel feedstuffs, it is important to complete a duty of care assessment (Revell and Revell 2006).
Concurrent with the investigations into tropical and subtropical forages was research focussed on temperate herbaceous forages. In our initial screening of 13 mainstream and alternative pasture species of southern Australia, using fermentation in vitro, we discovered that a legume biserrula (Biserrula pelecinus) produced 73% less CH4 than did lucerne, and 90% less CH4 than did the highest CH4-producing species, bladder clover (Trifolium spumosum; Banik et al. 2013). Subsequently, other mainstream pasture species were investigated, and it was shown that when subterranean clover (Trifolium subterraneum) was fed to sheep, CH4 production was reduced by 30% compared with feeding ryegrass (Muir et al. 2020). Moreover, the methanogenic potential of subterranean clover is found to be a heritable trait, so it can be manipulated by plant breeding (Kaur et al. 2017). Birds-foot trefoil (lotus) was also explored for its potential, and theoretical estimates suggest that it can reduce the CH4 emission intensity for wool and prime lamb by increasing liveweight gain and fecundity (Doran-Browne et al. 2015). For some of the temperate forage species, the mitigation effect may be linked to their primary chemical composition and to enhancing productivity, thus reducing CH4 emission intensity, whereas in others, such as biserrula, the effect may be linked to the presence of specific anti-methanogenic PSCs (Banik et al. 2016).
In addition to the mainstream species, some alternative forages that are aimed at filling seasonal feed gaps in temperate parts of Australia were also investigated. Local varieties of turnip (Brassica rapa), chicory or plantain (Plantago lanceolata) were found to produce ~25% less CH4 (mL/g dry matter incubated) in vitro than did lucerne (Durmic et al. 2016). Feeding forage brassicas to cattle was found to reduce CH4 yield (g CH4/kg dry-matter intake) by 5%, and CH4 emission intensity (g/kg energy-corrected milk) by 10% (Williams et al. 2016). The mechanism of these effects is largely unknown, but it is likely to be a combination of primary chemical constituents and their PSC.
During this period of exploration of plant bioactivity, it became evident that, while some variation in methanogenic potential was related to plant species, there was also within-species variation. Often due to environmental factors, the same species can differ in primary chemicals, PSC composition, or simply, in moisture, consequently resulting in differences in methanogenic potential (Durmic et al. 2017). As we examined a core collection of biserrula, we also demonstrated variation among cultivars, growth stages and cutting treatments that were not influenced by environmental factors (Banik et al. 2019).
In addition to reducing CH4, most of the forages mentioned above presented fermentation profiles (as described by production of VFA and the acetate:propionate ratio) that were comparable or better than the respective controls (standard forages), implying that it is possible to target CH4 without impeding microbial fermentation and thus compromising animal productivity. Figure 1 presents examples of low-methanogenic forages in Australia and their effect on CH4, and outlines candidates that markedly reduce CH4 production, while maintaining or even promoting VFA production.
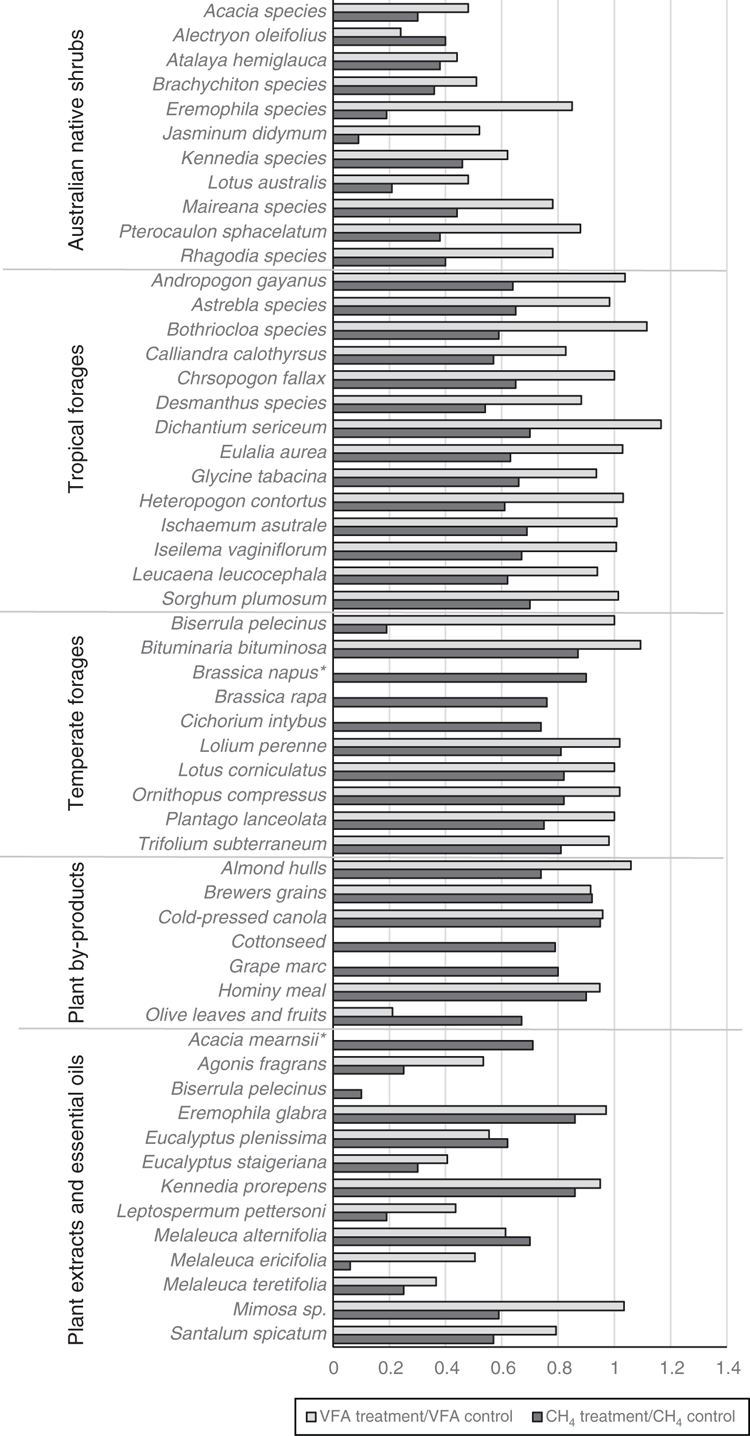
|
Bioactivity in plants by-products fed as supplements
Horticulture generates huge amounts of organic waste (prunings, leaves, seeds, fruits, peels, pulp, stones) that is a loss of valuable biomass and is an environmental burden. There is a need for cost-effective, sustainable and environmentally friendly processes for the utilisation of these products. This issue is particularly important in developing countries where livestock industries are constrained by fodder shortages and the high costs of conventional feeds. Horticultural by-products often retain a high nutrient content, so they are attractive as a supplementary feed in animal production. They can also be rich in PSC (Sagar et al. 2018), so many of these materials have been investigated globally for their potential to modulate rumen fermentation and mitigate enteric CH4 production (McGinn et al. 2009; Benchaar et al. 2013; Castillo-Lopez et al. 2017). The effects are mainly ascribed to increased intakes of fat and high-quality protein (Moate et al. 2011), or in some cases tannins or other PSC.
This concept is also relevant to Australia, where ~1500 kT of fruit and vegetable biomass is wasted each year during production, processing and packing stages, or is simply lost as food waste (ARCADIS 2019). The wine industry generates a by-product, grape marc, that is high in protein, fat, fibre and other nutrients and has been used as a feed supplement for cattle. It contains condensed tannins, which, in turn, can reduce enteric CH4 production when fed to ruminants (Goel and Makkar 2012).
By-products can contain a substantial amount of crude protein, increasing the excretion of ammonia, or products such grape marc have a high-water content, diluting out the bioactivity, causing product spoilage and increasing the cost of transport. Despite these issues, grape marc, in particular, continues to be a topic of interest because, as a major producer of wine, Australia generates ~200 kT of the by-product annually. However, there has been some inconsistencies in its mitigation potential, because only a limited reduction in CH4 production was seen when extracts of grape marc were tested in vitro (Hixson et al. 2018), whereas it reduced CH4 emissions by 20% when fed to lactating cows (Moate et al. 2014). This disagreement could be explained by the difference between in vitro and in vivo methodologies, or by variations in the chemical profiles of various types of grape marc (Russo et al. 2017). The high fibre content and low digestibility of grape marc reduces milk yield when fed to dairy cattle, when used to replace high energy supplements (Moate et al. 2020). However, when grape marc is substituted for feed with a similar energy value, CH4 emissions are reduced, with little change in productivity (Black et al. 2021).
