Enzyme systems for effective dag removal from cattle hides
Laura Navone A B and Robert E. Speight AA Queensland University of Technology, 2 George Street, Brisbane, Qld 4000, Australia.
B Corresponding author. Email: laura.navone@qut.edu.au
Animal Production Science 59(7) 1387-1398 https://doi.org/10.1071/AN18194
Submitted: 13 March 2018 Accepted: 31 August 2018 Published: 16 October 2018
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
The effective removal of recalcitrant manure balls (dags) composed of dung, hair, soil, urine, sugars and straw from the hides of cattle remains a significant issue for the livestock industry. Dags must be removed to reduce the likelihood of microbial meat contamination and irreversible damage during leather processing. Current removal methods require extensive washing over many hours per animal resulting in high water use, costs and stress to the animal. Enzymes can be highly effective catalysts for the breakdown of biomass but previous research into the enzymatic removal of dags has had limited success. This work investigates the latest commercial enzyme preparations and classes of enzymes never previously tested for dag removal in new formulations. Cellulase, xylanase, laccase and α-amylase enzymes were applied to target the lignocellulosic and starch components of the dags. Protease enzymes that targeted the interaction between the dag and the hair, were also investigated as a novel approach for dag removal from cattle. Our results show that the application of a protease with keratinolytic activity is crucial for dag removal, weakening the framework of hairs at the point of attachment between the hair and the dag, as well as potentially degrading adhesive protein that may hold the structure together. The addition of a reducing agent and surfactant to the treatment facilitated optimal decomposition of the dag structure. Implementation of these enzymatic dag removal systems could significantly reduce the time, water use, animal stress and costs of cleaning cattle in the red meat industry.
Additional keywords: keratinase, livestock cleaning.
Introduction
Dags must ideally be removed before livestock processing to minimise risks to food safety and leather quality. The recalcitrant nature of dags necessitates extensive washing leading to high costs and cattle stress. The problem is aggravated with long haired breeds in regions with rainy winter seasons, when animals become wet and muddy. Dags are mainly composed of lignocellulosic material (cellulose, hemicellulose and lignin) and other minor components, like feed proteins, hair, lipids, carbohydrates and minerals (Covington et al. 1999). They are attached to the hides through the hair alone and adherence of the dag to the epidermis is not observed (Covington et al. 1999).
Enzymes capable of degrading the constituents of biomass have been implemented in industries such as pulp and paper processing, textiles, food, agriculture and biofuels (Kuhad et al. 2011). The application of enzymes for the degradation of biomass associated with dags has the potential to generate numerous benefits compared with current methods. Extensive commercial development of biomass-degrading enzymes over recent decades has been largely motivated by the development of lignocellulosic biofuel production processes (Limayem and Ricke 2012). Enzymes have also been developed for animal feed, with phytases, proteases and carbohydrases being the most extensively used. As such, highly optimised commercial enzymes that target specific dag components (cellulose, lignin, xylan, protein and starch) are now commercially available. We also investigated the addition of surfactants to the enzymatic treatments. Surfactants are amphiphilic agents that modify the interfacial tension of water, they are used in soaps, detergents, emulsifiers, dispersing and wetting agents, animal feed and some groups of antiseptics (Mittal and Fendler 1982; Siyal et al. 2017).
A previously untested alternative for dag removal is to target the hair component of the dag at the point of attachment using hair active protease enzymes called keratinases. The hypothesis being that these enzymes could selectively weaken or remove the hair at the dag attachment site as well as attack the grain protein component internal to the dag structure. Hair is composed of keratin, a fibrous and recalcitrant structural protein that is also a component of skin, feathers, horns, hooves, cloves, nails and beaks (McKittrick et al. 2012). The recalcitrant structure of keratin is due to the high degree of cross-linking by disulfide bonds, hydrogen bonds, and hydrophobic interactions (Lange et al. 2016). Keratinases are capable of catalysing the cleavage and hydrolysis of highly stable keratin proteins that common proteases, like pepsin and papain, are not capable of degrading (Riffel and Brandelli 2006). Furthermore, reducing agents that cleave disulfide bonds, are necessary for complete keratin breakdown by facilitating keratinase access to the peptide bonds of keratin (Vignardet et al. 2001). Here we show that proteases are an essential component of enzyme cocktails to achieve dag removal from hides.
Methods
Cellulase, xylanase and α-amylase activity determination
Ronozyme Multigrain was a generous donation from DSM Nutritional Products (Wagga Wagga, NSW, Australia). Accellerase 1500 and Spezyme LT 300 were a generous donation from Connell Bros Co. (Melbourne, Vic., Australia). The dinitrosalicylic acid reducing sugar assay (DNS assay) was performed for cellulase, xylanase and α-amylase activity determinations (Miller 1959). Diluted enzyme (40 μL) in DNS assay buffer (100 mM acetate buffer pH 5.0, 20 mM CaCl2, 0.01% Tween 20) was pipetted in 200-μL wells of a 96-well PCR plate then 100 μL of enzyme substrate solution was added and the sample was mixed. Incubation was performed for 20 min at 37°C. After incubation, 60 μL of the DNS stop reagent was added to each sample and incubated at 100°C for 5 min. Samples were transferred to a 96-well spectrometer plate and the optical density measured at 530 nm. Determinations for each enzyme dilution were performed by triplicate. For blank determinations, 100 μL of substrate was added after addition of DNS stop reagent and treated the same way as enzyme dilution samples. One per cent carboxymethylcellulose, 0.4% xylan and 0.2% starch were used as the substrate solution for cellulase, xylanase and α-amylase activity determination, respectively. One unit of enzyme activity was defined as the amount of enzyme that releases 1 µmol of reducing sugar equivalents from the respective substrate per minute under the assay conditions used. For enzyme activity determination in the presence of surfactants, each enzyme was incubated in 50 mM acetate buffer pH 5.0 with 5% surfactant (Triton X-100, Saponin or Brij58). After 1 h of incubation at room temperature, the DNS assay was performed as previously indicated. Cellulase, xylanase and α-amylase activities were also determined by the DNS protocol after 16 h of dag treatment. For this, 40 μL of diluted dag treatment supernatant in 100 μL of assay buffer was pipetted in 200-μL wells of a 96-well PCR plate and the DNS protocol was performed as described. Protein concentrations were determined using the Bradford assay (Bradford 1976). Statistical analysis was performed using one-tailed distribution t-test, differences among means with P ≤ 0.01 were accepted as representing statistically significant differences.
