Probiotics and gut health: linking gut homeostasis and poultry productivity
S. Shini A B and W. L. Bryden
A B and W. L. Bryden  A
A
A School of Agriculture and Food Sciences, University of Queensland, Gatton, Qld 4343, Australia.
B Corresponding author. Email: s.shini@uq.edu.au
Animal Production Science 62(12) 1090-1112 https://doi.org/10.1071/AN20701
Submitted: 30 December 2020 Accepted: 05 April 2021 Published: 17 June 2021
Journal compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
The use of probiotics in poultry production has increased rapidly, and this movement has been promoted by global events, such as the prohibition or decline in the use of antibiotic growth promotants in poultry feeds. There has been a persistent search for alternative feed additives, and probiotics have shown that they can restore the composition of the gut microbiota, and produce health benefits to the host, including improvements in performance. Probiotics have shown potential to increase productivity in poultry, especially in flocks challenged by stressors. However, the outcomes of probiotic use have not always been consistent. There is an increasing demand for well defined products that can be applied strategically, and currently, probiotic research is focusing on delineating their mechanisms of action in the gut that contribute to an improved efficacy. In particular, mechanisms involved in the maintenance and protection of intestinal barrier integrity and the role of the gut microbiota are being extensively investigated. It has been shown that probiotics modulate intestinal immune pathways both directly and through interactions with the gut microbiota. These interactions are key to maintaining gut homeostasis and function, and improving feed efficiency. Research has demonstrated that probiotics execute their effects through multiple mechanisms. The present review describes recent advances in probiotic use in poultry. It focuses on the current understanding of gut homeostasis and gut health in chickens, and how it can be assessed and improved through supplementation of poultry diets with probiotics in poultry diets. In particular, cellular and molecular mechanisms involved in the maintenance and protection of gut barrier structure and function are described. It also highlights important factors that influence probiotic efficacy and bird performance.
Keywords: microbiota, mode of action, mucosal integrity, intestinal health, immunomodulation, necrotic enteritis, broiler performance.
Introduction
The gastrointestinal tract (GIT) or gut of all species is a tubular organ of great structural and functional complexity, as has been detailed elsewhere (Scanes and Pierzchala-Koziec 2014; Denbow 2015; Furness and Cottrell 2017; Pluske et al. 2019). An important part of the GIT is the small intestine, the segment where the majority of the digestion and absorption of nutrients takes place. Briefly, the intestinal mucosa (which consists of epithelium with a core of lamina propria) projects into the gut lumen through villi, and each villus serves as a functional absorptive unit (Fig. 1). The chicken intestinal epithelium is a single layer of columnar cells, and comprises enterocytes (absorptive cells), goblet (mucus-producing) and enteroendocrine cells, and various intraepithelial immune cells. The mucosa is covered by mucus (a complex hydrated gel that protects epithelial cells from chemical, enzymatic, microbial, and mechanical damage) and mucus-linked specific commensal bacteria. An intact epithelial barrier is essential for gut physiology and immunity or GIT homeostasis (de Santa Barbara et al. 2003; Kastl et al. 2020). The epithelial cells linked by intercellular junctions and the mucus layer are crucial components of the intestinal barrier that selectively permits the movement of ions, nutrients and water, but restricts the translocation of microbes and toxins from the lumen, thus playing a fundamental role in maintaining gut health of the host. However, the entire integrity of the barrier is sustained by a complex network of regulatory pathways that interact with the microbiota, luminal contents or digesta, and host mucosa.
The GIT ecosystem is a delicate balance among the microbiota, the intestinal epithelium and host immunity. The gut lumen wall covered by the mucus layer surrounds the intestinal contents, a mixture of nutrients, metabolites, and a diverse microbial community, that work in concert to maintain GIT homeostasis or health. Shifts in intestinal microbial composition of a chicken can be caused by stress emanating from diet, such as changes in dietary ingredients, antigens in the feed, microorganisms associated with the diet, pathogens contaminating the diet, and litter consumption (Drew et al. 2004; Choct 2009; Pan and Yu 2014; Antonissen et al. 2016; Borda-Molina et al. 2018). It appears that chicken can consume as much as 4% of their diet as litter (Jesse 2004). Water quality and housing conditions can also influence chicken gut microbiota (Diaz Carrasco et al. 2019). Structural modifications of the intestinal mucosa occur in response to infection and inflammation (Kaldhusdal et al. 1995; Jou et al. 1998; Olkowski et al. 2008; Vancamelbeke and Vermeire 2017; Shini et al. 2021). When gut homeostasis is disturbed, perturbations of the epithelium may also occur and contribute to the pathophysiology of intestinal disease, and from time-to-time systemic disorders. Moreover, subclinical infections, and chronic, low-grade intestinal inflammation, may also cause similar alterations of the epithelial barrier without clinical manifestations, and these may be difficult to diagnose.
The future Nobel laureate Elie Metchnikoff, a Russian working in Paris at the turn of the 20th century, was the first to suggest a role for the gut microbiota in health. However, a brief note in Nature in 1973 by the Finnish scientists Esko Nurmi and Marjatta Rantala, describing their research to control Salmonella infections in poultry with bacterial cultures, rekindled interest in the use of direct-fed microbials or probiotics, in animals (Nurmi and Rantala 1973). Rob Cumming and his group at the University of New England pioneered the application of ‘competitive exclusion’ (Lloyd et al. 1977; Soerjadi et al. 1978) in Australia. Since then, many investigators have shown that probiotics can restore the composition of the gut microbiome, and introduce beneficial functions to microbial communities, and in doing so, they prevent or reduce gut inflammation and intestinal infection and improve bird performance (Eckert et al. 2010; Mountzouris et al. 2010; Cengiz et al. 2015; Huff et al. 2015; Latorre et al. 2015; Park and Kim 2015; Forte et al. 2016; Bai et al. 2017; Pereira et al. 2019; Yadav and Jha 2019; Shini et al. 2020b; Zaghari et al. 2020).
The use of probiotics in poultry production has increased rapidly. This reflects the efficacy of most probiotic products in the field, acceptance by consumers of a product that is widely consumed in society, and the need to find additional feed additives following the banning or decline in the use of antibiotic growth promotants (AGPs) globally. Interestingly, probiotics, not unlike AGPs, do improve animal performance and both classes of feed additives initiate these improvements through reducing inflammation in the gut (Niewold 2007; Mountzouris et al. 2019). There is an increasing demand by feed companies and poultry producers for well defined antibiotic alternatives that can be applied strategically without significant increase in feed costs. Currently, probiotic research is focussed on delineating intestinal mechanisms of action with the objective to improve efficacy and feed utilisation.
The present review describes recent advances in probiotic use in poultry, and examples relating to the control of necrotic enteritis (see Box 1, Fig. 2) are cited frequently. It focuses on our current understanding of gut health, how to assess it in chickens, and highlights important mechanisms of probiotic action that appear to improve gut health. In particular, cellular and molecular mechanisms involved in the maintenance and protection of gut barrier structure and function are described.
| Box 1. Necrotic enteritis |
| Necrotic enteritis (NE) is a significant enteric infection of poultry, primarily seen in broiler chickens, but the disease has also been reported for commercial layers raised on the ground, cage reared replacement pullets and turkeys. Other avian species can be affected. In broiler chickens, Clostridium perfringens, a normal occupant of the digestive tract, is the major aetiologic agent for NE (Parish 1961; Long and Truscott 1976; Kaldhusdal and Hofshagen 1992). However, NE is a multifactorial disease and it occurs when gut conditions are altered in favour of clostridia proliferation, leading to the onset of NE. For example, coccidiosis (a parasitic disease of the intestinal tract of poultry caused by coccidian protozoa of Eimeria species) is believed to be one of the major predisposing factors for NE outbreaks (Al-Sheikhly and Al-Saieg 1980; Hermans and Morgan 2003; Collier et al. 2008). Other predisposing factors include the addition of fishmeal to the diet (Wu et al. 2010; Wu et al. 2014; Rodgers et al. 2015), feedstuffs containing high amounts of water-soluble non-starch polysaccharides, such as barley, rye and wheat (Kaldhusdal and Hofshagen 1992; Riddell and Kong 1992; Kaldhusdal and Skjerve 1996; Annett et al. 2002; Jia et al. 2009); in general, any factor that induces stress and immunosuppression in chickens and disrupts the homeostasis of the gut ecosystem contributes to the risk of NE in a flock. |
| NE is characterised by distended intestines containing gas and or fluids, and lesions such as patches of necrotic tissue on the intestinal mucosa (Fig. 2). NE often develops as an acute disease, with birds dying within a day after clinical signs are observed (e.g. ruffled feathers, depression, and diarrhoea; Fig. 2). Flock mortality can be as high as 10–30%, if untreated. The subclinical form of NE is associated with subtle clinical signs or no clinical signs; however, chronic damage of the intestinal mucosa (i.e. intestinal lesions) can be found after necropsy or histopathology (Kaldhusdal and Hofshagen 1992; Gholamiandehkordi et al. 2007; Park et al. 2008; Wu et al. 2010; Smyth 2016; Kogut et al. 2018; Shini et al. 2020b). Subclinical NE is difficult to diagnose, but can spread through flocks, resulting in substantial production losses (Skinner et al. 2010) due to malabsorption and reduced performance. |
| Historically, NE was effectively controlled by adding antimicrobial growth promoters (AGP) to broiler feed (Prescott et al. 1978; Elwinger et al. 1992, 1998). However, it has become one of the most significant broiler diseases globally, after the banning or reduced use of most AGPs in poultry diets. Current strategies focus on introducing alternatives to AGPs and managing predisposing factors, rather than trying to eliminate Clostridium. In the case of NE outbreaks associated with clinical signs and increased mortality, birds can still be treated therapeutically with antibiotics such as bacitracin, lincomycin, oxytetracycline or virginiamycin. |
Gut health
‘Gut health’ is a term that is used very frequently in the human and animal literature, especially when describing the outcomes of probiotic use. However, the definition is often very broad and nebulous. In the human health literature, the role of probiotics is seen as facilitating gut homeostasis, primarily through interactions with the gut microbiota (Watnick and Jugder 2020). As the gut is a very dynamic environment, the term gut homeostasis may be a more precise way in which to describe such an environment than the more general term, gut health; the terms are used here interchangeably.
Importance of gut health
The GIT has a central role in nutrient digestion and absorption, host metabolism and energy generation, intestinal barrier integrity, mucosal immunity and providing a niche for a stable microbiota (Kogut and Arsenault 2016). For poultry, a ‘healthy gut’ means the absence of abnormal or damaged structures and functions, so that the bird is protected from pathogens and is able to digest feed and absorb nutrients efficiently to achieve optimum performance. There is increasing evidence that probiotics can make an important contribution to keeping a gut healthy by the maintenance of gut homeostasis (Bajagai et al. 2016; Cameron and McAllister 2019; Zommiti et al. 2020). Optimal gut health is crucial not only for the health, performance and welfare of production animals, but it also contributes to the environment because it improves feed efficiency, reduces use of AGPs, and sustains food safety and human health.
An undeniable feature of probiotic use is the broad spectrum of applications that have resulted in positive animal performance outcomes. Increases in egg and milk production, gut health and disease reduction, leg health and lameness reduction, reproductive health, meat quality and metabolic homeostasis, have all been demonstrated experimentally following feed supplementation with probiotics (Mountzouris et al. 2010; Wideman et al. 2012; Shini et al. 2013, 2020b; Zheng et al. 2014, 2016; Latorre et al. 2015; Bajagai et al. 2016; Angelakis 2017; Gadde et al. 2017; Cameron and McAllister 2019; Park et al. 2020; Wang et al. 2020; Zommiti et al. 2020). Together, this is a very strong endorsement for the role of probiotics in promoting gut health, and animal health and productivity. Moreover, probiotic use will also have a positive impact on animal and bird welfare (Bryden et al. 2021). Importantly, there is increasing evidence that the gut microbiota plays a central role not only in physical, but also mental wellbeing, with obvious implications for bird health and welfare (Kraimi et al. 2019). Nevertheless, as we discuss below, there are instances when probiotics do not show any effect in improving animal and bird productivity.
Assessment of gut health
One difficulty to improving gut health with probiotics has been the lack of reliable methods or biomarkers to assess intestinal health or test probiotic efficacy. This assessment is of a particular importance in the case of subclinical infections and chronic intestinal inflammation (Kogut and Arsenault 2016; Kogut et al. 2018; De Meyer et al. 2019; Shini et al. 2020b). A bird with a subclinical infection or intestinal inflammation does not display signs of the disease, but epithelial damage and malabsorption can occur. For example, during subclinical necrotic enteritis (NE), birds may appear normal or have very mild clinical signs; however, a damaged intestinal epithelium can be found after necropsy and histopathology or electron microscopy (Kaldhusdal and Hofshagen 1992; Teshfam and Rahbari 2003; Gomide et al. 2004; Zekarias et al. 2005; Gholamiandehkordi et al. 2007; Park et al. 2008; Wu et al. 2010; Smyth 2016; Kogut et al. 2018; Shini et al. 2019, 2020a, 2020b, 2021). Subclinical intestinal infections are difficult to diagnose, but can spread through flocks; therefore, they are one of the most important problems in the poultry industry because of high economic losses. A standard evaluation methodology for intestinal health status needs to be established.
What to assess and how? There are visual clues and aspects of performance efficiency that might suggest gut health problems in a flock. Observations of compromised chicken performance, increased flock morbidity, evidence of diarrhoea or birds with pasty vents (Fig. 2), and the occurrence of wet droppings and wet litter (Dunlop et al. 2016; Kaldhusdal et al. 2016; De Cesare et al. 2017; Kumar et al. 2018; Shini et al. 2020b) may indicate disturbances to gut health and should be investigated to minimise the possibility of reduced flock welfare and productivity. In addition to performance and visual signs, necropsies of dead or culled birds can indicate the presence of any clinical or subclinical gut problems. As presented in Fig. 2, small and apparently normal birds may appear ‘healthy,’ but their intestinal mucosa might be ‘disturbed’, and this can be determined only at necropsy.
