Comparison of gastrointestinal transit times in stabled Thoroughbred horses fed freshly cut pasture and three conserved forage-based diets
Karlette A. Fernandes A , Chris W. Rogers A B D , Erica K. Gee A , Gareth Fitch A , Charlotte F. Bolwell A , Sandra Kittelmann C , Emma N. Bermingham
A B D , Erica K. Gee A , Gareth Fitch A , Charlotte F. Bolwell A , Sandra Kittelmann C , Emma N. Bermingham  C and David G. Thomas B
C and David G. Thomas B
A School of Veterinary Science, Massey University, Palmerston North, New Zealand.
B School of Agriculture and Environment, Massey University, Palmerston North, New Zealand.
C AgResearch, Grasslands Research Centre, Palmerston North, New Zealand.
D Corresponding author. Email: c.w.rogers@massey.ac.nz
Animal Production Science 62(12) 1192-1202 https://doi.org/10.1071/AN20695
Submitted: 24 December 2020 Accepted: 20 August 2021 Published: 16 December 2021
Journal compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Context: The type of forage offered to horses varies in physical form, moisture content and nutrient quality, and these variables could affect the intake, passage rate and digestibility of the forage consumed.
Aims: To investigate the changes in passage rate of digesta through the gastrointestinal tract in horses fed four different forage-based diets (diet effect).
Methods: Thoroughbred mares (n = 6) were stabled in loose boxes for 6 weeks. During Weeks 1, 3 and 5 (washout periods), all horses were fed freshly cut pasture, either in restricted quantities (Week 1) or ad libitum (Weeks 3 and 5). Using a 3 × 3 Latin square design during Weeks 2, 4 and 6, each pair of horses was abruptly transitioned to one of three conserved forage-based diets (chopped ensiled forage fed exclusively or with oats, or perennial ryegrass hay with oats) fed ad libitum. At the beginning of each week, indigestible polyethylene markers (n = 200) were administered to the horses via a nasogastric tube, followed immediately by transition to the new diet.
Key results: There was a significant diet effect on the daily dry-matter intake of feed (P < 0.0001), percentage of time spent eating (P < 0.001), frequency of voiding faeces (P < 0.05) and quantity of faeces voided (P < 0.0001). There was a significant horse effect on the daily dry-matter intake of feed (P < 0.0001) and quantity of faeces voided (P < 0.0001), but no differences in the percentage of time spent eating or the frequency of voiding faeces. There were significant diet and horse effects on the time to recovery of the first marker in the faeces (P < 0.01 and P < 0.01 respectively) and the mean retention time of markers in the gastrointestinal tract (P < 0.05 and P < 0.001 respectively). Mean retention time was negatively correlated with feed intake and quantity of faeces voided (r2 = –0.51 and r2 = –0.64 respectively).
Conclusions: Longer mean retention time was associated with a greater fibre content in the diet and a restricted feed supply, thus supporting the hypothesis that horses alter mean retention time on the basis of a nutrient absorption optimisation model.
Implications: Feed composition, but also the quantities offered, may alter measurement of apparent feed digestibility in horses.
Keywords: conserved forage, faeces, feed intake, gastrointestinal transit time, mean retention time, pasture, passage rate of digesta, Thoroughbred horses.
Introduction
Horses are hindgut fermenters adapted to eating a high-forage diet (Janis 1976). When kept on pasture, horses spend most (~70%) of their time grazing a variety of forage species (Crowell-Davis et al. 1985; Randall et al. 2014). In New Zealand, most horses are kept on pasture all year round (Fernandes et al. 2014b; Verhaar et al. 2014; Rogers et al. 2020), but it is common practice to intensively manage racehorses and some competition horses in stables for parts of the day, with variable turnout periods for grazing (Rogers et al. 2007; Stowers et al. 2009; Verhaar et al. 2014; Wood et al. 2019). A variety of conserved forages, grain and grain by-products are frequently incorporated into the diet of many horses, especially when pasture is unavailable or inadequate in quantity and nutritive value, or to provide additional dietary energy for performance (Goodwin et al. 2002; Harris 2009; Hoffman et al. 2009). Gastrointestinal disorders may occur in horses and ponies, with some animals being more prone than others (Clarke et al. 1990; Hudson et al. 2001; Julliand 2005). A common recommendation to prevent gastrointestinal disturbances in horses is to avoid abrupt dietary changes and to maintain high proportions of forage in the diet (Julliand et al. 2001). Although several studies have investigated aspects of conserved forages fed to horses (Drogoul et al. 2001; Müller et al. 2008; Muhonen et al. 2009, 2010), the effect of different conserved forages on the passage rate of digesta and the microbial population in the hindgut is still poorly understood (Fernandes et al. 2014a; Garber et al. 2020).
Many types of conserved forages are fed to horses, and vary in physical form, such as, for example, long-stemmed, chaffed or pelleted forages, the nutrient quality of which can vary depending on several factors (Müller and Uden 2007). For example, conserved forages may consist of variable grass and legume species such as ryegrass (Lolium perenne), clover (Trifolium repens), timothy (Phleum pratense), lucerne (Medicago sativa) or a blend of multiple forage species, and these may be harvested at different stages of maturity (i.e. early, middle or late stages), resulting in a variable nutrient quality of the forage (Lewis et al. 1995; Hoffman et al. 2001; Müller and Uden 2007).
Traditionally, the most common method for conservation of forage was in the form of hay. More recently, feeding horses ensiled forages (such as haylage) is becoming increasingly popular (Müller and Uden 2007). Several authors have reported potential benefits associated with ensiled forages, including higher voluntary feed intake, palatability and digestibility (Müller and Uden 2007; Ragnarsson and Lindberg 2010), reduced dust particles due to the higher moisture content (Clements and Pirie 2007), and easier transportation and convenient storage due to polyethylene-wrapped packaging (Patel et al. 2014; Verhaar et al. 2014), than with hay.
Passage rate of digesta (feed) through the gastrointestinal tract has been previously investigated, and is best described by measuring the mean retention time (MRT) of indigestible markers that travel through the gastrointestinal tract with the feed consumed (Pearson and Merritt 1991; Cuddeford et al. 1995; Pearson et al. 2001). The transit time (T1) is described as the time when the first marker is recovered in the faeces, and is an indication of the minimum time between ingestion of the feed and voiding of faeces (Udén et al. 1982).
Differences in the passage rate of digesta through the gastrointestinal tract have been reported in equids (e.g. horses, ponies and donkeys) fed different diets, i.e. 24–26 h when grazing or fed cut pasture (Grace et al. 2003) versus 26–77 h when fed different types of conserved forages (Cuddeford et al. 1995; Pearson et al. 2001; Moore-Colyer et al. 2003), and 43–52 h when fed ground-pelleted hay versus 27–46 h when fed chopped hay (Drogoul et al. 2000). The physical form of the forage and the particle size of digesta were identified as dominant effects (Drogoul et al. 2000; Moore-Colyer et al. 2003). Furthermore, increasing the proportion of forage in the diet at the expense of grain reduced the passage rate of digesta through the gastrointestinal tract, from 42 h for a 50:50 hay and barley diet, to 30 h for a 100% hay diet (Drogoul et al. 2001). In ponies fed ad libitum versus restricted quantities, the passage rate increased from 21 to 31 h for chopped alfalfa, and from 32 to 36 h for chopped oat straw (Pearson et al. 2001). However, while the passage rate of different types of forage and mixed forage–grain diets has been investigated in horses, following an adaption period after dietary change, the passage rate (MRT and T1) immediately following abrupt transition between forage-based diets has not been investigated. It is of importance to know that this as passage rate will affect the composition of the hindgut microbiota and this may contribute to the manifestation of gastrointestinal disorders with acute changes in diet.
