Feed conversion efficiency in sheep genetically selected for resistance to gastrointestinal nematodes
G. F. Ferreira A , G. Ciappesoni A , D. Castells B , F. Amarilho-Silveira C , E. A. Navajas A , D. Giorello A , G. Banchero A and I. De Barbieri
A and I. De Barbieri  A D
A D
A Instituto Nacional de Investigación Agropecuaria, Ruta 5 km 386, 45000, Tacuarembó, Uruguay.
B Secretariado Uruguayo de la Lana, Ruta 7 km 140, 94101, Florida, Uruguay.
C Universidade Federal do Rio Grande do Sul, Avenuenida Bento Gonçalves, 7712, 91509-900, RS, Brasil.
D Corresponding author. Email: idebarbieri@inia.org.uy
Animal Production Science 61(8) 754-760 https://doi.org/10.1071/AN20121
Submitted: 24 March 2020 Accepted: 12 February 2021 Published: 10 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Context: It has been indicated that there might be an antagonism between selection for gastrointestinal nematode resistance and high productivity and feed conversion efficiency in ruminants.
Aims: This study aimed to determine whether genetic selection for resistance to gastrointestinal nematodes would alter the feed intake and feed efficiency of sheep with or without an infection of Haemonchus contortus.
Methods: Sixty-seven Corriedale lambs (357 ± 14 days old) derived from flocks genetically selected to be resistant (n= 29) or susceptible (n = 38) to gastrointestinal nematodes (GIN) were evaluated for individual dry-matter intake (DMI), feed conversion ratio (FCR) and residual feed intake (RFI). Considering bodyweight (BW), GIN line and sires, males were allotted to one of three outdoor pens and females to one of two, each pen being equipped with five automated feeding systems and two automatic weighing platforms to record individual feed intake and BW. Feed (lucerne haylage, crude protein 20.5%, metabolisable energy 9.2 MJ/kg DM) and water were offered ad libitum. The experiment was conducted in two periods. First, animals were maintained worm-free (14 days of acclimatisation and 44 days of records) and then, in Period 2 (42 days), animals were artificially infected with 6000 L3 of Haemonchus contortus. Worm egg counts were recorded on Days 9, 23, 27, 30, 42 post-infection. While DMI, FCR, average daily gain and BW were analysed using a generalised linear model including dams age, pen and GIN line as fixed effects, RFI was analysed including only GIN line.
Key results: In both periods, GIN line did not have a significant (P > 0.05) effect on DMI, FCR, RFI, average daily gain or BW. Worm egg count was different (P < 0.05) on Day 23 post-infection (Period 2), being higher in susceptible line.
Conclusions: The most important finding of this study is that breeding GIN-resistant animals would not have a negative effect on feed conversion efficiency when evaluated as FCR or RFI in 1-year old lambs fed ad libitum with a high-protein diet.
Implications: Breeding for resistance to internal nematodes does not affect feed efficiency or productivity.
Keywords: feed conversion efficiency, nematodes, gastrointestinal nematodes.
Introduction
In Uruguay individual worm egg counts (WEC) are used for the genetic evaluation of resistance to gastrointestinal nematodes (GIN) in sheep. The objective is to contribute to genetically improve productivity by breeding animals more resistant to GIN, on the basis of expected progeny difference (EPD) for WEC, reducing the use of chemical drugs and contamination of pastures with gastrointestinal nematodes (Castells 2008). Infection by GIN decreases animal voluntary feed intake (Parkins and Holmes 1989) and promotes changes in the metabolism of proteins, energy and some minerals to increase the immune response at the intestinal level. Reduced body growth, wool production and reproductive performance have been attributed to reduced feed intake and greater demand for nutrients by the immune system (Walkden-Brown and Kahn 2002). In particular, increased protein requirements have been observed. Furthermore, this higher demand of nutrients varies with animal genetic resistance to GIN, age and GIN challenge, with resistant lambs having higher requirements of energy (4%) and protein (5%) when they were GIN challenged (Liu et al. 2005).
Favourable and unfavourable genetic correlations between resistance to GIN and productive traits have been reported. In Corriedale, negative (favourable) genetic correlations have been estimated between fleece and bodyweights with WEC (Castells 2008), although these associations were unfavourable according to Morris et al. (2000). Estimates in Merino show no genetic association or were unfavourable (Eady et al. 2003; Safari et al. 2005). Masters and Ferguson (2019) concluded in their review that sheep producing high fleece weights would be less able to supply the increased demand of nutrients necessary for an adequate immune response to infections.
Genetic selection of animals for a greater production associated with a greater feed efficiency may reduce the available resources for the animal to respond to all the demands of the immune system, ontogenic growth, social behaviour and reproduction (Greer 2008; Rauw 2012). This type of focussed selection can reduce the ability of the individuals to respond to stressors and adapt to changing environmental conditions, leading to an increased risk of diseases and, ultimately, a poorer robustness of the animal. Selection for more productive sheep, in terms of heavier body and fleece and finer fibre diameter, is indicated by the genetic trends of the Corriedale breed in the Uruguayan genetic evaluation (www.geneticaovina.com.uy). Consequently, an increase of feed intake might be expected as a correlated response (Fogarty et al. 2009). Considering the relevance of feed costs in the production system and the potential genetic variation of feed conversion efficiency, including this trait in current genetic evaluations is an alternative to decrease feed costs without reducing production performance (Cammack et al. 2005; Paganoni et al. 2017).