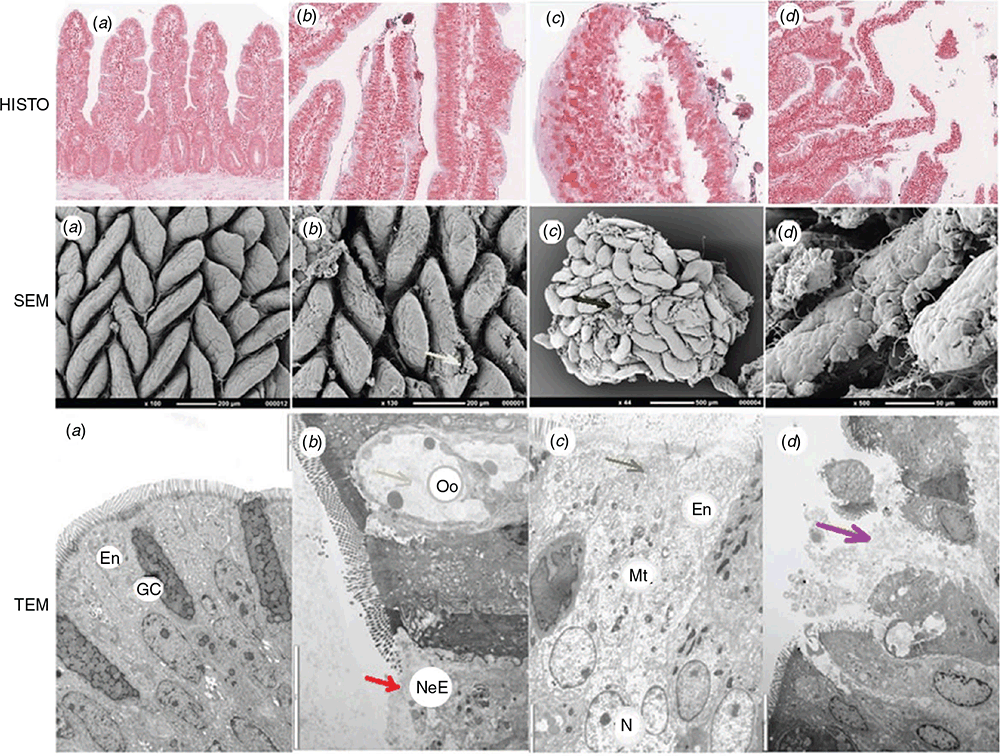
In addition to observations, intestinal health can be assessed using invasive tools that are necessary for a definite diagnosis, such as blood testing (haematology and serology), necropsy and histopathology or even electron microscopy for ultrastructure evaluations (Chen et al. 2015; Smyth 2016; Baxter et al. 2019; Shini et al. 2019, 2020a, 2020b, 2021). These direct tests provide more conclusive details. Studies conducted with a broiler model of subclinical NE (Shini et al. 2020b) have demonstrated that histopathology of the ileal mucosa helps quantify villus morphological alterations (Fig. 3, HISTO: a–d), and demonstrate focal erosion of epithelial cells and the fusion of adjacent villi, in otherwise apparently healthy birds. Histopathology of NE-challenged birds helped detect the successful infection with Eimeria, and oocyst formation underneath the epithelium (Fig. 3, HISTO: b, c). Consequently, the epithelial detachment in sheets and mucus production occurred and predisposed colonisation by Clostridium perfringens (Cp) deeper into the mucosa and crypts, causing focal necrosis (Shini et al. 2020a; Fig. 3 HISTO: c, d, h, l). The presence of Cp, intimately associated with necrotic lesions of intestinal mucosa, was also essential for the diagnosis of NE, and Gram-staining of ileal tissue permitted identification and enumeration of Cp (Fig. 3, HISTO: b, d; Shini et al. 2020b). The electron microscopy study of ileal tissue allowed the examination of enterocyte condition and demonstrated the value of this approach, as birds with subclinical NE displayed only very mild or no signs of the disease. In terms of ultrastructural examination of mucosa, the condition of epithelium and enterocytes, and their content and features, such as mitochondria, lysosomes, endoplasmic reticulum, tight junction (TJ) and microvilli can show the state of mucosa health (Fig. 3 SEM: a–d; TEM: a–d, 4). Other cells and structural components of the intestinal mucosal barrier such as mucus-producing cells (goblet cells), immune-competent cells, i.e. intraepithelial lymphocytes and tissue-related immune cells (macrophages and heterophils), and microbial presence, provide evidence of involvement of inflammatory and immune defence mechanisms in the pathogenesis of NE (Shini et al. 2020a, 2020b, 2021).
When evaluating intestinal health, both the intestinal wall and the luminal contents must be considered. The luminal contents or digesta contain a variety of microbial and chemical components such as bacteria, nutrients, endogenous secretions, other dietary ingredients, antigens and substances delivered with diet or generated in the GIT tract by digestion and microbial metabolism; all are potential biomarkers of gut health. In the case of intestinal disturbances, inflammatory metabolites can be assessed in the digesta or excreta. Most of these components are known to participate in the stimulation of gut mucosal defence, and mucosa growth and regeneration by directly stimulating enterocytes or acting on the enteric nervous system and modulating a variety of GIT functions (Furness and Cottrell 2017; Pluske et al. 2019). Metabolome analysis using biological specimens, such as digesta, excreta, blood or gut tissue, can target metabolites and explore disease-related or dysregulated metabolic pathways and their biological implications for gut health (Celi et al. 2019). However, here is a need for reliable and rapid tests for gut health that can be conducted on farm. In this regard, excreta is an invaluable source of information of clinical and subclinical infections. Faecal droppings can be checked for abnormalities in colour, consistency and content, such as excessive water, fat, mucus, gas bubbles and undigested feed particles. Inflammatory metabolites in excreta show promise as potential biomarkers for the evaluation of intestinal barrier function in broilers; metabolites include ovotransferrin (Goossens et al. 2018), cloacal immunoglobulin A (IgA); (Baxter et al. 2019), fibronectin, intestinal alkaline phosphatase and lipocalin-2 (Barekatain et al. 2020). Detection of such biomarkers could be incorporated into an excreta dipstick to be used on-farm for a quick gut health screening test.
Two simple, but very important, physicochemical parameters of digesta that affect microbial activity, digestion and absorption in the small intestine, are viscosity and pH. Many grains (wheat, barley, rye, triticale and oats) contain soluble fibre or non-starch polysaccharides (NSP), that interact with mucus and secretions in the GIT to form a hydrocolloidal layer and impair digestion and absorption (Knudsen 2014; Bederska-Łojewska et al. 2017). Moreover, prolonged exposure to NSP can induce a low-grade sterile inflammatory response (Rubartelli et al. 2013). Intestinal viscosity may also influence the host resistance to diseases. The supplementation of diets rich in viscous grains with probiotics and exogenous enzymes, such as xylanase, partially breaks down NSP, and reduces digesta viscosity, and decreases the proliferation of undesirable microbes such as Escherichia coli and Clostridium perfringens (Latorre et al. 2015), and parasites such as Ascaridia galli (Dänicke et al. 2009). The measurement of digesta viscosity is a possible biomarker of gut health in poultry. However, greater standardisation of how digesta viscosity is measured and the results interpreted is required (Bedford 2018). The pH decreases as the digesta passes from the crop into the proventriculus and gizzard, and then becomes progressively less acidic when it reaches the small intestine (Jeurissen et al. 2002; Denbow 2015). Many probiotics decrease the ileal pH in broilers, and this mechanism is related to increases in commensal microbial growth or destruction of pathogenic bacteria (see section Mechanisms of probiotic action). However, it has been suggested that digesta pH measurement must be conducted in a manner that avoids artefacts that can be introduced by exposing digesta to air or touching the intestinal wall with the pH probe (Jeurissen et al. 2002).
Finally, an increase of metabolites or nutrient concentration in the excreta can reflect gut problems and therefore could serve as biomarkers. For example, excess of undigested feed particles, or starch, fat or unabsorbed bile acids/salts in excreta might indicate problems with digestion and absorption or permeability in the small intestine. In the case of bile, the majority of bile acids (over 90%) are actively absorbed in the distal ileum, and only less than 10% are lost in the excreta (Scanes and Pierzchala-Koziec 2014; Ticho et al. 2019); an excess in excreta could be an indicator of reduced enterohepatic recycling. However, a word of caution with the use of excreta biomarkers. The avian excreta sample is a mixture of faeces and urine, and contains metabolites from the ileum, and those excreted in urine or generated by bacterial fermentation in the caeca. Thus, avian excreta values will therefore reflect both GIT and post-absorptive metabolism problems. For example, calcium concentrations in excreta reflect both incomplete feed digestibility and urinary excretion. The relative importance of both routes of excretion will depend on gut homeostasis and the metabolic status of the bird (Li et al. 2017).
Poultry house dust may be another source in which to measure markers for subclinical infection. Ahaduzzaman et al. (2021) found that in the case of NE-subclinical and NE-clinical flocks, high levels of Eimeria spp. and C. perfringens were detected in dust after inoculation, followed by a gradual decline over time, while in the control flock, C. perfringens and netB were detected at low levels. Recent research has also shown improved litter quality and decreased ammonia emissions after feeding probiotics to poultry. Dietary B. subtilis supplementation reduced ammonia emission in laying hens, by improving the activity of enzymes and N utilisation (Zhang et al. 2012). Park et al. (2016) also reported that a shift of excreta faecal microbial composition following E. faecium supplementation in laying hens and B. subtilis in broilers (Park and Kim 2015) was accompanied by increased nutrient retention and reduction in nutrient excretion, leading to improved nutrient digestibility and reduced excreta ammonia emissions. Ducatelle et al. (2018) proposed that volatile organic compounds (VOCs) from chicken excreta were potential novel markers of intestinal health, whereas previously, VOCs were considered only for their contribution to malodourous and environmental pollution. Although, it had been shown that different VOC profiles occur in chickens with and without Campylobacter infection (Garner et al. 2008). Six compounds (hexanal, E-2-octenal, pyrrole, ethyl ethanoate, methyl alcohol and 2-heptanone) were identified and used together, to classify excreta samples as positive or negative for Campylobacter. These biogases were recommended as biomarkers for Campylobacter infection.
Gut microbiota
We have known for many years that there is microbial activity throughout the avian digestive tract from the presence of volatile or short-chain fatty acids (Annison et al. 1968). With the advent of molecular techniques, it has been shown that there is a diverse microbiota throughout the avian GIT (Rychlik 2020), which has a determinant role in gut health (Broom 2019). The ‘elephant in the room’ in relation to gut homeostasis is the microbiota with its greatest concentration in the caeca. In birds, the caeca are important sites of fermentation. Compared with other parts of GIT, the caecum contains a more diverse and stable microbial community, including anaerobes (Oakley et al. 2014). However, gut health evaluations target the distal portion of the small intestine or ileum. This section of the GIT has a vital role in digestion, absorption and mucosal immunity (see Box 2) and it is rich in microbiota (less rich and diverse microbial populations than in caeca). The importance of gut microbes to bird performance and productivity is starting to be unravelled using bacterial 16S rRNA (rRNA) gene sequencing (Stanley et al. 2014; Kollarcikova et al. 2019), but our understanding of the diversity of the microbiota from different GIT segments is still very limited. However, the composition and diversity of mucosa-associated microbiota seems to be a more interesting indicator of intestinal homeostasis, due to its close proximity to the intestinal tissue and its important biological role in the development of mucosal immunity (Shang et al. 2018) and bird physiology through the endocrine responses of the microbiota (Villageliũ and Lyte 2017).
| Box 2. Ileum |
| The ileum is the most distal section of the small intestine and extends from vitelline diverticulum (formerly Meckel’s diverticulum) to the ileo–caecal junction. It has an important role in digestion and absorption, and mucosal immunity. The ileum exhibits several unique features of the mucosal immune system, or gut-associated lymphoid tissues (GALT). These features include lymphoid cells located in the epithelial lining (intraepithelial lymphocytes and Paneth cells), the immune cells in the lamina propria, as well as specialised lymphoid structures, such as Peyer’s patches covered by microfold cells (M-cells), which all play roles in the regulation and stimulation of gut immune defence (Allaire et al. 2018). Conditions in the ileum are more favourable for microbial growth (including growth of probiotic bacteria) than in the more proximal small intestine (duodenum and jejunum). The pH (close to neutral), and a longer transit time through the ileum create a suitable environment for microbial growth and metabolism (Booijink et al. 2010; Gerritsen et al. 2011). Lu et al. (2003) studied ileal microbiota and found that Lactobacillus was the major group (70%), followed by members of the family Clostridiaceae (11%), Streptococcus (6.5%) and Enterococcus (6.5%). The presence of segmented filamentous bacteria (SFB), Candidatus savagella, a unique group of commensal bacteria, attached to the ileal epithelium during the first 3–4 weeks of a bird’s life, reinforces the ileum’s critical role in innate and adaptive immunity (Goodwin et al. 1991; Ericsson et al. 2014). Hence, many researchers select the ileum as an organ of interest, when evaluating gut health. Likewise, those interested in nutrient metabolism often sample from the ileum as it is the last segment of the small intestine where digestion and absorption is essentially complete. However, the jejunum is the segment of small intestine where most absorption occurs, and the caecum, a part of large intestine, contains the highest microbial cell densities in the GIT. |
A diverse and normal (or commensal) microflora (microbiome) is associated with the epithelial and mucosal surfaces of a chicken’s body (including the skin, gut, cloaca, oral cavity, upper respiratory tract, oviduct and lungs), which is a complex invisible organ that is integrated into the biology of the host. The role is to maintain homeostasis and protect against invading microbes. This normal microflora is usually stable, with specific genera populating various body regions during particular periods in the bird’s life. It is represented by a large variety of microbial communities, involving several thousands of different taxonomic units of bacteria, fungi, viruses, bacteriophages, archaea and eukaryotes. The chick’s microbiome is partly acquired from the hen and egg, but is greatly influenced by environmental factors (Ding et al. 2017). The bacterial species found in the chicken gut include four predominant bacterial phyla (Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria), which account for more than 99.42% of the total sequences (Oakley et al. 2014; Sun et al. 2018). Lactobacilli, Streptococci and Escherichia coli are found in small numbers in the gut. The gut microbiota is constantly reshaped by contact with the outside environment and might be damaged when its collective population structure is altered (Baquero and Nombela 2012). Particular interest has been paid to the bacterial communities or ‘microbiome’ of the gut, as it confers health benefits to the host, such as aiding in the digestion and absorption of nutrients, contributing to the construction of the intestinal epithelial barrier, the development and function of the host immune system, and competing with pathogenic microbes to prevent their harmful propagation (Kogut and Arsenault 2016; Shang et al. 2018; Kogut 2019). The gut microbiota is altered by diet, pathogens, antimicrobials, and other nutritional, hormonal, or both internal and external behavioural factors (Oakley et al. 2014; Diaz Carrasco et al. 2019; Haberecht et al. 2020). Both pathogenic and beneficial microbes can be found in the lumen of the gut or on the gut’s mucosal surface (Jeurissen et al. 2002). The composition of microflora at each site could be slightly different and determined by different factors (Shang et al. 2018); however, they influence each other and are collectively responsible for the gut health status. In saying this, when sampling, care should be taken to assess the microbiota on both sites, as there could be significant differences between lumen and mucosa sampling sites.
Discussions of the microbiota normally centre on single organisms, with the implication that ‘bugs act alone’. However, there is increasing evidence that many bacteria form biofilms and the ecological niche, so formed, provides mutual benefits to the participating bacteria. Biofilm formation is usually considered a precursor to intestinal disease, but this is hotly debated (Tytgat et al. 2019). Low doses of antibiotics induce bacterial biofilm, and antibiotic resistance and infection recurrence can be connected to biofilm formation. Biofilm-forming bacteria are highly organised in multicellular bacterial structures on the intestinal absorption surfaces, thus preventing normal functions in the ileal mucosa. For C. perfringens, biofilm formation could play a role in the development of NE because a biofilm can help bacteria adhere to surfaces, and this facilitates colonisation and infection (Charlebois et al. 2014). In humans, there is evidence for anti-biofilm activity of probiotic spore-forming bacilli (Bacillus amyloliquefaciens B-1895) against clinical and animal urinary tract infection isolates such as P. mirabilis (Algburi et al. 2020).
Gut dysbiosis, resulting from changes in composition and function of the gut microbiota and disruption of gut barrier function, has been reported in poultry. It has been triggered by non-infectious and infectious factors, including the ban on AGPs in poultry feed and administration of antibiotics at therapeutic doses (Ducatelle et al. 2018). Metabolite concentrations affected by gut microbiota dysbiosis, such as short-chain fatty acids (SCFAs) and secondary bile acids influence host metabolism. Microbial fermentation of carbohydrates results in the production of a range of SCFAs, predominately acetate, propionate, butyrate and lactate (Annison et al. 1968; Macfarlane and Macfarlane 2012). The SCFAs, especially butyrate, are the preferred substrate for the epithelial cells, and are associated with cell proliferation, differentiation, and apoptosis, increased MUC2 gene expression, and antioxidant activity; all play a part in the integrity of the gut barrier (Lee and Hase 2014). Therefore, disturbances of gut health will be reflected in increased or decreased intestinal permeability to metabolites generated in the gut. High serum d-lactate concentrations are indicative of increased intestinal permeability in laying hens (Lei et al. 2013), and increased d-lactate concentrations have been observed after lipopolysaccharide challenge in broilers (Wu et al. 2013). Gut dysbiosis also generates toxic metabolites from protein fermentation and the impaired intestinal barrier may permit translocation of these toxins into the systemic circulation (Yadav and Jha 2019).
There is no doubt that feed additives and supplements such as probiotics, prebiotics, organic acids, and exogenous enzymes can modulate the intestinal microbial community of the host to promote health (Adeola and Cowieson 2011; Kim et al. 2011; Teng and Kim 2018; Araujo et al. 2019; Pereira et al. 2019; Yadav and Jha 2019; Haberecht et al. 2020) and are especially recommended for use in poultry to build or re-establish normal flora during periods of stress and other challenges that cause immunosuppression.