Feeding ruminants plant oils is another effective way of reducing enteric CH4 production, while utilising oil-rich waste products generated during the oil-extraction process. Olive cake, cashew nut shell, hazelnut pericarps, and the seeds from sunflower, flax and canola have been identified as CH4 mitigators (Beauchemin et al. 2009; Watanabe et al. 2010; Niderkorn et al. 2020). The anti-methanogenic action in these involves direct removal of hydrogen during fermentation (Eugène et al. 2008; Rasmussen and Harrison 2011). The products considered in Australia for CH4 mitigation include brewers’ grains, cold-pressed canola, hominy meal, pequi oil, almond hulls and cottonseed (Moate et al. 2011; Durmic et al. 2014, Duarte et al. 2017; Williams et al. 2018). Among these, almond hulls have been shown to reduce CH4 production by 25% (Durmic et al. 2014), while preliminary investigations into olive leaves and fruits suggested a 50% reduction in CH4 production in vitro (Shakeri et al. 2017), and a recent study linked the effect to polyphenol content and shift in bacterial populations (Lee et al. 2021).
Whole plant extracts and essential oils
The exploration of CH4 mitigation strategies extended from feeding whole plants or plant products, to a quest for specific bioactive molecules of plant origin. Early reports from Europe showed that extracts from flavouring oils, particularly garlic, reduced CH4 emissions (Busquet et al. 2005a, 2005b; Chaves et al. 2008).
In Australia, extracts from two native forage plants (i.e. Tar Bush or Kennedia prorepens, as well as biserrula), significantly reduced CH4 production in vitro (Durmic et al. 2014; Banik et al. 2016). In biserrula, selected fractions were tested against methanogens in pure culture and found to inhibit some key ones, including those found in Australian grazing sheep (Banik et al. 2016). More research is needed to identify specific anti-methanogenic compounds from a variety of candidate plants that can be tested in vivo.
Significant research has been dedicated to studying the anti-methanogenic effects of essential oils, and, globally, those from clove, white thyme, citronella, peppermint, anise and cinnamon have been reported to reduce CH4 emissions (Patra and Yu 2012; Benchaar 2016; Günal et al. 2017). Essential oils from Australian plants gained attention in the late 1990s as potent antimicrobials for human pathogens (Hammer et al. 1999), leading to assessment of their value for CH4 mitigation. Essential oils from swamp paperbark (Melaleuca ericifolia), honey myrtle (M. teretifolia) and lemon-scented teatree (Leptospermum petersonii) were found to be very potent and inhibiting CH4 production by up to 75% (in vitro), although they also inhibited microbial fermentation (VFA) at the doses tested (Durmic et al. 2014). Subsequently, we identified optimal doses that did not affect overall rumen fermentation, but were still effective at reducing CH4 production (Jahani-Azizabadi et al. 2019). In vitro work is continuing to identify the mechanisms that explain the effect and to optimise the doses of pure active ingredient, after which we will move to in vivo testing.
Pure plant compounds: tannins, saponins and other PSC
Tannins and saponins are often abundant in plants of low methanogenic potential, with condensed tannins becoming a major focus for anti-methanogenic compound research (Waghorn 2008; Patra and Saxena 2011; Rira et al. 2013). In vivo studies confirmed the potency of condensed tannins, with emission reductions of more than 50% having been reported (Carulla et al. 2005; Lima et al. 2019). Tannins can act directly, affecting methanogens and protozoa, and preventing methanogens from attaching to protozoa, or act indirectly by inhibiting overall rumen microbial activity, with the subsequent consequence of reducing animal productivity (Kumar and Vaithiyanathan 1990; Ku-Vera et al. 2020).
Saponins have been also considered for CH4 mitigation because they can control ruminal protozoa and thus reduce the number of methanogens that are directly associated with them (Patra and Saxena 2009; Jayanegara et al. 2012). However, the results with saponin-rich plant sources have been variable, with effects ranging from no CH4 reduction to moderate reduction in vitro and in vivo (Goel and Makkar 2012; Liu et al. 2019; Molina-Botero et al. 2019). These disagreements could be explained by variation in the saponin source. The most promising candidates reported around the world appear to be Yucca schidigera, Saponaria officinalis, Medicago sativa, Camellia sinensis, Enterolobium cyclocarpum and Quillaja Saponaria, inhibiting CH4 production by up to 40% (Rodríguez and Fondevila 2012; Patra et al. 2017).
In Australia, Ramírez-Restrepo et al. (2016) reported an 18% reduction in total daily CH4 emissions (g/day) and a 22% reduction in yield (g/kg dry-matter intake) after feeding steers with tea-seed saponins in combination with Rhodes grass and grain concentrate
Investigation of the active components of essential oils has progressed, also leading to the discovery of some potent pure compounds from these that inhibit CH4 production in vitro and in vivo, including thymol, carvacrol, cinnamaldehyde, garlic organosulfur compounds, citral, limonene, linalool, α- and β-pinene (Busquet et al. 2005a; Cardozo et al. 2006; Macheboeuf et al. 2008; Joch et al. 2016; Ma et al. 2016). Similar work is currently being conducted on compounds found in Australian plant essential oils.
There are advantages in working with pure compounds. The structure is well known and the effects on CH4 production can be attributed specifically to the compound itself, presenting opportunities to investigate mechanisms of action. They are more attractive than mixed compounds or whole plants from a commercialisation perspective, because purity can be verified for drug acceptability and efficacy. Once identified, they can be obtained from natural sources, using scaled-up extraction processes, or even synthesised. However, anti-methanogenic effects might be less efficient with a single compound than with a combination of compounds, some of which may not even be identified (Patra et al. 2017).
Commercial plant-based products
Plant bioactivity has been explored globally, with a view to the development of commercial products. One of the first, based on essential oil compounds, was Crina® Ruminant (Akzo Nobel Ltd, Netherlands) that had positive effects on animal production, but with limited effects on CH4 mitigation (Beauchemin and McGinn 2006; Tomkins et al. 2015; Patra et al. 2017). Another essential oil product, Agolin® Ruminant (Agolin SA, Switzerland), was more effective and became the first feed additive certified for CH4 mitigation in ruminants (Carbon Trust Assurance Ltd, https://agolin.ch/certifications/). It contains compounds from coriander seed, eugenol, geranyl acetate and geraniol. In vitro, Agolin® Ruminant reduced CH4 production by 30% (Durmic et al. 2014), and when fed to dairy cattle at a rate of 1 g/head daily, it decreased CH4 production by more than 10% without affecting animal productivity (Belanche et al. 2020). A recently emerged product that is showing promise, MootralTM (Mootral, Switzerland), a combination of extracts from garlic and bitter orange, persistently reduced CH4 production in vitro by 70% (Eger et al. 2018). Activo® Premium (EW Nutrition, Germany), a mix of microencapsulated PSC, has been reported to reduce enteric CH4 production in sheep by 26%, while improving rumen fermentation, digestibility and protein synthesis (Soltan et al. 2018). All of these products are commercially available as feed additives in Australia, but, in parallel, the work is progressing towards developing local products.
There are some obvious advantages when using a commercial product for CH4 mitigation, including the following: it is backed up by extensive research and development; it has passed regulatory requirements; and it is easy to adopt and apply. However, these products do come at a cost, so their use is often limited to intensive industries that can reliably incorporate such additives in the feedlots.
Advantages and limitations of plant bioactivity
Plant-based approaches to CH4 mitigation in ruminants may offer several benefits and advantages. Many of the plant sources under consideration are already a major part of ruminant diets, so there is little imposition on the animal and they qualify as ‘natural’. The anti-methanogenic PSC are present, abundant and diverse in some of these plants. In addition to reducing CH4 production, some bioactive plants and plant-based products have other beneficial properties. They can improve animal feed intake and utilisation, enhance fermentability and digestibility, reduce protein degradability, and increase animal productivity (Aerts et al. 1999; Akanmu and Hassen 2018). A wide variety of these plants and PSC are also effective in managing animal digestive disorders, such as lactic acidosis, controlling animal diseases (i.e. worms), or enhancing animal reproduction (Kotze et al. 2009; Hutton et al. 2010; Durmic and Blache 2012). Many of the bioactive plants that have been investigated in Australia are native (unbred) plants, grown locally; so, they contribute to the preservation of biodiversity and thus a more ‘ecologically friendly’ animal production system. Adding these native forages to the production system has also been reported to add value to the feed-base (Vercoe et al. 2009; Revell et al. 2013) and improve overall farm profitability (Monjardino et al. 2010). Further, if plant-based bioactive compounds are derived from organic waste that would otherwise end up as landfill, then the CH4 mitigation achieved by feeding them to livestock is accompanied by a reduction in environmental pollution and gas emissions from the secondary fermentation.