Previous reviews indicated that there might be an antagonism between selection for high productivity and increasing feed conversion efficiency and metabolic process linked with health traits, which may lead to metabolic changes reducing robustness (Greer 2008; Rauw 2012; Cantalapiedra-Hijar et al. 2018). However, some studies in sheep have reported favourable consequences on feed intake and wool and body growth of genetic resistance to GIN (Doyle et al. 2011), although mechanisms explaining animal performance might be different among GIN lines. Our hypothesis was that animals genetically resistant to GIN would not present differences in feed intake, productive performance and feed conversion efficiency from susceptible animals, when they are free of parasite infection. However, they would have a lower feed conversion ratio (FCR) and residual feed intake (RFI) under a parasite challenge with Haemonchus contortus.
Materials and methods
The experiment was conducted at INIA’s Experimental Unit La Magnolia, located in Tacuarembó, Uruguay (31°42′32″S, 55°49′36″W). All applied protocols were approved by INIA Animal Ethics Committee (Approval numbers INIA_2018.2 and INIA_2018.3).
Animals and experimental design
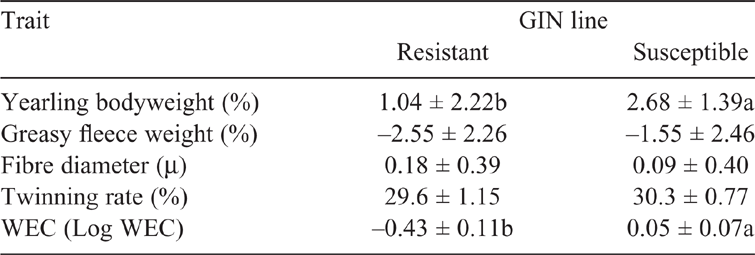
This study included 67 Corriedale lambs (41 non-castrated males; 26 females) from two genetically divergent selection lines (GIN lines; resistant (R), 29; susceptible (S), 38) for GIN resistance, based on animal selection by WEC EPD (Castells 2005). In 1998, the Uruguayan Wool Secretariat initiated a genetic program to develop divergent lines for resistance to GIN in the Corriedale breed, in which breeding animals were selected for high and low WEC EPD. The first R and S progenies were born in 2000 and 2003 respectively (Castells and Gimeno 2011). Lambs evaluated in our study were born in 2017 and were sired by three rams from the R line and two rams from the S line, with 6–31 progeny per sire. Males represented 63% and 61% of the animals of the R and S line respectively.
Lambs grazed together from birth until the onset of the experiment and were exposed to natural paddock infection of GIN. Three WEC were performed between weaning and the experiment, as part of the data recording for the genetic evaluation. Although there are no stool cultures of all WEC of these lambs, previous studies (Castells 2008; Goldberg et al. 2012) and our own data (surveyed in the stud-flocks since 2017) indicate that the most prevalent nematode is Haemonchus sp., followed by Trichostrongylus sp. Average WEC EPD of the R lambs was in the bottom 1% of the population genetic evaluated, while for the S lambs, the average EPD was in the highest 25% (Table 1). No differences (P > 0.05) were found between GIN lines for fleece weight, fibre diameter or twinning rate EPDs; however, animals from S line had higher EPD for bodyweight.
Within the present experiment, two consecutive feed efficiency trials were conducted, without and with parasite challenge. In the first period, animals were kept worm-free, by drenching them at the beginning with a combination of 0.1% abamectin with 1% derquantel (Startect®, Zoetis, Montevideo, Uruguay) at a dose of 1 mL/5 kg of bodyweight (BW), in accordance with the individual BW. Ten days later, a WEC analysis was performed and it confirmed that lambs were free of GIN. In Period 2, a second feed efficiency trial was conducted, in which animals were artificially infected. The parasite challenge was performed by an artificial infection with 6000 L3 larvae of Haemonchus contortus, through a daily oral dose of 2000 L3 for three consecutive days (Days 0–2, D0–D2). Mono parasite-specific strains were recovered from faecal not stool culture by using the Henriksen and Korsholm technique (Fiel et al. 2011). After D0, FAMACHA© (for monitoring the animals; Van Wyk and Bath 2002) was performed on Days 9, 17, 23, 30, 37 and 42 along with an individual WEC on Days 9, 23, 30, 37 and 42, by using the modified McMaster technique (Roberts and O’Sullivan 1949). At the end of Period 2, all animals were drenched with an oral anthelmintic (Raider®, Cibeles, Montevideo, Uruguay; 0.2% ivermectin and 8% levamisole) according to their BW (dose 1 mL/10 kg of BW). To study differential responses after infection, on the basis of the prepatent period of the parasite (Méndez y Cabo 1980), Period 2 was divided into two subperiods (2.a and 2.b), with Period 2.a being from D0 to D23 and Period 2.b from D24 to D42 post-infection.
