Dietary chromium-methionine supplementation and broiler (22–43 days) responses during heat stress. 1. Growth performance and carcass yield, metabolisable energy and serum biochemistry
Felipe Santos Dalólio A E , Luiz Fernando Teixeira Albino A , Jadir Nogueira da Silva A , Alba Kyonara Alves Tenório Fireman B , Álvaro Mário Burin
A E , Luiz Fernando Teixeira Albino A , Jadir Nogueira da Silva A , Alba Kyonara Alves Tenório Fireman B , Álvaro Mário Burin A Centre for Agrarian Sciences, Federal University of Viçosa, Viçosa, MG 36570-900, Brazil.
B Zinpro Animal Nutrition, Piracicaba, SP 13416-310, Brazil.
C Department of Animal Science, University of São Paulo, Piracicaba, SP 13418-900, Brazil.
D Department of Animal Science, Federal University of Sergipe, Nossa Senhora da Glória, SE 4960-000, Brazil.
E Corresponding author. Email: felipesantos181@hotmail.com
Animal Production Science 61(6) 586-595 https://doi.org/10.1071/AN20140
Submitted: 11 March 2020 Accepted: 19 October 2020 Published: 14 January 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Context: Chromium (Cr) is considered a beneficial trace element. It has been reported that supplementation with Cr in the diet promotes improvements in the productive variables of broilers reared under heat stress (HS).
Aim: The study aimed to evaluate dose response of Cr as chromium-methionine (CrMet) supplementation on metabolisable energy, serum biochemistry, growth performance and carcass yield of broilers.
Methods: Three hundred and thirty-six 22-day-old male broiler chickens were randomly assigned to four blocks, six treatments (0, 0.10, 0.20, 0.40, 0.80 and 1.20 mg/kg dry matter (DM) Cr as CrMet), eight repetitions with seven birds per experimental unit, subjected to HS (33°C for 12h/day) from 22 to 43 days. The supplemented CrMet level for each variable studied was estimated using linear and quadratic regressions.
Key results: The bodyweight was quadratically affected at 35 and 43 days (P < 0.01), as well as bodyweight gain (P = 0.02) and feed conversion ratio (P = 0.01) from 22 to 43 days. A linear improvement (P = 0.03) was observed in the feed conversion ratio from 22 to 28 days and bodyweight gain for 22 to 35 days (P = 0.02). The nitrogen-corrected apparent metabolisable energy and the coefficient of metabolisation of energy were quadratically affected (P < 0.01 and P < 0.01, respectively) by CrMet levels in the diet. A quadratic response was observed on total serum cholesterol (P < 0.01), serum glucose (P = 0.07) and triacylglycerol (P < 0.01). The abdominal fat deposition was quadratically affected (P < 0.01) by CrMet levels in the diet.
Conclusions: The supplementation of 0.77 mg/kg DM Cr as CrMet improves performance, carcass characteristics and serum biochemistry parameters of broiler chickens reared under heat stress.
Implications: The results indicate that CrMet can be supplemented in the diet for broilers reared under heat stress to improve productivity of broiler chickens.
Keywords: broilers, carcass, chickens, chromium methionine, Cr, heat stress, poultry nutrition, trace mineral supplementation, tropical poultry production.
Introduction
Heat stress (HS) is a major concern in poultry production, mainly in tropical regions, due to its negative effects on feed intake (FI), bodyweight (BW) gain and carcass characteristics, as well as increased feed conversion ratio (FCR) and animal mortality which leads to financial losses (Li et al. 2018). Providing nutritional strategies to broiler chickens can help mitigate detrimental effects from exposure to HS. Chromium (Cr) is one micronutrient that stands out among the supplementation alternatives (Samanta et al. 2008; Sahin et al. 2017).
Cr has been proven to be beneficial in poultry production. Studies with dietary Cr supplementation have found improved nutrient metabolism, serum biochemistry, such as the reduction of TRI and cholesterol, and performance of broiler chickens exposed to HS (Sahin et al. 2002; Amatya et al. 2004; Zha et al. 2009; Toghyani et al. 2012; Zhang and Kim 2014; Jahanian and Rasouli 2015; Rao et al. 2016). The improvement in the productivity and biochemical parameters in broilers may be a result of Cr effect on cell sensitivity to insulin caused by the low-molecular weight Cr-binding-substance (LMWCr) (Vincent 2017). The LMWCr is a saturated oligopeptide composed of glycine, cysteine, glutamate and aspartate (Davis and Vincent 1997).
Currently nitrogen-corrected apparent metabolisable energy (AMEn) is the reference used by the poultry industry to formulate commercial diets for least-cost, since it accurately indicates nutrient-use efficiency in broilers. According to Yaghobfar (2016) the AMEn is not used with 100% of efficiency for production, because during metabolism, around 15% of the energy is wasted as heat. Cr supplementation can increase villus height, improves villus height to crypt depth ratio and decrease heat production in the jejunal mucosa of birds reared under HS (Li et al. 2018). In addition, Cr improves apparent digestibility of protein and carbohydrate, and optimises lipid metabolism by increased activity of the lipoprotein lipase with decreased cholesterol, low-density lipoprotein, triacylglycerol (TRI) and glucose (GLU), and increased high-density lipoprotein levels in association with greater feed efficiency (Króliczewska et al. 2004; Ahmed et al. 2005). Thus, Cr supplementation can promote a lower caloric increment in the diet associated with improved performance. However, there are currently no studies evaluating the effects of Cr supplementation on the AMEn values of broilers subjected to HS.
In addition to the positive effects on poultry performance, Cr supplementation can help improve meat quality by increasing lean meat yield and reducing abdominal fat (Toghyani et al. 2010; Rajalekshmi et al. 2014; Huang et al. 2016), which are carcass traits valued by consumers, who are increasingly well informed and eager to consume good-quality meat.
The aim of this study was to evaluate the dose response of dietary chromium methionine (CrMet) supplementation on metabolisable energy, serum biochemistry, performance and carcass yield (CY) of broiler chickens subjected to HS from 22 to the 43 days of age.
Materials and methods
All procedures utilised in the present study were approved by the Ethics and Research Committee of the Federal University of Viçosa, Viçosa, Brazil. The Ethics Committee approved this study (approval number 15/2016).