Plant-based CH4 mitigation strategies, while having many advantages, also have certain limitations and present challenges. Propagation and utilisation of low methanogenic forages may be restricted to a single geographical location, climate or season, with constraints in their nutritional profile, supply, biomass, or because the strategy to incorporate them is not feasible for all animal production systems. Tannin- and saponin-rich browse and extracts are too often restricted in their application due to the depression of feed intake, fermentation and milk yield (Busquet et al. 2005b; Hess et al. 2006; Tan et al. 2011; Castro-Montoya et al. 2012). The use of industry by-products is often limited because their high water content can lead to spoilage, as well as increased cost of transport and processing. High content of protein, tannin, sugar or lignin may also affect the animal directly, by inhibiting rumen function, or lead to increased GHG emissions from manure of animals fed these by-products (Hünerberg et al. 2014). Vegetable oils and fats that remain in oil by-products can also have negative effects on milk production (Martin et al. 2008), whereas tannins and terpenes may leave residues and taint the animal products (Mason et al. 2017). Although essential oils are considered natural, with a history of use in traditional medicine, some toxic effects have been recorded in livestock (Horky et al. 2019).
However, the main problems of progressing plant-based approaches for CH4 mitigation are confirmation of effectiveness in vivo and finding the optimal dose and mode of delivery. Many reports have failed to demonstrate in vivo efficacy of promising candidates that have emerged from in vitro screening (Meale et al. 2014; Benchaar 2016). For those that are shown to be effective in vivo, doses are often too high, so there are adverse effects on rumen microbes, or the feeding requirements are simply impractical (Benchaar et al. 2008; Macheboeuf et al. 2008; Grainger et al. 2009). There is also the effect of the interactions among host species, genotype, rumen conditions and animal diet, limiting extrapolation to specific production systems and situations (Calsamiglia et al. 2007; Patra and Saxena 2009; Castro-Montoya et al. 2012). For example, some plant-based feed additives are most effective when combined with a high-fibre diet (Shakeri et al. 2017), whereas others are better suited to combination with concentrate rations (Calsamiglia et al. 2007). Furthermore, most in vivo data in the literature are derived from short-term trials. As a result, extrapolation to production systems becomes difficult because the rumen microbes can adapt to the PSC (Moss et al. 2000; Busquet et al. 2005a; Pellikaan et al. 2011), or degrade them into metabolites with less bioactivity (Malecky et al. 2012; Ghaffari et al. 2015). These adaptations can explain reduced efficacity over time of candidate PSC in vivo (Benchaar et al. 2008). We also need to take testing beyond laboratory-based or animal house-type in vivo studies and assess candidates and plant-based strategies under commercial conditions. This issue becomes evident when we consider extensive grazing systems in which the amount of bioactive compound that an animal ingests is unpredictable. Moreover, in some production systems, the application of plant bioactivity may not be practical or cost-effective.
Options for plant bioactivity-based CH4 mitigation in Australia
Table 1 and Figs 1 and 2 present an overview of benefits and limitations of plant-based mitigation strategies for Australian ruminant industries, summarised from information provided by Black et al. (2015, 2021) and other literature cited in the present review. Briefly, in Fig. 1, we have summarised the information on the level of CH4 reduction, as well as the effect on rumen fermentation (VFA production) gathered in Australia and for different sources of plant bioactivity. In Table 1, we have then summarised the extent (moderate–high) and the type (specific–nonspecific) of effect on CH4 for each category. In there, we have also evaluated the other benefits to animal production, such as good agronomic properties and nutritive value of the plant material fed, effect on fermentation and animal health, and, consequently, on animal productivity and welfare. We have also considered the barriers and limitations to adoption for each practice. Finally, we sourced information from Black et al. (2021) to plot these mitigation categories according the predicted time to practical application, barrier/cost to implement, and the national CH4 mitigation potential in Australia based on 25% reduction in CH4 emissions across all Australian ruminants and 10% adoption. We then used the information on the barriers and limitations, whether the methodology is something that producers are familiar with, whether the plant used in the strategy has good agronomic properties, biomass and NV, so as to estimate and present ‘likelihood of adoption’ (low–high).

|
Given the Australian focus on grazing livestock, changing forage species available for consumption seems the obvious first option. The wide range of eco-climatic zones, from tropical to temperate, to hostile, dry environments, will also dictate the strategies that are most applicable. The natural, sustainable, cost-effective solutions for CH4 mitigation in grazing ruminants are therefore likely to be low-methanogenic browse, i.e. native forage shrubs in low- to medium-rainfall zones; mainstream/alternative herbaceous plants for temperate climates; tropical or rangeland plants for the northern regions of Australia.
Temperate forages such as subterranean clover and lucerne are highly ranked in terms of national mitigation potential and practicality; they achieve only a moderate reduction in CH4 production, but they offer a significant reduction in CH4 emission intensity due to their high nutritive value and positive effect on other fermentation pathways. They are familiar to producers, and despite requiring high inputs for sourcing, establishment, cultivation and maintenance, they are likely to have a high adoption rate, and as such can contribute significantly to national CH4 mitigation overall (Fig. 2). By contrast, the current estimates for Australian native forage shrubs predict that these have a smaller role in national mitigation, as they are geographically contained and need to be grazed in a mix to offset any deficiencies in nutritive profile or negative effects on the rumen, animal health and productivity. Greater implementation is also limited by our incomplete agronomic knowledge of the species and insufficient analysis of the anti-methanogenic properties in a wider range of native plants that are naturally present in the feedbase. These limitations extend the timeline of more widespread adoption, which is currently limited to areas of marginal land and as a drought reserve (Fig. 2). Research is required to overcome these knowledge gaps, so the shrubs are contributing more to the feedbase in a variety of regions. As some of them have a strong, direct anti-methanogenic effect, and many are found to be naturally present and already grazed in Australian rangelands, predictions of ruminant emissions from Australian rangelands, and the value of our native plants, may need to be revisited and altered when more information becomes available.
Tropical forages (e.g. Leucaena) generally elicit moderate reductions in enteric CH4 production, but have the potential to increase animal productivity by 20% and therefore significantly reduce CH4 emission intensity, when compared with the standard practice of grazing Rhodes grass pastures (Harrison et al. 2015). Despite barriers due to high establishment costs, complex management, and issues with anti-nutritive factors and toxicity (Table 1), they have the advantage of providing good biomass and nutritive profiles (Taylor et al. 2016), resulting in moderate prospects for practical application.
Industry by-products are already valued as a feed supplement in Australia, and some of these also bring a desired reduction in CH4 production (Table 1). However, the distribution and use of the by-products is limited to farms that are in relatively close proximity to the site of generation, resulting in a relatively low likelihood of adoption (Fig. 2). As a high-energy supplement, their most obvious application is intensive systems (feedlot) and high-performance animals (dairy cattle). Moreover, the anti-methanogenic effect is often non-specific and some of the by-products may have negative effects on the animal; so, further research is needed to find the optimal inclusion levels that balance these positive and negative effects. In this category, by-products of oil extraction processes or brewing industries have been reported to have some potential for practical application in intensive farming systems and have been shown to reduce CH4 emission by 15–20% (Moate et al. 2011). Other by-products should be assessed further for their benefits and their potential to address feed shortages and contribute to agricultural waste management in Australia.
While we already have access to some of the imported commercial plant-based products, our products are yet to be developed, and are yet to be investigated for our conditions and systems. Also, we do not know how much they could realistically reduce CH4 and what the cost of this strategy would be.