Laccase activity determination
Laccase was a generous donation from Connell Bros Co. and laccase activity was determined by a continuous spectrophotometric rate determination method. Briefly, 0.5 mL of diluted enzyme in assay buffer (100 mM potassium phosphate buffer pH 6.5) was incubated with 2.2 mL of assay buffer at 30°C. The reaction was started by addition of 0.3 mL of 0.216 mM syringaldazine and the absorbance was measured for 10 min at 530 nm. Blanks were performed with 0.5 mL of deionised water with no addition of enzyme. One unit of enzyme activity is defined as the amount of enzyme that catalyses the conversion of 1 µmole of substrate per minute.
Treatment of dags with enzymes
At least three pieces of dag of ~5–8 cm2 were cut from hides and incubated with individual enzymes (Spezyme LT 300, Accellerase 1500, Ronozyme Multigrain and laccase), combination of enzymes or combination of enzymes and surfactants (Lecithin, Tween 20, Tween 80, Triton X-100, Saponin, Brij58, Genapol X-80) in 50 mM acetate buffer pH 5 in a final volume of 100 mL. At least three pieces of dag of ~5–8 cm2 were incubated with Ronozyme ProAct with or without reducing agents (1% sodium sulfite or 2% sodium thioglycolate) and with or without 5% Triton X-100 in 100 mM Tris-Base buffer pH 10 in a final volume of 100 mL. Control experiments were performed in buffer solution with no added enzymes or surfactants. In all cases, the dags pieces were incubated for 16 h at room temperature and decomposition was analysed by spatula testing, conferring a score according to ease of dag disruption, or by determination of total sugars in solution. Ronozyme ProAct was a generous donation from DSM Nutritional Products.
Determination of sugar concentrations in solution after dag treatment
Total sugars in solution resulting from polysaccharide degradation (e.g. cellulose, starch) before (0 h) and after (16 h) of dag treatment were determined using the DNS protocol (Miller 1959). For this, 40-uL supernatant aliquots from each treatment were pipetted in 200-μL wells of a 96-well PCR plate. Then 100 μL of DNS assay buffer was added and the sample was mixed. Posteriorly, 60 μL of the DNS stop reagent was added to each sample and incubated at 100°C for 5 min. Samples were transferred to a 96-well spectrometer plate and the optical density measured at 530 nm. Determinations for each aliquot were performed in triplicate. Blanks were performed with dag treatment buffer (50 mM acetate buffer pH 5) in the presence of 5% surfactant (Triton X-100, Saponin or Brij58). Concentrations are shown as mmoles of sugar per g of dag. Statistical analysis was performed using one-tailed distribution t-test, differences among means with P ≤ 0.05 were accepted as representing statistically significant differences.
Determination of soluble peptide concentrations in solution after protease treatment
Soluble peptides in solution after treatment of hair samples of 0.01 g from cow hides (Bos taurus) with 2 U/mL of Ronozyme ProAct in 5 mL of 100 mM TRIS-HCl buffer pH 10, for 16 h at 22°C or 37°C at 200 rpm, with or without reducing agents (1% sodium sulfite or 2% sodium thioglycolate), and soluble peptides in solution resulting from protein degradation after dag treatment with 10 U/mL of Ronozyme ProAct with or without reducing agents (1% sodium sulfite or 2% sodium thioglycolate) and with or without 5% Triton X-100 in 100 mM Tris-Base buffer pH 10 in a final volume of 100 mL, were assessed using the Bradford assay (Bradford 1976). Control samples for dag treatment measured peptides in solution after dag incubation in buffer solution with no added enzyme, with or without reducing agents or surfactants. Soluble peptide concentrations after dag treatment are shown as µg of peptide per g of dag. Control hair samples were incubated in reaction buffer without enzyme for 16 h at 22°C or 37°C at 200 rpm. Experiments were conducted in triplicate. Statistical analysis was performed using one-tailed distribution t-test, differences among means with P < 0.05 were accepted as representing statistically significant differences.
Scanning electron microscopy
Hair samples from the inside of dag samples treated with Ronozyme ProAct for 16 h at room temperature, with or without reducing agent (1% sodium sulfite or 2% sodium thioglycolate), were washed with water, air-dried, fixed in a sample holder stub and gold coated using a Leica EM SCD005 Gold Coater (~10 nm). Secondary electron images were obtained with a Zeiss ∑igma Field Emission Scanning Electron Microscope. Images were obtained under vacuum using 2-kV accelerating voltage.
Hide treatment with keratinase
A small piece of hide (15 cm × 9 cm) with dags attached was treated with 10 U/mL Ronozyme ProAct in 2% sodium thioglycolate and 5% Triton X-100 for 16 h at room temperature with very low shaking. The treatment was performed in a container and the hide was completely covered with the enzyme solution.
Results
Specific activity determination of biomass degradation enzymes
Commercial enzymes for biomass (mainly lignocellulosic material) degradation were obtained from two companies, namely, Ronozyme Multigrain from DSM, Accellerase 1500, Spezyme LT 300 and Laccase from Dupont (Table 1).