Probiotics, performance and health
In the past 20 years, there has been an exponential increase in the use of probiotics in animal agriculture. The estimated global market value of probiotic supplementation in animal feed is some US$4.6 billion (Marketsand Markets 2019), and the benefits of probiotic application have been demonstrated for animal and bird performance, as described above. Nevertheless, Applegate et al. (2010) raised important areas of concern for the use of probiotics by industry, including unfamiliarity with product, overselling of product effects, product inconsistency, lack of documented physiological and microbial effects in vivo, and lack of documented persistence. In the intervening decade, research has improved industry’s confidence in the application of probiotics. However, there are still many areas of uncertainty and this is exacerbated by the number of probiotic products on the market with impressive claims of efficacy, many with little proof. Some areas of probiotic use and application that require further delineation are discussed in this section.
Probiotics and AGPs
The Food and Drug Administration (FDA) recommends ‘overall reduction in use of all classes of medically important antimicrobials in food-producing animals; complete restriction of use of all classes of medically important antimicrobials in food-producing animals for growth promotion, and complete restriction of use of all classes of medically important antimicrobials in food-producing animals for prevention of infectious diseases that have not yet been clinically diagnosed’ (FDA 2017). Concerns about drug-resistant superbugs and the lack of new antibiotics for treating human and animal diseases, have led to the reduction in the use, or in some countries, explicit banning, of antibiotics and other antimicrobial agents in food-producing animals. This situation has created a void for those involved in animal agriculture and promoted a research environment seeking to find alternative strategies for the maintenance of gut homeostasis. Probiotics have shown many positive effects in maintaining and improving gut health by reducing inflammation, as do antibiotics (Niewold 2007; Mountzouris et al. 2019). However, it is likely that both groups of compounds reduce inflammation by different mechanisms. It should be pointed out that probiotics are not a therapeutic replacement or substitute for antibiotics or other antimicrobials; it will take more than one product (probiotic, prebiotic, phytogenic, organic acid, essential oil or a combination thereof) to replace the beneficial effects that AGPs delivered in the past.
Increasing evidence suggests that probiotics assist in the maintenance of intestinal integrity. There is a plethora of terminology that is used to describe probiotic effects on intestinal mucosa, including, maintenance, improvement, enhancement, alleviation, control and prevention of infections. All of these terms indicate that the probiotic has either prevented a pathogen from causing intestinal damage (maintained mucosa), or regenerated or improved damaged mucosa. However, there is still much to be explored on probiotic- and host-related factors that affect the outcomes of probiotic use. The benefit of probiotic use is most obvious in chickens challenged by stress such as infection rather than when chickens are maintained under optimal conditions, as is also the case with AGPs. Previous research conducted with challenged chickens (by heat or cold stress) or with experimentally infected birds, showed that probiotics improved growth or feed conversion ratio (FCR; Mountzouris et al. 2010; Huff et al. 2015; Park and Kim 2015; Abudabos et al. 2016; Shini et al. 2020b). In most of cases, intestinal health of challenged birds was maintained or improved, and this could have been the reason for improved performance in many trials with challenged birds. We have demonstrated with electron microscopy that birds challenged with subclinical NE and fed the probiotic Bacillus amyloliquefaciens strain H57 (H57), maintained the cellular architecture of the ileal epithelium comparable to control birds, whereas birds without H57 supplementation had a damaged and disintegrated mucosa (Shini et al. 2021); NE-challenged and H57-ed birds also showed a significant decrease in FCR against NE (1.28 vs 1.36), and achieved a FCR similar to control birds (1.28 vs 1.27 respectively; Shini et al. 2020b).
Do probiotics always work?
A large number of investigators have used diets supplemented with probiotics and studied their efficacy on bird performance; however, the outcomes have not always been consistent (Bajagai et al. 2016). There have been mixed reports on the effects of probiotics on broiler performance. Some investigators have reported positive effects on bird performance (Palamidi et al. 2016; De Cesare et al. 2017; Pereira et al. 2019), while others have not seen any significant probiotic effect (Olnood et al. 2015b; de Souza et al. 2018; Zarei et al. 2018; Araujo et al. 2019). Similar outcomes have been reported in humans, with investigators explaining that ‘probiotics don’t work for everyone,’ and suggesting factors such as strain of probiotic organism, dose, route and period of administration, and health conditions of the host (Islam 2016), as being responsible for these discrepancies. These factors could also apply to production animals.
While effects of probiotics in ameliorating gut infections are well established (Higgins et al. 2010; Menconi et al. 2011; Huff et al. 2015; Forte et al. 2016; de Souza et al. 2018; Shini et al. 2020b, 2021), other beneficial effects on liver, bone and muscle, egg and meat quality (Watkins and Kratzer 1983; Mutuş et al. 2006; Lutful Kabir 2009; Wideman et al. 2012; Zheng et al. 2014, 2016; Cengiz et al. 2015; Bai et al. 2017; Yan et al. 2019) have been reported and should be considered when probiotics are applied. All these effects contribute to enhanced performance and profitability, and improved health and welfare of flocks. However, many of these claimed benefits of probiotic use are difficult to quantify in a production setting and require further investigation.
Is it best to use multi-strain probiotics?
Commercially available probiotics contain different bacterial species and strains that are often isolated from a variety of habitats. Some products contain single species (strains), some contain multiple species (strains), with others containing multiple isolates of the same species (Smith 2014; Bajagai et al. 2016; Aalaei et al. 2019). There are other products that contain probiotic organisms in combination with prebiotics, organic acids, essential oils and phytogenics, but discussion of these mixtures is outside the scope of the present review. The probiotic strain is considered essential for effectiveness. A single-strain probiotic can be very effective if the bacterial strain is carefully selected for the circumstances under which it will be used. The selection of a probiotic strain should take into account the relevant functional properties of the strain, and whether it can be considered safe for poultry and human consumption; this principle would also apply when designing a multi-strain (multi-species) probiotic. Multiple species probiotics most frequently have various combinations of Lactobacilli (often a few different species or strains), Streptococcus species, Bifidobacterium species, Enterococcus species and Bacillus species. The relevance on using a multi-strain (multi-species) probiotic lies in the fact that some bacterial strains (species) perform some functions, whereas others perform different functions, all contributing to an overall beneficial outcome to meet the expectations for the use of the probiotic. Moreover, not all isolates or strains of the same bacterial species have equal efficacy as a probiotic (Liu et al. 2010). Bacillus spp. probiotics have been used successfully due to spore formation and their survival rate and persistence in the GIT (Cutting 2011). Many Bacillus spp. produce enzymes and bacteriocins, potentially enhancing their mode of action in the gut (Cutting 2011). There are many commercial products that are single- or multi-strain probiotics, but the benefits of using more than one strain or species in a single product has not been clearly established (Zhao et al. 2013). However, single-strain probiotics have unique properties, and using a single-strain probiotic makes it easier to differentiate the effectiveness and understand the mode of action of a probiotic. Therefore, single-strain probiotics are easier to patent, whereas multi-strain probiotics are not, often lacking clinical studies for the correct combination of strains. Examples of microorganisms in single- and multi-strain/species probiotic products, commercially used in animal feeds, including poultry, have been detailed in a report by Bajagai et al. (2016), along with the effect on poultry (broilers and laying hens) performance.
What other factors determine probiotic efficacy?
There is general agreement among researchers and manufacturers for more quality control studies regarding formulation, route of administration and delivery system, dose and dosage regimen of strain-specific probiotic products. These studies should be undertaken before the release of a commercial product (Weese and Martin 2011) and are crucial for appropriate application and achieving the desired health and performance outcomes (Kechagia et al. 2013; de Simone 2019).
Administration of probiotics on farms can be via feed or drinking water; adding to feed is the most commonly used method for poultry. Heat-stable probiotic products can be added to feed before pelleting or applied post-pelleting (spray-coating), or delivered through litter. Olnood et al. (2015b) showed that different routes (feed, water, litter, and gavage) for administering L. johnsonii did not significantly influence broiler growth performance. Furthermore, there were no statistically significant differences among the various routes of administration on the gut microflora, but individual oral application (gavage) resulted in the greatest reduction in intestinal counts of Enterobacteria and C. perfringens, an outcome regarded as a key attribute for probiotic application in poultry diets (Olnood et al. 2015b). In another study, administration in drinking water appeared to be superior over supplementation in feed, in terms of performance and immune competence of birds (Karimi Torshizi et al. 2010).
As for dosage, FAO/WHO guidelines emphasise that ‘when administered in adequate amounts, probiotics confer a health benefit to the host’ (FAO/WHO 2006; Morelli and Capurso 2012). The dose of a probiotic is the number of viable microbial cells in a given product, derived by plate counting the total number of colony-forming units (CFUs) in a given volume, and expressed as CFU/g or mL. The minimum effective concentration of probiotics in feed or water is debated; however, it is generally accepted that probiotic products should have a minimum concentration of 106 CFU/g or mL. For humans, it is recommended that a total of 108–109 probiotic microorganisms need to be taken daily for the probiotic to be effective (Kechagia et al. 2013; Forssten and Ouwehand 2020). However, clinical studies have shown that the concentration of a probiotic needs to be 106 CFU/mL in the small intestine and 108 CFU/g in the colon to obtain a clinical effect in the gut (Minelli and Benini 2008). For poultry, probiotic application is based on the manufacturer’s recommendations for dose and duration of administration. In a recent study (S. Shini, unpubl. data), a diet supplemented with graded doses of H57 at 0, 106, 107 and 108 CFU/g feed was fed to broiler chickens and half of the birds were challenged with subclinical NE. The data showed that there was a dose effect. Birds challenged with NE and fed H57 at 107 and 108 CFU/g feed, had similar growth rates, and these birds performed better than did birds fed H57 at 0 and 106 CFU/g feed. FCR was significantly improved in birds challenged with NE and fed the highest dose of H57. From these data, it appears that there is a threshold dose for performance in challenged birds and it was concluded that the dose of 107 CFU/g feed would be satisfactory for field application, as it improves the performance of challenged birds and is more economical. At necropsy, the lower dose (106 CFU/g feed) of H57 also showed benefits on mucosal health of challenged birds. Tomaszewska et al. (2018) demonstrated that the influence of probiotic administration on tibia geometry was also dose-dependent in female turkeys.
Other factors that could influence probiotic efficacy are poultry species, the breed, age, and health or disease conditions. Most probiotic work with poultry is undertaken with broilers, but the same response may not occur in other avian species (Edens 2003). Similar improved growth rates from probiotic use are seen in chickens and turkeys (Smith 2014), but less in ducks where overfeeding causes significant changes to metabolism, microbial diversity and growth (Even et al. 2018). The use of different broiler breeds could also have been the reason for contrasting results when using the same probiotic. For example, studies with an E. faecium strain showed different results on performance when conducted with different broiler breeds; Cao et al. (2013) found an effect on growth in male Cobb broilers, while Zhao et al. (2013) did not find any effect in Ross broilers. The use of probiotics has shown to be more effective in young birds, as this coincides with the critical period of GIT microbial colonisation (Applegate et al. 2010). This is very important for chicks hatched from artificially incubated eggs, thus lacking contact with hens, resulting in delayed development of the intestinal microflora. As birds grow, the microflora becomes more diverse and tends to become relatively stable. Diet content is also likely to be a contributing factor to probiotic efficacy (Lutful Kabir 2009).
The results of many clinical investigations in humans suggest that probiotics may be useful in preventing and treating various health conditions and diseases; however, efficacy depends on the health and disease status, including clinical indications (acute or chronic). There is evidence that probiotics are effective for acute infectious diarrhoea, antibiotic associated diarrhoea, Clostridium difficile associated diarrhoea, hepatic encephalopathy, ulcerative colitis, irritable bowel syndrome, functional gastrointestinal disorders, and necrotising enterocolitis; conversely, there is evidence that probiotics are not effective for acute pancreatitis and Crohn’s disease (Wilkins and Sequoia 2017). In poultry, enteric diseases have become a major concern after the exclusion of AGPs, and feed additives including probiotics have had varying degrees of success in combating these diseases, probably due to the factors outlined above, and the stress and disease challenges faced by birds on farm.
Mechanisms of probiotic action
Probiotics contain live bacterial cultures, but how they induce their effects remains unclear. This is a complex area of biology in which there are multiple players; consequently, many possible modes of action and pathways are involved. Several mechanisms have been established or proposed and it is likely that the beneficial effects of probiotics are mediated by multiple mechanisms, and some of these mechanisms are outlined below.
Modulation of intestinal microbiota
The gut microbiota is one component of an animal’s microbiota that colonises all epithelial and mucosal surfaces of the GIT. Overall health and wellbeing is dependent on a balanced gut microbiota and delivery to the host of microbial-derived metabolites. Probiotics are considered to be one of the best alternatives to antibiotics, especially in terms of modulating the intestinal microbiota. The inclusion of probiotics in the diet has shown to help maintain or rebuild the intestinal microbiome by stimulating the growth of beneficial indigenous microorganisms such as Lactobacilli and Bifidobacteria (Gagliardi et al. 2018). These bacteria have the ability to limit the direct contact of pathogenic bacteria with the epithelium by competitive exclusion for access to mucosal surfaces, nutrients, and the creation of harsh environmental conditions in the lumen for pathogens. Competitive exclusion has been found to be effective in inhibiting intestinal colonisation by Salmonella, Shigella, Clostridium and Listeria (Naidu et al. 1999; Bermudez-Brito et al. 2012; Park and Kim 2015). A probiotic strain of L. plantarum has been reported to induce MUC2 and MUC3 mucins, thus inhibiting the adhesion of pathogenic E. coli (Mack et al. 1999). It has been observed that many probiotic bacteria show a greater capacity to adhere to the chicken’s intestinal mucosa than do pathogens and, therefore, displace them (Collado et al. 2005). It has also been demonstrated that many probiotics favour the survival of beneficial bacteria such as Lactobacilli because they decrease intestinal pH (Olnood et al. 2015a; Shini et al. 2020b; Zaghari et al. 2020). Lactic acid production is increased by B. amyloliquefaciens, which most probably explains the drop in ileum pH (Wu et al. 2011; Salim et al. 2013; Shini et al. 2020b) associated with feeding this probiotic species.
Probiotics can modify the ecosystem of the GIT by the production and release of enzymes, bacteriocins, and other secondary compounds into the gut. For example, many Bacillus spp., including B. amyloliquefaciens, produce large quantities of extracellular enzymes such as amylase, protease and lipase, and some cellulases and xylanases (Elshaghabee et al. 2017), that can assist by increasing nutrient digestibility and deactivating anti-nutritional factors present in poultry diets (Yadav and Jha 2019). Similar enzymes are routinely added to poultry diets to improve nutrient digestibility and to degrade anti-nutritive factors (Bedford 2000, 2018; Ravindran 2013). Probiotics have also been shown to increase the abundance of segmented filamentous bacteria (SFB), the indigenous bacteria that are found in ileal microflora of chicks (Shini et al. 2021). Liao et al. (2012) manipulated the colonisation of SFB in chickens by feeding Lactobacillus delbrueckii at hatch, resulting in SFB colonisation occurring 4 days earlier, with implications for regulation of the immune response, and this mechanism is explored below.