Future work
The outcomes from the research undertaken have moved us closer towards practical solutions to mitigating methane. While some strategies for enteric CH4 mitigation in Australia exist, more are yet to be researched and established. Focusing on plant bioactivity is clearly an option worthy of further investigation and investment. Australia is well positioned to explore and exploit its own plant resources as a means of balancing out the environmental effects of its livestock. While doing so, it may also address some other issues, including animal productivity, farm profitability, animal health, the security of plant biodiversity and the management of organic waste. While there seems to be an enormous potential for Australian plants and PSC, most of the exciting candidates are yet to be investigated in detail in vivo and under commercial settings; so, their potential for commercialisation is not clear. Also, the long-term impacts on palatability, intake, performance, and the quality of animal products, need to be investigated. Moreover, they need to be carefully assessed with regards to seasonal availability, total CH4 emission and to rule out any toxicity to the animals and humans needs. Balancing these issues will almost universally depend on finding optimal doses and delivery methods, and then developing and adopting plant-based mitigation strategies for whole-farm enterprises. Clearly, Australia must align with global efforts to find effective mitigation strategies, and start developing locally relevant approaches tailored to our animals, climate, national profile, capacities and needs.
Data availability statement
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Zoey Durmic was Associate Editors of Animal Production Science at the time of submission, but was blinded from the peer-review process for this paper.
Declaration of funding
This research did not receive any specific funding, but we have drawn on data that was generated through the projects funded through the Reducing Emissions from Livestock Research Program (Department of Agriculture, Fisheries and Forestry, Australia) and the National Livestock Methane Program (Meat and Livestock Australia).
References
Aerts RJ, Barry TN, McNabb WC (1999) Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agriculture, Ecosystems & Environment 75, 1–12.| Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages.Crossref | GoogleScholarGoogle Scholar |
AGEIS (2017) ‘Australian Greenhouse Emissions Information System National Greenhouse Gas Inventory: Kyoto Protocol Classifications.’ Available at http://ageis.climatechange.gov.au/ [Verified May 2021]
Akanmu AM, Hassen A (2018) The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Animal Production Science 58, 900–908.
| The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility.Crossref | GoogleScholarGoogle Scholar |
ARCADIS (2019) National Food Waste Assessment – final report. Available at https://www.environment.gov.au/protection/waste/publications/national-food-waste-assessment-final-report [Verified October 2020]
Banik BK, Durmic Z, Erskine W, Ghamkhar K, Revell C (2013) In vitro ruminal fermentation characteristics and methane production differ in selected key pasture species in Australia. Crop and Pasture Science 64, 935–942.
| In vitro ruminal fermentation characteristics and methane production differ in selected key pasture species in Australia.Crossref | GoogleScholarGoogle Scholar |
Banik BK, Durmic Z, Erskine W, Revell CK, Vadhanabhuti J, McSweeney CS, Padmanabha J, Flematti GR, Algreiby AA, Vercoe PE (2016) Bioactive fractions from the pasture legume Biserrula pelecinus L. have an anti-methanogenic effect against key rumen methanogens. Anaerobe 39, 173–182.
| Bioactive fractions from the pasture legume Biserrula pelecinus L. have an anti-methanogenic effect against key rumen methanogens.Crossref | GoogleScholarGoogle Scholar | 27060275PubMed |
Banik BK, Durmic Z, Erskine W, Revell C (2019) Anti-methanogenic advantage of biserrula (Biserrula pelecinus) over subterranean clover (Trifolium subterraneum) from in vitro fermentation is maintained across growth stages and cutting treatments. Crop and Pasture Science 70, 263–272.
| Anti-methanogenic advantage of biserrula (Biserrula pelecinus) over subterranean clover (Trifolium subterraneum) from in vitro fermentation is maintained across growth stages and cutting treatments.Crossref | GoogleScholarGoogle Scholar |
Beauchemin KA, McGinn SM (2006) Methane emissions from beef cattle: effects of fumaric acid, essential oil, and canola oil. Journal of Animal Science 84, 1489–1496.
| Methane emissions from beef cattle: effects of fumaric acid, essential oil, and canola oil.Crossref | GoogleScholarGoogle Scholar | 16699105PubMed |
Beauchemin KA, McGinn SM, Benchaar C, Holtshausen L (2009) Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: effects on methane production, rumen fermentation, and milk production. Journal of Dairy Science 92, 2118–2127.
| Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: effects on methane production, rumen fermentation, and milk production.Crossref | GoogleScholarGoogle Scholar | 19389969PubMed |
Beauchemin KA, Ungerfeld EM, Eckard RJ, Wang M (2020) Review: fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation. Animal 14, s2–s16.
| Review: fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation.Crossref | GoogleScholarGoogle Scholar | 32024560PubMed |
Beijer WH (1952) Methane fermentation in the rumen of cattle. Nature 170, 576–577.
| Methane fermentation in the rumen of cattle.Crossref | GoogleScholarGoogle Scholar | 13002369PubMed |
Belanche A, Newbold CJ, Morgavi DP, Bach A, Zweifel B, Yáñez-Ruiz DR (2020) A meta-analysis describing the effects of the essential oils blend Agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals 10, 620
| A meta-analysis describing the effects of the essential oils blend Agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows.Crossref | GoogleScholarGoogle Scholar |
Benchaar C (2016) Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows. Animal 10, 418–425.
| Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows.Crossref | GoogleScholarGoogle Scholar | 26888487PubMed |
Benchaar C, McAllister TA, Chouinard PY (2008) Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. Journal of Dairy Science 91, 4765–4777.
| Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts.Crossref | GoogleScholarGoogle Scholar | 19038952PubMed |
Benchaar C, Hassanat F, Gervais R, Chouinard PY, Julien C, Petit HV, Massé DI (2013) Effects of increasing amounts of corn dried distillers grains with solubles in dairy cow diets on methane production, ruminal fermentation, digestion, N balance, and milk production. Journal of Dairy Science 96, 2413–2427.
| Effects of increasing amounts of corn dried distillers grains with solubles in dairy cow diets on methane production, ruminal fermentation, digestion, N balance, and milk production.Crossref | GoogleScholarGoogle Scholar | 23462175PubMed |
Black J, Davison T, Fennessy P, Cohn P, Sedger A, Empson M (2015) National Livestock Methane Program National Needs and Gaps Analysis. B.CCH.6000 final report. Available at https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/Environment-On-Farm/National-Livestock-Methane-Program-National-Needs-and-Gaps-Analysis/3196 [Verified October 2020]
Black JL, Davison TM, Box I (2021) Methane emissions from ruminants in Australia: mitigation potential and applicability of mitigation strategies. Animals (Basel) 11, 951
| Methane emissions from ruminants in Australia: mitigation potential and applicability of mitigation strategies.Crossref | GoogleScholarGoogle Scholar | 33805324PubMed |
Blaxter KL, Czerkawski J (1966) Modifications of the methane production of the sheep by supplementation of its diet. Journal of the Science of Food and Agriculture 17, 417–421.
| Modifications of the methane production of the sheep by supplementation of its diet.Crossref | GoogleScholarGoogle Scholar | 5913171PubMed |
Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, López S (2012) Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology 176, 78–93.
| Manipulation of rumen fermentation and methane production with plant secondary metabolites.Crossref | GoogleScholarGoogle Scholar |
Busquet M, Calsamiglia S, Ferret A, Cardozo PW, Kamel C (2005a) Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. Journal of Dairy Science 88, 2508–2516.
| Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture.Crossref | GoogleScholarGoogle Scholar | 15956313PubMed |
Busquet M, Calsamiglia S, Ferret A, Carro MD, Kamel C (2005b) Effect of garlic oil and four of its compounds on rumen microbial fermentation. Journal of Dairy Science 88, 4393–4404.
| Effect of garlic oil and four of its compounds on rumen microbial fermentation.Crossref | GoogleScholarGoogle Scholar | 16291631PubMed |
Busquet M, Calsamiglia S, Ferret A, Kamel C (2006) Plant extracts affect in vitro rumen microbial fermentation. Journal of Dairy Science 89, 761–771.
| Plant extracts affect in vitro rumen microbial fermentation.Crossref | GoogleScholarGoogle Scholar | 16428643PubMed |
Calsamiglia S, Busquet M, Cardozo PW, Castillejos L, Ferret A (2007) Invited review: essential oils as modifiers of rumen microbial fermentation. Journal of Dairy Science 90, 2580–2595.
| Invited review: essential oils as modifiers of rumen microbial fermentation.Crossref | GoogleScholarGoogle Scholar | 17517698PubMed |
Cardozo PW, Calsamiglia S, Ferret A, Kamel C (2006) Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. Journal of Animal Science 84, 2801–2808.
| Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet.Crossref | GoogleScholarGoogle Scholar | 16971582PubMed |
Carulla JE, Kreuzer M, Machmüller A, Hess HD (2005) Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Australian Journal of Agricultural Research 56, 961–970.
| Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep.Crossref | GoogleScholarGoogle Scholar |
Castillo-Lopez E, Jenkins CJR, Aluthge ND, Tom W, Kononoff PJ, Fernando SC (2017) The effect of regular or reduced-fat distillers grains with solubles on rumen methanogenesis and the rumen bacterial community. Journal of Applied Microbiology 123, 1381–1395.
| The effect of regular or reduced-fat distillers grains with solubles on rumen methanogenesis and the rumen bacterial community.Crossref | GoogleScholarGoogle Scholar | 28891118PubMed |
Castro-Montoya J, De Campeneere S, Van Ranst G, Fievez V (2012) Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production. Animal Feed Science and Technology 176, 47–60.
| Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production.Crossref | GoogleScholarGoogle Scholar |
Charmley E, Stephens ML, Kennedy PM (2008) Predicting livestock productivity and methane emissions in northern Australia: development of a bio-economic modelling approach. Australian Journal of Experimental Agriculture 48, 109–113.
| Predicting livestock productivity and methane emissions in northern Australia: development of a bio-economic modelling approach.Crossref | GoogleScholarGoogle Scholar |
Chaves AV, He ML, Yang WZ, Hristov AN, McAllister TA, Benchaar C (2008) Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria. Canadian Journal of Animal Science 88, 117–122.
| Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria.Crossref | GoogleScholarGoogle Scholar |
Cottle DJ, Nolan JV, Wiedemann SG (2011) Ruminant enteric methane mitigation: a review. Animal Production Science 51, 491–514.
| Ruminant enteric methane mitigation: a review.Crossref | GoogleScholarGoogle Scholar |
Dalzell SA, Burnett DJ, Dowsett JE, Forbes VE, Shelton HM (2012) Prevalence of mimosine and DHP toxicity in cattle grazing Leucaena leucocephala pastures in Queensland, Australia. Animal Production Science 52, 365–372.
| Prevalence of mimosine and DHP toxicity in cattle grazing Leucaena leucocephala pastures in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Dong H, MacDonald JD, Ogle SM, Sanchez MJS, Rocha MT (2019) Volume 4. Agriculture, forestry and other land use. In ‘Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories’. (Eds E Calvo Buendia, K Tanabe, A Kranjc, J Baasansuren, M Fukuda, S Ngarize, A Osako, Y Pyrozhenko, P Shermanau, S Federici) (IPCC: Switzerland)
Doran-Browne N, Behrendt R, Kingwell R, Eckard R (2015) Modelling the potential of birdsfoot trefoil (Lotus corniculatus) to reduce methane emissions and increase production on wool and prime lamb farm enterprises. Animal Production Science 55, 1097–1105.
| Modelling the potential of birdsfoot trefoil (Lotus corniculatus) to reduce methane emissions and increase production on wool and prime lamb farm enterprises.Crossref | GoogleScholarGoogle Scholar |
Duarte AC, Durmic Z, Vercoe PE, Chaves AV (2017) Dose-response effects of dietary pequi oil on fermentation characteristics and microbial population using a rumen simulation technique (Rusitec). Anaerobe 48, 59–65.
| Dose-response effects of dietary pequi oil on fermentation characteristics and microbial population using a rumen simulation technique (Rusitec).Crossref | GoogleScholarGoogle Scholar | 28668707PubMed |
Durmic Z, Blache D (2012) Bioactive plants and plant products: effects on animal function, health and welfare. Animal Feed Science and Technology 176, 150–162.
| Bioactive plants and plant products: effects on animal function, health and welfare.Crossref | GoogleScholarGoogle Scholar |
Durmic Z, Hutton P, Revell DK, Emms J, Hughes S, Vercoe PE (2010) In vitro fermentative traits of Australian woody perennial plant species that may be considered as potential sources of feed for grazing ruminants. Animal Feed Science and Technology 160, 98–109.
| In vitro fermentative traits of Australian woody perennial plant species that may be considered as potential sources of feed for grazing ruminants.Crossref | GoogleScholarGoogle Scholar |
Durmic Z, Moate PJ, Eckard R, Revell DK, Williams R, Vercoe PE (2014) In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. Journal of the Science of Food and Agriculture 94, 1191–1196.
| In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation.Crossref | GoogleScholarGoogle Scholar | 24105682PubMed |
Durmic Z, Moate PJ, Jacobs JL, Vadhanabhuti J, Vercoe PE (2016) In vitro fermentability and methane production of some alternative forages in Australia. Animal Production Science 56, 641–645.
| In vitro fermentability and methane production of some alternative forages in Australia.Crossref | GoogleScholarGoogle Scholar |
Durmic Z, Ramirez-Restrepo CA, Gardiner C, O’Neill CJ, Hussein E, Vercoe PE (2017) Differences in the nutrient concentrations, in vitro methanogenic potential and other fermentative traits of tropical grasses and legumes for beef production systems in northern Australia. Journal of the Science of Food and Agriculture 97, 4075–4086.
| Differences in the nutrient concentrations, in vitro methanogenic potential and other fermentative traits of tropical grasses and legumes for beef production systems in northern Australia.Crossref | GoogleScholarGoogle Scholar | 28205235PubMed |
Eger M, Graz M, Riede S, Breves G (2018) Application of MootralTM reduces methane production by altering the archaea community in the rumen simulation technique. Frontiers in Microbiology 9, 2094
| Application of MootralTM reduces methane production by altering the archaea community in the rumen simulation technique.Crossref | GoogleScholarGoogle Scholar | 30233557PubMed |
EPA (2003) Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2001. Available at https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2001 [Verified October 2020]
Eugène M, Massé D, Chiquette J, Benchaar C (2008) Meta-analysis on the effects of lipid supplementation on methane production in lactating dairy cows. Canadian Journal of Animal Science 88, 331–337.
| Meta-analysis on the effects of lipid supplementation on methane production in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar |
Ghaffari MH, Durmic Z, Real D, Vercoe P, Smith G, Oldham C (2015) Furanocoumarins in tedera do not affect ruminal fermentation in continuous culture. Animal Production Science 55, 544–550.
| Furanocoumarins in tedera do not affect ruminal fermentation in continuous culture.Crossref | GoogleScholarGoogle Scholar |
Goel G, Makkar HP (2012) Methane mitigation from ruminants using tannins and saponins. Tropical Animal Health and Production 44, 729–739.
| Methane mitigation from ruminants using tannins and saponins.Crossref | GoogleScholarGoogle Scholar | 21894531PubMed |
Grainger C, Clarke T, Auldist MJ, Beauchemin KA, McGinn SM, Waghorn GC, Eckard RJ (2009) Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Canadian Journal of Animal Science 89, 241–251.
| Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows.Crossref | GoogleScholarGoogle Scholar |
Grainger C, Williams R, Eckard RJ, Hannah MC (2010) A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain. Journal of Dairy Science 93, 5300–5308.
| A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain.Crossref | GoogleScholarGoogle Scholar | 20965346PubMed |
Günal M, Pinski B, AbuGhazaleh AA (2017) Evaluating the effects of essential oils on methane production and fermentation under in vitro conditions. Italian Journal of Animal Science 16, 500–506.
| Evaluating the effects of essential oils on methane production and fermentation under in vitro conditions.Crossref | GoogleScholarGoogle Scholar |
Hammer KA, Carson CF, Riley TV (1999) Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology 86, 985–990.
| Antimicrobial activity of essential oils and other plant extracts.Crossref | GoogleScholarGoogle Scholar | 10438227PubMed |
Hammond KJ, Burke JL, Koolaard JP, Muetzel S, Pinares-Patiño CS, Waghorn GC (2013) Effects of feed intake on enteric methane emissions from sheep fed fresh white clover (Trifolium repens) and perennial ryegrass (Lolium perenne) forages. Animal Feed Science and Technology 179, 121–132.
| Effects of feed intake on enteric methane emissions from sheep fed fresh white clover (Trifolium repens) and perennial ryegrass (Lolium perenne) forages.Crossref | GoogleScholarGoogle Scholar |
Hariadi BT, Santoso B (2010) Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. Journal of the Science of Food and Agriculture 90, 456–461.
| Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid.Crossref | GoogleScholarGoogle Scholar | 20355068PubMed |
Harrison MT, McSweeney C, Tomkins NW, Eckard RJ (2015) Improving greenhouse gas emissions intensities of subtropical and tropical beef farming systems using Leucaena leucocephala. Agricultural Systems 136, 138–146.
