Suitability of litter amendments for the Australian chicken meat industry
S. A. Cockerill A , P. F. Gerber B , S. W. Walkden-Brown B and M. W. Dunlop A C
A , P. F. Gerber B , S. W. Walkden-Brown B and M. W. Dunlop A C
A Department of Agriculture and Fisheries, Queensland Government, EcoSciences Precinct, 41 Boggo Road, Dutton Park, Qld 4102, Australia.
B Animal Science, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
C Corresponding author. Email: mark.dunlop@daf.qld.gov.au
Animal Production Science 60(12) 1469-1481 https://doi.org/10.1071/AN19587
Submitted: 15 October 2019 Accepted: 10 March 2020 Published: 19 May 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
The Australian chicken meat indutstry is rapidly expanding due to the increasing consumption of chicken meat. As a result, the industry has growing issues of sourcing new bedding materials and disposing of spent litter, which can be attributed, in part, to a lack of widespread litter re-use for rearing chickens. According to insights and perspectives recently gathered from industry stakeholders, it is believed that re-using litter will become more common in the future, so as to reduce production costs and ease pressures on both the supply of new bedding materials and disposal of spent litter. However, there are potential risks that need to be addressed if litter re-use increases, particularly with regard to the production and mitigation of ammonia, which can negatively affect chicken health if not managed correctly. The present review discusses the potential benefits reported for different types of litter amendments, which have the primary goal of reducing ammonia volatilisation, but may also contribute to improvements in bird performance, welfare, pathogen loads, fertiliser value of spent litter, and reduced costs associated with purchasing new bedding materials. Acidifiers have been shown to be the most effective of all amendment types, with sodium bisulfate or alum being among the most commonly tested products mentioned in research literature. Litter amendments are currently rarely used in Australia, but it is hoped that the information provided in the present review, based mostly on overseas usage and research, will help inform future decision-making on the use of these products in Australian poultry production systems.
Additional keywords: alum, ammonia, broiler, poultry, sodium bisulfate, welfare.
Introduction
Consumption of chicken meat in Australia in 2018–2019 was anticipated to be 47.7 kg per person per year, which is significantly greater than the consumption of other competing meats, and is expected to rise a further 8% by 2022 (ACMF 2018). This forecast growth of Australian chicken meat production may require modification to current management practices, including sourcing bedding material and litter re-use (Watson and Wiedemann 2019). One of the most significant environmental issues that the chicken meat industry faces is the accumulation of wastes, specifically manure and litter (Bolan et al. 2010).
In Australia, the dominant litter-management practice is to source new bedding for each flock (Wiedemann 2015). Litter re-use is currently not a widespread practice, even though it has the potential to reduce costs and improve environmental sustainability (Wiedemann 2015). Using new bedding for each flock results in approximately one million tonnes of spent chicken litter being produced annually (Biomass Producer 2013). This method comes with significant financial and environment implications, with the constant need to not only source new material but to dispose of spent litter (Watson and Wiedemann 2019).
In contrast to the Australian situation, in the United States of America (USA) and Brazil, which are the two global leaders in chicken meat production, litter re-use for multiple consecutive flocks is widely utilised for two specific reasons, namely, to reduce production costs and environmental impact (Roll et al. 2011; ACMF 2018). Significant research has been conducted investigating how the addition of amendments to litter used across multiple flocks can improve production and welfare, predominantly by reducing ammonia concentrations. It is vital that the Australian industry understands these practices if there is ever the need to expand litter re-use in this country.
In addition to providing information on amendments and their application in other countries, it is important to understand current Australian attitudes to litter re-use and the role of amendments now and into the future. To this end, semi-structured interviews were conducted with selected key stakeholders of the Australian chicken meat industry. The interviews used a series of open questions to garner the level of knowledge, experience and perspectives on using litter amendments in Australia, rather than collecting statistics on industry practices. Receiving similar feedback from multiple respondents was considered to be indicative of the relative importance of the responses.
The objectives of the present consultation and review were to identify industry attitudes and practices regarding re-use of litter and the role of litter amendments. We also sought to identify litter amendment products that are available to be used in meat chicken production in Australia, and to discuss the factors that influence their suitability and ability to reduce risks associated with ammonia, improve litter properties, reduce costs, add value to the production system, and contribute to disease and pathogen management.
Industry perspective on litter re-use and amendments
The industry questionnaire and interviews were completed by 12 people representing key stakeholders of the Australian chicken meat industry. Respondents were selected on the basis of recommendations by industry representative bodies and individuals, as people with previous experience or knowledge of litter re-use practices and litter amendments. They included veterinarians, farming managers, service people, litter contractors or poultry consultants. Each was aligned with at least one of seven integrator companies and represented national or state-specific experiences, views and trends for New South Wales, Victoria, South Australia, Tasmania and Queensland (respondents who commented on national perspectives indicated that their responses were also representative of Western Australia). Due to some respondents having limited experiences with litter re-use or amendments, and alignment with a specific integrator, it could be expected that some responses were subject to some biases. The responses showed low rates of litter re-use due to a preference for full litter clean-out at the end of each grow-out, so as to enable thorough cleaning, disinfection and placement of new bedding between each flock. While respondents commented that new bedding materials were becoming more difficult and expensive to source, this has not resulted in uptake of litter re-use in most regions. Litter re-use has been adopted on some farms in response to shortages of new bedding supplies, but even these use only partial litter re-use practices, where new bedding is placed in the brooding section and re-used litter is used only in the non-brooding section of the shed.
Concerns about litter re-use were concentrated on ammonia, disease and pathogen carry-over, odour, additional labour requirements, and inadequate time for litter-treatment processes to be completed effectively due to quick turn-around between flocks. These concerns appear to have outweighed the potential benefits observed by those who re-use litter, which included cost-effectiveness and having warmer, drier and better insulating litter. Litter re-use is effectively a pre-requisite to using litter amendments, so it is not surprising that with such limited litter re-use, and effectively no brooding on used litter, there is currently no routine use of litter amendments in Australia.
Half of the respondents indicated that uptake of litter re-use practices and use of litter amendments was likely in the future due to challenges with sourcing new bedding, increasing costs, and increasing challenges with selling or disposing of spent litter (50% indicated more likely to uptake litter re-use, 17% indicated less likely, 25% said no change to current levels of re-use and 8% did not respond to this question). To support industry uptake, sound scientific evidence, proof of cost effectiveness and suitability for Australian chicken meat production practices would be necessary. The following review regarding ammonia and litter amendments is seen as the first essential step for compiling knowledge about litter amendments and identifying where further research is required to support uptake by the Australian chicken meat industry when litter re-use becomes the preferred practice.
Review of ammonia formation, effects and mitigation strategies
Ammonia (NH3) formation in meat chicken houses
Chicken excreta is high in uric acid, which is nitrogen rich. Uric acid undergoes a stepwise enzymatic degradation during microbial decomposition in the litter, resulting in the production of ammonia (Carlile 1984; Naseem and King 2018). The products from this chemical process have been described previously (Eqn 1; Groot Koerkamp 1994), as follows:

Once NH3 is produced in litter, NH3 volatilisation occurs, which is the primary cause of NH3 being present in its gaseous form within the atmosphere of the production shed (Naseem and King 2018). This process exists as an equilibrium between NH3 and ammonium (NH4+; Eqn 2; Groot Koerkamp 1994), as follows:

Incorporating both Eqns 1 and 2, Fig. 1 shows the general process of where NH3 comes from and how it diffuses and disperses within a production shed, resulting in detectable concentrations (Elliott and Collins 1982). Gaining an understanding on this process is vital for the development of counter measures to reduce in-house NH3 concentration.
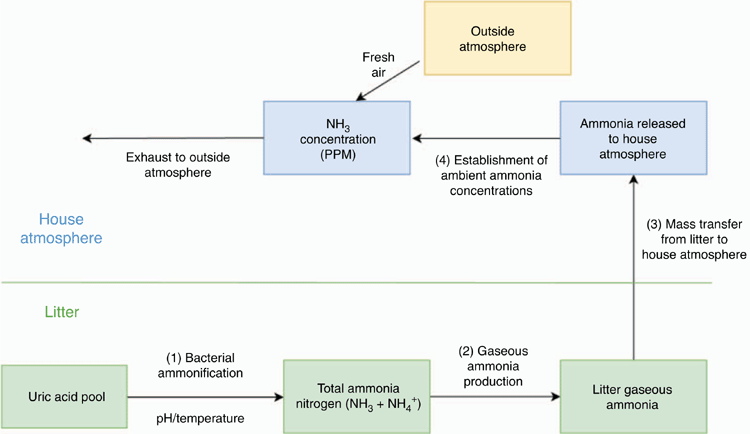
|
Factors that affect the NH3–NH4+ equilibrium
As NH3 volatilisation exists as an equilibrium with NH4+ concentration, there are many influential factors that can shift the reaction to the more desired product, NH4+ (Groot Koerkamp 1994). These factors include pH, moisture content, temperature, feed, bacterial processes and the control of the atmosphere through ventilation (Groot Koerkamp 1994; Moore et al. 2008). The dominant controlling factor in NH3 volatilisation is pH, as it has been well documented that NH3 production increases in basic conditions (pH of >7.0; Moore et al. 2008), with virtually no NH3 being released with even slightly acidic litter (pH of ≤6.0; Elliott and Collins 1982). A more acidic environment involving the increased presence of hydrogen ions pushes the equilibrium towards the production of NH4+.