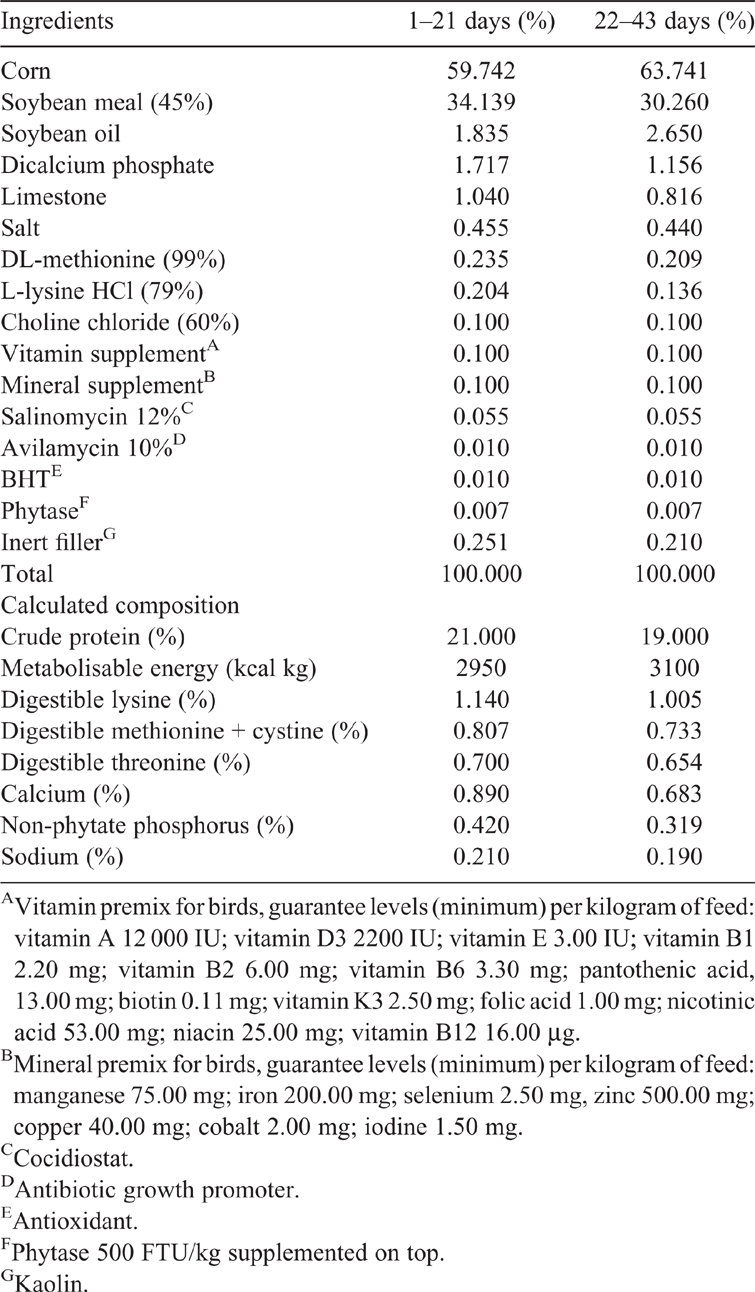
Three hundred and thirty-six 22-day-old male Cobb 500 broilers with average BW of 858.20 g (±5%) were used in the study. The experiment was started with the animals after 21 days of age because HS of 33°C causes deleterious damages in the productivity of the birds due to the increase of the muscular protein deposition that increases the production of endogenous heat. Animals were kept at thermal comfort temperature from Day 1 to Day 21, as recommended in the Cobb 500 breed line manual (Cobb-Vantress 2013). Birds were fed the same basal diet according to nutritional recommendations by Rostagno et al. (2011) for 1-to-21 days of age (Table 1).
|
During the experimental period from 22 to 43 days of age, broilers were kept in climatic chambers under HS at 33.0 ± 0.8°C for 12 h (07:00 to 18:59 hours). After this period, they were kept at 23.0 ± 0.8°C (19:00 to 06:59 hours). Relative humidity was kept at 65.0 ± 3.5% throughout the experimental period. Mash feeds and water were available for ad libitum consumption. Mortality was recorded daily. The light program used was 20 h of artificial light and 4 h of dark from 22 to 43 days of age, with three fluorescent lamps per chamber.
Environmental conditions inside the chambers were monitored twice a day (07:00 and 18:00 hours), using dry-bulb, wet-bulb and black-globe thermometers placed in the middle of the chambers, 1.20 m above the ground. Throughout the experimental period, dry bulb temperature and relative air humidity data were recorded every 5 min, on a daily basis, using Testo T/R, model H1 ( ± 0.1°C (temperature) and 1% (humidity) resolution; ± 0.5°C (temperature) and ± 1% (humidity) accuracy) and Hobo H08 Pro (–30°C to + 50°C (temperature) and 0 to 100% (humidity) resolution; ± 0.2°C (temperature) and ± 3% (humidity)) dataloggers. These data were converted to the Black Globe Temperature and Humidity Index (BGTHI) as suggested by Buffington et al. (1981).
The experiment used a completely randomised block design with four blocks (each block comprised a climatic chamber), six treatments, eight repetitions (two repetitions per block) and seven animals per experimental unit. Treatments consisted of the basal diet with one of six CrMet (Availa Cr 1000, Zinpro Corporation, Eden Prairie, MN, USA), levels (Table 1) formulated for 22- to 43-day-old broilers, according to nutritional recommendations by Rostagno et al. (2011). The six CrMet inclusion concentrations were 0.00, 0.10, 0.20, 0.40, 0.80 and 1.20 mg/kg. The hypothesis to assess the doses of CrMet used in the study was based on a previously published review article by Dalólio et al. (2018) in which the average maximum dose of 1.08 mg/kg of Cr, regardless of the source used, was optimum to improve performance and carcass characteristics.