As part of a larger study to examine the effect of an abrupt change of forage diets on the hindgut microbiota, the current study described here aimed to investigate the changes in T1 and MRT when horses were fed four different forage-based diets (diet effect), and to examine variation among horses (horse effect) on the passage rate by using indigestible markers.
Materials and methods
Animals and experimental design
The use of animals, experimental procedures and collection of the faecal samples for the study were approved by the Massey University Animal Ethics Committee (MUAEC), Massey University, Palmerston North, New Zealand (Protocol number 14/35). The management of the horses used in the study (including feeding, housing, husbandry and welfare) were in accordance with the guidelines set within the code of ethical conduct for the use of live animals for research, testing and teaching. A veterinarian examined the horses on a weekly basis to ensure that all horses remained clinically normal during the study period.
Using a 3 × 3 replicated Latin square design, stabled horses were abruptly transitioned to three randomly allocated conserved forage-based diet treatments. Between dietary-treatment periods, each of 7 days in duration, the horses were provided with a washout period of 7 days, during which freshly cut pasture was fed ad libitum to each horse. Feed and faecal samples were collected at regular intervals following each dietary transition, and data were recorded to investigate the differences in gastrointestinal transit times between horses and diets.
Six (non-pregnant) Thoroughbred mares were enrolled in the study (mean age ± standard deviation (s.d.), 13.5 ± 3.7 years, and mean bodyweight (BW) ± s.d., 528 ± 26 kg, weighed at the beginning of the study). Prior to the study, the horses were maintained on a commercial Thoroughbred stud farm (Palmerston North, Manawatu, New Zealand), and managed as a cohort on predominantly perennial ryegrass–clover pasture (typically ~80–95% perennial ryegrass (Lolium perenne) and ~5–20% white clover (Trifolium repens)). The horses had received an annual vaccination (Equivac 2 in 1, Pfizer Animal Health, Australia) and the most recent anthelmintic treatment had been administered 1 week before the commencement of the study. At the start of the study, all horses were examined by a veterinarian and were of similar body condition (body condition score (BCS) = 5, on a 9-point scale; Henneke et al. 1983), as assessed by the author (K. A. Fernandes). The horses were reported to have been in good health during the 6 months preceding the study.
Three days before the start of the study, the horses were transported from the stud farm to the trial site. On arrival, the horses were transferred into individual, adjacent, outdoor holding paddocks (15 × 15 m, containing ryegrass–clover pasture, ~1000 kg dry matter (DM)/ha) to facilitate adaptation to the new environment. A fresh faecal sample was collected (from a recently voided faecal mass) from each horse within 2 h of arrival, and examined for faecal egg count. During the 3-day adaptation period, each horse was offered ~12 kg DM of ryegrass–clover hay per day, fed twice daily, in addition to the limited amount of grazing available in the paddocks.
Housing and stable management during the trial
On Day 1 of the trial, the horses were individually stabled in 3 × 3.5 m loose boxes, with sawdust bedding to a depth of 8–10 cm. The loose boxes were adjacent to each other and the top half of the stable door was kept open at all times, allowing visual contact among all horses. The horses were turned out individually each day during the trial into two 6 × 8 m concrete yards, for 30 min in the morning and afternoon. The general health and appetite of the horses were observed daily. BCS (1–9; Henneke et al. 1983) and BW (measured using walk-on scales, TruTest economy plus-700, Auckland, New Zealand, accuracy 0.5 kg) of the horses were recorded on the 1st, 4th and 7th day of each treatment block.
The horses were provided ad libitum access to water and a 500 g trace-mineral salt block (Summit Littlix multi-mineral salt block, Dominion Salt Ltd, Mount Maunganui, New Zealand). Cut grass and hay was provided in hay nets (large size nylon-rope hay net, 107 cm in length, with 15 × 15 cm mesh openings) and the chopped ensiled forage diet, oats and some cut pasture were provided in a feed bin.
Diets and feeding management
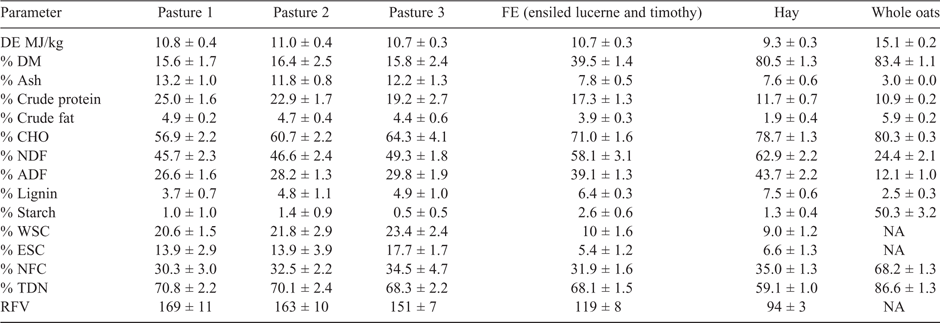
The horses were offered three forage-based diets, with freshly cut pasture being fed to the horses as a washout diet between the dietary treatment blocks (Diets P1, P2 and P3 were fed during Weeks 1, 3 and 5 of the trial respectively) over the 6-week period. The three treatment diets were a commercial chopped ensiled forage (Diet FE), the same commercial chopped ensiled forage mixed with whole oats (Diet FE+O), and a perennial ryegrass hay fed with whole oats (Diet H+O). The nutrient composition of the dietary components is provided in Table 1.
During Weeks 1, 3 and 5 of the trial, the horses were fed freshly cut grass obtained from ryegrass–clover pasture (typically comprising of ~80–95% perennial ryegrass (Lolium perenne) and ~5–20% white clover (Trifolium repens), sourced from two 2 ha paddocks on an adjacent dairy farm (No. 4 Dairy Farm, Massey University, Palmerston North, New Zealand). Prior to Day 1 of the trial, the pasture had not been grazed for a 6–8-week period (previously grazed by cattle and never by horses) and had an average sward height of 15–20 cm (~3000–3500 kg DM/ha). The grass was cut using a sickle bar mower to a height of 3–5 cm above the ground, between 0800 hours and 0900 hours (AM pasture cut) and between 1600 hours and 1700 hours (PM pasture cut) each day. The cut grass was immediately transported to the trial site and stored in a feed room for less than 12 h before feeding.
The horses were provided with two hay nets containing ~10–15 kg and one bucket containing ~5–8 kg of cut pasture (fresh weight). As per individual horse requirements, the hay nets and feed buckets were refilled (either when empty or at ~4-h intervals at 0800 hours, 1200 hours, 1600 hours, 2000 hours and 2400 hours) to provide 1.3–2.3% BW of feed (DM basis). Due to logistic difficulties, the quantity of cut pasture offered during Diet Period P1 was restricted compared with ad libitum access to feed in Diet Periods P2 and P3.
During Weeks 2, 4 and 6, one pair of horses was randomly allocated to one of the following diets: FE, FE+O or H+O according to a 3 × 3 Latin-square design. Diet FE was a commercially available chopped ensiled forage diet (FibreEzy® (FE), Fibre Fresh Feeds Ltd, Reporoa, New Zealand), and comprised lucerne (Medicago sativa; alfalfa; 50%) and timothy (Phleum pratense; 50%) grass, chopped into 1–5 cm stubbles, and ensiled with molasses (1%). Diet FE was offered at a minimum of ~2.5–3.0% BW (DM basis), as two feeds at 0800 hours and 2000 hours, (~20 kg Diet FE/horse.day, on as-fed basis). To ensure ad libitum feeding, additional quantities of Diet FE were provided when less than 25% of the feed offered was remaining in the bucket.
