Effects of phytase supplementation on growth performance, plasma biochemistry, bone mineralisation and phosphorus utilisation in pre-lay pullets fed various levels of phosphorus
Mingyan Jing A , Shusheng Zhao A , Anna Rogiewicz B , Bogdan A. Slominski B and James D. House A B C
A B C
A Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB, R3T 2N2, Canada.
B Department of Animal Science, University of Manitoba, Winnipeg, MB, R3T 2N2, Canada.
C Corresponding author. Email: James.House@umanitoba.ca
Animal Production Science 61(6) 568-576 https://doi.org/10.1071/AN20265
Submitted: 9 June 2020 Accepted: 24 November 2020 Published: 23 February 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Context: Reducing the environmental impact of animal production is becoming a really hot topic, especially with raised concerns over excessive flows of nitrogen and phosphorus (P) to the environment.
Aims: The present study was conducted to determine the effects of phytase supplementation on growth, plasma biochemistry, bone mineralisation and P utilisation of pre-lay pullets fed varying levels of non-phytate P.
Methods: A total of 240 Lohmann pullet chicks were randomly allocated to one of six dietary treatments with eight replicate cages (5 birds per cage) per treatment. Six treatments included three phytase-free diets and three diets supplemented with 1000 U/kg phytase; the non-phytate P levels were 2.75–2.50–2.25, 3.75–3.50–3.25 and 4.75–4.50–4.25 g/kg in the former, and 1.75–1.50–1.25, 2.75–2.50–2.25 and 3.75–3.50–3.25 g/kg in the latter, for the age of 0–4, 4–8 and 8–16 weeks respectively.
Key results: No significant differences were found for growth performance, plasma biochemistry (calcium, P, alkaline phosphatase and albumin) and bone mineralisation among dietary treatments, but P retention (%) was different (P < 0.001). Analysis of planned contrasts showed that phytase supplementation increased phytate P retention (P < 0.001), and improving the utilisation of phytate P tended most efficiently under low P conditions. Total P retention rate was reduced slightly by phytase supplementation (P < 0.05).
Conclusions: The results indicated that dietary non-phytate P level could possibly be reduced to 1.75, 1.50 and 1.25 g/kg for 0–4, 4–8 and 8–16 weeks of age respectively after phytase supplementation, without compromising pullet growth and performance during the pre-laying period.
Implications: The results of this study will contribute to decreasing P excretion by poultry and reducing the potential environmental impact with land application of manure.
Keywords: characteristics of bones, growth, phosphorus retention, phytase.
Introduction
A large proportion (~80%) of phosphorus (P) in many plant seeds is in the form of phytate (myo-inositol hexakisphosphate; Marounek et al. 2008). Monogastric animals, including poultry, have a very limited capacity to hydrolyse phytate, due to an insufficient amount of endogenous phytase in their gastrointestinal tracts. To overcome this problem, supplementation of inorganic P or non-phytate P (NPP) to diets has become necessary to meet the animal’s nutritional needs. The results of many studies (Gordon and Roland 1997; Keshavarz 1998, 2000a; Boling et al. 2000; Jing et al. 2018a) indicate that the NPP requirement for laying hens may be lower than the 2.5 g/kg minimum recommended by the National Research Council (NRC 1994). For the pre-laying rations, Keshavarz (2000b) suggested that the NPP levels could be reduced from 4.0, 3.5 and 3.0 g/kg (NRC 1994) to 2.0, 1.5 and 1.0 g/kg for the period of 0–6, 7–12 and 13–18 weeks of age respectively without negative impacts on bird performance. Results from our recent study indicated that 2.00, 1.75 and 1.50 g/kg NPP for 0–4, 5–8 and 9–16 weeks of age respectively was adequate to support healthy growth and development of pre-lay pullets (Jing et al. 2018b).
In contrast, supplementation of microbial phytase to poultry diets is a practical method of improving P utilisation, given its ability to hydrolyse phytate to inositol and inorganic P. This allows a lower amount of P to be used in the diet, thus reducing P excretion (Viveros et al. 2002; Dersjant‐Li et al. 2015). The use of phytase in laying hen and broiler diets has been widely reported (Boling et al. 2000; Lim et al. 2003; Selle and Ravindran 2007; Li et al. 2016). However, relatively limited information is available in the literature to position responses of pre-lay pullets to exogenous phytase supplementation. We hypothesised that phytase supplementation, along with coordinated reductions in dietary NPP supply, would have no effects on the growth of pre-lay pullets and lead to reduced P excretion. Therefore, the present study was conducted to determine the effect of including microbial phytase in diets containing varying levels of NPP on growth, plasma biochemistry, bone quality and P utilisation in pullets from 0 to 16 weeks of age.