Chemical composition of diets and Cr analysed are shown in Table 2. To determine the dry matter (DM) in basal diet, samples were dried at 105°C for 16 h (AOAC 2006; method 934.01) in a drying oven. Nitrogen (N) was determined by the total combustion of sample, Dumas method (AOAC 2006; method 968.06) to calculate the CP content (N × 6.25). Ether extract (EE) was determined by Soxhlet method (method 920.39) and the ash content was measured by burning the samples at 650°C overnight (method 942.05). Crude fibre (CF) was determined by AOAC International 2006, (method 962.09). The calcium (Ca) and phosphorus (P) were determined by AOAC International 2006 (method 984.01) and (method 965.17), respectively. The Cr quantification was performed using atomic absorption spectrophotometry (Williams et al. 1962).

|
The total excreta collection method, as described by Sibbald and Slinger (1963), was used to determine the apparent metabolisable energy (AME) and AMEn values. Excreta were collected in each experimental unit, from broilers from 38 to 42 days of age, using metal trays coated with plastic material. Samples were collected twice a day to avoid fermentation; FI was measured during the collection period. The collected excreta were placed in plastic bags, properly identified, weighed and stored in a freezer. They were homogenised at the end of the collection period and aliquots were taken and placed in a forced air circulation oven at 55°C for pre-drying purposes. Diet and excreta samples were analysed for gross energy (GE), DM, and nitrogen (N). Excreta were oven-dried and ground in a mill grinder (Retsch ZM 100, F. Kurt Retsch GmbH and Co. KG, Haan, Germany) until they passed through a 0.5-mm mesh sieve, before chemical analysis. Samples oven-dried (Uniterm, Russel-Lindsey Engineering Ltd, Birmingham, England, UK) at 105°C for 24 h (AOAC 2006; method 934.01) to determine the DM content. A Parr adiabatic bomb calorimeter was used to determine GE using benzoic acid as an internal standard (Model 6200, Parr Instruments, Moline, IL). Nitrogen was determined based on the combustion method (AOAC 2006; method 968.06). AME and AMEn values were calculated based on N and GE results of feed and excreta, using the equations by Matterson et al. (1965): AME (kcal kg–1) = (GEingested – GEexcreted)/dry matteringested; AMEn (kcal kg–1) = (GEingested − (GEexcreted + 8.22(Ningested − Nexcreted))/dry matteringested. The metabolisability coefficient was calculated by dividing AMEn by GE (CAMEn = AMEn/GE) to evaluate the use of diets.
After fasting for 12 h, two 43-day-old birds per experimental unit (total of 96 birds) were selected based on average experimental unit BW (±5%) to determine the serum concentration of total proteins (TP), albumin (AB), uric acid (UA), total cholesterol (TC), GLU and TRI (Harr 2002). Blood was collected through cardiac venous puncture and stored in polyethylene tubes (Parasuraman et al. 2010; Qin et al. 2020). During the blood collection process by cardiac puncture, no animals were killed. After clot formation, the blood was centrifuged under refrigeration (3220g for 15 min at ~4°C) to obtain serum. Serum readings were performed in the Elitech Flexor EL 200 autoanalyser using Elitech commercial kits.
On Day 28, 35 and 43 birds and left-over feed were weighed to measure BW, FI, bodyweight gain (BWG) and FCR, adjusted for mortality. Daily birds were monitored for mortality.
Two birds per experimental unit (total of 96 birds) were selected within the upper and lower 5% of each pen average weight at 43 days of age for processing yield evaluations of carcass, breast, thigh and drumstick, wing, abdominal fat and viscera (liver, gizzard and heart). After fasting for 8 h, the birds were placed in appropriate plastic boxes and transported to a processing room lit by blue artificial light.
Birds individually passed electrical stunning (45 V for 3 s), bled for 3 min after carotid and jugular veins cut, scalded at 60°C for 45 s, and mechanically defeathered. Carcasses were manually eviscerated and then statically chilled in slush ice for 3 h before processing. Breast fillets, thigh and drumstick, wing, abdominal fat and viscera were manually deboned from the carcasses. After evisceration, CY was calculated based on post-fasting BW: CY % = (carcass weight × 100/BW) taking into consideration head, neck and legs. Carcass parts and viscera yields were calculated based on carcass weight: % PY = (part weight × 100/carcass weight).
Data were subjected to variance and regression analyses based on the following statistical model:

where Yij = the value of the response variable at the ith level of dietary CrMet concentration and the jth block; µ = a constant common to all the observations; CrMeti = is the fixed effect of the dietary CrMet concentration level, i = 6; Blockj = is the random effect of the block, j = 4; eij = is the random residual associated to the Yij.
The residuals from the models for all the variables were submitted to assumptions tests. Residual normality was verified based on the visualisation of histograms, quantile-quantile plots and the Shapiro-Wilk’s test. Residuals independence was verified through graphics of predicted versus residual values. Homogeneity of variances was verified through box-plot graphs and the Levene’s test. In general, all the assumptions were met for all the variables with some exceptions. When an assumption was not met, an exclusion of extreme values was performed based on the standardised residuals, where values outside the range of ± 3 were excluded (representing three standard deviations from the mean, which is zero). Such approach solved the problems and resulted in meeting the assumptions.
Following, the regression effects (linear and quadratic) were tested using orthogonal contrasts. When a quadratic effect was obtained, the point of maximum/minimum dietary CrMet concentration level was calculated solving the following equation:

where maximum or minimum CrMet = maximum or minimum CrMet supplementation level; β1 = linear coefficient of the regression; β2 = quadratic coefficient of the regression.
After performing the linear regressions for all the variables, non-linear regressions were performed when the variables presented P-values < 0.10 with linear broken-line (LBL) and quadratic broken-line (QBL) models tested. The LBL regression model was expressed as:
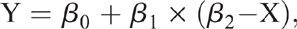
where (β2 – X) = 0 for X > β2; Y = the response variable; X = the dietary CrMet concentration; β0 = the value of the response variable at the plateau; β1 = the slope; β2 = the dietary CrMet concentration at the break point.
The QBL regression model was expressed as:

where (β2 – X) = 0 for X > β2; Y = the response variable; X = the dietary CrMet concentration; β0 = the value at the plateau; β1 = the slope; β2 = the dietary CrMet concentration at the break point.