Diet FE+O comprised the same chopped ensiled forage provided in Diet FE, mixed with whole oats (Avena sativa), and Diet H+O comprised perennial ryegrass hay fed with whole oats. The perennial ryegrass hay typically contained ~80% perennial ryegrass (Lolium perenne), and small proportions of white (Trifolium repens) and red (Trifolium pratense) clovers, herbs and weeds. The hay was harvested and processed as one batch at the same location (Manawatu, New Zealand), and was stored in a dry covered shed for ~6 months before the study period. Both forage components of the diets FE+O and H+O were fed ad libitum at ~2% BW (DM basis)/horse.day.
The quantity of oats offered was calculated on the basis of 50% of the minimum daily digestible energy requirements for maintenance for a 500 kg horse (~35 MJ/horse.day; National Research Council 2007), equivalent to 2.5 kg DM/horse.day, divided into two feeds. Diet FE+O was provided in a feed bin twice daily at 0800 hours and 2000 hours, and to ensure ad libitum feeding, additional quantities of the chopped ensiled forage were provided when less than 25% of feed was remaining in the bucket. Hay was offered in two hay nets (weighing ~5–8 kg, as-fed basis) at 0800 hours each day (similar to the procedure described for Diet P). The hay nets were replenished at 2000 hours with additional quantities of hay, so as to provide ad libitum access to feed.
Refusals were collected twice daily (morning between 0700 hours and 0800 hours and evening between 1900 hours and 2000 hours) and weighed (TruTest-703 Electronic Scales, Auckland, New Zealand) to determine the amount of feed consumed (Glunk et al. 2013b). DM intake (DMI, kg/day) of each diet was calculated as the difference between the weight of feed consumed and the weight of the refusals (including spillage) measured on an as-is basis, and the values were corrected to a DM basis for each feed.
Feed samples
Representative feed samples of each diet fed to the horses were collected to determine the nutrient content. One pooled sample (~1 kg wet weight) of Diet P1 (n = 7), and two samples (representing the AM and PM cuts) for Diets P2 (n = 14) and P3 (n = 14) were collected each day. The pasture samples consisted of ~10 smaller subsamples from different locations within the freshly cut pasture. One sample (~200 g) was collected on alternate days for the chopped ensiled forage used in Diets FE and FE+O (n = 9) and hay (~500 g) used in Diet H+O (n = 9), and one sample of whole oats (200 g) was collected per week (n = 3). The feed samples were weighed and stored at –20°C until analysis.
At the end of the trial, the feed samples (n = 56) were lyophilised (FD18, Cuddon Freeze Dry, Blenheim, New Zealand) and ground to pass through a 1 mm screen (Cyclotec 1093 Sample Mill, Foss, Hillerod, Denmark). The processed samples were analysed for nutrient content by a commercial forage testing laboratory (Equi-Analytical, Ithaca, NY, USA). The method of analysis used was a combination of near-infrared and plasma spectrophotometer techniques to analyse DM, crude protein, neutral and acid detergent fibres, lignin, water-soluble and ethanol-soluble carbohydrates, starch, fat, ash and minerals.
Indigestible markers
The passage rate of a diet (time taken for a diet to transit through the gastrointestinal tract) was estimated using solid-phase indigestible markers according to previously described methods (Blaxter et al. 1956; McGreevy et al. 2001; Pearson et al. 2006; Rosenfeld et al. 2006). The solid-phase markers used in the present trial were hollow cylindrical pieces (4–5 mm length, 5 mm outer diameter, ~40 mg weight) prepared from polyethylene tubing (Ledathene, Leda, Wellington, New Zealand). At 0800 hours on the first day of each treatment block (Days 1, 8, 15, 22, 29 and 36), the horses were intubated and 200 polyethylene markers were administered via a nasogastric tube with 1–2 L of water. Green- and blue-coloured polyethylene markers were used on alternate 7-day treatment blocks.
Faecal samples
Faecal matter was collected from all horses at hourly intervals from 6 h after nasogastric administration of the markers, until 96 h post-administration of markers. The total wet weight of the faeces voided (kg FW/day) by each horse was recorded at the end of each day. The markers were retrieved manually by sifting through the faecal matter collected during the first 4 days of each week, and the number of markers recovered was recorded to estimate gastrointestinal transit time.
Determination of gastrointestinal transit time
The percentage of markers recovered in the faeces of each horse was calculated by dividing the number of markers recovered by the number of markers administered. The transit time delay of the markers was measured as the time (h) post-administration of markers to the first appearance of a marker in the faeces (T1; Moore-Colyer et al. 2003; Rosenfeld et al. 2006). The MRT (h) of the markers in the gastrointestinal tract for each diet was calculated by an equation previously described for particulate-phase MRT in ruminants and horses (Faichney 1975; Rodrigues et al. 2012).
Behaviour monitoring
During the first 4 days (Days 1–4, 8–12, 15–18, 22–25, 29–32 and 36–39) of each treatment block, the frequency of voiding faeces was recorded and the behaviour of horses was monitored by using an instantaneous-scan sampling technique (Bateson 1991), by using an ethogram to describe eating/drinking, standing alert, resting, abnormal behaviours, and other behaviours. Every hour, the behaviour of the horses was observed from a distance of 10–20 m outside the loose box and one scan sample per horse was recorded (n = 96 per horse per week, total n = 3456 observations). If an hourly observation was not recorded, it was considered as a missing value for all six horses at that time point.
Statistical analyses
The distribution of variables in the dataset was tested for normality using the Shapiro–Wilk test, and the data are presented as mean ± s.d. for parametric data or median ± interquartile range for non-parametric data.
Significant differences among groups (diets and horses) were determined using an ANOVA for parametric data, followed by a post hoc Bonferroni test (DMI, quantity of faeces voided, T1, and MRT), and the Kruskal–Wallis test for non-parametric data (BW and percentage of time spent eating). Count-time data (cumulative recovery of markers and frequency of voiding faeces) were tested for significant effects of diet and horse by using Kaplan–Meier survival functions and the Log-rank test (Mantel–Cox test).
The DMI (g DM/kg BW0.75) and the quantity of faeces voided (FW) by each horse on each diet were calculated as average of the first 4 days in each dietary treatment block (n = 36 observations), and the relationships of DMI and FW with MRT (h) were compared using the Spearman’s rank correlation. The variables g DM/kg BW0.75, kg FW and percentage of time spent eating (of 6 horses on 6 diets) were ranked as low or high, and MRT was ranked as short or long. The ranked variables were compared using multiple correspondence analysis in two dimensions, with normalisation on principal coordinates. The data were analysed in STATA version 12.1 (Stata Corporation, College Station, TX, USA) with significance set at P < 0.05.
Results
The horses remained clinically normal during the 6-week study period. The faecal egg counts at the start of the study were between 0 and 50 eggs per gram of faeces. There was no change in body condition (BCS 5 (range 5–6)) or bodyweight (528 ± 10 vs 548 ± 12) throughout the trial.