|
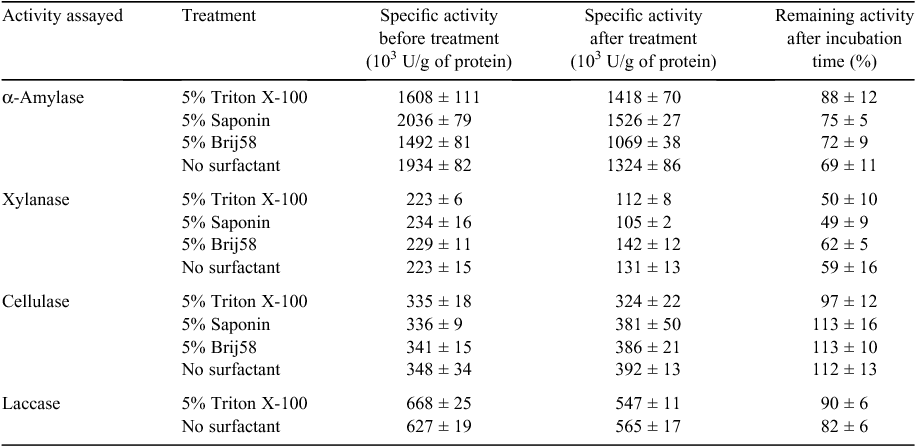
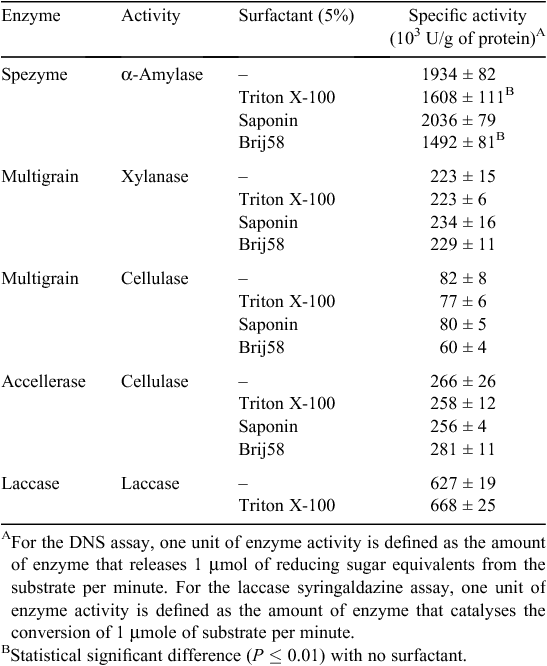
Xylanase, celullase and α-amylase specific activities were measured for Ronozyme Multigrain, Accellerase 1500 and Spezyme LT 300, respectively using the DNS protocol for reducing sugar quantification (Miller 1959). Ronozyme Multigrain was also reported by the manufacturer to have cellulase activity, therefore, it was also assayed using the DNS assay method with carboxy methyl cellulose as the substrate. Activities are expressed as enzyme units per g of protein as determined by the DNS and syringaldazine assays for carbohydrases and laccases respectively (Table 2). The specific activity of each commercial formulation was also determined in the presence of surfactants at a concentration of 5% (weight to volume) to test the effect on enzyme activity (Table 2). Addition of Triton X-100 and Brij58 showed a statistical significant negative effect on α-amylase activity when compared with the activity with no surfactant. No statistically significant difference was observed for cellulase and xylanase activities in the presence of each surfactant.
|
Enzymatic treatment of dags
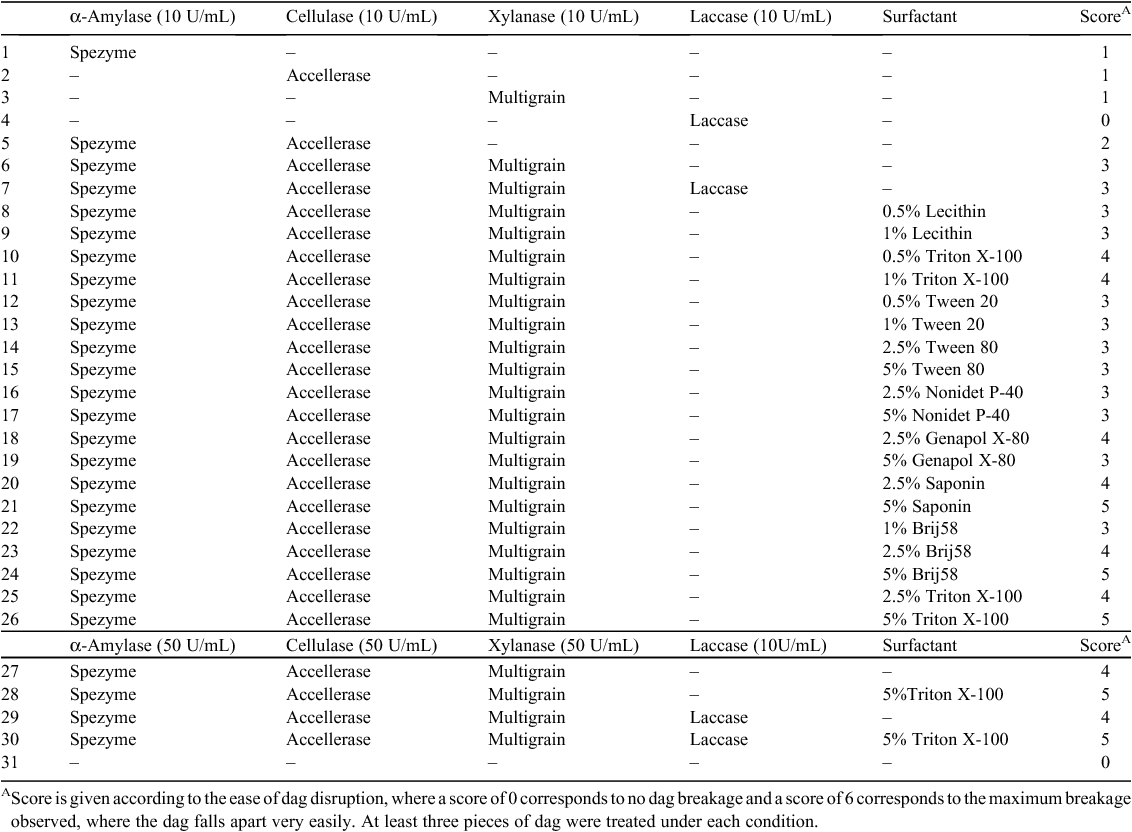
Dag samples were treated with single enzymes or combinations of enzymes according to Table 3. The effect on dag decomposition by enzymes was initially evaluated by spatula testing, with an objective score from 1 to 6 given according to the observed increasing ease of disruption. The addition of the individual enzymes Spezyme LT 300, Accellerase 1500 or Ronozyme Multigrain that display α-amylase, cellulase (endoglucanase and β-glucosidase) or xylanase and β-glucanase activities respectively, had an effect on dag decomposition when compared with the control sample. However, the combination of enzymes had a greater effect on decomposition; the best results were observed when the three enzymes Spezyme LT 300, Accellerase 1500 or Ronozyme Multigrain were used in combination in Treatment 6 (Fig. 1b , Table 3). The addition of laccase to this combination did not appear to improve dag decomposition in Treatment 7 (Fig. 1b , Table 3).