Materials and methods
Animals and housing
All animal care procedures followed a protocol approved by the University of Manitoba’s Animal Care Protocol Management and Review Committee, in accordance with the Canadian Council on Animal Care guidelines (CCAC 2009). A total of 240 Lohmann LSL-Lite pullet chicks were obtained from a local commercial hatchery on the day of hatch and randomly placed into groups of five birds per cage (68 cm wide, 37 cm high and 98 cm deep). Every cage was considered an experimental unit, and there were eight replicate units per dietary treatment (40 pullets per treatment). Lighting and room temperature were well controlled to conform to the recommended housing programs for this strain (Lohmann Tierzucht, Cuxhaven, Germany). Feed and water were offered for ad libitum consumption throughout the experiment.
Experimental design and diets
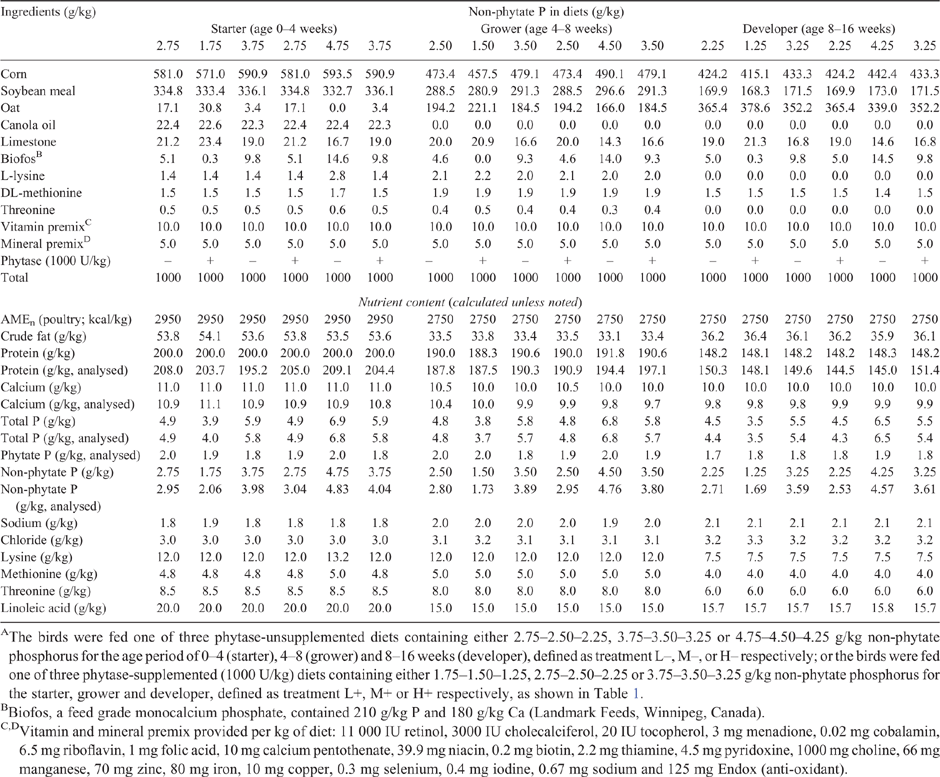
A three-phase feeding regimen was applied and identified as 0–4 weeks of age for phase I, 4–8 weeks of age for phase II and 8–16 weeks of age for phase III. Corn-soybean meal-oat-based diets were formulated to meet or exceed the nutrient specifications for each phase, as indicated in the management guide for Lohmann LSL-Lite pullets (Tierzucht 2015). The experimental design and diet composition are shown in Tables 1 and 2 respectively. Six dietary treatments were employed, which included three phytase-supplemented (1000 U/kg) diets (L+, M+ and H+), and three diets without phytase addition (L–, M– and H–). The former contained 1.0 g/kg lower NPP, as compared with the latter, based on the assumption that the supplementation of phytase is suggested to release ~1.0 g/kg NPP in the laying hen diet (Slominski 2011). Specifically, pullets in treatment L– were fed diets with no supplemental phytase containing a sequence of 2.75, 2.50 and 2.25 g/kg NPP, and pullets in treatment L+ were fed phytase-supplemented diets containing a sequence of 1.75, 1.50 and 1.25 g/kg NPP, for the age phase of 0–4, 4–8 and 8–16 weeks respectively. The NPP level was increased by an increment of 1.0 g/kg in each phase, thus making the other two phytase-unsupplemented treatments M– and H–, where the NPP levels were 3.75–3.50–3.25 g/kg and 4.75–4.50–4.25 g/kg, and two phytase-supplemented treatments M+ and H+ where the NPP levels were 2.75–2.50–2.25 g/kg and 3.75–3.50–3.25 g/kg for the three phases respectively. The phytase product (Bio-Phytase 5000) was obtained from Canadian Bio-Systems (Calgary, Canada), and contained a minimum phytase activity of 5000 units (FTU)/g (EC 3.1.3.26). Diets were analysed for crude protein (method 990.03), calcium (Ca; method 968.08) and total P (method 965.17) according to the Association of Official Analytical Chemists (AOAC International 2005). Phytate P was analysed as described by Haug and Lantzsch (1983). The phytate P extraction was conducted by adding 10 mL 0.2 N HCl to 0.1 g ground sample