Maintenance of intestinal barrier integrity
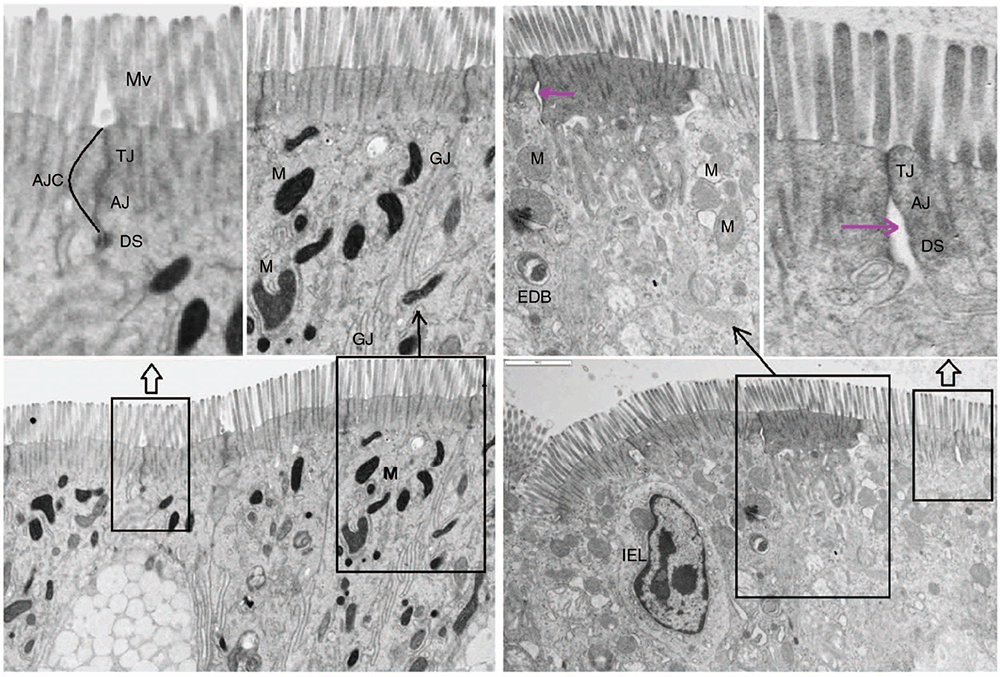
The intestinal epithelium is a dynamic bidirectional layer that functions as a frontier-barrier, accepting or refusing movement of intraluminal particles into adjacent enterocytes and underlying microvasculature. The junctional complexes between enterocytes maintain the integrity of the epithelial barrier by regulating paracellular permeability and are composed of TJ, gap junctions, adherens junctions (AJ), and desmosomes as shown in Fig. 4. The TJ and AJ constitute the apical junctional complex, the primary structure that regulates the intestinal barrier. Tight junctions include four integral transmembrane proteins (occludin, claudin, junctional adhesion molecule, and tricellulin) that interact with the actin cytoskeleton (Ulluwishewa et al. 2011). The DS and GJ are involved in cell–cell adhesion, and intracellular communication respectively. The paracellular barrier in normal intestinal tissue is characterised by high expression levels of TJ proteins and a low paracellular permeability (or a tight epithelium), while mucosal inflammation is frequently associated with decreased expression of junctional proteins (or a leaky epithelium). The integrity of the mucosal barrier is important, for the digestion and absorption of nutrients, and it is considered the first line of defence against pathogens and toxic molecules. The disruption of its integrity is the primary cause of disturbed physiology, and inefficient feed utilisation. Hence, there is great interest to understand how probiotics can assist in improving gut mucosal integrity, barrier function and nutrient metabolism.
Several mechanisms appear to be associated with the role of probiotics in improving mucosal barrier integrity, and protecting it from a variety of insults. It has been suggested that probiotics enhance the mucosal barrier by increasing the production of mucus, inhibiting bacterial translocation, and strengthening TJ in a manner similar to that of normal gut microbiota (La Fata et al. 2018). There is also a growing body of evidence indicating that bioactive molecules released by some probiotics activate various cell-signalling pathways that strengthen the TJ and preserve mucosal barrier function (Rao and Samak 2013). Changes in absorption occurring in coccidiosis and NE have been linked to changes in intestinal permeability, a feature of intestinal barrier function (Williams 2005; Ducatelle et al. 2018). Many luminal and systemic factors can independently influence barrier function and cause leakage of plasma proteins and diarrhoea (Quigley 2016; Camilleri 2019). Electron microscopy using a NE broiler model (co-infection with coccidiosis and Cp) demonstrated enterocyte injury and loss of cellular integrity (damage of intercellular connections) within the epithelium due to erosions and ulcerations caused by Eimeria oocytes and Cp (Figs 3, 4). It was thought that barrier dysfunction contributed to diarrhoea via a leak flux mechanism. Birds challenged with NE and treated with a probiotic H57 had preserved ileal mucosa and normal enterocytes and TJ (Shini et al. 2020a).
Coccidiosis and NE-related diarrhoea and malabsorption have been associated with reduced nutrient digestion and performance (Turk 1972; Witlock and Ruff 1977). However, epithelial damage in Eimeria infected birds appears to be less severe than in NE birds; therefore, a higher absorptive area is available, contributing to a slightly better performance in Eimeria-infected birds than in NE-challenged birds (Shini et al. 2020b). In our experiments with H57, it was demonstrated that the probiotic can maintain the intestinal epithelial barrier by improving epithelial cell morphology, in particular mitochondria, and preserving the enterocyte apical junctional complex (which includes TJ; Fig. 4; Shini et al. 2021). Mitochondria have a crucial role in the regulation of gut functions such as intestinal barrier integrity and mucosal immune responses (Jackson and Theiss 2020). Enterocyte mitochondria are involved in the regulation of numerous aspects of cellular activity, including, but not limited to, apoptosis, Ca2+ signalling, and redox homeostasis of the cell (Kang and Pervaiz 2012). Our findings suggest that the maintenance of the epithelial barrier is an energy-dependent process, thus swollen, irregular, vacuolated, or cristae-damaged mitochondria (observed in NE birds), are associated with loss of ATP (ATP) generation and release of oxygen radicals in enterocytes. Subsequent apoptotic necrosis of enterocytes and impaired energy metabolism of epithelial cells evoke a variety of insults, allowing spread of infection. Similar degenerative changes and mitochondrial dysfunction, including oxidative stress and impaired ATP production, are also observed in the intestines of patients with inflammatory bowel disease. In an experiment with broiler chicks, an early infection with Salmonella enterica induced paracellular transport leakage and mucosal barrier dysfunction, but these adverse effects were prevented by the administration of a probiotic culture derived from poultry gut proprietary strains of lactic acid bacteria (Prado-Rebolledo et al. 2017). It has also been shown that B. subtilis 747 improved epithelial barrier integrity of chickens by elevating occludin concentrations in the TJ (Park et al. 2020).
Modulation of inflammatory and immune responses
Probiotics influence host immunity, without invading host tissues. Probiotics are living microorganisms and as such the intestinal mucosa responds to probiotic exposure through innate and acquired immune mechanisms, as it does for other ‘foreign agents.’ However, the response to commensal and probiotic bacteria is a ‘good thing,’ and it accounts for a substantial part of their immunomodulatory effects. The reaction starts with an inflammatory response that activates immune cells, cytokines and chemokines, acute phase proteins and other signalling mediators to protect the body (Abdulkhaleq et al. 2018). Inflammation is a complex and fine-tuned mechanism and may act as both a ‘friend and foe’ (Kjekshus 2015). Normally, it is followed by resolution, and this is an active and highly regulated cellular and molecular process required to protect against deleterious consequences of the inflammatory response (Serhan et al. 2007). Unresolved inflammation can lead to intestinal or system diseases, and increases in energy expenditure for maintaining a chronic immune response. Hence, it affects bird health and performance. The exposure to pathogens induces the intestinal defence mechanisms and the first response is the innate (non-specific) response, including the inflammatory response. Innate immune cells (including dendritic cells) can respond and detect microbial fragments through pattern recognition receptors, and mount a robust immune response against pathogens. In fact, most probiotics appear to be potentially well tolerated by immune cells (Plaza-Díaz et al. 2017). Probiotics are considered to be regulators of inflammation (Lescheid 2014; Peng et al. 2020). Multiple mechanisms of action have been suggested to explain the protective and anti-inflammatory effects of probiotics in modulating intestinal inflammation. Probiotics enhance production of SCFAs with anti-inflammatory properties (e.g. butyrate), as well as increase synthesis of antimicrobial peptides (bacteriocins) that influence inflammation resolution pathways in the mucosa (Wang et al. 2016; Tarradas et al. 2020). It has been proposed that probiotics that target the innate immune system and stimulate it, could be more advantageous. This is because this type of response is quick, multicomponent and non-specific (Swaggerty et al. 2019), offering local and systemic benefits, especially for young birds. Subsequently, components of acquired (specific) immune response would be induced, such as B- and T-lymphocytes and their products, (antibodies and interleukins), and a more specific immune response mounted.
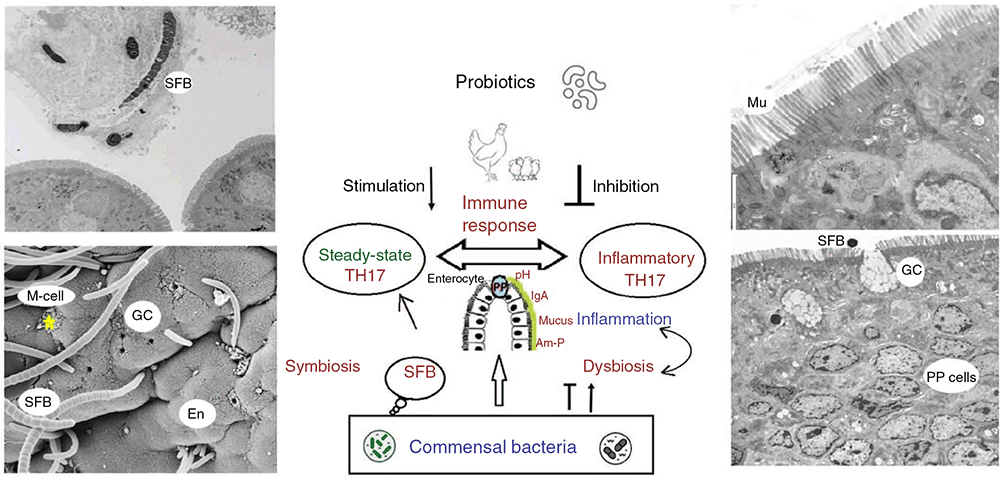
The intestinal surface is also protected from invasion of foreign antigens by an efficient protective layer of ‘partners in slime’, including a mucus layer (which contains mucin and glycocalyx layers) and chemical barriers such as antimicrobial proteins (Johansson et al. 2013). Other mucosal partners, including antigen specific secretory IgA, and mucus associated microbiota (commensal bacteria, e.g. SFB), all play an important role in the initiation of an integration of an immune response (Fig. 5). In experiments with the probiotic H57, we demonstrated that birds exposed to NE and supplemented with H57 produced a better immune response than NE-challenged birds not supplemented with H57; more precisely, birds treated with H57 had increased abundance of goblet cells and intraepithelial lymphocytes (Shini et al. 2020b), and elevated levels of defensin (Gal-6) mRNA expression (S. Shini, unpubl. data). An exciting aspect of the experiments with H57 was finding SFB, and evaluating its abundance and morphology. Interestingly, there were more SFB attached to villi in birds exposed to ‘foreign microorganisms’ (pathogens and the probiotic), i.e. NE, NE-H57 and H57 birds, than in control birds. However, the abundance was lower in H57 birds than in NE-exposed birds (Shini et al. 2021). It appeared that H57 regulates the abundance of SFB, in NE-challenged H57-fed birds, most probably by suppressing pathogenic Th17 cells and inducing steady-state Th17 cells (Fig. 5).
Segmented filamentous bacteria (SFB) are intestinal commensal microorganisms with important roles in host immunology and physiology (Box 3; Ivanov and Littman 2010; Ericsson et al. 2014), and they are host specific. SFB are found on the ileal mucosa (Fig. 3), and participate in the activation of naïve CD4 T+ cells to become Th17 cells, which are capable of producing IL-17 cytokines with a proinflammatory role in the generation of the mucosal (IgA) immune response (Sczesnak et al. 2011). Probiotics favouring SFB could therefore have an effect in stimulating the immune response in the gut. There is evidence that probiotics have an anti-inflammatory effect, which is a consequence of downregulation of IL-17 production and other proinflammatory Th17-secreted cytokines (Tanabe 2013). IL-17 is beneficial in controlling dysbiosis in the gut, but may be harmful if dysregulated (Fig. 5). In the case of overgrown SFB, accumulation of Th17 cells in the ileum could lead to damaging inflammatory effects, as was the case with NE-challenged birds. Supplementation with the probiotic H57 limited the SFB expansion and the Th17 associated proinflammatory response in NE-H57 birds (Fig. 5). It is suggested that probiotics (through beneficial bacteria, and SFB) induce a moderate steady-state Th17 cellular response, and an appropriate production of IL-17, while pathogenic-induced inflammatory Th17 cells are involved in the development of inflammation and dysbiosis of resident microbes (Tanabe 2013; Flannigan and Denning 2018).
| Box 3. Segmented filamentous bacteria |
| Candidatus savagella or segmented filamentous bacteria (SFB), a genetic relative of the genus Clostridium, are unique microorganisms identified as Gram-positive, anaerobic, spore-forming bacteria, colonising the ileum of many young vertebrates, including chickens (Klaasen et al. 1992; Ericsson et al. 2014). SFB are host-specific indigenous bacteria ranging from 0.7 to1.8 µM in diameter and up to 80 µM in length and have important roles in modulating host immunology and physiology (Ivanov and Littman 2010; Ericsson et al. 2014). SFB are involved in regulating postnatal development and maturation of immune responses in the gut of mammals and some birds (chicken and turkey). SFB have a unique morphology, life cycle and binding location. They intimately interact with the host, most notably, firmly attaching to epithelial cells of the distal ileum by one end of the filament (holdfast) to form multicellular filaments, as well as single ‘holdfasts’ and spores, i.e. vegetative and dormant reproductive cells (Chase and Erlandsen 1976). |
| SFB have gained attention due to their capacity to induce and stimulate multiple types of intestinal lymphoid tissue (Peyer’s patches and IEL). SFB are found attached to the ileal mucosa (Fig. 3), and stimulate helper 17 (Th17), which are capable of producing IL-17 cytokines with a proinflammatory role in the generation of the mucosal (IgA) immune response (Ivanov and Littman 2010; Ericsson et al. 2014; Hedblom et al. 2018). As part of the commensal microbiota, SFB have a key role in post-hatching maturation of gut immunity, especially mucosal immunity, and participate in the activation of naïve CD4+ T cells to become Th17 cells, which are capable of producing IL-17 cytokines (Sczesnak et al. 2011). Chicken SFB (Fig. 5) have an important role in the critical transition period from innate and maternal immunity to adaptive immunity, especially during the first 3 weeks post-hatch. In chickens, B. amyloliquefaciens, (H57) reduced the SFB expansion and potentially down-regulated the Th17 associated proinflammatory response (Shini et al. 2021). |
Investigations with Salmonella have found that probiotics stimulate the gut immune system, protecting against infection, by improving the phagocytic activity of peritoneal and spleen macrophages, increasing the antibody response, and improving protection against Salmonella infection (Martin Manuel et al. 2017). Lactobacillus sp. was used as a vehicle for an orally administered avian influenza virus vaccine, and was found to be effective in inducing the systemic and mucosal immune responses with higher anti-haemagglutinin-specific IgA and IgG concentrations (Wang et al. 2013). It was suggested that the acquired immune responses were dependent on the Lactobacillus as a recombinant. Lee et al. (2010) studied the effects of Bacillus spp. as direct-fed microbials on immune characteristics in broiler chickens, and found that cytokine mRNA concentrations (IL-13, IL-6, IL-17 and IL-10) in intraepithelial lymphocytes were increased, decreased or unchanged, depending on the strain used. It was suggested that the direct-fed microbials in the present study showed immunomodulating effects and enhanced host protective immunity against enteric pathogens in broiler chickens. Earlier studies by (Chichlowski et al. 2007) had suggested decreased expression of IL-6 (a proinflammatory cytokine), and increased expression of IL-10 (an anti-inflammatory cytokine). Whereas Haghighi et al. (2008) associated the repression of IL-12 and IFN-gamma (proinflammatory cytokines) expression with the effect of probiotic-mediated reduction and intestinal colonisation of Salmonella typhimurium. Further studies are required to determine the precise action of probiotics on the immune response. These findings will be key to delineating the immune pathways modulated by probiotics, and in identifying more adequate approaches to optimise gut health and performance in poultry.
Improvement of oxidative status
Several studies claim that probiotic supplementation improves antioxidant activity and reduces cell damage caused by oxidation (Aluwong et al. 2013; Persichetti et al. 2014; Wang et al. 2017). Probiotic lactic acid bacteria modulate the redox status of the host via their metal ion-chelating ability, antioxidant systems, regulation of signalling pathways, enzyme producing ROS, and intestinal microbiota. An increase in the activity of antioxidative enzymes protects cells from oxidative stress-induced damage. Most of investigators measure the antioxidant activity using the content of serum (total antioxidant capacity), superoxide dismutase (SOD) and glutathione peroxidase to assess the antioxidant effect of probiotics. However, most of studies have been conducted in vitro and, it is difficult to relate these results to reactions in vivo.