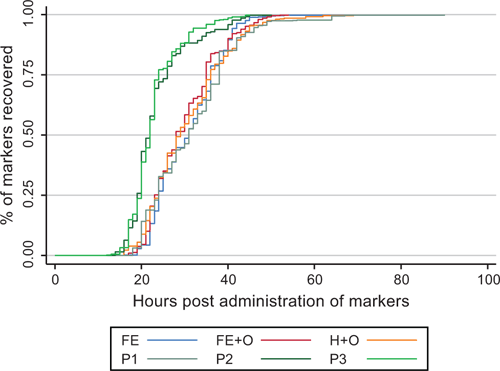
Overall, 75–95% of the markers administered to the horses were recovered from the faeces collected during the first 96 h. The log-rank test indicated a significant effect of diet on the cumulative percentage of markers recovered in the faeces within the 4-day collection interval (P < 0.001; Fig. 1). Diet P1 (restricted cut pasture) differed from Diet Periods P2 and P3 (ad libitum cut pasture; P < 0.001), but there was no difference between Diet Periods P2 and P3 (P = 0.168). There was a trend for a difference among Diets FE, FE+O and H+O (P = 0.061). There was a significant horse effect on the cumulative percentage of markers recovered in the faeces (P < 0.001). There appeared to be greater variation in marker recovery curves for some diets (Diets P2 and FE+O vs Diet P3) and horses (H4, H5 and H6 vs H2) (P < 0.001 for each comparison; Figs S1 and S2, available as Supplementary material to this paper).
|
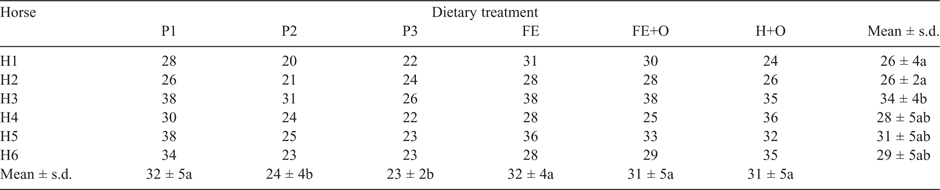
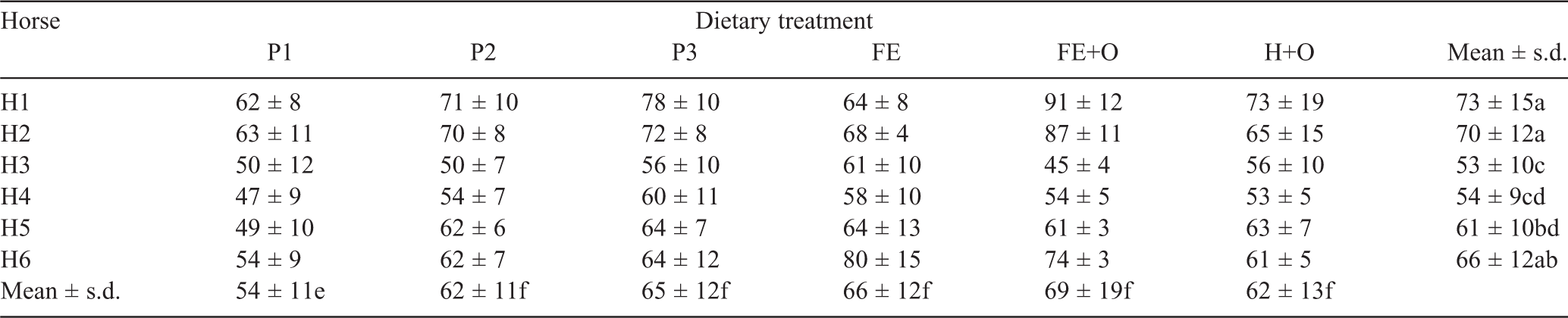
The mean time in hours (h) ± s.d. for recovery of the first marker for each diet across all horses was 19 ± 4 (P1), 14 ± 3 (P2), 15 ± 3 (P3), 21 ± 5 (FE), 19 ± 4 (FE+O) and 17 ± 2 (H+O), and ranged for horses from 15 ± 3 (Horse 6) to 22 ± 5 (Horse 3). Both diet (P < 0.01) and horse (P < 0.01) had a significant effect on the time at which the first marker was recovered. Retention time (h) of the markers in the gastrointestinal tract for each horse, stratified by diet, is presented in Table 2. There was a significant effect of diet (P < 0.05) and horse (P < 0.001) on the MRT.
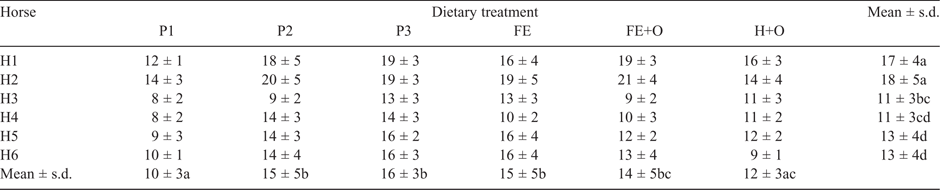
The horses voided between 8 and 21 kg/day of faeces (FW) at the rate of one faecal output every 2 h. The quantity of faeces voided per day differed significantly among diets (P < 0.0001) and among horses (P < 0.0001). Horses 1 and 2 voided a greater weight of faeces (12–21 kg/day) when compared with the other horses (8–16 kg/day; Table 3). The Kaplan–Meier survival functions indicated a diet effect (P = 0.046) on the cumulative frequency of faeces voided, with no difference observed among horses.
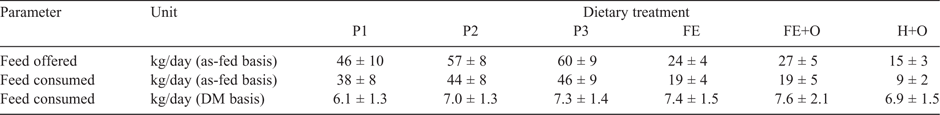
All horses consumed between 1.1% and 1.5% BW of a feed (DM basis), equivalent to 64–91 MJ of digestible energy per day. The quantity of feed offered to, and consumed by, the horses is presented in Table 4. The DMI (kg/day) differed significantly among horses (P < 0.0001) and diets (P < 0.0001), with a significant (P < 0.0001) diet × horse interaction. The among-horse effect remained significant when DMI was expressed as %BW and BW0.75 (P < 0.0001 for each comparison). Within each dietary treatment week, the horses consumed ~1–5% less feed on the first day than on the subsequent days in the week, but this reduction in feed intake was not significant. The quantity of feed (g DM/kg BW0.75.day) consumed was highest for the diets containing chopped ensiled forage (Diets FE and FE+O), followed by the Diets P2, P3 and H+O, and lowest for Diet P1. Across all diets, Horses 1 and 2 consumed higher quantities of feed than the other horses (Table 5). MRT was negatively correlated with kg DMI/day (r = –0.45), g DM/kg BW0.75 (r = –0.51) and kg FW (r = –0.64). Multiple correspondence analysis showed that horses that consumed more feed and voided more faeces had a shorter MRT than did horses that consumed less feed and voided less faeces, with 80% of the variation being explained on one principal coordinate.
When observed at hourly-intervals over the first four days of each treatment block, horses were most often found to be resting (43–56% of total observations), eating (24–39%) or standing alert (12–24%), with a similar pattern observed across all diets (Fig. S3). There was a significant difference in the percentage of time spent eating among diets (P < 0.001), with no significant differences among horses (P = 0.96). Horses were observed to be eating more often during Diet Periods P2 and P3, when compared with Diet Period P1 (P < 0.01) and this finding was consistent with the amount of feed offered during the diet periods. Horses spent more time eating Diet H+O than Diets FE and FE+O (P < 0.01). Some diets (Diets P1, FE and FE+O) appeared to show more variation among horses in time budgets.
Discussion
The present study investigated the passage rate of digesta through the gastrointestinal tract after dietary transition. The results obtained supported the hypothesis that diet quantity and composition, and horse, have significant effects on the passage rate of digesta. The proportion of polyethylene markers recovered in the faeces was equal to, or higher than, the recovery rates reported in other studies (McGreevy et al. 2001; Boscan et al. 2006). This high percentage of marker recovery and the frequency of faecal collection (hourly intervals) indicated that a high precision could be attained when estimating the MRT of the diets used in the present study (Rosenfeld et al. 2006).
The dietary transitions may have affected the feed intake of the horses in the present study, as small decreases in DMI were observed on the days the diets were changed; however, these were limited to within 1–5% of subsequent days. Ideally, to quantify the effect of diet and horse, transit time would have been monitored once the horses were habituated to the diet. However, the objective of the larger study was to quantify the effect of diet change (pasture to forage to pasture) on the faecal microbiota. Thus, we were interested in the transit time of digesta during the period of first introducing the new diet, so we could relate these data to any changes in microbiota.