Detrimental effects of NH3 on chickens and humans
The damaging effects of NH3 on both birds and workers are well documented and it is recommended that in-house concentration should be <25 ppm (25 µL per L of air), with the ideal concentration being <10 ppm (Naseem and King 2018). Australian welfare standards require NH3 to be <20 ppm, with some farming schemes requiring the concentration to be <15 ppm (RSPCA 2013; Animal Health Australia 2017).
Effects of NH3 exposure are dependent on the concentration and duration of exposure. Even at low concentrations, workers can experience acute effects such as irritation to the upper respiratory tract, nose and eyes (Naseem and King 2018). Ammonia can also contribute to the production of secondary particulate matter that has a diameter of <2.5 µm (PM2.5) through the formation of ammonium sulfate ((NH4)2SO4), ammonium bisulfate (NH4HSO4) and ammonium nitrate (NH4NO3) (Erisman and Schaap 2004; Fine et al. 2008). These particles can penetrate into the lower respiratory tract of humans, potentially resulting in chronic respiratory disease (Naseem and King 2018). Naseem and King (2018) observed that chronic cough, phlegm, bronchitis and chest tightness were all higher in poultry workers and chicken catchers than in a control group, which consisted of non-exposed workers.
Ammonia can have detrimental effects on chicken production. Miles et al. (2004) observed that final bodyweight was 6% and 9% lower in the groups exposed to 50 ppm and 75 ppm of NH3 respectively, during the first 4 weeks of production than it was in the control group. Mortality was also observed to be 13.9% for chicks exposed to an NH3 concentration of 75 ppm, compared with the 5.8% at 0 ppm (Miles et al. 2004).
Ammonia can also affect chicken health and welfare. Ammonia causes damage in the respiratory tract, including partial loss of tracheal cilia at a concentration of 25 ppm (Anderson et al. 1966), and complete deciliation of the epithelium of the upper portion of trachea at a concentration of 100 ppm (Oyetunde et al. 1978). Those changes weaken the defence mechanisms of the respiratory system and are related to increased susceptibility to bacterial and viral airborne infections (Anderson et al. 1966; Quarles and Kling 1974; Oyetunde et al. 1978; Beker et al. 2004). Above concentrations of 25 ppm, NH3 causes inflammation of the cornea and conjunctiva, and exposures to 50–75 ppm of NH3 can lead to a significant corneal ulceration after 7 days of exposure (Valentine 1964; Miles et al. 2006; Olanrewaju et al. 2007). Chickens under trial and commercial grow-out conditions have shown signs of healing when no longer exposed to NH3 concentrations of >25 ppm; however, complete return to normal cannot be assumed when there is severe damage (Miles et al. 2006).
In addition to respiratory and ocular issues, Zhang et al. (2011) found that foot pads can become damaged due to increased concentration of NH3 in litter. If damage becomes too significant, quality of life can begin to diminish due to lack of mobility. Although not as severe, gaseous NH3 emissions can have some residual effects on the environment such as potentially contributing to acid rain through associated reactions that form sulfur dioxide (SO2) and nitrogen oxide (NOx) (ApSimon et al. 1987; Menz and Seip 2004). Ammonia emissions can also contribute to a decrease of pH in soil through nitrification (Menz and Seip 2004). Local water systems are also at risk of increased nitrates due to runoff (Naseem and King 2018).
Current management strategies to mitigate NH3-related issues in Australia
In Australia, the detrimental effects of high NH3 concentration are, for the most part, controlled through ventilation and husbandry practices, including brooding on new bedding materials. Previous investigations into NH3 concentrations on Australian meat chicken farms with full cleanout have shown that concentrations are generally <15 ppm, peaking between 3 and 5 weeks of age, and declining thereafter when measured at 30 cm and 150 cm above ground level, but persisting at higher concentrations when measured at 5 cm above ground level (Islam et al. 2010). In a separate study, NH3 concentrations were found to be significantly higher in sheds with re-used pasteurised litter, particularly during the first 2 weeks of the grow-out, but were still acceptable, being <20 ppm (Walkden-Brown et al. 2010). However, a further study found markedly elevated NH3 concentrations in sheds with re-used litter (40–47 ppm at 7 days) relative to those with new litter (5.9–17.6 ppm; Cressman et al. 2014).
As outlined in Fig. 1, even with NH3 volatilisation occurring rapidly, acceptably low concentration of NH3 within the poultry house can be maintained with sufficient ventilation (Elliott and Collins 1982; Carlile 1984). Increasing ventilation, especially during brooding when the farmer is trying to heat the shed to 30–34 °C, can significantly increase energy costs because heat energy exhausted from the house needs to be replaced with supplemental heating (using electric or gas heaters). This is particularly so during the winter months in colder regions, making the approach impractical at these times. Reducing NH3 volatilisation at the litter level will reduce the need for ventilation to control the in-house NH3 concentration, which may be in excess of that required for chicken comfort, litter moisture and relative humidity control.
The most influential factors in reducing NH3 volatilisation are based around litter properties such as pH and moisture content, regardless of whether the litter is being re-used or not. Litter re-use is not the primary management practice utilised in Australia. Instead, use of new bedding materials for every flock is preferred (Wiedemann 2015). This strategy comes with some benefits, primarily reduced risks associated with NH3, but it comes at a significant financial cost (Watson and Wiedemann 2019). It is feasible that litter re-use will become more common in the future as a way to reduce production costs associated with new bedding as well as shed ventilation and heating, which are associated with maintaining low in-house NH3 concentration by dilution with fresh air.
Review of litter amendments and their effects
Litter amendment products
The present review focuses on the acidifying products, as these are the most widely used in commercial meat chicken production because they are reliable, effective for NH3 reduction and affordable (Choi and Moore 2008). However, amendments are not limited to acidifiers, with other amendments such as inhibitors, adsorbents and alkalinisers being potentially viable options that have not been widely adopted in commercial meat chicken production yet.
Acidifiers
The most common form of litter amendments is a group of agents known as ‘acidifiers’. These acidifying agents act to decrease the pH of the litter to below 7.0, thus creating an environment where the NH3–NH4+ equilibrium outlined in Eqn 2 favours the production of NH4+ (Groot Koerkamp 1994; Moore et al. 2000). Three examples of acidifying agents that are commonly used in other countries are alum (aluminium sulfate), sodium bisulfate and sulfuric acid. When applied to the litter, the hydrogen ions released are attracted to the partially negatively charged nitrogen of NH3 and combine to produce NH4+. Ammonium can react with sulfate, another component in most of the acidifiers, to produce (NH4)2SO4, which is commonly found in fertilisers (Hadlocon and Zhao 2015). A major drawback of chemical acidifiers is that their effectiveness is limited to the availability of the reactants. Once these are used, pH will begin to become more basic with the constant addition of bird excreta. For example, Walkden-Brown et al. (2010) found that application of either alum or sodium bisulfate at the rate of 0.425 kg/m2 to re-used litter before placement of chickens, induced large reductions (55–75%) in NH3 production from litter at 7 days and 14 days after placement, but the reductions were much smaller (but still significant) thereafter, up to 42 days post application.
In addition to controlling NH3 emissions, acidifiers have been shown to reduce pests and pathogens in re-used litter. Specifically, alum, sodium bisulfate and acidified clay have been shown to control adult and larval darkling beetles after 34 days of chick placement (McWard and Taylor 2000) and to reduce infectious laryngotracheitis virus to non-detectable levels in litter, as determined by a bioassay (Giambrone et al. 2008). Litter treatment with alum (0.97 kg/m2) and sodium bisulfate (0.27 kg/m2) also contributed to a statistically significant reduction in Eimeria oocyst count, from an average of 9373 oocysts/g in untreated litter, to an average of 7100 oocysts/g in the treatment groups after 6 weeks of chick placement (Sahoo et al. 2017).