Feed efficiency trials in Periods 1 and 2
Individual feed intake and BW were recorded automatically during the feed efficiency trials. Considering sex of the lamb, BW, GIN line and sires, the lambs (RFID-tagged) were allotted to one of five outdoor pens of 150 m2 (minimum 12.5 m2 per animal), with access to shade (roof), food and water ad libitum. The floor of the pens consisted of an area of concrete under the roof and an area of dirt without pasture. Because males were non-castrated, they were allocated in three pens, while females were in two separated pens. Both GIN lines (proportionally) and more than one sire were represented in each pen. To diminish dominance and to help shy feeders to express potential feed intake, heavy lambs were separated from light animals in different pens.
Each animal was monitored daily, visually and throughout an online software that identified the entrance of the lamb to the feeder and the trough (which was associated with a scale to automatically record BW). Each pen had five individual automated feeders and two weighing platforms (Intergado®, Belo Horizote, MG, Brazil) that were equipped with an electronic tag reader, precision scale, and were connected to a central computer, allowing the control of dry-matter intake (DMI) and BW of the animals on a daily basis. The feed offered was haylage of lucerne (Festín®: DM, 38%; crude protein, 20.5%; metabolisable energy, 9.2 MJ/kg DM; neutral detergent fibre, 42.9%; acid detergent fibre, 35.4%).
In Period 1, a 58-day feed efficiency trial was conducted, including 14 days of acclimatisation to feed and facilities, and 44 days of records. Lambs were 357 ± 14 days old at the beginning of the experiment, at the start of the acclimatisation, and the average BWs were 28.5 ± 4.5 kg and 26.5 ± 3.3 kg for males and females respectively. In Period 2, the feed efficiency trial started immediately after Period 1 and lasted 42 days, given that the animals were already acclimatised. Male and female lambs began the second period test with an average BW of 34.3 ± 6.2 and 32.4 ± 5.3 kg respectively.
Measurement of rib-eye area and fat thickness
Measurements of rib-eye area (REA) and fat thickness (FT) were performed at the end of each period. The animals were scanned with an Aloka SSD 500V W/2X real-time scanner (Tokyo, Japan) using a 3.5 MHz linear probe (UST-5511U-3.5, 18 cm, Aloka, Tokyo, Japan). The probe was placed perpendicular to the spine between the thoracic vertebrae 12 and 13, before each measurement, the wool of the lamb was separated, and vegetable oil was used as a coupling between the skin and the probe. Once a satisfactory image was obtained on the site, it was captured on video. Subsequently, REA and FT were measured by image analysis with BioSoftToolbox®II for Beef C 2007–2012 (Biotronic, Inc. Software, Aspen, IA, USA).
Statistical analyses and calculations
The ADG was calculated by linear regression of the daily BW records during the period of each feed efficiency trial; the model of the regression equation corresponds with y = β0 + β1x, where y = daily BW (kg), β0 = regression intercept, β1 = average daily gain (kg/day); x = experimental day. FCR was calculated as FCR = observed DMI (kg/day) / ADG (kg/day), expressed in kg of DM/kg BW gain.
The model used to calculate RFI (Koch et al. 1963) was, y = BW0.75 + ADG + DA + Pen + ε (RFI), where y = observed average daily DM feed intake, BW0.75 is the metabolic average BW (kg, covariate), ADG is the average daily gain, previously calculated by linear regression (g/day, covariate), DA is the effect of the dam’s age (4 levels), Pen is the effect (5 levels) of the pen (includes the effect of sex) and RFI is the residual error (difference between the observed and expected DMI). Data on REA and FT were not included in the model because preliminary analysis indicated that they were not significant (data not shown).
Evaluated traits (DMI, FCR, ADG, BW0.75, REA and FT) were analysed using a generalised linear model (GLM) that included DA, Pen and GIN line, as fixed effects. RFI was analysed only including GIN line as fixed effect and for REA and FT at the end of each period; the initial measures of the period were utilised as covariates. Additionally, the WEC data at different days post-infection (9, 23, 30, 37 and 42 days) were normalised by the following logarithmic transformations: LogWEC = Loge(WEC+50), and analysed with a repeated-measures model (compound symmetry structure) with the fixed effects of DA, Pen, Days post infection, GIN line and the interaction of Day and GIN line. Statistical analyses were performed using software SAS program version 9.4 for Windows (Copyright © 2012 SAS Inst., Cary, NC, USA). The Tukey test was used to compare the means with an α of 5% of significance. Four animals were removed from the dataset, two presented FAMACHA© 4 (anaemic) at D37 and were drenched, and the other two were considered outliers for feed conversion efficiency (atypical FCR with r2 for ADG of <0.38).