Dry-matter intake for these horses at ~1.3% BW was lower than that reported for similar cohorts of horses, ~2% BW (Grace et al. 2002a, 2002b, 2003). The DMI may have been reduced as an indirect effect of keeping the mares in a loose box/stable, as opposed to the pasture environment, within which they were usually managed. There are indications within the literature that for horses usually managed with a pasture-based system, there is a moderate reduction in DMI with the confinement to a loose box/stable environment (Grace et al. 2003). Irrespective of the diet offered, the average DMI did not decrease below 1.3% BW, indicating that the horses consumed sufficient DM (~7 kg DM/horse.day; DE ~64–91 MJ/day) to meet or exceed NRC recommended maintenance requirements (National Research Council 2007). The constant BW and BCS of all horses throughout the study period supported the calculation that even with these lower DMI, maintenance or greater digestible energy was being consumed.
Only two horses (H1 and H5) exhibited abnormal behaviours (box walking and weaving respectively), which were observed rarely, irrespective of the diet. The weaving and box walking in these horses are typically expressed in anticipation of more (new) food, or less frequently, due to confinement in stables (Cooper et al. 2005). Despite the occasional recording of abnormal behaviours, the time spent eating for Horses 1 and 5 did not differ from that of the other horses, and their BWs remained constant during the study.
Shorter MRTs have been reported after feeding ad libitum than restricted quantities of alfalfa and oat straw (Pearson et al. 2001). Similar reductions in T1 and MRT were observed in the present study when horses were fed ad libitum (P2 and P3) compared with restricted (P1) quantities of cut pasture. The MRT increased from 23–24 h to 32 h when the level of cut pasture was inadvertently restricted and became similar to the MRTs observed when the horses were fed ad libitum conserved forage (31–32 h). The adaptation of horses to the confinement of the stable environment during Week 1 may have confounded the results of the study, but the clustering of marker–recovery curves confirmed that the level of feeding (ad libitum vs restricted), and hence feed intake, had a significant effect on, and perhaps drives the passage rate of digesta through the gastrointestinal tract.
The MRT were consistent among the periods when the horses were fed ad libitum cut pasture (P2 and P3), and these values were similar to a previous report of weanling Thoroughbred horses fed cut pasture in stables or when grazing ad libitum in paddocks (Grace et al. 2003). The comparable MRTs reported between the studies is noteworthy, considering the differences in the age of horses (adult vs weanling) and the type of particulate phase markers (polyethylene markers vs mobile nylon-bag technique) used. There may also be a breed-effect difference in MRT. When compared on a per kilogram metabolic BW, Welsh-cross pony geldings that were fed at restricted levels consumed similar quantities of ensiled forage (62 vs 66 g DM/kg BW0.75.day) as the Thoroughbred mares fed ad libitum in the present study, indicating that the relative rate of feed intake may have been slower in our horses than in the ponies. Therefore, given the variability in the feed intake of different equid types (e.g. horses, ponies and donkeys; Cuddeford et al. 1995; Drogoul et al. 2001; Pearson et al. 2001), some caution is warranted when comparing the results obtained in the present study with previous investigations, the majority of which were conducted on ponies (Cuddeford et al. 1995; Pearson et al. 2001; Moore-Colyer et al. 2003).
The inconsistencies in the feed intake and passage rate of digesta reported in previous work may have been due to differences in the feeding and management of the animals used in the studies (de Fombelle et al. 2004; Rodrigues et al. 2012; Jensen et al. 2014). A large variation in voluntary feed intake and passage rate of digesta was also observed among the horses in the present study, even though the experimental design controlled for breed, diet and management of the horses. The MRT varied by up to 10 h among the horses, with the longest MRT being observed for Horse 3, and this slower passage rate appeared to be linearly related to the DM of feed consumed (Horse 3 consistently consumed less DM than did other horses in the study). Perhaps other horse-specific factors, such as the rate of DMI, individual metabolic rates and reproductive status, also influence the feeding behaviour of horses, but the effects of these factors on the DMI and passage rate of digesta are poorly understood, and may require further investigation.
The model for DMI (g DM/kg BW0.75.day) showed a significant diet × horse interaction, such that some horses were observed eating Diets FE and FE+O more frequently than Diet H+O. Horses are reported to prefer forages harvested at early maturities (Staniar et al. 2010; Särkijärvi et al. 2012), and preferentially consume greater quantities of lucerne forage than other forage species (LaCasha et al. 1999; Rodiek and Jones 2012), and ensiled forage when compared with hay prepared from the same forage species (Müller and Uden 2007). The preference of some horses for diets containing ensiled forage (45% moisture) when compared with hay (10% moisture) may be due to the greater palatability of the chopped ensiled forage diet and the shorter time required to consume it (chopped forage of low DM content takes less time to chew and mix with saliva than does long-stem forage of a higher DM content). Differences in the fibre length of the forages (cut pasture, chopped ensiled forage and long-stem hay) may have had some influence on the MRTs of digesta (Moore-Colyer et al. 2003), but the effects of diet on the passage rate of digesta observed in the present study appeared to be more likely due to differences in other horse-specific factors that may have influenced the feed-intake levels, such as individual preferences or palatability of a diet. Nonetheless, the rate of adaptability to a diet, its palatability and individual preferences of horses are poorly understood and merit further investigation.
Some horses in the present study had higher DMIs and shorter MRTs than did others. The underlying reason for the inter-horse variation may be similar to that observed for an inter-species variation, where donkeys were found to consume less DM than were ponies, and, consequently, had longer retention times and higher apparent digestibility of the feed consumed (Cuddeford et al. 1995; Pearson et al. 2001; Boscan et al. 2006). DMI is negatively correlated with the apparent digestibility of feed (Morel 2010), and this relationship supports the argument that longer retention time allows more time for microbial fermentation in the hindgut and increases the apparent digestibility of the feed consumed (Cuddeford et al. 1995). This physiological strategy may be beneficial to the survival of horses under feral conditions, where decreases in pasture growth rates, and thus pasture availability in summer and winter, may reduce the quantity of feed consumed; however, a consequent increase in the MRT of digesta may allow better digestibility of the feed consumed, which may then meet the daily energy requirements for maintaining BW (Kuntz et al. 2006).
However, this potential survival strategy could be a problem when managing obese domestic horses and ponies (easy-keepers), when kept on restricted access to pasture (shorter grazing turnout periods or limited sward height) designed to reduce their caloric intake (Geor 2010). Horses and ponies are capable of increasing their forage consumption during restricted grazing periods by increasing the rate of feed intake, with reports of ~40% of daily DMI consumed by ponies during a 3-h restricted grazing turnout (Ince et al. 2005), and greater mean rates of DMI observed in horses during 3- and 6-h restricted grazing turnouts than during 24-h access to grazing (Glunk et al. 2013a). Although restricting pasture access decreases the total intake of pasture, the compensatory increase in the rate of feed intake (during the turnout period) and the longer MRT of digesta (due to the restricted feeding time) may enable horses and ponies to maintain BW even when the quantity of feed is restricted (Dugdale et al. 2011; Argo et al. 2012; Glunk et al. 2013a).
Variation in feed intake affects the passage rate of digesta and the apparent digestibility; therefore, these factors should be taken into consideration when formulating specific feeding regimens for horses and ponies, and when comparing the rate of change in microbiota populations when horses transition between diets. The differences observed in the feed intake levels and MRTs may explain some of the previously reported variation in the population of hindgut or faecal microbiota (Dougal et al. 2014; Fernandes et al. 2014a), and perhaps explain the increased susceptibility of some horses and ponies to gastrointestinal disturbances. This hypothesis requires further investigation.