|
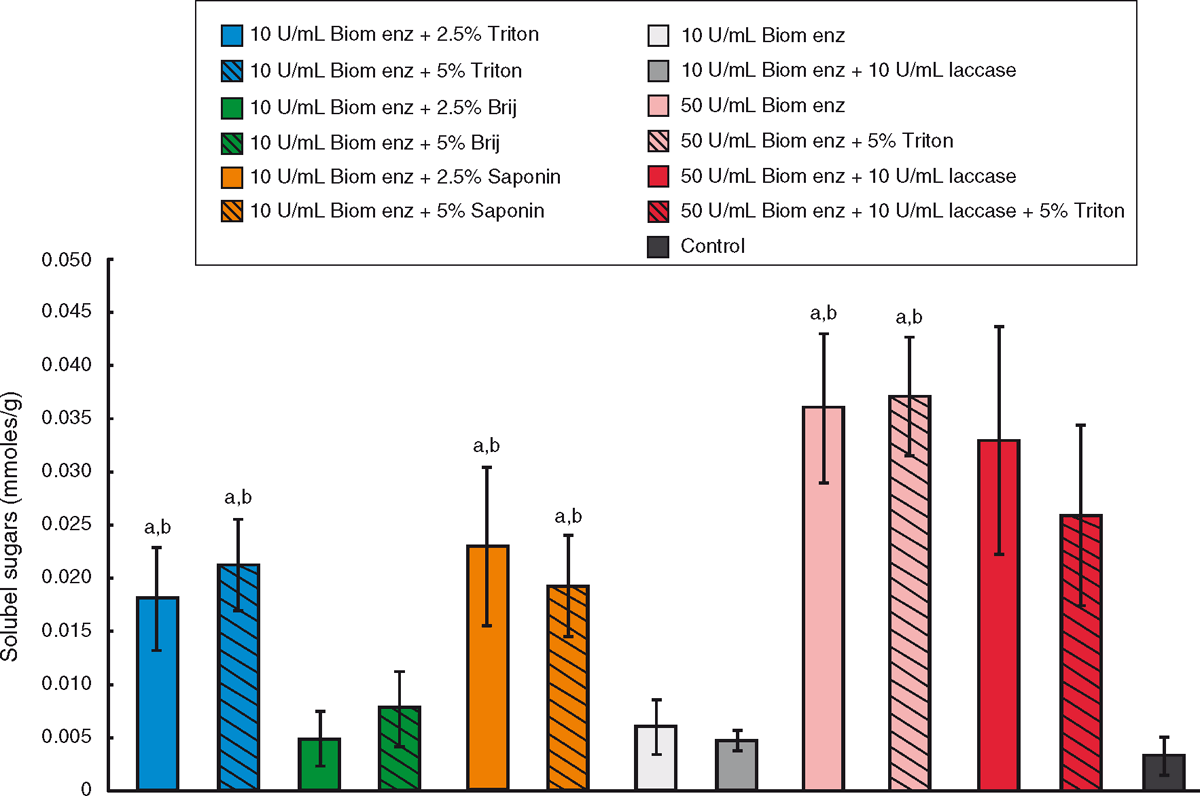
Following these results, dags were treated with a combination of enzymes and surfactants (Table 3). An improved degradation effect was observed when Triton X-100, Saponin or Brij58 surfactants were added to the mixture. These samples became muddier and fell apart more easily than dag samples treated with enzymes only (Fig. 1, Table 3). These results suggest the addition of surfactants could be aiding the permeabilisation of the dag structure, facilitating entry of the enzymes and contact with their substrates.
Thirteen different treatments from the enzyme and surfactant combinations from Table 3 were analysed using the DNS protocol to test for enzymatic release of reducing sugars. Sugars in solution derived from the carbohydrate fraction of dag samples after enzyme treatment were measured as a quantitative indication of dag decomposition (Fig. 2). According to our results, the addition of Triton X-100 or Saponin to the enzyme treatment improved the release of sugars into solution. As enzymatic activity is not enhanced in the presence of surfactants (Table 2), this effect is likely to be due to the surfactant having a physical effect on the dag, such as increasing permeability. The presence of 10 U/mL of laccase enzyme did not improve the amount of sugars obtained. When 50 U/mL of each enzyme was used for dag treatment the amount of sugars in solution increased when compared with treatment with 10 U/mL of each enzyme. The observed dag decomposition and the ease of disruption with a spatula however did not show an improvement. The spatula testing score given to dags treated with 10 U/mL or 50 U/mL of each enzyme was the same in both cases (Table 3). This result implies that the degradation of the carbohydrate/lignocellulose component of the dag may not be the key pathway towards structural weakening of the dag.
Analysis of enzymatic activity after dag treatment
Xylanase (Ronozyme Multigrain), celullase (Accellerase 1500 and Ronozyme Multigrain), α-amylase (Spezyme LT 300) and laccase activities were determined after dag treatment to test the stability of the enzymatic preparation during degradation (Table 4). Treatments with a combination of enzymes and 5% surfactant (Triton X-100, Saponin or Brij58) were selected for each activity determination.
A decrease in α-amylase and xylanase activities was observed after 16 h of dag treatment at room temperature (Table 4). α-Amylase and xylanase activities decreased 31% and 41%, respectively, after incubation without surfactant (Table 4). Cellulase activity did not appear to be affected under this condition. Laccase activity was not notably affected, retaining 90% of activity after dag treatment without surfactant. Furthermore, the presence of 5% surfactant (Triton X-100, Saponin or Brij58) did not have a marked effect on stability of the enzymes (Table 4).
Dag treatment with protease
The use of protease enzymes to attack the interaction between the hair and the dag and also degrade the feed protein component of the dag was investigated. Protease enzymes had not previously been specifically tested for dag degradation.
The commercial protease Ronozyme ProAct was obtained from DSM. In previous work, we had shown that this enzyme was very effective for keratin degradation and its activity can be improved in the presence of reducing agents (specific activity with no reducing agent 262 ± 5 × 103 U/g, in the presence of 1% sodium sulfite 338 ± 2 × 103 U/g or in the presence of 2% sodium thioglycolate 684 ± 60 × 103 U/g, where one keratinase unit is defined as an increase of 0.1 in absorption units at 595 nm after incubation with keratin azure for 1 h at 37°C) (Navone and Speight 2018).