Aluminium sulfate (alum)
Alum (aluminium sulfate, Al2(SO4)3) is one of the more commonly used acidifiers in chicken meat production in the USA. It is available in both dry and liquid form. Choi and Moore (2008) observed the effects of both dry and liquid alum treatment at low (0.49 kg/m2) and high (0.98 kg/m2) rates applied to poultry litter under laboratory conditions. The amendments were mixed into the litter and, after a 42 days period, NH3 concentrations were recorded. It was determined that the low- and high-rate dry alum reduced NH3 by 77% and 96% respectively. Liquid alum also reduced NH3 by 89–96% at low and high rates respectively (Choi and Moore 2008). These results demonstrated that alum had the capacity to significantly reduce the amount of NH3 volatilised from litter. Even though this was a controlled experiment without constant presence of birds, the results clearly showed potential for reduction in NH3 volatilisation.
Eugene et al. (2015) conducted a study to assess alum treatments in an entire meat chicken house over the course of four flocks compared with a control. In this study, litter was de-caked between sequential grow-outs in both the control and treated poultry houses. Alum was added to litter at a rate of 2.37 kg/m2 (0.18–0.19 kg/bird), which was higher than the recommended amount but was chosen so that the alum would continue to be active throughout the entire grow-out to produce 3.63 kg meat chickens. Using alum reduced daily in-house NH3 emissions by 42% with an overall emission reduction of 47% (Eugene et al. 2015; although it should be recognised that this trial did not have shed replication, which means that there is potential for the results to be attributed to a shed effect rather than the treatment alone). These results, while lower than those recorded by Choi, showed that significant NH3 reductions occur with alum application to re-used litter and throughout the production cycle where birds are consistently dropping waste. It was also estimated that 287 kg more nitrogen was present in the alum-treated litter at the end of the flock than there was in the control, increasing its agronomic value as a fertiliser (Eugene et al. 2015).
In a study by Worley et al. (2000), alum was applied at the ‘recommended’ rate of 0.98 kg/m2 and half-rate of 0.49 kg/m2 in separate poultry houses. In-house NH3 concentration was maintained <25 ppm by adjusting minimum ventilation rates. Higher application rate of alum was found to increase NH4+ content of litter by ~25% compared with the half-rate, which was indicative of more NH3 being retained in the litter in NH4+ form (Worley et al. 2000). There were no significant differences in the amount of total nitrogen in litter, bodyweight, feed conversion, mortality or production costs between the two rates (Worley et al. 2000). These results indicated that it is likely that reductions in NH3 emissions were improved with a high-rate alum application; however, the low-rate application showed very comparable results at a reduced cost. The authors also suggested further strategies to reduce the cost of using alum by only treating the brooding section in the shed where chicks are at the highest level of risk (Worley et al. 2000).
Alum has also been shown to reduce pathogen loading in litter sourced from a commercial meat chicken house. After the fifth flock of use, litter was removed and placed in test chambers with alum added at a rate of 10% of total weight (Rothrock et al. 2008). Initial concentrations of Campylobacter jejuni for the test and control chambers were found to be 9.3 × 107 cells/g and 6.3 × 107 cells/g respectively. After 4 weeks, the concentration of C. jejuni in the test chamber was below the detectable limits (104 cells/g) and the control was 1.9 × 107 cells/g, indicating that the addition of alum can potentially result in a three-log reduction in the concentration of C. jejuni (Rothrock et al. 2008). As with NH3 reduction, alum has a diminished effectiveness with regards to Campylobacter inhibition once the pH rises (Rothrock et al. 2008). Line and Bailey (2006) found that alum treatment was able to delay the onset of Campylobacter incidence when applied to five separate production sheds. It was determined that alum treatment did not delay the onset of Salmonella incidence and, therefore, was not an effective method of control (Line and Bailey 2006; Chung et al. 2015; Sahoo et al. 2017).
Sodium bisulfate
Sodium bisulfate (NaHSO4) is another acidifier that is commonly used in chicken meat production in the USA. When applied to litter, NaHSO4 dissociates to Na+, H+ and SO4–,with the hydrogen ion causing the pH in the litter to be reduced (Jones-Hamilton AG 2018). Hunolt et al. (2015) conducted a controlled 14-day laboratory experiment and a field study using different strategies of NaHSO4 application to reduce NH3 volatilisation. Fresh manure was added every 48 h to simulate bird excretion. Initial NaHSO4 application was applied at a rate of 0.48 kg/m2, with re-applications being at a rate of 0.24 kg/m2. Ammonia volatilisation was observed at a 269% increase when manure remained untreated, as opposed to the test that utilised NaHSO4 re-application (Hunolt et al. 2015). Treated litter had a nitrogen concentration of 26.5 g/kg compared with 24.6 g/kg for untreated litter, indicating an increase in nitrogen retention (Hunolt et al. 2015). A single initial application of NaHSO4 resulted in NH3 concentrations being significantly lower than in the control test (Hunolt et al. 2015). These results suggested that a single application of NaHSO4 could be enough to reduce NH3 concentration; however, re-application would result in further reductions, but at an increased cost.
Hunolt et al. (2015) also conducted a field experiment where NaHSO4 was applied to a single commercial meat chicken house. For three consecutive flocks, NaHSO4 was applied at a rate of 0.24 kg/m2, with two re-applications being performed at 5 and 10 days. For the first three flocks, there was no significant difference in nitrogen and NH4+ concentrations of the litter (Hunolt et al. 2015); however, after the fourth flock, 42.5 g/kg of nitrogen was recorded in the treated litter, compared with 38.6 g/kg in the control litter (Hunolt et al. 2015). Ammonium concentration of the treated litter was 15.3 g/kg after the fourth flock, which again was more than the 13.2 g/kg in the control (Hunolt et al. 2015). These results indicated that there was some reduction in NH3 volatilisation that was inferred by increased retention of nitrogen and NH4+; however, there was no replication of the treatment sheds, which may have reduced the strength of the conclusions that can be drawn from this trial. Reduction in NH3 volatilisation can be linked to a reduction in pH caused by an acidifier. In this field study, the lowest pH achieved was 6.46, which is still close to neutral and potentially explains why the results did not indicate significant reductions in NH3 volatilisation.
Pope and Cherry (2000) conducted a study in meat chicken houses utilising the same application rate of 0.24 kg/m2. In this study, mean in-house NH3 concentration of 6.2 ppm was recorded after application. This was significantly lower than the mean concentration of 62.3 ppm measured in the control houses (Pope and Cherry 2000). However, after 2 weeks, the NH3 concentration in the control houses had reduced to 19.8 ppm but in the test shed it had increased to 10.7 ppm (Pope and Cherry 2000). These results indicated that NaHSO4 has the capability to reduce NH3 concentration to an acceptable level for the first 2 weeks of the grow-out.
Total bacterial count showed that NaHSO4 was able to inhibit bacterial presence immediately after application (1.45 × 108 colony-forming units per gram (cfu/g) for control and 9.2 × 106 cfu/g for treatment; Pope and Cherry 2000). However, 1 week after application, there was no significant difference between test and control, indicating that the long-term effect of NaHSO4 on bacterial concentration is limited. Line and Bailey (2006) observed that there was a delay of onset of Campylobacter when NaHSO4 was applied to litter and that there was no effect on Salmonella, which is a result similar to what they observed with alum treatment.
Sulfuric acid
Sulfuric acid has been used as a direct litter treatment mostly in laboratory studies, but has also been used in the form of acidified clay in commercial use. In a study by Williams and Macklin (2013), sulfuric acid showed capacity to reduce NH3 concentration when applied directly to litter that was sourced from meat chicken houses. Three rates of sulfuric acid treatments were applied, namely, 0.098, 0.195 and 0.293 L/m2, which resulted in NH3 concentrations of 2.7, 2.4 and 1.1 ppm respectively, after 96 h. At the same time, the control concentration was 20.8 ppm, which showed that a significant reduction occurred when sulfuric acid was used. For the same treatments, a Salmonella cocktail was added to the litter before addition of sulfuric acid. After 96 h, there was no Salmonella recorded in any of the treatments, whereas the control had a concentration of Salmonella of 2.7 colony-forming units (cfu/g; Williams and Macklin 2013). Due to the short duration of this experiment, it is unknown whether these effects on NH3 and Salmonella concentrations would be ongoing. It is expected that if the pH remained acidic, these inhibitory effects would continue, but it should be noted that the pH of litter for the lowest sulfuric acid rate began to increase at the end of the 96-h period (Williams and Macklin 2013).
In a controlled pen experiment, acidified clay was found to reduce NH3 emissions significantly for a period of 30 days after application, with the pH of the treated litter remaining less than the control for 3 weeks after application (McWard and Taylor 2000). Two compositions of acidified clay were used (36% and 46% sulfuric acid by weight) and results indicated that there was no significant difference between the two and that they were equally effective in reducing NH3 emissions. Alum and sodium bisulfate were also assessed and it was observed that there was no significant difference in the reduction of NH3 for all three amendments when compared with the control, indicating that all three methods were potentially viable when being applied at a rate of 0.45–0.48 kg/m2.