Conclusions
The results of the present study highlighted that diet-specific factors (such as feed type, composition and intake level) influence the MRT of digesta in the gastrointestinal tract. As expected, there was among-horse variation due to horse-specific factors (such as eating behaviour, voluntary DMI and quantity of faeces voided). The level of feed intake appears to drive the passage rate of digesta, and thus, reduced intake may potentially increase the apparent digestibility of the feed consumed, due to the greater time available for microbial fermentation.
Data availability
The data that support this study will be shared on reasonable request to the corresponding author.
Conflict of interest
Chris Rogers is an Associate Editor of Animal Production Science but was blinded from the peer-review process for this paper.
Declaration of funding
The work was funded a grant from the Ministry of Business, Innovation and Employment – Science and Innovation Group (Callaghan Innovation). The author (K. A. Fernandes) acknowledges the receipt of a Massey University Doctoral Research Scholarship.
Acknowledgements
The authors acknowledge the research facilities and equipment provided by Massey Equine & Farm Services, Massey University No. 4 Dairy Farm and AgResearch, and the assistance provided by the Massey Equine staff and undergraduate students, to conduct the research trial. We especially thank Laure Gomita (Intern – National Institute of Agricultural Research, INRA-France) for assistance with sample collection and management of the horses, Christa Bodaan (Equine Surgery Resident, Massey University, New Zealand) for assistance with the nasogastric intubations, and Paul Sirois (Director of Forage Laboratory Services and Equi-Analytical Laboratories at Dairy One) for consultation on feed sample analysis. The horses were provided by Goodwood Stud (Palmerston North, New Zealand) and the commercial chopped ensiled forage was provided by Fibre Fresh Feeds Ltd (Reporoa, New Zealand), for which we are grateful.
References
Argo CM, Curtis GC, Grove-White D, Dugdale AHA, Barfoot CF, Harris PA (2012) Weight loss resistance: a further consideration for the nutritional management of obese Equidae. Veterinary Journal (London, England) 194, 179–188.| Weight loss resistance: a further consideration for the nutritional management of obese Equidae.Crossref | GoogleScholarGoogle Scholar |
Bateson P (1991) Assessment of pain in animals. Animal Behaviour 42, 827–839.
| Assessment of pain in animals.Crossref | GoogleScholarGoogle Scholar |
Blaxter KL, Graham NM, Wainman FW (1956) Some observations on the digestibility of food by sheep, and on related problems. British Journal of Nutrition 10, 69–91.
| Some observations on the digestibility of food by sheep, and on related problems.Crossref | GoogleScholarGoogle Scholar |
Boscan P, Van Hoogmoed LM, Farver TB, Snyder JR (2006) Evaluation of the effects of the opioid agonist morphine on gastrointestinal tract function in horses. American Journal of Veterinary Research 67, 992–997.
| Evaluation of the effects of the opioid agonist morphine on gastrointestinal tract function in horses.Crossref | GoogleScholarGoogle Scholar | 16740092PubMed |
Clarke L, Roberts M, Argenzio R (1990) Feeding and digestive problems in horses: physiologic responses to a concentrated meal. The Veterinary Clinics of North America. Equine Practice 6, 433–450.
| Feeding and digestive problems in horses: physiologic responses to a concentrated meal.Crossref | GoogleScholarGoogle Scholar | 2202501PubMed |
Clements J, Pirie R (2007) Respirable dust concentrations in equine stables. Part 1: validation of equipment and effect of various management systems. Research in Veterinary Science 83, 256–262.
| Respirable dust concentrations in equine stables. Part 1: validation of equipment and effect of various management systems.Crossref | GoogleScholarGoogle Scholar | 17477944PubMed |
Cooper JJ, McAll N, Johnson S, Davidson HPB (2005) The short-term effects of increasing meal frequency on stereotypic behaviour of stabled horses. Applied Animal Behaviour Science 90, 351–364.
| The short-term effects of increasing meal frequency on stereotypic behaviour of stabled horses.Crossref | GoogleScholarGoogle Scholar |
Crowell-Davis SL, Houpt KA, Carnevale J (1985) Feeding and drinking behavior of mares and foals with free access to pasture and water. Journal of Animal Science 60, 883–889.
| Feeding and drinking behavior of mares and foals with free access to pasture and water.Crossref | GoogleScholarGoogle Scholar | 3988655PubMed |
Cuddeford D, Pearson RA, Archibald RF, Muirhead RH (1995) Digestibility and gastro-intestinal transit time of diets containing different proportions of alfalfa and oat straw given to Thoroughbreds, Shetland ponies, Highland ponies and donkeys. Animal Science 61, 407–417.
| Digestibility and gastro-intestinal transit time of diets containing different proportions of alfalfa and oat straw given to Thoroughbreds, Shetland ponies, Highland ponies and donkeys.Crossref | GoogleScholarGoogle Scholar |
de Fombelle A, Veiga L, Drogoul C, Julliand V (2004) Effect of diet composition and feeding pattern on the prececal digestibility of starches from diverse botanical origins measured with the mobile nylon bag technique in horses. Journal of Animal Science 82, 3625–3634.
| Effect of diet composition and feeding pattern on the prececal digestibility of starches from diverse botanical origins measured with the mobile nylon bag technique in horses.Crossref | GoogleScholarGoogle Scholar | 15537784PubMed |
Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Geor RJ, Nielsen BD, Schott HC, Elzinga S, Newbold CJ (2014) Characterisation of the faecal bacterial community in adult and elderly horses fed a high fibre, high oil or high starch diet using 454 pyrosequencing. Public Library of Science 9, e87424
| Characterisation of the faecal bacterial community in adult and elderly horses fed a high fibre, high oil or high starch diet using 454 pyrosequencing.Crossref | GoogleScholarGoogle Scholar |
Drogoul C, Poncet C, Tisserand JL (2000) Feeding ground and pelleted hay rather than chopped hay to ponies: 1-consequences for in vivo digestibility and rate of passage of digesta. Animal Feed Science and Technology 87, 117–130.
| Feeding ground and pelleted hay rather than chopped hay to ponies: 1-consequences for in vivo digestibility and rate of passage of digesta.Crossref | GoogleScholarGoogle Scholar |
Drogoul C, de Fombelle A, Julliand V (2001) Feeding and microbial disorders in horses: 2-effect of three hay:grain ratios on digesta passage rate and digestibility in ponies. Journal of Equine Veterinary Science 21, 487–491.
| Feeding and microbial disorders in horses: 2-effect of three hay:grain ratios on digesta passage rate and digestibility in ponies.Crossref | GoogleScholarGoogle Scholar |
Dugdale AHA, Curtis GC, Cripps PJ, Harris PA, Argo CM (2011) Effects of season and body condition on appetite, body mass and body composition in ad libitum fed pony mares. Veterinary Journal (London, England) 190, 329–337.
| Effects of season and body condition on appetite, body mass and body composition in ad libitum fed pony mares.Crossref | GoogleScholarGoogle Scholar |
Faichney G (1975) The use of markers to partition digestion within the gastro-intestinal tract of ruminants. Digestion and Metabolism in the Ruminant 1, 277–291.