and placed on the tube rotator at room temperature for 3 h. The suspension was filtered and 1 mL filtrate was transferred into a hydrolysis tube, followed by the addition of 2 mL of ferric ammonium sulfate solution. The mixture of this solution was boiled at 100°C for 30 min and left it at room temperature, and then centrifuged at 1008g at room temperature for 30 min. Then, 1 mL of this supernatant was mixed with 1.5 mL of 2,2′-bipyridine solution with a vortex. After the mixing, the mixture solution was incubated for 10 min and the light absorbance was measured with a spectrophotometer at 519 nm against deionised water. The NPP content was calculated by subtracting phytate P from total P.

|
|
Sample collection and analysis
Bodyweight and feed consumption were recorded every week for 16 weeks. At the mid (Week 8) and end (Week 16) of the experiment, 3-mL blood samples drawn from the wing vein from two birds per cage were collected for biochemical assays using an automated analyser (Cell-Dyn 3500 System; Abbott Laboratories, Abbott Park, IL, USA); and digestibility studies were conducted to determine mineral excretion and retention, as described previously (Jing et al. 2018b). Details of the procedures for sample preparation and determination of Ca and P were described previously by our research group (Neijat et al. 2011; Jing et al. 2018b). At the end of the experiment, two birds from each cage were killed by CO2 asphyxiation, and the left tibias were removed and stored frozen before the determination of bone mineral density and ash content, according to procedures described previously (Jing et al. 2018b).
Statistical analyses
The cage was considered as the experimental unit, and all calculations were generated based on cage averages. Data were analysed as a repeated measures design (on the cages) using the MIXED procedure of SAS (SAS Institute, Cary, NC, USA), including fixed effects of diet, age and diet × age, as well as the random effect of cage within diet. Bone measurements were conducted at the end of the study, so the analysis of these data considered only diet effects. Means were compared using Tukey’s test, with a significance level set at P < 0.05. When the overall ANOVA showed significant differences among diets, orthogonal polynomial contrasts were applied to further examine the distinct effects of dietary NPP and phytase, the two dietary factors. Any data values with standardised residuals >3 in absolute value were considered outliers and excluded from the analysis.
Results
Growth performance
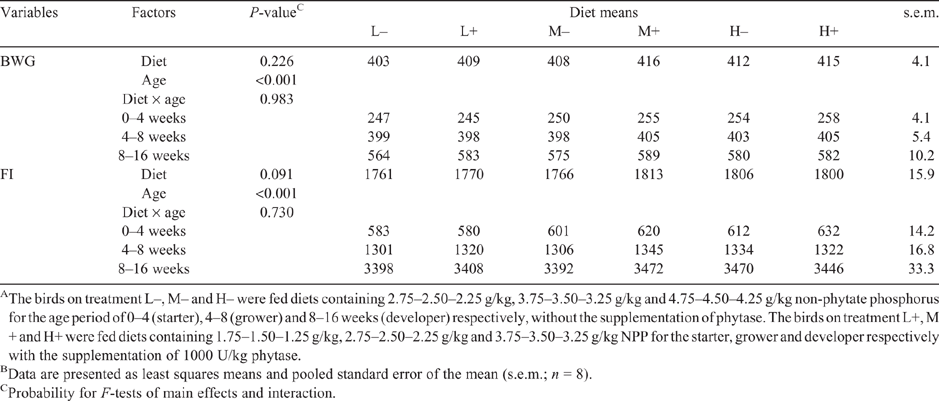
Bodyweight gain and feed intake during the entire experimental period are presented in Table 3. No significant differences (P > 0.05) were found for bodyweight gain and feed intake among diets. As expected, bodyweight gain and feed intake increased with increasing age (P < 0.001). There was no diet × age interaction for either parameter (P > 0.05).