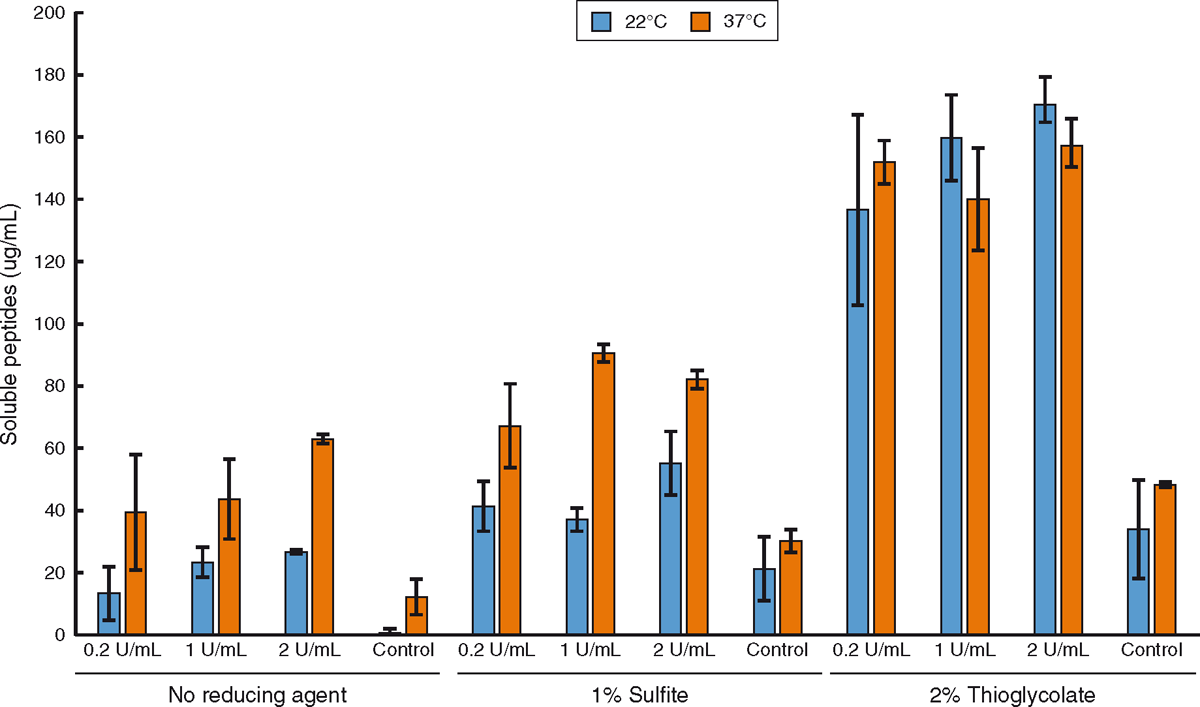
In this work, hair samples from cow hides were treated for 16 h with Ronozyme ProAct with or without the reducing agents 1% sodium sulfite or 2% sodium thioglycolate at 22°C and 37°C (Fig. 3). Ronozyme ProAct has been shown to be very effective on keratin degradation at 37°C (Navone and Speight 2018); however, lower temperatures had not been tested previously. This experiment was conducted to evaluate the degradation effect of Ronozyme ProAct at lower temperatures as the enzyme would likely be applied on farm during winter periods. The extent of hair degradation after enzymatic treatment in the presence of reducing agents was quantified by the measurement of released soluble peptides using the Bradford assay (Fig. 3). The decrease in temperature appeared to have little effect when samples were treated in the presence of 2% sodium thioglycolate implying that the rate of the enzyme catalysed reaction was not limiting. However, treatment at 22°C decreased keratin degradation compared with higher temperatures when no reducing agent was used or in the presence of 1% sodium sulfite. This decrease in activity at lower temperatures is characteristic of enzyme catalysed reactions. Each enzyme has a specific optimal temperature of activity. Moving above or below the optimal temperature will slow down the rate of catalysis in a way that is specific for each enzyme.
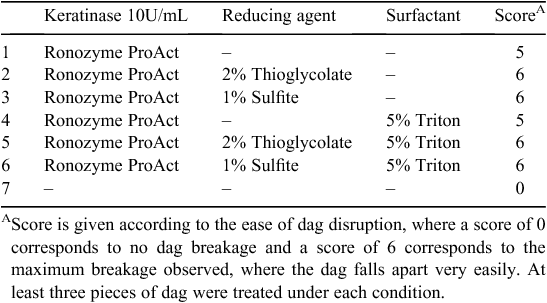
Following these results, dag samples were treated with Ronozyme ProAct with or without reducing agents according to Table 5. The effect on dag decomposition was evaluated by spatula testing, giving a score from 0 to 6 according to ease of disruption. Residual Ronozyme ProAct keratinase activity was determined after 16 h incubation at room temperature in the presence of 5% Triton X-100 and no changes in activity were observed implying that the enzyme is highly stable in the reaction conditions.
|
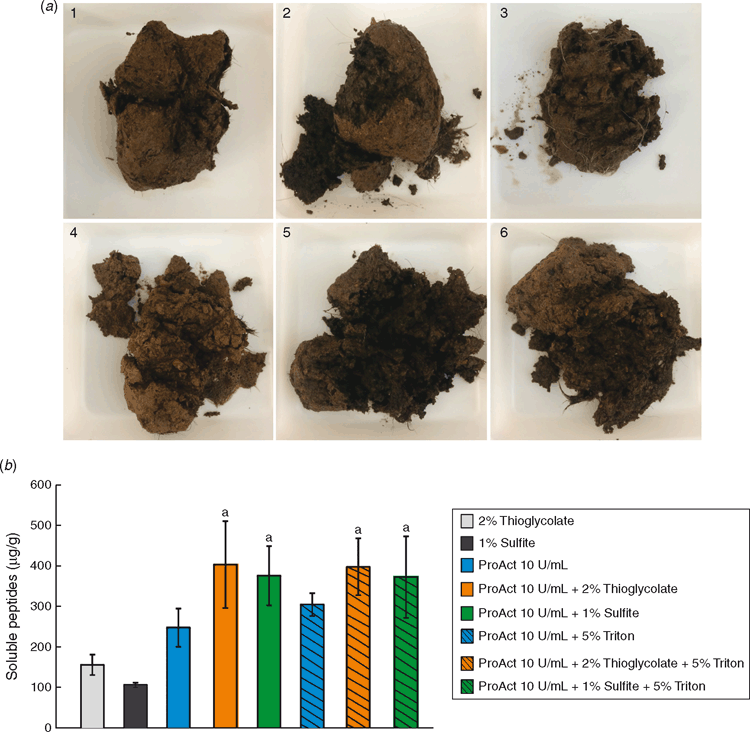
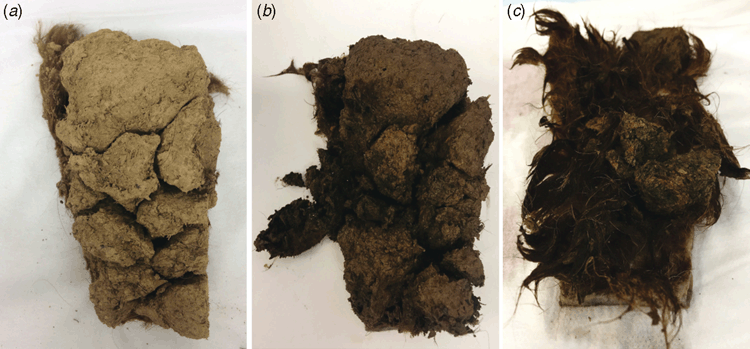
Dag decomposition was clearly evident after treatment with Ronozyme ProAct (Fig. 4a ). The presence of reducing agent further contributed to the degradation of the sample (Fig. 4a ). After 16 h of incubation, dag samples treated with keratinase and reducing agent fell easily apart into pieces during spatula testing. Hairs observed inside the dag appeared to be loose and unattached to the biomass.