Fernandes KA, Kittelman S, Rogers CW, Gee E, Bolwell C, Birmingham EN, Thomas DG (2014a) Faecal Microbiome of Forage-fed Horses in New Zealand and the Population Dynamics of Microbial Communities Following Dietary Change. PLoS One 9, e112846
| Faecal Microbiome of Forage-fed Horses in New Zealand and the Population Dynamics of Microbial Communities Following Dietary Change.Crossref | GoogleScholarGoogle Scholar | 25383707PubMed |
Fernandes KA, Rogers CW, Gee EK, Bolwell CF, Thomas DG (2014b) A cross-sectional survey of rider and horse demographics, and the feeding, health and management of Pony Club horses in New Zealand. In ‘Proceedings of the New Zealand Society of Animal Production. Napier’, 30 June 2013. Vol. 74, pp. 11–16. (NZSAP: New Zealand)
Garber A, Hastie P, McGuinness D, Malarange P, Murray J-A (2020) Abrupt dietary changes between grass and hay alter faecal microbiota of ponies. PLoS One 15, e0237869
| Abrupt dietary changes between grass and hay alter faecal microbiota of ponies.Crossref | GoogleScholarGoogle Scholar | 32810164PubMed |
Geor RJ (2010) Nutrition and Exercise in the Management of Horses and Ponies at High Risk for Laminitis. Journal of Equine Veterinary Science 30, 463–470.
| Nutrition and Exercise in the Management of Horses and Ponies at High Risk for Laminitis.Crossref | GoogleScholarGoogle Scholar |
Glunk EC, Pratt-Phillips SE, Siciliano PD (2013a) Effect of restricted pasture access on pasture dry matter intake rate, dietary energy intake, and fecal pH in horses. Journal of Equine Veterinary Science 33, 421–426.
| Effect of restricted pasture access on pasture dry matter intake rate, dietary energy intake, and fecal pH in horses.Crossref | GoogleScholarGoogle Scholar |
Glunk EC, Weber W, Martinson KL (2013b) The effect of hay net design on rate and amount of forage consumed by adult horses. Journal of Equine Veterinary Science 33, 362–363.
| The effect of hay net design on rate and amount of forage consumed by adult horses.Crossref | GoogleScholarGoogle Scholar |
Goodwin D, Davidson H, Harris P (2002) Foraging enrichment for stabled horses: effects on behaviour and selection. Equine Veterinary Journal 34, 686–691.
| Foraging enrichment for stabled horses: effects on behaviour and selection.Crossref | GoogleScholarGoogle Scholar | 12455839PubMed |
Grace N, Shaw HL, Gee EK, Firth EC (2002a) Determination of the digestible energy intake and apparent absorption of macroelements in pasture-fed lactating Thoroughbred mares. New Zealand Veterinary Journal 50, 182–185.
| Determination of the digestible energy intake and apparent absorption of macroelements in pasture-fed lactating Thoroughbred mares.Crossref | GoogleScholarGoogle Scholar | 16032268PubMed |
Grace ND, Gee EK, Firth EC, Shaw HL (2002b) Digestible energy intake, dry matter digestibility and mineral status of grazing New Zealand Thoroughbred yearlings. New Zealand Veterinary Journal 50, 63–69.
| Digestible energy intake, dry matter digestibility and mineral status of grazing New Zealand Thoroughbred yearlings.Crossref | GoogleScholarGoogle Scholar | 16032212PubMed |
Grace ND, Rogers CW, Firth EC, Faram TL, Shaw HL (2003) Digestible energy intake, dry matter digestibility and effect of increased calcium intake on bone parameters of grazing thoroughbred weanlings in New Zealand. New Zealand Veterinary Journal 51, 165–173.
| Digestible energy intake, dry matter digestibility and effect of increased calcium intake on bone parameters of grazing thoroughbred weanlings in New Zealand.Crossref | GoogleScholarGoogle Scholar | 16032319PubMed |
Harris P (2009) Feeding management of elite endurance horses. The Veterinary Clinics of North America. Equine Practice 25, 137–153.
| Feeding management of elite endurance horses.Crossref | GoogleScholarGoogle Scholar | 19303556PubMed |
Henneke DR, Potter GD, Kreider JL, Yeates BF (1983) Relationship between condition score, physical measurements and body fat percentage in mares. Equine Veterinary Journal 15, 371–372.
| Relationship between condition score, physical measurements and body fat percentage in mares.Crossref | GoogleScholarGoogle Scholar | 6641685PubMed |
Hoffman R, Wilson J, Kronfeld D, Cooper W, Lawrence L, Sklan D, Harris P (2001) Hydrolyzable carbohydrates in pasture, hay, and horse feeds: direct assay and seasonal variation. Journal of Animal Science 79, 500–506.
| Hydrolyzable carbohydrates in pasture, hay, and horse feeds: direct assay and seasonal variation.Crossref | GoogleScholarGoogle Scholar | 11219461PubMed |
Hoffman CJ, Costa LR, Freeman LM (2009) Survey of feeding practices, supplement use, and knowledge of equine nutrition among a subpopulation of horse owners in New England. Journal of Equine Veterinary Science 29, 719–726.
| Survey of feeding practices, supplement use, and knowledge of equine nutrition among a subpopulation of horse owners in New England.Crossref | GoogleScholarGoogle Scholar |
Hudson JM, Cohen ND, Gibbs PG, Thompson JA (2001) Feeding practices associated with colic in horses. Journal of the American Veterinary Medical Association 219, 1419–1425.
| Feeding practices associated with colic in horses.Crossref | GoogleScholarGoogle Scholar | 11724182PubMed |
Ince J, Longland A, Moore-Colyer M, Newbold C, Harris P (2005) A pilot study to estimate the intake of grass by ponies with restricted access to pasture. In ‘Proceedings of the British Society of Animal Science Annual Conference’, York, UK, April 2005. pp. 4–6. (BSAS: UK)
Janis C (1976) The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30, 757–774.
| The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion.Crossref | GoogleScholarGoogle Scholar | 28563331PubMed |
Jensen R, Austbø D, Bach KK, Tauson A (2014) The effect of dietary carbohydrate composition on apparent total tract digestibility, feed mean retention time, nitrogen and water balance in horses. Animal 8, 1788–1796.
| The effect of dietary carbohydrate composition on apparent total tract digestibility, feed mean retention time, nitrogen and water balance in horses.Crossref | GoogleScholarGoogle Scholar | 25018093PubMed |
Julliand V (2005) Impact of nutrition on the microflora of the gastro-intestinal tract in horses. In ‘Applied Equine Nutrition: Equine Nutrition Conference (ENUCO)’. (Ed. A Lindner) pp. 85–103. (Wageningen Academic Publishers: The Netherlands)
Julliand V, de Fombelle A, Drogoul C, Jacotot E (2001) Feeding and microbial disorders in horses: 3-effects of three hay:grain ratios on microbial profile and activities. Journal of Equine Veterinary Science 21, 543–546.
| Feeding and microbial disorders in horses: 3-effects of three hay:grain ratios on microbial profile and activities.Crossref | GoogleScholarGoogle Scholar |
Kuntz R, Kubalek C, Ruf T, Tataruch F, Arnold W (2006) Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. The Journal of Experimental Biology 209, 4557–4565.
| Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake.Crossref | GoogleScholarGoogle Scholar | 17079725PubMed |
LaCasha PA, Brady HA, Allen VG, Richardson CR, Pond KR (1999) Voluntary intake, digestibility, and subsequent selection of matua bromegrass, coastal bermudagrass, and alfalfa hays by yearling horses. Journal of Animal Science 77, 2766–2773.
| Voluntary intake, digestibility, and subsequent selection of matua bromegrass, coastal bermudagrass, and alfalfa hays by yearling horses.Crossref | GoogleScholarGoogle Scholar | 10521039PubMed |
Lewis LD, Knight A, Lewis B, Lewis C (1995) ‘Equine clinical nutrition: feeding and care.’ (Wiley- Blackwell: Baltimore, MD, USA)
McGreevy PD, Webster AJF, Nicol CJ (2001) Study of the behaviour, digestive efficiency and gut transit times of crib-biting horses. The Veterinary Record 148, 592–596.
| Study of the behaviour, digestive efficiency and gut transit times of crib-biting horses.Crossref | GoogleScholarGoogle Scholar | 11386445PubMed |
Moore-Colyer MJ, Morrow H, Longland A (2003) Mathematical modelling of digesta passage rate, mean retention time and in vivo apparent digestibility of two different lengths of hay and big-bale grass silage in ponies. British Journal of Nutrition 90, 109–118.