|
Plasma biochemistry
Diet had no effect (P > 0.05) on plasma biochemical indices, except uric acid (Table 4). Contrast analysis showed that plasma uric acid was not influenced by phytase supplementation (P > 0.05), but linearly reduced (P < 0.01) with increasing levels of NPP in diets devoid of phytase (data not shown). Most plasma biochemical constituents were differentially affected by age (P < 0.01). No diet × age interaction effect was observed to be significant for any of the studied variables (P > 0.05). Additional plasma biochemistry data can be found in Table S1, available as Supplementary Material to this paper.

|
Bone mineralisation
Parameters of bone mineralisation, including tibia bone mineral density, tibia weight and tibia ash at 16 weeks of age, were not different among diets (P > 0.05; Table 5).

|
Calcium and P retention
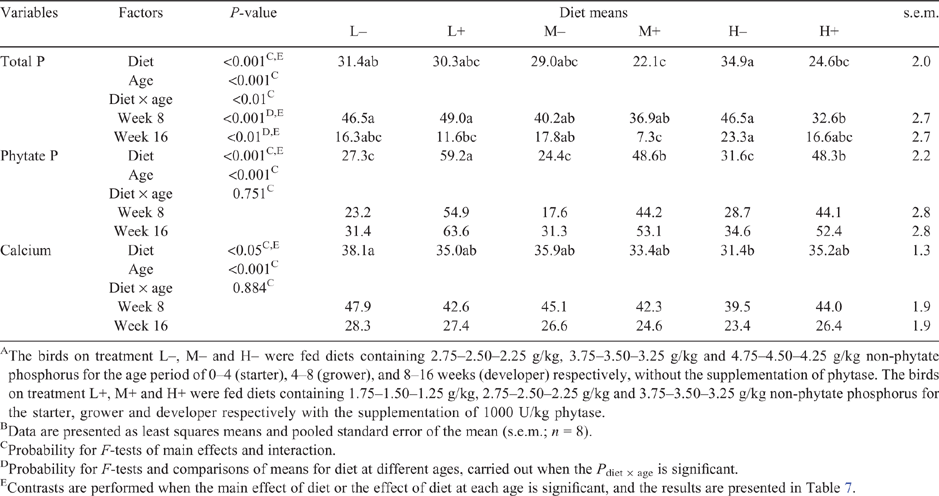
Retention (%) of total P, phytate P and Ca is shown in Table 6 and Table 7. Considering the characteristics of the experimental design, contrast analysis in Table 7 was performed on the basis of the results of Table 6 to further evaluate the distinct effects of phytase and NPP level, the two dietary factors studied. Data of intake, excretion and retention (mg/bird·day) of total P, phytate P and Ca can be found in Table S2a and Table S2b, available as Supplementary Material to this paper.

|
Calcium retention was different (P < 0.05; Table 6) between L– and H–, the two dietary regimens devoid of phytase. This could be closely associated with the variation of analysed dietary Ca for the age period of 4–8 weeks, being highest in the former (10.4 g/kg) and lower in the latter (9.8 g/kg), as shown in Table 2. Furthermore, contrast analysis (Table 7) consistently showed that the retention of calcium was not affected by phytase supplementation (P > 0.05), and the changing trend followed its analysed levels in the three phytase-unsupplemented diets. Total P and phytate P retention was different among diets (P < 0.001; Table 6). Contrast analysis further showed that phytase supplementation increased phytate P retention, but reduced total P retention (P < 0.05; Table 7). Phytate P retention (%) of pullets fed the three phytase-supplemented diets increased, on average, by 24.2% compared with that of pullets fed the three phytase-unsupplemented diets. Furthermore, when phytase was included in the diets, the retention of phytate P tended to have quadratic responses to the gradient of dietary NPP (P = 0.064; Table 7), being highest in the birds from the treatment L+; however, no significant differences were found between M+ and H+ (Table 6). There was a 6.1% reduction in total P retention (%) of the three phytase-supplemented diets compared with the three phytase-unsupplemented diets. The age of the bird was observed to have an effect on those parameters of nutrient flow (P < 0.001), and an interaction effect between diet and age was also observed for total P retention (P < 0.01).