Results
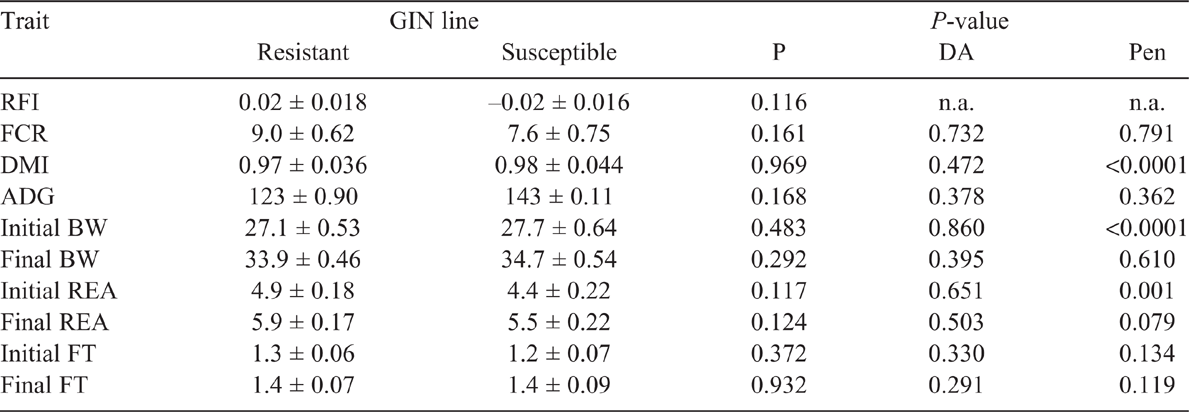
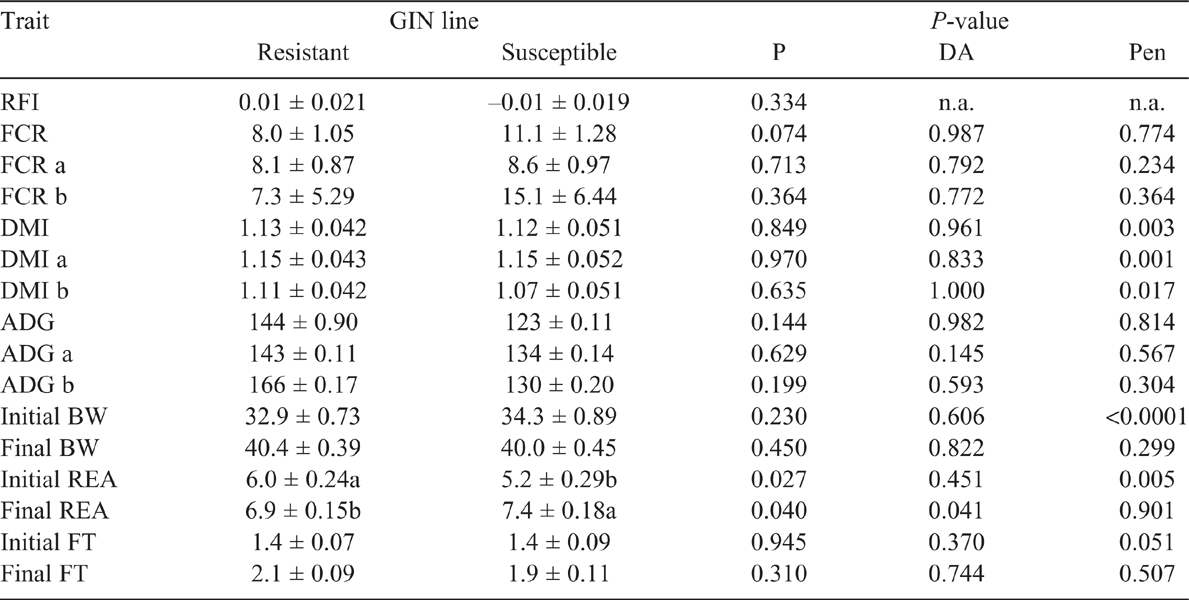
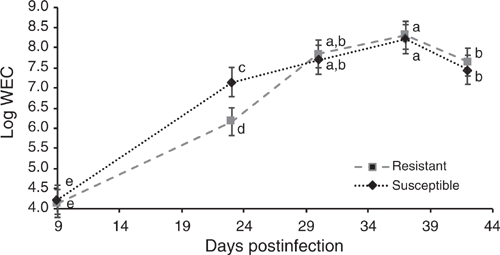
Residual feed intake did not differ between the GIN lines in the two periods studied (P > 0.05), without and with parasite challenge. The FCR in both periods, with or without infection, did not differ between R and S animals (P > 0.05). However, in Period 2, with H. contortus infection, R animals tended to have a lower FCR than did S ones, 8.0 and 11.1 respectively (P = 0.074). There were no statistical differences in DMI, ADG or final BW and FT in the two periods (P > 0.05; Tables 2, 3). The average DMI of all lambs was 0.99 and 1.14 kg of DM/day for Periods 1 and 2 respectively. REA at the end of Period 2 was larger in S lambs than in the R group (P < 0.05). In WEC evaluation, only the GIN line has no significant effect (P > 0.05), all the other factors (DA, Pen, Day post infection and the interaction Day and GIN line) had a significant effect (P < 0.01). Comparing the GIN lines on the same day post-infection, S animals presented a higher WEC (P = 0.0001) than did R animals only on Day 23, with no significant differences in WEC on Days 9, 30, 37 and 42 (Fig. 1). Back-transformed values of LogWEC indicated that WEC values on Day 23 were 976 and 1772 for the R and S lines respectively, while on Day 37, these values were 4557 and 4186 WEC for the same lines.
|
Discussion
The first part of our hypothesis that animals genetically resistant to GIN would not present differences in feed intake, productive performance and feed conversion efficiency when they are free of parasite infection was accepted. However, the argument that GIN-resistant animals would have lower feed conversion efficiency than susceptible animals under a parasite challenge with Haemonchus contortus was not supported by the results obtained here.