| Mathematical modelling of digesta passage rate, mean retention time and in vivo apparent digestibility of two different lengths of hay and big-bale grass silage in ponies.Crossref | GoogleScholarGoogle Scholar |
Morel P (2010) Effect of age, live weight and dry matter intake on total tract nutrient digestibility in adult mares. In ‘Proceedings of the New Zealand Society of Animal Production. Vol. 70’, Palmerston North, New Zealand. pp. 152–156. (NZSAP: New Zealand)
Muhonen S, Julliand V, Lindberg JE, Bertilsson J, Jansson A (2009) Effects on the equine colon ecosystem of grass silage and haylage diets after an abrupt change from hay. Journal of Animal Science 87, 2291–2298.
| Effects on the equine colon ecosystem of grass silage and haylage diets after an abrupt change from hay.Crossref | GoogleScholarGoogle Scholar | 19329474PubMed |
Muhonen S, Wartena FC, Wesker A, Julliand V (2010) Effect of three different forage-based diets on microbial flora, pH and viscosity of the equine hindgut. In ‘The impact of nutrition on the health and welfare of horses’. (Eds A Ellis, A Longland, M Coenen, N Miraglia) pp. 196–198. (Wageningen Academic Publishers: The Netherlands)
Müller CE, Uden P (2007) Preference of horses for grass conserved as hay, haylage or silage. Animal Feed Science and Technology 132, 66–78.
| Preference of horses for grass conserved as hay, haylage or silage.Crossref | GoogleScholarGoogle Scholar |
Müller CE, von Rosen D, Udén P (2008) Effect of forage conservation method on microbial flora and fermentation pattern in forage and in equine colon and faeces. Livestock Science 119, 116–128.
| Effect of forage conservation method on microbial flora and fermentation pattern in forage and in equine colon and faeces.Crossref | GoogleScholarGoogle Scholar |
National Research Council (2007) ‘Nutrient requirements of horses.’ (National Academies Press: Washington, DC, USA)
Patel D, Thomas D, Gee E, Bolwell C, Rogers C (2014) Voluntary feed intake of ensiled lucerne/timothy and red clover hay by adult thoroughbred geldings. In ‘Proceedings of the Australasian Equine Science Symposium. Vol. 5’, Gold Coast, Qld, Australia. pp. 18. (AESS: Australia)
Pearson RA, Merritt JB (1991) Intake, digestion and gastrointestinal transit-time in resting donkeys and ponies and exercised donkeys given ad-libitum hay and straw diets. Equine Veterinary Journal 23, 339–343.
| Intake, digestion and gastrointestinal transit-time in resting donkeys and ponies and exercised donkeys given ad-libitum hay and straw diets.Crossref | GoogleScholarGoogle Scholar | 1959523PubMed |
Pearson RA, Archibald RF, Muirhead RH (2001) The effect of forage quality and level of feeding on digestibility and gastrointestinal transit time of oat straw and alfalfa given to ponies and donkeys. British Journal of Nutrition 85, 599–606.
| The effect of forage quality and level of feeding on digestibility and gastrointestinal transit time of oat straw and alfalfa given to ponies and donkeys.Crossref | GoogleScholarGoogle Scholar |
Pearson RA, Archibald RF, Muirhead RH (2006) A comparison of the effect of forage type and level of feeding on the digestibility and gastrointestinal mean retention time of dry forages given to cattle, sheep, ponies and donkeys. British Journal of Nutrition 95, 88–98.
| A comparison of the effect of forage type and level of feeding on the digestibility and gastrointestinal mean retention time of dry forages given to cattle, sheep, ponies and donkeys.Crossref | GoogleScholarGoogle Scholar |
Ragnarsson S, Lindberg JE (2010) Impact of feeding level on digestibility of a haylage-only diet in Icelandic horses. Journal of Animal Physiology and Animal Nutrition 94, 623–627.
| Impact of feeding level on digestibility of a haylage-only diet in Icelandic horses.Crossref | GoogleScholarGoogle Scholar | 19912427PubMed |
Randall L, Rogers C, Hoskin S, Swainson N (2014) Preference for different pasture grasses by horses in New Zealand. In ‘Proceedings of the New Zealand Society of Animal Production. Vol. 74’, Napier, New Zealand. pp. 79–84. (NZSAP: New Zealand)
Rodiek AV, Jones BE (2012) Voluntary Intake of Four Hay Types by Horses. Journal of Equine Veterinary Science 32, 579–583.
| Voluntary Intake of Four Hay Types by Horses.Crossref | GoogleScholarGoogle Scholar |
Rodrigues LM, de Almeida FQ, Pereira MB, Miranda ACT, Guimarães A, de Andrade AM (2012) Roughage digestion evaluation in horses with total feces collection and mobile nylon bags. Revista Brasileira de Zootecnia 41, 341–346.
| Roughage digestion evaluation in horses with total feces collection and mobile nylon bags.Crossref | GoogleScholarGoogle Scholar |
Rogers CW, Gee EK, Firth EC (2007) A cross-sectional survey of Thoroughbred stud farm management in the North Island of New Zealand. New Zealand Veterinary Journal 55, 302–307.
| A cross-sectional survey of Thoroughbred stud farm management in the North Island of New Zealand.Crossref | GoogleScholarGoogle Scholar | 18059648PubMed |
Rogers CW, Gee EK, Bolwell CF, Rosanowski SM (2020) Commercial equine production in New Zealand 2: growth and development of the equine athlete. Animal Production Science 60, 2155–2163.
| Commercial equine production in New Zealand 2: growth and development of the equine athlete.Crossref | GoogleScholarGoogle Scholar |
Rosenfeld I, Austbo D, Volden H (2006) Models for estimating digesta passage kinetics in the gastrointestinal tract of the horse. Journal of Animal Science 84, 3321–3328.
| Models for estimating digesta passage kinetics in the gastrointestinal tract of the horse.Crossref | GoogleScholarGoogle Scholar | 17093224PubMed |
Särkijärvi S, Sormunen-Cristian R, Heikkilä T, Rinne M, Saastamoinen M (2012) Effect of grass species and cutting time on in vivo digestibility of silage by horses and sheep. Livestock Science 144, 230–239.
| Effect of grass species and cutting time on in vivo digestibility of silage by horses and sheep.Crossref | GoogleScholarGoogle Scholar |
Staniar W, Bussard J, Repard N, Hall M, Burk A (2010) Voluntary intake and digestibility of teff hay fed to horses. Journal of Animal Science 88, 3296–3303.
| Voluntary intake and digestibility of teff hay fed to horses.Crossref | GoogleScholarGoogle Scholar | 20581289PubMed |
Stowers NL, Rogers CW, Hoskin SO (2009) Management of weanlings on commercial Throughbred stud farms in the North Island of New Zealand. In ‘Proceedings of the New Zealand Society of Animal Production. Vol. 69’, Christchurch, New Zealand. pp. 4–9. (NZSAP: New Zealand)
Udén P, Rounsaville T, Wiggans G, Van Soest P (1982) The measurement of liquid and solid digesta retention in ruminants, equines and rabbits given timothy (Phleum pratense) hay. British Journal of Nutrition 48, 329–339.
| The measurement of liquid and solid digesta retention in ruminants, equines and rabbits given timothy (Phleum pratense) hay.Crossref | GoogleScholarGoogle Scholar |
Verhaar N, Rogers CW, Gee E, Bolwell C, Rosanowski SM (2014) The feeding practices and estimated workload in a cohort of New Zealand competition horses. Journal of Equine Veterinary Science 34, 1257–1262.
| The feeding practices and estimated workload in a cohort of New Zealand competition horses.Crossref | GoogleScholarGoogle Scholar |
Wood IJ, Lancaster BE, Rogers CW (2019) The feeding and management of Thoroughbred and Standardbred Racehorses displaying clinical signs of recurrent exertional rhabdomyolysis. New Zealand Journal of Animal Science and Production 79, 26–31.