Acidified clay has also shown ability to reduce the frequency of occurrence of Salmonella in litter over a short time span. Acidified clay was applied to re-used litter at a low (0.360 kg/m2) and high (1.631 kg/m2) rate. After 11 days, a significantly lower frequency of occurrence of S. enterica serovar enteritidis in birds was observed, being 0% and 2.5% respectively, for low and high dose, when compared with the control where a frequency of occurrence of 27.5% was observed (Vicente et al. 2007). A replicate experiment was conducted using new litter (instead of re-used), and prevalence of S. enteritidis increased for the control (46%), low dose (23%) and high dose (18%; Vicente et al. 2007). Overall, results from these trials indicated that S. enteritidis is a greater risk for chicks grown on new bedding (Vicente et al. 2007). These results indicated that sulfuric acid amendments have the capability to reduce Salmonella concentrations; however, this was not the case for alum and sodium bisulfate amendments.
Inhibitors
An inhibitor, in essence, is an addition to a reaction that prevents a specific step, reducing the amount of the end product. A primary step in the overall breakdown of uric acid into NH3 is facilitated by the enzyme urease, which converts urea to NH3 (Singh et al. 2009). N-(n-butyl)thiophosphorictriamde (NBPT) is an example of a strong urease inhibitor due to its ability to block active sites in three places (Singh et al. 2009).
Singh et al. (2009) performed several experiments on the effectiveness of NBPT in NH3 reduction. A 21-day cage experiment conducted with two applications of NBPT at 0 days and 7 days resulted in a reduction in total ammoniacal nitrogen of 10% when the treatment was compared with the control (Singh et al. 2009). Other experiments performed showed minimal reduction in NH3 production; however, this was attributed to low moisture content as NBPT relies on hydrogen ions to be effective (Singh et al. 2009).
StalosanF (Vilofoss, Fredericia, Denmark) is another example of an inhibitor that can potentially reduce NH3 volatilisation. While containing no ‘active’ ingredient, it is primarily made of phosphates, with other ingredients including clay, iron and copper and has a natural pH of 4. StalosanF has been trialled in Australia using different application methods and comparing to a control (Nutrifoss 2014). The first method applied StanlosanF by spreading throughout the entire house 3 days before chick placement at a rate of 100 g/m2, and then reapplying on 7, 14 and 21 days of the grow-out to re-used litter that was in the in the non-brooding area. The second method applied StalosanF 3 days before chick placement at a rate 50 g/m2 with no re-applications. All sheds, including the control, used partial litter re-use practices where new bedding was placed in the brooding section and re-used litter was used in the non-brooding section of the shed. Results indicated that the first method consistently reduced in-house NH3 concentrations throughout the course of the experiment when compared with the second method and the control, with immediate reductions of between 44–74% being reported on re-applications, although the results were not statistically analysed or peer reviewed (Nutrifoss 2014). After 21 days, it was determined that ventilation was sufficient to comply with RSPCA requirements for NH3 concentration. Overall, based on this limited trial, it was suggested that application of StalosanF was able to reduce NH3 concentration during the early stage of the grow-out (Nutrifoss 2014).
Adsorbers
Adsorbers have been used in the waste-management industry for the purpose of reducing undesired products by binding to a specific additive (Li et al. 2008). Clinoptilolite is a naturally occurring zeolite with a high affinity for the absorption of NH3 and NH4+ (McCrory and Hobbs 2001). An application of 5 kg/m2 to litter resulted in a 35% reduction of aerial NH3 emissions (McCrory and Hobbs 2001). This shows that clinoptilolite has the capacity to reduce NH3 when applied in litter; however, the application rate used was quite high and would require a large quantity to be used in meat chicken houses.
Karamanlis et al. (2008) studied the addition of clinoptilolite to both the feed and litter in simulated meat chicken production compartments, each containing 650 birds. It was determined that adding clinoptilolite to litter reduced NH3 concentration compared with the control; however, NH3 reduction was not observed when clinoptilolite was added to feed.
In an Australian pen study, Walkden-Brown et al. (2013) found that the adsorbent agents bentonite and zeolite added at a rate of 1.56 kg/m2 to re-used litter before chick placement significantly reduced NH3 production but to a far lesser extent than alum or sodium bisulfate. However, there appeared to be a more sustained effect over the full grow-out period of 42 days rather than just in the early brooding period. Ammonia reduction over the total period was 40% and 32% for bentonite and zeolite respectively.
These results provided some evidence that both inhibitors and adsorbents can be successful at reducing NH3 concentrations in simulated meat chicken houses, but would require more extensive research to observe their effects in commercial production houses. StalosanF, although not being the subject of a significant amount of research, is commercially available in Australia and, therefore, is an option for future use.
Alkalinisers
Alkaline materials such as quicklime (CaO) and hydrated lime (Ca(OH)2) have been extensively used to inactivate pathogens in manure and sewage sludge before land application (USEPA 1999; EFSA 2010). Quicklime reacts with water to produce heat and hydrated lime (Maguire et al. 2006). Quicklime and hydrated lime have been shown to inactivate foodborne pathogens (Bennett et al. 2003; Stringfellow et al. 2010) and several viruses such as influenza virus and Newcastle disease virus (Ruenphet et al. 2019) by increasing alkalinity to a pH 12 or above and reducing water activity in treated litter (Maguire et al. 2006; Alphin et al. 2009).
In contrast to the previous amendments, treatment with lime promotes a rapid volatilisation of NH3 due to reduction of NH4+ concentration in treated litter (Bennett et al. 2003; Maguire et al. 2006; Ruiz et al. 2008; Stringfellow et al. 2010). Therefore, lime should not be applied when NH3 concentrations are high, but to control pathogens and volatilise nitrogen in re-used litter between production cycles.
Addition of 10–15% quicklime on the basis of the weight of the litter has been shown to reduce the total plate counts from 793 000 cfu/mL to 6500 cfu/mL in a laboratory study using litter from commercial poultry houses (Maguire et al. 2006) and from 1 600 000 cfu/mL to 1000 cfu/mL in a field trial in commercial meat chicken sheds (Ruiz et al. 2008).
Laboratory studies found that the addition of 5–20% hydrated lime to poultry litter that was experimentally contaminated with S. enteritidis significantly reduced the recovery incidence of Salmonella within 24 h (Bennett et al. 2003). Likewise, the addition of 5–10% quicklime to litter reduced S. typhimurium concentrations to undetectable levels under laboratory conditions, even when samples were enriched (Stringfellow et al. 2010). However, when bacterial counts were performed after 0.2% or 5% of hydrated lime was applied to turkey litter under commercial conditions, there was a significant reduction in total aerobic bacteria counts but not in concentrations of Campylobacter and Salmonella (Bennett et al. 2005).
Bird performance and welfare
Acidifying litter amendments have demonstrated the capacity to reduce NH3 volatilisation in meat chicken houses. As Moore et al. (2008) and Naseem and King (2018) discussed, low in-house NH3 concentration provides a healthier living environment. Understanding whether the improvement in living conditions results in an improvement to bird performance and welfare is vital when considering whether inclusion of litter amendments in Australian practices will be beneficial and viable.
Oviedo-Rondón et al. (2013) reported that applying sodium bisulfate throughout an entire shed significantly reduced NH3 emissions (a 47% decrease for the highest rate utilised), and that there was no statistical difference in bird performance when sodium bisulfate application was used only in the brooding section. Final bodyweights from every application rate did not show significant differences, indicating that higher application rates of sodium bisulfate did not necessarily directly translate to an increased bird production, despite lower NH3 concentrations. Mortality and other health attributes were also shown to be unaffected by increased rates of sodium bisulfate application (Oviedo-Rondón et al. 2013). It is known that birds are at their greatest risk of exposure to high NH3 when they are young, particularly in the brooder area (Miles et al. 2004). Therefore, it is perhaps relevant that the control for this experiment was a low-rate sodium bisulfate application specifically to the brooding section and that NH3 concentrations were assessed on the basis of results obtained throughout the entire shed (Oviedo-Rondón et al. 2013). The authors suggested the low-rate sodium bisulfate application to the brooding section reduced the NH3 emissions sufficiently, thus providing the birds with an acceptable living environment during their most important stage of development. This may account for the lack of statistical difference in bird performance with respects to application rate, as the lowest rate applied still provided the birds with comfortable living conditions. It should also be noted that the highest concentration of NH3 observed was 28.1 ppm (Oviedo-Rondón et al. 2013), which is just above the currently accepted limit in production (Naseem and King 2018). The lack of improvement in flock performance when using varying rates of sodium bisulfate may simply be explained by chicks never being subjected to detrimental concentrations of NH3, even in the control.