The GIN lines did not show differences on RFI or FCR regardless of the presence or absence of GIN. Therefore, it can be assumed that breeding sheep more resistant to GIN would not affect feed conversion efficiency, implying, for instance, that the costs of immunity or of the disease were not relevant enough to affect RFI, DMI or ADG in our study. Our results are supported by Liu et al. (2005) and Doyle et al. (2011), who reported that increased genetic resistance to GIN did not have any unfavourable consequences on animal performance (BW gain, fleece weight, wool growth) or voluntary feed intake, thus not affecting feed conversion efficiency with or without parasite challenge. Interestingly, in our study in Period 2, after animals were challenged, a tendency for an improved feed conversion efficiency was observed in those animals less susceptible to the disease. The tendency for a greater feed conversion into animal product in R lambs could be a consequence of spared energy after a less intense immune response to infection, particularly a reduced intestine cellular response and proliferation (Gill et al. 1992; Greer et al. 2005) compared with that observed in S animals. Indeed, Poppi and McLennan (2010) explained that parasitised animals have extra requirements of amino acids and show inefficiencies in the use of protein and energy, because they must prioritise reparation of digestive tract, replacement of plasma and mucous proteins and develop an immune response to parasites. In summary, even though a tendency for an improved FCR in GIN-resistant animals was detected, the most important finding of the present study is that breeding GIN-resistant animals would not have a negative effect on feed conversion efficiency when evaluated as FCR or RFI.
It has been suggested that the development of GIN immunity in sheep is metabolically more expensive than the subsequent expression of immunity (Wagland et al. 1984). This might also explain the lack of differences between S and R animals. The lambs in our experiment were 1-year old when immunity would be already established (Cuquerella 1992). If this experiment were conducted with younger lambs, the results could have been different. Indeed, the parasite immunity (known as protection) starts to develop between 6 and 9 months of age (Donald and Waller 1973). At this age, it could have been possible to find differences in animal performance and feed conversion efficiency, since large amount of nutrients are used for the development of immunity. In accordance with our results, experiments conducted with 10- and 18-month-old Merino wethers of divergent lines (Liu et al. 2005) indicated that without parasitic challenge, the cost of immunity was not large enough to alter the performance of the animals.
In addition to the effect of age and cost of immunity, it has been observed that high levels of crude protein in the diet (e.g. 19%) might minimise the effects of parasites (Datta et al. 1998; Steel 2003). In GIN infection, access to high dietary protein improves the immune response, since parasitised animals allocate more protein for mucoprotein production and replace detached epithelial cells (Liu et al. 2005) than do worm-free animals or animals with low parasite loads. This protein is diverted from production processes to give priority to maintenance, synthesis of proteins in plasma and blood, repair and integrity of the mucous membranes of the gastrointestinal tract and maintenance of the immune response (Parkins and Holmes 1989; McRae et al. 2015). The access of animals to diets with high protein would benefit consumption, in addition to conferring better resilience to susceptible animals (Kahn et al. 2003) by increasing protein synthesis (Kyriazakis et al. 1994; Doyle et al. 2014). Our animals were fed a diet with a high protein content (20.5%) and that might explain why R and S animals presented no differences in WEC counts from D23 to D42. The initial different response (D23), can be partially attributed to differences in the immune system response between the GIN lines. In this sense, Escribano et al. (2019), working with the same divergent lines of sheep, found that the earlier response to infection of R animals is related to higher levels of IgA in saliva and plasma, along with a more developed T-helper 2 lymphocyte response and a greater cytokine production from the beginning of the infection, while the immune response is developed 3 weeks after infection in S line. Supporting this, Liu et al. (2005) found that R animals had an immune response developed earlier (after infection), with a higher proportion of globulin in relation to albumin than in S animals. Moreover, they also reported that the increased nutrient demands to the parasite challenge of R sheep is modulated by the age, being irrelevant in elder sheep. Also, it has to be considered that three consecutive doses of 2000 L3 larvae of Haemonchus contortus used in our experiment would not be as effective to express the differences to GIN resistance between GIN lines as would natural infections of trickle or continuous infections with smaller doses of larvae throughout Period 2 (Emery et al. 2016). In summary, despite the large difference in WEC EPD (Table 1) and in phenotypic WEC (data not shown) measured from weaning to the start of study between GIN lines, differences on animal performances and WEC (after infection in Period 2) were not substantial. Animal age and the diet could have mediated to minimise the phenotypic expression of the divergent selection on GIN infection and animal performance.