Cao et al. (2019) used the supernatant from ileum mucosa to determine the myeloperoxidase, nitric oxide, SOD, catalase and malondialdehyde after treatments with dietary L. plantarum 1.2567. They observed significantly improved anti-inflammatory and antioxidant activity in broiler chickens with Cp-induced NE. Another investigator used two different probiotics (Bacillus subtilis and B. cereus) and did not find any antioxidant effect using total antioxidant capacity, SOD, and hydrogen peroxide measurements (Abudabos et al. 2016). A probiotic containing both Clostridium butyricum and a combination of Saccharomyces boulardii and Pediococcus acidilactici was beneficial when added to the diets of the laying hen, as it enhanced intestinal development and improved antioxidant activity in the gut (Xiang et al. 2019). The probiotic treated hens exhibited decreased levels of reactive oxygen species (ROS) in ileum and caecum, and reduced malondialdehyde in serum (Xiang et al. 2019).
In our experiments with H57, we did not measure any antioxidative biomarker; however, we assessed the effect of probiotic H57 indirectly by evaluating enterocyte morphology and mitochondrial abundance and morphology. The ultramicroscopy of the ileal epithelium showed severe enterocyte damage, and loss of cellular integrity in NE birds. Mitochondria, in particular, had morphological alterations; they were irregular in form, containing electron-lucent regions of matrix, swollen or damaged cristae, most probably due to oxidative stress, resulting in increased ROS exposure and lowered ATP production (Figs 3, 4). In control, H57 and NE-H57 birds, most mitochondria were round or elongated, with mild or no structural damage, and displayed well preserved structure (Fig. 4). Mitochondrial damage and dysfunction were found in NE birds, which impaired ATP production, causing cell necrosis. Dietary addition of H57 appears to maintain mitochondrial morphology and improve their efficiency in terms of mitochondrial ROS production and scavenging, most probably due to release of enzymes and improved digestion and absorption of nutrients (Shini et al. 2019).
Modification of digestion and metabolism
Since the early studies of Marie Coates and her colleagues with germ-free birds, there has been an appreciation of the importance of the gut microflora to the nutrition of the host (Coates 1986). Many commensal bacteria, including probiotics, produce enzymes to breakdown feed substrates to permit bacterial metabolism, and these enzymes may also facilitate digestion by the host (Bajagai et al. 2016; Rowland et al. 2018). In addition to enzymes, probiotics secrete many other compounds that can interact directly or indirectly with the nutritional status of the host. The SCFA butyrate is a good example and can act as an energy source for enterocytes as mentioned above. As discussed in the present review, probiotics improve the integrity of epithelial barrier, consequently increasing nutrient absorption and, ultimately, improving feed efficiency. Probiotics can indirectly modify the gut homeostasis by helping in the assimilation of nutrients through activation of the transport system from ATP generated by improved digestion. A significant sign of reduced digestion and absorption is the change in excreta quality (also discussed above) and the presence of watery excreta or undigested feed particles in the excreta. An absence of pasty vent in chickens indicates normal digestion and absorption. De Cesare et al. (2017) demonstrated that supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) at the recommended dietary dosage of 1 × 109 CFU/kg feed significantly improved nutrient digestibility, particularly crude fat and protein, and reduced the incidence of pasty vent in broiler chickens. In our study with H57, broiler chicks exposed to NE and fed the probiotic H57 had a significant improvement of FCR, and a low pasty vent occurrence (Shini et al. 2020b). The modification of digestion and metabolism by probiotics is very complex, with both direct and indirect aspects (the probiotic itself or probiotic-derived products) and occurs in conjunction with the activities and actions of the normal microbiota and microbiota-related products. It involves many pathways, which influence the overall metabolism of the host and are responsible for feed efficiency.
Concluding overview and future directions
Following the reduction or ban of AGPs in poultry feed, there has been worldwide interest in the application of alternative feed additives, especially probiotics. Recent advances on the effects of probiotics in improving poultry gut health and bird productivity have been discussed and the following was concluded from the review:
-
Gut health is a complex phenomenon that includes all the biological structures of the intestinal tract and their functions, along with the genetic and physiological makeup of the host, the diet being consumed, and the intestinal microbiota. The dynamic interactions of these different components determine gut homeostasis and the health status of the gut.
-
Achievement of gut homeostasis involves several physiological, microbiological, immunological and physical functions that maintain intestinal dynamic balance or homeostasis.
-
Determination of gut health or homeostasis is difficult to ascertain, especially when disturbances are chronic or in the case of subclinical disease. Physical appearance of the bird may give an indication that there is GIT disturbance. Excreta is an invaluable source of information of clinical and subclinical infections and biomarkers are being developed for leaky gut.
-
Biomarkers are indirect measures but for determination of subtle changes in gut structure, electron microscopy is the method of choice. Using electron microscopy has also provided insights into how the action of a probiotic maintains gut wall integrity.
-
The mechanisms of probiotic action are complex and include local and systemic changes, and corresponding effects on the gut microbiota, intestinal barrier integrity, immunity, oxidation status and feed efficiency.
-
Gut homeostasis is an important link to bird productivity, but there are circumstances when productivity gains are not seen when diets are supplemented with probiotics. Many factors are likely to contribute to this outcome, but a key factor is a stressful environment; a situation in which probiotics enhance bird performance.
The increasing demand for probiotics by the poultry industry demonstrates the benefits that probiotics can bring to poultry productivity. Despite the great deal of research that has been published on probiotics and avian gut health, as outlined above, many questions remain unanswered. Advances in microbial and molecular methods will increase our understanding of the factors that determine gut homeostasis, in particular the interactions of diet, indigenous microbiota and host metabolism, especially of the intestinal mucosa. Such studies should clarify mechanisms of probiotic action and define mechanistic attributes of different probiotic organisms. This should provide insights into why probiotics are not uniformly successful in the field and inform the development of next-generation probiotics. It will also provide basic information for studies that combine different feed additives to achieve gut homeostasis. To accomplish these outcomes, there will need to be rigorous in vitro and in vivo testing under standard conditions that represent field conditions. This will be greatly enhanced by the development of relevant biomarkers that access the efficacy of probiotics. It is important that future specific probiotic products are designed for strategic field application.
Conflicts of interest
Both authors are members of the Editorial Board of Animal Production Science. Neither were involved in the review and editorial process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
Funding for the research work on probiotic Bacillus amyloliquefaciens strain H57 was provided by an Advance Queensland Innovation Partnership grant awarded to the Queensland University of Technology and the University of Queensland, along with their collaborating industry partners Ridley AgriProducts Pty Ltd, Bioproton Pty Ltd and Kennedy Creek Lime Pty Ltd.
Acknowledgements
The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis, St Lucia Campus, and the Poultry Research Group, Gatton Campus, University of Queensland. WLB had the great privilege to receive his initial training in poultry science and nutrition by Professor Rob Cumming and Professor David Farrell, respectively.
References
Aalaei M, Khatibjoo A, Zaghari M, Taherpou K, Akbari-Gharaei M, Soltani M (2019) Effect of single- and multi-strain probiotics on broiler breeder performance, immunity and intestinal toll-like receptors expression. Journal of Applied Animal Research 47, 236–242.| Effect of single- and multi-strain probiotics on broiler breeder performance, immunity and intestinal toll-like receptors expression.Crossref | GoogleScholarGoogle Scholar |
Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM (2018) The crucial roles of inflammatory mediators in inflammation: a review. Veterinary World 11, 627–635.
| The crucial roles of inflammatory mediators in inflammation: a review.Crossref | GoogleScholarGoogle Scholar | 29915501PubMed |
Abudabos A, Alyemni A, Zakaria HAH (2016) Effect of two strains of probiotics on the antioxidant capacity, oxidative stress, and immune responses of Salmonella-challenged broilers. Brazilian Journal of Poultry Science 18, 175–180.
| Effect of two strains of probiotics on the antioxidant capacity, oxidative stress, and immune responses of Salmonella-challenged broilers.Crossref | GoogleScholarGoogle Scholar |
Adeola O, Cowieson AJ (2011) Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. Journal of Animal Science 89, 3189–3218.
| Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production.Crossref | GoogleScholarGoogle Scholar | 21512114PubMed |
Ahaduzzaman Md, Keerqin C, Kumar A, Musigwa S, Morgan N, Kheravii SK, Sharpe S, Williamson S, Wu S-B, Walkden-Brown SW, Gerber PF (2021) Detection and quantification of Clostridium perfringens and Eimeria spp. in poultry dust using real-time PCR under experimental and field conditions. Avian Diseases 65, 77–85.
Al-Sheikhly F, Al-Saieg A (1980) Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Diseases 24, 324–333.
| Role of coccidia in the occurrence of necrotic enteritis of chickens.Crossref | GoogleScholarGoogle Scholar | 6254485PubMed |
Algburi A, Alazzawi SA, Al-Ezzy AIA, Weeks R, Chistyakov V, Chikindas ML (2020) Potential probiotics Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 co-aggregate with clinical isolates of Proteus mirabilis and prevent biofilm formation. Probiotics and Antimicrobial Proteins 12, 1471–1483.
| Potential probiotics Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 co-aggregate with clinical isolates of Proteus mirabilis and prevent biofilm formation.Crossref | GoogleScholarGoogle Scholar | 31989448PubMed |
Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA (2018) The intestinal epithelium: central coordinator of mucosal immunity. Trends in Immunology 39, 677–696.
| The intestinal epithelium: central coordinator of mucosal immunity.Crossref | GoogleScholarGoogle Scholar | 29716793PubMed |
Aluwong T, Kawu M, Raji M, Dzenda T, Govwang F, Sinkalu V, Ayo J (2013) Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants 2, 326–339.
| Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 26784468PubMed |
Angelakis E (2017) Weight gain by gut microbiota manipulation in productive animals. Microbial Pathogenesis 106, 162–170.
| Weight gain by gut microbiota manipulation in productive animals.Crossref | GoogleScholarGoogle Scholar | 27836763PubMed |
Annett CB, Viste JR, Chirino-Trejo M, Classen HL, Middleton DM, Simko E (2002) Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathology 31, 598–601.
| Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A.Crossref | GoogleScholarGoogle Scholar | 12593744PubMed |
Annison EF, Hill KJ, Kenworthy R (1968) Volatile fatty acids in the digestive tract of the fowl. British Poultry Science 22, 207–216.
Antonissen G, Eeckhaut V, Van Driessche K, Onrust L, Haesebrouck F, Ducatelle R, Moore RJ, Van Immerseel F (2016) Microbial shifts associated with necrotic enteritis. Avian Pathology 45, 308–312.
| Microbial shifts associated with necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 26950294PubMed |
Applegate TJ, Klose V, Steiner T, Ganner A, Schatzmayr G (2010) Probiotics and phytogenics for poultry: myth or reality. Journal of Applied Poultry Research 19, 194–210.
| Probiotics and phytogenics for poultry: myth or reality.Crossref | GoogleScholarGoogle Scholar |
Araujo R, Polycarpo G, Barbieri A, Silva K, Ventura G, Polycarpo V (2019) Performance and economic viability of broiler chickens fed with probiotic and organic acids in an attempt to replace growth-promoting antibiotics. Brazilian Journal of Poultry Science 21, eRBCA-2018–0912
Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T (2017) Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poultry Science 96, 74–82.
| Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 27486257PubMed |
Bajagai YS, Klieve AV, Dart PJ, Bryden WL (2016) ‘Probiotics in animal nutrition: production, impact and regulation. FAO Animal Production and Health paper no. 179.’ (Ed. HPS Makkar) (FAO: Rome, Italy)
Baquero F, Nombela C (2012) The microbiome as a human organ. Clinical Microbiology and Infection 18, 2–4.
| The microbiome as a human organ.Crossref | GoogleScholarGoogle Scholar | 22647038PubMed |
Barekatain R, Howarth GS, Willson N-L, Cadogan D, Wilkinson S (2020) Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens. PLoS One 15, e0237505
| Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 32790727PubMed |
Baxter MFA, Latorre JD, Dridi S, Merino-Guzman R, Hernandez-Velasco X, Hargis BM, Tellez-Isaias G (2019) Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Frontiers in Veterinary Science 6, 144
| Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model.Crossref | GoogleScholarGoogle Scholar | 31143767PubMed |
Bederska-Łojewska D, Świątkiewicz S, Arczewska-Włosek A, Schwarz T (2017) Rye non-starch polysaccharides: their impact on poultry intestinal physiology, nutrients digestibility and performance indices – a review. Annals of Animal Science 17, 351–369.
| Rye non-starch polysaccharides: their impact on poultry intestinal physiology, nutrients digestibility and performance indices – a review.Crossref | GoogleScholarGoogle Scholar |
Bedford MR (2000) Exogenous enzymes in monogastric nutrition: their current value and future benefits. Animal Feed Science and Technology 86, 1–13.
| Exogenous enzymes in monogastric nutrition: their current value and future benefits.Crossref | GoogleScholarGoogle Scholar |
Bedford MR (2018) The evolution and application of enzymes in the animal feed industry: the role of data interpretation. British Poultry Science 59, 486–493.
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Annals of Nutrition & Metabolism 61, 160–174.
| Probiotic mechanisms of action.Crossref | GoogleScholarGoogle Scholar |
Booijink CC, El-Aidy S, Rajilic-Stojanovic M, Heilig HG, Troost FJ, Smidt H, Kleerebezem M, De Vos WM, Zoetendal EG (2010) High temporal and inter-individual variation detected in the human ileal microbiota. Environmental Microbiology 12, 3213–3227.
| High temporal and inter-individual variation detected in the human ileal microbiota.Crossref | GoogleScholarGoogle Scholar | 20626454PubMed |
Borda-Molina D, Seifert J, Camarinha-Silva A (2018) Current perspectives of the chicken gastrointestinal tract and its microbiome. Computational and Structural Biotechnology Journal 16, 131–139.
| Current perspectives of the chicken gastrointestinal tract and its microbiome.Crossref | GoogleScholarGoogle Scholar | 30026889PubMed |
Broom LJ (2019) Host-microbe interactions and gut health in poultry: focus on innate responses. Microorganisms 7, 139
| Host-microbe interactions and gut health in poultry: focus on innate responses.Crossref | GoogleScholarGoogle Scholar |
Bryden WL, Li X, Ruhnke I, Zhang D, Shini S (2021) Nutrition, feeding and laying hen welfare. Animal Production Science 61, 893–914.
| Nutrition, feeding and laying hen welfare.Crossref | GoogleScholarGoogle Scholar |
Cameron C, McAllister TA (2019) Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Beneficial Microbes 10, 773–799.
| Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets?Crossref | GoogleScholarGoogle Scholar |
Camilleri M (2019) Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 68, 1516–1526.
| Leaky gut: mechanisms, measurement and clinical implications in humans.Crossref | GoogleScholarGoogle Scholar | 31076401PubMed |
Cao GT, Zeng XF, Chen AG, Zhou L, Zhang L, Xiao YP, Yang CM (2013) Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Science 92, 2949–2955.
| Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88.Crossref | GoogleScholarGoogle Scholar | 24135599PubMed |
Cao L, Wu XH, Bai YL, Wu XY, Gu SB (2019) Anti-inflammatory and antioxidant activities of probiotic powder containing Lactobacillus plantarum 1.2567 in necrotic enteritis model of broiler chickens. Livestock Science 223, 157–163.
| Anti-inflammatory and antioxidant activities of probiotic powder containing Lactobacillus plantarum 1.2567 in necrotic enteritis model of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Celi P, Verlhac V, Pérez Calvo E, Schmeisser J, Kluenter A-M (2019) Biomarkers of gastrointestinal functionality in animal nutrition and health. Animal Feed Science and Technology 250, 9–31.