Soluble peptide concentrations after dag treatments were measured using the Bradford assay (Fig. 4b ). As expected from the studies performed with cattle hair (Fig. 3) where the presence of reducing agent improved keratin degradation by Ronozyme ProAct, the addition of sodium sulfite or sodium thioglycolate improved the amount of soluble peptides after dag treatment. The addition of 5% Triton X-100 did not appear to further improve the release of peptides into solution. However, as the presence of surfactant improved dag decomposition when treatment was performed with biomass-degrading enzymes, presumably by aiding permeabilisation of the structure, its incorporation into a keratinase dag cleaning formulation was considered.
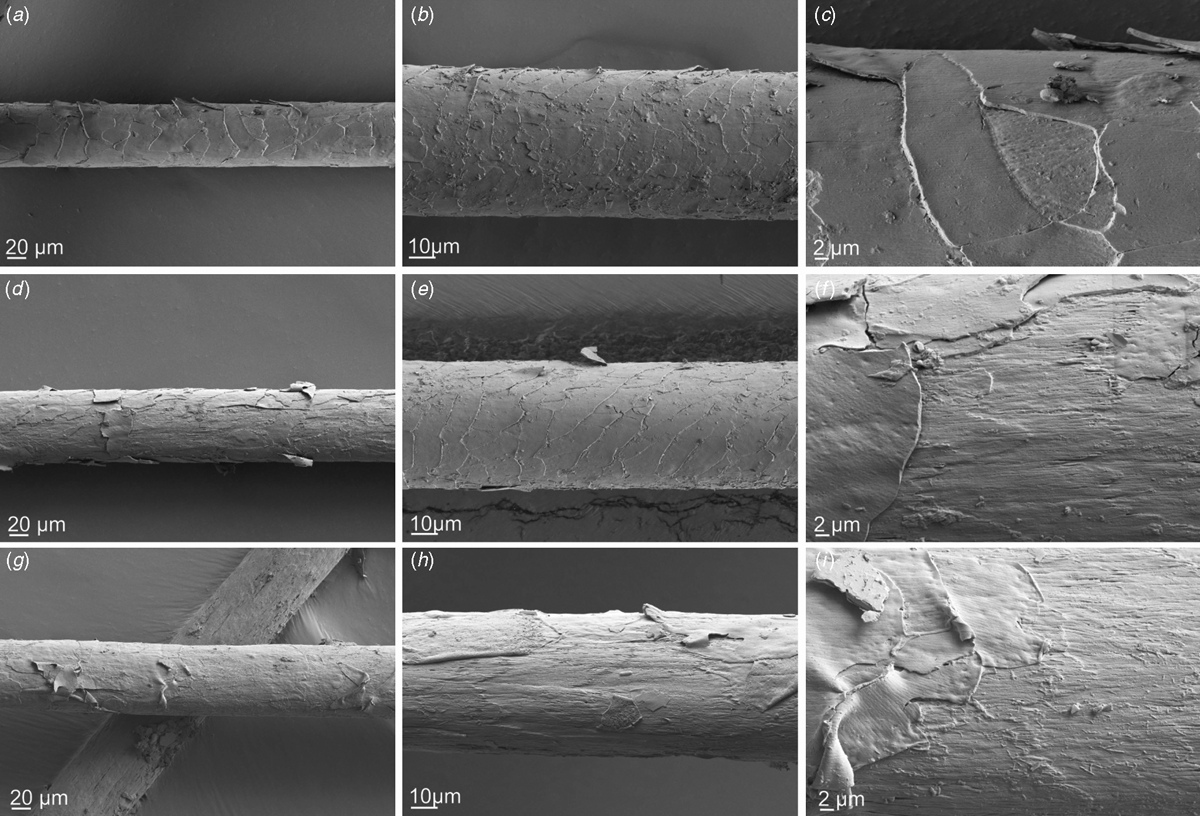
Hairs from the dag treatments with Ronozyme ProAct with and without reducing agents were collected from inside the structure after spatula testing and studied by scanning electron microscopy (Fig. 5). Hairs from dags treated with Ronozyme ProAct without reducing agent displayed cuticle lifting (Fig. 5a–c ), whereas hairs from dags treated with Ronozyme ProAct in the presence of 1% sodium sulfite displayed cuticle lifting and cortex degradation in some areas (Fig. 5d–f ). Hairs from dags treated with Ronozyme ProAct in the presence of 2% sodium thioglycolate were found to have more extensive cuticle lifting and cortex degradation in all areas (Fig. 5g–i ). This result clearly indicates that the enzymes are able to penetrate the dag in active form to aid dag deconstruction. Removal of the cuticle on the surface of the hair implies that the attachment between the dag and the hair (via the cuticle) has also been destroyed.
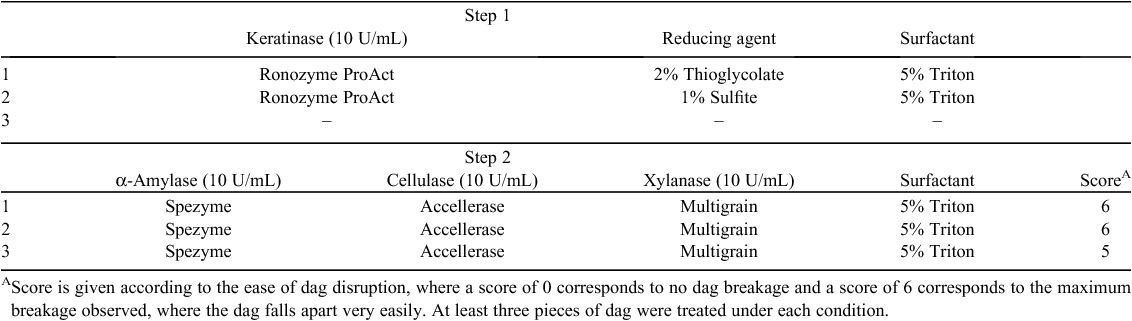
Dag samples were also treated in a two-step experiment, first with Ronozyme ProAct for 8 h and then with biomass-degrading enzymes for 16 h at room temperature (i.e. Spezyme LT 300, Accellerase 1500 and Ronozyme Multigrain) according to Table 6. Treatment with Ronozyme ProAct was performed at pH 10 with the two different reducing agents and both with 5% Triton X-100. The control sample was incubated with pH 10 buffer only. After 8 h the solution from each treatment was carefully removed and replaced with the combined biomass-degrading enzyme solution, again including 5% Triton X-100 at pH 5 and incubated for 16 h at room temperature. Enzymes were not added in a single step because of the proteolytic activity of Ronozyme ProAct that may degrade the other enzymes, and the requirement of different pH for optimal activity of the enzymes.