In some studies, use of sodium bisulfate as a litter amendment has been shown to improve bird welfare and performance when used at a low rate of application such as 0.24 kg/m2 (Terzich et al. 1998a, 1998b) or 0.25–0.15 kg/m2 (Toppel et al. 2019a, 2019b). Death rate due to ascites, which is a commonly found disease in meat chickens, was found to be 5.9% when sodium bisulfate was applied to litter, which was a significant reduction on the 31.5% for the control (Terzich et al. 1998a). The high mortality rate due to ascites in the control experiment indicated that the conditions of the experiment may have predisposed the birds to this condition. Other studies in more controlled experimental conditions found no difference in the mortality rates from treated and untreated groups (McWard and Taylor 2000; Nagaraj et al. 2007; Williams and Macklin 2013), although, in a field trial, the mortality rate was higher in sodium bisulfate group (2.79–2.88%) than in the control (2.03–2.27%; Toppel et al. 2019a, 2019b). Foot-pad lesions were at least 10% lower for groups treated once or twice with sodium bisulfate during the rearing period than they were in untreated groups (Nagaraj et al. 2007; Williams and Macklin 2013; Toppel et al. 2019b). Treatment with sodium bisulfate has been shown to increase bodyweight in some experimental studies (Terzich et al. 1998b; McWard and Taylor 2000), while other experimental studies (Nagaraj et al. 2007; Williams and Macklin 2013) and a field study at commercial farm with 240 000 birds (Toppel et al. 2019a, 2019b) found no difference between treated and control groups. Air-sac scores and damage to cells within trachea were found to be significantly improved for birds raised on the sodium bisulfate-treated litter (Terzich et al. 1998b).
Zhang et al. (2011) observed that alum treatment decreased NH3 emissions by 30% when compared with the control and was able to maintain a lower litter pH for 35 days after application. Despite these reductions, there was no significant improvement in performance, foot-pad scores or hock burns when stocking density was maintained between 12 and 20 birds/m2. Although there was no significant difference in foot-pad scores and hock burns between alum-treated litter and the control litter, the scores obtained were low when the stocking density was within a normal range. Contrary to the above results, a significant increase to overall bodyweight of meat chickens has been observed when litter was treated with alum (Moore et al. 2000). In total, 600 000 birds (from 10 sheds, half control and half treatment, over two farms and repeated for three grow-outs) were used throughout the course of a field trial in commercial meat chicken farms and the average weight of birds grown on alum treated litter was 1.73 kg compared with 1.66 kg for the control. It was determined that this increase in bird performance was most likely to be due to the 99% reduction of NH3 volatilisation caused by the alum treatment (Moore et al. 2000; Younis et al. 2016).
McWard and Taylor (2000) assessed meat chicken performance and welfare indicators such as bodyweight as well as foot-pad, carcass-quality, breast-blister and air-sac scores when alum, sodium bisulfate and acidified clay were applied to litter. Bodyweights for birds raised on amended litter were found to be 2.74, 2.70 and 2.66 kg for acidified clay, sodium bisulfate and alum respectively, which were all higher than the weight of birds in the control experiment (2.61 kg). Carcass-quality, breast-blister and foot-pad scores were all improved for birds that were raised on litter treated with acidified clay. Carcass quality was also improved when alum and sodium bisulfate amendments were used, but not breast blisters and air-sac scores.
Ruiz et al. (2008) showed that meat chickens reared for 42 days on used litter treated with up to 15% quicklime did not develop breast or footpad blisters, and that there were no negative effects on bird performance (bodyweight, feed consumption, feed conversion and mortality). This finding conflicts with preliminary poult-performance trials, which have shown that hydrated lime concentrations greater than 5% in litter caused ocular and respiratory irritation in poults during the first 48 h after placement (Bennett et al. 2005). However, in this same study, concentrations up to 5% lime improved poult performance.
In an Australian study with treatments applied on each of three farms, Cressman et al. (2014) found that addition of a locally available acidic-clay granular litter amendment to re-used litter reduced NH3 concentrations, but not litter pH. Addition of the amendment reduced early and total mortality, but also reduced bird weights at 35 days, possibly due to consumption of the amendment, and increased the severity of foot-pad lesions at 35 days. Thus, the effects were mixed.
Environmental effects of amendments
Spent litter has substantial agronomic value as a fertiliser because it contains useful nutrients, in particular nitrogen and phosphorous (Bolan et al. 2010; Poultry Hub 2018). Therefore, increasing the concentration of these nutrients in the litter would also increase its agronomic value as a fertiliser. Acidifiers and adsorbents inhibit nitrogen volatilisation and, thus, increase the nitrogen concentration and fertiliser value of spent litter (Redding 2013; Eugene et al. 2015), although the increase in nitrogen may not be sufficient to change nutrient management-planning calculations for crop needs or prevention of land-application impacts. As with any manure fertiliser, it is vital that application of any manure to agricultural land is managed properly, because runoff into natural water can have potentially adverse effects (Bolan et al. 2010). Moore et al. (2000) found that there was a 73% decrease in phosphorus runoff from alum-treated litter when compared with normal litter, over 3 years. It was determined that alum treatment not only enhanced production of the meat chickens once applied to litter, but also reduced potential environmental impacts (Moore et al. 2000). The reduction in soluble phosphorus, in particular, may actually enable land-application rates of manure to be increased, while still protecting the environment from nutrient runoff and ensuring sustainability (Moore 2011). Further research is required to confirm whether similar effects to runoff would occur if other amendments were used for litter treatment.
Liming litter has also been shown to decrease soluble phosphorus by more than 90% and can have an additional benefit of reducing soil acidity in areas with acid soil (Maguire et al. 2006; Ruiz et al. 2008). It may also provide an effective tool for reducing pathogen concentrations in poultry litter before land application (Bennett et al. 2005; Maguire et al. 2006; Ruiz et al. 2008; Lopes et al. 2013). The reduction of nitrogen in limed litter is associated with an increase in NH3 volatilisation (Ruiz et al. 2008) and could potentially reduce its value as an agricultural fertiliser (Moore et al. 2000).
Economic considerations
In a preliminary economic analysis, the cost of purchasing new bedding at the start of a grow-out was compared with strategies of re-using litter, with and without litter amendments, in either the full shed or just the brooding section. Prices for fresh bedding materials (Table S1 available as Supplementary Material to this paper) were obtained from Watson and Wiedemann (2019). The costing analysis included the costs of the raw materials but not the costs associated with machinery, labour, litter topping-up, shed heating, ventilation, therapeutic treatments or occasional replacement of spent litter with new bedding. Costs for litter amendments (Table S2) were obtained from Australian retailers if possible; however, several products are not readily available in Australia. For products sold in the USA, retail pricing was converted from US$ to AU$. It was assumed that in the event of wide adoption of litter amendments in Australia, the pricing in Australia would be similar to that in the USA.
Scenarios evaluated in the comparison included the following:
-
New bedding throughout the shed (no amendments)
-
New bedding in the brooding section, with re-used litter in the remaining half of the shed (i.e. partial litter re-use with no amendments)
-
Re-used litter, with amendments used throughout the shed
-
Re-used litter used throughout the shed, with litter amendment used only in the brooding half of the shed
-
New bedding in the brooding section, with re-used litter in the remaining half of the shed, and with litter amendments used throughout the shed (i.e. partial litter re-use with amendments)
-
New bedding in the brooding section, with re-used litter in the remaining half of the shed, and with litter amendments applied in only half of the shed (i.e. partial litter re-use with half-shed of amendments)
An assumption was made that litter amendments would be applied only once, before bird placement, according to recommendations in the literature or provided by manufacturers. It is recommended that some products be re-applied under certain circumstances, but re-application was not considered in the cost comparison.
It was found that the cost of re-using litter combined with a litter amendment was less than was that of purchasing new bedding materials in the case of wood shavings (Fig. 2). Cost comparisons with other bedding materials including sawdust, rice hulls, straw and recycled wood pallets are presented in Fig. S2–S5 of the Supplementary Material. Cost reduction was maximal when the amendment was applied only in the brooding section, which is where risks associated with NH3 are likely to be greatest. Overall, the costs associated with full litter re-use combined with amendments were similar to the costs of partial litter re-use, where new bedding is purchased for the brooding section and re-used litter is used in the non-brooding end of the shed. Litter amendments are not currently used with partial litter re-use because investing in new bedding in the brooding section reduces risks associated with NH3. Using litter amendments combined with purchasing new litter (for either the whole shed or half of the shed) increases costs associated with litter and, therefore, it is likely that it would be considered only if necessary, so as to address a specific situation where NH3 is posing a risk.