When linear polynomial regressions or non-linear regressions (LBL and QBL) were significant, the coefficient (R2) and root mean squared error (RMSE) were presented for comparisons. All the analyses were performed using SAS on demand for academics (SAS Institute Inc. 2012). Analysis of variance, regression and orthogonal contrasts were conducted using the SAS PROC MIXED. Assumptions of residual normality, homogeneity and independence were made using SAS PROC UNIVARIATE, SAS PROC GLM and SAS PROC REG respectively. Non-linear regression models (LBL and QBL) were performed using SAS PROC NLIN. Significance was considered at the level of P < 0.05 (5%) of probability.
Results
No Cr was detected in the control diet (Table 2). The Cr concentrations analysed in the experimental diets were close to the calculated concentrations for the experimental treatments.
Table 3 shows the effects of increasing dietary levels of CrMet on performance variables of broilers. The broiler BW was quadratically affected by CrMet levels at 35 and 43 days (P = 0.02 and P = 0.02 respectively). A quadratic effect was also observed on the BWG of broilers (P = 0.02) from 22 to 43 days. While the FCR responded linearly (P = 0.03) to increasing levels of CrMet from 22 to 28 days. From 22 to 43 days the FCR response to CrMet levels was quadratic (P < 0.01). FI was not affected (P > 0.05) by CrMet supplementation.
Effects of CrMet increasing dietary levels on metabolisable energy variables are presented in Table 4. An increasing quadratic effect of CrMet supplementation was observed on AMEn values (P < 0.01) and CAMEn (P < 0.01), without significant effect on AME (P = 0.13).
Table 5 shows the effects of increasing dietary levels of CrMet on serum biochemistry variables. Decreasing linear responses of CrMet levels in broilers diets were observed in TC (P < 0.01) and GLU (P < 0.01). While a quadratic effect of CrMet levels was observed in serum TRI (P < 0.01) of broilers, without responses in serum concentrations of TP, AB and UA (P > 0.05).
Effects of the increasing dietary levels of CrMet on CY variables are presented in Table 6. The CrMet supplementation in broiler diets affected the abdominal fat in a quadratic way (P < 0.01), while the relative heart weight has linearly decreased (P < 0.01) as the CrMet supplemented levels increased. No effect of CrMet supplemented levels was observed on carcass, breast, thighs, wings, liver and gizzard yields (P > 0.05).
The equations for each variable, with respective adjusted model are presented in the Table 7.
Two different models could adjust the BW data at 35 days of age, QR and LBL. CrMet supplementation levels estimated for the models, for having greater BW at 35 days, were 0.80 and 0.22 mg/kg, respectively. The LBL model was chosen, since it presented same R2 and better RMSE. For greater BW at 43 days of age, the CrMet supplementation level was 0.77 mg/kg, estimated according to the QR model. Three models could adjust the BWG data from 22 to 35 days of age, LR, LBL and QBL. CrMet supplementation levels estimated for the models, for having greater BWG from 22 to 35 days, were 0.25 and 0.37 mg/kg, the model LBL was properly chosen, since it presented same R2 and better RMSE. For the variable BWG from 22 to 43 days of age, the supplementation level was 0.71 mg/kg, estimated for the QR model. Two models could adjust the FCR data from 22 to 35 days of age, LBL and QBL. CrMet supplementation levels estimated for the models, for better FCR from 22 to 35 days, were 0.13 and 0.19 mg/kg, the model LBL and QBL have the same R2 and RMSE. Thus, for the better FCR in the period of 22 to 35 days, the level of 0.13 mg/kg is recommended for the lowest inclusion level. Data from FCR variable from 22 to 43 days of age were adjusted for QR model, which predicts a CrMet supplementation level of 0.68 mg/kg.
The CrMet level which was observed to give better AMEn was 0.12 mg/kg, as estimated by the quadratic model. The fitted model for CAMEn was QR, indicating that the optimal level of CrMet was 0.21 mg/kg, as estimated by the equation.
Data from the variable TC were adjusted for three different models, QR, LBL and QBL, with the best supplementation levels of CrMet to reach the lower serum concentration of TC at 0.91, 0.11 and 0.14 mg/kg, respectively, according to the different models. In this situation, the model LBL is chosen as suitable since it presents R2 and RMSE values more appropriate and for the low level of inclusion to reach the lower TC serum concentration. Data from the variable GLU were adjusted for models LBL and QBL, through which two levels were found as better supplementation of CrMet: 0.64 and 0.16 mg/kg, respectively. The choice for the model QBL was justified for having a more suitable R2 and RMSE. The model QR had adjusted well the data from variable TRI. The best level of CrMet supplementation predicted for the model for lower serum concentration of GLU was 0.71 mg/kg.
The two carcass variables affected by CrMet supplementation levels were abdominal fat (AF) and relative heart weight. Data from AF were adjusted for QR model, which estimated a CrMet supplementation level of 0.75 mg/kg. Although the relative heart weight data were adjusted for LBL and QBL models, with the CrMet supplementation levels estimated at 0.43 and 0.77 mg/kg respectively.
Discussion
Average BGTHI (84.56) was measured in the experimental period comprising the application of HS (33°C/12h) in association with 65% relative humidity. Vaz et al. (2009) evaluated the dietary CrMet supplementation of broiler chickens subjected to HS and observed that environments presenting BGTHI 82.9 (or higher) are stressful for 22-day-old (or older) birds. This outcome indicated that the HS applied during the 22–43 day period was enough to cause discomfort for the broilers, since it simulated thermal amplitude variations that often happen in poultry production worldwide.
In this study, increasing CrMet levels quadratically influenced BW, BWG and FCR in the whole period (22–43 days), without affecting FI. The optimal CrMet levels which improved BW, BWG and FCR were 0.77, 0.71 and 0.68 mg/kg respectively. Similar results have been observed in several studies conducted with heat-stressed broiler chickens fed diets supplemented with Cr. Noori et al. (2012) observed that supplementation of 0.80 mg/kg of CrMet increased BWG of broilers from 21 to 42 days, reared under HS. Jahanian and Rasouli (2015) also observed that CrMet supplementation of 0.50 mg/kg increased BWG and improved FCR of broilers from 1 to 42 days, reared under HS. Conversely, Vaz et al. (2009) and Ghazi et al. (2012) conducted studies with heat-stressed broiler chickens fed diets supplemented with CrMet, without observing any effects on BWG, FI and FCR throughout the trials.