|
The treatment with biomass-degrading enzymes after protease/keratinase treatment in the presence of reducing agents did not appear to further improve the degree of dag degradation compared with the keratinase alone (Tables 5, 6). Soluble peptide concentrations were measured after step 1 and 2, with no further release of soluble peptides into solution observed after treatment with biomass-degrading enzymes (data not shown).
Hide treatment with keratinase
A small piece of hide (15 cm × 9 cm) with dags attached was treated with 10 U/mL Ronozyme ProAct in the presence of 2% sodium thioglycolate and 5% Triton X-100 (Fig. 6). After 16 h of treatment at room temperature, dags appeared loosely attached to the hide and easily came off with 3 min of low pressure water washing at the sink (Fig. 6c ).
Discussion
In this work we studied the dag decomposition ability of commercial enzymes for biomass degradation. Each of these enzymes was assayed for specific activity and applied in different combinations and concentrations to dag samples. Decomposition was assessed with spatula testing and quantified by measurement of enzyme-derived products in solution after treatment. Individual treatment of dag with biomass-degrading enzymes (α-amylase, cellulase and xylanase) had some effect on decomposition, however, the degree of breakdown was enhanced when these enzymes were applied in combination. From the spatula testing however it appeared that the extent of degradation was not sufficient for the reliable removal of dags from cattle. Higher concentrations of enzymes might be needed to archive this goal but the experiments showed that a 5-fold increase in enzyme, while increasing the amount of soluble sugars, did not increase dag degradation and would add additional costs. No suitable treatments, enzymatic or otherwise, currently exist to effectively remove dags from cattle. Previous enzymatic removal investigations applied research grade enzymes that were inefficient for dag removal compared with the current state of the art commercial enzymes tested here. Also, keratinases were not previously considered, nor was a formulation including other important additives like surfactants and reducing agents (Slattery et al. 2005; Cassells and Haritos 2009). Enzymes had been tested in UK conditions resulting in patentable technology (Covington and Evans 2003). Commercial application of the technology however has not been realised and the patents have been withdrawn.
In this study, the addition of surfactants to the enzymatic formulation appeared to facilitate the permeabilisation of the dag structure enhancing enzymatic degradation. Triton X-100 and Saponin showed the best results when compared with other surfactants. Saponin is a food grade surfactant that could be easily incorporated in an enzymatic formulation for animal applications (Oakenfull 1981).
An alternative approach for dag decomposition that consisted of targeting the interaction between the hair and the dag as well as any residual feed protein that may act like glue in the dag was also pursued in this work. Hair is mainly composed of the structural protein keratin. Keratins contain a high degree of disulfide bonding, which confers rigidity and chemical resistance. Keratinases capable of de-hairing cattle and goat hides have been reported (Huang et al. 2003; Vijayaraghavan et al. 2014; Khandelwal et al. 2015) and could be applied in a controlled fashion to dags to weaken or break the dag–hair interaction, allowing dag removal from the hides. It is also possible that protein from partially digested grains and rumen microbes in manure could act as an adhesive within the dag, binding the other components together. The enzymes tested in this work displayed both casein and keratin hydrolysis activities and so can degrade the protein component as well as hair keratin. To target the hair and grain protein component of the dag, Ronozyme ProAct, a commercial protease from DSM-Novozymes, previously shown in our laboratory to be very effective towards keratin breakdown (Navone and Speight 2018) was tested. Sodium sulfite or sodium thioglycolate were also included as reducing agents to the treatment of dag with keratinase. Sodium sulfite is a reducing agent used in some biological systems for keratin degradation (Grumbt et al. 2013), and sodium thioglycolate is commonly applied in hair cosmetics for hair waving and straightening (Wickett 1983; Williams and Daniels 1989; Makino 2009). In both cases the agents reduce disulfide bonds in keratin making the structure more amenable to enzyme hydrolysis.
Significant dag breakdown was found when a mixture of keratinase, reducing agent and surfactant was applied to dag samples. Similar dag decomposition results were observed using either sodium sulfite or sodium thioglycolate; enhanced results were observed with sodium thioglycolate during hair treatments at lower temperature (22°C). Sodium sulfite is a food grade compound and its inclusion in the formulation for animal applications might be straightforward from a regulatory perspective. It should be noted that Ronozyme ProAct is approved for use with livestock, being a registered food additive, as are most of the enzymes tested in this study.
The results suggest a possible hypothesis for dag formation and degradation. According to this hypothesis, the dag biomass (mainly lignocellulosic material) accumulates on a patch of hair fibres. The mixture of manure, soil, urine, straw and partially digested grains initially deposits on hide hairs and continues to build on layer after layer until the ball-like aggregate is formed. The long hair fibres within the dag are the scaffold that support the structure with the lignocellulose components held together by constituents in the dag that can act like a glue (sugars, starch and protein would all act in this way once dried). Given that amylase for starch degradation was relatively ineffective compared with protease/keratinase we suggest that the presence of feed protein contributes to the generation of an adhesive component that helps the layers of biomass adhere into a compact, recalcitrant dag aggregate. A Meat and Livestock Australia report detected 13–17% of protein and 10% starch components in dags (Slattery et al. 2005). Our results suggest that using proteases with keratinolytic activity to attack the hair fibre scaffold within the dag, the point of attachment between the hair and the dag, and also the adhesive protein component, breaks down the dag framework structure and facilitates decomposition and release. The addition of surfactants aids the permeation of the treatment solution and accessibility of the keratinase to internal hairs. The combination of keratinase and biomass-degrading enzymes did not seem to further improve dag breakdown. As the majority of the lignocellulose particles present in dags have been through animal digestion, they are already too small to provide structural strength and degrading them even further with biomass-degrading enzymes should not have a noticeable effect. The key strategy is therefore to attack the structural framework and the binding agent: the hairs and the feed protein component respectively.