At the end of the Infection Period 2 (after parasitic challenge) BW, BW gain and FT were not different between the GIN lines, but REA was affected by the nematode infection and, therefore, affected body composition of the animals. This particular result might be explained, first, by the genetic correlations between WEC and weight traits that have been reported to be not significantly different from zero or, when significant, they are favourable (negative; Brown and Fogarty 2017; Hollema et al. 2018). Similarly, small significant favourable correlations of muscle and fat traits with WEC have been estimated, although not always being different from zero. However, evidence of genotype by environment interaction has been reported for WEC and muscle depth in sheep (Pollott and Greeff 2004). Variations in larval challenges is one of the environmental factors explaining differences in the genetic correlations between those traits. In low-burden environments, selection for genetic resistance to WEC would not affect muscle development, while in high-burden environment, it could be favourably affected (Pollott and Greeff 2004). In our study, as expected, average WEC EPD of R and S animals were significantly different, as they were in the top 1% and lower 75% of the Uruguayan Corriedale genetic evaluation. In addition, in Period 2, worm burden in the first 30 days was similar to that considered as low-medium WEC environment by Pollott and Greeff (2004), while in the last 12 days, it would be a high WEC. Therefore, a positive genetic correlation between REA and WEC in a low-burden environment can partially explain the larger REA of GIN-susceptible animals.
Second, the larger REA of S lambs at the end of Period 2 could be explained, at least partially, by differential portioning of nutrients between the GIN lines. Because of a nematode infection, protein and energy requirements of sheep increase, and a differential response could be expected by R and S lambs. Liu et al. (2005), comparing unselected and selected GIN resistant animals, found a larger increase in protein (5%) and energy (4%) requirements in the line resistant to GIN, as previously stated. Those extra requirements of R animals, in addition to an earlier immune response (Escribano et al. 2019) expressed by a smaller WEC at D23, might indicate a different portioning (trade-off) of nutrients towards offsetting parasite infection. Partitioning of nutrients is affected by nematode infection, which reduces metabolisable protein supply, increasing protein demand, decreasing protein deposition and rates of protein synthesis in wool and muscle (Coop and Sykes 2002). Therefore, an earlier immune response associated with extra requirements of R sheep may explain the different muscle development between the GIN lines detected here.
This study has provided additional evidence that selection for resistance to GIN may not have negative effects on DMI, ADG, BW and feed conversion efficiency measured as FCR or RFI in 1-year old animals fed with a high-protein diet. Although animals from divergent lines selected for 17 years by GIN resistance were evaluated here, further research considering larger number of animals and sires, younger ages, different diets, continuous (trickle) infections and periods of evaluation, overcoming some of the constrains of this study, will contribute to enhancing the understanding of the associations among these traits.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the assistance of the Agricultural Technician Fernando Rovira, the staff of the Experimental Unit La Magnolia, and INIA Tacuarembó. This research has received funding from the European Union’s Horizon 2020 research and innovation program under the Grant Agreement n°772787 (Smarter) and from the Instituto Nacional de Investigación (INIA_CL_38: Rumiar).
References
Brown DJ, Fogarty NM (2017) Genetic relationships between internal parasite resistance and production traits in Merino sheep. Animal Production Science 57, 209–215.| Genetic relationships between internal parasite resistance and production traits in Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Cammack KM, Leymaster K, Jenkins TG, Nielsen MK (2005) Estimates of genetic parameters for feed intake, feeding behavior, and daily gain in composite ram lambs. Journal of Animal Science 83, 777–785.
| Estimates of genetic parameters for feed intake, feeding behavior, and daily gain in composite ram lambs.Crossref | GoogleScholarGoogle Scholar | 15753331PubMed |
Cantalapiedra-Hijar G, Abo-Ismail M, Carstens GE, Guan LL, Hegartty R, Kenny DA, McGee M, Plastow G, Relling A, Ortigues-Marty I (2018) Review: biological determinants of between-animal variation in feed efficiency of growing beef cattle. Animal 12, s321–s335.
| Review: biological determinants of between-animal variation in feed efficiency of growing beef cattle.Crossref | GoogleScholarGoogle Scholar | 30139392PubMed |
Castells D (2005) Adaptación de genotipos a ambientes adversos: resistencia genética de los ovinos a parásitos gastrointestinales. Agrociencia 9, 587–593.
Castells D (2008) Evaluación de resistencia genética de ovinos Corriedale a los nematodos gastrointestinales en Uruguay: Heredabilidad y Correlaciones genéticas entre el recuento de huevos de nematodos y caracteristicas productivas. MSc Thesis, Universidad de la República, Uruguay.
Castells D, Gimeno D (2011) Selection of Corriedale sheep for resistance or susceptibility to nematode infection in Uruguay. In ‘Proceedings of the 23rd International Conference of the World Association for the Advancement of Veterinary Parasitology (WAVVP)’, Buenos Aires, Argentina. Available at http://helminto.inta.gob.ar/WAAVP23/session_o.html [Verified 23 February 2021]
Coop RL, Sykes AR (2002) Interactions between gastrointestinal parasites and nutrients. In ‘Sheep Nutrition’. (Eds M Freer, H Dove) pp. 213–331. (CAB International: Wallingford, UK)
Cuquerella A (1992) Respuesta humoral en hemoncosis ovina, dinámica de la producción de anticuerpos y caracterización antigénica de Haemonchus contortus. PhD Thesis, Universidad Complutense de Madrid, España.