The positive effects on broiler performance can be further explained by the fact that CrMet linearly reduced levels of serum glucose, increasing the use of GLU up-take and amino acids for animal growth purposes. Sahin et al. (2002) observed linear increased sensibility to insulin promoted by Cr-picolinate (Cr-Pic) supplementation levels (0; 0.20; 0.40 and 1.20 mg/kg) with improving the performance of broilers reared under HS. Sahin et al. (2010) also observed linear increase in performance of meat-type quails reared under HS and fed diets supplemented with Cr-Pic levels (0, 0.40 and 0.80 mg/kg). Once absorbed, Cr promotes cascades of cellular signals that activate GLU transporter-2,4 (GLUT-2,4) and amino acids transporters, also activating the phosphotyrosine phosphatase in lipocytes, diversifying and optimising the metabolism of broiler chickens fed diets supplemented with Cr (Davis et al. 1996; Sahin et al. 2017).
The improvement in broiler performance with CrMet supplementation can be partially explained by the better use of dietary AMEn observed in the present study. The CrMet levels quadratically influenced AMEn and CAMEn. The optimal CrMet levels which improved AMEn and CAMEn were 0.12 and 0.21 mg/kg respectively. These results suggest that CrMet supplementation may improve broilers metabolism. Sahin et al. (2002) reported higher serum concentrations of triiodothyronine (T3) and thyroxine (T4) in broilers fed diets with Cr supplementation which positively influenced broilers growth. Thus, increased T3 and T4 levels can improve energy utilisation by the broilers. Moreover, Ahmed et al. (2005) recorded increased apparent protein and carbohydrate metabolism in broiler chickens fed diets supplemented with Cr chloride (CrCl3).
According to Li et al. (2018) the Cr-Pic supplementation increased the villus height to crypt depth ratio and decreased the heat shock protein-70 expression in the jejunal mucosa of ducks subjected to HS. Amatya et al. (2004) and Carvalho et al. (2015) also observed that the supplementation of Cr for heat-stressed birds increased the retention of essential trace elements (Cu, Fe, Zn and Mn) that synergistically work by stimulating nutrient metabolism (Cu and Fe play an important role in oxygen transport by haemoglobin, whereas Zn and Mn are essential enzymatic co-factors) improving broiler and laying hens performance. Thus, the association of all these factors suggests CrMet supplementation may have reduced the mucosal damage of broilers under HS and favoured the increase of AMEn and CAMEn, indicating a higher retention of N for growth in relation to gross energy of the diet.
The improvement in AMEn values and the reduction in serum GLU observed in our study indicated metabolic optimisation of broilers exposed to heat. According to Vincent (2010) HS reduces Cr plasmatic concentration and deposition in tissues, besides increasing urinary excretion. Thus, the ability of the LMWCr to increase the sensitivity of target cells to insulin recognition may be impaired. Karami et al. (2018) found increased blood insulin concentrations of laying hens fed diets containing 0.40 mg/kg CrMet. This increase indicates a better uptake of GLU by tissues, low adipose tissue synthesis and increased use of amino acids for protein synthesis purposes, a fact that explains the decreased serum TC, GLU and TRI levels observed in the current study.
CrMet supplementation up to 0.71 mg/kg reduced serum concentrations of TRI, GLU and TC in broilers reared under HS. Similar results were observed by Noori et al. (2012), the supplementation with 0.80 mg/kg CrMet decreased TRI and TC concentrations in broiler chickens. According to Samanta et al. (2008) broiler chickens fed diets supplemented with 0.50 mg/kg of Cr-Pic decreased plasma GLU and TC levels. Based on Souza et al. (2010), Cr appears to inhibit the hydroxy-methyl-glutaryl-CoA reductase, an enzyme responsible for enabling cholesterol synthesis and reducing serum TC levels in the body. Other studies (Toghyani et al. 2012; Habibian et al. 2013; Xiao et al. 2017) also recorded similar results on serum biochemical profiles in broilers fed diets supplemented with Cr.
Brooks et al. (2016) recorded increased liver glycogen, as well as a trend of decreased non-esterified fatty acids in the plasma of broiler chickens fed diets supplemented with 0.43 to 0.45 mg/kg of chromium-propionate (Cr-Prop) from 22 to 42 days. In addition, broilers subjected to HS presented increased sodium-dependent GLU transporter (SGLT-1) activity, which increased GLU uptake levels (Garriga et al. 2006). Hence, these factors lead to higher GLU retention in the muscles, lower serum GLU concentrations, decreased gluconeogenesis process in the liver, as well as lower GLU and amino acid transformations into fat to be deposited in the abdominal cavity of the animals. In addition, there are reports that Cr inhibits the production of tumour necrosis factor-α (TNF-α), which appears to inhibit lipogenesis in fat cells due to decreased lipoprotein lipase, GLUT4 and acetyl-coA-synthetase levels (Sethi and Hotamisligil 1999; Jain and Kannan 2001), leading to decreased abdominal fat levels. The association between these factors reduced AF in broiler chickens in the current study.
In the present study, the supplementation with 0.75 mg/kg of CrMet reduced the AF of the broilers at 43 days old. Ebrahimzadeh et al. (2013) also found decreased AF in broiler chickens fed diets supplemented with 0.80 mg/kg of CrMet. Toghyani et al. (2012) also observed a decrease in the AF of the broilers fed diets supplemented with 1.50 mg/kg of Cr-nicotinic acid. Besides decreased AF, Zha et al. (2009) observed increased breast meat yield and higher breast meat concentration in broilers fed diets supplemented with 0.50 mg/kg Cr-Pic. Habibian et al. (2013) observed higher breast yield in heat-stressed broiler chickens supplemented with 1.20 mg/kg CrMet. However, in the present study no effect was observed of CrMet supplementation on CY and cuts.
In this study, CrMet supplementation up to 0.43 mg/kg promoted reduction in the relative heart weight of broilers. Conversely, Sahin et al. (2002) observed linear increased heart, gizzard and liver yield in heat-stressed broiler chickens fed diets containing Cr-Pic. However, Habibian et al. (2013) and Zhang and Kim (2014) did not find effects of CrMet supplementation on the relative weight of broiler chicken hearts, gizzards or livers.