Discussion
In striving for sustainable agricultural practices, maintaining competitive feed costs and performance while minimising environmental impacts is challenging the poultry sector. The essential mineral P is a prime example of a nutrient of concern, given the potential for excess environmental P. To partially address this concern, focus has turned to P supply strategies, including the investigation of responses of birds to P supply and phytase supplementation in diets. The present study was designed to examine the influence of phytase supplementation on performance, clinical biochemistry, bone mineralisation and P utilisation of pre-lay pullets fed diets containing varying levels of NPP.
In our previous study in which supplemental phytase was not included (Jing et al. 2018b), a significant reduction in bodyweight gain, feed intake and plasma P was observed at the age of 8 weeks, when pullets were fed 2.00, 1.75 and 1.50 g/kg NPP (the lower end) for the age period of 0–4, 5–8 and 9–16 weeks respectively. However, the present study showed that supplementing phytase to diets containing lower NPP resulted in growth performance and plasma P comparable to those obtained from pullets that were fed diets containing high NPP. This was most obvious when the birds were offered 1.75, 1.50 and 1.25 g/kg NPP for the three age periods respectively, an NPP regimen even lower than that used in our previous study. These results indicated that phytase supplementation corrected the reduction in pullet performance and plasma P of birds fed low P diets. Furthermore, no changes were found in plasma biochemical indices among treatments. This might indicate that birds function normally under the current dietary regimens. Particularly, the current data showed that plasma uric acid decreased with increasing levels of NPP exclusively in the phytase-free groups. Our previous study also showed reduced uric acid in plasma of pullets as dietary NPP increased (Jing et al. 2018b). This indicates that dietary P may regulate intestinal and/or renal uric acid transporters, such as adenosine triphosphate-binding cassette transporter G2 (Bhatnagar et al. 2016). The mechanism underlying dietary P regulation of uric acid metabolism is not yet known. Studies in humans showed an inverse relationship between P excretion and uric acid excretion (Sakoh et al. 2016). The authors suggested a possible role of parathyroid hormone and fibroblast growth factor 23 in regulating P and uric acid homeostasis (Sakoh et al. 2016; Sugimoto et al. 2017). Punna and Roland (1999) indicated that the addition of phytase in pullet diets from Day 1 of age through the first lay cycle (Week 21 to 36) could prevent reductions in performance of pullets fed 1.0 g/kg NPP diets. Further investigations of the effect of pre-lay diet on subsequent laying performance and egg quality are still warranted.
No significant differences were found in the parameters used to assess bone mineralisation (tibia bone mineral density, tibia weight, tibia ash percent) in the current study. These results could be interpreted as: (i) any potential bone mineralisation compromise under the lowest NPP regimen (1.75–1.50–1.25 g/kg) was corrected with phytase supplementation; or (ii) bone mineralisation could be well maintained at this level of NPP and supplemental phytase had no further effects. The latter could be supported to some extent by previous research. Our earlier pullet study showed that bone quality was not affected when lowering dietary NPP from 5.00, 4.75 and 4.50 g/kg to 2.00, 1.75 and 1.50 g/kg for the age period of 0–4, 5–8 and 9–16 weeks respectively (Jing et al. 2018b). Keshavarz (2000b) also reported that no significant differences were found for tibia weight and tibia ash when pullets were fed different NPP ranging from 2.0, 1.5 and 1.0 g/kg (the lower end) to 4.0, 3.5 and 3.0 g/kg (the higher end) for the period of 0–6, 7–12 and 13–18 weeks of age respectively, and phytase did not have an effect on bone quality. However, subsequent effects of this low NPP regimen in the pre-lay ration on bone quality during the laying period still need to be investigated. An early study by Douglas and Harms (1986) showed that bone ash percentage measured at 20 weeks was not affected by feeding less P during the pre-lay period.