Conclusion
Is this work, biomass-degrading enzymes and a protease with keratinolytic activity for dag decomposition and removal from cattle hides were evaluated. The treatment with enzymes targeting lignocellulosic material and starch had an effect on dag deconstruction, which was enhanced when the enzymes (cellulase, α-amylase and xylanase) were added in combination, however, variable results were observed. The treatment with keratinase showed an extensive degree of dag breakdown that was not further improved with a two-step treatment using keratinase and biomass-degrading enzymes. As a result of this work, we propose an enzymatic system for the decomposition of dags that involves a keratinase enzyme (Ronozyme ProAct), a reducing agent and a surfactant (patent application submitted by Meat and Livestock Australia). According to our results, 2% sodium thioglycolate or 1% sodium sulfite can be incorporated as reducing agent and 5% Triton X-100 or saponin as surfactant in a future formulation. Assessment of the proposed dag cleaning formulation on hide samples from more geographic areas with different feeding regimens to more fully assess the effect of dag variability (e.g. to assess changes in composition, protein type (e.g. wheat or sorghum) hardness and size) should be conducted in future studies. This work provides a solution to the problem of dag removal leading to improved animal welfare and reducing significant costs to the industry.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the Central Analytical Research Facility, operated by the Institute for Future Environments (QUT). Access to CARF is supported by funding from the Science and Engineering Faculty (QUT). This work was funded by Meat and Livestock Australia.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.| A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar |
Cassells, J, Haritos, V (2009) Assessment of an enzyme mixture for removal of dags from feedlot cattle. MLA Final Report B.FLT.0226. CSIRO Entomology, Canberra, ACT.
Covington AD, Evans CS (2003) Cleaning animal skins Patent WO 2003080873 A1.
Covington AD, Tozan M, Evans CS (1999) Enzymatic removal of dung from animal hides and skins. In ‘Proceedings of the XXV International Union of Leather Technologists and Chemists Congress, Chennai, India. pp. 355–361.
Grumbt M, Monod M, Yamada T, Hertweck C, Kunert J, Staib P (2013) Keratin degradation by dermatophytes relies on cysteine dioxygenase and a sulfite efflux pump. The Journal of Investigative Dermatology 133, 1550–1555.
| Keratin degradation by dermatophytes relies on cysteine dioxygenase and a sulfite efflux pump.Crossref | GoogleScholarGoogle Scholar |
Huang Q, Peng Y, Li X, Wang H, Zhang Y (2003) Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus. Current Microbiology 46, 0169–0173.
| Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus.Crossref | GoogleScholarGoogle Scholar |
Khandelwal HB, More SV, Kalal K, Laxman RS (2015) Eco-friendly enzymatic dehairing of skins and hides by C. brefeldianus protease. Clean Technologies and Environmental Policy 17, 393–405.
| Eco-friendly enzymatic dehairing of skins and hides by C. brefeldianus protease.Crossref | GoogleScholarGoogle Scholar |
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Research 2011, 1–10.
| Microbial cellulases and their industrial applications.Crossref | GoogleScholarGoogle Scholar |
Lange L, Huang Y, Busk PK (2016) Microbial decomposition of keratin in nature – a new hypothesis of industrial relevance. Applied Microbiology and Biotechnology 100, 2083–2096.
| Microbial decomposition of keratin in nature – a new hypothesis of industrial relevance.Crossref | GoogleScholarGoogle Scholar |
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Progress in Energy and Combustion Science 38, 449–467.
| Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects.Crossref | GoogleScholarGoogle Scholar |
Makino Y (2009) Reducing agent for straightening curly hair and process for straightening curly hair. Patent US 7635465 B2.
McKittrick J, Chen P-Y, Bodde S, Yang W, Novitskaya E, Meyers M (2012) The structure, functions, and mechanical properties of keratin. JOM 64, 449–468.
| The structure, functions, and mechanical properties of keratin.Crossref | GoogleScholarGoogle Scholar |
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31, 426–428.
| Use of dinitrosalicylic acid reagent for determination of reducing sugar.Crossref | GoogleScholarGoogle Scholar |
Mittal KL, Fendler EJ (Eds) (1982) ‘Solution behavior of surfactants. Theoretical and applied aspects.’ Vol. 1. (Plenum Press: New York)
Navone L, Speight R (2018) Understanding the dynamics of keratin weakening and hydrolysis by proteases. PLoS One 13, e0202608
| Understanding the dynamics of keratin weakening and hydrolysis by proteases.Crossref | GoogleScholarGoogle Scholar |
Oakenfull D (1981) Saponins in food – a review. Food Chemistry 7, 19–40.
| Saponins in food – a review.Crossref | GoogleScholarGoogle Scholar |
Riffel A, Brandelli A (2006) Keratinolytic bacteria isolated from feather waste. Brazilian Journal of Microbiology 37, 395–399.
| Keratinolytic bacteria isolated from feather waste.Crossref | GoogleScholarGoogle Scholar |
Siyal F, Babazadeh D, Wang C, Arain M, Saeed M, Ayasan T, Zhang L, Wang T (2017) Emulsifiers in the poultry industry. World’s Poultry Science Journal 73, 611–620.
| Emulsifiers in the poultry industry.Crossref | GoogleScholarGoogle Scholar |
Slattery, B, Davis, J, Carmody, B (2005) Use of enzymes for removing feedlot dags from the live animal. MLA Final Report FLOT.214. Rutherglen Research Institute, Rutherglen, Vic.
Vignardet C, Guillaume Y, Michel L, Friedrich J, Millet J (2001) Comparison of two hard keratinous substrates submitted to the action of a keratinase using an experimental design. International Journal of Pharmaceutics 224, 115–122.
| Comparison of two hard keratinous substrates submitted to the action of a keratinase using an experimental design.Crossref | GoogleScholarGoogle Scholar |
Vijayaraghavan P, Lazarus S, Vincent SGP (2014) De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: biosynthesis and properties. Saudi Journal of Biological Sciences 21, 27–34.
| De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: biosynthesis and properties.Crossref | GoogleScholarGoogle Scholar |
Wickett RR (1983) Kinetic studies of hair reduction using a single fiber technique. Journal of the Society of Cosmetic Chemists 34, 301–316.
Williams BW, Daniels PM (1989) Rearranger process and composition for permanent waving process. Patent US 4883657 A.