According to Huang et al. (2016), the discrepancy among studies may be explained by differences in sources, Cr concentration, different basal diets and experimental treatments. Besides that, variations in facilities environment and hygiene, local sanitary challenges, severity of the stressor agent (heat or cold), dietary features (inclusion of enzymes, especially phytase, electrolyte balance, and use of synthetic amino acids) and statistical model used can change productive responses.
According to Cemin et al. (2017) it is possible to compare models through the values of R2 and RMSE. In general, the QR and QBL models obtained better R2 and RMSE values to establish the CrMet requirements for the significant variables under study. However, there was a divergence between CrMet supplementation levels estimated in different models for the same characteristic. This was expected because QR models establish the optimal level of response for each variable (Pesti et al. 2009), whereas the use of non-linear models such as QBL establishes the inflection point (plateau) of the response curve for each variable.
The CrMet supplementation levels varied according to each productive characteristic and between the different types of model adopted. For the performance variables, CrMet supplementation with 0.77 mg/kg allowed the highest BW, BWG and better FCR in the total 22 to 43 days of age of the chickens. For the AMEn and CAMEn the supplementation of 0.21 mg/kg of CrMet provided better utilisation of metabolisable energy of the diet in broilers reared under HS conditions. For the serum biochemistry variables, supplementation with up to 0.71 mg/kg of CrMet was satisfactory for the maximum reduction in the concentration of TRI, GLU and TC. For carcass characteristics, supplementation with 0.75 mg/kg resulted in a lower AF content in the carcass. In this sense, supplementation with CrMet provides higher productive efficiency in broiler chickens reared in HS conditions.
Conclusions
Supplementation CrMet improved energy metabolisation, serum biochemistry, performance and reduced abdominal fat of broiler chickens reared in HS and harvested at 43 days. The supplementation of 0.77 mg/kg of CrMet is recommended to provide satisfactory performance of broiler chickens under HS with adequate carcass traits.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), for granting the scholarship; to Federal University of Viçosa (UFV), National Council for Scientific and Technological Development (CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Minas Gerais Research Support Foundation (FAPEMIG – Fundação de Amparo à Pesquisa de Minas Gerais), for supporting the development of the research; and to Zinpro Corporation, for funding it.
References
Ahmed N, Haldar S, Pakhira MC, Ghosh TK (2005) Growth performances, nutrient utilization and carcass traits in broiler chickens fed with a normal and a low energy diet supplemented with inorganic chromium (as chromium chloride hexahydrate) and a combination of inorganic chromium and ascorbic acid. Journal of Agricultural Science 143, 427–439.| Growth performances, nutrient utilization and carcass traits in broiler chickens fed with a normal and a low energy diet supplemented with inorganic chromium (as chromium chloride hexahydrate) and a combination of inorganic chromium and ascorbic acid.Crossref | GoogleScholarGoogle Scholar |
Amatya JL, Haldar S, Ghosh TK (2004) Effects of chromium supplementation from inorganic and organic sources on nutrient utilization, mineral metabolism and meat quality in broiler chickens exposed to natural heat stress. Animal Science 79, 241–253.
| Effects of chromium supplementation from inorganic and organic sources on nutrient utilization, mineral metabolism and meat quality in broiler chickens exposed to natural heat stress.Crossref | GoogleScholarGoogle Scholar |
AOAC (2006) ‘Official methods of analysis’, 18th edn. (Association of Official Analytical Chemists: Arlington, VA, USA)
Brooks MA, Grimes JL, Lloyd KE, Krafka K, Lamptey A, Spears JW (2016) Chromium propionate in broilers: effect on insulin sensitivity. Poultry Science 95, 1096–1104.
| Chromium propionate in broilers: effect on insulin sensitivity.Crossref | GoogleScholarGoogle Scholar | 26933236PubMed |
Buffington DE, Collasso-Arocho A, Canton GH, Pitt D (1981) Black globe-humidity index (BGHI) as comfort equation for dairy cows. Transactions of the ASAE. American Society of Agricultural Engineers 24, 711–714.
| Black globe-humidity index (BGHI) as comfort equation for dairy cows.Crossref | GoogleScholarGoogle Scholar |
Carvalho LSS, Rosa DRV, Litz FH, Fagundes NS, Fernandes EA (2015) Effect of the inclusion of organic copper, manganese, and zinc in the diet of layers on mineral excretion, egg production, and eggshell quality. Brazilian Journal of Poultry Science 17, 87–92.
| Effect of the inclusion of organic copper, manganese, and zinc in the diet of layers on mineral excretion, egg production, and eggshell quality.Crossref | GoogleScholarGoogle Scholar |
Cemin HS, Vieira SL, Stefanello C, Kipper M, Kindlein L, Helmbrecht A (2017) Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed. PLoS One 12, e0179665
| Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed.Crossref | GoogleScholarGoogle Scholar | 28636626PubMed |
Cobb-Vantress (2013) ‘Broiler management guide.’ (Cobb-Vantress Inc.: Siloam Springs, AR, USA) Available at: https://www.cobb-vantress.com/ [Verified 30 October 2016]
Dalólio FS, Albino LFT, Silva JN, Campos PHRF, Lima HJD, Moreira J, Ribeiro Junior V (2018) Dietary chromium supplementation for heat-stressed broilers. World’s Poultry Science Journal 74, 101–116.
| Dietary chromium supplementation for heat-stressed broilers.Crossref | GoogleScholarGoogle Scholar |
Davis CM, Vincent JB (1997) Isolation and characterisation of a biologically active form of chromium oligopeptide from bovine liver. Archives of Biochemistry and Biophysics 339, 335–343.
| Isolation and characterisation of a biologically active form of chromium oligopeptide from bovine liver.Crossref | GoogleScholarGoogle Scholar | 9056266PubMed |
Davis CM, Sumrall KH, Vincent JB (1996) The biologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP). Biochemistry 35, 12963–12969.
| The biologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP).Crossref | GoogleScholarGoogle Scholar | 8841143PubMed |
Ebrahimzadeh SK, Farhoomand P, Noori K (2013) Effects of chromium methionine supplementation on performance, carcass traits, and the Ca and P metabolism of broiler chickens under heat-stress conditions. Journal of Applied Poultry Research 22, 382–387.