Results of the digestibility assay demonstrated that phytase supplementation significantly improved the utilisation of phytate P. The retention (%) of phytate P, on average, was 52.0% and 27.8% respectively in the three phytase-supplemented diets and three phytase-unsupplemented diets. Without considering dietary NPP level, phytase supplementation, overall, contributed to a 24.2% increase in the availability of phytate P. Additionally, the current results also indicated that the extent of enhancement in phytate P utilisation was influenced by dietary P status. Phytate P retention responded in a quadratic manner to increasing levels of dietary NPP, being highest in the lowest NPP regimen and comparable between the other two NPP regimens, suggesting that the action of phytase was most efficient in birds under low P conditions. Other studies have shown that high levels of dietary inorganic P reduced phytate P availability in chickens, possibly due to the inhibitory effect of P on phytase activity, reflecting a negative feedback mechanism elicited by excessive amounts of dietary P (Ravindran et al. 2000; Ankra-Badu et al. 2004). Further studies are still needed to examine the impact of dietary inorganic P supply on the P-releasing efficacy of phytase. In contrast, in agreement with the results of our previous study (Jing et al. 2018b), the current data showed that phytate P retention (%) was higher at Week 16 than Week 8, indicating that phytate P could be more efficiently utilised by older pullets than young pullets. Other researchers reported that the utilisation of phytate P increased as the age of laying hens increased (Maddaiah et al. 1964; Marounek et al. 2008), possibly due to more endogenous phytase present in the gastrointestinal tract of older birds (Marounek et al. 2010). The current data showed that endogenous phytase generally contributed 27.8% of the phytate P utilisation, based on the calculation of the average of phytate P retention rates derived from the three phytase-unsupplemented diets. In the present study, it was hypothesised that supplementation of phytase could provide 1.0 g/kg NPP to the birds (Slominski 2011). However, this assumption may not be entirely accurate based on the results of total P retention, as the latter differed slightly between the phytase-supplemented and phytase-unsupplemented diets. This difference may be due to the influence of various factors related to phytase, diets and animals used in research (Leske and Coon 1999; Slominski 2011; Dersjant-Li et al. 2015). Interestingly, phytase supplementation led to a decrease in total P retention when the birds were fed certain levels of NPP, which was supported by results of planned contrasts between the average of two phytase-present dietary regimens (M+ and H+), and the average of two phytase-absent dietary regimens (L– and M–), where the NPP levels were 2.75–2.50–2.25 or 3.75–3.50–3.25 g/kg. It was inferred that 2.75, 2.50 and 2.25 g/kg NPP for the age of 0–4, 4–8 and 8–16 weeks respectively was adequate for pullet growth and bone health, based on the results of our current and previous study (Jing et al. 2018b), as well as other research reports (Keshavarz 2000b). The present data suggested that the reduced retention of total P could be an indication of a reduction in P availability, when phytase was supplemented to diets that contained adequate or sufficient NPP. In other words, the addition of phytase without properly reducing dietary NPP could yield negative environmental impacts. Our results differ from those of Keshavarz (2000b), who reported that phytase did not have an effect on total P retention in pullets fed various NPP regimens. The role of dietary NPP status may need to be considered in evaluating the effect of phytase on P utilisation.
In conclusion, the present study showed that adding microbial phytase (1000 U/kg) to the pre-lay ration significantly improved phytate P availability. Moreover, supplementing phytase to a diet containing low levels of NPP resulted in performance and bone mineralisation comparable to that obtained from pullets fed higher levels of NPP. These results confirmed that dietary NPP inclusion could be reduced through phytase supplementation, and under the present experimental conditions, its level could be reduced to 1.75, 1.50 and 1.25 g/kg for 0–4, 4–8 and 8–16 weeks of age respectively without affecting bone mineralisation and growth of pre-lay pullets. Additionally, the present study indicated that the effect of phytase on P utilisation might be mediated by dietary P status.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors greatly thank Manitoba Agriculture, Food and Rural Initiatives, and Manitoba Egg Farmers for their generous financial support. Statistical assistance from G. H. Crow and L. Onischuk (both from Department of Animal Science, University of Manitoba) is highly appreciated.