| Improving greenhouse gas emissions intensities of subtropical and tropical beef farming systems using Leucaena leucocephala.Crossref | GoogleScholarGoogle Scholar |
Hess HD, Tiemann TT, Noto F, Carulla JE, Kreuzer M (2006) Strategic use of tannins as means to limit methane emission from ruminant livestock. International Congress Series 1293, 164–167.
| Strategic use of tannins as means to limit methane emission from ruminant livestock.Crossref | GoogleScholarGoogle Scholar |
Hixson JL, Durmic Z, Vadhanabhuti J, Vercoe PE, Smith PA, Wilkes EN (2018) Exploiting compositionally similar grape marc samples to achieve gradients of condensed tannin and fatty acids for modulating in vitro methanogenesis. Molecules (Basel, Switzerland) 23, 1793
| Exploiting compositionally similar grape marc samples to achieve gradients of condensed tannin and fatty acids for modulating in vitro methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Horky P, Skalickova S, Smerkova K, Skladanka J (2019) Essential oils as a feed additives: pharmacokinetics and potential toxicity in monogastric animals. Animals (Basel) 9, 352
| Essential oils as a feed additives: pharmacokinetics and potential toxicity in monogastric animals.Crossref | GoogleScholarGoogle Scholar |
Hristov AN, Ott T, Tricarico J, Rotz A, Waghorn G, Adesogan A, Dijkstra J, Montes F, Oh J, Kebreab E, Oosting SJ, Gerber PJ, Henderson B, Makkar HPS, Firkins JL (2013) SPECIAL TOPICS: mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options. Journal of Animal Science 91, 5095–5113.
| SPECIAL TOPICS: mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options.Crossref | GoogleScholarGoogle Scholar | 24045470PubMed |
Hünerberg M, Little SM, Beauchemin KA, McGinn SM, O’Connor D, Okine EK, Harstad OM, Kröbel R, McAllister TA (2014) Feeding high concentrations of corn dried distillers’ grains decreases methane, but increases nitrous oxide emissions from beef cattle production. Agricultural Systems 127, 19–27.
| Feeding high concentrations of corn dried distillers’ grains decreases methane, but increases nitrous oxide emissions from beef cattle production.Crossref | GoogleScholarGoogle Scholar |
Hutton PG, Durmic Z, Vercoe PE (2010) Investigating Eremophila glabra as a bioactive agent for preventing lactic acidosis in sheep. Animal Production Science 50, 449–453.
| Investigating Eremophila glabra as a bioactive agent for preventing lactic acidosis in sheep.Crossref | GoogleScholarGoogle Scholar |
Jahani-Azizabadi J, Durmic Z, Vadhanabhuti J, Vercoe PE (2019) Effect of some Australian native shrubs essential oils on in vitro rumen microbial fermentation of a high-concentrate diet. The Journal of Animal & Plant Sciences 29, 8–15.
Jayanegara A, Leiber F, Kreuzer M (2012) Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. Journal of Animal Physiology and Animal Nutrition 96, 365–375.
| Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments.Crossref | GoogleScholarGoogle Scholar | 21635574PubMed |
Joch M, Cermak L, Hakl J, Hucko B, Duskova D, Marounek M (2016) In vitro screening of essential oil active compounds for manipulation of rumen fermentation and methane mitigation. Asian–Australasian Journal of Animal Sciences 29, 952–959.
| In vitro screening of essential oil active compounds for manipulation of rumen fermentation and methane mitigation.Crossref | GoogleScholarGoogle Scholar | 26954157PubMed |
Johnson KA, Johnson DE (1995) Methane emissions from cattle. Journal of Animal Science 73, 2483–2492.
| Methane emissions from cattle.Crossref | GoogleScholarGoogle Scholar | 8567486PubMed |
Kaur P, Appels R, Bayer PE, Keeble-Gagnere G, Wang J, Hirakawa H, Shirasawa K, Vercoe P, Stefanova K, Durmic Z, Nichols P, Revell C, Isobe SN, Edwards D, Erskine W (2017) Climate clever clovers: new paradigm to reduce the environmental footprint of ruminants by breeding low methanogenic forages utilizing haplotype variation. Frontiers in Plant Science 8, 1463
| Climate clever clovers: new paradigm to reduce the environmental footprint of ruminants by breeding low methanogenic forages utilizing haplotype variation.Crossref | GoogleScholarGoogle Scholar | 28928752PubMed |
Kotze AC, O’Grady J, Emms J, Toovey AF, Hughes S, Jessop P, Bennell M, Vercoe PE, Revell DK (2009) Exploring the anthelmintic properties of Australian native shrubs with respect to their potential role in livestock grazing systems. Parasitology 136, 1065–1080.
| Exploring the anthelmintic properties of Australian native shrubs with respect to their potential role in livestock grazing systems.Crossref | GoogleScholarGoogle Scholar | 19523255PubMed |
Ku-Vera JC, Jiménez-Ocampo R, Valencia-Salazar SS, Montoya-Flores MD, Molina-Botero IC, Arango J, Gómez-Bravo CA, Aguilar-Pérez CF, Solorio-Sánchez FJ (2020) Role of Secondary Plant Metabolites on Enteric Methane Mitigation in Ruminants. Frontiers in Veterinary Science 7,
| Role of Secondary Plant Metabolites on Enteric Methane Mitigation in Ruminants.Crossref | GoogleScholarGoogle Scholar | 33195495PubMed |
Kumar R, Vaithiyanathan S (1990) Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves. Animal Feed Science and Technology 30, 21–38.
| Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves.Crossref | GoogleScholarGoogle Scholar |
Lee SJ, Kim HS, Eom JS, Choi YY, Jo SU, Chu GM, Lee Y, Seo J, Kim KH, Lee SS (2021) Effects of Olive (Olea europaea L.) Leaves with Antioxidant and Antimicrobial Activities on In Vitro Ruminal Fermentation and Methane Emission. Animals (Basel) 11, 2008
| Effects of Olive (Olea europaea L.) Leaves with Antioxidant and Antimicrobial Activities on In Vitro Ruminal Fermentation and Methane Emission.Crossref | GoogleScholarGoogle Scholar | 34359136PubMed |
Li X (2013) Eremophila glabra reduces methane production in sheep. PhD, The University of Western Australia, Perth, WA, Australia.
Li X, Durmic Z, Liu S, McSweeney CS, Vercoe PE (2014) Eremophila glabra reduces methane production and methanogen populations when fermented in a Rusitec. Anaerobe 29, 100–107.
| Eremophila glabra reduces methane production and methanogen populations when fermented in a Rusitec.Crossref | GoogleScholarGoogle Scholar | 24225531PubMed |
Lima PR, Apdini T, Freire AS, Santana AS, Moura LML, Nascimento JCS, Rodrigues RTS, Dijkstra J, Garcez Neto AF, Queiroz MAÁ, Menezes DR (2019) Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Animal Feed Science and Technology 249, 10–17.
| Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep.Crossref | GoogleScholarGoogle Scholar |
Liu Y, Ma T, Chen D, Zhang N, Si B, Deng K, Tu Y, Diao Q (2019) Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in dorper crossbred ewe. Animals (Basel) 9, 29
| Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in dorper crossbred ewe.Crossref | GoogleScholarGoogle Scholar |
Ma T, Chen D, Tu Y, Zhang N, Si B, Deng K, Diao Q (2016) Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. Journal of Animal Science and Biotechnology 7, 1
| Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes.Crossref | GoogleScholarGoogle Scholar | 26779340PubMed |
Macheboeuf D, Morgavi DP, Papon Y, Mousset JL, Arturo-Schaan M (2008) Dose–response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Animal Feed Science and Technology 145, 335–350.
| Dose–response effects of essential oils on in vitro fermentation activity of the rumen microbial population.Crossref | GoogleScholarGoogle Scholar |
Malecky M, Albarello H, Broudiscou LP (2012) Degradation of terpenes and terpenoids from Mediterranean rangelands by mixed rumen bacteria in vitro. Animal 6, 612–616.
| Degradation of terpenes and terpenoids from Mediterranean rangelands by mixed rumen bacteria in vitro.Crossref | GoogleScholarGoogle Scholar | 22436277PubMed |
Martin C, Rouel J, Jouany JP, Doreau M, Chilliard Y (2008) Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil. Journal of Animal Science 86, 2642–2650.
| Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil.Crossref | GoogleScholarGoogle Scholar | 18469051PubMed |
Mason SE, Mullen KAE, Anderson KL, Washburn SP, Yeatts JL, Baynes RE (2017) Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle. Food Additives & Contaminants: Part A 34, 740–749.
| Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle.Crossref | GoogleScholarGoogle Scholar |
McAllister TA, Newbold CJ (2008) Redirecting rumen fermentation to reduce methanogenesis. Australian Journal of Experimental Agriculture 48, 7–13.
| Redirecting rumen fermentation to reduce methanogenesis.Crossref | GoogleScholarGoogle Scholar |
McGinn SM, Chung Y-H, Beauchemin KA, Iwaasa AD, Grainger C (2009) Use of corn distillers’ dried grains to reduce enteric methane loss from beef cattle. Canadian Journal of Animal Science 89, 409–413.
| Use of corn distillers’ dried grains to reduce enteric methane loss from beef cattle.Crossref | GoogleScholarGoogle Scholar |
McSweeney C, Tomkins N (2015) B.CCH.6510_Final_Report. Available at https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/Environment-On-Farm/Impacts-of-Leucaena-plantations-on-greenhouse-gas-emissions-in-northern-Australian-cattle-production-systems/3039
Meale SJ, Chaves AV, Baah J, McAllister TA (2012) Methane production of different forages in in vitro ruminal fermentation. Asian-Australasian Journal of Animal Sciences 25, 86–91.
| Methane production of different forages in in vitro ruminal fermentation.Crossref | GoogleScholarGoogle Scholar | 25049482PubMed |
Meale SJ, Chaves AV, McAllister TA, Iwaasa AD, Yang WZ, Benchaar C (2014) Including essential oils in lactating dairy cow diets: effects on methane emissions. Animal Production Science 54, 1215–1218.
| Including essential oils in lactating dairy cow diets: effects on methane emissions.Crossref | GoogleScholarGoogle Scholar |
Min BR, Solaiman S, Waldrip HM, Parker D, Todd RW, Brauer D (2020) Dietary mitigation of enteric methane emissions from ruminants: a review of plant tannin mitigation options. Animal Nutrition
| Dietary mitigation of enteric methane emissions from ruminants: a review of plant tannin mitigation options.Crossref | GoogleScholarGoogle Scholar | 33005757PubMed |
Moate PJ, Williams SRO, Grainger C, Hannah MC, Ponnampalam EN, Eckard RJ (2011) Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows. Animal Feed Science and Technology 166–167, 254–264.
| Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows.Crossref | GoogleScholarGoogle Scholar |
Moate PJ, Williams SRO, Torok VA, Hannah MC, Ribaux BE, Tavendale MH, Eckard RJ, Jacobs JL, Auldist MJ, Wales WJ (2014) Grape marc reduces methane emissions when fed to dairy cows. Journal of Dairy Science 97, 5073–5087.
| Grape marc reduces methane emissions when fed to dairy cows.Crossref | GoogleScholarGoogle Scholar | 24952778PubMed |
Moate PJ, Jacobs JL, Hixson JL, Deighton MH, Hannah MC, Morris GL, Ribaux BE, Wales WJ, Williams SRO (2020) Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows. Animals 10, 976
| Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows.Crossref | GoogleScholarGoogle Scholar |
Molina-Botero IC, Arroyave-Jaramillo J, Valencia-Salazar S, Barahona-Rosales R, Aguilar-Pérez CF, Ayala Burgos A, Arango J, Ku-Vera JC (2019) Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers. Animal Feed Science and Technology 251, 1–11.
| Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers.Crossref | GoogleScholarGoogle Scholar |
Monjardino M, Revell D, Pannell DJ (2010) The potential contribution of forage shrubs to economic returns and environmental management in Australian dryland agricultural systems. Agricultural Systems 103, 187–197.
| The potential contribution of forage shrubs to economic returns and environmental management in Australian dryland agricultural systems.Crossref | GoogleScholarGoogle Scholar |
Moss AR, Jouany J-P, Newbold J (2000) Methane production by ruminants: its contribution to global warming. Annales de Zootechnie 49, 231–253.
| Methane production by ruminants: its contribution to global warming.Crossref | GoogleScholarGoogle Scholar |
Muir SK, Kennedy AJ, Kearney G, Hutton P, Thompson AN, Vercoe P, Hill J (2020) Offering subterranean clover can reduce methane emissions compared with perennial ryegrass pastures during late spring and summer in sheep. Animal Production Science 60, 1449–1458.
| Offering subterranean clover can reduce methane emissions compared with perennial ryegrass pastures during late spring and summer in sheep.Crossref | GoogleScholarGoogle Scholar |
Navarro-Villa A, O’Brien M, López S, Boland TM, O’Kiely P (2011) In vitro rumen methane output of red clover and perennial ryegrass assayed using the gas production technique (GPT). Animal Feed Science and Technology 168, 152–164.
| In vitro rumen methane output of red clover and perennial ryegrass assayed using the gas production technique (GPT).Crossref | GoogleScholarGoogle Scholar |
Niderkorn V, Barbier E, Macheboeuf D, Torrent A, Mueller-Harvey I, Hoste H (2020) In vitro rumen fermentation of diets with different types of condensed tannins derived from sainfoin (Onobrychis viciifolia Scop.) pellets and hazelnut (Corylus avellana L.) pericarps. Animal Feed Science and Technology 259, 114357
| In vitro rumen fermentation of diets with different types of condensed tannins derived from sainfoin (Onobrychis viciifolia Scop.) pellets and hazelnut (Corylus avellana L.) pericarps.Crossref | GoogleScholarGoogle Scholar |
Oskoueian E, Abdullah N, Oskoueian A (2013) Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Research International 2013, 349129
| Effects of flavonoids on rumen fermentation activity, methane production, and microbial population.Crossref | GoogleScholarGoogle Scholar | 24175289PubMed |
Pal K, Patra AK, Sahoo A, Kumawat PK (2015) Evaluation of several tropical tree leaves for methane production potential, degradability and rumen fermentation in vitro. Livestock Science 180, 98–105.
| Evaluation of several tropical tree leaves for methane production potential, degradability and rumen fermentation in vitro.Crossref | GoogleScholarGoogle Scholar |
Patra AK, Saxena J (2009) The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutrition Research Reviews 22, 204–219.
| The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production.Crossref | GoogleScholarGoogle Scholar | 20003589PubMed |
Patra AK, Saxena J (2011) Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. Journal of the Science of Food and Agriculture 91, 24–37.
| Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition.Crossref | GoogleScholarGoogle Scholar | 20815041PubMed |
Patra AK, Yu Z (2012) Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Applied and Environmental Microbiology 78, 4271–4280.
| Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations.Crossref | GoogleScholarGoogle Scholar | 22492451PubMed |
Patra A, Park T, Kim M, Yu Z (2017) Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. Journal of Animal Science and Biotechnology 8, 13
| Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances.Crossref | GoogleScholarGoogle Scholar | 28149512PubMed |
Pellikaan WF, Stringano E, Leenaars J, Bongers DJGM, van Laar-van Schuppen S, Plant J, Mueller-Harvey I (2011) Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES). Animal Feed Science and Technology 166–167, 377–390.
| Evaluating effects of tannins on extent and rate of in vitro gas and CH4 production using an automated pressure evaluation system (APES).Crossref | GoogleScholarGoogle Scholar |
Piñeiro-Vázquez AT, Canul-Solis JR, Jiménez-Ferrer GO, Alayón-Gamboa JA, Chay-Canul AJ, Ayala-Burgos AJ, Aguilar-Pérez CF, Ku-Vera JC (2018) Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage. Asian-Australasian Journal of Animal Sciences 31, 1738–1746.
| Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage.Crossref | GoogleScholarGoogle Scholar | 29103289PubMed |
Ramírez-Restrepo CA, Tan C, O’Neill CJ, López-Villalobos N, Padmanabha J, Wang J, McSweeney CS (2016) Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation. Animal Feed Science and Technology 216, 58–67.
| Methane production, fermentation characteristics, and microbial profiles in the rumen of tropical cattle fed tea seed saponin supplementation.Crossref | GoogleScholarGoogle Scholar |
Rasmussen J, Harrison A (2011) The benefits of supplementary fat in feed rations for ruminants with particular focus on reducing levels of methane production. ISRN Veterinary Science 2011, 613172
| The benefits of supplementary fat in feed rations for ruminants with particular focus on reducing levels of methane production.Crossref | GoogleScholarGoogle Scholar | 23738103PubMed |
Rea S, Bowman JP, Popovski S, Pimm C, Wright AG (2007) Methanobrevibacter millerae sp. nov. and Methanobrevibacter olleyae sp. nov., methanogens from the ovine and bovine rumen that can utilize formate for growth. International Journal of Systematic and Evolutionary Microbiology 57, 450–456.
| Methanobrevibacter millerae sp. nov. and Methanobrevibacter olleyae sp. nov., methanogens from the ovine and bovine rumen that can utilize formate for growth.Crossref | GoogleScholarGoogle Scholar | 17329767PubMed |
Revell D, Revell C (2006) Implications of ‘duty of care’ for the development of new pasture species. In ‘Groundbreaking Stuff. Proceedings of the 13th Australian Agronomy Conference’, 10–14 September 2006, Perth, WA, Australia. (Eds N Turner, T Acuna) pp. 1–9.