| Effects of chromium methionine supplementation on performance, carcass traits, and the Ca and P metabolism of broiler chickens under heat-stress conditions.Crossref | GoogleScholarGoogle Scholar |
Garriga C, Hunter RR, Amat C, Planas JM, Mitchell MA, Moretó M (2006) Heat stress increase apical glucose transport in the chicken jejunum. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 290, 195–201.
| Heat stress increase apical glucose transport in the chicken jejunum.Crossref | GoogleScholarGoogle Scholar |
Ghazi SH, Habibian M, Moeini MM (2012) Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress. Biological Trace Element Research 146, 309–317.
| Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress.Crossref | GoogleScholarGoogle Scholar | 22127829PubMed |
Habibian M, Ghazi S, Moeini M (2013) Lack of effect of dietary chromium supplementation on growth performance and serum insulin, glucose, and lipoprotein levels in broilers reared under heat stress condition. Biological Trace Element Research 153, 205–211.
| Lack of effect of dietary chromium supplementation on growth performance and serum insulin, glucose, and lipoprotein levels in broilers reared under heat stress condition.Crossref | GoogleScholarGoogle Scholar | 23591960PubMed |
Harr KE (2002) Clinical chemistry of companion avian species: a review. Veterinary Clinical Pathology 31, 140–151.
| Clinical chemistry of companion avian species: a review.Crossref | GoogleScholarGoogle Scholar | 12189602PubMed |
Huang Y, Yang J, Xiao F, Lloyd K, Lin X (2016) Effects of supplemental chromium source and concentration on growth performance, carcass traits, and meat quality of broilers under heat stress conditions. Biological Trace Element Research 170, 216–223.
| Effects of supplemental chromium source and concentration on growth performance, carcass traits, and meat quality of broilers under heat stress conditions.Crossref | GoogleScholarGoogle Scholar | 26184120PubMed |
Jahanian R, Rasouli E (2015) Dietary chromium methionine supplementation could alleviate immunosuppressive effects of heat stress in broiler chicks. Journal of Animal Science 93, 3355–3363.
| Dietary chromium methionine supplementation could alleviate immunosuppressive effects of heat stress in broiler chicks.Crossref | GoogleScholarGoogle Scholar | 26440004PubMed |
Jain SK, Kannan K (2001) Chromium chloride inhibits oxidative stress and TNF-alpha secretion caused by exposure to high glucose in cultured U937 monocytes. Biochemical and Biophysical Research Communications 289, 687–691.
| Chromium chloride inhibits oxidative stress and TNF-alpha secretion caused by exposure to high glucose in cultured U937 monocytes.Crossref | GoogleScholarGoogle Scholar | 11726202PubMed |
Karami M, Torki M, Mohammadi H (2018) Effects of dietary supplemental chromium methionine, zinc oxide, and ascorbic acid on performance, egg quality traits, and blood parameters of laying hens subject to heat stress. Journal of Applied Animal Research 46, 1174–1184.
| Effects of dietary supplemental chromium methionine, zinc oxide, and ascorbic acid on performance, egg quality traits, and blood parameters of laying hens subject to heat stress.Crossref | GoogleScholarGoogle Scholar |
Króliczewska B, Zawadski W, Dobrzanski Z, Kaczmarek-Oliwa A (2004) Changes in selected serum parameters of broiler chicken fed supplemental chromium. Journal of Animal Physiology and Animal Nutrition 88, 393–400.
| Changes in selected serum parameters of broiler chicken fed supplemental chromium.Crossref | GoogleScholarGoogle Scholar | 15584948PubMed |
Li R, Zhou Y, Li Y, Guo L, Zhang Y, Qi Z (2018) Effects of chromium picolinate supplementation on growth performance, small intestine morphology and antioxidant status in ducks under heat stress conditions. International Journal of Morphology 36, 226–234.
| Effects of chromium picolinate supplementation on growth performance, small intestine morphology and antioxidant status in ducks under heat stress conditions.Crossref | GoogleScholarGoogle Scholar |
Matterson LD, Potter LM, Stutz MW, Singsen EP (1965) ‘The metabolizable energy of feeds ingredients for chickens.’ (The University of Connecticut, Agricultural Experiment Station, Connecticut, New England)
Noori K, Farhoomand P, Ebrahimzadeh SK (2012) Effect of chromium methionine supplementation on performance and serum metabolites in broiler chickens thermoneutral and under heat-stress conditions. Iranian Journal of Applied Animal Science 2, 79–82.
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. Journal of Pharmacology & Pharmacotherapeutics 1, 87–93.
| Blood sample collection in small laboratory animals.Crossref | GoogleScholarGoogle Scholar |
Pesti GM, Vedenov D, Cason JA, Billard L (2009) A comparison of methods to estimate nutritional requirements from experimental data. British Poultry Science 50, 16–32.
| A comparison of methods to estimate nutritional requirements from experimental data.Crossref | GoogleScholarGoogle Scholar | 19234926PubMed |
Qin S, Zhang L, Ma F, Che Y, Wang H, Shi Z (2020) Dietary zinc and growth, carcass characteristics, immune responses, and serum biochemistry of broilers. Animal Production Science 60, 815–822.
| Dietary zinc and growth, carcass characteristics, immune responses, and serum biochemistry of broilers.Crossref | GoogleScholarGoogle Scholar |
Rajalekshmi M, Sugumar C, Chirakkal H, Rao SVR (2014) Influence of chromium propionate on the carcass characteristics and immune response of commercial broiler birds under normal rearing conditions. Poultry Science 93, 574–580.
| Influence of chromium propionate on the carcass characteristics and immune response of commercial broiler birds under normal rearing conditions.Crossref | GoogleScholarGoogle Scholar | 24604850PubMed |
Rao SVR, Prakash B, Raju MVLN, Panda AK, Kumari RK (2016) Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biological Trace Element Research 172, 511–520.
| Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer.Crossref | GoogleScholarGoogle Scholar | 26743864PubMed |
Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RFM, Lopes DC, Ferreira AS, Barreto SLT (Eds) (2011) ‘Brazilian tables for poultry and swine-Composition of feedstuffs and nutritional requirements’, 3rd edn. (Viçosa: Brasil)
Sahin K, Sahin N, Onderic M, Gursu F, Cikim G (2002) Optimal dietary concentration of chromium for alleviating the effect of heat stress on growth, carcass qualities, and some serum metabolites of broiler chickens. Biological Trace Element Research 89, 53–64.
| Optimal dietary concentration of chromium for alleviating the effect of heat stress on growth, carcass qualities, and some serum metabolites of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 12413051PubMed |
Sahin N, Akdemir F, Tuzcu M, Hayrli A, Smith MO, Sahin K (2010) Effects of supplemental chromium sources and levels on performance, lipid peroxidation, and proinflammatory markers in heat-stressed quails. Animal Feed Science and Technology 159, 143–149.
| Effects of supplemental chromium sources and levels on performance, lipid peroxidation, and proinflammatory markers in heat-stressed quails.Crossref | GoogleScholarGoogle Scholar |
Sahin N, Hayrli A, Orhan C, Tuzcu M, Akdemir F, Komorowski JR, Sahin K (2017) Effects of supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poultry Science 96, 4317–4324.
| Effects of supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress.Crossref | GoogleScholarGoogle Scholar | 29053811PubMed |
Samanta S, Haldar S, Bahadur V, Ghosh TK (2008) Chromium picolinate can ameliorate the negative effects of heat stress and enhance performance, carcass and meat traits in broiler chickens by reducing the circulatory cortisol level. Journal of the Science of Food and Agriculture 88, 787–796.
| Chromium picolinate can ameliorate the negative effects of heat stress and enhance performance, carcass and meat traits in broiler chickens by reducing the circulatory cortisol level.Crossref | GoogleScholarGoogle Scholar |
SAS Institute Inc. (2012). SAS on demand for academics. Release 9.04.01M5P09132017. SAS Institute Inc., Cary, NC, USA. Available at https://odamid.oda.sas.com/SASStudio/ [Verified 22 October 2020]
Sethi JK, Hotamisligil GS (1999) The role of TNFα in adipocyte metabolism. Seminars in Cell & Developmental Biology 10, 19–29.
| The role of TNFα in adipocyte metabolism.Crossref | GoogleScholarGoogle Scholar |
Sibbald IR, Slinger SJ (1963) A biological assay for metabolizable energy in poultry feed ingredients together with findings which demonstrate some of the problems associated with the evaluation of fats. Poultry Science 59, 1275–1279.
| A biological assay for metabolizable energy in poultry feed ingredients together with findings which demonstrate some of the problems associated with the evaluation of fats.Crossref | GoogleScholarGoogle Scholar |
Souza LMG, Murakami AE, Fernandes JIM, Guerra RLH, Martins EM (2010) Influência do cromo no desempenho, na qualidade da carne e no teor de lipídeos no plasma sanguíneo de frangos de corte. Revista Brasileira de Zootecnia 39, 808–814.
| Influência do cromo no desempenho, na qualidade da carne e no teor de lipídeos no plasma sanguíneo de frangos de corte.Crossref | GoogleScholarGoogle Scholar |
Toghyani M, Gheisari AA, Khodami A, Toghyani M, Mohammadrezaei M, Bahadoran R (2010) Effect of dietary chromium yeast on thigh meat quality of broiler chicks in heat stress condition. International Journal of Nutrition and Food Engineering 4, 920–923.
| Effect of dietary chromium yeast on thigh meat quality of broiler chicks in heat stress condition.Crossref | GoogleScholarGoogle Scholar |
Toghyani M, Toghyani M, Shivazad M, Gheisari AA, Bahadoran R (2012) Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biological Trace Element Research 146, 171–180.
| Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents.Crossref | GoogleScholarGoogle Scholar | 22006223PubMed |
Vaz RGM, Oliveira RFM, Donzele JL, Albino LFT, Oliveira WP, Silva BAN (2009) Levels of dietary chromium in rations for male broilers kept under heat stress from one to 42 days of age. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 61, 484–490.
| Levels of dietary chromium in rations for male broilers kept under heat stress from one to 42 days of age.Crossref | GoogleScholarGoogle Scholar |
Vincent JB (2010) Chromium: celebrating 50 years as an essential element? Dalton Transactions 39, 3787–3794.
| Chromium: celebrating 50 years as an essential element?Crossref | GoogleScholarGoogle Scholar | 20372701PubMed |
Vincent JB (2017) New evidence against chromium as an essential trace element. The Journal of Nutrition 147, 2212–2219.
| New evidence against chromium as an essential trace element.Crossref | GoogleScholarGoogle Scholar | 29021369PubMed |
Williams CH, David DJ, Iismaa O (1962) The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. The Journal of Agricultural Science 59, 381–385.
| The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry.Crossref | GoogleScholarGoogle Scholar |
Xiao F, Ao D, Zhou B, Spears JW, Lin X, Huang Y (2017) Effects of supplemental chromium propionate on serum lipids, carcass traits, and meat quality of heat-stressed broilers. Biological Trace Element Research 176, 401–406.
| Effects of supplemental chromium propionate on serum lipids, carcass traits, and meat quality of heat-stressed broilers.Crossref | GoogleScholarGoogle Scholar | 27660074PubMed |
Yaghobfar A (2016) The efficiency of AMEn and TMEn utilization for NE in broiler diets. Brazilian Journal of Poultry Science 18, 47–56.
| The efficiency of AMEn and TMEn utilization for NE in broiler diets.Crossref | GoogleScholarGoogle Scholar |
Zha LY, Zeng JW, Chu XW, Mao LM, Luo HJ (2009) Efficacy of trivalent chromium on growth performance, carcass characteristics and tissue chromium in heat-stressed broilers chicks. Journal of the Science of Food and Agriculture 89, 1782–1786.
| Efficacy of trivalent chromium on growth performance, carcass characteristics and tissue chromium in heat-stressed broilers chicks.Crossref | GoogleScholarGoogle Scholar |
Zhang S, Kim IH (2014) Effects of Cr-methionine supplementation on growth performance, relative organ weight, immune hormones, and meat quality of broiler chicks under heat stress. Indian Journal of Animal Sciences 84, 511–515.