Datta FU, Nolan JV, Rowe JB, Gray GD (1998) Protein supplementation improves the performance of parasitised sheep fed a straw based diet. International Journal for Parasitology 28, 1269–1278.
| Protein supplementation improves the performance of parasitised sheep fed a straw based diet.Crossref | GoogleScholarGoogle Scholar | 9762574PubMed |
Donald A, Waller P (1973) Gastro-intestinal nematode parasite populations in ewes and lams and the origin and time course of infective larval availability in pastures. International Journal for Parasitology 3, 219–233.
| Gastro-intestinal nematode parasite populations in ewes and lams and the origin and time course of infective larval availability in pastures.Crossref | GoogleScholarGoogle Scholar | 4706572PubMed |
Doyle EK, Kahn LP, McClure SJ, Lea JM (2011) Voluntary feed intake and diet selection of Merino sheep divergently selected for genetic difference in resistance to Haemonchus contortus. Veterinary Parasitology 177, 316–323.
| Voluntary feed intake and diet selection of Merino sheep divergently selected for genetic difference in resistance to Haemonchus contortus.Crossref | GoogleScholarGoogle Scholar | 21330058PubMed |
Doyle EK, Kahn LP, McClure SJ (2014) Nutrient partitioning of Merino sheep divergently selected for genetic difference in resistance to Haemonchus contortus. Veterinary Parasitology 205, 175–185.
| Nutrient partitioning of Merino sheep divergently selected for genetic difference in resistance to Haemonchus contortus.Crossref | GoogleScholarGoogle Scholar | 25027755PubMed |
Eady S, Woolaston R, Barger I (2003) Comparison of genetic and nongenetic strategies for control of gastrointestinal nematodes of sheep. Livestock Production Science 81, 11–23.
| Comparison of genetic and nongenetic strategies for control of gastrointestinal nematodes of sheep.Crossref | GoogleScholarGoogle Scholar |
Emery DL, Hunt PW, Le Jambre LF (2016) Haemonchus contortus: the then and now, and where to from here? International Journal for Parasitology 46, 755–769.
| Haemonchus contortus: the then and now, and where to from here?Crossref | GoogleScholarGoogle Scholar | 27620133PubMed |
Escribano C, Saravia A, Costa M, Castells D, Ciappesoni G, Riet-correa F, Freire T (2019) Resistance to Haemonchus contortus in Corriedale sheep is associated to high parasite- specific IgA titer and a systemic Th2 immune response. Scientific Reports 9, 19579
| Resistance to Haemonchus contortus in Corriedale sheep is associated to high parasite- specific IgA titer and a systemic Th2 immune response.Crossref | GoogleScholarGoogle Scholar | 31862904PubMed |
Fiel CA, Steffan PE, Ferreyra DA (2011) ‘Diagnóstico las parasitosis más frecuentes los rumiantes técnicas diagnóstico e interpretación de resultados.’ (Abad Benjamin: Buenos Aires, Argentina)
Fogarty NM, Safari E, Mortimer SI, Greeff JC, Hatcher S (2009) Heritability of feed intake in grazing Merino ewes and the genetic relationships with production traits. Animal Production Science 49, 1080–1085.
| Heritability of feed intake in grazing Merino ewes and the genetic relationships with production traits.Crossref | GoogleScholarGoogle Scholar |
Gill HS, Watson DL, Brandon MR (1992) In vivo inhibition by a monoclonal antibody to CD4+ T cells of humoral and cellular immunity in sheep. Immunology 77, 38–42.
Goldberg V, Ciappesoni G, Aguilar I (2012) Genetic parameters for nematode resistance in periparturient ewes and post-weaning lambs in Uruguayan Merino sheep. Livestock Science 147, 181–187.
| Genetic parameters for nematode resistance in periparturient ewes and post-weaning lambs in Uruguayan Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Greer AW (2008) Trade-offs and benefits: implications of promoting a strong immunity to gastrointestinal parasites in sheep. Parasite Immunology 30, 123–132.
| Trade-offs and benefits: implications of promoting a strong immunity to gastrointestinal parasites in sheep.Crossref | GoogleScholarGoogle Scholar | 18186772PubMed |
Greer AW, Stankiewicz M, Jay NP, McAnulty RW, Sykes AR (2005) The effect of concurrent corticosteroid induced immuno-suppression and infection with the intestinal parasite Trichostrongylus colubriformis on food intake and utilization in both immunologically naïve and competent sheep. Animal Science 80, 89–99.
| The effect of concurrent corticosteroid induced immuno-suppression and infection with the intestinal parasite Trichostrongylus colubriformis on food intake and utilization in both immunologically naïve and competent sheep.Crossref | GoogleScholarGoogle Scholar |
Hollema BL, Bijma P, van der Werf JHJ (2018) Sensitivity of the breeding values for growth rate and worm egg count to environmental worm burden in Australian Merino sheep. Journal of Animal Breeding and Genetics 135, 357–365.
| Sensitivity of the breeding values for growth rate and worm egg count to environmental worm burden in Australian Merino sheep.Crossref | GoogleScholarGoogle Scholar | 29993145PubMed |
Kahn LP, Knox MR, Gray GD, Lea JM, Walkden-Brown SW (2003) Enhancing immunity to nematode parasites in single-bearing Merino ewes through nutrition and genetic selection. Veterinary Parasitology 112, 211–225.