References
Ankra-Badu GA, Aggrey SE, Pesti GM, Bakalli RI, Edwards HM (2004) Modeling of parameters affecting phytate phosphorus bioavailability in growing birds. Poultry Science 83, 1083–1088.| Modeling of parameters affecting phytate phosphorus bioavailability in growing birds.Crossref | GoogleScholarGoogle Scholar | 15285496PubMed |
AOAC International (2005) ‘Association of official analytical chemists. Official methods of analysis.’ 18th edn. (AOAC International: Washington, DC)
Bhatnagar V, Richard EL, Wu W, Nievergelt CM, Lipkowitz MS, Jeff J, Maihofer AX, Nigam SK (2016) Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clinical Kidney Journal 9, 444–453.
| Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling.Crossref | GoogleScholarGoogle Scholar | 27274832PubMed |
Boling SD, Douglas MW, Johnson ML, Wang X, Parsons CM, Koelkebeck KW, Zimmerman RA (2000) The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens. Poultry Science 79, 224–230.
| The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens.Crossref | GoogleScholarGoogle Scholar | 10735751PubMed |
CCAC (2009) ‘Guidelines on: the care and use of farm animals in research, teaching and testing.’ Vol. II. (Canadian Council on Animal Care: Ottawa)
Dersjant-Li Y, Awati A, Schulze H, Partridge G (2015) Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. Journal of the Science of Food and Agriculture 95, 878–896.
| Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors.Crossref | GoogleScholarGoogle Scholar | 25382707PubMed |
Douglas CR, Harms RH (1986) Phosphorus requirement of commercial Leghorn pullets from 8 to 20 weeks. Poultry Science 65, 2366–2368.
| Phosphorus requirement of commercial Leghorn pullets from 8 to 20 weeks.Crossref | GoogleScholarGoogle Scholar | 3575224PubMed |
Gordon RW, Roland DA (1997) Performance of commercial laying hens fed various phosphorus levels with and without supplemental phytase. Poultry Science 76, 1172–1177.
| Performance of commercial laying hens fed various phosphorus levels with and without supplemental phytase.Crossref | GoogleScholarGoogle Scholar | 9251148PubMed |
Haug W, Lantzsch H-J (1983) Sensitive method for the rapid determination of phytate in cereals and cereal products. Journal of the Science of Food and Agriculture 34, 1423–1426.
| Sensitive method for the rapid determination of phytate in cereals and cereal products.Crossref | GoogleScholarGoogle Scholar |
Jing M, Zhao S, Rogiewicz A, Slominski BA, House JD (2018a) Assessment of the minimal available phosphorus needs of laying hens: implications for phosphorus management strategies. Poultry Science 97, 2400–2410.
| Assessment of the minimal available phosphorus needs of laying hens: implications for phosphorus management strategies.Crossref | GoogleScholarGoogle Scholar | 29617962PubMed |
Jing M, Zhao S, Rogiewicz A, Slominski BA, House JD (2018b) Assessment of the minimal available phosphorus needs of pullets during the pre-laying period. Poultry Science 97, 557–567.
| Assessment of the minimal available phosphorus needs of pullets during the pre-laying period.Crossref | GoogleScholarGoogle Scholar | 29077938PubMed |
Keshavarz K (1998) Further investigations on the effect of dietary manipulation of protein, phosphorus, and calcium for reducing their daily requirement for laying hens. Poultry Science 77, 1333–1346.
| Further investigations on the effect of dietary manipulation of protein, phosphorus, and calcium for reducing their daily requirement for laying hens.Crossref | GoogleScholarGoogle Scholar | 9733121PubMed |
Keshavarz K (2000a) Nonphytate phosphorus requirement of laying hens with and without phytase on a phase feeding program. Poultry Science 79, 748–763.
| Nonphytate phosphorus requirement of laying hens with and without phytase on a phase feeding program.Crossref | GoogleScholarGoogle Scholar | 10824965PubMed |
Keshavarz K (2000b) Reevaluation of nonphytate phosphorus requirement of growing pullets with and without phytase. Poultry Science 79, 1143–1153.
| Reevaluation of nonphytate phosphorus requirement of growing pullets with and without phytase.Crossref | GoogleScholarGoogle Scholar | 10947183PubMed |
Leske KL, Coon CN (1999) A bioassay to determine the effect of phytase and phytate-phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poultry Science 78, 1151–1157.
| A bioassay to determine the effect of phytase and phytate-phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens.Crossref | GoogleScholarGoogle Scholar | 10472841PubMed |
Li W, Angel R, Kim SW, Brady K, Yu S, Plumstead PW (2016) Impacts of dietary calcium, phytate, and nonphytate phosphorus concentrations in the presence or absence of phytase on inositol hexakisphosphate (IP6) degradation in different segments of broilers digestive tract. Poultry Science 95, 581–589.