Revell DK, Norman HC, Vercoe PE, Phillips N, Toovey A, Bickell S, Hulm E, Hughes S, Emms J (2013) Australian perennial shrub species add value to the feed base of grazing livestock in low- to medium-rainfall zones. Animal Production Science 53, 1221–1230.
| Australian perennial shrub species add value to the feed base of grazing livestock in low- to medium-rainfall zones.Crossref | GoogleScholarGoogle Scholar |
Rira M, Marie-Magdeleine C, Archimède H, Morgavi DP, Doreau M (2013) Effect of condensed tannins on methane emission and ruminal microbial populations. In ‘Energy and protein metabolism and nutrition in sustainable animal production, Vol. 134’. (Eds JW Oltjen, ELH Kebreab) pp. 501–502. (Wageningen Academic Publishers: Wageningen, The Netherlands)
Rodríguez R, Fondevila M (2012) Effect of saponins from Enterolobium cyclocarpum on in vitro microbial fermentation of the tropical grass Pennisetum purpureum. Journal of Animal Physiology and Animal Nutrition 96, 762–769.
| Effect of saponins from Enterolobium cyclocarpum on in vitro microbial fermentation of the tropical grass Pennisetum purpureum.Crossref | GoogleScholarGoogle Scholar | 21605176PubMed |
Russo VM, Jacobs JL, Hannah MC, Moate PJ, Dunshea FR, Leury BJ (2017) In vitro evaluation of the methane mitigation potential of a range of grape marc products. Animal Production Science 57, 1437–1444.
| In vitro evaluation of the methane mitigation potential of a range of grape marc products.Crossref | GoogleScholarGoogle Scholar |
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Comprehensive Reviews in Food Science and Food Safety 17, 512–531.
| Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization.Crossref | GoogleScholarGoogle Scholar | 33350136PubMed |
Shakeri P, Durmic Z, Vadhanabhuti J, Vercoe PE (2017) Products derived from olive leaves and fruits can alter in vitro ruminal fermentation and methane production. Journal of the Science of Food and Agriculture 97, 1367–1372.
| Products derived from olive leaves and fruits can alter in vitro ruminal fermentation and methane production.Crossref | GoogleScholarGoogle Scholar | 27376199PubMed |
Silivong P, Hervaseng B, Preston TR (2013) Methane production from Jack fruit, Muntingia, Leucaena, Gliricidia (Gliricidia sepium), Mimosa (Mimosa pigra) and Acacia auriculoformis foliages in an in vitro incubation with potassium nitrate as source of NPN. Livestock Research for Rural Development 25, 15
Soliva CR, Zeleke AB, Clément C, Hess HD, Fievez V, Kreuzer M (2008) In vitro screening of various tropical foliages, seeds, fruits and medicinal plants for low methane and high ammonia generating potentials in the rumen. Animal Feed Science and Technology 147, 53–71.
| In vitro screening of various tropical foliages, seeds, fruits and medicinal plants for low methane and high ammonia generating potentials in the rumen.Crossref | GoogleScholarGoogle Scholar |
Soltan YA, Natel AS, Araujo RC, Morsy AS, Abdalla AL (2018) Progressive adaptation of sheep to a microencapsulated blend of essential oils: ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Animal Feed Science and Technology 237, 8–18.
| Progressive adaptation of sheep to a microencapsulated blend of essential oils: ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis.Crossref | GoogleScholarGoogle Scholar |
Suybeng B, Charmley E, Gardiner CP, Malau-Aduli BS, Malau-Aduli AEO (2019) Methane emissions and the use of Desmanthus in beef cattle production in northern Australia. Animals (Basel) 9, 542
| Methane emissions and the use of Desmanthus in beef cattle production in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Swainson N, Muetzel S, Clark H (2018) Updated predictions of enteric methane emissions from sheep suitable for use in the New Zealand national greenhouse gas inventory. Animal Production Science 58, 973–979.
| Updated predictions of enteric methane emissions from sheep suitable for use in the New Zealand national greenhouse gas inventory.Crossref | GoogleScholarGoogle Scholar |
Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, Ho YW (2011) Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Animal Feed Science and Technology 169, 185–193.
| Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro.Crossref | GoogleScholarGoogle Scholar |
Tavendale MH, Meagher LP, Pacheco D, Walker N, Attwood GT, Sivakumaran S (2005) Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Animal Feed Science and Technology 123–124, 403–419.
| Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis.Crossref | GoogleScholarGoogle Scholar |
Taylor CA, Harrison MT, Telfer M, Eckard R (2016) Modelled greenhouse gas emissions from beef cattle grazing irrigated leucaena in northern Australia. Animal Production Science 56, 594–604.
| Modelled greenhouse gas emissions from beef cattle grazing irrigated leucaena in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Tiemann TT, Lascano CE, Wettstein HR, Mayer AC, Kreuzer M, Hess HD (2008) Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal 2, 790–799.
| Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs.Crossref | GoogleScholarGoogle Scholar | 22443605PubMed |
Tomkins NW, Denman SE, Pilajun R, Wanapat M, McSweeney CS, Elliott R (2015) Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Animal Feed Science and Technology 200, 25–34.
| Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay.Crossref | GoogleScholarGoogle Scholar |
Vercoe PE, Durmic Z, Revell DK (2009) Rumen microbial ecology: helping to change landscapes. Optiones Mediterraneennes. A85 225–236, 20103102279
Waghorn G (2008) Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production: progress and challenges. Animal Feed Science and Technology 147, 116–139.
| Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production: progress and challenges.Crossref | GoogleScholarGoogle Scholar |
Watanabe Y, Suzuki R, Koike S, Nagashima K, Mochizuki M, Forster RJ, Kobayashi Y (2010) In vitro evaluation of cashew nut shell liquid as a methane-inhibiting and propionate-enhancing agent for ruminants. Journal of Dairy Science 93, 5258–5267.
| In vitro evaluation of cashew nut shell liquid as a methane-inhibiting and propionate-enhancing agent for ruminants.Crossref | GoogleScholarGoogle Scholar | 20965342PubMed |
Whitelaw FG, Eadie JM, Bruce LA, Shand WJ (1984) Methane formation in faunated and ciliate-free cattle and its relationship with tureen volatile fatty acid proportions. British Journal of Nutrition 52, 261–275.
| Methane formation in faunated and ciliate-free cattle and its relationship with tureen volatile fatty acid proportions.Crossref | GoogleScholarGoogle Scholar |
Williams SRO, Moate PJ, Deighton MH, Hannah MC, Wales WJ, Jacobs JL (2016) Milk production and composition, and methane emissions from dairy cows fed lucerne hay with forage brassica or chicory. Animal Production Science 56, 304–311.
| Milk production and composition, and methane emissions from dairy cows fed lucerne hay with forage brassica or chicory.Crossref | GoogleScholarGoogle Scholar |
Williams SRO, Chaves AV, Deighton MH, Jacobs JL, Hannah MC, Ribaux BE, Morris GL, Wales WJ, Moate PJ (2018) Influence of feeding supplements of almond hulls and ensiled citrus pulp on the milk production, milk composition, and methane emissions of dairy cows. Journal of Dairy Science 101, 2072–2083.
| Influence of feeding supplements of almond hulls and ensiled citrus pulp on the milk production, milk composition, and methane emissions of dairy cows.Crossref | GoogleScholarGoogle Scholar | 29290453PubMed |
Wright AD, Williams AJ, Winder B, Christophersen CT, Rodgers SL, Smith KD (2004) Molecular diversity of rumen methanogens from sheep in Western Australia. Applied and Environmental Microbiology 70, 1263–1270.
| Molecular diversity of rumen methanogens from sheep in Western Australia.Crossref | GoogleScholarGoogle Scholar | 15006742PubMed |