Potential implementation of litter amendments in Australian production systems
With an emphasis being placed on reducing our carbon footprint, and reducing production costs in the face of the increasing cost of sourcing new bedding, there is increasing interest in adoption of litter re-use (Biomass Producer 2013; ACMF 2018). If litter re-use were to become a more common practise, products such as litter amendments may be required to assist with maintaining litter characteristics and reducing NH3 volatilisation. The emission of NH3 cannot be attributed to one specific cause, but rather to a variety of influential factors, and, therefore, a combination of management strategies is likely to be more successful in mitigating the effects of NH3 (Cohuo-Colli et al. 2018).
Widespread implementation of litter amendments as a prevention strategy is unlikely to occur, and is not warranted, if the current production system based on brooding on new litter is maintained. However, tactical use of amendments in situations of high NH3 concentration could be useful to provide immediate relief. Sodium bisulfate has been shown to be safe for implementation during flock grow-out and could be utilised is cases of high NH3 concentration (Purswell et al. 2013; Hunolt et al. 2015). Widespread adoption of litter amendments in Australia is likely to be closely tied to increases in adoption of litter re-use strategies.
Another cost-saving compromise to full implementation of litter amendments would be limiting use of amendments to just the brooding section. Worley et al. (2000) found that implementation of alum to just the brooding section produced similar results to whole-house application. As birds are at their highest health risk due to the effects of NH3 during brooding, it is vital to control NH3 during this time (Worley et al. 2000). In a similar vein, the levels of amendment used may be varied to suit likely temperature conditions and ventilation rates and costs during brooding.
The use of amendments would never remove the need for ventilation; however, their use may reduce costs associated with the amount of ventilation needed to maintain NH3 concentrations. This is particularly the case in winter when the cost of heating can be more significant. While there has been minimal research with regards to litter amendments and their application specifically in Australian systems, findings from other countries are likely to be applicable to Australian systems, perhaps with some prior testing and modification.
Conclusions
Responses from a questionnaire indicated that the Australian chicken meat industry is likely to increase uptake of litter re-use practices in the future. Ammonia volatilisation from litter is one of the many challenges experienced while safely and efficiently rearing meat chickens. Ammonia has the potential to adversely affect performance and welfare of the chickens, and also employee safety, if not appropriately managed (Naseem and King 2018). Risks associated with NH3 increase if litter is re-used for multiple grow-outs. It is vital to control NH3 volatilisation, and this appears to be possible and affordable with the use of litter amendments, as supported by considerable research. Our preliminary economic comparison showed that re-using litter with judicious use of litter amendments can reduce costs compared with purchasing new bedding materials. Litter amendments have shown the ability in some studies to not only reduce NH3 volatilisation but also improve production and welfare of the birds, particularly by increasing bodyweight and improving health of foot pads and the respiratory system. Acidifiers have shown to be the most effective of all amendment types, with sodium bisulfate and alum being among the most commonly tested products mentioned in research literature. Other amendments that do not come under the classification of an acidifier have shown some promising results with regards to NH3 management, but lack the depth of research and require additional research evaluation. There has been minimal research regarding the use of litter amendments in Australian chicken meat production. While the research summarised in this review provides useful guidance, further research will be required to quantify the efficacy and cost effectiveness of using litter amendments in Australia. Uptake will be dependent on demonstrating economic and other benefits under local conditions in large-scale studies.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This research has been supported by funding from AgriFutures Australia, through the Chicken Meat R&D program, the University of New England, Armidale, and the Department of Agriculture and Fisheries, Queensland Government.
References
ACMF (2018) ‘Australian industry facts & figures.’ Available at https://www.chicken.org.au/facts-and-figures/ [Verified 29 October 2018].Alphin RL, Johnson KJ, Ladman BS, Benson ER (2009) Inactivation of avian influenza virus using four common chemicals and one detergent. Poultry Science 88, 1181–1185.
| Inactivation of avian influenza virus using four common chemicals and one detergent.Crossref | GoogleScholarGoogle Scholar | 19439628PubMed |
Anderson DP, Beard CW, Hanson RP (1966) Influence of poultry house dust, ammonia, and carbon dioxide on the resistance of chickens to Newcastle disease virus. Avian Diseases 10, 177–188.
| Influence of poultry house dust, ammonia, and carbon dioxide on the resistance of chickens to Newcastle disease virus.Crossref | GoogleScholarGoogle Scholar | 5963856PubMed |
Animal Health Australia (2017) ‘Proposed draft Australian animal welfare standards and guidelines for poultry (Public consultation version: Nov 2017).’ Animal Health Australia, Braddon ACT. Available at http://www.animalwelfarestandards.net.au/poultry/poultry-public-consultation [Verified 24 April 2020].
ApSimon HM, Kruse M, Bell JNB (1987) Ammonia emissions and their role in acid deposition. Atmospheric Environment 21, 1939–1946.
Beker A, Vanhooser SL, Swartzlander JH, Teeter RG (2004) Atmospheric ammonia concentration effects on broiler growth and performance. Journal of Applied Poultry Research 13, 5–9.
| Atmospheric ammonia concentration effects on broiler growth and performance.Crossref | GoogleScholarGoogle Scholar |
Bennett DD, Higgins SE, Moore RW, Beltran R, Caldwell DJ, Byrd JA, Hargis BM (2003) Effects of lime on Salmonella enteritidis survival in vitro. Journal of Applied Poultry Research 12, 65–68.
| Effects of lime on Salmonella enteritidis survival in vitro.Crossref | GoogleScholarGoogle Scholar |
Bennett DS, Higgins SE, Moore R, Byrd JA, Beltran R, Corsiglia C, Caldwell D, Hargis BM (2005) Effect of addition of hydrated lime to litter on recovery of selected bacteria and poult performance. Journal of Applied Poultry Research 14, 721–727.
| Effect of addition of hydrated lime to litter on recovery of selected bacteria and poult performance.Crossref | GoogleScholarGoogle Scholar |
Biomass Producer (2013) ‘Poultry litter.’ Available at http://biomassproducer.com.au/producing-biomass/biomass-types/animal-waste/poultry-litter [Verified 26 March 2019].
Bolan NS, Szogi AA, Chuasavathi T, Seshadri B, Rothrock MJ, Panneerselvam P (2010) Uses and management of poultry litter. World’s Poultry Science Journal 66, 673–698.
| Uses and management of poultry litter.Crossref | GoogleScholarGoogle Scholar |
Carlile FS (1984) Ammonia in poultry houses: a literature review. World’s Poultry Science Journal 40, 99–113.
| Ammonia in poultry houses: a literature review.Crossref | GoogleScholarGoogle Scholar |
Choi IH, Moore PA (2008) Effect of various litter amendments on ammonia volatilization and nitrogen content of poultry litter. Journal of Applied Poultry Research 17, 454–462.
| Effect of various litter amendments on ammonia volatilization and nitrogen content of poultry litter.Crossref | GoogleScholarGoogle Scholar |
Chung TH, Park C, Choi IH (2015) Effects of Korean red ginseng marc with aluminum sulfate against pathogen populations in poultry litters. Journal of Ginseng Research 39, 414–417.
| Effects of Korean red ginseng marc with aluminum sulfate against pathogen populations in poultry litters.Crossref | GoogleScholarGoogle Scholar | 26869836PubMed |
Cohuo-Colli JM, Salinas-Ruíz J, Hernández-Cázares AS, Hidalgo-Contreras JV, Brito-Damián VH, Velasco-Velasco J (2018) Effect of litter density and foot health program on ammonia emissions in broiler chickens. Journal of Applied Poultry Research 27, 198–205.
| Effect of litter density and foot health program on ammonia emissions in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Cressman MD, Moeller S, Walkden-Brown SW, Zerby H (2014) ‘Impacts of litter heaping and turning +/− litter amendment on broiler performance and welfare’. PIX2014, 28 May 2014, Poultry Information Exchange litter management proceedings. pp. 55–65. (Gold Coast Convention Centre: Gold Coast, Qld)
EFSA (2010) Scientific opinion on lime treatment of solid pig and poultry manure. EFSA Journal 8, 16
| Scientific opinion on lime treatment of solid pig and poultry manure.Crossref | GoogleScholarGoogle Scholar |
Elliott HA, Collins NE (1982) Factors affecting ammonia release in broiler houses. Transactions of the ASAE. American Society of Agricultural Engineers 25, 413–418.
| Factors affecting ammonia release in broiler houses.Crossref | GoogleScholarGoogle Scholar |
Erisman JW, Schaap M (2004) The need for ammonia abatement with respect to secondary PM reductions in Europe. Environmental Pollution 129, 159–163.
| The need for ammonia abatement with respect to secondary PM reductions in Europe.Crossref | GoogleScholarGoogle Scholar | 14749079PubMed |
Eugene B, Moore PA, Li H, Miles D, Trabue S, Burns R, Buser M (2015) Effect of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions. Journal of Environmental Quality 44, 1530–1540.
| Effect of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions.Crossref | GoogleScholarGoogle Scholar | 26436270PubMed |
Fine PM, Sioutas C, Solomon PA (2008) Secondary particulate matter in the United States: insights from the particulate matter supersites program and related studies. Journal of the Air and Waste Management Association 58, 234–253.
| Secondary particulate matter in the United States: insights from the particulate matter supersites program and related studies.Crossref | GoogleScholarGoogle Scholar | 18318339PubMed |
Giambrone JJ, Fagbohun O, Macklin KS (2008) Management practices to reduce infectious laryngotracheitis virus in poultry litter. Journal of Applied Poultry Research 17, 64–68.
| Management practices to reduce infectious laryngotracheitis virus in poultry litter.Crossref | GoogleScholarGoogle Scholar |
Groot Koerkamp PWG (1994) Review on emissions of ammonia from housing systems for laying hens in relation to sources, processes, building design and manure handling. Journal of Agricultural Engineering Research 59, 73–87.
| Review on emissions of ammonia from housing systems for laying hens in relation to sources, processes, building design and manure handling.Crossref | GoogleScholarGoogle Scholar |
Hadlocon LJ, Zhao L (2015) Production of ammonium sulfate fertilizer using acid spray wet scrubbers. Agricultural Engineering International: CIGR Journal 2015, 41–51.