| Biomarkers of gastrointestinal functionality in animal nutrition and health.Crossref | GoogleScholarGoogle Scholar |
Cengiz Ö, Köksal BH, Tatlı O, Sevim Ö, Ahsan U, Üner AG, Ulutaş PA, Beyaz D, Büyükyörük S, Yakan A, Önol AG (2015) Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poultry Science 94, 2395–2403.
| Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers.Crossref | GoogleScholarGoogle Scholar | 26240393PubMed |
Charlebois A, Jacques M, Archambault M (2014) Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Frontiers in Microbiology 5, 183
| Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials.Crossref | GoogleScholarGoogle Scholar | 24795711PubMed |
Chase DG, Erlandsen SL (1976) Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. Journal of Bacteriology 127, 572–583.
| Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum.Crossref | GoogleScholarGoogle Scholar | 931952PubMed |
Chen J, Tellez G, Richards JD, Escobar J (2015) Identification of potential biomarkers for gut barrier failure in broiler chickens. Frontiers in Veterinary Science 2, 1–10.
| Identification of potential biomarkers for gut barrier failure in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Chichlowski M, Croom J, McBride BW, Daniel L, Davis G, Koci MD (2007) Direct-fed microbial primalac and salinomycin modulate whole-body and intestinal oxygen consumption and intestinal mucosal cytokine production in the broiler chick. Poultry Science 86, 1100–1106.
| Direct-fed microbial primalac and salinomycin modulate whole-body and intestinal oxygen consumption and intestinal mucosal cytokine production in the broiler chick.Crossref | GoogleScholarGoogle Scholar | 17495079PubMed |
Choct M (2009) Managing gut health through nutrition. British Poultry Science 50, 9–15.
| Managing gut health through nutrition.Crossref | GoogleScholarGoogle Scholar | 19234925PubMed |
Coates ME (1986) The biologist’s debt to the domestic fowl. British Poultry Science 27, 3–10.
| The biologist’s debt to the domestic fowl.Crossref | GoogleScholarGoogle Scholar | 3708405PubMed |
Collado MC, Gueimonde M, Hernández M, Sanz Y, Salminen S (2005) Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. Journal of Food Protection 68, 2672–2678.
| Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion.Crossref | GoogleScholarGoogle Scholar | 16355841PubMed |
Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR (2008) Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Veterinary Immunology and Immunopathology 122, 104–115.
| Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth.Crossref | GoogleScholarGoogle Scholar | 18068809PubMed |
Cutting SM (2011) Bacillus probiotics. Food Microbiology 28, 214–220.
| Bacillus probiotics.Crossref | GoogleScholarGoogle Scholar | 21315976PubMed |
Dänicke S, Moors E, Beineke A, Gauly M (2009) Ascaridia galli infection of pullets and intestinal viscosity: consequences for nutrient retention and gut morphology. British Poultry Science 50, 512–520.
| Ascaridia galli infection of pullets and intestinal viscosity: consequences for nutrient retention and gut morphology.Crossref | GoogleScholarGoogle Scholar | 19735021PubMed |
De Cesare A, Sirri F, Manfreda G, Moniaci P, Giardini A, Zampiga M, Meluzzi A (2017) Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS One 12, e0176309
| Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 28472118PubMed |
De Meyer F, Eeckhaut V, Ducatelle R, Dhaenens M, Daled S, Dedeurwaerder A, De Gussem M, Haesebrouck F, Deforce D, Van Immerseel F (2019) Host intestinal biomarker identification in a gut leakage model in broilers. Veterinary Research 50, 46
| Host intestinal biomarker identification in a gut leakage model in broilers.Crossref | GoogleScholarGoogle Scholar | 31215487PubMed |
de Santa Barbara P, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cellular and Molecular Life Sciences: CMLS 60, 1322–1332.
| Development and differentiation of the intestinal epithelium.Crossref | GoogleScholarGoogle Scholar | 12943221PubMed |
de Simone C (2019) The unregulated probiotic market. Clinical Gastroenterology and Hepatology 17, 809–817.
| The unregulated probiotic market.Crossref | GoogleScholarGoogle Scholar | 29378309PubMed |
de Souza LFA, Araújo DN, Stefani LM, Giometti IC, Cruz-Polycarpo VC, Polycarpo G, Burbarelli MF (2018) Probiotics on performance, intestinal morphology and carcass characteristics of broiler chickens raised with lower or higher environmental challenge. Austral Journal of Veterinary Sciences 50, 35–41.
| Probiotics on performance, intestinal morphology and carcass characteristics of broiler chickens raised with lower or higher environmental challenge.Crossref | GoogleScholarGoogle Scholar |
Denbow DM (2015) Gastrointestinal anatomy and physiology. In ‘Sturkie’s Avian Physiology’. 6th edn. (Ed. CG Scanes) pp. 337–366. (Elsevier Inc.: New York, NY, USA)
Diaz Carrasco JM, Casanova NA, Fernández Miyakawa ME (2019) Microbiota, gut health and chicken productivity: what is the connection? Microorganisms 7, 374–389.
| Microbiota, gut health and chicken productivity: what is the connection?Crossref | GoogleScholarGoogle Scholar |
Ding J, Dai R, Yang L, He C, Xu K, Liu S, Zhao W, Xiao L, Luo L, Zhang Y, Meng H (2017) Inheritance and establishment of gut microbiota in chickens. Frontiers in Microbiology 8, 1967
| Inheritance and establishment of gut microbiota in chickens.Crossref | GoogleScholarGoogle Scholar | 29067020PubMed |
Drew MD, Syed NA, Goldade BG, Laarveld B, Van Kessel AG (2004) Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poultry Science 83, 414–420.
| Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 15049494PubMed |
Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F, Van Immerseel F (2018) Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Veterinary Research 49, 43
| Biomarkers for monitoring intestinal health in poultry: present status and future perspectives.Crossref | GoogleScholarGoogle Scholar | 29739469PubMed |
Dunlop MW, Moss AF, Groves PJ, Wilkinson SJ, Stuetz RM, Selle PH (2016) The multidimensional causal factors of ‘wet litter’ in chicken-meat production. The Science of the Total Environment 562, 766–776.
| The multidimensional causal factors of ‘wet litter’ in chicken-meat production.Crossref | GoogleScholarGoogle Scholar | 27110988PubMed |
Eckert NH, Lee JT, Hyatt D, Stevens SM, Anderson S, Anderson PN, Beltran R, Schatzmayr G, Mohnl M, Caldwell DJ (2010) Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. Journal of Applied Poultry Research 19, 59–67.
| Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets.Crossref | GoogleScholarGoogle Scholar |
Edens F (2003) An alternative for antibiotic use in poultry: probiotics. Brazilian Journal of Poultry Science 5, 75–97.
| An alternative for antibiotic use in poultry: probiotics.Crossref | GoogleScholarGoogle Scholar |
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Frontiers in Microbiology 8, 1490
| Bacillus as potential probiotics: status, concerns, and future perspectives.Crossref | GoogleScholarGoogle Scholar | 28848511PubMed |
Elwinger K, Schneitz C, Berndtson E, Fossum O, Teglöf B, Engstöm B (1992) Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Veterinaria Scandinavica 33, 369–378.
| Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks.Crossref | GoogleScholarGoogle Scholar | 1488953PubMed |
Elwinger K, Berndtson E, Engström B, Fossum O, Waldenstedt L (1998) Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens. Acta Veterinaria Scandinavica 39, 433–441.
| Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 9926457PubMed |
Ericsson AC, Hagan CE, Davis DJ, Franklin CL (2014) Segmented filamentous bacteria: commensal microbes with potential effects on research. Comparative Medicine 64, 90–98.
Even M, Davail S, Rey M, Tavernier A, Houssier M, Bernadet MD, Gontier K, Pascal G, Ricaud K (2018) Probiotics strains modulate gut microbiota and lipid metabolism in mule ducks. The Open Microbiology Journal 12, 71–93.
| Probiotics strains modulate gut microbiota and lipid metabolism in mule ducks.Crossref | GoogleScholarGoogle Scholar | 29755604PubMed |
FAO/WHO (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. A report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live lactic acid bacteria, Cordoba, Argentina, 1–4 October 2001, Rome, Italy. Available at http://www.fao.org/3/a-a0512e.pdf [Verified 2 November 2020]
FDA (2017) Fact sheet. Veterinary feed directive final rule and next steps. Available at https://www.fda.gov/animal-veterinary/development-approval-process/fact-sheet-veterinary-feed-directive-final-rule-and-next-steps [Verified October 2020]
Flannigan KL, Denning TL (2018) Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity. Immunology 154, 537–546.
| Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity.Crossref | GoogleScholarGoogle Scholar |
Forssten S, Ouwehand CA (2020) Dose–response recovery of probiotic strains in simulated gastro-intestinal passage. Microorganisms 8, 112
| Dose–response recovery of probiotic strains in simulated gastro-intestinal passage.Crossref | GoogleScholarGoogle Scholar |
Forte C, Acuti G, Manuali E, Casagrande Proietti P, Pavone S, Trabalza-Marinucci M, Moscati L, Onofri A, Lorenzetti C, Franciosini MP (2016) Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poultry Science 95, 2528–2535.
| Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens.Crossref | GoogleScholarGoogle Scholar | 27143778PubMed |
Furness JB, Cottrell JJ (2017) Signalling from the gut lumen. Animal Production Science 57, 2175–2187.
| Signalling from the gut lumen.Crossref | GoogleScholarGoogle Scholar |
Gadde U, Kim WH, Oh ST, Lillehoj HS (2017) Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Animal Health Research Reviews 18, 26–45.
| Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review.Crossref | GoogleScholarGoogle Scholar | 28485263PubMed |
Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, Bonfiglio G, Trancassini M, Passariello C, Pantanella F, Schippa S (2018) Rebuilding the gut microbiota ecosystem. International Journal of Environmental Research and Public Health 15, 1679
| Rebuilding the gut microbiota ecosystem.Crossref | GoogleScholarGoogle Scholar |
Garner CE, Smith S, Elviss NC, Humphrey TJ, White P, Ratcliffe NM, Probert CS (2008) Identification of Campylobacter infection in chickens from volatile faecal emissions. Biomarkers 13, 413–421.
| Identification of Campylobacter infection in chickens from volatile faecal emissions.Crossref | GoogleScholarGoogle Scholar | 18484355PubMed |
Gerritsen J, Smidt H, Rijkers GT, de Vos WM (2011) Intestinal microbiota in human health and disease: the impact of probiotics. Genes & Nutrition 6, 209–240.
| Intestinal microbiota in human health and disease: the impact of probiotics.Crossref | GoogleScholarGoogle Scholar |
Gholamiandehkordi AR, Timbermont L, Lanckriet A, Van Den Broeck W, Pedersen K, Dewulf J, Pasmans F, Haesebrouck F, Ducatelle R, Van Immerseel F (2007) Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathology 36, 375–382.
| Quantification of gut lesions in a subclinical necrotic enteritis model.Crossref | GoogleScholarGoogle Scholar | 17899461PubMed |
Gomide MH, Sterzo EV, Macari M, Boleli IC (2004) Use of scanning electron microscopy for the evaluation of intestinal epithelium integrity. Brasilian Journal of Animal Science 33, 1500–1505.
Goodwin MA, Cooper GL, Brown J, Bickford AA, Waltman WD, Dickson TG (1991) Clinical, pathological, and epizootiological features of long-segmented filamentous organisms (bacteria, LSFOs) in the small intestines of chickens, turkeys, and quails. Avian Diseases 35, 872–876.
| Clinical, pathological, and epizootiological features of long-segmented filamentous organisms (bacteria, LSFOs) in the small intestines of chickens, turkeys, and quails.Crossref | GoogleScholarGoogle Scholar | 1786018PubMed |
Goossens E, Debyser G, Callens C, De Gussem M, Dedeurwaerder A, Devreese B, Haesebrouck F, Flügel M, Pelzer S, Thiemann F, Ducatelle R, Van Immerseel F (2018) Elevated faecal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens. Veterinary Research 49, 51
| Elevated faecal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 29925427PubMed |
Haberecht S, Bajagai YS, Moore RJ, Van TTH, Stanley D (2020) Poultry feeds carry diverse microbial communities that influence chicken intestinal microbiota colonisation and maturation. AMB Express 10, 143
| Poultry feeds carry diverse microbial communities that influence chicken intestinal microbiota colonisation and maturation.Crossref | GoogleScholarGoogle Scholar | 32803529PubMed |
Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S (2008) Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Veterinary Microbiology 126, 225–233.
| Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection.Crossref | GoogleScholarGoogle Scholar | 17681719PubMed |
Hedblom GA, Reiland HA, Sylte MJ, Johnson TJ, Baumler DJ (2018) Segmented filamentous bacteria: metabolism meets immunity. Frontiers in Microbiology 9, 1991
| Segmented filamentous bacteria: metabolism meets immunity.Crossref | GoogleScholarGoogle Scholar | 30197636PubMed |
Hermans PG, Morgan KL (2003) The epidemiology of necrotic enteritis in broiler chickens Research in Veterinary Science 74, 19
| The epidemiology of necrotic enteritis in broiler chickensCrossref | GoogleScholarGoogle Scholar |
Higgins JP, Higgins SE, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Vicente JL, Hargis BM, Tellez G (2010) Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella enteritidis in neonatal broilers. Poultry Science 89, 243–247.
| Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella enteritidis in neonatal broilers.Crossref | GoogleScholarGoogle Scholar | 20075275PubMed |
Huff GR, Huff WE, Rath NC, El-Gohary FA, Zhou ZY, Shini S (2015) Efficacy of a novel prebiotic and a commercial probiotic in reducing mortality and production losses due to cold stress and Escherichia coli challenge of broiler chicks. Poultry Science 94, 918–926.
| Efficacy of a novel prebiotic and a commercial probiotic in reducing mortality and production losses due to cold stress and Escherichia coli challenge of broiler chicks.Crossref | GoogleScholarGoogle Scholar | 25743418PubMed |
Islam SU (2016) Clinical Uses of Probiotics. Medicine 95, e2658
| Clinical Uses of Probiotics.Crossref | GoogleScholarGoogle Scholar | 26844491PubMed |
Ivanov II, Littman DR (2010) Segmented filamentous bacteria take the stage. Mucosal Immunology 3, 209–212.
| Segmented filamentous bacteria take the stage.Crossref | GoogleScholarGoogle Scholar | 20147894PubMed |
Jackson DN, Theiss AL (2020) Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 11, 285–304.
| Gut bacteria signaling to mitochondria in intestinal inflammation and cancer.Crossref | GoogleScholarGoogle Scholar | 30913966PubMed |
Jesse LG (2004) Alternative litter materials for growing poultry. North Carolina Poultry Industry Newsletter 1, 1–5.
Jeurissen SH, Lewis F, van der Klis JD, Mroz Z, Rebel JM, ter Huurne AA (2002) Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Current Issues in Intestinal Microbiology 3, 1–14.