| Enhancing immunity to nematode parasites in single-bearing Merino ewes through nutrition and genetic selection.Crossref | GoogleScholarGoogle Scholar | 12591197PubMed |
Koch R, Swiger L, Chambers D, Gregory K (1963) Efficiency of feed use in beef cattle. Journal of Animal Science 22, 486–494.
| Efficiency of feed use in beef cattle.Crossref | GoogleScholarGoogle Scholar |
Kyriazakis I, Oldham JD, Coop RL, Jackson F (1994) The effect of subclinical intestinal nematode infection on the diet selection of growing sheep. British Journal of Nutrition 72, 665–677.
| The effect of subclinical intestinal nematode infection on the diet selection of growing sheep.Crossref | GoogleScholarGoogle Scholar |
Liu SM, Smith TL, Karlsson LJE, Palmer DG, Besier RB (2005) The costs for protein and energy requirements by nematode infection and resistance in Merino sheep. Livestock Production Science 97, 131–139.
| The costs for protein and energy requirements by nematode infection and resistance in Merino sheep.Crossref | GoogleScholarGoogle Scholar |
Masters DG, Ferguson M (2019) A review of the physiological changes associated with genetic improvement in clean fleece production. Small Ruminant Research 170, 62–73.
| A review of the physiological changes associated with genetic improvement in clean fleece production.Crossref | GoogleScholarGoogle Scholar |
McRae KM, Stear MJ, Good B, Keane OM (2015) The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunology 37, 605–613.
| The host immune response to gastrointestinal nematode infection in sheep.Crossref | GoogleScholarGoogle Scholar | 26480845PubMed |
Méndez M, Cabo JL (1980) Determination of the prepatent period of Haemonchus contortus in sheep. Ciencia y Tecnica en la Agricultura, Veterinaria 2, 19–30.
Morris CA, Vlassoft A, Bisset SA, Baker RL, Watson TG, West CJ, Wheerler M (2000) Continued selection of Romney sheep for resistance or susceptibility to nematode infection: estimates of direct and correlated responses. Animal Science 70, 17–27.
| Continued selection of Romney sheep for resistance or susceptibility to nematode infection: estimates of direct and correlated responses.Crossref | GoogleScholarGoogle Scholar |
Paganoni B, Rose G, Macleay C, Jones C, Brown DJ, Kearney G, Ferguson M, Thompson AN (2017) More feed efficient sheep produce less methane and carbon dioxide when eating high-quality pellets. Journal of Animal Science 95, 3839–3850.
| More feed efficient sheep produce less methane and carbon dioxide when eating high-quality pellets.Crossref | GoogleScholarGoogle Scholar | 28992015PubMed |
Parkins JJ, Holmes HP (1989) Effects of gastrointestinal helminth parasites on ruminant nutrition. Nutrition Research Reviews 2, 227–246.
| Effects of gastrointestinal helminth parasites on ruminant nutrition.Crossref | GoogleScholarGoogle Scholar | 19094355PubMed |
Pollott GE, Greeff JC (2004) Genotype × environment interactions and genetic parameters for fecal egg count and production traits of Merino sheep 1. Journal of Animal Science 82, 2840–2851.
| Genotype × environment interactions and genetic parameters for fecal egg count and production traits of Merino sheep 1.Crossref | GoogleScholarGoogle Scholar | 15484934PubMed |
Poppi DP, McLennan SR (2010) Nutritional research to meet future challenges. Animal Production Science 50, 329–338.
| Nutritional research to meet future challenges.Crossref | GoogleScholarGoogle Scholar |
Rauw WM (2012) Feed Efficiency and Animal Robustness. In ‘Feed Efficiency in the Beef Industry’ (Ed. RA Hill) pp. 105–122. (Wiley-Blackwell: Iowa, USA)
Roberts F, O’Sullivan P (1949) Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Australian Journal of Agricultural Research 1, 99–102.
| Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle.Crossref | GoogleScholarGoogle Scholar |
Safari E, Fogarty NM, Gilmour AR (2005) A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livestock Production Science 92, 271–289.
| A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep.Crossref | GoogleScholarGoogle Scholar |
Steel JW (2003) Effects of protein supplementation of young sheep on resistance development and resilience to parasitic nematodes. Australian Journal of Experimental Agriculture 43, 1469–1476.
| Effects of protein supplementation of young sheep on resistance development and resilience to parasitic nematodes.Crossref | GoogleScholarGoogle Scholar |
Van Wyk JA, Bath GF (2002) The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Veterinary Research 33, 509–529.
Wagland BM, Steel JW, Windon RG, Dineen JK (1984) The response of lambs to vaccination and challenge with Trichostrongylus colubriformis: effect of plane of nutrition on, and the inter-relationship between, immunological responsiveness and resistance. International Journal for Parasitology 14, 39–44.
| The response of lambs to vaccination and challenge with Trichostrongylus colubriformis: effect of plane of nutrition on, and the inter-relationship between, immunological responsiveness and resistance.Crossref | GoogleScholarGoogle Scholar | 6706464PubMed |
Walkden-Brown SW, Kahn LP (2002) Nutritional modulation of resistance and resilience to gastrointestinal nematode infection. Journal of Animal Science 15, 912–924.