Hunolt AE, Maguire RO, Ogejo JA, Badgley BD, Frame WH, Reiter MS (2015) Multiple applications of sodium bisulfate to broiler litter affect ammonia release and litter properties. Journal of Environmental Quality 44, 1903–1910.
| Multiple applications of sodium bisulfate to broiler litter affect ammonia release and litter properties.Crossref | GoogleScholarGoogle Scholar | 26641342PubMed |
Islam AFMF, Dunlop M, Wells B, Walkden-Brown SW (2010) Spatial and temporal variation in ammonia concentrations in broiler sheds: effects of chicken age and shed type. In ‘Australian poultry science symposium’, (Ed. P Selle) 1–3 February 2010, Sydney, NSW, Australia.
Jones-Hamilton AG (2018) ‘PLT: poultry litter treatment product data sheet for broilers.’ Available at https://www.joneshamiltonag.com/product/plt/ [Verified 26 March 2019].
Karamanlis X, Fortomaris P, Arsenos G, Dosis I, Papaioannou D, Batzios C, Kamarianos A (2008) The effect of a natural zeolite (clinoptilolite) on the performance of broiler chickens and the quality of their litter. Asian–Australasian Journal of Animal Sciences 21, 1642–1650.
| The effect of a natural zeolite (clinoptilolite) on the performance of broiler chickens and the quality of their litter.Crossref | GoogleScholarGoogle Scholar |
Li H, Xin H, Liang Y, Burns RT (2008) Reduction of ammonia emissions from stored laying hen manure through topical application of zeolite, Al+Clear, Ferix-3, or poultry litter treatment. Journal of Applied Poultry Research 17, 421–431.
| Reduction of ammonia emissions from stored laying hen manure through topical application of zeolite, Al+Clear, Ferix-3, or poultry litter treatment.Crossref | GoogleScholarGoogle Scholar |
Line JE, Bailey JS (2006) Effect of on-farm litter acidification treatments on Campylobacter and Salmonella populations in commercial broiler houses in northeast Georgia. Poultry Science 85, 1529–1534.
| Effect of on-farm litter acidification treatments on Campylobacter and Salmonella populations in commercial broiler houses in northeast Georgia.Crossref | GoogleScholarGoogle Scholar | 16977837PubMed |
Lopes M, Roll VFB, Leite FL, Dai Prá MA, Xavier EG, Heres T, Valente BS (2013) Quicklime treatment and stirring of different poultry litter substrates for reducing pathogenic bacteria counts. Poultry Science 92, 638–644.
| Quicklime treatment and stirring of different poultry litter substrates for reducing pathogenic bacteria counts.Crossref | GoogleScholarGoogle Scholar | 23436514PubMed |
Maguire RO, Hesterberg D, Gernat A, Anderson K, Wineland M, Grimes J (2006) Liming poultry manures to decrease soluble phosphorus and suppress the bacteria population. Journal of Environmental Quality 35, 849–857.
| Liming poultry manures to decrease soluble phosphorus and suppress the bacteria population.Crossref | GoogleScholarGoogle Scholar | 16585628PubMed |
McCrory DF, Hobbs PJ (2001) Additives to reduce ammonia and odor emissions from livestock wastes: a review. Journal of Environmental Quality 30, 345–355.
| Additives to reduce ammonia and odor emissions from livestock wastes: a review.Crossref | GoogleScholarGoogle Scholar | 11285894PubMed |
McWard GW, Taylor DR (2000) Acidified clay litter amendment. Journal of Applied Poultry Research 9, 518–529.
| Acidified clay litter amendment.Crossref | GoogleScholarGoogle Scholar |
Menz FC, Seip HM (2004) Acid rain in Europe and the United States: an update. Environmental Science and Policy 7, 253–265.
| Acid rain in Europe and the United States: an update.Crossref | GoogleScholarGoogle Scholar |
Miles DM, Branton SL, Lott BD (2004) Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poultry Science 83, 1650–1654.
| Atmospheric ammonia is detrimental to the performance of modern commercial broilers.Crossref | GoogleScholarGoogle Scholar | 15510548PubMed |
Miles DM, Miller WW, Branton SL, Maslin WR, Lott BD (2006) Ocular responses to ammonia in broiler chickens. Avian Diseases 50, 45–49.
| Ocular responses to ammonia in broiler chickens.Crossref | GoogleScholarGoogle Scholar | 16617980PubMed |
Moore PA Jr (2011) Improving the sustainability of animal agriculture by treating manure with alum. In ‘Environmental chemistry of animal manure’. (Ed. Z He) pp. 349–382 (Nova Science Publishers Inc.: New York)
Moore PA, Daniel TC, Edwards DR (2000) Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminium sulfate. Journal of Environmental Quality 29, 37–49.
| Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminium sulfate.Crossref | GoogleScholarGoogle Scholar |
Moore PA, Jr, Miles DM, Burns RT, Pote DH, Berg WK (2008) Evaluation of ammonia emissions from broiler litter. In ‘Livestock environment VIII: proceedings of the 8th international symposium’. Available at https://www.scopus.com/inward/record.uri?eid=2-s2.0-63149194977&partnerID=40&md5=b5962ac9654244cbc22e97789cbaec76 [Verified 26 March 2019]
Nagaraj M, Wilson CAP, Saenmahayak B, Hess JB, Bilgili SF (2007) Efficacy of a litter amendment to reduce pododermatitis in broiler chickens. 1. Journal of Applied Poultry Research 16, 255–261.
| Efficacy of a litter amendment to reduce pododermatitis in broiler chickens. 1.Crossref | GoogleScholarGoogle Scholar |
Naseem S, King AJ (2018) Ammonia production in poultry houses can affect health of humans, birds, and the environment: techniques for its reduction during poultry production. Environmental Science and Pollution Research International 25, 15269–15293.
| Ammonia production in poultry houses can affect health of humans, birds, and the environment: techniques for its reduction during poultry production.Crossref | GoogleScholarGoogle Scholar | 29705898PubMed |
Nutrifoss (2014) ‘Australian poultry trial multi batch litter management.’ Available at http://nutrifoss.com.au/publications/ [Verified 31 March 2019]
Olanrewaju H, Miller W, Maslin W, Thaxton J, Dozier WA, Purswell J, Branton S (2007) Interactive effects of ammonia and light intensity on ocular, fear and leg health in broiler chickens. International Journal of Poultry Science 6, 762–769.
| Interactive effects of ammonia and light intensity on ocular, fear and leg health in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Oviedo-Rondón EO, Shah SB, Grimes JL, Westerman PW, Campeau D (2013) Live performance of roasters raised in houses receiving different acidifier application rates. Journal of Applied Poultry Research 22, 922–928.
| Live performance of roasters raised in houses receiving different acidifier application rates.Crossref | GoogleScholarGoogle Scholar |
Oyetunde OO, Thomson RG, Carlson HC (1978) Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne 19, 187–193.