Jia W, Slominski BA, Bruce HL, Blank G, Crow G, Jones O (2009) Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poultry Science 88, 132–140.
| Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge.Crossref | GoogleScholarGoogle Scholar | 19096067PubMed |
Johansson MEV, Sjövall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. Nature Reviews. Gastroenterology & Hepatology 10, 352–361.
| The gastrointestinal mucus system in health and disease.Crossref | GoogleScholarGoogle Scholar |
Jou TS, Schneeberger EE, Nelson WJ (1998) Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. The Journal of Cell Biology 142, 101–115.
| Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases.Crossref | GoogleScholarGoogle Scholar | 9660866PubMed |
Kaldhusdal M, Hofshagen M (1992) Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poultry Science 71, 1145–1153.
| Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1641378PubMed |
Kaldhusdal M, Skjerve E (1996) Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway. Preventive Veterinary Medicine 28, 1–16.
| Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway.Crossref | GoogleScholarGoogle Scholar |
Kaldhusdal M, Evensen Ø, Landsverk T (1995) Clostridium perfringens necrotizing enteritis of the fowl: a light microscopic, immunohistochemical and ultrastructural study of spontaneous disease. Avian Pathology 24, 421–433.
| Clostridium perfringens necrotizing enteritis of the fowl: a light microscopic, immunohistochemical and ultrastructural study of spontaneous disease.Crossref | GoogleScholarGoogle Scholar | 18645799PubMed |
Kaldhusdal M, Benestad SL, Løvland A (2016) Epidemiologic aspects of necrotic enteritis in broiler chickens: disease occurrence and production performance. Avian Pathology 45, 271–274.
| Epidemiologic aspects of necrotic enteritis in broiler chickens: disease occurrence and production performance.Crossref | GoogleScholarGoogle Scholar | 26956946PubMed |
Kang J, Pervaiz S (2012) Mitochondria: redox metabolism and dysfunction. Biochemistry Research International 2012, 896751
| Mitochondria: redox metabolism and dysfunction.Crossref | GoogleScholarGoogle Scholar | 22593827PubMed |
Karimi Torshizi MA, Moghaddam AR, Rahimi S, Mojgani N (2010) Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. British Poultry Science 51, 178–184.
| Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response.Crossref | GoogleScholarGoogle Scholar | 20461578PubMed |
Kastl AJ, Terry NA, Wu GD, Albenberg LG (2020) The structure and function of the human small intestinal microbiota: current understanding and future directions. Cellular and Molecular Gastroenterology and Hepatology 9, 33–45.
| The structure and function of the human small intestinal microbiota: current understanding and future directions.Crossref | GoogleScholarGoogle Scholar | 31344510PubMed |
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM (2013) Health benefits of probiotics: a review. ISRN Nutrition 2013, 481651
| Health benefits of probiotics: a review.Crossref | GoogleScholarGoogle Scholar | 24959545PubMed |
Kim GB, Seo YM, Kim CH, Paik IK (2011) Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poultry Science 90, 75–82.
| Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers.Crossref | GoogleScholarGoogle Scholar | 21177446PubMed |
Kjekshus J (2015) Inflammation: friend and foe. EBioMedicine 2, 634–635.
| Inflammation: friend and foe.Crossref | GoogleScholarGoogle Scholar | 26288833PubMed |
Klaasen HLBM, Koopman JP, Poelma FGJ, Beynen AC (1992) Intestinal, segmented, filamentous bacteria FEMS Microbiology Reviews 8, 165–179.
| Intestinal, segmented, filamentous bacteriaCrossref | GoogleScholarGoogle Scholar |
Knudsen KEB (2014) Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Science 93, 2380–2393.
| Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets.Crossref | GoogleScholarGoogle Scholar |
Kogut MH (2019) The effect of microbiome modulation on the intestinal health of poultry. Animal Feed Science and Technology 250, 32–40.
| The effect of microbiome modulation on the intestinal health of poultry.Crossref | GoogleScholarGoogle Scholar |
Kogut MH, Arsenault RJ (2016) Editorial: Gut Health: the new paradigm in food animal production. Frontiers in Veterinary Science 3, 71
| Editorial: Gut Health: the new paradigm in food animal production.Crossref | GoogleScholarGoogle Scholar | 27630994PubMed |
Kogut MH, Genovese KJ, Swaggerty CL, He H, Broom L (2018) Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poultry Science 97, 2339–2346.
| Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal.Crossref | GoogleScholarGoogle Scholar | 29618086PubMed |
Kollarcikova M, Kubasova T, Karasova D, Crhanova M, Cejkova D, Sisak F, Rychlik I (2019) Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poultry Science 98, 2347–2353.
| Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota.Crossref | GoogleScholarGoogle Scholar | 30624758PubMed |
Kraimi N, Dawkins M, Gebhardt-Henrich SG, Velge P, Rychlik I, Volf J, Creach P, Smith A, Colles F, Leterrier C (2019) Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: a review. Physiology & Behavior 210, 112658
| Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: a review.Crossref | GoogleScholarGoogle Scholar |
Kumar S, Chen C, Indugu N, Werlang GO, Singh M, Kim WK, Thippareddi H (2018) Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS One 13, e0192450
| Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens.Crossref | GoogleScholarGoogle Scholar | 30576333PubMed |
La Fata G, Weber P, Mohajeri MH (2018) Probiotics and the gut immune system: indirect regulation. Probiotics and Antimicrobial Proteins 10, 11–21.
| Probiotics and the gut immune system: indirect regulation.Crossref | GoogleScholarGoogle Scholar | 28861741PubMed |
Latorre JD, Hernandez-Velasco X, Bielke LR, Vicente JL, Wolfenden R, Menconi A, Hargis BM, Tellez G (2015) Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. British Poultry Science 56, 723–732.
| Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet.Crossref | GoogleScholarGoogle Scholar | 26539833PubMed |
Lee WJ, Hase K (2014) Gut microbiota-generated metabolites in animal health and disease. Nature Chemical Biology 10, 416–424.
| Gut microbiota-generated metabolites in animal health and disease.Crossref | GoogleScholarGoogle Scholar | 24838170PubMed |
Lee KW, Lee SH, Lillehoj HS, Li GX, Jang SI, Babu US, Park MS, Kim DK, Lillehoj EP, Neumann AP, Rehberger TG, Siragusa GR (2010) Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poultry Science 89, 203–216.
| Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 20075271PubMed |
Lei K, Li YL, Yu DY, Rajput IR, Li WF (2013) Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poultry Science 92, 2389–2395.
| Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens.Crossref | GoogleScholarGoogle Scholar | 23960122PubMed |
Lescheid D (2014) Probiotics as regulators of inflammation: a review. Functional Foods in Health and Disease 4, 299–311.
| Probiotics as regulators of inflammation: a review.Crossref | GoogleScholarGoogle Scholar |
Li X, Zhang D, Bryden WL (2017) Calcium and phosphorus metabolism and nutrition of poultry: are current diets formulated in excess? Animal Production Science 57, 2304–2310.
| Calcium and phosphorus metabolism and nutrition of poultry: are current diets formulated in excess?Crossref | GoogleScholarGoogle Scholar |
Liao N, Yin Y, Sun G, Xiang C, Liu D, Yu HD, Wang X (2012) Colonization and distribution of segmented filamentous bacteria (SFB) in chicken gastrointestinal tract and their relationship with host immunity. FEMS Microbiology Ecology 81, 395–406.
| Colonization and distribution of segmented filamentous bacteria (SFB) in chicken gastrointestinal tract and their relationship with host immunity.Crossref | GoogleScholarGoogle Scholar | 22429007PubMed |
Liu Y, Fatheree NY, Mangalat N, Rhoads JM (2010) Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. American Journal of Physiology. Gastrointestinal and Liver Physiology 299, G1087–G1096.
| Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation.Crossref | GoogleScholarGoogle Scholar | 20798357PubMed |
Lloyd AB, Cumming RB, Kent RD (1977) Prevention of Salmonella typhimurium infection in poultry by pretreatment of chickens and poults with intestinal extracts. Australian Veterinary Journal 53, 82–87.
| Prevention of Salmonella typhimurium infection in poultry by pretreatment of chickens and poults with intestinal extracts.Crossref | GoogleScholarGoogle Scholar | 324465PubMed |
Long JR, Truscott RB (1976) Necrotic enteritis in broiler chickens. III. Reproduction of the disease. Canadian Journal of Comparative Medicine 40, 53–59.
Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD (2003) Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Applied and Environmental Microbiology 69, 6816–6824.
| Diversity and succession of the intestinal bacterial community of the maturing broiler chicken.Crossref | GoogleScholarGoogle Scholar | 14602645PubMed |
Lutful Kabir SM (2009) The role of probiotics in the poultry industry. International Journal of Molecular Sciences 10, 3531–3546.
| The role of probiotics in the poultry industry.Crossref | GoogleScholarGoogle Scholar | 20111681PubMed |
Macfarlane GT, Macfarlane S (2012) Bacteria, colonic fermentation, and gastrointestinal health. Journal of AOAC International 95, 50–60.
| Bacteria, colonic fermentation, and gastrointestinal health.Crossref | GoogleScholarGoogle Scholar | 22468341PubMed |
Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. The American Journal of Physiology 276, G941–G950.
MarketsandMarkets (2019) Probiotics in animal feed market by livestock (poultry, ruminants, swine, aquaculture, pets), source (bacteria [Lactobacilli, Streptococcus thermophilus, Bifidobacteria] and yeast & fungi), form (dry and liquid), and region: global forecast to 2025. Report. Available at https://www.marketsandmarkets.com/Market-Reports/probiotics-animal-feed-market-85832335.html [Verified 2 December 2020]
Martin Manuel P, Elena B, Carolina MG, Gabriela P (2017) Oral probiotics supplementation can stimulate the immune system in a stress process. Journal of Nutrition & Intermediary Metabolism 8, 29–40.
| Oral probiotics supplementation can stimulate the immune system in a stress process.Crossref | GoogleScholarGoogle Scholar |
Menconi A, Wolfenden AD, Shivaramaiah S, Terraes JC, Urbano T, Kuttel J, Kremer C, Hargis BM, Tellez G (2011) Effect of lactic acid bacteria probiotic culture for the treatment of Salmonella enterica serovar Heidelberg in neonatal broiler chickens and turkey poults. Poultry Science 90, 561–565.
| Effect of lactic acid bacteria probiotic culture for the treatment of Salmonella enterica serovar Heidelberg in neonatal broiler chickens and turkey poults.Crossref | GoogleScholarGoogle Scholar | 21325226PubMed |
Minelli EB, Benini A (2008) Relationship between number of bacteria and their probiotic effects. Microbial Ecology in Health and Disease 20, 180–183.
| Relationship between number of bacteria and their probiotic effects.Crossref | GoogleScholarGoogle Scholar |
Morelli L, Capurso L (2012) FAO/WHO Guidelines on probiotics: 10 years later. Journal of Clinical Gastroenterology 46, S1–S2.
| FAO/WHO Guidelines on probiotics: 10 years later.Crossref | GoogleScholarGoogle Scholar | 22955349PubMed |
Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K (2010) Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poultry Science 89, 58–67.
| Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition.Crossref | GoogleScholarGoogle Scholar | 20008803PubMed |
Mountzouris KC, Palamidi I, Paraskeuas V, Griela E, Fegeros K (2019) Dietary probiotic form modulates broiler gut microbiota indices and expression of gut barrier genes including essential components for gut homeostasis. Journal of Animal Physiology and Animal Nutrition 103, 1143–1159.
| Dietary probiotic form modulates broiler gut microbiota indices and expression of gut barrier genes including essential components for gut homeostasis.Crossref | GoogleScholarGoogle Scholar | 31087706PubMed |
Mutuş R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS (2006) The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poultry Science 85, 1621–1625.
| The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers.Crossref | GoogleScholarGoogle Scholar | 16977848PubMed |
Naidu AS, Bidlack WR, Clemens RA (1999) Probiotic spectra of lactic acid bacteria (LAB). Critical Reviews in Food Science and Nutrition 39, 13–126.
| Probiotic spectra of lactic acid bacteria (LAB).Crossref | GoogleScholarGoogle Scholar | 10028126PubMed |
Niewold TA (2007) The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action: a hypothesis. Poultry Science 86, 605–609.
| The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action: a hypothesis.Crossref | GoogleScholarGoogle Scholar | 17369528PubMed |
Nurmi E, Rantala M (1973) New aspects of Salmonella infection in broiler production. Nature 241, 210–211.
| New aspects of Salmonella infection in broiler production.Crossref | GoogleScholarGoogle Scholar | 4700893PubMed |
Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, Lee MD, Collett SR, Johnson TJ, Cox NA (2014) The chicken gastrointestinal microbiome. FEMS Microbiology Letters 360, 100–112.
| The chicken gastrointestinal microbiome.Crossref | GoogleScholarGoogle Scholar | 25263745PubMed |
Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Laarveld B, Sawicki G (2008) Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Research in Veterinary Science 85, 543–553.
| Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue.Crossref | GoogleScholarGoogle Scholar | 18359497PubMed |
Olnood CG, Beski SSM, Choct M, Iji PA (2015a) Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Animal Nutrition 1, 184–191.
| Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 29767136PubMed |
Olnood CG, Beski SSM, Iji PA, Choct M (2015b) Delivery routes for probiotics: effects on broiler performance, intestinal morphology and gut microflora. Animal Nutrition 1, 192–202.
| Delivery routes for probiotics: effects on broiler performance, intestinal morphology and gut microflora.Crossref | GoogleScholarGoogle Scholar | 29767168PubMed |
Palamidi I, Fegeros K, Mohnl M, Abdelrahman WHA, Schatzmayr G, Theodoropoulos G, Mountzouris KC (2016) Probiotic form effects on growth performance, digestive function, and immune related biomarkers in broilers. Poultry Science 95, 1598–1608.
| Probiotic form effects on growth performance, digestive function, and immune related biomarkers in broilers.Crossref | GoogleScholarGoogle Scholar | 26944970PubMed |
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5, 108–119.
| Intestinal microbiome of poultry and its interaction with host and diet.Crossref | GoogleScholarGoogle Scholar | 24256702PubMed |
Parish WE (1961) Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii. Journal of Comparative Pathology 71, 377–393.
| Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii.Crossref | GoogleScholarGoogle Scholar | 14483884PubMed |
Park JH, Kim IH (2015) The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium. Journal of Animal Physiology and Animal Nutrition 99, 326–334.
| The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium.Crossref | GoogleScholarGoogle Scholar | 25244020PubMed |
Park SS, Lillehoj HS, Allen PC, Park DW, FitzCoy S, Bautista DA, Lillehoje EP (2008) Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Diseases 52, 14–22.
| Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 18459290PubMed |
Park JW, Jeong JS, Lee SI, Kim IH (2016) Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poultry Science 95, 2829–2835.
| Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens.Crossref | GoogleScholarGoogle Scholar | 27422665PubMed |
Park I, Lee Y, Goo D, Zimmerman NP, Smith AH, Rehberger T, Lillehoj HS (2020) The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poultry Science 99, 725–733.
| The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima.Crossref | GoogleScholarGoogle Scholar | 32036975PubMed |
Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, Padilla J, Akmel A, Bhatti J, Rahaman SO, Biswas D (2020) Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Comprehensive Reviews in Food Science and Food Safety 19, 1908–1933.
| Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods.Crossref | GoogleScholarGoogle Scholar | 33337097PubMed |
Pereira R, Bortoluzzi C, Durrer A, Fagundes NS, Pedroso AA, Rafael JM, Perim JEdL, Zavarize KC, Napty GS, Andreote FD, Costa DP, Menten JFM (2019) Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. Journal of Animal Physiology and Animal Nutrition 103, 72–86.
| Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy.Crossref | GoogleScholarGoogle Scholar | 30485573PubMed |
Persichetti E, De Michele A, Codini M, Traina G (2014) Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 30, 936–938.
| Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay.Crossref | GoogleScholarGoogle Scholar | 24985014PubMed |
Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A (2017) Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 9, 555
| Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases.Crossref | GoogleScholarGoogle Scholar |
Pluske JR, Miller DW, Sterndale SO, Turpin DL (2019) Associations between gastrointestinal-tract function and the stress response after weaning in pigs. Animal Production Science 59, 2015–2022.
| Associations between gastrointestinal-tract function and the stress response after weaning in pigs.Crossref | GoogleScholarGoogle Scholar |
Prado-Rebolledo OF, Delgado-Machuca JdJ, Macedo-Barragan RJ, Garcia-Márquez LJ, Morales-Barrera JE, Latorre JD, Hernandez-Velasco X, Tellez G (2017) Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens. Avian Pathology 46, 90–94.
| Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 27545145PubMed |
Prescott JF, Sivendra R, Barnum DA (1978) The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. The Canadian Veterinary Journal 19, 181–183.