| Impacts of dietary calcium, phytate, and nonphytate phosphorus concentrations in the presence or absence of phytase on inositol hexakisphosphate (IP6) degradation in different segments of broilers digestive tract.Crossref | GoogleScholarGoogle Scholar | 26740131PubMed |
Lim HS, Namkung H, Paik IK (2003) Effects of phytase supplementation on the performance, egg quality, and phosphorous excretion of laying hens fed different levels of dietary calcium and nonphytate phosphorous. Poultry Science 82, 92–99.
| Effects of phytase supplementation on the performance, egg quality, and phosphorous excretion of laying hens fed different levels of dietary calcium and nonphytate phosphorous.Crossref | GoogleScholarGoogle Scholar | 12580249PubMed |
Maddaiah VT, Kurnick AA, Reid BL (1964) Phytic acid studies. Proceedings of the Society for Experimental Biology and Medicine 115, 391–393.
| Phytic acid studies.Crossref | GoogleScholarGoogle Scholar | 14121619PubMed |
Marounek M, Skřivan M, Dlouhá G, Břeňová N (2008) Availability of phytate phosphorus and endogenous phytase activity in the digestive tract of laying hens 20 and 47 weeks old. Animal Feed Science and Technology 146, 353–359.
| Availability of phytate phosphorus and endogenous phytase activity in the digestive tract of laying hens 20 and 47 weeks old.Crossref | GoogleScholarGoogle Scholar |
Marounek M, Skřivan M, Rosero O, Rop O (2010) Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat-maize-soybean diet. Journal of Animal and Feed Sciences 19, 430–439.
| Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat-maize-soybean diet.Crossref | GoogleScholarGoogle Scholar |
Neijat M, House JD, Guenter W, Kebreab E (2011) Calcium and phosphorus dynamics in commercial laying hens housed in conventional or enriched cage systems. Poultry Science 90, 2383–2396.
| Calcium and phosphorus dynamics in commercial laying hens housed in conventional or enriched cage systems.Crossref | GoogleScholarGoogle Scholar | 21934024PubMed |
NRC (1994) ‘Nutrient requirements of domestic animals. Nutrient requirements of poultry.’ 9th rev. edn. (National Research Council, National Academy Press: Washington, DC)
Punna S, Roland DA (1999) Influence of supplemental microbial phytase on first cycle laying hens fed phosphorus-deficient diets from day one of age. Poultry Science 78, 1407–1411.
| Influence of supplemental microbial phytase on first cycle laying hens fed phosphorus-deficient diets from day one of age.Crossref | GoogleScholarGoogle Scholar | 10536789PubMed |
Ravindran V, Cabahug S, Ravindran G, Selle PH, Bryden WL (2000) Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorus levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. British Poultry Science 41, 193–200.
| Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorus levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention.Crossref | GoogleScholarGoogle Scholar | 10890216PubMed |
Sakoh T, Nakayama M, Tsuchihashi T, Yoshitomi R, Tanaka S, Katafuchi E, Fukui A, Shikuwa Y, Anzai N, Kitazono T, Tsuruya K (2016) Associations of fibroblast growth factor 23 with urate metabolism in patients with chronic kidney disease. Metabolism: Clinical and Experimental 65, 1498–1507.
| Associations of fibroblast growth factor 23 with urate metabolism in patients with chronic kidney disease.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Ravindran V (2007) Microbial phytase in poultry nutrition. Animal Feed Science and Technology 135, 1–41.
| Microbial phytase in poultry nutrition.Crossref | GoogleScholarGoogle Scholar |
Slominski BA (2011) Recent advances in research on enzymes for poultry diets. Poultry Science 90, 2013–2023.
| Recent advances in research on enzymes for poultry diets.Crossref | GoogleScholarGoogle Scholar | 21844268PubMed |
Sugimoto R, Watanabe H, Ikegami K, Enoki Y, Imafuku T, Sakaguchi Y, Murata M, Nishida K, Miyamura S, Ishima Y, Tanaka M, Matsushita K, Komaba H, Fukagawa M, Otagiri M, Maruyama T (2017) Downregulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney International 91, 658–670.
| Downregulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism.Crossref | GoogleScholarGoogle Scholar | 27988213PubMed |
Tierzucht L Management guide, cage housing. Available at http://www.winmixsoft.com/files/info/Lohman-lsl-classic-management-guide-eng.pdf. [Verified 23 March 2015]
Viveros A, Brenes A, Arija I, Centeno C (2002) Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poultry Science 81, 1172–1183.
| Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus.Crossref | GoogleScholarGoogle Scholar | 12211310PubMed |