Pope MJ, Cherry TE (2000) An evaluation of the presence of pathogens on broilers raised on poultry litter treatment®-treated litter. Poultry Science 79, 1351–1355.
| An evaluation of the presence of pathogens on broilers raised on poultry litter treatment®-treated litter.Crossref | GoogleScholarGoogle Scholar | 11020084PubMed |
Poultry Hub (2018) ‘Poultry litter.’ Available at http://www.poultryhub.org/production/husbandry-management/housing-environment/poultry-litter/ [Verified 31 March 2019]
Purswell JL, Davis JD, Kiess AS, Coufal CD (2013) Effects of frequency of multiple applications of litter amendment on litter ammonia and live performance in a shared airspace. Journal of Applied Poultry Research 22, 469–473.
| Effects of frequency of multiple applications of litter amendment on litter ammonia and live performance in a shared airspace.Crossref | GoogleScholarGoogle Scholar |
Quarles CL, Kling HF (1974) Evaluation of ammonia and infectious bronchitis vaccination stress on broiler performance and carcass quality. Poultry Science 53, 1592–1596.
| Evaluation of ammonia and infectious bronchitis vaccination stress on broiler performance and carcass quality.Crossref | GoogleScholarGoogle Scholar |
Redding MR (2013) Bentonite can decrease ammonia volatilisation losses from poultry litter: laboratory studies. Animal Production Science 53, 1115–1118.
| Bentonite can decrease ammonia volatilisation losses from poultry litter: laboratory studies.Crossref | GoogleScholarGoogle Scholar |
Roll VFB, Dai Prá MA, Roll AP (2011) Research on Salmonella in broiler litter reused for up to 14 consecutive flocks. Poultry Science 90, 2257–2262.
| Research on Salmonella in broiler litter reused for up to 14 consecutive flocks.Crossref | GoogleScholarGoogle Scholar |
Rothrock MJ, Cook KL, Warren JG, Sistani K (2008) The effect of alum addition on microbial communities in poultry litter. Poultry Science 87, 1493–1503.
| The effect of alum addition on microbial communities in poultry litter.Crossref | GoogleScholarGoogle Scholar | 18648040PubMed |
RSPCA (2013) Meat chickens RSPCA approved farming scheme standards. RSPCA Australia, Deakin West, ACT. Available at https://rspcaapproved.org.au/join/#standards [Verified 31 March 2019]
Ruenphet S, Punyadarsaniya D, Jantafong T, Takehara K (2019) Stability and virucidal efficacies using powder and liquid forms of fresh charcoal ash and slaked lime against Newcastle disease virus and avian influenza virus. Veterinary World 12, 1–6.
| Stability and virucidal efficacies using powder and liquid forms of fresh charcoal ash and slaked lime against Newcastle disease virus and avian influenza virus.Crossref | GoogleScholarGoogle Scholar | 30936648PubMed |
Ruiz V, Ruiz D, Gernat AG, Grimes JL, Murillo JG, Wineland MJ, Anderson KE, Maguire RO (2008) The effect of quicklime (CaO) on litter condition and broiler performance. Poultry Science 87, 823–827.
| The effect of quicklime (CaO) on litter condition and broiler performance.Crossref | GoogleScholarGoogle Scholar | 18420971PubMed |
Sahoo SP, Kaur D, Sethi APS, Sharma A, Chandra M, Chandrahas (2017) Effect of chemically amended litter on litter quality and broiler performance in winter. Journal of Applied Animal Research 45, 533–537.
| Effect of chemically amended litter on litter quality and broiler performance in winter.Crossref | GoogleScholarGoogle Scholar |
Singh A, Casey KD, King WD, Pescatore AJ, Gates RS, Ford MJ (2009) Efficacy of urease inhibitor to reduce ammonia emission from poultry houses. Journal of Applied Poultry Research 18, 34–42.
| Efficacy of urease inhibitor to reduce ammonia emission from poultry houses.Crossref | GoogleScholarGoogle Scholar |
Stringfellow K, Caldwell D, Lee J, Byrd A, Carey J, Kessler K, McReynolds J, Bell A, Stipanovic R, Farnell M (2010) Pasteurization of chicken litter with steam and quicklime to reduce Salmonella typhimurium. Journal of Applied Poultry Research 19, 380–386.
| Pasteurization of chicken litter with steam and quicklime to reduce Salmonella typhimurium.Crossref | GoogleScholarGoogle Scholar | 32336902PubMed |
Terzich M, Quarles C, Goodwin MA, Brown J (1998a) Effect of Poultry Litter Treatment® (PLT®) on death due to ascites in broilers. Avian Diseases 42, 385–387.
| Effect of Poultry Litter Treatment® (PLT®) on death due to ascites in broilers.Crossref | GoogleScholarGoogle Scholar | 9645331PubMed |
Terzich M, Quarles C, Goodwin MA, Brown J (1998b) Effect of Poultry Litter Treatment® (PLT®) on the development of respiratory tract lesions in broilers. Avian Pathology 27, 566–569.
| Effect of Poultry Litter Treatment® (PLT®) on the development of respiratory tract lesions in broilers.Crossref | GoogleScholarGoogle Scholar | 18484274PubMed |
Toppel K, Kaufmann F, Schon H, Gauly M, Andersson R (2019a) Effect of pH-lowering litter amendment on animal-based welfare indicators and litter quality in a European commercial broiler husbandry. Poultry Science 98, 1181–1189.
| Effect of pH-lowering litter amendment on animal-based welfare indicators and litter quality in a European commercial broiler husbandry.Crossref | GoogleScholarGoogle Scholar | 30325450PubMed |
Toppel K, Kaufmann F, Schön H, Gauly M, Andersson R (2019b) Effect of pH-lowering litter amendment on animal-based welfare indicators and litter quality in a European commercial broiler husbandry. Poultry Science 98, 1181–1189.
| Effect of pH-lowering litter amendment on animal-based welfare indicators and litter quality in a European commercial broiler husbandry.Crossref | GoogleScholarGoogle Scholar | 30325450PubMed |
USEPA (1999) ‘Biosolids generation, use, and disposal in the United States.’ (United States Environmental Protection Agency, Washington, DC) Available at https://www.epa.gov/biosolids/biosolids-generation-use-and-disposal-united-states [Verified 31 March 2019]
Valentine H (1964) A study of the effect of different ventilation rates on the ammonia concentrations in the atmosphere of broiler houses. British Poultry Science 5, 149–159.
| A study of the effect of different ventilation rates on the ammonia concentrations in the atmosphere of broiler houses.Crossref | GoogleScholarGoogle Scholar |
Vicente JL, Higgins SE, Hargis BM, Tellez G (2007) Effect of poultry guard litter amendment on horizontal transmission of Salmonella enteritidis in broiler chicks. International Journal of Poultry Science 6, 314–317.
| Effect of poultry guard litter amendment on horizontal transmission of Salmonella enteritidis in broiler chicks.Crossref | GoogleScholarGoogle Scholar |
Walkden-Brown SW, Islam AFMF, Dunlop M, Wells B (2010) ‘Optimising methods for multiple batch litter use by broilers.’ (Australian Poultry CRC Armidale, NSW)
Walkden-Brown SW, Islam AFMF, Van Den Heuvel A, Cressman MD, Redding MR (2013) Effects of various additives to reused broiler litter on litter ammonia production, chicken welfare and performance’. In ‘Australian poultry science symposium’, pp. 175–178 Sydney, NSW, Australia, 17–20 February 2013. Available at https://poultry-research.sydney.edu.au/publications/ [Verified 24 April 2019]
Watson K, Wiedemann SG (2019) ‘Review of fresh litter supply, management and spent litter utilisation. Vol. project no PRJ-010655.’ (AgriFutures Australia: Canberra, ACT). Available at https://www.agrifutures.com.au/product/review-of-fresh-litter-supply-management-and-spent-litter-utilisation/ [Verified 6 August 2019].
Wiedemann SG (2015) ‘Litter reuse: an evidence-based guide to reusing litter. Vol. project PRJ-006440, publication 14/095.’ (Rural Industries Reserach and Development Corporation (RIRDC): Canberra, ACT). Available at https://www.agrifutures.com.au/product/litter-reuse-an-evidence-based-guide-to-reusing-litter/ [Verified 19 March 2019].
Williams ZT, Macklin KS (2013) Reduction of Salmonella and ammonia emissions in broiler litter using sulfuric acid and aluminum sulfate. International Journal of Poultry Science 12, 328–334.
| Reduction of Salmonella and ammonia emissions in broiler litter using sulfuric acid and aluminum sulfate.Crossref | GoogleScholarGoogle Scholar |
Worley JW, Cabrera ML, Risse LM (2000) Reduced levels of alum to amend broiler litter. Applied Engineering in Agriculture 16, 441–444.
| Reduced levels of alum to amend broiler litter.Crossref | GoogleScholarGoogle Scholar |
Younis MEM, Bazh EKA, Ahmed HA, Elbestawy AR (2016) Broiler performance, carcass characters and litter composition after management of fresh litter with two types of acidifier amendments. International Journal of Agriculture Innovation and Research 4, 1050–1056.
Zhang H-f, Jiao H-c, Song Z-g, Lin H (2011) Effect of alum-amended litter and stocking density on ammonia release and footpad and hock dermatitis of broilers. Agricultural Sciences in China 10, 777–785.
| Effect of alum-amended litter and stocking density on ammonia release and footpad and hock dermatitis of broilers.Crossref | GoogleScholarGoogle Scholar |