Quigley EM (2016) Leaky gut: concept or clinical entity? Current Opinion in Gastroenterology 32, 74–79.
| Leaky gut: concept or clinical entity?Crossref | GoogleScholarGoogle Scholar | 26760399PubMed |
Rao RK, Samak G (2013) Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Current Nutrition and Food Science 9, 99–107.
| Protection and restitution of gut barrier by probiotics: nutritional and clinical implications.Crossref | GoogleScholarGoogle Scholar | 24353483PubMed |
Ravindran V (2013) Feed enzymes: the science, practice, and metabolic realities. Journal of Applied Poultry Research 22, 628–636.
| Feed enzymes: the science, practice, and metabolic realities.Crossref | GoogleScholarGoogle Scholar |
Riddell C, Kong XM (1992) The influence of diet on necrotic enteritis in broiler chickens. Avian Diseases 36, 499–503.
| The influence of diet on necrotic enteritis in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 1417581PubMed |
Rodgers NJ, Swick RA, Geier MS, Moore RJ, Choct M, Wu SB (2015) A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Diseases 59, 38–45.
| A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 26292532PubMed |
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K (2018) Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition 57, 1–24.
| Gut microbiota functions: metabolism of nutrients and other food components.Crossref | GoogleScholarGoogle Scholar | 28393285PubMed |
Rubartelli A, Lotze MT, Latz E, Manfredi A (2013) Mechanisms of sterile inflammation. Frontiers in Immunology 4, 398
| Mechanisms of sterile inflammation.Crossref | GoogleScholarGoogle Scholar | 24319446PubMed |
Rychlik I (2020) Composition and function of chicken gut microbiota. Animals (Basel) 10, 103
| Composition and function of chicken gut microbiota.Crossref | GoogleScholarGoogle Scholar |
Salim HM, Kang HK, Akter N, Kim DW, Kim JH, Kim MJ, Na JC, Jong HB, Choi HC, Suh OS, Kim WK (2013) Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poultry Science 92, 2084–2090.
| Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 23873556PubMed |
Scanes CG, Pierzchala-Koziec K (2014) Biology of the gastrointestinal tract in poultry. Avian Biology Research 7, 193–222.
| Biology of the gastrointestinal tract in poultry.Crossref | GoogleScholarGoogle Scholar |
Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, Littman DR, Ivanov II (2011) The genome of Th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host & Microbe 10, 260–272.
| The genome of Th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment.Crossref | GoogleScholarGoogle Scholar |
Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL (2007) Resolution of inflammation: state of the art, definitions and terms. The FASEB Journal 21, 325–332.
| Resolution of inflammation: state of the art, definitions and terms.Crossref | GoogleScholarGoogle Scholar | 17267386PubMed |
Shang Y, Kumar S, Oakley B, Kim WK (2018) Chicken gut microbiota: importance and detection technology. Frontiers in Veterinary Science 5, 254
| Chicken gut microbiota: importance and detection technology.Crossref | GoogleScholarGoogle Scholar | 30406117PubMed |
Shini S, Shini A, Blackall PJ (2013) The potential for probiotics to prevent reproductive tract lesions in free-range laying hens. Animal Production Science 53, 1298–1308.
| The potential for probiotics to prevent reproductive tract lesions in free-range laying hens.Crossref | GoogleScholarGoogle Scholar |
Shini S, Aland RC, Bryden WL (2019) The abundance and morphology of mitochondria in enterocytes of chickens exposed to necrotic enteritis and treated with a probiotic. In ‘Proceedings of the 7th International Conference on Poultry Intestinal Health’, 3–5 April 2019, Rome, Italy. p. 242.
Shini S, Aland RC, Dart PJ, Callaghan MJ, Speight RE, Bryden WL (2020a) Ultrastructural changes in the ileal mucosa of broilers exposed to necrotic enteritis and Bacillus amyloliquefaciens H57. In ‘Proceedings of the 31st Annual Australian Poultry Science Symposium’, 16–19 February 2020, Sydney, Australia. p. 130. (Poultry Research Foundation, University of Sydney: Sydney, NSW, Australia)
Shini S, Zhang D, Aland RC, Li X, Dart PJ, Callaghan MJ, Speight RE, Bryden WL (2020b) Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poultry Science 99, 4278–4293.
| Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency.Crossref | GoogleScholarGoogle Scholar | 32867972PubMed |
Shini S, Aland RC, Bryden WL (2021) Avian intestinal ultrastructure changes provide insight into the pathogenesis of enteric diseases and probiotic mode of action. Scientific Reports 11, 167
| Avian intestinal ultrastructure changes provide insight into the pathogenesis of enteric diseases and probiotic mode of action.Crossref | GoogleScholarGoogle Scholar | 33420315PubMed |
Skinner JT, Bauer S, Young V, Pauling G, Wilson J (2010) An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Diseases 54, 1237–1240.
| An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 21313845PubMed |
Smith J (2014) A review of avian probiotics. Journal of Avian Medicine and Surgery 28, 87–94.
| A review of avian probiotics.Crossref | GoogleScholarGoogle Scholar | 25115036PubMed |
Smyth JA (2016) Pathology and diagnosis of necrotic enteritis: is it clear-cut? Avian Pathology 45, 282–287.
| Pathology and diagnosis of necrotic enteritis: is it clear-cut?Crossref | GoogleScholarGoogle Scholar | 26981703PubMed |
Soerjadi AS, Lloyd AB, Cumming RB (1978) Streptococcus faecalis, a bacterial isolate which protects young chickens from enteric invasion by Salmonella. Australian Veterinary Journal 54, 549–550.
| Streptococcus faecalis, a bacterial isolate which protects young chickens from enteric invasion by Salmonella.Crossref | GoogleScholarGoogle Scholar | 111659PubMed |
Stanley D, Hughes RJ, Moore RJ (2014) Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Applied Microbiology and Biotechnology 98, 4301–4310.
| Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease.Crossref | GoogleScholarGoogle Scholar | 24643736PubMed |
Sun J, Wang Y, Li N, Zhong H, Xu H, Zhu Q, Liu Y (2018) Comparative analysis of the gut microbial composition and meat flavor of two chicken breeds in different rearing patterns. BioMed Research International 2018, 4343196
| Comparative analysis of the gut microbial composition and meat flavor of two chicken breeds in different rearing patterns.Crossref | GoogleScholarGoogle Scholar | 30643812PubMed |
Swaggerty CL, Callaway TR, Kogut MH, Piva A, Grilli E (2019) Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms 7, 65
| Modulation of the immune response to improve health and reduce foodborne pathogens in poultry.Crossref | GoogleScholarGoogle Scholar |
Tanabe S (2013) The effect of probiotics and gut microbiota on Th17 cells. International Reviews of Immunology 32, 511–525.
| The effect of probiotics and gut microbiota on Th17 cells.Crossref | GoogleScholarGoogle Scholar | 24094077PubMed |
Tarradas J, Tous N, Esteve-Garcia E, Brufau AJ (2020) The control of intestinal inflammation: a major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry. Microorganisms 8, 148
| The control of intestinal inflammation: a major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry.Crossref | GoogleScholarGoogle Scholar |
Teng P-Y, Kim WK (2018) Review: roles of prebiotics in intestinal ecosystem of broilers. Frontiers in Veterinary Science 5, 245
| Review: roles of prebiotics in intestinal ecosystem of broilers.Crossref | GoogleScholarGoogle Scholar | 30425993PubMed |
Teshfam M, Rahbari S (2003) Alteration in small intestinal structure induced by experimental subclinical coccidiosis in chicken. Journal of Applied Animal Research 24, 33–39.
| Alteration in small intestinal structure induced by experimental subclinical coccidiosis in chicken.Crossref | GoogleScholarGoogle Scholar |
Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA (2019) Intestinal absorption of bile acids in health and disease. Comprative Physiology 10, 21–56.
| Intestinal absorption of bile acids in health and disease.Crossref | GoogleScholarGoogle Scholar |
Tomaszewska E, Kwiecień M, Dobrowolski P, Klebaniuk R, Muszyński S, Olcha M, Blicharski T, Grela ER (2018) Dose-dependent effects of probiotic supplementation on bone characteristics and mineralisation in meat-type female turkeys. Animal Production Science 58, 507–516.
| Dose-dependent effects of probiotic supplementation on bone characteristics and mineralisation in meat-type female turkeys.Crossref | GoogleScholarGoogle Scholar |
Turk DE (1972) Protozoan parasitic infections of the chick intestine and protein digestion and absorption. The Journal of Nutrition 102, 1217–1221.
| Protozoan parasitic infections of the chick intestine and protein digestion and absorption.Crossref | GoogleScholarGoogle Scholar | 5057208PubMed |
Tytgat HLP, Nobrega FL, van der Oost J, de Vos WM (2019) Bowel biofilms: tipping points between a healthy and compromised gut? Trends in Microbiology 27, 17–25.
| Bowel biofilms: tipping points between a healthy and compromised gut?Crossref | GoogleScholarGoogle Scholar | 30219265PubMed |
Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of Nutrition 141, 769–776.
| Regulation of tight junction permeability by intestinal bacteria and dietary components.Crossref | GoogleScholarGoogle Scholar | 21430248PubMed |
Vancamelbeke M, Vermeire S (2017) The intestinal barrier: a fundamental role in health and disease. Expert Review of Gastroenterology & Hepatology 11, 821–834.
| The intestinal barrier: a fundamental role in health and disease.Crossref | GoogleScholarGoogle Scholar |
Villageliũ DN, Lyte M (2017) Microbial endocrinology: why the intersection of microbiology and neurobiology matters to poultry health. Poultry Science 96, 2501–2508.
| Microbial endocrinology: why the intersection of microbiology and neurobiology matters to poultry health.Crossref | GoogleScholarGoogle Scholar | 29050443PubMed |
Wang Z, Yu Q, Fu J, Liang J, Yang Q (2013) Immune responses of chickens inoculated with recombinant Lactobacillus expressing the haemagglutinin of the avian influenza virus. Journal of Applied Microbiology 115, 1269–1277.
| Immune responses of chickens inoculated with recombinant Lactobacillus expressing the haemagglutinin of the avian influenza virus.Crossref | GoogleScholarGoogle Scholar | 23937220PubMed |
Wang S, Zeng X, Yang Q, Qiao S (2016) Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. International Journal of Molecular Sciences 17, 603
| Antimicrobial peptides as potential alternatives to antibiotics in food animal industry.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Wu Y, Wang Y, Fu A, Gong L, Li W, Li Y (2017) Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Applied Microbiology and Biotechnology 101, 3015–3026.
| Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production.Crossref | GoogleScholarGoogle Scholar | 27957629PubMed |
Wang J, Ishfaq M, Guo Y, Chen C, Li J (2020) Assessment of probiotic properties of Lactobacillus salivarius isolated from chickens as feed additives. Frontiers in Veterinary Science 7, 415
| Assessment of probiotic properties of Lactobacillus salivarius isolated from chickens as feed additives.Crossref | GoogleScholarGoogle Scholar | 32766298PubMed |
Watkins BA, Kratzer FH (1983) Effect of oral dosing of Lactobacillus strains on gut colonization and liver biotin in broiler chicks. Poultry Science 62, 2088–2094.
| Effect of oral dosing of Lactobacillus strains on gut colonization and liver biotin in broiler chicks.Crossref | GoogleScholarGoogle Scholar | 6415641PubMed |
Watnick PI, Jugder B-E (2020) Microbial control of intestinal homeostasis via enteroendocrine cell innate immune signalling. Trends in Microbiology 28, 141–149.
| Microbial control of intestinal homeostasis via enteroendocrine cell innate immune signalling.Crossref | GoogleScholarGoogle Scholar | 31699645PubMed |
Weese JS, Martin H (2011) Assessment of commercial probiotic bacterial contents and label accuracy. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne 52, 43–46.
Wideman RF, Hamal KR, Stark JM, Blankenship J, Lester H, Mitchell KN, Lorenzoni G, Pevzner I (2012) A wire-flooring model for inducing lame-ness in broilers: evaluation of probiotics as a prophylactic treatment. Poultry Science 91, 870–883.
| A wire-flooring model for inducing lame-ness in broilers: evaluation of probiotics as a prophylactic treatment.Crossref | GoogleScholarGoogle Scholar | 22399726PubMed |
Wilkins T, Sequoia J (2017) Probiotics for gastrointestinal conditions: a summary of the evidence. American Family Physician 96, 170–178.
Williams RB (2005) Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathology 34, 159–180.
| Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity.Crossref | GoogleScholarGoogle Scholar | 16191699PubMed |
Witlock DR, Ruff MD (1977) Comparison of the intestinal surface damage caused by Eimeria mivati, E. necatrix, E. maxima, E. brunetti, and E. acervulina by scanning electron microscopy. The Journal of Parasitology 63, 193–199.
| Comparison of the intestinal surface damage caused by Eimeria mivati, E. necatrix, E. maxima, E. brunetti, and E. acervulina by scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar | 859075PubMed |
Wu SB, Rodgers N, Choct M (2010) Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Diseases 54, 1058–1065.
| Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 20945788PubMed |
Wu BQ, Zhang T, Guo LQ, Lin JF (2011) Effects of Bacillus subtilis KD1 on broiler intestinal flora. Poultry Science 90, 2493–2499.
| Effects of Bacillus subtilis KD1 on broiler intestinal flora.Crossref | GoogleScholarGoogle Scholar | 22010234PubMed |
Wu QJ, Zhou YM, Wu YN, Zhang LL, Wang T (2013) The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Veterinary Immunology and Immunopathology 153, 70–76.
| The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 23453767PubMed |
Wu SB, Stanley D, Rodgers N, Swick RA, Moore RJ (2014) Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Veterinary Microbiology 169, 188–197.
| Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 24522272PubMed |
Xiang Q, Wang C, Zhang H, Lai W, Wei H, Peng J (2019) Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals (Basel) 9, 1110
| Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens.Crossref | GoogleScholarGoogle Scholar |
Yadav S, Jha R (2019) Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. Journal of Animal Science and Biotechnology 10, 2
| Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry.Crossref | GoogleScholarGoogle Scholar | 30651986PubMed |
Yan FF, Mohammed AA, Murugesan GR, Cheng HW (2019) Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poultry Science 98, 1083–1089.
| Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes.Crossref | GoogleScholarGoogle Scholar | 30476217PubMed |
Zaghari M, Sarani P, Hajati H (2020) Comparison of two probiotic preparations on growth performance, intestinal microbiota, nutrient digestibility and cytokine gene expression in broiler chickens. Journal of Applied Animal Research 48, 166–175.
| Comparison of two probiotic preparations on growth performance, intestinal microbiota, nutrient digestibility and cytokine gene expression in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Zarei A, Lavvaf A, Motamedi Motlagh M (2018) Effects of probiotic and whey powder supplementation on growth performance, microflora population, and ileum morphology in broilers. Journal of Applied Animal Research 46, 840–844.
| Effects of probiotic and whey powder supplementation on growth performance, microflora population, and ileum morphology in broilers.Crossref | GoogleScholarGoogle Scholar |
Zekarias B, Stockhofe-Zurwieden N, Post J, Balk F, van Reenen C, Gruys E, Rebel JMJ (2005) The pathogenesis of and susceptibility to malabsorption syndrome in broilers is associated with heterophil influx into the intestinal mucosa and epithelial apoptosis. Avian Pathology 34, 402–407.
| The pathogenesis of and susceptibility to malabsorption syndrome in broilers is associated with heterophil influx into the intestinal mucosa and epithelial apoptosis.Crossref | GoogleScholarGoogle Scholar | 16236573PubMed |
Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li C, Ma JY, Bi YZ (2012) Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poultry Science 91, 2755–2760.
| Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens.Crossref | GoogleScholarGoogle Scholar | 23091128PubMed |
Zhao X, Guo Y, Guo S, Tan J (2013) Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Applied Microbiology and Biotechnology 97, 6477–6488.
| Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 23666481PubMed |
Zheng A, Luo J, Meng K, Li J, Zhang S, Li K, Liu G, Cai H, Bryden WL, Yao B (2014) Proteome changes underpin improved meat quality and yield of chickens (Gallus gallus) fed the probiotic Enterococcus faecium. BMC Genomics 15, 1167
| Proteome changes underpin improved meat quality and yield of chickens (Gallus gallus) fed the probiotic Enterococcus faecium.Crossref | GoogleScholarGoogle Scholar | 25532559PubMed |
Zheng A, Luo J, Meng K, Li J, Bryden WL, Chang W, Zhang S, Wang LXN, Guohua Liu G, Yao B (2016) Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus). BMC Genomics 17, 89
| Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus).Crossref | GoogleScholarGoogle Scholar | 26830196PubMed |
Zommiti M, Chikindas ML, Ferchichi M (2020) Probiotics – live biotherapeutics: a story of success, limitations, and future prospects – not only for humans. Probiotics and Antimicrobial Proteins 12, 1266–1289.
| Probiotics – live biotherapeutics: a story of success, limitations, and future prospects – not only for humans.Crossref | GoogleScholarGoogle Scholar | 31376026PubMed |